Abstract
Novel primary immunodeficiency disorders are being identified with next generation sequencing technologies. We describe one patient with cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) haploinsufficiency who had recurrent enhancing brain lesions, nodular pulmonary infiltrates, hepatosplenomegaly, immune cytopenias, as well as progressive hypogammaglobulinemia and lymphopenia. We describe a second patient with activated p110δ syndrome (APDS) / p110δ activating mutation causing senescent T cells, lymphadenopathy, and immunodeficiency (PASLI) in association with recurrent respiratory tract infections, Epstein-Barr virus infection, lymphadenopathy, elevated serum IgM, and progressive lymphopenia. These presentations highlight the need for astute clinical judgment in the evaluation of patients with potential primary immunodeficiency disorders.
Keywords: CTLA-4, PIK3CD, autoimmunity, immune dysregulation, immune deficiency
Introduction
Novel primary immunodeficiency disorders present a unique challenge for clinicians [1]. The clinical phenotype may include a wide age range in terms of time of onset of symptoms and features of immune dysregulation such as organ specific extra nodal lymphocytic infiltration, lymphadenopathy, splenomegaly and autoimmunity [2–3]. There may also be clinical phenotypes marked by recurrent respiratory tract infections, persistent Epstein-Barr virus, and lymphadenopathy [4–5].
Cytotoxic T-lymphocyte Associated Molecule 4 (CTLA-4) serves as a negative regulator of T-lymphocyte responses [6]. Germline mutations in CTLA4 which abrogate functional CTLA-4 results in a haploinsufficiency state and a clinical phenotype marked by lymphocytic infiltration of the brain, lung, and gut as well as autoimmune cytopenias and risk for endocrinopathies in addition to progressive hypogammaglobulinemia, lymphopenia, and infections consequent to a B cell dysfunction [2–3].
PI3K proteins downstream of costimulatory receptors are essential for directing the growth and activity of lymphocytes [7]. Gain-of-function mutations in PI3KCD leads to increased catalytic activity of the p110δ subunit of PI3Kδ which results in activated p110δ delta syndrome (APDS) / p110δ activating mutation causing senescent T cells, lymphadenopathy, and immunodeficiency (PASLI), a syndrome of disrupted of B and T cell development and a clinical phenotype of recurrent respiratory tract infections, viral infections, lymphadenopathy, abnormal antibody production, and lymphopenia [4–5].
We describe the discovery of CTLA-4 haploinsufficiency and PASLI/APDS in two pediatric patients. The identification of these cases should promote increased awareness of the phenotypic heterogeneity of novel forms of primary immunodeficiency syndromes so early definitive treatment can be expedited.
Case Reports
Patient 1
A previously healthy 11 year-old Caucasian female was referred for an incidental finding of bilateral papilledema, a brain MR with foci of leptomeningeal enhancement, as well as neutropenia. The past medical history was non-contributory with the exception of a six month history of recurrent rashes, abdominal pain, vomiting, and diarrhea. The family history was notable for a maternal history of vitiligo, hypothyroidism, a maternal grandmother with hypothyroidism, and a maternal great grandmother with multiple sclerosis. The physical exam was notable for hepatosplenomegaly. Laboratory examination documented severe neutropenia (absolute neutrophil count 224 / uL). CT imaging documented pulmonary nodules as well as adenopathy. A bone marrow examination and bronchoscopy were normal. A cerebrospinal fluid examination demonstrated a lymphocytic pleocytosis (white blood cell count 200 / uL with 80% lymphocytes) as well as an elevated protein level of 71 mg/dL (normal 12-60). Infection was excluded. A lung and lymph node biopsy documented follicular bronchiolitis and follicular lymphoid hyperplasia; respectively.
Secondary to complaints of low grade fevers, aphthous ulcerations, arthralgias, abdominal pain, and diarrhea additional evaluation was completed. Laboratory examination documented an absence of autoantibodies with the exception of a positive direct antiglobulin test. Normal quantitative immunoglobulins were documented including a IgA 126 mg / dL (normal 69-309), IgG 1400 mg / dL (normal 613-1295), IgM 171 mg / dL (normal 53-334), and IgE <2 IU / mL (normal 2-393). Lymphocyte immunophenotyping results are provided in Table 1. Biopsies of the gastrointestinal tract documented chronic inflammation of the esophagus, gastric mucosa, and colon. Intramucosal lymphoid nodules were documented in the colon. A diagnosis of vasculitis was made and she demonstrated improvement with corticosteroids.
Table 1.
Lymphocyte immunophenotyping of patients.
| Pt 1 | Pt 1 | Pt 2 | Pt 2 | Normal range | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Marker | % | Abs #/µl |
% | Abs #/µl |
% | Abs #/µl |
% | Abs #/µl |
% | Abs #/µl |
| CD19+ B | 33 | 454 | 34 | 170 | 12 | 167 | 4 | 30 | 8–20 | 5–50 |
| Immature/Transitional B* | 1 | 14 | 4 | 47 | 2–11 | 1–36 | ||||
| Memory B** | 0.5 | 6 | 0.5 | 5 | 0.8–3.6 | 12–68 | ||||
| CD3+ T | 63 | 871 | 60 | 304 | 68 | 910 | 54 | 520 | 66–82 | 820–2400 |
| CD4+ T | 42 | 572 | 38 | 190 | 31 | 410 | 23 | 220 | 35–53 | 440–1600 |
| CD8+ T | 16 | 219 | 17 | 86 | 36 | 477 | 31 | 290 | 18–36 | 180–850 |
| NK (CD16+CD56+) | 0 | 5 | 7 | 33 | 18 | 246 | 41 | 390 | 5–16 | 80–340 |
| WBC # | 2100 | 2900 | 5400 | 3000 | 3500–10900 | |||||
| Lympho # | 1380 | 508 | 1340 | 700 | 1500–6500 | |||||
| Age at analysis | 12 years | 18 years | 5 years | 15 years | ||||||
Immature/Transitional B cells = CD20+CD10+
Memory B cells = CD20+CD27+
At 12 years of age she developed severe headache, decreased vision, and seizures. MR imaging demonstrated enhancement in the temporal and parietal lobe consistent with her diagnosis of vasculitis. A cerebrospinal fluid examination was completed demonstrating a similar lymphocytic pleocytosis. Antineuronal antibody testing was negative. Faint oligoclonal bands were documented; however, they are absent on subsequent evaluation. Infection was excluded. She was treated with corticosteroids in addition to intravenous immunoglobulin with improvement in her clinical symptoms.
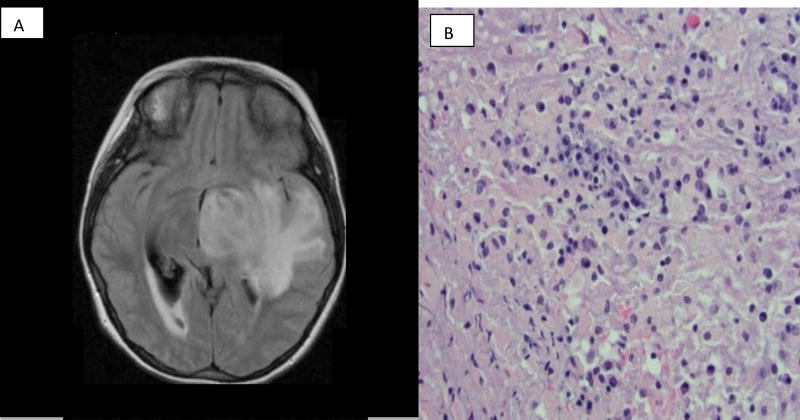
At 15 years of age she developed gum bleeding in association with severe thrombocytopenia (platelet count < 10,000 / uL) consistent with immune thrombocytopenia. Treatment with corticosteroids and intravenous immunoglobulin led to improvement. She then developed severe headaches and vomiting in association with a large area of enhancement in the temporal and parietal lobe on MR imaging as seen in Figure 1. A brain biopsy demonstrated lymphocytic infiltration as depicted in Figure 1. Improvement was documented following treatment with corticosteroids, cyclophosphamide, and rituximab.
Figure 1.
Brain lesion as documented by brain MR imaging and brain biopsy in Case Report 1. A) Brain MR imaging documenting large left temporal and parietal lesion with enhancement and mass effect. B) Brain biopsy results demonstrating lymphocytic infiltration.
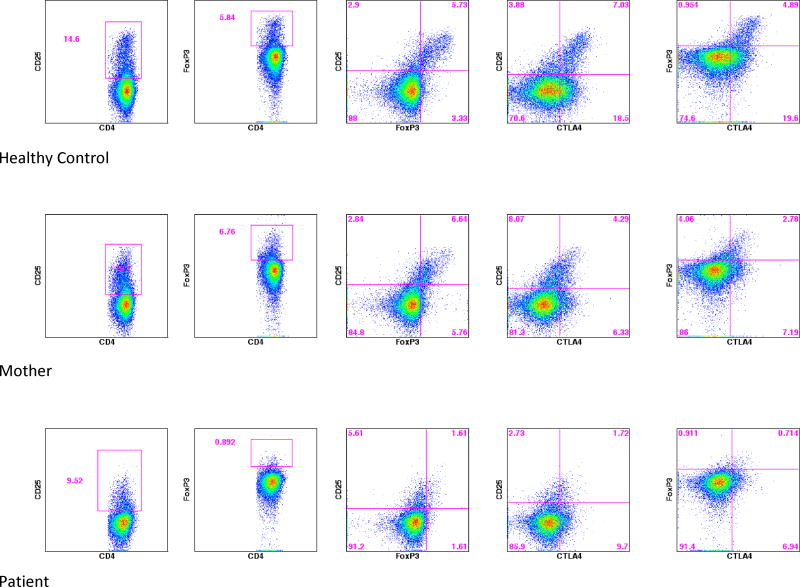
She is now 21 years of age with persistent neurological complaints and corticosteroid dependence. Laboratory examination documented hypogammaglobulinemia as demonstrated by a IgG 210 mg / dL (normal 751-1560), IgM 55 mg / dL (normal 46-304), IgA < 7 mg / dL (82-453), and IgE <0.25 IU / mL. Progressive lymphopenia was also noted as depicted in Table 1. In addition to CD4 lymphopenia memory B cells were notably diminished. Given the phenotypic overlap with recently described cases of CTLA-4 haploinsufficiency targeted Sanger sequencing of CTLA4 was completed. Sanger sequencing confirmed a heterozygous mutation in CTLA4, a single base transition, c.151C>T causing substitution of a stop codon for arginine at amino acid 51 (R51X). This mutation is predicted to cause nonsense mediated decay of the CTLA4 transcript and subsequent haploinsufficiency of the protein. The same mutation (R51X) was present in the mother, grandmother, and absent in the father. Figure 2 demonstrates analysis of her regulatory T cell phenotype and intracellular CTLA-4 staining documenting prominent aberrations when compared to a healthy control and her mildly symptomatic mother who demonstrated similar, but less prominent aberrations in regulatory T cell phenotype and intracellular CTLA-4 staining.
Figure 2.
Regulatory T cell phenotype and intracellular CTLA-4 staining in Case Report 1.
Patient 2
A previously healthy 2 year-old Hispanic female was referred for evaluation of fever, hepatosplenomegaly, anemia, and thrombocytopenia. The past medical and family history was non-contributory. The physical exam was notable for splenomegaly. Laboratory examination documented anemia and thrombocytopenia. A bone marrow examination was normal. Infectious mononucleosis was suspected based on EBV serologic testing (positive EBV viral capsid IgM antibody) and EBV polymerase chain reaction (1,010 copies / uL).
At 5 years of age she continued to have recurrent upper and lower respiratory tract infections prompting additional laboratory evaluation. CT imaging documented mild axillary adenopathy and bronchial wall thickening. Laboratory examination demonstrated abnormal quantitative immunoglobulins including a serum IgA<7 mg / dL (normal 22-157), IgG 1450 mg / dL (normal 423-1090), and IgM 302 mg / dL (normal 45-190). Lymphocyte immunophenotyping results are provided in Table 1. In addition to CD4 lymphopenia memory B cells were notably diminished; however, transitional B cells were elevated. Antibody responses to tetanus toxoid and Haemophilus influenzae Type b were documented; however, levels of antibodies to pneumococcal capsular polysaccharides were low. She was treated with immunoglobulin replacement with improvement in her clinical symptoms.
She is now 15 years of age and continues to receive subcutaneous immunoglobulin replacement therapy. Recent CT imaging documented adenopathy, a slightly enlarged spleen (12 cm) and bronchial wall thickening. An absence of autoantibodies was documented. Notably, her serum IgM remains elevated. Analysis for genetic causes of hyper IgM syndromes were negative. Given the phenotypic overlap with recently described cases of APDS/PASLI targeted Sanger sequencing of the PI3KCD gene was completed. Sanger sequencing confirmed a heterozygous mutation in PI3KCD. Mutation analysis of PI3KCD demonstrates a single base transition, c.3061 G>A causing substitution of glutamic acid for lysine at amino acid 1021 (E1021K) of the p110δ protein, the catalytic subunit of PI3Kδ. This mutation is predicted to cause increased PI3Kδ activity. The same mutation (E1021K) was absent in the mother.
Discussion
Next generation sequencing techniques continue to reveal the diagnosis of a variety of novel primary immunodeficiency disorders [8]. The phenotypes associated with these novel primary immunodeficiencies are diverse as depicted in Table 2. These genetic defects affect T and B cell development and function compromising mechanisms of central and peripheral tolerance as well as host responses to pathogens. CTLA-4 haploinsufficiency and APDS/PASLI are two recently described immune dysregulation syndromes which provide clinical examples of how alterations in lymphocyte homeostasis may result in perturbations in tolerance and host response to infectious pathogens.
Table 2.
Clinical features of CTLA-4 haploinsufficiency and APDS
| Gene (year) |
Mode of Inheritance |
Age at onset |
Clinical features of immune dysregulation and autoimmunity | Infectious complications | Investigational therapy |
|---|---|---|---|---|---|
|
| |||||
| CTLA4 (2014) | Autosomal dominant | 0–72 years | Infections: respiratory | Viruses: EBV LPD and viremia, CMV viremia | mTOR pathway inhibitors |
| Lymphocytic infiltrates: CNS, GI, lung, bone marrow, kidney, liver | CTLA4-Ig | ||||
| Lymphadenopathy, hepatosplenomegaly | |||||
| Loss of function | Enteropathy: mild to severe, growth failure | Other: Pneumocystis jirovecii | |||
| Autoimmunity: hematologic, GI, arthritis, other | |||||
| Hypogammaglobulinemia: +/− , variable | |||||
| Lymphopenia: +/− , variable | |||||
|
| |||||
| PIK3CD (2013) | Autosomal dominant | 0–22 years | Infections: respiratory & abscesses | Viruses: EBV LPD and viremia, CMV viremia & lymphadenitis, severe HSV, VZV, plantar warts | mTOR pathway inhibitors |
| Lymphocytic infiltrates: Mucosal nodular lymphoid aggregates | P110δ inhibitors | ||||
| Lymphadenopathy, hepatosplenomegaly | |||||
| Gain of function | Bronchiectasis +/− | Other: Cryptosporidium | |||
| Autoimmunity: hematologic, GI, liver | |||||
| Hypogammaglobulinemia: +/−, also hyper IgM | |||||
| Lymphopenia: +/− | |||||
CTLA-4 haploinsufficiency patients develop autoimmunity including organ specific lymphocytic infiltration with notable extranodal targets of the brain, lung, and gut [2–3]. Autoimmunity including autoimmune cytopenias and endocrinopathies are well described [2–3]. Affected patients demonstrate laboratory abnormalities including CD4+ T cell lymphopenia as well as diminished numbers and function of regulatory T cells [2–3]. B cells may also be diminished as the disease progresses with patient’s age resulting in hypogammaglobulinemia and abnormal antibody responses. CTLA-4 haploinsufficiency was considered because of the central nervous system involvement, responsiveness to immunosuppression, disease onset in early adolescence, as well as evolving lymphopenia and hypogammaglobulinemia. As expected our patients’ regulatory T cell numbers and CTLA-4 expression was diminished. Of note, her mother carried the same mutation and was also mildly affected (e.g., vitiligo, anti-parietal cell antibodies, diminished regulatory T cells) suggesting the presence of phenotypic variability that may be secondary to variable penetrance or epigenetic modifiers.
Downstream of molecules such as CTLA-4 are signaling molecules including PI3K proteins. PI3K proteins are essential for directing the growth and activity of lymphocytes [7]. Alterations in PI3K signaling lead to perturbations in T and B cell function and the clinical phenotype of APDS/PASLI consisting of recurrent respiratory tract infections, viral infections, lymphadenopathy, abnormal antibody production, and lymphopenia [4–5]. APDS/PASLI was considered because of the clinical features of recurrent sinopulmonary infections, EBV infection, lymphadenopathy, as well as laboratory features of abnormal antibody production including an elevation in serum IgM and lymphopenia.
Therapeutic options for patients with CTLA-4 haploinsufficiency include m-TOR inhibitors. Our patient with CTLA-4 haploinsufficiency has been started on Rapamycin, an m-TOR inhibitor, and has demonstrated clinical improvement. Abatacept which is a CTLA-4 fusion protein currently utilized to treat autoimmune disease [9] including rheumatoid arthritis may also have additional benefits for patients with CTLA-4 haploinsufficiency. For patients with gain-of-function mutations in PI3KCD m-TOR inhibitors and a variety of selective p110δ inhibitors are under evaluation. Of note, p110δ inhibitors are being developed and evaluated in the treatment of various cancers as well as inflammatory and autoimmune diseases [10].
Increased awareness and early identification of patients with these novel primary immunodeficiency disorders will expedite the use of therapeutic interventions including the use of immunomodulatory agents and targeted small molecules which may prevent escalation of autoimmune disease and curtail the development of sequelae such as infections and malignancy.
Acknowledgments
Funding source:
No current external funding sources were utilized for this study.
This research was supported by the Intramural Research Program of the NIH and NIAID.
Footnotes
Financial disclosure:
The authors have no financial disclosures.
Conflict of interest statement:
The authors have no conflict of interests to disclose.
Abbreviations:
None
References
- 1.Subbarayan A, Colarusso G, Hughes SM, et al. Clinical features that identify children with primary immunodeficiency diseases. Pediatrics. 2011;127(5):810–6. doi: 10.1542/peds.2010-3680. [DOI] [PubMed] [Google Scholar]
- 2.Schubert D, Bode C, Kenefeck R, et al. Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nat Med. 2014;20(12):1410–6. doi: 10.1038/nm.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuehn HS, Ouyang W, Lo B, et al. Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science. 2014;345(6204):1623–7. doi: 10.1126/science.1255904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angulo I, Vadas O, Garcon F, et al. Phosphoinositide 3-kinase δ gene mutation predisposes to respiratory infection and airway damage. Science. 2013;342(6160):866–71. doi: 10.1126/science.1243292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lucas CL, Kuehn HS, Zhao F, et al. Dominant-activating germline mutations in the gene encoding the PI(3)K catalytic subunit p110δ result in T cell senescence and human immunodeficiency. Nat Immunol. 2014;15(1):88–97. doi: 10.1038/ni.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gardner D, Jeffrey LE, Sansom DM. Understanding the CD28/CTLA-4 (CD152) pathway and its implications for costimulatory blockade. Am J Transplant. 2014;14(9):1985–91. doi: 10.1111/ajt.12834. [DOI] [PubMed] [Google Scholar]
- 7.Okkenhaug K. Signaling by the phosphoinositide 3-kinase family in immune cells. Annu Rev Immunol. 2013;31:675–704. doi: 10.1146/annurev-immunol-032712-095946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chinen J, Notarangelo LD, Shearer WT. Advances in basic and clinical immunology in 2014. J Allergy Clin Immunol. 2015;135(5):1132–1141. doi: 10.1016/j.jaci.2015.02.037. [DOI] [PubMed] [Google Scholar]
- 9.Selmi C, Generali E, Massarotti M, et al. New treatments for inflammatory rheumatic disease. Immunol Res. 2014;60(2–3):277–88. doi: 10.1007/s12026-014-8565-5. [DOI] [PubMed] [Google Scholar]
- 10.Dienstmann R, Rodon J, Serra V, et al. Picking the point of inhibition: a comparative review of PI3K/AKT/mTOR pathway inhibitors. Mol Cancer Ther. 2014;13(5):1021–31. doi: 10.1158/1535-7163.MCT-13-0639. [DOI] [PubMed] [Google Scholar]