Abstract
Non-small-cell lung cancer (NSCLC) is one of the most common malignancies that is responsible for a high level of cancer-associated mortalities worldwide. Previous evidence has shown that Calotropin is an upstream activator of protein kinase B, which can further inhibit the growth and promote the apoptosis of NSCLC cells. In the present study, the efficacy of Calotropin on growth, aggressiveness and apoptosis of NSCLC cells was investigated, as well as the potential underlying mechanism. The results demonstrated that Calotropin inhibited H358 cell growth, migration and invasion. Flow cytometry assay showed that Calotropin promoted the apoptosis of H358 cells in vitro. Western blot analysis demonstrated that Calotropin inhibited fibronectin (FN), Vimentin (VIM) and E-cadherin (Eca) protein expression levels in H358 cells in vitro. In addition, Calotropin treatment upregulated pro-apoptosis gene expression, including caspase-3, caspase-8 and apoptotic protease activating factor-1, and downregulated anti-apoptosis gene expression, including P53, B-cell lymphoma (Bcl) 2 and Bcl-2-like protein 2 in H358 cells. The results also revealed that the expression levels of cytotoxic T-lymphocyte associated antigen 4 (CTLA-4) were decreased by Calotropin treatment in H358 cells. Analyses of the underlying mechanism indicated that Calotropin inhibited transforming growth factor-β (TGF-β) and extracellular signal-regulated kinase (ERK) expression. Overexpression of CTLA-4 inhibited Calotropin-mediated downregulation of TGF-β and ERK expression in H358 cells. In vivo assay revealed that Calotropin administration significantly inhibited tumor growth and prolonged animal survival over the 120-day observation period. Immunohistochemistry demonstrated that the number of apoptotic cells increased and the expression levels of CTLA-4 were decreased in the Calotropin-treated tumor group when compared with control. In addition, the expression levels of TGF-β and ERK were downregulated in the Calotropin-treated tumor group compared with control. In conclusion, the results of the present study indicated that Calotropin administration regulated NSCLC apoptosis by downregulating the CTLA-4-mediated TGF-β/ERK signaling pathway, suggesting that Calotropin may be a potential anti-cancer agent for the treatment of NSCLC.
Keywords: calotropin, non-small-cell lung cancer, apoptosis, cytotoxic T-lymphocyte associated antigen 4, transforming growth factor-β/extracellular signal-regulated kinase
Introduction
Non-small cell lung cancer (NSCLC) is one of the most common types of human cancers and is characterized by rapid growth, migration, invasion and reoccurrence (1,2). NSCLC includes adenocarcinoma, large cell carcinoma and squamous cell carcinoma, which is also the most frequent type of lung cancer that accounts for ~80% of whole lung cancer cases (3–5). Lung cancer is a respiratory disease that is the leading cause global cancer-associated mortalities due to poor air contamination caused by worldwide industrial pollution (6). Risk assessment of lung resection for lung cancer according to pulmonary function has been systematically reviewed (7). Despite the increasing number of therapeutic improvements for NSCLC proposed, the poor survival rate of patients with NSCLC was reported to be <15% over 5 years, which has caused a number of problems in clinical practice (8,9). Therefore, more efficient anti-NSCLC agents are required to explore alternative treatments for patients with NSCLC in preclinical and clinical trials.
Tumor apoptosis is a hallmark in the pathogenesis and treatment of patients with cancer (10). Calotropin is an active compound isolated from Asclepias curasavica L., which exerts strong inhibitory effects on cisplatin-induced resistance in NSCLC cells (11). A previous report has also revealed that Calotropin could inhibit the Wnt signaling pathway by increasing casein kinase 1a activity in colon cancer cells (12). A molecular mechanism study revealed that Calotropin regulated the apoptosis of tumor cells by inducing cell cycle arrest at the G2/M phase through decreasing the expression levels of cyclins, cyclin dependent kinase (CDK)-1 and CDK2 (11). In addition, cytotoxicity assays have indicated that Calotropin promoted caspase activation by downregulating the expression levels of anti-apoptotic proteins in K562 cells (13). These reports suggested that Calotropin may serve an important role in improving resistance via apoptosis in NSCLC cells.
In the present study, the inhibitory effects of Calotropin on the growth and aggressiveness of NSCLC cells were investigated. The efficacy of Calotropin for NSCLC cell apoptosis was analyzed in vitro and in vivo. The CTLA-4-mediated transforming growth factor-β (TGF-β)/extracellular signal-regulated kinase (ERK) signaling pathway was also studied in NSCLC cells following treatment with Calotropin. In vivo experiments revealed the inhibitory effects of Calotropin for tumor growth and survival rate by promoting the apoptosis of NSCLC cells. These results were suggestive of the important role of Calotropin in decreasing the CTLA-mediated TGF-β/ERK signaling pathway and also supported the strategy of potential anti-cancer drugs that target the TGF-β/ERK signaling pathway.
Materials and methods
Ethics statement
The present study was approved by the Ethics Committee of The Fourth People's Hospital of Guiyang (Guizhou, China), and was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of China (14). All surgical procedures and euthanasia were performed under IV sodium pentobarbital anesthesia (35 mg/kg), and all efforts were made to minimize suffering.
Cells culture
H358 cells were purchased from American Type Culture Collection (Manassas, VA, UA). H358 cells were cultured in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 3 mM L-glutamine, 50 µg/ml gentamicin (BioWhittakerä; Lonza Group, Ltd., Basal, Switzerland) and 1% penicillin/streptomycin. Cells were cultured at 37°C for 48 h with 5% CO2 until forming 90% confluence.
Reverse transcription-quantitative polymerase chain (RT-qPCR)
Total RNA was extracted from H358 cells (1×107) following treatment with Calotropin (0.50 mg/ml) for 48 h at 37°C using the RNAeasy Mini kit (Qiagen, Inc., Valencia, CA, USA). Total RNA (1 µg) was transcribed into cDNA at 37°C for 2 h using the QuantiNova Reverse Transcription kit (Qiagen, Inc.) and the quality was confirmed by electrophoresis. The cDNA (10 ng) was subjected to RT-qPCR using the SYBR Green Master Mix system (Bio-Rad Laboratories, Inc., Hercules, CA, USA). PCR amplification was preliminary denaturation at 95°C for 60 sec, followed by 45 cycles of 95°C for 30 sec, annealing at 58°C for 30 sec, and 72°C for 30 sec in a total volume of 20 µl containing 50 ng of genomic DNA, 200 µM dNTP, 2.5 units of Taq DNA polymerase, and 200 µM of each primer. All of the forward and reverse primers were synthesized by Invitrogen (Table I; Thermo Fisher Scientific, Inc.). Relative mRNA expression was calculated using the 2−ΔΔCq method (15) and the results are expressed as the n-fold way compared with the control.
Table I.
Sequences of primers used in the present study.
| Sequence | ||
|---|---|---|
| Gene name | Forward | Reverse |
| Bcl-w | 5′-AGCTCCTGCACCAGGAAAC-3′ | 5′-GCCAGCTCCACAGACATAAC-3′ |
| Bcl-2 | 5′-CGTCATAACTAAAGACACCCC-3′ | 5′-TTCATCTCCAGTATCCGACT-3′ |
| Caspase-3 | 5′-ATGGAGAACAACAAAACCTCAGT-3′ | 5′-TTGCTCCCATGTATGGTCTTTAC-3′ |
| Caspase-8 | 5′-CACTAGAAAGGAGGAGATGGAAAG-3′ | 5′-CTATCCTGTTCTCTTGGAGAGTCC-3′ |
| P53 | 5′-ATTTCACCCTTAAGATCCGTGGG-3′ | 5′-AGACTGGCCCTTCTTGGTCT-3′ |
| Apaf-1 | 5′-TATTGTGATATTGTTTTAAATTTGA-3′ | 5′-CAAAACATAACTAAACCTCAAAAACA-3′ |
| β-actin | 5′-ACGGTCAGGTCATCACTATCG-3′ | 5′-GGCATAGAGGTCTTTACGGATG-3′ |
Bcl-w, Bcl-2-like protein 2; Bcl-2, B-cell lymphoma 2; Apaf-1, apoptotic protease activating factor 1.
Overexpression of CTLA-4
H358 cells were cultured until 90% confluence and the media was then removed. Cells were then transfected with pedue12.4-CTLA-4 (100 pmol, Invitrogen; Thermo Fisher Scientific, Inc.) or pedue12.4-vector (control, 100 pmol, Invitrogen; Thermo Fisher Scientific, Inc.) using Lipofectamine 2000™ (Invitrogen; Thermo Fisher Scientific, Inc.). Overexpression was then assessed in H358 cells and those with stable CTLA-4-overexpression were selected for subsequent experiments using the Guided Screening system, as previously described (16). After 72-h transfection, protein expression levels of ERK1/2, phosphorylated (p)-ERK and TGF-β were analyzed in the two groups of cells by western blotting following treatment with Calotropin (0.50 mg/ml) for 48 h at 37°C.
MTT cytotoxicity assays
H358 cells were incubated with Calotropin (0.50 mg/ml) in 96-well plates for 24, 48 and 72 h at 37°C in triplicate for each condition; PBS was added instead of Calotropin as the control. At each time point, 20 µl of MTT (5 mg/ml) in PBS solution was added to each well, the plate was further incubated for 4 h at 37°C. The majority of the medium was then removed and 100 µl of dimethylsulfoxide was added into the wells to solubilize the crystals. The optical density (OD) was measured using a Bio-Rad Laboratories, Inc. ELISA reader at a wavelength of 450 nm.
Cell invasion and migration assays
H358 cells were treated with Calotropin (0.50 mg/ml) for 24 h at 37°C and non-treated cells were used as the control. The migration and invasion of H358 cells was evaluated using a 6-well culture plate with chamber inserts (BD Biosciences, San Jose, CA, USA). For migration assays, 1×104/well H358 cells were placed into the upper chamber with the non-coated membrane and DMEM medium containing 5% FBS was added into the lower chamber for 48 h at 37°C. For invasion assays, cells (1×104/well) were placed in the upper chamber of BD BioCoat Matrigel Invasion Chambers (BD Biosciences) for 48 h at 37°C. Cells were fixed with 10% paraformaldehyde for 30 min at 37°C and stained with 0.5% crystal violet (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 30 min at 37°C. The migration and invasion of H358 cells was assessed by counting the number of cells in at least three randomly stained microscope fields for every membrane under a light microscope (magnification, ×40).
Flow cytometry analysis
H358 cells were cultured until 90% confluence was reached. Apoptosis was assessed following H358 cell incubation with Calotropin (0.50 mg/ml) for 48 h at 37°C. H358 cells were trypsinized using 1% trypsin for 10 min at 37°C and collected following incubation. The cells were then washed in cold PBS, adjusted to 1×106 cells/ml with PBS, labeled with an Annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) kit (BD Bioscience), and analyzed with a FACScan flow cytometer (BD Bioscience). Data was analyzed using a FlowJo software (version 8.0.2; FlowJo LLC, Ashland, OR, USA).
Western blot analysis
H358 cells were treated with Calotropin (0.50 mg/ml) for 24 h. The cells (1×106) were harvested, lysed in Radioimmunoprecipitation Assay buffer and then homogenized at 4°C for 10 min. Protein concentration was measured by a BCA protein assay kit (Thermo Scientific, Pittsburgh PA, USA). Protein (10 µg) was separated on 12% SDS-PAGE assays followed by protein transfer onto PVDF membranes (EMD Millipore, Billerica, MA, USA). Membranes were blocked with 5% BSA at 4°C for 12 h and then incubated with rabbit anti-mouse ERK1/2 (1:1,000, cat. no. ab93125), fibronectin (FN; 1:1,000, cat. no. ab2413), Vimentin (VIM; 1:1,000, cat. no. ab92547), E-cadherin (Eca; 1:1,1000, cat. no. ab6528), caspase-3 (cap-3; 1:1,000, cat. no. ab13585), B-cell lymphoma 2 (Bcl-2; 1:500, cat. no. ab32124), Bcl-2-like protein 2 (Bcl-w; 1:500, cat. no. ab38629), caspase-8 (cap-8; 1:1,000, cat. no. ab25901), cyclins (1:1,000, cat. no. ab74632), CDK1 (1:1,000, cat. no. ab18), CDK2 (1:1,000, cat. no. ab32147), P53 (1:1,000, cat. no. ab1101), cytochrome c (Cyto c; 1:1,000, cat. no. ab13575), c-Jun N-terminal kinase (JNK; 1:1,000, cat. no. ab176662) and TGF-β (1:1,000, cat. no. ab31013; all Abcam, Shanghai, China) for 12 h at 4°C. The membrane was then incubated with HRP-conjugated goat anti-rabbit IgG (1:2,000; ab6721; Abcam) at 4°C for 2 h. Analysis of protein expression was then performed using a chemiluminescence detection system. The density of the bands was analyzed by Quantity one software version 4.62 (Bio-Rad Laboratories, Inc.).
Animal study
A total of 80 specific pathogen-free female nude (6–8 weeks old, weight 30–35 g) mice were purchased from SLAC, Shanghai Laboratory Animal Center Co., Ltd. (Shanghai, China). All mice were housed at 23±1°C and relative humidity of 50±5% with a 12-h light/dark cycle. All were provided with free to access food and water. Mice were subcutaneously injected with H358 cells (1×108 cells) and were divided into 2 groups (n=40/group). Treatments commenced 6 days following tumor implantation once the tumor diameter had reached 5–8 mm. Mice were then intravenously injected every day for 7 days with Calotropin (5.0 mg/kg); PBS injections served as the control. Data for short term tumor volume was recorded for a total of 25 days. The tumor volumes were calculated as previously described (17). The survival rates of mice were assessed over 120 days' observation.
Immunohistochemistry
Tumor xenografts were excised from mice following 7 days of treatment with Calotropin or PBS, fixed using 10% formaldehyde for 30 min at 37°C, dehydrated, embedded in paraffin wax and then cut into serial sections of 4-µm thickness. The sections were de-paraffinized in xylene and then antigen retrieval was performed in tumor sections using Antigen Retrieval Buffer (100X Tris-EDTA Buffer, pH 9.0; ab93684). Tumor sections were blocked with 5% BSA (Sigma-Aldrich; Merck KGaA) and then incubated with rabbit anti-mouse cluster of differentiation (CD)-3 (1:1,000, cat. no. ab16044; Abcam) and CD8 (1:1,000, cat. no. ab22378; Abcam), cap-3 (1:1,000, cat. no. ab25901; Abcam) or cap-8 (1:1,000, cat. no. ab13585; Abcam) primary antibodies for 12 h at 4°C. Then, a horseradish peroxidase-conjugated polyclonal anti-goat IgG (1:2,000, ab150077, Abcam) was used to incubate samples for 1 h at room temperature. A Ventana Benchmark automated staining system was used for analyzing protein expression (Bio-Rad Laboratories, Inc.). Images were observed using a fluorescence microscope (Leica Microsystems GmbH, Wetzlar, Germany). Images were captured on MicroChemi version 4.2 (https://dnr-is.com).
Terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end labeling (TUNEL) assay
TUNEL staining was performed to analyze apoptotic cells in tumor tissues using the ApopTag kit (EMD Millipore) according to the manufacturer's instructions. Briefly, paraffin tumor sections were treated with immunohistochemistry for BrdU (dilution 1:1,000; Sigma-Aldrich; Merck KGaA) as described previously (18). Statistical quantification of TUNEL-positive tumor cells was calculated to analyze the efficacy of Calotropin for inhibition of tumor growth in three randomly select fields.
Analysis of lymphocytes infiltration
Tumor tissue was removed, fixed in 10% formalin for 30 min at 37°C and embedded in paraffin to generate tumor sections (4 µm). Sections were stained with hematoxylin and eosin for 2 h at 37°C, and then examined under a light microscope.
Cytotoxic T lymphocyte (CTL) assay
On day 25, tumors were excised and splenocytes were isolated from experimental mice following 7 days of calotropin treatment and used as effector cells following restimulation with concanavalin A and mixture of heat shock protein/peptides in vitro for 4 days at 37°C. A lactate dehydrogenase assay was used to analyze the anti-tumor-specific CTL response to H358 cells. H358 cells (target cells) were seeded in 96-well plates. Pierce™ LDH Cytotoxicity Assay Kit (cat no. 88953; Thermo Fisher Scientific, Inc.) was used to analyze CTL. The simulative splenocytes were plated in 96-well plates in triplicate with varying effector cell:target cell ratios of 5:1, 10:1 and 15:1. Following 4 h incubation at 37°C, 150 µl supernatant was obtained using centrifugation at 4,000 × g for 10 min at room temperature and then analyzed in a Well scan at OD 490 nm (Bio-Rad Laboratories, Inc.). The effect of CTL was calculated as follows: Percentage cytotoxicity (%)=100× [(experimental release-spontaneous release)/(maximum release-spontaneous release)].
ELISA
The expression levels of TGF-β in calotropin-treated cells were analyzed with a Mouse TGF-beta 1 DuoSet ELISA kit (cat. no. DY1679; Bio-Rad Laboratories, Inc.), according to the manufacturer's instructions. The results were analyzed using an ELISA reader system (Bio-Rad Laboratories, Inc.).
Statistical analysis
Data are presented as the mean ± standard error mean of triplicate experiments. SPSS software version 17.0 (SPSS Inc., Chicago, IL, USA) was used for all data analyses. Unpaired data was determined by a Student's t test and comparisons between multiple groups were assessed via one-way analysis of variance followed by Dunnett's test. *P<0.05 was considered to indicated a statistically significant difference.
Results
Analysis of the effects of Calotropin on NSCLC cell growth, migration and invasion in vitro
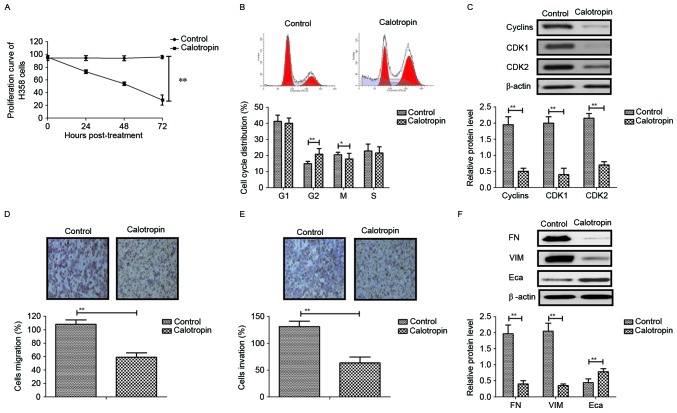
As shown in Fig. 1A, Calotropin (0.50 mg/ml) markedly suppressed H358 cell growth in a time-dependent manner compared with the control. Cell cycle experiments demonstrated that Calotropin arrested H358 cells at the G2/M phase (Fig. 1B). Western blot analysis revealed that Calotropin significantly decreased the expression levels of cyclins, CDK1 and CDK2 (Fig. 1C). The migration and invasion assays demonstrated that Calotropin markedly inhibited the aggressiveness of H358 cells (Fig. 1D and E). Western blotting observed that Calotropin inhibited FN and VIM, and promoted Eca protein expression levels in H358 cells in vitro (Fig. 1F). These results suggested that Calotropin may significantly inhibit NSCLC cell growth, migration and invasion in vitro.
Figure 1.
Effects of Calotropin (0.50 mg/ml) on NSCLC cell growth, migration and invasion in vitro. (A) Calotropin suppressed H358 cell growth in a time-dependent manner following 72 h post-treatment. (B) Calotropin arrested H358 cells at the G2/M phase. (C) Calotropin inhibited the protein expression levels of cyclins, CDK1 and CDK2 in H358 cells. Calotropin inhibited the (D) migration and (E) invasion of H358 cells. (F) Calotropin (0.50 mg/ml) also inhibited the protein expression levels of FN, VIM and Eca in H358 cells. Magnification, ×40. Data are presented as the mean ± standard error mean. *P<0.05 and **P<0.01, as indicated. NSCLC, non-small-cell lung cancer; CDK, cyclin-dependent kinase; FN, fibronectin; VIM, vimentin; Eca, E-cadherin.
Analysis of the effects of Calotropin on NSCLC cell apoptosis in vitro
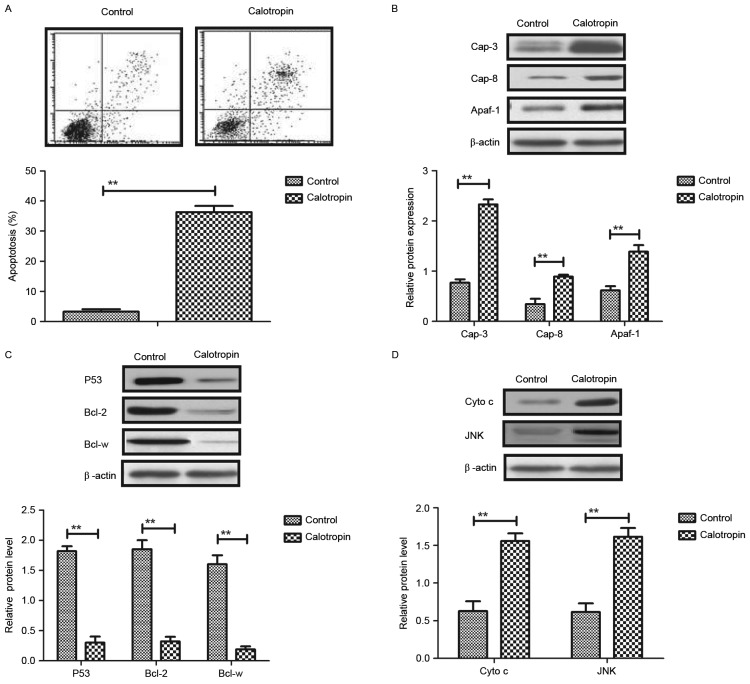
As shown in Fig. 2A, Calotropin significantly promoted the apoptosis of H358 cells detected by Annexin V-FITC and PI staining using flow cytometry analysis. Western blotting demonstrated that Calotropin treatment upregulated the pro-apoptosis protein expression levels of caspase-3, caspase-8 and apoptotic protease activating factor (Apaf)-1 in H358 cells (Fig. 2B). However, the anti-apoptosis protein expression levels of P53, Bcl-2 and Bcl-w were downregulated by Calotropin in H358 cells (Fig. 2C). Cyto c and JNK expression levels are associated with the apoptosis of lung tumor cells (19,20). Therefore, the present study investigated Cyto c and JNK expression levels in H358 cells following treatment with Calotropin. The results demonstrated that Cyto c and JNK protein expression levels were upregulated in Calotropin-treated H358 cells (Fig. 2D). These results indicated that Calotropin promoted the apoptosis of H358 cells via the upregulation of caspase-3, caspase-8 and apoptotic protease activating factor (Apaf)-1 expression.
Figure 2.
Effects of Calotropin (0.50 mg/ml) on NSCLC cell apoptosis in vitro. (A) Calotropin promoted the apoptosis of H358 cells, as detected by Annexin V-fluorescein isothiocyanate and propidium iodide staining, and flow cytometry analysis. (B) Calotropin promoted the protein expression levels of caspase-3, caspase-8 and Apaf-1 in H358 cells. (C) Calotropin treatment inhibited the protein expression levels of P53, Bcl-2 and Bcl-w in H358 cells. (D) Calotropin promoted the protein expression levels of Cyto c and JNK. Data are presented as the mean ± standard error mean. **P<0.01, as indicated. NSCLC, non-small-cell lung cancer; Cap, caspase; Apaf-1, apoptotic protease activating factor 1; Bcl-2, B-cell lymphoma 2; Bcl-w, Bcl-2-like protein 2; Cyto c, cytochrome c; JNK, c-Jun N-terminal kinase.
Analysis of the effects of Calotropin on CTLA expression and the TGF-β/ERK signaling pathway in NSCLC cells
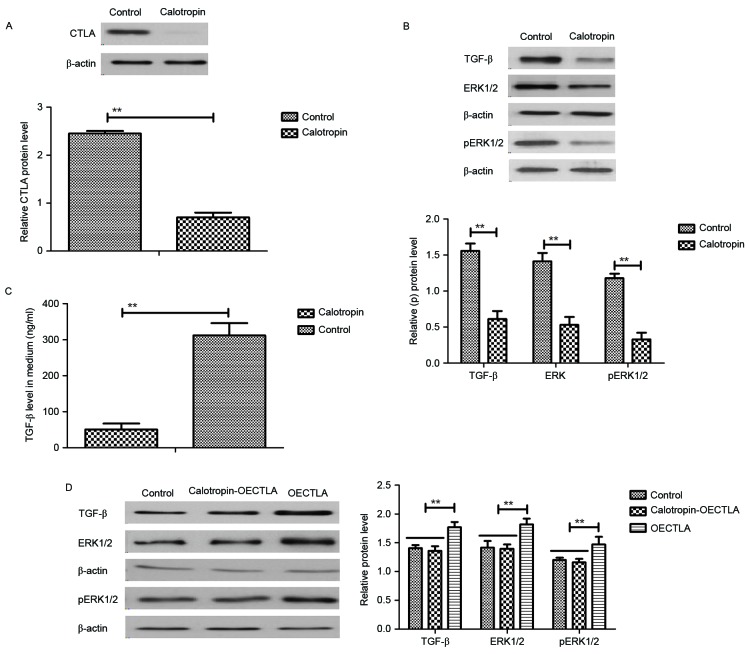
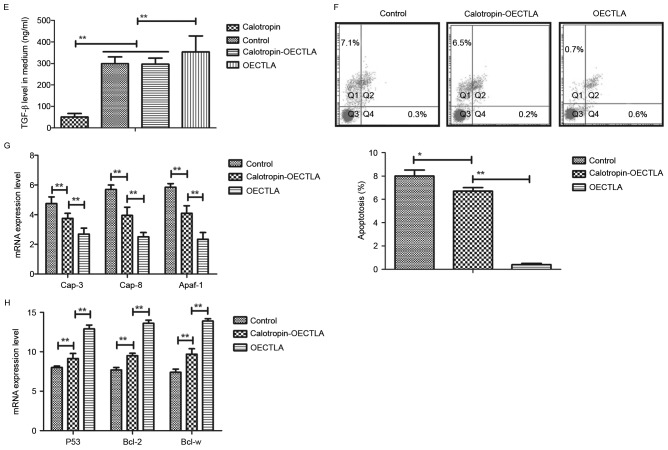
In order to analyze the inhibitory effects of Calotropin on the apoptosis of H358 cells, the present study investigated CTLA expression and the TGF-β/ERK signaling pathway. The results demonstrated that Calotropin decreased the CTLA expression levels in H358 cells following 24 h incubation (Fig. 3A). In addition, TGF-β and ERK1/2 expression levels as well as ERK1/2 phosphorylation were downregulated by Calotropin treatment in H358 cells (Fig. 3B). Calotropin treatment decreased the extracellular TGF-β contents in H358 cell media (Fig. 3C). It was also revealed that overexpression of CTLA (OECTLA) promoted TGF-β and ERK1/2 protein expression as well as ERK1/2 phosphorylation, and inhibited the downregulation of TGF-β and ERK1/2 stimulated by Calotropin in H358 cells (Fig. 3D). Extracellular TGF-β contents levels of TGF-β were also increased by OECTLA in H358 cells (Fig. 3E). Flow cytometry analysis also indicated that OECTLA inhibited Calotropin-induced H358 cell apoptosis (Fig. 3F). The results demonstrated that OECTLA ameliorated the Calotropin-mediated upregulation of caspase-3, caspase-8 and Apaf-1 mRNA levels (Fig. 3G), and downregulation of P53, Bcl-2 and Bcl-w mRNA levels in H358 cells (Fig. 3H). These results indicated that Calotropin may induce apoptosis through the CTLA-mediated TGF-β/ERK signaling pathway.
Figure 3.
Effects of Calotropin (0.50 mg/ml) on CTLA expression and the TGF-β/ERK signaling pathway in NSCLC cells. (A) Calotropin decreased the CTLA protein expression levels in H358 cells. (B) Calotropin inhibited the protein expression levels of TGF-β, as well as ERK1/2 protein expression and phosphorylation in H358 cells. (C) Calotropin treatment decreased the extracellular TGF-β contents in the cell media of H358 cells. (D) OECTLA inhibited the Calotropin-mediated downregulation of TGF-β protein expression, and ERK1/2 protein expression and phosphorylation in H358 cells. (E) OECTLA also abolished the Calotropin-induced inhibition of TGF-β secretion in the cell media of H358 cells. (F) OECTLA inhibited Calotropin-induced H358 cell apoptosis. (G) OECTLA inhibited Cap-3, Cap-8 and Apaf-1 mRNA levels in H358 cells. (H) OECTLA promoted P53, Bcl-2 and Bcl-w mRNA levels in H358 cells. Data are presented as the mean ± standard error mean. *P<0.05 and **P<0.01, as indicated. CTLA, cytotoxic T-lymphocyte associated antigen 4; NSCLC, non-small-cell lung cancer; TGF-β, transforming growth factor-β; ERK, extracellular signal-regulated kinase; p-, phosphorylated; OECTLA, overexpression of CTLA; Cap, caspase; Apaf-1, apoptotic protease activating factor 1; Bcl-2, B-cell lymphoma 2; Bcl-w, Bcl-2-like protein 2.
In vivo anti-cancer efficacy of Calotropin on NSCLC-bearing mice
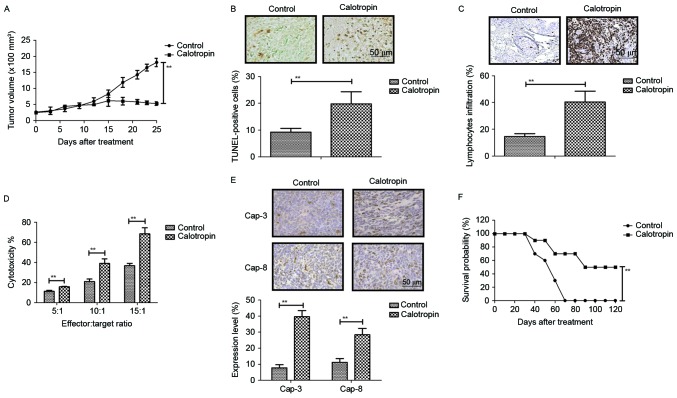
The present study investigated the anti-tumor effects of Calotropin in H358-bearing mice. The data demonstrated that Calotropin administration markedly inhibited tumor growth during the 25-day short term observation period when compared with PBS-treated mice (Fig. 4A). It was also observed that the number of apoptotic cells was increased in Calotropin-treated tumors (Fig. 4B). Immunohistochemistry revealed that the amount of lymphocyte infiltration was upregulated in tumors following treatment with Calotropin (Fig. 4C). CTL immune response targeting of H358 cells showed that Calotropin promoted the CTL response in H358-bearing mice (Fig. 4D). Immunohistochemistry for caspase-3 and caspase-8 revealed that Calotropin significantly promoted these tumor-apoptosis markers in experimental mice (Fig. 4E). Long-term observation demonstrated that Calotropin significantly prolonged the survival of H358-bearing mice (Fig. 4F). These results suggested that Calotropin may be a potential anti-cancer drug for NSCLC.
Figure 4.
In vivo anti-cancer efficacy of Calotropin (5.0 mg/kg) on NSCLC-bearing mice. (A) Calotropin administration significantly inhibited tumor growth over a 25-day short term observation period. (B) Calotropin administration increased the number of apoptotic cells in Calotropin-treated tumors. (C) Calotropin administration also increased lymphocyte infiltration in tumors excised from NSCLC-infected mice. (D) Calotropin promoted the CTL immune response of target H358 cells. (E) Calotropin promoted the expression levels of Cap-3 and Cap-8 in the tumors excised from NSCLC-bearing mice. Magnification, 40×. (F) Calotropin treatment also prolonged the survival of mice injected with NSCLCs over the 120-day observation period. Data are presented as the mean ± standard error mean. **P<0.01, as indicated. NSCLC, non-small-cell lung cancer; CTL, cytotoxic T-lymphocyte; Cap, caspase; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling.
Discussion
Lung cancer is a major public health problem and is the leading cause of cancer-associated mortality in the world (21). Clinical investigation has revealed that NSCLC is accountable for ~95% of all lung cancer cases that result in a high incidence and mortality rate (22). Systematic review and meta-analysis have demonstrated that drug-induced apoptosis contribute to the inhibition of tumor cell growth and aggressiveness (23). In addition, it has been previously reported that Calotropin extracted from Asclepias curassavica induces cell cycle arrest and apoptosis in cisplatin-resistant lung cancer cells (11). In the present study, the efficacy of Calotropin on the inhibition of growth and aggressiveness of NSCLC cells was investigated. The pro-apoptosis capacity and potential mechanism underlying Calotropin-mediated apoptosis in NSCLC cells were also analyzed. The results demonstrated that Calotropin inhibited H358 cell growth, migration and invasion by decreasing FN and VIM, and increasing Eca expression levels (24,25). It was also revealed that Calotropin treatment promoted apoptosis by upregulating pro-apoptosis gene and downregulating anti-apoptosis protein expression. In vivo assay showed that Calotropin administration significantly inhibited tumor growth and prolonged animals survival over the 120-day observation period.
CTLA-4 is a multifunctional protein that serves an important role in the delivery of signaling molecules during cell communication processes (26,27). Currently, CTLA-4 expression represents the initial activation checkpoint in molecular terms and is regarded as an essential regulator of self-reactivity in the immune system, which has exerted anti-tumor effects in the treatment of human colon cancer (28). In addition, Klyushnenkova et al (26) have demonstrated that a CTLA-4 blockade prevents immune tolerance by targeting CD25+ regulatory T cells in a human leukocyte antigen-antigen D related transgenic mouse model of prostate cancer. Furthermore, the CTLA-4 blockade stimulates T cell activation, which has been identified to have an essential role in neoplasm regression in the adaptive immune system by generating natural killer cells and CTL responses targeting tumor cells (29). Li et al (30) have proposed that the anti-tumor immunological responses may be achieved through anti-CTLA-4 antibody incubation in a prostate cancer murine model. In the present study, the results revealed that Calotropin administration significantly downregulated CTLA-4 expression in NSCLC cells. These findings suggested that Calotropin administration significantly downregulated CTLA-4 through the TGF-β/ERK signaling pathway in NSCLC cells and tumor tissues.
The TGF-β signaling pathway is an essential regulator of a number of cellular processes involved in carcinogenesis, by regulating the genetic variation of runt-related transcription factor (RUNX)-1, RUNX2, RUNX3, mitogen-activated protein kinase 1 and eukaryotic translation initiation factor 4E (31,32). TGF-β is also regarded as a T cell regulator in colon cancer and may suppress tumor progression in colon cancer (33,34). In addition, previous studies have demonstrated that the anticancer activity of buttermilk against sw480 colon cancer cells is associated with caspase-independent cell death via the attenuation of the Wnt, protein kinase B (AKT) and ERK signaling pathways, and the reactivation of ERK and AKT confers the apoptotic resistance of colon cancer cells (35,36). Furthermore, a previous study also revealed that growth suppression in colon cancer cells is associated with RAS/ERK by bone morphogenetic protein-mothers against decapentaplegic homolog signaling (37). A number of proteins exert divergent effects in the colon mucosa through the regulation of the ERK/Ras-related C3 botulinum toxin substrate/JNK-dependent signaling pathways in colon cancer cells (38). The results of the present study revealed that Calotropin treatment decreased CTLA-4 expression levels in H358 cells. Mechanistic analyses indicated that Calotropin inhibited TGF-β and ERK expression levels, which further led to tumor inhibition and the prolonged survival of tumor-bearing mice.
In conclusion, the results of the present study demonstrated that Calotropin treatment upregulated pro-apoptosis gene expression, including caspase-3, caspase-8 and Apaf-1; however, it downregulated anti-apoptosis gene expression, including P53, Bcl-2 and Bcl-w in H358 cells, which is in agreement with previous reports (39,40). It was also indicated that overexpressing CTLA-4 abolished the Calotropin-mediated downregulation of TGF-β and ERK expression in H358 cells. Notably, it was revealed that the TGF-β/ERK signaling pathway may be involved in the Calotropin-mediated apoptosis of NSCLC cells, which also inhibited tumor growth and prolonged the survival of NSCLC-bearing mice. These results indicated that Calotropin may suppress the growth, aggressiveness and apoptosis of NSCLC cells by regulating metastasis-associated genes and the CTLA-4-mediated TGF-β/ERK signaling pathway, which provides a potential therapeutic strategy for the treatment of NSCLC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
Authors' contributions
LT performed the experiments. XX analyzed all the data from the experiments. ZZ designed the experiments for the present study. All authors read and approved the final paper.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of The Fourth People's Hospital of Guiyang (Guizhou, China), and was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of China.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Paesmans M, Grigoriu B, Ocak S, Roelandts M, Lafitte JJ, Holbrechts S, Berghmans T, Meert AP, Moretti L, Danyi S, et al. Systematic qualitative review of randomised trials conducted in nonsmall cell lung cancer with a noninferiority or equivalence design. Eur Respir J. 2015;45:511–524. doi: 10.1183/09031936.00092814. [DOI] [PubMed] [Google Scholar]
- 2.D'Antonio C, Milano A, Righini R, Onesti CE, Bassanelli M, Falcone R, Paris I, Lauro S, Marchetti P. Pharmacogenomics in lung cancer chemotherapy: A review of what the oncologist should know. Anticancer Res. 2014;34:5241–5250. [PubMed] [Google Scholar]
- 3.Brody H. Lung cancer. Nature. 2014;513:S1. doi: 10.1038/507S1a. [DOI] [PubMed] [Google Scholar]
- 4.Moro-Sibilot D, Smit E, de Castro Carpeño J, Lesniewski-Kmak K, Aerts JG, Villatoro R, Kraaij K, Nacerddine K, Dyachkova Y, Smith KT, et al. Non-small cell lung cancer patients with brain metastases treated with first-line platinum-doublet chemotherapy: Analysis from the European FRAME study. Lung Cancer. 2015;90:427–432. doi: 10.1016/j.lungcan.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Barnett SA, Downey RJ, Zheng J, Plourde G, Shen R, Chaft J, Akhurst T, Park BJ, Rusch VW. Utility of routine PET imaging to predict response and survival after induction therapy for non-small cell lung cancer. Ann Thorac Surg. 2016;101:1052–1059. doi: 10.1016/j.athoracsur.2015.09.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fenton-Ambrose L, Kazerooni EA. Preventative care: Lung-cancer screens now worth the cost. Nature. 2014;514:35. doi: 10.1038/514035b. [DOI] [PubMed] [Google Scholar]
- 7.Sawabata N, Nagayasu T, Kadota Y, Goto T, Horio H, Mori T, Yamashita S, Iwasaki A. Risk assessment of lung resection for lung cancer according to pulmonary function: Republication of systematic review and proposals by guideline committee of the Japanese association for chest surgery 2014. Gen Thorac Cardiovasc Surg. 2015;63:14–21. doi: 10.1007/s11748-014-0475-x. [DOI] [PubMed] [Google Scholar]
- 8.Sekiguchi Y, Shimada A, Imai H, Wakabayashi M, Sugimoto K, Nakamura N, Sawada T, Komatsu N, Noguchi M. Patient with refractory multiple myeloma developing eosinophilia after lenalidomide treatment and lung cancer 9 months later: Case report and review of the literature. Indian J Hematol Blood Transfus. 2014;30(Suppl 1):S264–S270. doi: 10.1007/s12288-014-0355-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schild SE, Rule WG, Ashman JB, Vora SA, Keole S, Anand A, Liu W, Bues M. Proton beam therapy for locally advanced lung cancer: A review. World J Clin Oncol. 2014;5:568–575. doi: 10.5306/wjco.v5.i4.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kato T, Fujii T, Ide M, Takada T, Sutoh T, Morita H, Yajima R, Yamaguchi S, Tsutsumi S, Asao T, et al. Effect of long interval between hyperthermochemoradiation therapy and surgery for rectal cancer on apoptosis, proliferation and tumor response. Anticancer Res. 2014;34:3141–3146. [PubMed] [Google Scholar]
- 11.Mo EP, Zhang RR, Xu J, Zhang H, Wang XX, Tan QT, Liu FL, Jiang RW, Cai SH. Calotropin from Asclepias curasavica induces cell cycle arrest and apoptosis in cisplatin-resistant lung cancer cells. Biochem Biophys Res Commun. 2016;478:710–715. doi: 10.1016/j.bbrc.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Park HY, Toume K, Arai MA, Sadhu SK, Ahmed F, Ishibashi M. Calotropin: A cardenolide from calotropis gigantea that inhibits Wnt signaling by increasing casein kinase 1alpha in colon cancer cells. Chembiochem. 2014;15:872–878. doi: 10.1002/cbic.201300786. [DOI] [PubMed] [Google Scholar]
- 13.Wang SC, Lu MC, Chen HL, Tseng HI, Ke YY, Wu YC, Yang PY. Cytotoxicity of calotropin is through caspase activation and downregulation of anti-apoptotic proteins in K562 cells. Cell Biol Int. 2009;33:1230–1236. doi: 10.1016/j.cellbi.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Davey G, Wu Z. Attitudes in China toward the use of animals in laboratory research. Altern Lab Anim. 2007;35:313–316. doi: 10.1177/026119290703500305. [DOI] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Renshaw A, Elsheikh TM. A validation study of the Focalpoint GS imaging system for gynecologic cytology screening. Cancer Cytopathol. 2013;121:737–738. doi: 10.1002/cncy.21336. [DOI] [PubMed] [Google Scholar]
- 17.Zhuang T, Djemil T, Qi P, Magnelli A, Stephans K, Videtic G, Xia P. Dose calculation differences between Monte Carlo and pencil beam depend on the tumor locations and volumes for lung stereotactic body radiation therapy. J Appl Clin Med Phys. 2013;14:4011. doi: 10.1120/jacmp.v14i2.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalyuzhny AE. Combination of TUNEL assay with immunohistochemistry for simultaneous detection of DNA fragmentation and oxidative cell damage. Methods Mol Biol. 2011;682:15–27. doi: 10.1007/978-1-60327-409-8_2. [DOI] [PubMed] [Google Scholar]
- 19.Gharavi N, El-Kadi AO. Expression of cytochrome P450 in lung tumor. Curr Drug Metab. 2004;5:203–210. doi: 10.2174/1389200043489045. [DOI] [PubMed] [Google Scholar]
- 20.Song W, Ma Y, Wang J, Brantley-Sieders D, Chen J. JNK signaling mediates EPHA2-dependent tumor cell proliferation, motility, and cancer stem cell-like properties in non-small cell lung cancer. Cancer Res. 2014;74:2444–2454. doi: 10.1158/0008-5472.CAN-13-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magnuson WJ, Yeung JT, Guillod PD, Gettinger SN, Yu JB, Chiang VL. Impact of deferring radiation therapy in patients with epidermal growth factor receptor-mutant non-small cell lung cancer who develop brain metastases. Int J Radiat Oncol Biol Phys. 2016;95:673–679. doi: 10.1016/j.ijrobp.2016.01.037. [DOI] [PubMed] [Google Scholar]
- 22.Jiang SY, Zhao J, Wang MZ, Huo Z, Zhang J, Zhong W, Xu Y. Small-cell lung cancer transformation in patients with pulmonary adenocarcinoma: A case report and review of literature. Medicine (Baltimore) 2016;95:e2752. doi: 10.1097/MD.0000000000002752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shivapurkar N, Reddy J, Chaudhary PM, Gazdar AF. Apoptosis and lung cancer: A review. J Cell Biochem. 2003;88:885–898. doi: 10.1002/jcb.10440. [DOI] [PubMed] [Google Scholar]
- 24.Drebert Z, MacAskill M, Doughty-Shenton D, De Bosscher K, Bracke M, Hadoke PWF, Beck IM. Colon cancer-derived myofibroblasts increase endothelial cell migration by glucocorticoid-sensitive secretion of a pro-migratory factor. Vascul Pharmacol. 2017;89:19–30. doi: 10.1016/j.vph.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim CW, Go RE, Lee HM, Hwang KA, Lee K, Kim B, Lee MY, Choi KC. Cigarette smoke extracts induced the colon cancer migration via regulating epithelial mesenchymal transition and metastatic genes in human colon cancer cells. Environ Toxicol. 2017;32:690–704. doi: 10.1002/tox.22271. [DOI] [PubMed] [Google Scholar]
- 26.Klyushnenkova EN, Riabov VB, Kouiavskaia DV, Wietsma A, Zhan M, Alexander RB. Breaking immune tolerance by targeting CD25+ regulatory T cells is essential for the anti-tumor effect of the CTLA-4 blockade in an HLA-DR transgenic mouse model of prostate cancer. Prostate. 2014;74:1423–1432. doi: 10.1002/pros.22858. [DOI] [PubMed] [Google Scholar]
- 27.Duraiswamy J, Freeman GJ, Coukos G. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors-response. Cancer Res. 2014;74:633–635. doi: 10.1158/0008-5472.CAN-13-2752. [DOI] [PubMed] [Google Scholar]
- 28.Son CH, Bae JH, Shin DY, Lee HR, Choi YJ, Jo WS, Jung Ho M, Kang CD, Yang K, Park YS. CTLA-4 blockade enhances antitumor immunity of intratumoral injection of immature dendritic cells into irradiated tumor in a mouse colon cancer model. J Immunother. 2014;37:1–7. doi: 10.1097/CJI.0000000000000007. [DOI] [PubMed] [Google Scholar]
- 29.Gardner D, Jeffery LE, Sansom DM. Understanding the CD28/CTLA-4 (CD152) pathway and its implications for costimulatory blockade. Am J Transplant. 2014;14:1985–1991. doi: 10.1111/ajt.12834. [DOI] [PubMed] [Google Scholar]
- 30.Li F, Guo Z, Yu H, Zhang X, Si T, Liu C, Yang X, Qi L. Anti-tumor immunological response induced by cryoablation and anti-CTLA-4 antibody in an in vivo RM-1 cell prostate cancer murine model. Neoplasma. 2014;61:659–671. doi: 10.4149/neo_2014_081. [DOI] [PubMed] [Google Scholar]
- 31.Slattery ML, Herrick JS, Lundgreen A, Wolff RK. Genetic variation in the TGF-β signaling pathway and colon and rectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2011;20:57–69. doi: 10.1158/1055-9965.EPI-10-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slattery ML, Lundgreen A, Herrick JS, Caan BJ, Potter JD, Wolff RK. Associations between genetic variation in RUNX1, RUNX2, RUNX3, MAPK1 and eIF4E and riskof colon and rectal cancer: Additional support for a TGF-β-signaling pathway. Carcinogenesis. 2011;32:318–326. doi: 10.1093/carcin/bgq245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Becker C, Fantini MC, Neurath MF. TGF-beta as a T cell regulator in colitis and colon cancer. Cytokine Growth Factor Rev. 2006;17:97–106. doi: 10.1016/j.cytogfr.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Becker C, Fantini MC, Schramm C, Lehr HA, Wirtz S, Nikolaev A, Burg J, Strand S, Kiesslich R, Huber S, et al. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity. 2004;21:491–501. doi: 10.1016/j.immuni.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 35.Kuchta-Noctor AM, Murray BA, Stanton C, Devery R, Kelly PM. Anticancer activity of buttermilk against SW480 colon cancer cells is associated with caspase-independent cell death and attenuation of Wnt, Akt, and ERK signaling. Nutr Cancer. 2016;68:1234–1246. doi: 10.1080/01635581.2016.1206580. [DOI] [PubMed] [Google Scholar]
- 36.Wang CY, Guo ST, Wang JY, Yan XG, Farrelly M, Zhang YY, Liu F, Yari H, La T, Lei FX, et al. Reactivation of ERK and Akt confers resistance of mutant BRAF colon cancer cells to the HSP90 inhibitor AUY922. Oncotarget. 2016;7:49597–49610. doi: 10.18632/oncotarget.10414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beck SE, Jung BH, Del Rosario E, Gomez J, Carethers JM. BMP-induced growth suppression in colon cancer cells is mediated by p21WAF1 stabilization and modulated by RAS/ERK. Cell Signal. 2007;19:1465–1472. doi: 10.1016/j.cellsig.2007.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grijelmo C, Rodrigue C, Svrcek M, Bruyneel E, Hendrix A, de Wever O, Gespach C. Proinvasive activity of BMP-7 through SMAD4/src-independent and ERK/Rac/JNK-dependent signaling pathways in colon cancer cells. Cell Signal. 2007;19:1722–1732. doi: 10.1016/j.cellsig.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 39.de Oca J, Azuara D, Sanchez-Santos R, Navarro M, Capella G, Moreno V, Sola A, Hotter G, Biondo S, Osorio A, et al. Caspase-3 activity, response to chemotherapy and clinical outcome in patients with colon cancer. Int J Colorectal Dis. 2008;23:21–27. doi: 10.1007/s00384-007-0362-3. [DOI] [PubMed] [Google Scholar]
- 40.Vadde R, Radhakrishnan S, Reddivari L, Vanamala JK. Triphala extract suppresses proliferation and induces apoptosis in human colon cancer stem cells via suppressing c-Myc/Cyclin D1 and elevation of Bax/Bcl-2 ratio. Biomed Res Int. 2015;2015:649263. doi: 10.1155/2015/649263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.