Abstract
Castration-resistant prostate cancer (CRPC) is difficult to treat in current clinical practice. Hypoxia is an important feature of the CRPC microenvironment and is closely associated with the progress of CRPC invasion. However, no research has been performed on the immune escape of CRPC from NK cells. The present study focused on this subject. Firstly, when the CRPC cell lines C4-2 and CWR22Rv1 were induced by hypoxia, the expression of the UL16 binding protein (ULBP) ligand family of natural killer (NK) group 2D (NKG2D; ULBP-1, ULBP-2 and ULBP-3) and MHC class I chain-related proteins A and B (MICA/MICB) decreased. NKG2D is the main activating receptor of NK cells. Tumor cells were then co-cultured with NK cells to conduct NK cell-mediated cytotoxicity experiments, which revealed the decreased immune cytolytic activity of NK cells on hypoxia-induced CRPC cells. In exploring the mechanism behind this observation, an increase in programmed death-ligand 1 (PD-L1) expression in CRPC cells induced by hypoxia was observed, while the addition of PD-L1 antibody effectively reversed the expression of NKG2D ligand and enhanced the cytotoxic effect of NK cells on CRPC cells. In the process of exploring the upstream regulatory factors of PD-L1, inhibition of the Janus kinase (JAK)1,2/signal transducer and activator of transcription 3 (Stat3) signaling pathway decreased the expression of PD-L1 in CRPC cells. Finally, it was observed that combined inhibition of JAK1,2/PD-L1 or Stat3/PD-L1 was more effective than inhibition of a single pathway in enhancing the immune cytolytic activity of NK cells. Taking these results together, it is thought that combined inhibition of the JAK1,2/PD-L1 and Stat3/PD-L1 signaling pathways may enhance the immune cytolytic activity of NK cells toward hypoxia-induced CRPC cells, which is expected to provide novel ideas and targets for the immunotherapy of CRPC.
Keywords: hypoxia; castration-resistant prostate cancer; natural killer cell cytotoxicity; programmed death-ligand 1; natural killer group 2D; Janus kinase1,2/signal transducer and activator of transcription 3
Introduction
With continuous promotion of castration and anti-androgen therapy, clinical treatment of androgen independent protate cancer or castration-resistant prostate cancer (CRPC) has become difficult. It is not uncommon that CRPC develops metastases that chemotherapy and radiotherapy have limited effects on, which seriously affects patients' quality of life. Therefore, research on mechanisms of CRPC progression seems particularly important (1–3). The tumor microenvironment is essential for tumor genesis and tumor development (4,5), with hypoxia a strong research topic in recent years. Hypoxia can induce vascular formation in tumors, and is also widely involved in tumor formation, development, metastasis and recurrence (6–8). Hypoxia accelerates epithelial-mesenchymal transition, invasion, and metastasis in prostate cancer. Also, hypoxia may lead to a decreased sensitivity to radiotherapy and chemotherapy in prostate cancer treatment (9–12). However, there have been scant studies on hypoxia-induced immune evasion in prostate cancer. Therefore, we carried out this study to discover the role of hypoxia in tumor immune regulation.
Hypoxia is involved in immune evasion of a variety of tumors (13) involving many types of immune cells, including T cells, natural killer (NK) cells, macrophages and dendritic cells, that can inhibit or kill tumors (13). Hypoxia may lead to upregulation of the expression of stem cell marker Nanog and transforming growth factor beta 1, resulting in low immune killing capacity of T lymphocytes and macrophages against tumor cells (14). It was discovered in a lung cancer and melanoma study that hypoxia could induce miR-210 expression, which decreased tumor cell susceptibility to antigen-specific cytotoxic T lymphocytes and led to tumor formation and development (15). The NK cell mediated immune response can kill tumor cells directly with no dependence on antibodies or complements, which is a unique advantage in tumor immunity. By improving immune killing ability of NK cells against tumor cells, tumor formation and development can be effectively controlled. Suppression of expression of NK cell activating receptors MICA and MICB on the tumor cell surface by hypoxia can cause immune evasion from NK cells in pancreatic cancer, osteosarcoma, multiple myeloma and other malignant tumors (16–19). The role of hypoxia regarding NK cell immune evasion in prostate cancer is rarely reported. In a study of DU145 and PC3 in prostate cancer cells, hypoxia inhibited the expression of NKG2D ligands on the surface of the tumor cells, thereby inhibiting the killing of tumor cells by activated NK cells (20,21).
The mechanism of hypoxia-mediated immune evasion is unknown. Many studies have indicated that programmed cell death ligand 1 (PD-L1) plays an important role in tumor immune evasion (22,23). A study of non-small cell lung cancer showed that tumor cells overexpress PD-L1, thereby binding PD-1 receptors on the surface of T cells and inhibiting T cell immune attack, resulting in immune evasion (24,25). Studies of ovarian cancer, melanoma, bladder cancer, laryngeal squamous cell carcinoma and other malignant tumors have indicated that downregulation of PD-1/PD-L1 improves tumor susceptibility to immune cells (26–30). PD-1 is expressed in multiple immune cells, including T cells, B cells, NK cells and dendritic cells (31,32). The effect of PD-L1 in immune evasion of CRPC from NK cells, which is rarely studied, became the research direction of these experiments.
NKG2D is an important receptor for activation of NK cells. The upregulation of expression of NKG2D ligands, MHC class I chain-related proteins A and B (MICA and MICB) and UL16-binding proteins ULBP-1, ULBP-2, ULBP-3, can promote immune cytolytic activity of NK cells to tumors (33). This receptor has become the subject of enhancing the anti-tumor immunity of NK cells in this study. Previous work has shown that the combination of PD-1 and PD-L1 constitutes the PD-1-PD-L1 signaling pathway; in addition to this pathway's immunosuppressive effects through T cells (34), it can also inhibit the anti-tumor activity of NK cells by inhibiting the function of NKG2D ligands on the surface of tumor cells (35). Interestingly, based on a review of the literature, we found that the JAK1,2/Stat3 signaling pathway is the upstream regulator of PD-L1, which also includes other widely studied regulators such as Akt, MAPK, MEK, NFκB, and mTOR (36–40).
We selected CRPC cell lines C4-2 and CWR22Rv1 as subjects of study. After induction of the cells by hypoxia, we assayed NK cell mediated cytotoxicity and colony formation to determine whether there were any changes in CRPC cell immune susceptibility to NK cells. We investigated the underlying molecular mechanisms of the changes to seek an effective way to enhance the killing ability of NK cells against CRPC cells, with the aim of inhibiting or killing tumors and offering a new approach to CRPC immunotherapy.
Materials and methods
Cell culture
The C4-2 cells used in the assays were generously donated by the China Center for Type Culture Collection (CCTCC). The CWR22Rv1 and NK92 cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) and the cell culture medium was RPMI 1,640 medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10% charcoal stripped fetal bovine serum (Thermo Fisher Scientific, Inc.). We used an anoxic incubator (1%O2, 5%CO2, 94%N2) (Sanyo, Osaka, Japan) and a normoxic incubator (21%O2, 5%CO2, 74%N2) (Sanyo). C4-2 and CWR22Rv1 lines of CRPC cells grown inananoxic incubator for 24 h served as anoxic cells. NK92 cells were cultured in Minimum Essential Medium containing sodium bicarbonate (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), IL-2 (Bio-Techne, Minneapolis, MN, USA), inositol, folic acid, 12.5% horse serum (Sigma-Aldrich; Merck KGaA), 2-mercaptoethanol (Bio-Rad Laboratories, Inc., Hercules, CA, USA), and 12.5% FBS (HyClone; GE Healthcare Life Sciences, Logan, UT, USA).
NK cell mediated cytotoxicity assay
Tumor cells were seeded at 2,000 cells/well in a 96-well plate and cultured overnight. After aspirating all the medium, NK cells were added at a ratio of tumor cells to NK cells of 1:1, 1:5, or 1:15; the inhibitors PD-L1 Ab (329710; BioLegend, Inc., San Diego, CA, USA), JAK inhibitor 1 (CAS457081-03-7; EMD Millipore, Billerica, MA, USA), and Stattic were added at a ratio of 1:1,000 at the same time. Cells were cultured for 4 h, then a 50 µl aliquot of medium was used in a LDH cytotoxic assay using the LDH cytotoxic assay kit (88954; Thermo Fisher Scientific, Inc.). The experimental release was corrected by subtracting the amount released spontaneously in cells at corresponding dilutions. The percentage cytotoxicity was expressed as
and used to calculate the immune killing ability of NK cells against tumor cells.
Colony formation assay
Using the gradient dilution cell-count method, we seeded 200 cells in 6 cm dishes (REF353002; Corning Incorporated, Corning, NY, USA). After overnight culture, tumor cells and NK cells were incubated for 4 h at 1:1, 1:5 and 1:15 ratios. After aspirating all the medium and removing NK cells, tumor cells were continuously cultured for 14 days after replacing the conventional culture medium. Then the target cells were fixed with formaldehyde for 15 min, stained with crystal violet for 30 min, and observed and photographed under the microscope. The protocol was repeated three times in each group and the results were averaged.
Total RNA extraction and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
When the adherent tumor cells had grown over about 70% of the surface, PD-L1 Ab, JAK inhibitor 1, Stattic, LY294002, SB203580, and U0126 were added separately at a ratio of 1:1,000, then cells were cultured for 6 h, and total RNA was extracted. We used Superscript III transcriptase (Invitrogen; Thermo Fisher Scientific, Inc.) to reverse-transcribe total RNA (1 µg). Following primer setup, (the primer solutions included RT buffer, dNTPs, RT random primers, MultiScribe reverse transcriptase and RNase-free water), we conducted qPCR with a Bio-Rad CFX96 reaction system (reactions included cDNA, RNAase-free water, SYBR Green and primers; Table I). Using GAPDH as a reference, the expression of target gene mRNA was measured by the intensity of green fluorescence.
Table I.
Primer sequences used for reverse transcription-quantitative polymerase chain reaction.
| Gene | Direction | Sequence |
|---|---|---|
| GAPDH | Forward | 5′-AACGGATTTGGTCGTATTGGG-3′ |
| Reverse | 5′-CCTGGAAGATGGTGATGGGAT-3′ | |
| PD-L1 | Forward | 5′-GCTATGGTGGTGCCGACTAC-3′ |
| Reverse | 5′-TTGGTGGTGGTGGTCTTACC-3′ | |
| MICA | Forward | 5′-ACTGCTTGAGCCGCTGAGA-3′ |
| Reverse | 5′-GAGGTGCAAAAGGGAAGATGC-3′ | |
| MICB | Forward | 5′-GGGGCGCAGGTGACTAAAT-3′ |
| Reverse | 5′-CCTACGTCGCCACCTTCTCA-3′ | |
| ULBP-1 | Forward | 5′-CAGCAGACGATGAGGACATT-3′ |
| Reverse | 5′-GACAGAAAGTGGCAGAAGGTG-3′ | |
| ULBP-2 | Forward | 5′-CATTACTTCTCAATGGGAGACTGT-3′ |
| Reverse | 5′-TGTGCCTGAGGACATGGCGA-3′ | |
| ULBP-3 | Forward | 5′- ATTCTTCCGTACCTGCTATT-3′ |
| Reverse | 5′-GCTATCCTTCTCCCACTTCT-3′ |
PD-L1, programmed death-ligand 1; MICA, MHC class I chain-related protein A; MICB, MHC class I chain-related protein B; ULBP, UL16 binding protein.
Western blot analysis
Tumor cells were collected after centrifugation and the supernatant removed. Then cell lysate was added, the protein concentration measured, sodium dodecyl sulphate-polyacrylamide gel electrophoresis performed, and the protein transferred to polyvinylidene difluoride membranes (EMD Millipore). After adding blocking solution and incubating with primary antibodies (1:1,000), the membranes were incubated with horseradish peroxidase labeled secondary antibody (1:5,000), followed by capture of images with an ECL imaging system (Thermo Fisher Scientific, Inc.). Primary antibodies used in the assay included p-HIF-1α (EP1215Y; ab210073; Abcam, Cambridge, UK), p-PD-L1 (MAB1086; R&D Systems, Inc., Minneapolis, MN, USA), JAK1 (pY1022, A7125; Assay Biotech, Fremont, CA, USA), p-JAK2 (pY1007 + 1008, 601–670; Abbomax, Inc., San Jose, CA, USA), p-MAPK (9101S; Cell Signaling Technology, Inc., Danvers, MA, USA), p-MEK (Ser 217/221, 9121; Cell Signaling Technology, Inc.), p-Akt (S473, 9271; Cell Signaling Technology, Inc.), p-Stat3 (Y705, ab76315; Abcam), p-NFκB (S536, ab86299; Abcam), and GAPDH (2118S; Cell Signaling Technology, Inc.).
Signaling pathway inhibitors
We added appropriately diluted inhibitors of various cell signaling pathways, JAK inhibitor 1 (5 µM) (CAS457081-03-7; EMD Millipore), Stattic (10 µM) (CAS19983-44-9; EMD Millipore), PD-L1 Ab (329710; BioLegend, Inc.), LY294002, SB203580 (both Sigma-Aldrich; Merck KGaA) and U0126 (Cell Signaling Technology, Inc.), which respectively inhibit JAK1, JAK2, Stat3, Akt, MAPK and MEK, to hypoxia-treated cells before co-incubation with tumor cells.
Statistical analysis
All data were reported as the mean ± standard deviation of 3 experimental repeats., and were analyzed using SPSS 19.0 software (IBM Corp., Armonk, NY, USA). The differences between two groups were analyzed by a two-tailed Student's t-test. One-way analysis of variance followed by Fisher's least significant difference post hoc test were used for comparisons among multiple groups. P<0.05 was considered to indicate a statistically significant difference.
Results
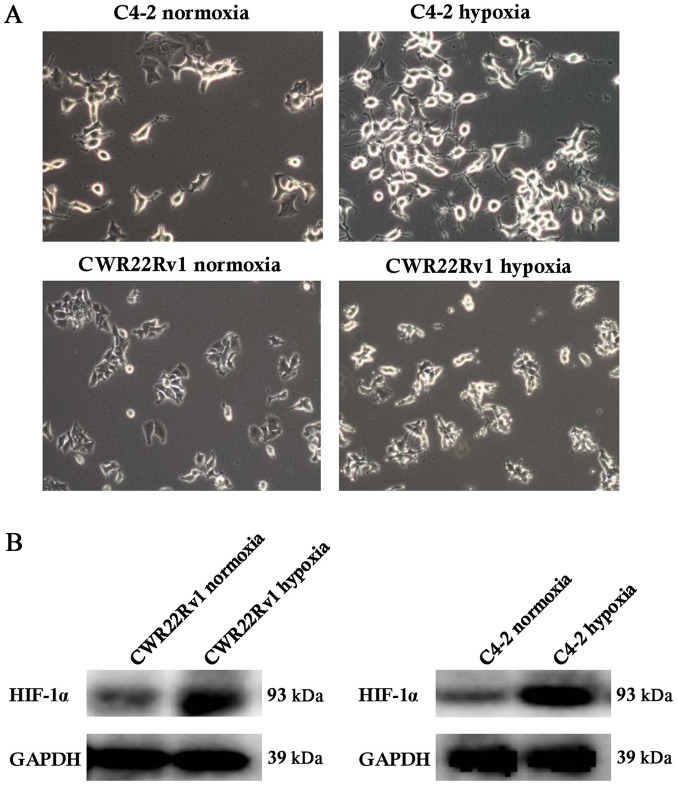
Hypoxia-induced C4-2 and CWR22Rv1 cells showed changes in shape and increased expression of hypoxia-inducible factor-1α (HIF-1α)
After culture of C4-2 and CWR22Rv1 lines of CRPC cells in a hypoxic incubator for 24 h, the expected changes in cell morphology, such as to spindle-shaped and polygonal cells, and an increase in cell refractive index were observed (Fig. 1A). Normoxic cultured cells were the control group. We observed significantly upregulated expression of HIF-1α in tumor cells exposed to hypoxia. While HIF-1α can be easily degraded by the intracellular oxygen-dependent ubiquitin-proteasome pathway under normoxic conditions, under hypoxic conditions, its expression is stable (33), suggesting that the assayed C4-2 and CWR22Rv1 cells were effectively induced by hypoxia (Fig. 1B).
Figure 1.
Hypoxia induction in the C4-2 and CWR22Rv1 lines of castration-resistant prostate cancer cells. (A) A total of 24 h following hypoxia induction, the cells appeared polygonal and spindle-shaped, and the refractive index of the cells increased (magnification, ×40). (B) A significantly increased expression of HIF-1α was detected by western blotting following hypoxia induction. HIF, hypoxia-inducible factor.
Hypoxia reduced expression of NKG2D activating ligandsin C4-2 and CWR22Rv1 cell lines and led to increased tolerance to immune killing of NK cells
We also detected changes in expression of genes encoding NKG2D ligands in hypoxia-induced C4-2 and CWR22Rv1 cells. Gene expression of these ligands (ULBP-1, ULBP-2, ULBP-3, MICA and MICB, all found on the surface of target cells) significantly decreased (Fig. 2A). In hypoxia-induced CRPC cells co-cultured for 4 h with various ratios of NK cells, lactate dehydrogenase release showed that the killing ability of NK cells significantly decreased against hypoxia induced target cells (Fig. 2B). The colony formation assay suggested that as the ratio of co-cultured NK cells to tumor cells increased, the clone-forming ability of the hypoxia group showed no obvious changes, while a significant decrease was observed in the normoxia group. These results indicated that hypoxia increased survival of target cells co-cultured with NK cells, which was consistent with the findings in the NK cell mediated cytotoxicity assay (Fig. 2C). In conclusion, hypoxia can lead to CRPC cell immune tolerance to NK cells.
Figure 2.
NKG2D ligand gene expression in the hypoxia-induced CRPC cell lines C4-2 and CWR22Rv1, and the effects of hypoxia on NK cell mediated cytotoxicity and clone formation. (A) Following hypoxia induction, gene expression of NKG2D ligand, which promotes NK cell activation, was significantly decreased. (B) Following hypoxia induction, the killing ability of NK cells against CRPC cells was significantly decreased. (C) The ratio of co-cultured NK cells to tumor cells and the CRPC cell clone-forming ability under hypoxia. **P<0.01 and ***P<0.001 vs. normoxia. NKG2D, natural killer group 2D; NK cells, natural killer cells; CRPC, castration-resistant prostate cancer.
Hypoxia-induced C4-2, and CWR22Rv1 CRPC cells had increased PD-L1 expression, resulting in immune escape from NK cells
In further study on the mechanisms affecting expression of PD-L1, both PD-L1 gene and protein expression in C4-2 and CWR22Rv1 cells were higher after hypoxia induction than under normoxic conditions (Fig. 3A). To determine whether hypoxia induces CRPC cells to evade NK cell immunity, we added PD-L1 antibodies to hypoxia-induced CRPC cells and detected upregulation of NKG2D ligand expression (Fig. 3B). We assayed NK cell mediated cytotoxicity, and discovered that addition of PD-L1 antibodies significantly increased the susceptibility of hypoxia-induced CRPC cells to NK cell immunity (Fig. 3C), which confirmed that PD-L1 protein is involved in hypoxia-induced CRPC cell immune evasion.
Figure 3.
Expression of PD-L1 gene in the hypoxia-induced castration-resistant prostate cancer cell lines C4-2 and CWR22Rv1, and alterations in NKG2D ligand gene expression and NK cell mediated cytotoxicity following the addition of PD-L1 antibodies. (A) Following hypoxia induction, the expression of PD-L1 significantly increased. In the hypoxia-induced group, adding PD-L1 antibodies (B) reversed the expression of natural killer group 2D ligand genes and (C) enhanced NK cell immune killing ability against tumor cells. *P<0.05, **P<0.01 and ***P<0.001 vs. normoxia and as indicated. NK cells, natural killer cells; PD-L1, programmed death-ligand 1; MICA, MHC class I chain-related protein A; MICB, MHC class I chain-related protein B; ULBP, UL16 binding protein.
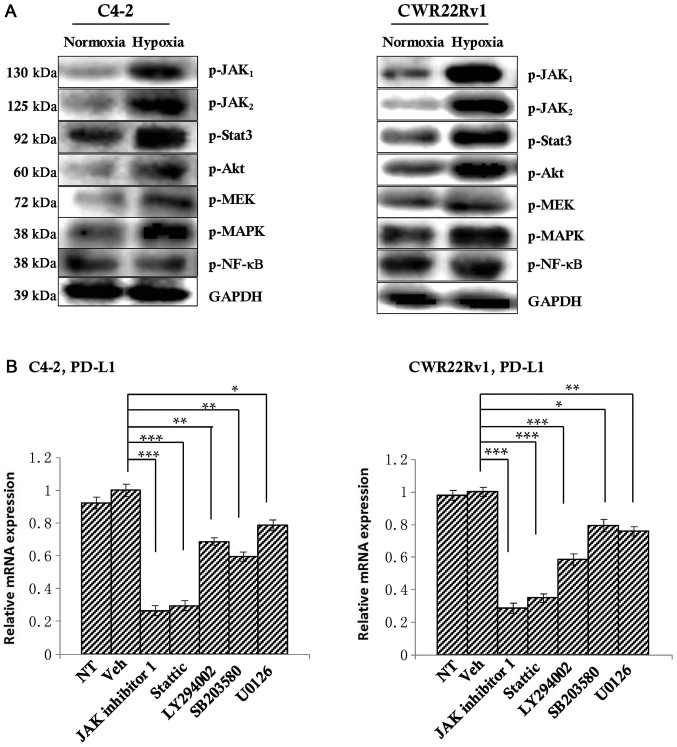
PD-L1 expression is inhibited by blocking the JAK1,2/Stat3 signaling pathway
To explore the mechanism behind PD-L1 regulation of CRPC cells against NK cell immunity, we selected well-studied signaling pathways of PD-L1 regulation, including JAK1, JAK2, Stat3, PI3K/Akt, MAPK, MEK, and NFκB (34–39). We first detected differences in expression of these molecular markers in both normoxia and hypoxia groups by western blotting, and found that the expression of JAK1, JAK2, Stat3, Akt, MAPK, MEK in hypoxia group was increased compared with the normoxia group (Fig. 4A); thus, we think that these proteins may be involved in the regulation of PD-L1 under hypoxic conditions in the tumor. To observe their regulation of PD-L1, we added inhibitors of these proteins' corresponding signaling pathways JAK inhibitor 1 (which can simultaneously inhibit the expression of JAK 1 and JAK 2), Stattic, LY294002, SB203580 and U0126 at a ratio of 1:1,000 to hypoxic tumor cells. After 6 h, the level of PD-L1 was detected by RT-qPCR. The untreated group served as a control. The results showed that JAK inhibitor 1 and Stattic significantly down-regulate PD-L1 expression. PI3k inhibitor LY294002 could also significantly reduce the expression of PD-L1, but JAK inhibitor 1 and Stattic inhibited PD-L1 more. So we chose JAK inhibitor 1 and Stattic as the research subjects. Of course, given this result, PI3k inhibitor LY294002 will also be our future research direction (Fig. 4B).
Figure 4.
Detection of the upstream regulatory factors of PD-L1. (A) Western blotting indicated an upward tendency in the expression of p-JAK1, p-JAK2, p-Akt, p-Stat3, p-MAPK and p-MEK in the hypoxia group. (B) Once the corresponding inhibitors were added, PD-L1 expression in all of the above signaling pathways decreased to varying degrees, with significant decreases observed following the addition of JAK inhibitor 1 and Stattic, suggesting that the inhibition of the JAK1,2/Stat3 signaling pathway may inhibit PD-L1 expression. *P<0.05, **P<0.01 and ***P<0.001, as indicated. PD-L1, programmed death-ligand 1; JAK, Janus kinase; Akt, protein kinase B; Stat, signal transducer and activator of transcription; NF, nuclear factor; MAPK, mitogen-activated protein kinase; MEK, MAPK kinase; p-, phosphorylated; NT, not treated.
JAK1,2/Stat3 signaling pathway inhibition increases the susceptibility of CRPC cells to NK cell immunity, with a combination of PD-L1 antibodies and JAK inhibitor 1/Stattic more effective either alone. CRPC cells in the hypoxia group were used as the study subject, and the JAK1,2 inhibitor JAK inhibitor 1 or the Stat3 inhibitor Stattic were added. The untreated group was used as the control. RT-qPCR showed increased expression of NKG2D ligands (Fig. 5A), suggesting that inhibition of the JAK1,2/Stat3 signaling pathway may improve the immune cytolytic activity of NK cells toward CRPC cells under hypoxic conditions.
Figure 5.
Effects of JAK1,2 and Stat3 single inhibition and JAK1,2/PD-L1 and Stat3/PD-L1 combined inhibition on NK cell mediated cytotoxicity. (A) Inhibition of the expression of JAK1,2 or Stat3 upregulated NKG2D ligand expression in castration-resistant prostate cancer cells under hypoxia. (B) NK cell cytotoxic effects on tumor cells were significantly increased following the addition of JAK inhibitor 1 or Stattic in the hypoxia group. (C) Combinations of JAK inhibitor 1/PD-L1 antibody or Stattic/PD-L1 antibody were superior to single applications, indicating that combined inhibition of the PD-L1 and JAK1,2/Stat3 signaling pathways enhanced NK cell immune killing ability against hypoxia-induced CRPC cells. *P<0.05, **P<0.01 and ***P<0.001, as indicated. JAK, Janus kinase; Stat, signal transducer and activator of transcription; NKG2D, natural killer group 2D; NK cells, natural killer cells; PD-L1, programmed death-ligand 1; MICA, MHC class I chain-related protein A; MICB, MHC class I chain-related protein B; ULBP, UL16 binding protein; NT, not treated.
Since blocking the JAK1,2/Stat3 signaling pathway can inhibit PD-L1 expression in hypoxic CRPC cells and may upregulate expression of NKG2D ligands of CRPC cells, to test whether this also enhances the immune killing function of NK cells against tumors by down-regulating PD-L1, we used hypoxic CRPC cells as targets and added the same JAK1,2/Stat3 signaling pathway inhibitors to an NK cell mediated cytotoxicity assay. Both JAK inhibitor 1 and Stattic significantly increased susceptibility of CRPC cells to NK cell immunity compared to an untreated group (Fig. 5B). Finally, we added combinations of PD-L1 antibody/JAK inhibitor 1 and PD-L1 antibody/Stattic to the NK cell mediated cytotoxicity assay and discovered that combined treatments were superior to each single application. Combined treatments achieved better results in enhancing NK cell immune killing function against CRPC cells, which could provide new approaches to CRPC targeted therapy (Fig. 5C).
Discussion
In clinical practice, the incidence of both local progression and distant metastasis in CRPC patients has significantly increased, seriously affecting patients' quality of life (40,41). Although the mechanisms behind this increase are not yet fully understood, hypoxia, as an important feature of the tumor microenvironment, plays an essential role (42,43). In this study, CRPC cell lines C4-2 and CWR22Rv1 were grown in an incubator under conditions of hypoxia. We observed changes in the morphology of these cells to polygonal and spindle shapes, and high expression of HIF-1α that was dependent on the oxygen concentration, consistent with hypoxia-induced changes in the cells, which may provide fundamental data for further exploration of the effects of hypoxia on tumor immunity. In the hypoxic microenvironment of prostate cancer, HIF-1α and related proteins present a major research direction. Our focus on the JAK1,2/Stat3-PD-L1 signaling pathway has not been studies before, and our results suggest this pathway was upregulated in a hypoxic microenvironment, resulting in immune escape of CRPC from NK cells.
The occurrence and development of tumors are closely related to the immunity of the human body, in which the direct killing function of NK cells against tumors has a unique advantage; however, there are scant studies on the mechanisms of NK cell immunity against CRPC. Our study took this as a starting point to explore the mechanisms of NK cell immunity against CRPC in a hypoxic microenvironment. Firstly we examined the expression of ligands of NKG2D, a major activating receptor of NK cells, which include the ULBP family (ULBP-1, ULBP-2 and ULBP-3) and MICA/MICB (44). Expression of all these markers showed a downward trend, consistent with NKG2D playing an important role in tumor immunity of NK cells. CRPC cells also had stronger immune tolerance to NK cells in a hypoxic microenvironment, confirming a stronger immune evasion function under these conditions.
To explore the mechanisms of immune evasion, we selected PD-L1, a relatively well-studied protein in tumor targeted therapy (45,46), as a research subject. PD-L1 expression of hypoxia-induced CRPC cells significantly increased, and by adding PD-L1 antibodies, the expression of NKG2D ligands was reversed, while the immune killing ability of NK cells against tumor cells was significantly enhanced. The combination of PD-1 and PD-L1 constitutes the PD-1-PD-L1 signaling pathway, thus affecting immune cytolytic activity of immune cells to tumor cells, which is mainly reflected in the following aspects: (1) Inhibition of function of TIL cells by (a) inhibiting the activation of TIL cells, (b) influencing Th cell differentiation, (c) inhibiting the production of effector cytokines, (d) promoting the secretion of suppressive cytokines, and (e) increasing TIL apoptosis, thus resulting in tumor immune escape (34); (2) Inhibiting the function of NK cells, thereby reducing their anti-tumor effect (35); (3) Expression of PD-L1 on TAM, which can cause synergistic stimulation, inhibiting the immune susceptibility of tumor cells (47).
During examination of PD-L1 upstream regulatory factors, we found that the JAK1,2/Stat3 signaling pathways played an important role, and by adding appropriate antibodies (JAK inhibitor 1 or PD-L1 antibody), PD-L1 expression of CRPC cells was inhibited under hypoxic conditions. The JAK1,2/Stat3 signaling pathway is widely involved in proliferation, differentiation, migration and other processes of cells (48). It plays an important role in occurrence and development of many tumors including prostate cancer (49–53). The results of our study also indicated that in a hypoxic microenvironment, the JAK1,2/Stat3 signaling pathway promotes immune evasion of NK cells by CRPC cells. The current targeted immunotherapy of PD-1/PD-L1 against tumors has achieved good results, effectively reducing tumor size and prolonging the survival of patients. However, clinical observation shows that there is still some degree of immunosuppression, which may be related to the complex immune escape mechanism of the tumor itself. Combined administration of drugs through multiple ways may be an effective choice for tumor targeted immunotherapy (54). Thus, we studied the combined inhibition of JAK1,2/PD-L1 and Stat3/PD-L1, and observed that a combined inhibition was more effective in enhancing NK cell cytotoxicity toward hypoxia-induced CRPC cells. Given these results, we may develop a combination inhibitor of JAK1,2/Stat3 and PD-L1, and conduct related animal studies and clinical trials, thereby providing a more effective means of clinical immunotherapy of CRPC.
Acknowledgements
The authors would like to thank Dr. Soo Ok Lee (Department of Urology, The Second Affiliated Hospital of Soochow University, Suzhou, Jiangsu, China) for assisting with the preparation of the manuscript.
Funding
The present study was supported by the Pre Research Fund Project of The Second Affiliated Hospital of Soochow University (grant no. SDFEYBS1707), The National Natural Science Foundation of China (grant nos. 81472776 and 81773221), and by Preponderant Discipline Construction Funding of the Second Affiliated Hospital of Soochow University (grant no. XKQ2015008).
Availability of data and materials
The analyzed datasets generated during the study are available from the corresponding author on reasonable request.
Authors' contributions
LJX, QM and JZ performed the experiments and statistical analyses, and created the figures. JL and BXX contributed to the generation of the knockdown cell lines. JG and CYS provided and performed the staining of human tissues. YCZ and YBZ assisted with the interpretation of data and reviewed the manuscript. DRY and YXS conceived the idea and wrote the manuscript. All authors reviewed and agreed to the information in this manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Cornford P, Bellmunt J, Bolla M, Briers E, De Santis M, Gross T, Henry AM, Joniau S, Lam TB, Mason MD, et al. EAU-ESTRO-SIOG Guidelines on prostate cancer. Part II: Treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. 2017;71:630–642. doi: 10.1016/j.eururo.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Lowrance WT, Roth BJ, Kirkby E, Murad MH, Cookson MS. Castration-resistant prostate cancer: AUA Guideline Amendment 2015. J Urol. 2016;195:1444–1452. doi: 10.1016/j.juro.2015.10.086. [DOI] [PubMed] [Google Scholar]
- 3.Crawford ED, Higano CS, Shore ND, Hussain M, Petrylak DP. Treating patients with metastatic castration resistant prostate cancer: A comprehensive review of available therapies. J Urol. 2015;194:1537–1547. doi: 10.1016/j.juro.2015.06.106. [DOI] [PubMed] [Google Scholar]
- 4.Qiu J, Jiang W, Yang Y, Feng C, Chen Z, Guan G, Zhuo S, Chen J. Monitoring changes of tumor microenvironment in colorectal submucosa using multiphoton microscopy. Scanning. 2015;37:17–22. doi: 10.1002/sca.21174. [DOI] [PubMed] [Google Scholar]
- 5.Casey SC, Amedei A, Aquilano K, Azmi AS, Benencia F, Bhakta D, Bilsland AE, Boosani CS, Chen S, Ciriolo MR, et al. Cancer prevention and therapy through the modulation of the tumor microenvironment. Semin Cancer Biol. 2015;35(Suppl):S199–S223. doi: 10.1016/j.semcancer.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolff M, Kosyna FK, Dunst J, Jelkmann W, Depping R. Impact of hypoxia inducible factors on estrogen receptor expression in breast cancer cells. Arch Biochem Biophys. 2017;613:23–30. doi: 10.1016/j.abb.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Wu X, Qiao B, Liu Q, Zhang W. Upregulation of extracellular matrix metalloproteinase inducer promotes hypoxia-induced epithelial-mesenchymal transition in esophageal cancer. Mol Med Rep. 2015;12:7419–7424. doi: 10.3892/mmr.2015.4410. [DOI] [PubMed] [Google Scholar]
- 8.Clavo B, Robaina F, Fiuza D, Ruiz A, Lloret M, Rey-Baltar D, Llontop P, Riveros A, Rivero J, Castañeda F, et al. Predictive value of hypoxia in advanced head and neck cancer after treatment with hyperfractionated radio-chemotherapy and hypoxia modification. Clin Transl Oncol. 2017;19:419–424. doi: 10.1007/s12094-016-1541-x. [DOI] [PubMed] [Google Scholar]
- 9.Li W, Dong Y, Zhang B, Kang Y, Yang X, Wang H. PEBP4 silencing inhibits hypoxia-induced epithelial-to-mesenchymal transition in prostate cancer cells. Biomed Pharmacother. 2016;81:1–6. doi: 10.1016/j.biopha.2016.03.030. [DOI] [PubMed] [Google Scholar]
- 10.Li M, Wang YX, Luo Y, Zhao J, Li Q, Zhang J, Jiang Y. Hypoxia inducible factor-1α-dependent epithelial to mesenchymal transition under hypoxic conditions in prostate cancer cells. Oncol Rep. 2016;36:521–527. doi: 10.3892/or.2016.4766. [DOI] [PubMed] [Google Scholar]
- 11.Wang W, Liu M, Guan Y, Wu Q. Hypoxia-responsive Mir-301a and Mir-301b promote radioresistance of prostate cancer cells via downregulating NDRG2. Med Sci Monit. 2016;22:2126–2132. doi: 10.12659/MSM.896832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nomura T, Yamasaki M, Hirai K, Inoue T, Sato R, Matsuura K, Moriyama M, Sato F, Mimata H. Targeting the Vav3 oncogene enhances docetaxel-induced apoptosis through the inhibition of androgen receptor phosphorylation in LNCaP prostate cancer cells under chronic hypoxia. Mol Cancer. 2013;12:27. doi: 10.1186/1476-4598-12-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barsoum IB, Koti M, Siemens DR, Graham CH. Mechanisms of hypoxia-mediated immune escape in cancer. Cancer Res. 2014;74:7185–7190. doi: 10.1158/0008-5472.CAN-14-2598. [DOI] [PubMed] [Google Scholar]
- 14.Hasmim M, Noman MZ, Messai Y, Bordereaux D, Gros G, Baud V, Chouaib S. Cutting edge: Hypoxia-induced Nanog favors the intratumoral infiltration of regulatory T cells and macrophages via direct regulation of TGF-β1. J Immunol. 2013;191:5802–5806. doi: 10.4049/jimmunol.1302140. [DOI] [PubMed] [Google Scholar]
- 15.Noman MZ, Buart S, Romero P, Ketari S, Janji B, Mari B, Mami-Chouaib F, Chouaib S. Hypoxia-inducible miR-210 regulates the susceptibility of tumor cells to lysis by cytotoxic T cells. Cancer Res. 2012;72:4629–4641. doi: 10.1158/0008-5472.CAN-12-1383. [DOI] [PubMed] [Google Scholar]
- 16.Lu Y, Hu J, Sun W, Duan X, Chen X. Hypoxia-mediated immune evasion of pancreatic carcinoma cells. Mol Med Rep. 2015;11:3666–3672. doi: 10.3892/mmr.2015.3144. [DOI] [PubMed] [Google Scholar]
- 17.Yamada N, Yamanegi K, Ohyama H, Hata M, Nakasho K, Futani H, Okamura H, Terada N. Hypoxia downregulates the expression of cell surface MICA without increasing soluble MICA in osteosarcoma cells in a HIF-1α-dependent manner. Int J Oncol. 2012;41:2005–2012. doi: 10.3892/ijo.2012.1630. [DOI] [PubMed] [Google Scholar]
- 18.Sarkar S, Germeraad WT, Rouschop KM, Steeghs EM, van Gelder M, Bos GM, Wieten L. Hypoxia induced impairment of NK cell cytotoxicity against multiple myeloma can be overcome by IL-2 activation of the NK cells. PLoS One. 2013;8:e64835. doi: 10.1371/journal.pone.0064835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Labiano S, Palazon A, Melero I. Immune response regulation in the tumor microenvironment by hypoxia. Semin Oncol. 2015;42:378–386. doi: 10.1053/j.seminoncol.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton TK, Hu N, Kolomitro K, Bell EN, Maurice DH, Graham CH, Siemens DR. Potential therapeutic applications of phosphodiesterase inhibition in prostate cancer. World J Urol. 2013;31:325–330. doi: 10.1007/s00345-012-0848-7. [DOI] [PubMed] [Google Scholar]
- 21.Siemens DR, Hu N, Sheikhi AK, Chung E, Frederiksen LJ, Pross H, Graham CH. Hypoxia increases tumor cell shedding of MHC class I chain-related molecule: Role of nitric oxide. Cancer Res. 2008;68:4746–4753. doi: 10.1158/0008-5472.CAN-08-0054. [DOI] [PubMed] [Google Scholar]
- 22.Barsoum IB, Smallwood CA, Siemens DR, Graham CH. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res. 2014;74:665–674. doi: 10.1158/0008-5472.CAN-14-2598. [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Jiang CC, Jin L, Zhang XD. Regulation of PD-L1: A novel role of pro-survival signalling in cancer. Ann Oncol. 2016;27:409–416. doi: 10.1093/annonc/mdv615. [DOI] [PubMed] [Google Scholar]
- 24.Shi MH, Xing YF, Zhang ZL, Huang JA, Chen YJ. Effect of soluble PD-L1 released by lung cancer cells in regulating the function of T lymphocytes. Zhonghua Zhong Liu Za Zhi. 2013;35:85–88. doi: 10.3760/cma.j.issn.0253-3766.2013.02.002. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 25.Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, Mikse OR, Cherniack AD, Beauchamp EM, Pugh TJ, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013;3:1355–1363. doi: 10.1158/2159-8290.CD-13-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahoney KM, Freeman GJ, McDermott DF. The next immune-checkpoint inhibitors: PD-1/PD-L1 blockade in melanoma. Clin Ther. 2015;37:764–782. doi: 10.1016/j.clinthera.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mandai M, Hamanishi J, Abiko K, Matsumura N, Baba T, Konishi I. Anti-PD-L1/PD-1 immune therapies in ovarian cancer: Basic mechanism and future clinical application. Int J Clin Oncol. 2016;21:456–461. doi: 10.1007/s10147-016-0968-y. [DOI] [PubMed] [Google Scholar]
- 28.Bardoli AD, Afshar M, Viney R, Foster M, Porfiri E, Zarkar A, Stevenson R, James ND, Bryan RT, Patel P. The PD-1/PD-L1 axis in the pathogenesis of urothelial bladder cancer and evaluating its potential as a therapeutic target. Future Oncol. 2016;12:595–600. doi: 10.2217/fon.15.337. [DOI] [PubMed] [Google Scholar]
- 29.Brower V. Anti-PD-L1 antibody active in metastatic bladder cancer. Lancet Oncol. 2015;16:e11. doi: 10.1016/S1470-2045(14)71167-2. [DOI] [PubMed] [Google Scholar]
- 30.Vassilakopoulou M, Avgeris M, Velcheti V, Kotoula V, Rampias T, Chatzopoulos K, Perisanidis C, Kontos CK, Giotakis AI, Scorilas A, et al. Evaluation of PD-L1 expression and associated tumor-infiltrating lymphocytes in laryngeal squamous cell carcinoma. Clin Cancer Res. 2016;22:704–713. doi: 10.1158/1078-0432.CCR-15-1543. [DOI] [PubMed] [Google Scholar]
- 31.Yao S, Chen L. PD-1 as an immune modulatory receptor. Cancer J. 2014;20:262–264. doi: 10.1097/PPO.0000000000000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang BY, Zhan YP, Zong WJ, Yu CJ, Li JF, Qu YM, Han S. The PD-1/B7-H1 pathway modulates the natural killer cells versus mouse glioma stem cells. PLoS One. 2015;10:e0134715. doi: 10.1371/journal.pone.0134715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joo HY, Yun M, Jeong J, Park ER, Shin HJ, Woo SR, Jung JK, Kim YM, Park JJ, Kim J, Lee KH. SIRT1 deacetylates and stabilizes hypoxia-inducible factor-1α (HIF-1α) via direct interactions during hypoxia. Biochem Biophys Res Commun. 2015;462:294–300. doi: 10.1016/j.bbrc.2015.04.119. [DOI] [PubMed] [Google Scholar]
- 34.Doi T, Ishikawa T, Okayama T, Oka K, Mizushima K, Yasuda T, Sakamoto N, Katada K, Kamada K, Uchiyama K, et al. The JAK/STAT pathway is involved in the upregulation of PD-L1 expression in pancreatic cancer cell lines. Oncol Rep. 2017;37:1545–1554. doi: 10.3892/or.2017.5399. [DOI] [PubMed] [Google Scholar]
- 35.Bellucci R, Martin A, Bommarito D, Wang K, Hansen SH, Freeman GJ, Ritz J. Interferon-γ-induced activation of JAK1 and JAK2 suppresses tumor cell susceptibility to NK cells through upregulation of PD-L1 expression. Oncoimmunology. 2015;4:e1008824. doi: 10.1080/2162402X.2015.1008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atefi M, Avramis E, Lassen A, Wong DJ, Robert L, Foulad D, Cerniglia M, Titz B, Chodon T, Graeber TG, et al. Effects of MAPK and PI3K pathways on PD-L1 expression in melanoma. Clin Cancer Res. 2014;20:3446–3457. doi: 10.1158/1078-0432.CCR-13-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang L, Huang F, Mei J, Wang X, Zhang Q, Wang H, Xi M, You Z. Posttranscriptional control of PD-L1 expression by 17β-estradiol via PI3K/Akt signaling pathway in ERα-positive cancer cell lines. Int J Gynecol Cancer. 2017;27:196–205. doi: 10.1097/IGC.0000000000000875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang X, Zhou J, Giobbie-Hurder A, Wargo J, Hodi FS. The activation of MAPK in melanoma cells resistant to BRAF inhibition promotes PD-L1 expression that is reversible by MEK and PI3K inhibition. Clin Cancer Res. 2013;19:598–609. doi: 10.1158/1078-0432.CCR-12-2731. [DOI] [PubMed] [Google Scholar]
- 39.Gowrishankar K, Gunatilake D, Gallagher SJ, Tiffen J, Rizos H, Hersey P. Inducible but not constitutive expression of PD-L1 in human melanoma cells is dependent on activation of NF-κB. PLoS One. 2015;10:e0123410. doi: 10.1371/journal.pone.0123410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hernandez RK, Cetin K, Pirolli M, Quigley J, Quach D, Smith P, Stryker S, Liede A. Estimating high-risk castration resistant prostate cancer (CRPC) using electronic health records. Can J Urol. 2015;22:7858–7864. [PubMed] [Google Scholar]
- 41.Kamoto T. Evaluation and diagnosis for castration resistant prostate cancer: CRPC. Nihon Rinsho. 2014;72:2103–2107. (In Japanese) [PubMed] [Google Scholar]
- 42.Walsh JC, Lebedev A, Aten E, Madsen K, Marciano L, Kolb HC. The clinical importance of assessing tumor hypoxia: Relationship of tumor hypoxia to prognosis and therapeutic opportunities. Antioxid Redox Signal. 2014;21:1516–1554. doi: 10.1089/ars.2013.5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jensen LD. The circadian clock and hypoxia in tumor cell de-differentiation and metastasis. Biochim Biophys Acta. 2015;1850:1633–1641. doi: 10.1016/j.bbagen.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 44.Lanier LL. NKG2D receptor and its ligands in host defense. Cancer Immunol Res. 2015;3:575–582. doi: 10.1158/2326-6066.CIR-15-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swaika A, Hammond WA, Joseph RW. Current state of anti-PD-L1 and anti-PD-1 agents in cancer therapy. Mol Immunol. 2015;67:4–17. doi: 10.1016/j.molimm.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 46.Ohaegbulam KC, Assal A, Lazar-Molnar E, Yao Y, Zang X. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol Med. 2015;21:24–33. doi: 10.1016/j.molmed.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cai B, Cai JP, Luo YL, Chen C, Zhang S. The specific roles of JAK/STAT signaling pathway in sepsis. Inflammation. 2015;38:1599–1608. doi: 10.1007/s10753-015-0135-z. [DOI] [PubMed] [Google Scholar]
- 48.Teng Y, Ross JL, Cowell JK. The involvement of JAK-STAT3 in cell motility, invasion, and metastasis. JAKSTAT. 2014;3:e28086. doi: 10.4161/jkst.28086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O'Shea JJ, Holland SM, Staudt LM. JAKs and STATs in immunity, immunodeficiency, and cancer. N Engl J Med. 2013;368:161–170. doi: 10.1056/NEJMra1202117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang SW, Sun YM. The IL-6/JAK/STAT3 pathway: Potential therapeutic strategies in treating colorectal cancer (Review) Int J Oncol. 2014;44:1032–1040. doi: 10.3892/ijo.2014.2259. [DOI] [PubMed] [Google Scholar]
- 51.Liu RY, Zeng Y, Lei Z, Wang L, Yang H, Liu Z, Zhao J, Zhang HT. JAK/STAT3 signaling is required for TGF-β-induced epithelial-mesenchymal transition in lung cancer cells. Int J Oncol. 2014;44:1643–1651. doi: 10.3892/ijo.2014.2310. [DOI] [PubMed] [Google Scholar]
- 52.Lapeire L, Hendrix A, Lambein K, Van Bockstal M, Braems G, Van Den Broecke R, Limame R, Mestdagh P, Vandesompele J, Vanhove C, et al. Cancer-associated adipose tissue promotes breast cancer progression by paracrine oncostatin M and Jak/STAT3 signaling. Cancer Res. 2014;74:6806–6819. doi: 10.1158/0008-5472.CAN-14-0160. [DOI] [PubMed] [Google Scholar]
- 53.Duzagac F, Inan S, Simsek Ela F, Acikgoz E, Guven U, Khan SA, Rouhrazi H, Oltulu F, Aktug H, Erol A, Oktem G. JAK/STAT pathway interacts with intercellular cell adhesion molecule (ICAM) and vascular cell adhesion molecule (VCAM) while prostate cancer stem cells form tumor spheroids. J BUON. 2015;20:1250–1257. [PubMed] [Google Scholar]
- 54.Kim JM, Chen DS. Immune escape to PD-L1/PD-1 blockade: Seven steps to success (or failure) Ann Oncol. 2016;27:1492–1504. doi: 10.1093/annonc/mdw217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed datasets generated during the study are available from the corresponding author on reasonable request.