Introduction
G protein-coupled receptors (GPCRs) are a family of 7 transmembrane-spanning proteins that collectively serve as the largest group of therapeutic targets. Within cardiology, GPCRs such as alpha and beta adrenergic receptors, the angiotensin type I receptor, as well as the P2Y12 receptor, are the targets of a variety of widely used medications. Canonically, ligands for GPCRs have been characterized as agonists, which promote or stabilize conformational changes in the receptor that result in activation of heterotrimeric G proteins and generation of second messenger systems, or antagonists that block such activation. Work over the past two decades, however, has found that ligands can induce distinct “active” receptor conformations which activate only specific subsets of a given receptor’s functional repertoire1. In particular, ligands have been identified that exhibit “bias” or “functional selectivity” towards specific G proteins or even other signal transducers such as β-arrestins. Further exploration of this “biased” signaling biology has led to important changes in the way pharmacologic agents are developed and screened. Although these terms have been used interchangeably to characterize a variety of GPCR signaling and biological functions, for the purpose of this review we will utilize the terms “bias” or “functional selectivity” to refer specifically to the ability of a GPCR ligand to stimulate signaling through one signal transducer over another (e.g. β-arrestin vs. G protein).
Beyond the recognition and development of these biased agonists, researchers have discovered novel mechanisms by which these ligands interact with receptors and engender unique functional profiles. The majority of pharmacologic GPCR ligands target orthosteric binding sites, i.e. the binding site of the endogenous ligand on a given receptor. Recent work has also identified ligands that bind to allosteric, or topographically distinct, binding sites on the receptors. It is perhaps not surprising that allosteric ligands have been identified that stabilize biased receptor conformations and thus function as biased allosteric modulators.
Biased agonists in cardiovascular pharmacology
Several examples of biased agonists have been identified or developed for cardiovascular indications. For instance, carvedilol, a widely-used beta adrenergic receptor blocker, has been observed to function as an antagonist for G protein-mediated signaling and an agonist for β-arrestin-mediated signaling at beta adrenergic receptors2. While the clinical implications of this discovery have yet to be fully determined, this work helped form the framework for exploring this signaling physiology more broadly and with other receptors.
The angiotensin type I receptor (AT1R) is an important GPCR in cardiovascular biology and pharmacology and ligands targeting this receptor are widely used for a variety of conditions including hypertension and chronic systolic heart failure. Recent work has shown that AT1R β-arrestin-bias mediates the Frank-Starling mechanism of force generation3 and that β-arrestin biased agonists at the AT1R stimulate cardiac contractility while also serving to antagonize some of the deleterious effects of AT1R signaling mediated through G proteins (Gq). One such biased agonist at the AT1R, TRV120027, was subsequently developed and has progressed through clinical trials for the treatment of acute decompensated heart failure (ADHF). Although the results of BLAST-AHF, a phase IIb clinical trial utilizing TRV120027 in the treatment of ADHF failed to meet its primary endpoint, the experience with this agent nevertheless demonstrated that biased agonists can function as novel GPCR ligands and possess unique pharmacologic profiles relative to their unbiased counterparts. Additionally, it may be that the properties of β-arrestin-biased AT1R agonists are more appropriate for the treatment of chronic heart failure as opposed to ADHF4.
Biased agonists targeting the μ-opioid receptor have also reached the late stages of clinical development. Work over the past two decades has demonstrated that subsets of the effects of μ-opioid receptor agonists such as morphine are mediated distinctly by G proteins or β-arrestins such that many of the common side effects of these agents including gastrointestinal and respiratory dysfunction appear to be secondary to β-arrestin-dependent signaling, whereas the analgesic effect of these agents appears to be mediated by G protein-dependent signaling (Gi). Biased agents, acting as agonists for G protein-dependent signaling and antagonists for β-arrestin-dependent signaling, are currently in clinical development for the treatment of moderate to severe acute pain. One such agent, oliceridine, has reached the late stages of clinical development, and others are also in pre-clinical development5.
The recognition that ligands can selectively activate a subset of a given GPCR’s functional profile has altered the way drug discovery efforts are conceived and performed. Several orthosteric biased agonists have reached the late stages of clinical development or are currently under pre-clinical development for a variety of conditions. These agents may offer distinct pharmacologic outcomes relative to their unbiased counterparts, but the true clinical impact of these agents remains to be determined.
Allosteric drugs
In addition to these orthosteric biased agonists, recent work has also recognized the existence of allosteric biased modulators. As a class, allosteric modulators offer several distinct benefits over orthosteric ligands: 1) these ligands may avoid challenging chemical spaces associated with orthosteric binding sites of specific GPCRs; 2) they may offer enhanced receptor subtype selectivity by avoiding interaction with a highly conserved orthosteric binding site; 3) they may possess decreased potential for adverse side effects due to a ceiling level to their effects; and 4) they may allow for conservation of patterns of activity of the endogenous ligand without desensitizing the receptor itself. Both positive allosteric modulators, which potentiate the activity of orthosteric agonists, and negative allosteric modulators, which non-competitively decrease the activity of orthosteric agonists, have been reported. Additional, more complex allosteric ligands such as bitopic ligands, which may bind to both orthosteric and allosteric sites, have also been identified. It is precisely the increased complexity and functionality of these biased allosteric ligands that makes them attractive pharmacologic targets in that such ligands could be designed to precisely target the desired functions downstream of a given receptor, while avoiding effects on non-target signaling or undesired functional activities of a given receptor.
Summary
Biased GPCR ligands represent a novel and functionally distinct group of GPCR ligands from their unbiased counterparts. They offer the potential for more targeted therapeutics with less untoward side effects. Several biased ligands have progressed through the late stages of clinical development and more biased ligands will continue to be developed for different GPCRs. Biased allosteric modulators may have even more refined functional profiles than their orthosteric counterparts, and offer the possibility of designing, developing, and producing safer, more targeted pharmacologic therapies.
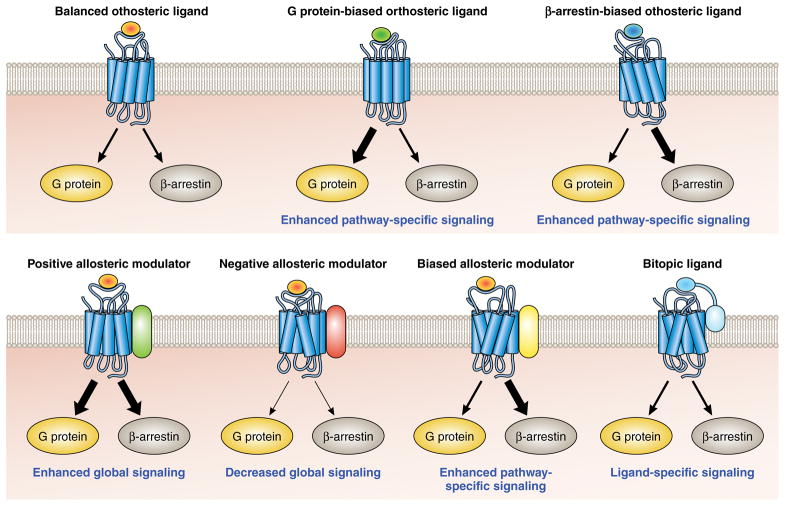
Figure 1. Potential pharmacologic mechanisms of biased G protein-coupled receptor signaling.
Multiple potential classes of biased pharmacologic compounds targeting GPCRs have been identified. These range from orthosteric ligands that interact with the binding site of the endogenous ligand, to allosteric modulators that bind a receptor at a site distinct from the endogenous ligand, to bitopic ligands that bind at both orthosteric and allosteric sites.
Glossary of terms
- Signaling bias
Alternatively labeled “functional selectivity”, this refers to a receptor’s ability to selectively engage or activate specific subsets of a given receptor’s downstream functions.
- Orthosteric ligand
A ligand that binds to the binding site of the endogenous ligand on a given receptor.
- Positive allosteric modulator
A ligand that binds a receptor at a site distinct from that of the endogenous ligand and which, upon binding to the receptor, potentiates the activity of the endogenous agonist.
- Negative allosteric modulator
A ligand that binds a receptor at a site distinct from that of the endogenous ligand and which, upon binding to the receptor, negatively modulates the activity of the endogenous agonist.
Footnotes
Conflict of Interest Disclosures: RJL and HAR report they are scientific co-founders and stockholders of Trevena, Inc. JWW has no potential conflicts to disclose.
References
- 1.Wisler JW, Xiao K, Thomsen AR, Lefkowitz RJ. Recent developments in biased agonism. Curr Opin Cell Biol. 2014;27:18–24. doi: 10.1016/j.ceb.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wisler JW, DeWire SM, Whalen EJ, Violin JD, Drake MT, Ahn S, Shenoy SK, Lefkowitz RJ. A unique mechanism of beta-blocker action: carvedilol stimulates beta-arrestin signaling. Proc Natl Acad Sci U S A. 2007;104:16657–16662. doi: 10.1073/pnas.0707936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abraham DM, Davis RT, 3rd, Warren CM, Mao L, Wolska BM, Solaro RJ, Rockman HA. beta-Arrestin mediates the Frank-Starling mechanism of cardiac contractility. Proc Natl Acad Sci U S A. 2016;113:14426–14431. doi: 10.1073/pnas.1609308113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryba DM, Li J, Cowan CL, Russell B, Wolska BM, Solaro RJ. Long-Term Biased beta-Arrestin Signaling Improves Cardiac Structure and Function in Dilated Cardiomyopathy. Circulation. 2017;135:1056–1070. doi: 10.1161/CIRCULATIONAHA.116.024482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manglik A, Lin H, Aryal DK, McCorvy JD, Dengler D, Corder G, Levit A, Kling RC, Bernat V, Hubner H, Huang XP, Sassano MF, Giguere PM, Lober S, Da D, Scherrer G, Kobilka BK, Gmeiner P, Roth BL, Shoichet BK. Structure-based discovery of opioid analgesics with reduced side effects. Nature. 2016;537:185–190. doi: 10.1038/nature19112. [DOI] [PMC free article] [PubMed] [Google Scholar]