Abstract
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) and other aryl hydrocarbon receptor (AhR) agonists have been shown to regulate bone development and remodeling in a species-, ligand-, and age-specific manner, however the underlying mechanisms remain poorly understood. In this study, we characterized the effect of 0.01–30 µg/kg TCDD on the femoral morphology of male and female juvenile mice orally gavaged every 4 days for 28 days and used RNA-Seq to investigate gene expression changes associated with the resultant phenotype. Micro-computed tomography revealed that TCDD dose-dependently increased trabecular bone volume fraction (BVF) 2.9- and 3.3-fold in male and female femurs, respectively. Decreased serum tartrate-resistant acid phosphatase (TRAP) levels, combined with a reduced osteoclast surface to bone surface ratio and repression of femoral proteases (cathepsin K, matrix metallopeptidase 13), suggests that TCDD impaired bone resorption. Increased osteoblast counts at the trabecular bone surface were consistent with a reciprocal reduction in the number of bone marrow adipocytes, suggesting AhR activation may direct mesenchymal stem cell differentiation towards osteoblasts rather than adipocytes. Notably, femoral expression of transmembrane glycoprotein NMB (Gpnmb; osteoactivin), a positive regulator of osteoblast differentiation and mineralization, was dose-dependently induced up to 18.8-fold by TCDD. Moreover, increased serum levels of 1,25-dihydroxyvitamin D3 were in accordance with the renal induction of 1α-hydroxylase Cyp27b1 and may contribute to impaired bone resorption. Collectively, the data suggest AhR activation tipped the bone remodeling balance towards bone formation, resulting in increased bone mass with reduced marrow adiposity.
Keywords: AhR, TCDD, Bone, Transmembrane glycoprotein NMB, Vitamin D
Graphical Abstract

INTRODUCTION
The skeletal system serves several vital functions in the body including: (i) providing structural support and the site of attachment for muscles, (ii) protecting internal organs such as the brain and bone marrow, and (iii) maintaining mineral (calcium, phosphorus) homeostasis and acid-base balance (Clarke, 2008). To preserve mechanical strength throughout life, bone undergoes continuous remodeling whereby old or microdamaged bone is resorbed by osteoclasts (i.e. bone resorption) and replaced with new bone formed by osteoblasts (i.e. bone formation). This remodeling is regulated by several hormones and cytokines including active vitamin D (1,25-dihydroxyvitamin D3), parathyroid hormone (PTH), calcitonin, receptor activator of nuclear factor KB ligand (RANKL), osteoprotegerin (OPG), tumor necrosis factor (TNF), interleukin-1 (IL-1), and IL-6 (Clarke, 2008).
During normal bone remodeling, resorption and formation are tightly coupled to prevent net changes in mass or mechanical strength. However, several factors including aging, hormonal changes (e.g. menopause), genetics, malabsorption (e.g vitamin D deficiency), drugs (e.g. glucocorticoids), and diseases (e.g. chronic kidney disease, hyperthyroidism) can disrupt this balance, leading to metabolic bone disorders (Feng and McDonald, 2011). The most prevalent metabolic bone disease is osteoporosis, which is characterized by decreased bone density, microarchitectural deterioration, and increased fracture risk (Eastell et al., 2016; Feng and McDonald, 2011). In contrast, osteosclerosis refers to a collection of hereditary and acquired disorders characterized by increased bone density (Ihde et al., 2011). This includes osteopetrosis, in which rare inherited mutations impair osteoclast function and thus resorption, resulting in increased bone mass (Feng and McDonald, 2011). Excessive bone accumulation produces not only brittle bones that are more susceptible to fractures, but also neurological complications from osseous nerve compression and anemia due to reduced marrow volume (Sobacchi et al., 2013).
To date, the role of environmental contaminants in the development of metabolic bone disorders has not been thoroughly investigated. However, several persistent environmental contaminants such as 2,3,7,8-tetrachlordibenzo-p-dioxin (TCDD) are potent activators of the aryl hydrocarbon receptor (AhR), which is known to regulate bone development, mineralization, and remodeling (Yu et al., 2017). The AhR is a basic helix-loop-helix Per-Arnt-Sim (PAS) transcription factor which elicits gene expression changes in a species-, sex-, age-, tissue-, and cell-specific manner (Denison and Nagy, 2003). Ligand binding initiates the dissociation of chaperone proteins, triggering translocation of the cytoplasmic AhR to the nucleus and heterodimerization with the aryl hydrocarbon receptor nuclear translocator (ARNT). The canonical pathway involves binding of the liganded AhR-ARNT complex to dioxin response elements (DREs) within the promoter region of target genes, leading to recruitment of transcriptional co-regulators and differential gene expression (Hankinson, 1995). However, an increasing number of studies report DRE-independent mechanisms of differential gene expression (Beischlag et al., 2008; Huang and Elferink, 2012).
Although several studies report that AhR activation alters bone mineralization, remodeling, and morphology, the resulting phenotypes differ between rodent models. For example, TCDD increases trabecular bone volume fraction (BVF) and mineral density (BMD) in adult mice (Herlin et al., 2013). In contrast, BMD is decreased in adult mice exposed to the less potent AhR agonist 3-methylcholanthrene (3MC), as well as in TCDD-treated mouse pups (Nishimura et al., 2009; Yu et al., 2015). In rats, BVF and BMD are also affected by AhR activation in a strain-, ligand-, and age-specific manner (Alvarez-Lloret et al., 2009; Finnila et al., 2010; Herlin et al., 2010; Jamsa et al., 2001; Lind et al., 2009). In vitro, TCDD impairs the differentiation and proliferation of various bone cells including osteoclast and osteoblast precursors (Carpi et al., 2009; Korkalainen et al., 2009; Yu et al., 2014a). Furthermore, both whole-body and osteoclast-specific AhR null mice exhibit increased BVF and reduced bone turnover, indicating AhR signaling plays a central role in normal bone development and homeostasis (Herlin et al., 2013; Yu et al., 2014b). In addition, AhR agonists affect the development of teeth, which can be considered mineralized exoskeleton. More specifically, TCDD impairs dentin formation in adult rat incisors, while polychlorinated dibenzodioxins (PCDDs) and dibenzofurans (PCDFs) have been associated with hypomineralization of tooth enamel in humans (Alaluusua et al., 1993; Alaluusua et al., 1999). However, the underlying mechanisms responsible for AhR-mediated alterations in bone morphology remain poorly understood.
The objectives of the current study were to (i) characterize the femoral phenotype in juvenile mice following repeated TCDD treatment, and (ii) investigate the underlying mechanisms by integrating dose-dependent femoral gene expression changes with serum biomarkers and metabolite levels. We report that TCDD dose-dependently increased femoral trabecular BVF while reducing bone marrow adiposity. Serum biomarker levels together with osteoclast counts indicate impaired bone resorption, while reciprocal changes in osteoblast and adipocyte counts suggest TCDD may alter mesenchymal stem cell (MSC) differentiation. Furthermore, induction of the positive osteoblastogenesis regulator transmembrane glycoprotein NMB (Gpnmb; aka osteoactivin) and dysregulation of vitamin D metabolism may play key roles in altering the balance between bone resorption and formation.
MATERIALS & METHODS
Animal Handling and Treatment
Postnatal day 25 (PND25) male and female C57BL/6 mice weighing within 10% of each other were obtained from Charles River Laboratories (Kingston, NY) and housed in Innovive Innocages (San Diego, CA) containing ALPHA-dri bedding (Shepherd Specialty Papers, Chicago, IL) in a 23°C environment with 30–40% humidity and a 12-hour (h) light/dark cycle (7am–7pm). Mice were provided Aquavive water (Innovive) and Harlan Teklad 22/5 Rodent Diet 8940 (Madison, WI) ad libitum, and were acclimated for 4 days (d) prior to treatment. (I) For the sex comparison study, male and female mice (PND28; n=8) were orally gavaged with sesame oil vehicle (Sigma-Aldrich, St. Louis, MO) or 30 µg/kg TCDD (AccuStandard, New Haven, CT) every 4d for a total of 28d (7 exposures; Supplementary Figure S1A). (II) For the dose-response study, only male mice (PND28; n=8) were orally gavaged with sesame oil vehicle, or 0.01, 0.03, 0.1, 0.3, 1, 3, 10, or 30 µg/kg TCDD every 4d for a total of 28d (7 exposures; Supplementary Figure S1B). (III) For the single dose study, male mice (PND28; n=5) were orally gavaged with a single dose of sesame oil vehicle or 30 µg/kg TCDD (1 exposure; Supplementary Figure S1C).
The doses used compensate for the relatively short study duration compared to lifelong cumulative human exposure from diverse AhR ligands, the bioaccumulative nature of halogenated AhR ligands, and differences in TCDD’s metabolism and half-life (humans: 1–11 years (Sorg et al., 2009; Wolfe et al., 1994), mice: 8–12d (Birnbaum, 1986; Gasiewicz et al., 1983)), and allow for computational modeling of dose-response curves. These doses result in mouse hepatic TCDD levels that span human background serum concentrations reported in the United States, Germany, Spain, and the United Kingdom to serum levels reported in Viktor Yushchenko 4–39 months following intentional poisoning (Nault et al., 2016a). All animal handling procedures were performed with the approval of the Michigan State University (MSU) Institutional Animal Care and Use Committee, in accordance with ethical guidelines and regulations.
Sample Collection
At 28d after the initial exposure (PND56) of the sex comparison and male dose-response studies, mice (fasted for 6h) were weighed and blood was collected from the submandibular vein prior to cervical dislocation. For the single dose study, animals were euthanized by cervical dislocation 7d after the initial exposure (PND35). Femurs were either: (i) cleaned of muscle and connective tissue, frozen in liquid nitrogen, and stored at −80°C, or (ii) fixed in 10% neutral buffered formalin and transferred to 70% ethanol 24 h later. Liver and kidneys were removed, frozen in liquid nitrogen, and stored at −80°C. Femurs, kidneys, and serum were collected in the sex comparison study, while femurs and livers were collected in the male dose-response study. For the single dose study, only femurs were collected. Sample sizes for the treatment groups of each endpoint measured are listed in Supplementary Table S1.
Micro-Computed Tomography
Formalin-fixed femurs were scanned using a GE Explore Locus micro-computed tomography (µCT) system with a voxel resolution of 20 µm obtained from 720 views, beam strength of 80 peak kV and 450 µA, and a beam angle increment of 0.5. Each scan consisted of bones from all experimental groups and a calibration phantom bone to maintain consistency throughout all scans. A fixed threshold of 496 was used to distinguish bone from marrow. Femur length was measured and a region of interest in the distal femur was analyzed and defined as 1% of the total length proximal to the growth plate and extending 2 mm toward the diaphysis and excluded the outer cortical bone. Trabecular bone mineral content (BMC), bone mineral density (BMD), bone volume fraction (BVF), thickness (Tb.Th.), spacing (Tb.Sp.), and number (Tb.N.) values were computed using GE Healthcare MicroView software for visualization and analysis of volumetric image data. Cortical BMC, BMD, inner and outer perimeter, and cortical and marrow area were measured in a 2 × 2 × 2 mm cube centered midway down the length of the bone using a threshold of 972 to distinguish bone from marrow. Estimation of the 10% benchmark dose (BMD10%) for the male dose-response morphology data was performed using the Benchmark Dose Software (BMDS v.2.6.0.1) developed by the U.S. EPA (https://www.epa.gov/bmds), where the model with the lowest Akaike’s Information Criterion (AIC) was selected as the best fitting model. If the ‘p-value for fit’ of the best fitting model was < 0.1, the fit was considered unacceptable and thus a BMD10% was not calculated.
Histopathology
Formalin-fixed femurs were vacuum infiltrated with paraffin using a Tissue-Tek VIP 2000 tissue processor (Sakura) and embedded with the Thermo Fisher HistoCentre III Embedding Center (Thermo Fisher, Waltham, Massachusetts) by the MSU Investigative Histopathology Laboratory (https://humanpathology.natsci.msu.edu/). Paraffin blocks were sectioned at 4–5 µm with a Reichert Jung 2030 rotary microtome (Reichert, Depew, New York) and dried for 2–24 h at 56°C to ensure adherence to slides. Slides were stained with hematoxylin and eosin (H&E) and photographed (10X magnification) using a Leitz Laborlux microscope and SPOT RT Color digital camera (Diagnostic Instruments, Inc.; Sterling Heights, Michigan). Adipocytes, greater than 15 µm diameter, were counted in the distal femur trabecular region (extending 2 mm from the growth plate toward the diaphysis). Area of analysis was determined using Image Pro-Plus software version 7.
For osteoclast and osteoblast quantification, slides were stained for tartrate-resistant acid phosphatase (TRAP) and counterstained with hematoxylin per manufacturer protocol (387A-IKT, Sigma). At least six images (40X magnification) per sample were taken at the distal metaphysis. TRAP-positive osteoclast surface along the bone surface was measured and expressed relative to the total bone surface. Osteoblast counts were performed by cellular morphology and consisted of cuboidal cells in direct contact with the bone surface. In addition, the ratio of osteoclasts to osteoblasts (OC/OB) was determined by dividing the number of osteoclasts by the number of osteoblasts. All measurements were performed using ImageJ (National Institute of Health, Bethesda, MD).
Serum Biochemistry
Serum TRAP (TRAcP 5b) activity was measured using a solid phase immunofixed enzyme activity assay (Immunodiagnostic Systems, Gaithersburg, MD), while serum amino-terminal propeptide of type 1 collagen (P1NP) and osteocalcin levels were quantified using enzyme immunoassays (Immunodiagnostic Systems and Alfa Aesar, Tewksbury, MA, respectively). Serum levels of free ionized calcium and phosphate were measured using colorimetric assays (Abcam, Cambridge, MA and Sigma-Aldrich, respectively). All colorimetric end products were measured using a Tecan (Männedorf, Switzerland) Infinite M200 plate reader. Serum levels of 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 were quantified using chemiluminescent immunoassays performed by MLabs (University of Michigan, Department of Pathology, Ann Arbor, MI).
RNA Extraction and Gene Expression Analysis
Frozen liver and kidney samples were homogenized in TRIzol using a Mixer Mill 300 tissue homogenizer (Retsch, Germany) and total RNA was isolated as previously described (Boverhof et al., 2005). Frozen femurs were crushed in liquid nitrogen with a Bessman Tissue Pulverizer (Spectrum Laboratories, Rancho Dominguez, CA) and total RNA was isolated using TRI Reagent (Molecular Research Center, Cincinnati, OH) according to the manufacturer’s instructions. RNA was quantified using a Nano-drop spectrophotometer (Thermo Scientific, Wilmington, DE) at 260 nm. Purity was assessed using the A260/A280 ratio and quality was analyzed using the Caliper LabChip GX (Perkin Elmer, Waltham, MA).
Dose-dependent gene expression in the male femur, and male and female liver was examined using RNA-Seq performed at the MSU Research Technology Support Facility (RTSF) Genomics Core (rtsf.natsci.msu.edu/genomics). Femoral libraries from three independent biological replicates (n=3) were prepared using the Illumina TruSeq RNA Sample Preparation Kit (Illumina, San Diego, CA). Libraries were quantified and sequenced as previously described, at a read depth of ~30M per sample (Fader et al., 2017a; Nault et al., 2015). Quality of reads was determined using FASTQC v0.11.3. Reads were mapped to the mouse reference genome (GRCm38 release 81) using Bowtie2 v2.2.6 and TopHat2 v2.1.0. For TCDD-mediated differential gene expression, fold changes were calculated relative to vehicle controls. Genes were considered differentially expressed if |fold change| ≥ 1.5 and statistical P1(t) value ≥ 0.8 at one or more doses. The male femur RNA-Seq dataset was deposited in the Gene Expression Omnibus (GEO; accession number GSE104551), while RNA-Seq datasets for the male liver (GSE87519) and female liver (GSE62902) were previously published (Fader et al., 2017a; Nault et al., 2015). The RNA-Seq results (fold changes, P1(t) values) for each gene discussed in the manuscript have been compiled in Supplementary Table S2. Dose-response modeling was performed using ToxResponse Modeler (Burgoon and Zacharewski, 2008), and median effective dose (ED50) values for differentially expressed genes (DEGs) exhibiting a sigmoidal response were reported in Supplementary Table S2. BMD10% and BMD10% lower confidence limits (BMDL) were calculated using BMDExpress (Yang et al., 2007) as previously described (Fader et al., 2015) and included in Supplementary Table S2.
Gene expression in male and female kidneys (vehicle vs. 30 µg/kg TCDD) was analyzed using quantitative real-time polymerase chain reaction (qRT-PCR). Total RNA was reverse transcribed by SuperScript II (Invitrogen) using oligo dT primer according to the manufacturer’s protocol. PCR amplification was conducted on a Bio-Rad CFX Connect Real-Time PCR Detection System. Gene expression relative to vehicle control was calculated using the 2−ΔΔCT method, where each sample was normalized to the geometric mean of 3 housekeeping genes (Gapdh, Ppia, and Pgk1) (Cui et al., 2009). Primer sequences are provided in Supplementary Table S3.
Enrichment Analysis
Male femur DEGs were analyzed for enriched functions using the Database for Annotation, Visualization, and Integrated Discovery (DAVID) v6.7. Only Gene Ontology (GO) Biological Prosses were considered. Enrichment scores (ES) ≥ 1.3 were considered significant, representing the −log scale geometric mean p-value of 0.05.
Putative DRE Identification and AhR Chromatin Immunoprecipitation (ChIP)
Putative DREs (pDREs) were previously identified (Nault et al., 2016b). Briefly, the regulatory region (10 kb upstream of the transcription start site together with 5′- and 3′-untranslated regions) and coding sequence of each gene was obtained from the University of California, Santa Cruz (UCSC) Genome Browser for mouse (mm10 GRCm38 build) and computationally searched for the DRE core consensus sequence 5′-GCGTG-3′. Each identified core was extended by 7 bp upstream and downstream, and the resulting 19 bp sequences were scored using a position weight matrix constructed from bona fide functional DREs. Matrix similarity scores (MSS) ≥ 0.856 were considered to be pDREs. Heat maps (Figures 6 and 7) indicate the presence/absence of pDREs within genes of interest while Supplementary Table S2 lists the number of pDREs and the location of the highest scoring pDRE for each gene discussed in the manuscript. UCSC genome browser tracks indicating pDRE locations within the mouse genome are available at http://dbzach.fst.msu.edu/index.php/supplementarydata.html.
Figure 6.
TCDD-elicited dose-dependent differential expression of femoral genes associated with heme biosynthesis, chemotaxis, ion transport, and bone remodeling. Male C57BL/6 mice were orally gavaged with sesame oil vehicle or 0.01–30 µg/kg TCDD every 4 days for 28 days. Color scale represents the log2(fold change) for differential gene expression, as determined by RNA-Seq analysis (3 biological replicates). Genes are listed by their official NCBI Entrez Gene symbol. The presence of putative dioxin response elements (pDREs; Matrix Similarity Score (MSS) ≥ 0.856) is indicated by a green box. Reads represent the maximum raw number of aligned reads to each transcript across doses indicating the potential level of femoral expression, where yellow represents a low level of expression (≤ 500 reads), and pink represents a higher level of expression (≥ 10,000).
Figure 7.
TCDD-elicited hepatic differential expression of genes involved in vitamin D metabolism and growth hormone signaling. Female and male C57BL/6 mice were orally gavaged with sesame oil vehicle or TCDD (0.01–30 µg/kg) every 4 days for 28 days. Color scale represents the log2(fold change) for differential gene expression, as determined by RNA-Seq analysis (5 biological replicates for females; 3 biological replicates for males). Genes are listed by their official NCBI Entrez Gene symbol. The presence of putative dioxin response elements (pDREs; Matrix Similarity Score (MSS) ≥ 0.856) and aryl hydrocarbon receptor (AhR) enrichment peaks (false discovery rate (FDR) ≤ 0.05) at 2 hours are shown as green boxes. Reads represent the maximum raw number of aligned reads to each transcript across doses indicating the potential level of hepatic expression, where yellow represents a low level of expression (≤ 500 reads), and pink represents a higher level of expression (≥ 10,000).
Hepatic AhR ChIP-Seq was previously performed on samples from female and male C57BL/6 mice 2 h following a single oral dose of 30 µg/kg TCDD (Fader et al., 2017a; Nault et al., 2016b). Supplementary Table S2 indicates whether AhR binding was detected within each hepatic gene discussed in the manuscript, while full ChIP-Seq datasets for the female and male liver are available on GEO (GSE97636 and GSE97634, respectively).
RESULTS
Micro-Computed Tomography
Long bones such as the femur consist of a shaft of dense cortical bone (diaphysis) surrounding the marrow, with a meshwork of spongy trabecular bone above and below (metaphysis) the epiphyseal plate at each end. Using high resolution µCT, the three-dimensional morphology of trabecular and cortical bone was evaluated in male and female mice gavaged with 30 µg/kg TCDD every 4d for 28d. In males, 30 µg/kg TCDD increased the trabecular BVF of the femur from 32% in controls to 94% in treated mice (2.9-fold increase). Similarly, the femoral trabecular BVF in female mice was increased from 28% in controls to 93% at 30 µg/kg TCDD (3.3-fold increase) (Figure 1A). This is consistent with a 5.6- and 5.0-fold increase in Tb.Th. in males and females, respectively (Figure 1C). Additionally, TCDD decreased Tb.Sp. 7.3- and 7.9-fold in males and females, respectively (Figure 1E), while Tb.N. was also decreased in both sexes (Figure 1G). Trabecular BMD was increased 2.5- and 2.7-fold in males and females, respectively, at 30 µg/kg TCDD (Figure 1I), while trabecular BMC was increased 2.6- and 2.2-fold, respectively (Figure 1K). TCDD-elicited changes in BVF, Tb.Th., Tb.Sp., BMD, and BMC were confirmed to be dose-dependent in male mice (Figure 1B,D,F,J,L), where BVF, Tb.Th., BMD, and BMC were increased at ≥ 10 µg/kg TCDD, while Tb.Sp. was decreased at ≥ 1 µg/kg TCDD. Using benchmark dose modeling software, the BMD10% values for male BVF, Tb.Sp., BMD, and BMC were determined to be 1.9 (Hill model), 0.6 (exponential model), 2.3 (Hill model), and 1.8 (exponential model) µg/kg TCDD, respectively. No model adequately fit the Tb.Th. or Tb.N. data. Representative isosurface images of the distal metaphysis region of the male femur demonstrate the dose-dependent effects of TCDD on the three-dimensional structure of mineralized trabecular bone (Figure 2). Male femur length was decreased ~3% at 30 µg/kg TCDD with no effect at ≤ 10 µg/kg (Supplementary Figure S2), and thus any effect on bone growth was negligible compared to altered trabecular morphology at ≥ 1 µg/kg.
Figure 1.
Micro-computed tomography evaluation of trabecular bone morphology in the femur following repeated TCDD dosing. In the sex comparison study (A,C,E,G,I,K), female (pink) and male (blue) C57BL/6 mice were orally gavaged with sesame oil vehicle or 30 µg/kg TCDD every 4 days for 28 days. In the dose-response study (B,D,F,H,J,L), male C57BL/6 mice were orally gavaged with sesame oil vehicle or 0.01–30 µg/kg TCDD every 4 days for 28 days. Bars represent the average of 7–8 biological replicates (A,C,E,G,I,K) or 6–8 biological replicates (B,D,F,H,J,L) + standard error of the mean. Statistical significance (* p ≤ 0.05) was determined using either a Student’s t-test (A,C,E,G,I,K) or a one-way ANOVA analysis followed by Dunnet’s post hoc test (B,D,F,H,J,L). Benchmark dose (BMD10%) values were determined using the U.S. EPA’s Benchmark Dose Software (BMDS).
Figure 2.
Representative isosurface images of trabecular bone morphology in the femur of male C57BL/6 mice orally gavaged with sesame oil vehicle or 0.01–30 µg/kg TCDD every 4 days for 28 days. Images were generated using GE Healthcare MicroView software and depict the three-dimensional structure of the mineralized trabecular bone within the distal metaphysis of the femur. Benchmark dose (BMD10%) values for trabecular spacing and bone volume fraction were determined to be 0.6 and 1.9 µg/kg TCDD, respectively.
µCT analysis of the cortical portion of the femur revealed modest changes. In the male dose-response study, the outer perimeter, marrow area, and BMD of the cortical bone were decreased at 30 µg/kg TCDD (Table 1). However, these minor changes were not confirmed in the male vs. female comparison. TCDD had no effect on cortical area, inner perimeter, or BMC in either the male dose-response study or the male vs. female comparison. These modest effects on cortical morphological parameters can likely be attributed to the lower metabolic activity of cortical compared to trabecular bone.
Table 1.
Micro-computed tomography evaluation of cortical bone morphology in the femur following repeated TCDD dosing
| Sex | TCDD (µg/kg) |
Inner Perimeter (mm) |
Outer Perimeter (mm) |
Marrow Area (mm2) |
Cortical Area (mm2) |
Mineral Density (mg/cm3) |
Mineral Content (µg) |
|
|---|---|---|---|---|---|---|---|---|
| Sex Comparison | Female | 0 | 3.54 ± 0.05 | 4.84 ± 0.04 | 0.89 ± 0.02 | 0.81 ± 0.02 | 667 ± 14 | 10.7 ± 0.5 |
| 30 | 3.47 ± 0.06 | 4.81 ± 0.03 | 0.87 ± 0.03 | 0.80 ± 0.02 | 669 ± 11 | 10.4 ± 0.3 | ||
| Male | 0 | 3.68 ± 0.06 | 5.02 ± 0.06 | 0.95 ± 0.02 | 0.84 ± 0.06 | 662 ± 16 | 10.6 ± 0.8 | |
| 30 | 3.66 ± 0.02 | 4.90 ± 0.01 | 0.95 ± 0.01 | 0.76 ± 0.01 | 635 ± 7 | 8.9 ± 0.3 | ||
| Dose Response | Male | 0 | 3.85 ± 0.06 | 4.95 ± 0.06 | 1.05 ± 0.04 | 0.69 ± 0.02 | 766 ± 16 | 9.6 ± 0.6 |
| 0.01 | 3.66 ± 0.05 | 4.84 ± 0.06 | 0.95 ± 0.02 | 0.72 ± 0.03 | 769 ± 22 | 10.4 ± 0.7 | ||
| 0.03 | 3.63 ± 0.09 | 4.90 ± 0.04 | 0.94 ± 0.04 | 0.74 ± 0.04 | 744 ± 12 | 10.4 ± 0.9 | ||
| 0.1 | 3.62 ± 0.06 | 4.93 ± 0.05 | 0.94 ± 0.03 | 0.78 ± 0.03 | 801 ± 22 | 12.2 ± 0.7 | ||
| 0.3 | 3.75 ± 0.04 | 4.97 ± 0.02 | 1.01 ± 0.02 | 0.76 ± 0.02 | 725 ± 27 | 10.4 ± 0.5 | ||
| 1 | 3.66 ± 0.08 | 4.88 ± 0.05 | 0.94 ± 0.03 | 0.73 ± 0.05 | 735 ± 13 | 10.1 ± 1.0 | ||
| 3 | 3.65 ± 0.05 | 4.86 ± 0.06 | 0.94 ± 0.02 | 0.73 ± 0.01 | 702 ± 10 | 9.7 ± 0.3 | ||
| 10 | 3.64 ± 0.07 | 4.86 ± 0.03 | 0.94 ± 0.03 | 0.75 ± 0.04 | 702 ± 19 | 9.9 ± 0.8 | ||
| 30 | 3.67 ± 0.08 | 4.75 ± 0.07* | 0.93 ± 0.03* | 0.63 ± 0.03 | 652 ± 14* | 7.2 ± 0.6 |
Statistical significance (* p ≤ 0.05) was determined using either a Student’s t-test (sex comparison study) or a one-way ANOVA analysis followed by Dunnet’s post hoc test (dose-response study).
To examine the rapidity of onset of these changes in bone morphology, µCT analysis was also performed on femurs collected from male mice 7d after a single oral gavage of 30 µg/kg TCDD. Trabecular BVF of the femur increased from 32% in controls to 68% (2.1-fold) in TCDD-treated mice (Figure 3A). Similarly, Tb.Th. was increased 2.1-fold (Figure 3B), while Tb.Sp. was decreased 2.3-fold by TCDD (Figure 3C). Additionally, trabecular BMD and BMC were increased 1.7- and 1.6-fold, respectively (Figure 3E,F). Representative isosurface images of the distal metaphysis region demonstrate the effects of a single TCDD dose on the trabecular microarchitecture of the femur (Figure 3G). These data indicate that TCDD-elicited alterations in trabecular morphology develop rapidly, where a single bolus dose of 30 µg/kg TCDD was sufficient to increase trabecular BVF (68%) and repeated TCDD dosing further exacerbated this phenotype (94%). Interestingly, the outer perimeter, cortical area, and BMC of the cortical bone were modestly increased at 30 µg/kg TCDD (Table 2), in contrast to the modest decreases observed in cortical bone following repeated dosing. The remainder of the manuscript will focus on TCDD-elicited changes after 28d of treatment.
Figure 3.
Micro-computed tomography evaluation of trabecular bone morphology in the femur following a single dose of TCDD. Male C57BL/6 mice were orally gavaged with a single dose of sesame oil vehicle or 30 µg/kg TCDD, and the femurs were collected 7 days later. (A–F) Bars represent the average of 5 biological replicates + standard error of the mean. Statistical significance (* p ≤ 0.05) was determined using a Student’s t-test. (G) Representative isosurface images of trabecular bone morphology were generated using GE Healthcare MicroView software and depict the three-dimensional structure of the mineralized trabecular bone within the distal metaphysis of the femur.
Table 2.
Micro-computed tomography evaluation of cortical bone morphology in the femur following a single dose of TCDD
| TCDD (µg/kg) |
Inner Perimeter (mm) |
Outer Perimeter (mm) |
Marrow Area (mm2) |
Cortical Area (mm2) |
Mineral Density (mg/cm3) |
Mineral Content (µg) |
|---|---|---|---|---|---|---|
| 0 | 3.58 ± 0.04 | 4.65 ± 0.02 | 0.93 ± 0.02 | 0.57 ± 0.02 | 672 ± 5 | 6.6 ± 0.4 |
| 30 | 3.66 ± 0.02 | 4.80 ± 0.03* | 0.97 ± 0.01 | 0.66 ± 0.02* | 695 ± 12 | 8.5 ± 0.4* |
Statistical significance (* p ≤ 0.05) was determined using a Student’s t-test
Bone Cell Composition
To investigate the effect of TCDD on bone cell composition, the number of osteoclasts on the trabecular bone surface was evaluated through staining for TRAP, which is secreted by osteoclasts to digest type I collagen within the bone matrix (Figure 4A). TRAP staining of the female femurs revealed a 1.3-fold decrease in the osteoclast surface to bone surface ratio at the distal metaphysis, while male femurs exhibited a non-significant 1.5-fold decrease (Figure 4C). Osteoblast counts relative to bone surface were increased 1.4-fold in TCDD treated male femurs. Paradoxically, the relative osteoblast number was unaffected in females (Figure 4A,D). The osteoclasts to osteoblasts ratio was decreased 1.5-fold in females, with a trending 1.7-fold (non-significant) decrease in males (Figure 4E). Consistent with a reciprocal relationship between osteoblasts and adipocytes, assessment of H&E-stained femurs revealed suppression of bone marrow adiposity at 30 µg/kg TCDD, with a 4.1-fold reduction in adipocyte (diameter > 15 µm) number in the distal trabecular region of both male and female mice (Figure 4B,F). Overall, the data suggest the bone remodeling balance was tipped towards bone formation, consistent with the increase in trabecular BVF.
Figure 4.
TCDD-elicited effects on osteoclast, osteoblast, and adipocyte counts in the distal trabecular region of the femur. Female and male C57BL/6 mice were orally gavaged with sesame oil vehicle or 30 µg/kg TCDD every 4 days for 28 days. (A) Representative microphotographs of tartrate-resistant acid phosphatase (TRAP)-stained femurs at 40X, where black arrows indicate osteoblasts and black arrow heads indicate osteoclasts. (B) Representative microphotographs of hematoxylin and eosin (H&E)-stained femurs at 10X, where white arrows indicate bone marrow adipocytes. Quantification of (C) TRAP-positive osteoclast surface along bone per µm bone surface, (D) osteoblast count per µm bone surface, (E) the ratio of osteoclasts to osteoblasts, and (F) adipocyte number per mm2 area. Bars represent the average of 6–8 biological replicates + standard error of the mean. Statistical significance (* p ≤ 0.05) was determined using a Student’s t-test.
Serum Biomarkers of Bone Turnover
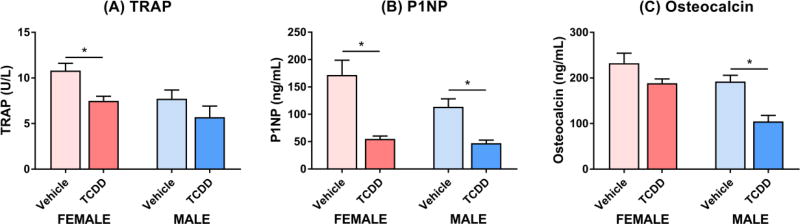
The effect of TCDD on bone turnover was further evaluated using serum biomarkers of resorption and formation. Serum levels of TRAP, a biomarker of osteoclastic activity and bone resorption (Wheater et al., 2013), were decreased 1.4-fold in females at 30 µg/kg TCDD, with a non-significant 1.4-fold decrease in males (Figure 5A). This is in accordance with decreases in the osteoclast surface to bone surface ratio, confirming bone resorption was impaired. P1NP and osteocalcin, which are released by osteoblasts during collagen deposition and matrix mineralization, were used as biomarkers of bone formation and osteoblastic activity (Wheater et al., 2013). TCDD decreased serum P1NP 2.4- and 3.1-fold in male and female mice, respectively (Figure 5B). Similarly, serum levels of osteocalcin were decreased 1.8-fold in male mice treated with 30 µg/kg TCDD, while females exhibited a non-significant 1.2-fold decrease (Figure 5C). However, given the observed increases in trabecular BVF and osteoblast counts, the decreased P1NP and osteocalcin levels following 28d of treatment may reflect negative feedback in response to enhanced bone formation earlier in the study. Despite changes in bone remodeling, serum concentrations of ionized calcium and inorganic phosphate were unaffected by TCDD in both sexes (Supplementary Figure S3).
Figure 5.
Serum levels of bone remodeling markers in female (pink) and male (blue) C57BL/6 mice orally gavaged with sesame oil vehicle or 30 µg/kg TCDD every 4 days for 28 days. (A) Tartrate-resistant acid phosphatase (TRAP) is secreted by osteoclasts to digest type I collagen and is therefore a marker of bone resorption. (B) Amino-terminal propeptide of type 1 collagen (P1NP) is a byproduct of osteoblast-mediated pro-collagen cleavage and is therefore a marker of bone formation. (C) Osteocalcin is also a marker of bone formation as it is secreted by osteoblasts. Bars represent the average of 6–8 biological replicates (A,C) or 5 biological replicates (B) + standard error of the mean. Statistical significance (* p ≤ 0.05) was determined using a Student’s t-test.
Femoral RNA-Seq Analysis
To investigate the underlying mechanisms responsible for the altered trabecular morphology, dose-dependent transcriptomic changes were analyzed in the femurs of male mice. RNA-Seq analysis detected 21,930 genes expressed in the femur, of which 1,720 were differentially expressed (|fold change| ≥ 1.5, P1(t) ≥ 0.8) at one or more doses. Cytochrome P450 1A1 (Cyp1a1) was induced 85.8-fold, as were other AhR target genes including AhR repressor (Ahrr; 10.3-fold), phosphoenolpyruvate carboxykinase 1 (Pck1; 4.7-fold), Cyp1b1 (3.9-fold), and NAD(P)H dehydrogenase quinone 1 (Nqo1; 1.4-fold).
DAVID analysis of the 1,720 DEGs identified 23 enriched functional clusters (ES ≥ 1.3) (Supplementary Figure S4). Of particular interest was TCDD-elicited differential gene expression associated with bone development and regulation of mineralization, ion transport, erythrocyte development, heme metabolism, chemotaxis, and inflammatory response. Several genes involved in heme biosynthesis were repressed, including the rate-limiting enzymes delta-aminolevulinate synthase 2 (Alas2; 1.6-fold) and ferrochelatase (Fech; 1.6-fold) (Fujiwara and Harigae, 2015), as well as hydroxymethylbilane synthase (Hmbs; 1.7-fold), uroporphyrinogen III synthase (Uros; 2.0-fold), and coproporphyrinogen oxidase (Cpox; 1.6-fold) (Figure 6). This repression of the heme biosynthetic pathway is consistent with hepatic repression of heme biosynthesis in TCDD-treated male mice (Fader et al., 2017a), and may impair erythrocyte formation within the bone marrow. TCDD also induced several chemokines (Ccl2, Ccl7, Ccl12, Cxcl2, Cxcl3, Cxcl13, Cxcl16, Xcl1) and chemokine receptors (Ccr1, Ccr7, Cxcr1, Cxcr2, Cx3cr1), while Ccr3 and Ccr9 were repressed. Similarly, benzo(a)pyrene has been reported to induce several inflammatory factors including Cxcl2 and Cxcl3 in freshly isolated mouse bone marrow cells through an AhR-dependent mechanism (N’Jai A et al., 2011). The expression of bicarbonate (e.g. Slc4a1, Slc4a8, Slc26a7), calcium (e.g. Atp2b4, Cacna1g, Cacna1e, Cacna1i), potassium (e.g. Atp1a3, Kcna2, Kcnj5, Slc12a5, Kcne4), and iron (e.g. Slc25a37, Slc11a2, Steap3, Trf) transporters were also altered (Figure 6). Genes associated with bone development and regulation of mineralization will be discussed below, specifically focusing on: (i) osteoblast and adipocyte differentiation, (ii) osteoclast formation and function, and (iii) vitamin D metabolism.
Osteoblast and Adipocyte Differentiation
Bone marrow MSCs are the common progenitor of both osteoblasts and adipocytes, where the differentiation balance between osteogenesis and adipogenesis is regulated by several lineage-specific transcription factors and signaling pathways. Peroxisome proliferator activated receptor ϒ (PPARϒ) and CCAAT/enhancer binding proteins (C/EBPs) dictate adipogenic differentiation, while runt related transcription factor 2 (RUNX2) and SP7 (aka osterix) are required for osteogenic differentiation. In addition, wingless-type MMTV integration site (Wnt) signaling promotes osteogenesis while inhibiting adipogenesis. Despite the substantial decrease in bone marrow adiposity, adipogenic lineage markers such as Cebpb and Cebpd were induced 3.9 and 1.6-fold in the male femur, respectively, while Pparg and Cebpa were unaffected by TCDD. TCDD also induced PPARϒ target genes including lipoprotein lipase (Lpl; 2.0-fold) and fatty acid binding protein 4 (Fabp4; 1.5-fold) (Figure 6). In contrast to the increased osteoblast count in the male femur, femoral expression of osteogenic transcription factors (Runx2, Sp7) and key players of the Wnt signaling pathway (Wnt10b, Lrp5, Lrp6, Ctnnb1) were unaffected by treatment.
Despite having little effect on Runx2 or Wnt signaling members, the expression of several genes involved in the regulation of osteoblast differentiation was altered. Notably, TCDD dose-dependently induced femoral expression of Gpnmb (aka osteoactivin) 18.8-fold, with a BMD10% of 0.84 µg/kg TCDD. GPNMB is a positive regulator of osteoblastogenesis where overexpression promotes the differentiation of osteoprogenitor cells to functional osteoblasts (Abdelmagid et al., 2008; Frara et al., 2016). Furthermore, TCDD induced several members of the S100 superfamily of calcium-binding proteins, including S100a8 (1.9-fold), S100a9 (1.8 fold), and S100a11 (1.9-fold). S100a8 and S100a9, which are involved in osteoblast differentiation and regulation of resorption (Zreiqat et al., 2007), were among the most abundantly expressed genes. In contrast, S100a4, a negative regulator of osteoblast differentiation and matrix mineralization, was repressed 2.2-fold (Figure 6) (Duarte et al., 2003).
Osteoclast Formation and Function
Osteoclast formation and activation are regulated by several cytokines including colony-stimulating factor (CSF1) and the ratio of RANKL to OPG. Osteoblast-secreted RANKL binds to receptor activator of NF-κB (RANK) on the surface of osteoclast precursors to initiate the formation of mature multinucleated osteoclasts, while macrophage CSF is required for the recruitment and differentiation of osteoclast precursors. Osteoblasts also secrete OPG, which serves as a decoy receptor to regulate the interaction between RANK and RANKL. Interestingly, male femoral Csf1 (encodes CSF) and Tnfsf11 (encodes RANKL) were induced 1.3- and 2.5 fold, respectively, while Tnfrsf11b (encodes OPG) expression was unaffected by TCDD (Figure 6).
During resorption, acidification of the osteoclast-bone surface interface is required for mineral solubilization and organic matrix digestion. Vacuolar H+-adenosine triphosphatase (V-type H+-ATPase), localized on the ruffled border of osteoclasts, secretes protons into the resorption pit. TCDD repressed femoral expression of the V0D2 subunit of this pump (Atp6v0d2; 1.5-fold), while 22 other subunits were unaffected. Additionally, carbonic anhydrase II (Car2), which generates most of the protons required by the V-type H+-ATPase, was repressed 1.7-fold (Figure 6). Interestingly, a Car2 mutation resulting in loss of function is associated with human ‘osteopetrosis with renal tubular acidosis’ (Stark and Savarirayan, 2009).
Following resorption pit acidification, osteoclasts secrete two major groups of endoproteases to remodel the extracellular organic matrix: (i) cathepsin cysteine proteases, and (ii) metalloproteinases consisting of matrix metalloproteinases (MMPs), a disintegrin and metalloproteinase domain (ADAMs), and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTSs). Femoral expression of Ctsk, the principal cysteine protease responsible for type I collagen degradation, was repressed 1.7-fold by TCDD in male mice. Similarly, Mmp13, which is required for collagen cleavage in the resorption pit, was repressed 1.7-fold. In contrast, TCDD induced femoral expression of several MMPs including Mmp8 (2.2-fold), Mmp9 (1.5-fold), Mmp15 (1.5-fold), and Mmp25 (2.4-fold), although their contribution to resorption remains unclear. Adam8, which plays a key role in osteoclast differentiation, was induced 2.1-fold. Additionally, femoral expression Adam19 and Adam21 was induced 1.7-fold, while Adam11 and Adam12 were repressed 1.8- and 1.6-fold, respectively. Femoral Adamts1 (2.0-fold), Adamts9 (1.6-fold), Adamts15 (2.2-fold), and Adamts20 (1.8-fold) were also induced (Figure 6). These results suggest TCDD altered protease-mediated remodeling of the organic bone matrix in the femur.
Vitamin D Metabolism
Bone resorption is regulated not only by locally produced cytokines, but also by hormones such as vitamin D. Vitamin D3 (cholecalciferol) is a prohormone consumed in the diet or synthesized in human skin, which undergoes two hydroxylation reactions before acquiring activity. The first hydroxylation is catalyzed by hepatic CYP2R1 to yield 25-hydroxyvitamin D3 (25-OH-D3), while CYP27A1 contributes to a lesser extent (Sawada et al., 2000; Zhu et al., 2013). TCDD dose-dependently repressed hepatic Cyp2r1 2.3-fold in females and 3.9-fold in males. Hepatic Cyp27a1 was also repressed 1.6- and 2.4-fold in female and male mice, respectively (Figure 7). Accordingly, serum 25-OH-D3 levels were reduced 1.4- and 1.3-fold in female and male mice, respectively, at 30 µg/kg TCDD (Figure 8A, Figure 9).
Figure 8.
The effect of TCDD on serum vitamin D metabolites and renal vitamin D metabolizing enzymes. Female (pink) and male (blue) C57BL/6 mice were orally gavaged with sesame oil vehicle or 30 µg/kg TCDD every 4 days for 28 days. Serum levels of (A) 25-OH-vitamin D3 and (B) 1,25-(OH)2-vitamin D3 were measured using chemiluminescent immunoassays, while renal expression of (C) Cyp27b1 and (D) Cyp24a1 was assessed through quantitative real-time polymerase chain reaction (qRT-PCR). Bars represent the average of 3–5 biological replicates (A,B), 4–5 biological replicates (C), or 5 biological replicates (D) + standard error of the mean. Statistical significance (*p ≤ 0.05) was determined using a Student’s t-test.
Figure 9.
TCDD-elicited dysregulation of vitamin D metabolism involves interactions between kidney, liver, and bone. Hepatic repression of Cyp2r1 and Cyp27a1 decreased serum levels of the pro-hormone form of vitamin D (25-OH-D3), while renal induction of Cyp27b1 increased serum levels of active vitamin D (1,25-(OH)2-D3). However, this induction of Cyp27b1 is not adequately explained by changes in fibroblast growth factor 23 (FGF23) or insulin-like growth factor 1 (IGF1) signaling, potentially suggesting direct AhR regulation. For genes and metabolites, red indicates increased levels, blue indicates decreased levels, and grey indicates not measured. Green circles indicate induction/activation, while orange rectangles indicate repression/inhibition.
Circulating 25-OH-D3 is bound to the vitamin D binding protein (DBP; aka group specific component, GC) to prolong its half-life and maintain stable stores. TCDD dose-dependently repressed hepatic Gc 1.4- and 4.3-fold in female and male mice, respectively (Figure 7), possibly decreasing 25-OH-D3 stability and serum levels. Beyond its role as a transport protein, GC also exerts immunomodulatory properties following transformation to macrophage activating factor (GC-MAF), which not only activates macrophages at sites of inflammation, but also promotes bone resorption (Adebanjo et al., 1998). Transformation to GC-MAF involves sequential deglycosylations mediated by galactosylceramidase (GALC) and neuraminidase 1 (NEU1), expressed by B cells and T cells, respectively (Yamamoto and Kumashiro, 1993). TCDD induced hepatic female and male Galc expression 1.3- and 1.7-fold, respectively, while Neu1 was induced 1.8-fold in both sexes (Figure 7). Increased Galc and Neu1 mRNA levels may be due to dose-dependent immune cell infiltration into the liver following treatment (Boverhof et al., 2005; Fader et al., 2017b). Despite the increased potential for transformation, Gc repression likely limits GC-MAF levels and may contribute to the TCDD-elicited dysregulation of bone resorption.
Following endocytic uptake into the proximal tubule of the kidney, 25-OH-D3 is further hydroxylated by CYP27B1 to yield 1α,25-dihydroxyvitamin D3 (1,25-(OH)2-D3, aka calcitriol), the hormonally active vitamin D form. Renal Cyp27b1 was induced 13.6- and 13.2-fold in females and males, respectively, at 30 µg/kg TCDD (Figure 8C). According, serum 1,25-(OH)2-D3 levels were increased 1.6-fold in female mice, while male levels exhibited a non-significant 1.3-fold increase (Figure 8B, Figure 9). Alternatively, 25-OH-D3 and 1,25-(OH)2-D3 can be hydroxylated by CYP24A1 to inactive 24,25-dihydroxyvitamin D3 and 1,24,25-trihydroxyvitamin D3, respectively. Renal Cyp24a1 was repressed 2.2-fold in male mice, while females displayed a non-significant 1.6-fold decrease, suggesting CYP24A1-mediated inactivation of vitamin D3 metabolites may be reduced (Figure 8D).
Renal Cyp27b1 expression and thus active vitamin D synthesis are tightly regulated by PTH, fibroblast growth factor 23 (FGF23), and insulin-like growth factor 1 (IGF1), as well as 1,25-(OH)2-D3 itself, to maintain mineral and bone homeostasis (Brenza and DeLuca, 2000). FGF23, which is secreted by osteocytes and osteoblasts, suppresses renal Cyp27b1 expression (Perwad et al., 2007). Fgf23 was dose-dependently induced 6.1-fold in the male femur (Figure 6), possibly by increased circulating 1,25-(OH)2-D3 which triggers negative feedback (Liu et al., 2006) (Figure 9). Active 1,25-(OH)2-D3 synthesis is also regulated by the growth hormone (GH)-IGF1 axis, whereby IGF1 induces renal Cyp27b1 expression (Gomez, 2006; Wei et al., 1998). TCDD dose-dependently repressed hepatic growth hormone receptor (Ghr) 2.4- and 8.7-fold in female and male mice, respectively. TCDD also repressed male hepatic Igf1 (8.0-fold), the acid labile subunit (Igfals; 37.9-fold), and the IGF binding protein 3 (Igfbp3; 1.7-fold). In contrast, hepatic Igf1 and Igfals were unaffected in female mice, while Igfbp3 was induced 1.6-fold (Figure 7). Thus, although the GH-IGF1 axis is compromised in males, IGF1 signaling is likely not responsible for Cyp27b1 induction (Figure 9).
The endocrine effects of 1,25-(OH)2-D3 on bone resorption are mediated through activation of the vitamin D receptor (Vdr), which was induced 2.2-fold in the male femur. In osteoblasts, VDR induces Tnfsf11, secreted phosphoprotein 1 (Spp1, aka osteopontin), progressive ankylosis (Ank), and ectonucleotide pyrophosphatase/phosphodiesterases (Enpp1 and Enpp3) to promote resorption and suppress mineralization. The induction of Tnfsf11 (2.5 fold) and Spp1 (1.6-fold) in the male femur is consistent with increased 1,25-(OH)2-D3, while Ank, Enpp1, and Enpp3 were unaffected (Figure 6).
DISCUSSION
Activation and knockout studies demonstrate the AhR plays an underappreciated role in the regulation of bone development and bone cell differentiation (Carpi et al., 2009; Korkalainen et al., 2009; Yu et al., 2014a; Yu et al., 2014b). Consequently, understanding the mechanisms through which AhR activation affects bone remodeling will help to elucidate the role of environmental contaminants in the development of metabolic bone disorders and may uncover novel therapeutic targets. In this study, we characterized the femoral phenotype resulting from repeated TCDD treatment of juvenile mice and investigated the dose-dependent femoral gene expression changes associated with this phenotype. TCDD-elicited alterations in the trabecular morphology of the femur were rapid and sensitive, with increased bone mass as early as 7d following a single dose and effects at doses as low as 1 µg/kg TCDD. Comparable effects in both sexes after 28d suggest the anti-estrogenic activity of TCDD contributes little to the underlying mechanism (Zacharewski and Safe, 1998). Our results are consistent with reported increases in trabecular BVF and BMD in adult mice treated with a total dose of 200 µg/kg TCDD over 10 weeks (Herlin et al., 2013).
Reduced serum levels of the resorption biomarker TRAP, combined with a reduction in the osteoclast surface to bone surface ratio, suggests TCDD impairs bone resorption. This is consistent with femoral repression of genes critical for osteoclast activity including enzymes involved in organic matrix degradation (Ctsk, Mmp13) and resorption pit acidification (Car2). Paradoxically, cytokines which promote osteoclast formation and function including Tnfsf11 (encodes RANKL) and Csf1 were induced in the femur, possibly in an attempt to overcome impaired resorption. Despite decreased serum levels of the bone formation biomarkers P1NP and osteocalcin, the osteoblast count at the trabecular bone surface was increased in male femurs. Given the increased trabecular BVF, TCDD appears to have tipped the bone remodeling balance towards bone formation, resulting in a net accumulation of mineralized bone. Interestingly, increased bone mass resulting from reduced bone resorption is characteristic of human osteopetrosis, where the fold increase in the trabecular BMD of patients with autosomal dominant osteopetrosis (~2- to 3-fold) is comparable to that reported in this study (Arruda et al., 2016).
Histopathological assessment of the femoral bone marrow compartment revealed TCDD decreased the number of bone marrow adipocytes in the distal trabecular region of both sexes. Epidemiological studies report an inverse relationship between BVF and bone marrow adiposity, with increased adipose tissue volume in the bone marrow of osteoporosis patients and impaired adipocyte differentiation in MSCs from patients with malignant infantile osteopetrosis (Justesen et al., 2001; Uckan et al., 2009). This inverse relationship may be due to MSC differentiation switching between osteogenic and adipocytic lineages, and/or altered levels of adipocyte-secreted adipokines (leptin and adiponectin) which affect osteoclast formation and function. TCDD decreases serum adiponectin levels in female mice despite no change in gonadal white adipose tissue weight (Nault et al., 2016a), suggesting reduced adipokine secretion may be due to fewer marrow adipocytes. Interestingly, AhR was recently identified as a key regulator of MSC multipotency during differentiation towards osteoblasts and adipocytes (Gérard et al., 2017), and therefore AhR activation may favor osteogenesis over adipogenesis. Consistent with this hypothesis, AhR has been identified as a negative regulator of adipocyte differentiation in both primary mouse embryo fibroblasts and human MSCs (Alexander et al., 1998; Podechard et al., 2009). MSC differentiation potential decreases drastically with age (Fafian-Labora et al., 2015; Kretlow et al., 2008), which may explain the negligible effects of TCDD on osteoblast and adipocyte differentiation markers at PND56. Furthermore, the fold change increase in the trabecular BVF of our juvenile mice was substantially greater than other studies demonstrating AhR-mediated BVF effects in adult rodents (≥ 8 weeks at the start of treatment) (Herlin et al., 2013; Jamsa et al., 2001; Yu et al., 2015), suggesting the sensitivity of bone to AhR ligands may decrease with age. MSC differentiation may therefore represent a key AhR target which contributes to this critical window of exposure.
Gpnmb (aka osteoactivin), a positive regulator of osteoblast differentiation and matrix mineralization, was the third most highly induced gene identified by RNA-Seq (18.8-fold) (Abdelmagid et al., 2008; Frara et al., 2016). The BMD10% for Gpnmb induction (0.84 µg/kg TCDD) was comparable to that of the most sensitive microarchitecture parameter (Tb.Sp.; 0.6 µg/kg TCDD), suggesting a mechanistic link to TCDD-elicited changes in trabecular morphology. Notably, Gpnmb is induced 3- to 4-fold in the long bones of a rat osteopetrosis model (Safadi et al., 2001). Furthermore, transgenic overexpression increases trabecular BVF and bone formation while decreasing resorption (Frara et al., 2016), consistent with the changes reported in this study. Gpnmb is also induced >1200-fold in the liver of TCDD-treated mice with AhR binding detected within its promoter (Fader et al., 2017a; Nault et al., 2016a), suggesting direct AhR regulation.
TCDD decreased serum levels of vitamin D precursor 25-OH-D3, while active 1,25-(OH)2-D3 levels were increased, consistent with previous studies (Nishimura et al., 2009). Renal induction of Cyp27b1 is likely responsible for 1,25-(OH)2-D3 accumulation, while decreased CYP24A1-mediation degradation may also contribute. Although hepatic and femoral expression of Igf1 and Fgf23 do not adequately explain Cyp27b1 induction, direct AhR-mediated activation cannot be excluded. Classically, 1,25-(OH)2-D3 promotes bone resorption by inducing osteoblast expression of Tnfsf11 (encodes RANKL), while simultaneously suppressing bone mineralization (Christakos et al., 2016). Paradoxically, active vitamin D analogs such as eldecalcitol have been shown to impair bone resorption in mice, leading to increased trabecular BVF and BMD (Harada et al., 2012). These changes are consistent with our reported results, however the mechanisms responsible for vitamin D-mediated impairment of bone resorption and increased BVF have not been elucidated.
In summary, TCDD altered the balance between bone resorption and formation in juvenile mouse femurs, resulting in increased bone mass with reduced marrow adiposity. Induction of Gpnmb was identified as a sensitive indicator of AhR activation within the femur, suggesting a mechanistic link that warrants further investigation. Our data also indicate that inappropriate AhR activation at an early age may alter the differentiation balance of MSCs, and therefore age may explain the contradictory reports of AhR-mediated changes in the bone morphology of adult rodents. It remains to be determined if the AhR represents a viable therapeutic target for the treatment of metabolic bone disorders.
Supplementary Material
HIGHLIGHTS.
TCDD increased trabecular bone volume fraction in male and female mouse femurs
Reduced osteoclast counts and serum TRAP levels indicate impaired resorption
Reciprocal changes in osteoblasts and adipocytes suggest altered MSC differentiation
Femoral induction of glycoprotein NMB may promote osteoblast differentiation/function
Increased 1,25-(OH)2-vitamin D3 levels were consistent with renal Cyp27b1 induction
Acknowledgments
This work was supported by the National Institute of Environmental Health Sciences Superfund Research Program [NIEHS SBRP P42ES04911] to TRZ. TRZ is partially supported by AgBioResearch at Michigan State University. KAF is supported by the Canadian Institutes of Health Research Doctoral Foreign Study Award [DFS-140386]. RN is supported by the National Institutes of Health Integrative Training in the Pharmacological Sciences Award [5T32GM092715].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
KAF, RN, SR, LRM, and TRZ have nothing to disclose.
References
- Abdelmagid SM, Barbe MF, Rico MC, Salihoglu S, Arango-Hisijara I, Selim AH, Anderson MG, Owen TA, Popoff SN, Safadi FF. Osteoactivin, an anabolic factor that regulates osteoblast differentiation and function. Exp Cell Res. 2008;314(13):2334–2351. doi: 10.1016/j.yexcr.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Adebanjo OA, Moonga BS, Haddad JG, Huang CL, Zaidi M. A possible new role for vitamin D-binding protein in osteoclast control: inhibition of extracellular Ca2+ sensing at low physiological concentrations. Biochem Biophys Res Commun. 1998;249(3):668–671. doi: 10.1006/bbrc.1998.9037. [DOI] [PubMed] [Google Scholar]
- Alaluusua S, Lukinmaa PL, Pohjanvirta R, Unkila M, Tuomisto J. Exposure to 2,3,7,8-tetrachlorodibenzo-para-dioxin leads to defective dentin formation and pulpal perforation in rat incisor tooth. Toxicology. 1993;81(1):1–13. doi: 10.1016/0300-483x(93)90152-i. [DOI] [PubMed] [Google Scholar]
- Alaluusua S, Lukinmaa PL, Torppa J, Tuomisto J, Vartiainen T. Developing teeth as biomarker of dioxin exposure. Lancet. 1999;353(9148):206. doi: 10.1016/S0140-6736(05)77214-7. [DOI] [PubMed] [Google Scholar]
- Alexander DL, Ganem LG, Fernandez-Salguero P, Gonzalez F, Jefcoate CR. Aryl-hydrocarbon receptor is an inhibitory regulator of lipid synthesis and of commitment to adipogenesis. J Cell Sci. 1998;111(Pt 22):3311–3322. doi: 10.1242/jcs.111.22.3311. [DOI] [PubMed] [Google Scholar]
- Alvarez-Lloret P, Lind PM, Nyberg I, Orberg J, Rodriguez-Navarro AB. Effects of 3,3’,4,4’,5-pentachlorobiphenyl (PCB126) on vertebral bone mineralization and on thyroxin and vitamin D levels in Sprague-Dawley rats. Toxicol Lett. 2009;187(2):63–68. doi: 10.1016/j.toxlet.2009.01.030. [DOI] [PubMed] [Google Scholar]
- Arruda M, Coelho MC, Moraes AB, de Paula Paranhos-Neto F, Madeira M, Farias ML, Vieira Neto L. Bone Mineral Density and Microarchitecture in Patients With Autosomal Dominant Osteopetrosis: A Report of Two Cases. J Bone Miner Res. 2016;31(3):657–662. doi: 10.1002/jbmr.2715. [DOI] [PubMed] [Google Scholar]
- Beischlag TV, Luis Morales J, Hollingshead BD, Perdew GH. The aryl hydrocarbon receptor complex and the control of gene expression. Crit Rev Eukaryot Gene Expr. 2008;18(3):207–250. doi: 10.1615/critreveukargeneexpr.v18.i3.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum LS. Distribution and excretion of 2,3,7,8-tetrachlorodibenzo-p-dioxin in congenic strains of mice which differ at the Ah locus. Drug Metab Dispos. 1986;14(1):34–40. [PubMed] [Google Scholar]
- Boverhof DR, Burgoon LD, Tashiro C, Chittim B, Harkema JR, Jump DB, Zacharewski TR. Temporal and dose-dependent hepatic gene expression patterns in mice provide new insights into TCDD-Mediated hepatotoxicity. Toxicol Sci. 2005;85(2):1048–1063. doi: 10.1093/toxsci/kfi162. [DOI] [PubMed] [Google Scholar]
- Brenza HL, DeLuca HF. Regulation of 25-hydroxyvitamin D3 1alpha-hydroxylase gene expression by parathyroid hormone and 1,25-dihydroxyvitamin D3. Arch Biochem Biophys. 2000;381(1):143–152. doi: 10.1006/abbi.2000.1970. [DOI] [PubMed] [Google Scholar]
- Burgoon LD, Zacharewski TR. Automated quantitative dose-response modeling and point of departure determination for large toxicogenomic and high-throughput screening data sets. Toxicol Sci. 2008;104(2):412–418. doi: 10.1093/toxsci/kfn083. [DOI] [PubMed] [Google Scholar]
- Carpi D, Korkalainen M, Airoldi L, Fanelli R, Hakansson H, Muhonen V, Tuukkanen J, Viluksela M, Pastorelli R. Dioxin-sensitive proteins in differentiating osteoblasts: effects on bone formation in vitro. Toxicol Sci. 2009;108(2):330–343. doi: 10.1093/toxsci/kfp021. [DOI] [PubMed] [Google Scholar]
- Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol Rev. 2016;96(1):365–408. doi: 10.1152/physrev.00014.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3(Suppl 3):S131–139. doi: 10.2215/CJN.04151206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Zhou J, Qiu J, Johnson MR, Mrug M. Validation of endogenous internal real-time PCR controls in renal tissues. Am J Nephrol. 2009;30(5):413–417. doi: 10.1159/000235993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- Duarte WR, Shibata T, Takenaga K, Takahashi E, Kubota K, Ohya K, Ishikawa I, Yamauchi M, Kasugai S. S100A4: a novel negative regulator of mineralization and osteoblast differentiation. J Bone Miner Res. 2003;18(3):493–501. doi: 10.1359/jbmr.2003.18.3.493. [DOI] [PubMed] [Google Scholar]
- Eastell R, O’Neill TW, Hofbauer LC, Langdahl B, Reid IR, Gold DT, Cummings SR. Postmenopausal osteoporosis. Nat Rev Dis Primers. 2016;2:16069. doi: 10.1038/nrdp.2016.69. [DOI] [PubMed] [Google Scholar]
- Fader KA, Nault R, Ammendolia DA, Harkema JR, Williams KJ, Crawford RB, Kaminski NE, Potter D, Sharratt B, Zacharewski TR. 2,3,7,8-Tetrachlorodibenzo-p-Dioxin Alters Lipid Metabolism and Depletes Immune Cell Populations in the Jejunum of C57BL/6 Mice. Toxicol Sci. 2015;148(2):567–580. doi: 10.1093/toxsci/kfv206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fader KA, Nault R, Kirby MP, Markous G, Matthews J, Zacharewski TR. Convergence of hepcidin deficiency, systemic iron overloading, heme accumulation, and REV-ERBalpha/beta activation in aryl hydrocarbon receptor-elicited hepatotoxicity. Toxicol Appl Pharmacol. 2017a;321:1–17. doi: 10.1016/j.taap.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fader KA, Nault R, Zhang C, Kumagai K, Harkema JR, Zacharewski TR. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD)-elicited effects on bile acid homeostasis: Alterations in biosynthesis, enterohepatic circulation, and microbial metabolism. Sci Rep. 2017b;7(1):5921. doi: 10.1038/s41598-017-05656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fafian-Labora J, Fernandez-Pernas P, Fuentes I, De Toro J, Oreiro N, Sangiao-Alvarellos S, Mateos J, Arufe MC. Influence of age on rat bone-marrow mesenchymal stem cells potential. Sci Rep. 2015;5:16765. doi: 10.1038/srep16765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, McDonald JM. Disorders of bone remodeling. Annu Rev Pathol. 2011;6:121–145. doi: 10.1146/annurev-pathol-011110-130203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnila MA, Zioupos P, Herlin M, Miettinen HM, Simanainen U, Hakansson H, Tuukkanen J, Viluksela M, Jamsa T. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure on bone material properties. J Biomech. 2010;43(6):1097–1103. doi: 10.1016/j.jbiomech.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Frara N, Abdelmagid SM, Sondag GR, Moussa FM, Yingling VR, Owen TA, Popoff SN, Barbe MF, Safadi FF. Transgenic Expression of Osteoactivin/gpnmb Enhances Bone Formation In Vivo and Osteoprogenitor Differentiation Ex Vivo. J Cell Physiol. 2016;231(1):72–83. doi: 10.1002/jcp.25020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T, Harigae H. Biology of Heme in Mammalian Erythroid Cells and Related Disorders. Biomed Res Int. 2015;2015:278536. doi: 10.1155/2015/278536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasiewicz TA, Geiger LE, Rucci G, Neal RA. Distribution, excretion, and metabolism of 2,3,7,8-tetrachlorodibenzo-p-dioxin in C57BL/6J, DBA/2J, and B6D2F1/J mice. Drug Metab Dispos. 1983;11(5):397–403. [PubMed] [Google Scholar]
- Gérard D, Schmidt F, Ginolhac A, Schmitz M, Halder R, Ebert P, Schulz MH, Sauter T, Sinkkonen L. Temporal epigenomic profiling identifies AHR as dynamic super-enhancer controlled regulator of mesenchymal multipotency. bioRxiv. 2017 doi: 10.1093/nar/gky1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez JM. The role of insulin-like growth factor I components in the regulation of vitamin D. Curr Pharm Biotechnol. 2006;7(2):125–132. doi: 10.2174/138920106776597621. [DOI] [PubMed] [Google Scholar]
- Hankinson O. The aryl hydrocarbon receptor complex. Annu Rev Pharmacol Toxicol. 1995;35:307–340. doi: 10.1146/annurev.pa.35.040195.001515. [DOI] [PubMed] [Google Scholar]
- Harada S, Mizoguchi T, Kobayashi Y, Nakamichi Y, Takeda S, Sakai S, Takahashi F, Saito H, Yasuda H, Udagawa N, et al. Daily administration of eldecalcitol (ED-71), an active vitamin D analog, increases bone mineral density by suppressing RANKL expression in mouse trabecular bone. J Bone Miner Res. 2012;27(2):461–473. doi: 10.1002/jbmr.555. [DOI] [PubMed] [Google Scholar]
- Herlin M, Finnila MA, Zioupos P, Aula A, Risteli J, Miettinen HM, Jamsa T, Tuukkanen J, Korkalainen M, Hakansson H, et al. New insights to the role of aryl hydrocarbon receptor in bone phenotype and in dioxin-induced modulation of bone microarchitecture and material properties. Toxicol Appl Pharmacol. 2013;273(1):219–226. doi: 10.1016/j.taap.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Herlin M, Kalantari F, Stern N, Sand S, Larsson S, Viluksela M, Tuomisto JT, Tuomisto J, Tuukkanen J, Jamsa T, et al. Quantitative characterization of changes in bone geometry, mineral density and biomechanical properties in two rat strains with different Ah-receptor structures after long-term exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicology. 2010;273(1–3):1–11. doi: 10.1016/j.tox.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Huang G, Elferink CJ. A novel nonconsensus xenobiotic response element capable of mediating aryl hydrocarbon receptor-dependent gene expression. Mol Pharmacol. 2012;81(3):338–347. doi: 10.1124/mol.111.075952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihde LL, Forrester DM, Gottsegen CJ, Masih S, Patel DB, Vachon LA, White EA, Matcuk GR., Jr Sclerosing bone dysplasias: review and differentiation from other causes of osteosclerosis. Radiographics. 2011;31(7):1865–1882. doi: 10.1148/rg.317115093. [DOI] [PubMed] [Google Scholar]
- Jamsa T, Viluksela M, Tuomisto JT, Tuomisto J, Tuukkanen J. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on bone in two rat strains with different aryl hydrocarbon receptor structures. J Bone Miner Res. 2001;16(10):1812–1820. doi: 10.1359/jbmr.2001.16.10.1812. [DOI] [PubMed] [Google Scholar]
- Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2(3):165–171. doi: 10.1023/a:1011513223894. [DOI] [PubMed] [Google Scholar]
- Korkalainen M, Kallio E, Olkku A, Nelo K, Ilvesaro J, Tuukkanen J, Mahonen A, Viluksela M. Dioxins interfere with differentiation of osteoblasts and osteoclasts. Bone. 2009;44(6):1134–1142. doi: 10.1016/j.bone.2009.02.019. [DOI] [PubMed] [Google Scholar]
- Kretlow JD, Jin YQ, Liu W, Zhang WJ, Hong TH, Zhou G, Baggett LS, Mikos AG, Cao Y. Donor age and cell passage affects differentiation potential of murine bone marrow-derived stem cells. BMC Cell Biol. 2008;9:60. doi: 10.1186/1471-2121-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind PM, Wejheden C, Lundberg R, Alvarez-Lloret P, Hermsen SA, Rodriguez-Navarro AB, Larsson S, Rannug A. Short-term exposure to dioxin impairs bone tissue in male rats. Chemosphere. 2009;75(5):680–684. doi: 10.1016/j.chemosphere.2008.12.024. [DOI] [PubMed] [Google Scholar]
- Liu S, Tang W, Zhou J, Stubbs JR, Luo Q, Pi M, Quarles LD. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol. 2006;17(5):1305–1315. doi: 10.1681/ASN.2005111185. [DOI] [PubMed] [Google Scholar]
- N’Jai AU, Larsen MC, Bushkofsky JR, Czuprynski CJ, Jefcoate CR. Acute disruption of bone marrow hematopoiesis by benzo(a)pyrene is selectively reversed by aryl hydrocarbon receptor-mediated processes. Mol Pharmacol. 2011;79(4):724–734. doi: 10.1124/mol.110.070631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nault R, Fader KA, Ammendolia DA, Dornbos P, Potter D, Sharratt B, Kumagai K, Harkema JR, Lunt SY, Matthews J, et al. Dose-Dependent Metabolic Reprogramming and Differential Gene Expression in TCDD-Elicited Hepatic Fibrosis. Toxicol Sci. 2016a;154(2):253–266. doi: 10.1093/toxsci/kfw163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nault R, Fader KA, Kirby MP, Ahmed S, Matthews J, Jones AD, Lunt SY, Zacharewski TR. Pyruvate Kinase Isoform Switching and Hepatic Metabolic Reprogramming by the Environmental Contaminant 2,3,7,8-Tetrachlorodibenzo-p-Dioxin. Toxicol Sci. 2016b;149(2):358–371. doi: 10.1093/toxsci/kfv245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nault R, Fader KA, Zacharewski T. RNA-Seq versus oligonucleotide array assessment of dose-dependent TCDD-elicited hepatic gene expression in mice. BMC Genomics. 2015;16(1):373. doi: 10.1186/s12864-015-1527-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura N, Nishimura H, Ito T, Miyata C, Izumi K, Fujimaki H, Matsumura F. Dioxin-induced up-regulation of the active form of vitamin D is the main cause for its inhibitory action on osteoblast activities, leading to developmental bone toxicity. Toxicol Appl Pharmacol. 2009;236(3):301–309. doi: 10.1016/j.taap.2009.01.025. [DOI] [PubMed] [Google Scholar]
- Perwad F, Zhang MY, Tenenhouse HS, Portale AA. Fibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin D-1alpha-hydroxylase expression in vitro. Am J Physiol Renal Physiol. 2007;293(5):F1577–1583. doi: 10.1152/ajprenal.00463.2006. [DOI] [PubMed] [Google Scholar]
- Podechard N, Fardel O, Corolleur M, Bernard M, Lecureur V. Inhibition of human mesenchymal stem cell-derived adipogenesis by the environmental contaminant benzo(a)pyrene. Toxicol In Vitro. 2009;23(6):1139–1144. doi: 10.1016/j.tiv.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Safadi FF, Xu J, Smock SL, Rico MC, Owen TA, Popoff SN. Cloning and characterization of osteoactivin, a novel cDNA expressed in osteoblasts. J Cell Biochem. 2001;84(1):12–26. doi: 10.1002/jcb.1259. [DOI] [PubMed] [Google Scholar]
- Sawada N, Sakaki T, Ohta M, Inouye K. Metabolism of vitamin D(3) by human CYP27A1. Biochem Biophys Res Commun. 2000;273(3):977–984. doi: 10.1006/bbrc.2000.3050. [DOI] [PubMed] [Google Scholar]
- Sobacchi C, Schulz A, Coxon FP, Villa A, Helfrich MH. Osteopetrosis: genetics, treatment and new insights into osteoclast function. Nat Rev Endocrinol. 2013;9(9):522–536. doi: 10.1038/nrendo.2013.137. [DOI] [PubMed] [Google Scholar]
- Sorg O, Zennegg M, Schmid P, Fedosyuk R, Valikhnovskyi R, Gaide O, Kniazevych V, Saurat JH. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) poisoning in Victor Yushchenko: identification and measurement of TCDD metabolites. Lancet. 2009;374(9696):1179–1185. doi: 10.1016/S0140-6736(09)60912-0. [DOI] [PubMed] [Google Scholar]
- Stark Z, Savarirayan R. Osteopetrosis. Orphanet J Rare Dis. 2009;4:5. doi: 10.1186/1750-1172-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uckan D, Kilic E, Sharafi P, Kazik M, Kaya F, Erdemli E, Can A, Tezcaner A, Kocaefe C. Adipocyte differentiation defect in mesenchymal stromal cells of patients with malignant infantile osteopetrosis. Cytotherapy. 2009;11(4):392–402. doi: 10.1080/14653240802582083. [DOI] [PubMed] [Google Scholar]
- Wei S, Tanaka H, Seino Y. Local action of exogenous growth hormone and insulin-like growth factor-I on dihydroxyvitamin D production in LLC-PK1 cells. Eur J Endocrinol. 1998;139(4):454–460. doi: 10.1530/eje.0.1390454. [DOI] [PubMed] [Google Scholar]
- Wheater G, Elshahaly M, Tuck SP, Datta HK, van Laar JM. The clinical utility of bone marker measurements in osteoporosis. J Transl Med. 2013;11:201. doi: 10.1186/1479-5876-11-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe WH, Michalek JE, Miner JC, Pirkle JL, Caudill SP, Patterson DG, Jr, Needham LL. Determinants of TCDD half-life in veterans of operation ranch hand. J Toxicol Environ Health. 1994;41(4):481–488. doi: 10.1080/15287399409531858. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Kumashiro R. Conversion of vitamin D3 binding protein (group-specific component) to a macrophage activating factor by the stepwise action of beta-galactosidase of B cells and sialidase of T cells. J Immunol. 1993;151(5):2794–2802. [PubMed] [Google Scholar]
- Yang L, Allen BC, Thomas RS. BMDExpress: a software tool for the benchmark dose analyses of genomic data. BMC Genomics. 2007;8:387. doi: 10.1186/1471-2164-8-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Du Y, Zhang X, Sun Y, Li S, Dou Y, Li Z, Yuan H, Zhao W. The aryl hydrocarbon receptor suppresses osteoblast proliferation and differentiation through the activation of the ERK signaling pathway. Toxicol Appl Pharmacol. 2014a;280(3):502–510. doi: 10.1016/j.taap.2014.08.025. [DOI] [PubMed] [Google Scholar]
- Yu H, Jiang L, Wan B, Zhang W, Gan C, Su N, He J, Huang J, Zhang K, Zhang Y. The role of aryl hydrocarbon receptor in bone remodeling. Prog Biophys Mol Biol. 2017 doi: 10.1016/j.pbiomolbio.2017.12.005. [DOI] [PubMed] [Google Scholar]
- Yu TY, Kondo T, Matsumoto T, Fujii-Kuriyama Y, Imai Y. Aryl hydrocarbon receptor catabolic activity in bone metabolism is osteoclast dependent in vivo. Biochem Biophys Res Commun. 2014b;450(1):416–422. doi: 10.1016/j.bbrc.2014.05.114. [DOI] [PubMed] [Google Scholar]
- Yu TY, Pang WJ, Yang GS. Aryl hydrocarbon receptors in osteoclast lineage cells are a negative regulator of bone mass. PLoS One. 2015;10(1):e0117112. doi: 10.1371/journal.pone.0117112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharewski T, Safe S. Antiestrogenic activity of TCDD and related compounds. In: Korach KS, editor. Reproductive and Developmental Toxicology. New York: Marcel Dekker, Inc; 1998. pp. 431–448. [Google Scholar]
- Zhu JG, Ochalek JT, Kaufmann M, Jones G, Deluca HF. CYP2R1 is a major, but not exclusive, contributor to 25-hydroxyvitamin D production in vivo. Proc Natl Acad Sci U S A. 2013;110(39):15650–15655. doi: 10.1073/pnas.1315006110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zreiqat H, Howlett CR, Gronthos S, Hume D, Geczy CL. S100A8/S100A9 and their association with cartilage and bone. J Mol Histol. 2007;38(5):381–391. doi: 10.1007/s10735-007-9117-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.