Abstract
Cognitive dysfunction is common in depression during both acute episodes and remission. Vortioxetine is a novel multimodal antidepressant that has improved cognitive function including executive function in depressed patients in randomised placebo-controlled clinical trials. However, it is unclear whether vortioxetine is able to target directly the neural circuitry implicated in the cognitive deficits in depression. Remitted depressed (n=48) and healthy volunteers (n=48) were randomised to receive 14 days treatment with 20 mg vortioxetine or placebo in a double-blind design. The effects of treatment on functional magnetic resonance imaging responses during an N-back working memory task were assessed at baseline and at the end of treatment. Neuropsychological measures of executive function, speed and information processing, attention and learning and memory were examined with the Trail Making Test (TMT), Rey Auditory Learning Test and Digit Symbol Substitution Test before and after treatment; subjective cognitive function was assessed using the Perceived Deficits Questionnaire (PDQ). Compared with placebo, vortioxetine reduced activation in the right dorsolateral prefrontal cortex and left hippocampus during the N-back task compared with placebo. Vortioxetine also increased TMT-A performance and self-reported cognitive function on the PDQ. These effects were seen across both subject groups. Vortioxetine modulates neural responses across a circuit subserving working memory in a direction opposite to the changes described in depression, when performance is maintained. This study provides evidence that vortioxetine has direct effects on the neural circuitry supporting cognitive function that can be dissociated from its effects on the mood symptoms of depression.
Introduction
Major depressive disorder (MDD) is associated with clinically significant and broad deficits in executive function, memory and learning.1 Recent research suggests that these deficits in cognitive function can persist even during periods of symptomatic remission from depression and may be difficult to resolve fully with available treatments.2, 3 These cognitive difficulties are an important mediator of functional impairment (such as workplace performance) in individuals with MDD4, 5, 6 and are therefore a priority for treatment development.
The neural bases of the cognitive impairment in depression have been studied in a variety of ways, usually using an established laboratory test and functional brain imaging. A particularly well-studied example is the N-back visual working memory task; this requires subjects to monitor a series of stimuli and indicate when the current stimulus matches the one from n steps earlier in the sequence.7 The working memory demands of this task are associated with increased engagement of a frontoparietal network and concomitant deactivation of the default-mode network (DMN) including the medial temporal lobe.8 This shift in activity from the DMN to the task-positive network is thought to underpin reallocation of available neuronal resources to regions required for working memory, thereby optimising performance.9
Depression has been associated with increased engagement of task-positive and reduced deactivation of DMN compared with healthy controls (HCs) when task performance is maintained. Specifically, depressed patients show increased activation in the cingulate10, 11 and frontal cortex10, 11, 12, 13 and attenuated deactivation in the medial temporal cortex.10, 14 Unmedicated patients in remission from depression and young volunteers with a family history of depression show a similar profile of neural hyperactivation in task-engaged areas with increasing working memory demands. The apparent difficulty that depressed patients have in switching off the DMN, coupled with the compensatory overactivation of the task-positive network, suggests an important cost to maintaining performance.10 Consistent with this, in studies where performance of the working task is impaired in depressed patients during functional magnetic resonance imaging (fMRI) acquisition, the converse pattern of reduced dorsolateral prefrontal cortex (dlPFC) activity is often seen.15 Taken together, these results support the idea that deficits in cognitive function represent an enduring or a possible trait vulnerability marker for depression.16, 17
The field has been slow to investigate the effects of different treatments on cognitive impairment in depression in adults of working age. Baune and Renger18 reviewed the literature and found evidence that selective serotonin reuptake inhibitors, tianeptine, duloxetine, bupropion and moclobemide ‘may exert certain improving effects on cognitive function in depression, such as in learning and memory and executive function’. However, the studies were often confounded by recovery from depression and cognitive function was rarely the primary outcome. Hence, many apparent effects of treatment could be pseudospecific, rather than an effect on cognition per se.
Vortioxetine is a new treatment for depression. Its principal actions are as a 5-HT3, 5-HT7 and 5-HT1D receptor antagonist, 5-HT1B receptor partial agonist, 5-HT1A receptor agonist and serotonin (5-HT) transporter inhibitor.19 The apparent functional effects are multimodal upon serotonergic, noradrenergic, dopaminergic, cholinergic, histaminergic, GABAergic and glutamatergic neurotransmission. Preclinical studies in animals suggested effects that improve procognitive function when compared with other antidepressants.20
In adult patients with MDD, vortioxetine is efficacious at doses up to 20 mg per day in short-term studies of 6–8 weeks duration.21, 22, 23 Given the pharmacological profile that has emerged from mechanistic preclinical studies, its effects on cognition have proved of particular interest. A recent study in depressed patients demonstrated a benefit of vortioxetine on cognitive function,24 consistent with that found using secondary analyses of a prior study in elderly depressed patients25 and in primary analysis in general adult patients.26 Path analysis of shared variance suggested that a large proportion of the improvement in cognitive function represented a direct and independent effect, rather than an epiphenomenon of broad-based symptom improvement in depression. However, a direct test of the hypothesis that vortioxetine improves cognitive function independent of a primary remediation of depression requires a different type of study.
We have therefore assessed the effects of vortioxetine in a double-blind placebo-controlled trial of neural and cognitive function in patients in remission from depression and in HCs. We used fMRI during the N-back test as well as a battery of neuropsychological measures applied previously in studies with depressed patients. We hypothesised that vortioxetine would modulate the blood-oxygen-level-dependent (BOLD) signal within neural structures previously identified as hyperactive in depressed patients. Specifically, we predicted a normalisation of the pattern of BOLD response in prespecified brain areas as the primary outcome. We also expected vortioxetine to improve performance on a subjective measure of cognitive difficulties assessed in the Perceived Difficulties Questionnaire (PDQ) and objective measures of cognition including the Trial Making Test (TMT) and Digit Symbol Substitution Test.
Materials and methods
Participants
Ninety-six participants were enrolled and dosed and all completed the study. All were recruited by advertisement and assessed for the presence of current and past psychiatric disorders using the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition.27
Forty-eight remitted MDD participants (27 women), mean age 35.6 years (range 20–53 years) and 48 HC participants (26 women), mean age 34.1 years (range 20–53 years), were recruited (see Supplementary Information for detailed information about inclusion and exclusion criteria and recruitment).
Participants were recruited from three academic sites in the United Kingdom (the Universities of Manchester and Oxford and the Institute of Psychiatry in London). All participants gave full informed written consent to the study, which was approved by a National Health Service ethics committee and received an honorarium for their participation.
Experimental design
In a randomised, double-blind, parallel-group, placebo-controlled study participants received a daily dose of vortioxetine (20 mg) or placebo for 13 or 14 days. Participants were instructed to take one capsule per day at their normal waking time around 0600–0900 hours. Measures (BOLD fMRI, neuropsychological assessments, questionnaires) were taken before (baseline) and on days 12 to 14 of drug administration (post treatment). No significant changes to the trial design or outcomes were made after trial initiation. The primary outcome of the trial, reported in this paper, was the fMRI data collected during the N-back task with the questionnaire and neuropsychological tests being secondary outcomes.
Questionnaire measures and neuropsychological tests
The National Adult Reading Test28 was used to estimate IQ at the screening session. Participant-rated baseline measures of anxiety (Spielberger State-Trait Anxiety Inventory, STAI),29 subjective mood (Watson et al.;30 subjective symptoms of depression (Beck Depression Inventory, BDI-II)31 and total score on the PDQ32 were assessed for all participants. The 17-item HAM-D17 (clinician rated) was also assessed. These scales were reassessed on the test day after 13–14 days of vortioxetine or placebo treatment. The Digit Symbol Substitution Test,33 Rey Auditory Verbal Learning Test34 and TMT35 were used to assess cognitive function (see Supplementary Information for more details about these tests).
N-back working memory task design
While in the scanner participants completed a letter variant of the N-back task.10 Working memory was manipulated by using three levels of complexity: blocks of 1-, 2- or 3-back trials, intermixed with a sensorimotor control task (0-back). The task is described in more detail in the Supplementary Information.
Data analysis
Details of the fMRI data acquisition preprocessing and first-level analyses are provided in the Supplementary Information.
Significant activations across the whole brain were identified using cluster-based thresholding of statistical images with a height threshold of Z=2.3 and a (whole-brain) corrected spatial extent threshold of P<0.05. Small volume corrections were applied to explore activation within predefined anatomical masks in the bilateral dlPFC, bilateral hippocampus, anterior cingulate cortex and precuneus (see Supplementary Materials). Finally, to exclude the influence of change in symptoms of depression on the neuroimaging outcomes, the group level analysis was repeated with the change in BDI between baseline and post treatment for each participant included as an additional covariate. To illustrate significant treatment effects, we extracted mean % signal change from the identified clusters. The maximum Z-score within each cluster is reported alongside the coordinates of the voxel with that score. The predefined primary analysis was a comparison of BOLD activity, within the anatomical structural masks, between the treatment groups in remitted subjects. Both this primary analysis and additional comparisons using all subjects were defined in a statistical analysis plan, which was finalised before database lock. Analyses of the behavioural, questionnaire and demographic data are described in the Supplementary Materials.
Results
Demographic and questionnaire measures at baseline
The subject groups were well matched for age, weight and IQ (Table 1). The proportion of women randomised to receive vortioxetine was somewhat larger than that randomised to receive placebo. The groups also differed on measures of personality, current/baseline mood and cognitive difficulties, with the remitted group scoring higher than the control group on trait anxiety, HAM-D17 (as per inclusion), BDI and the PDQ (see Supplementary Information for more details about participants and reported side effects).
Table 1. Demographic and clinical ratings at baseline.
| Variables |
Control |
Remitted |
Statistical comparison between remitted and control groups | ||
|---|---|---|---|---|---|
| Placebo | Vortioxetine | Placebo | Vortioxetine | ||
| N | 24 | 24 | 24 | 24 | |
| Age (years) | 33.8 (9.1) | 34.5 (8.9) | 38.1 (8.8) | 33.1 (9.0) | F<1 |
| Gender (m:f) | 13:11 | 9:15 | 13:11 | 8:16 | Wald χ2=0.03, d.f. =1, P>0.1 |
| Weight (kg) | 72.1 (12.8) | 73.5 (16.7) | 75.1 (16.6) | 71.4 (13.5) | F<1 |
| BMI (kg/m2) | 24.3 (3.4) | 25.8 (4.9) | 25.5 (4.0) | 24.9 (3.6) | F<1 |
| NART IQ score | 116.0 (7.8) | 115.9 (6.6) | 117.6 (6.0) | 118.1 (4.9) | F(1, 89)=2.3, P>0.1 |
| Trait-STAI score | 31.3 (4.8) | 31.3 (3.8) | 40.4 (6.8) | 38.3 (8.1) | F(1, 89)=41.1, P<0.001 |
| State-STAI score | 22.9 (3.5) | 24.6 (5.0) | 28.7 (6.9) | 29.5 (8.4) | F(1, 86)=16.5, P<0.001 |
| PANAS-positive score | 41.2 (5.6) | 37.3 (5.9) | 35.3 (8.1) | 34.3 (6.5) | F(1, 89)=10.8, P=0.001 |
| PANAS-negative score | 11.5 (1.4) | 11.1 (1.5) | 14.4 (3.5) | 13.1 (3.1) | F(1, 89)=21.9, P<0.001 |
| BDI score | 2.8 (1.3) | 3.3 (1.9) | 7.8 (6.7) | 6.9 (7.0) | F(1, 89)=17.1, P<0.001 |
| HAM-D17 baseline score | 0.3 (0.6) | 0.4 (0.7) | 1.6 (2.1) | 1.0 (1.0) | F(1, 89)=14.6, P<0.001 |
| Number of previous MDD episodes | — | — | 2.42 (0.8) | 2.83 (1.2) | |
| Total PDQ score | 12.8 (8.6) | 13.1 (9.0) | 28.2 (11.5) | 28.7 (10.3) | F(1, 89)=59.4, P<0.001 |
Abbreviations: BDI, Beck Depression Inventory; BMI, body mass index; d.f., degrees of freedom; HAM-D17, 17-item Hamilton Depression Rating Scale; MDD, major depressive disorder; MDE, major depressive episode; NART, National Adult Reading Test; PANAS, Positive and Negative Affect Scale; PDQ, Perceived Deficits Questionnaire; STAI, Spielberger State-Trait Anxiety Inventory.
Data are presented as mean (s.d.) and n for gender. Statistics reported are corrected for site and gender.
Effect of treatment on questionnaire measures
Depression ratings
There was no statistically significant effect of vortioxetine treatment on HAM-D17 scores, either combined or in either subject group individually (all P-values >0.14) (Table 2). However, vortioxetine did reduce the mean BDI score across all participants (main effect of treatment: F(1,88)=5.3, P=0.02) with this effect differing between the two subject groups (treatment × subject group interaction; F(1,88)=7.1, P=0.009). The remitted depressed group had a significant decrease in BDI, by a mean of 3.8 points (F(1,42)=7.4, P=0.009), whereas no effect was seen in control participants (F(1,42)=0.2, P=0.6). Inspection of the effect of treatment on the individual items from the BDI in the remitted group suggested that, relative to placebo, vortioxetine treatment resulted in the greatest improvement in self-reported concentration and interest (see Supplementary Information for additional analysis of subjective experience ratings).
Table 2. Change from baseline on questionnaire measures.
|
Control |
Remitted |
|||
|---|---|---|---|---|
| Placebo | Vortioxetine | Placebo | Vortioxetine | |
| State-STAI score | 2.1 (4.2) | −0.2 (4.8) | 0.4 (8.5) | −2.6 (6.6) |
| PANAS-positive score | −0.4 (5.6) | −1.6 (5.5) | −1.5 (5.8) | 2.8 (5.2) |
| PANAS-negative score | 0.08 (1.4) | −0.3 (1.4) | −0.2 (5.0) | −0.5 (2.2) |
| BDI score | 0.42 (2.8) | 0.46 (2.6) | 0.42 (5.3) | −3.08 (5.9) |
| HAM-D17 score | 0.17 (1.1) | 0.67 (1.6) | 0.83 (3.2) | 0.29 (1.7) |
| PDQ total score | −1.92 (5.7) | −5.42 (6.2) | −4.96 (6.6) | −7.58 (8.4) |
Abbreviations: BDI, Beck Depression Inventory; HAM-D17, 17-item Hamilton Depression Rating Scale; PANAS, Positive and Negative Affect Scale; PDQ, Perceived Deficits Questionnaire; STAI, Spielberger State-Trait Anxiety Inventory.
Note scores are calculated as post treatment—baseline; therefore, a reduction in symptoms is shown as a negative number. Data are presented as mean (s.d.).
PDQ
Vortioxetine decreased perceived cognitive deficits compared with placebo treatment (F(1,88)=5.9, P=0.02) and this did not interact with subject group (F(1,88)=0.1, P=0.7). However, when considering the two subject groups separately, there was a significant effect in the HC subjects (F(1,42)=5.2, P=0.03) but not the remitted depressed group (F(1,42)=1.8, P=0.2). Controlling for the effect of change in BDI did not change the pattern of results.
Neuropsychological task performance
DSST
There was no effect of subject group (remitted versus HCs) at baseline (F(1,89)=0.004, P=0.95) and performance was unaffected by vortioxetine compared with placebo across subject group as well as in each group considered separately (all P-values >0.8) (Supplementary Table 2).
TMT-A
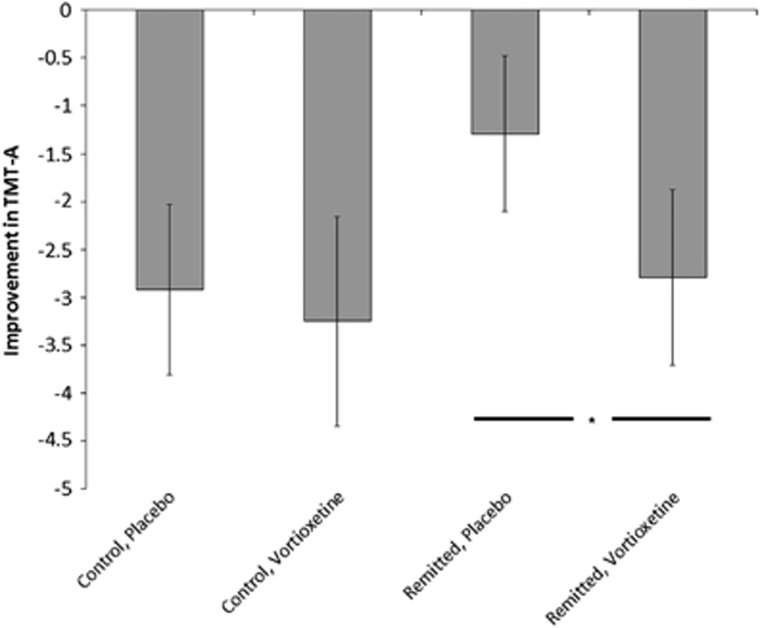
There were no differences in performance of this task between the remitted depressed and HC groups at baseline (F(1,89)=0.002, P=0.97). However, across both groups vortioxetine reduced the time needed to perform this task relative to placebo (F(1,88)=6.8, P=0.01; Figure 1). There was no subject group × treatment group interaction (F(1,88)=1.1, P=0.3). Analysis of the two subject groups separately revealed no significant effect of vortioxetine in controls (F(1,42)=1.4, P=0.24), although there was a significant reduction in the time to complete the task for remitted patients (F(1,42)=5.3, P=0.03). Controlling for the effect of change in BDI did not alter the pattern of results.
Figure 1.
Effect of vortioxetine on the Trail Making Test (TMT)-A test. The more negative the number, the greater the improvement across treatment. *P<0.05 for when the subject groups were analysed separately. Error bars represent s.e.m.
TMT-B
There were no differences in performance of this task between the remitted depressed and HC groups at baseline (F(1,89)=0.5, P=0.48). Vortioxetine again reduced the time taken to complete the task across both groups (F(1,88)=5.5, P=0.02) with no difference in this effect between subject groups (subject × treatment group interaction; F(1,88)=0.6, P=0.44). Analysing the groups separately revealed that vortioxetine significantly improved performance in HC subjects (F(1,42)=5.4, P=0.03) but had no effect in remitted subjects (F(1,42)=1.9, P=0.17). Controlling for the effect of improvement in BDI did not change the pattern of results.
RAVLT
There was no effect of subject group (remitted versus HCs) at baseline and performance was unaffected by vortioxetine administration across subject groups as well as in each group considered separately for both the acquisition and delayed recall scores of the RAVLT (all P-values >0.13).
fMRI data
Data from two participants (one from each group) were not included before datalock and unblinding, because of poor signal quality. There was no effect of subject group or treatment on participants’ behavioural performance of the N-back task (full analysis reported in Supplementary Information).
N-back minus 0-back
Effect of task
Across groups, performance of the N-back task was associated with increased neural responses in bilateral dlPFC, rostral medial PFC and bilateral posterior parietal cortex, consistent with previous reports (Supplementary Table 1 and Supplementary Figure 1). At the same time, the DMN was deactivated during the N-back compared with the 0-back in the inferior medial PFC, cingulate cortex and hippocampi. This network of response was not significantly different in the remitted depressed compared with HC participants.
Baseline difference between subject groups
No significant difference in activation between subject groups was found at baseline in any of the predefined anatomical masks. Similarly, whole-brain analysis revealed no baseline differences between subject groups.
Effect of treatment
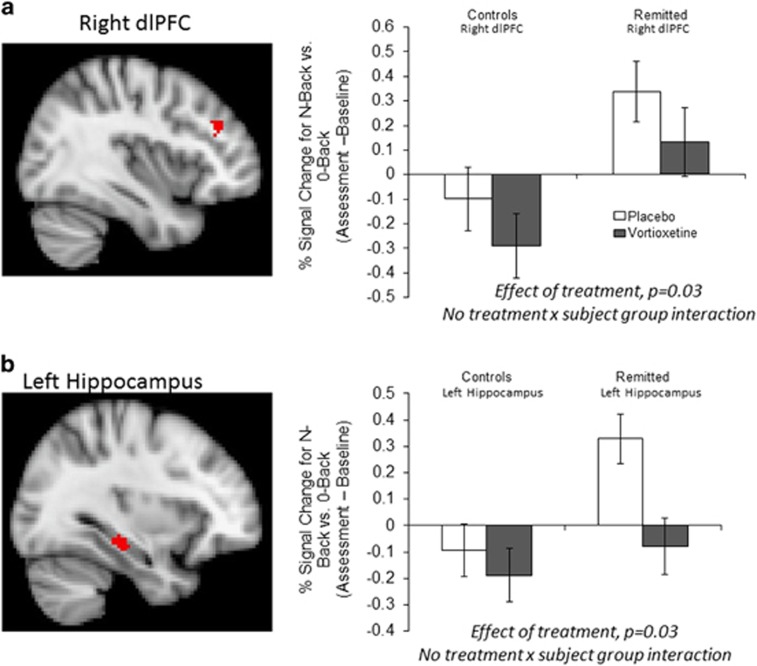
Across both groups vortioxetine significantly reduced BOLD signal within the right dlPFC and left hippocampus predefined anatomical masks (Table 3, Figure 2 and Supplementary Figure 2). Similar significant clusters were found when the change in BDI score was added to the analysis as a covariate. In the whole-brain analysis, vortioxetine additionally reduced response in the N-back task in the right insula, fusiform gyrus and lingual gyri (Table 3). These effects did not interact with subject group (remitted depressed versus HCs). However, in the analysis of the two subject groups separately, these effects of treatment within the predefined anatomical masks were largely seen in the remitted MDD group but not in the HC group (see Supplementary Results for additional analysis of the fMRI data).
Table 3. Differences in neural response to the effect of task contrast (i.e., N-back versus 0-back) during the N-back working memory task following vortioxetine versus placebo across all participants.
| Region/cluster |
Peak MNI coordinates |
Cluster size in voxels (mm3) | Peak Z-score | P-value | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Right dlPFCa | 38 | 38 | 28 | 48 | 3.34 | 0.030 |
| Left hippocampusa | −32 | −26 | −12 | 50 | 3.72 | 0.029 |
| Right posterior parietal cortex, extending into right lingual gyrus | 30 | −58 | 6 | 576 | 3.72 | 0.001 |
| Right temporal occipital fusiform cortex | 20 | −64 | −18 | 507 | 3.59 | 0.003 |
| Right insular cortex | 48 | 0 | 0 | 481 | 3.77 | 0.004 |
| Left lingual gyrus | −10 | −60 | −10 | 317 | 3.71 | 0.049 |
Abbreviations: dlPFC, dorsolateral prefrontal cortex; MNI, Montreal Neurological Institute.
Analyses restricted to predefined anatomical masks.
Figure 2.
Vortioxetine reduced blood-oxygen-level-dependent (BOLD) signal within (a) the right dorsolateral prefrontal cortex (dlPFC) and (b) left hippocampus across all participants. Red voxels show significant difference (cluster corrected P<0.05) between treatment groups in the change of N-back–0-back contrast across treatment within the prespecified anatomical masks across all subjects. Bar charts illustrate the mean (±s.e.m.) of the extracted signal change separated by treatment and subject group.
Discussion
The present results demonstrate that administration of vortioxetine modifies brain activation during performance of the N-back task in both remitted MDD patients and controls. Specifically, vortioxetine reduced neural activity in the right dlPFC and left hippocampus as well as across a network of temporal–parietal areas. This action of vortioxetine is opposite in direction of effect to the increases in BOLD signal described in MDD patients able to maintain task performance.10, 11, 12, 13, 14 We propose that it implies increased efficiency during effortful working memory performance. These neural changes were accompanied by improved subjective ratings of cognitive function as measured by the PDQ and improved performance in the TMT task following vortioxetine compared with placebo administration. These findings support a mechanism by which vortioxetine may improve executive function in MDD, unconfounded by syndromal depression.
Although cognitive impairments during remission are consistently reported in measures similar to those reported here,2, 17 the current sample of remitted depressed patients did not show objective deficits in performance or neural activation during the working memory task compared with controls. This may reflect both their age (younger than most clinical studies by a decade) and the method of recruitment (by advertisement rather than from a clinic sample). Nevertheless, the remitted depressed group reported significant subjective cognitive difficulties on the PDQ (as per inclusion criteria). Objective and subjective measures of cognitive function are often poorly correlated36 and subjective measures may well represent a more sensitive marker of the broader, everyday effects of cognitive function as they allow integration of information across situations and also reflect the effort required to optimise performance. While subjective cognitive complaints may be associated with subsyndromal symptoms of depression, the current finding that vortioxetine improved PDQ ratings across the groups (i.e. including the never depressed control group) compared with placebo administration suggests a direct effect of short-term vortioxetine on subjective cognition function. The absence of objective performance impairments, including for the N-back working memory task, had the advantage that imaging findings were easier to interpret.
Our primary outcome, the reduced BOLD signal in the dlPFC and hippocampus following short-term administration of vortioxetine, was chosen because these areas are known to show a hyperactive response during performance of the N-back task in depressed and remitted depressed patients when performance is maintained.10, 11, 12, 13, 14 The overactivity seen in depression during working memory has been proposed to reflect difficulty in switching off self-referential default-mode processing in this disorder.10 By contrast, overactivation of frontal and parietal areas is believed to represent an effortful compensatory mechanism to remediate this imbalance during maintained performance.10 Consistent with this, when the level of performance is not maintained a reduction in dlPFC activity has been reported.15 The observation that vortioxetine can reduce activation in the same network implies increased efficiency of this balance between DMN and task-positive networks during working memory performance. As such, vortioxetine may facilitate the ability to switch off the DMN and the need for overactivation of the dlPFC during executive function. The current results thereby provide a potential mechanism whereby vortioxetine can remediate cognitive deficits in depression.24, 25, 26
The pharmacological actions of vortioxetine consistent with an action on cognitive function include increased PFC monoamine levels and increased glutamatergic function in rodents.19 Improved procognition is demonstrated in object recognition and association learning in rodents following vortioxetine administration.37 The time course of our findings is consistent with improved executive function following vortioxetine administration at week 1 and week 8 of treatment in depression.26
A number of limitations to the current study should be acknowledged. The remitted depressed patients showed a small improvement in self-rated BDI mean scores with vortioxetine treatment. However, entering the BDI score as a covariate did not alter the pattern of results found for the questionnaire, cognitive task or fMRI data. Furthermore, other ratings of subjective state, mood and depression were not significantly affected by vortioxetine. Perhaps, most convincingly, the effects of vortioxetine on cognitive function were also seen in the HC group who had never experienced depression and whose BDI score was not affected by drug treatment. In summary, the cognitive effects of the medication were evident even in the absence of subjective or objective cognitive difficulty and in the absence of changes in mood or depression symptoms. As such, these results reinforce the findings from recent randomised controlled study data in depression where a relatively low proportion of variance in cognitive improvement following vortioxetine was explained by concurrent improvements in depressive symptom severity.26
Since the remitted depressed patients were not selected to, and did not show, deficits in objective neuropsychological task performance or overactivity in the working memory neural network compared with the HCs, we cannot generalise to a clinical group that may show impaired performance and function. Levels of performance on the Digit Symbol Substitution Test, TMT and Rey Auditory Learning Test appear to have been near ceiling levels across all groups and therefore may simply have been relatively insensitive to change. This limitation may explain why the current study did not demonstrate a beneficial effect of vortioxetine on the Digit Symbol Substitution Test, as has been reported in previous studies of acutely depressed patients,24, 25 although a small effect on the TMT task was found, perhaps because of differences in sensitivity to change in healthy people.
No active comparator group was used in the study, which prevents a direct comparison between the neurocognitive effects of vortioxetine with that of an alternative antidepressant. While a previous neuroimaging study of fluoxetine in depressed patients found no effect of treatment in the circuitry identified in this study,38 suggesting that the effects reported here are not common across all antidepressants, future studies would benefit from the inclusion of an active comparator.
Finally, 2 weeks is a relatively short period of treatment and most patients are likely to be treated chronically. Thus, it remains to be determined whether the effects of vortioxetine described here are maintained following longer term administration and are predictive of changes in cognitive function.
In conclusion, our results support direct effects of vortioxetine on the neural circuits important for executive function and working memory. It facilitated subjective and objective measures of cognitive function in remitted depressed patients and HCs with these effects being opposite in direction to those associated with depression when task performance is maintained. These effects could not be accounted for by changes in subjective mood in the remitted group, and were also observed in the HC group. They are therefore consistent with direct effects of the drug on the neural systems subserving executive function. The results from the imaging tasks suggest that vortioxetine may improve the efficiency and reduce the effort required to complete executive tasks. Given the impact of cognitive dysfunction on functional recovery in depression, these results hold promise for our understanding and treatment of cognitive dysfunction in depression. Finally it remains to be determined whether the effects of vortioxetine on cognition are specific to the cognitive deficits observed in depression or whether it also modulates similar cognitive deficits associated with other psychiatric disorders such as schizophrenia.
Acknowledgments
This study was sponsored by Lundbeck and managed by P1vital Limited. The authors acknowledge support from the NIHR Oxford cognitive health Clinical Research Facility. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Footnotes
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
MB is employed by P1vital and has received travel expenses from Lundbeck for attending conferences. JFD in the past 5 years has held grants from Servier, AZ and P1vital and given talks/advice for Servier, J&J, AZ and Lilly. Fees are paid as reimbursement for his time to the University of Manchester. CJH has received consultancy payments from Lundbeck, P1vital and Servier and is a shareholder in P1vital. She is a company director of Oxford Psychologists and holds shares in the same company. GG has held grants from Servier, received honoraria for speaking or chairing educational meetings from Abbvie, AZ, GSK, Lilly, Lundbeck, Medscape, Servier and advised AZ, Cephalon/Teva, Lundbeck, Merck, Otsuka, P1vital, Servier, Sunovion and Takeda, holds shares in P1vital and acted as an expert witness for Lilly. GRD is an employee of and is an owner holding shares in P1vital. SC has received travel expenses from Lundbeck for attending a conference and has received consultancy payments from P1vital. SRC, KGL and CKO are employed by Lundbeck. JB was employed by Lundbeck (at study development and initiation) and is now employed by Novartis. JS, RS, PH, and RM have no conflict of interest.
Supplementary Material
References
- Austin MP, Mitchell P, Goodwin GM. Cognitive deficits in depression: possible implications for functional neuropathology. Br J Psychiatry J Ment Sci 2001; 178: 200–206. [DOI] [PubMed] [Google Scholar]
- Baune BT, Miller R, McAfoose J, Johnson M, Quirk F, Mitchell D. The role of cognitive impairment in general functioning in major depression. Psychiatry Res 2010; 176: 183–189. [DOI] [PubMed] [Google Scholar]
- Gorwood P, Corruble E, Falissard B, Goodwin GM. Toxic effects of depression on brain function: impairment of delayed recall and the cumulative length of depressive disorder in a large sample of depressed outpatients. Am J Psychiatry 2008; 165: 731–739. [DOI] [PubMed] [Google Scholar]
- Buist-Bouwman MA, Ormel J, de Graaf R, de Jonge P, van Sonderen E, Alonso J et al. Mediators of the association between depression and role functioning. Acta Psychiatr Scand 2008; 118: 451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer TL, Kurian BT, Trivedi MH. Defining and measuring functional recovery from depression. CNS Drugs 2010; 24: 267–284. [DOI] [PubMed] [Google Scholar]
- Jaeger J, Berns S, Uzelac S, Davis-Conway S. Neurocognitive deficits and disability in major depressive disorder. Psychiatry Res 2006; 145: 39–48. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp 2005; 25: 46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousijn H, Rijpkema M, Qin S, van Wingen GA, Fernández G. Phasic deactivation of the medial temporal lobe enables working memory processing under stress. Neuroimage 2012; 59: 1161–1167. [DOI] [PubMed] [Google Scholar]
- Wirth M, Jann K, Dierks T, Federspiel A, Wiest R, Horn H. Semantic memory involvement in the default mode network: a functional neuroimaging study using independent component analysis. Neuroimage 2011; 54: 3057–3066. [DOI] [PubMed] [Google Scholar]
- Harvey P-O, Fossati P, Pochon J-B, Levy R, Lebastard G, Lehéricy S et al. Cognitive control and brain resources in major depression: an fMRI study using the n-back task. Neuroimage 2005; 26: 860–869. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Glahn DC, Peluso MAM, Hatch JP, Monkul ES, Najt P et al. Prefrontal hyperactivation during working memory task in untreated individuals with major depressive disorder. Mol Psychiatry 2007; 12: 158–166. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Srithiran A, Benitez J, Daskalakis ZZ, Oxley TJ, Kulkarni J et al. An fMRI study of prefrontal brain activation during multiple tasks in patients with major depressive disorder. Hum Brain Mapp 2008; 29: 490–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter H, Wolf RC, Spitzer M, Vasic N. Increased left prefrontal activation in patients with unipolar depression: an event-related, parametric, performance-controlled fMRI study. J Affect Disord 2007; 101: 175–185. [DOI] [PubMed] [Google Scholar]
- Rose EJ, Simonotto E, Ebmeier KP. Limbic over-activity in depression during preserved performance on the n-back task. Neuroimage 2006; 29: 203–215. [DOI] [PubMed] [Google Scholar]
- Garrett A, Kelly R, Gomez R, Keller J, Schatzberg AF, Reiss AL. Aberrant brain activation during a working memory task in psychotic major depression. Am J Psychiatry 2011; 168: 173–182. [DOI] [PubMed] [Google Scholar]
- Mannie ZN, Harmer CJ, Cowen PJ, Norbury R. A functional magnetic resonance imaging study of verbal working memory in young people at increased familial risk of depression. Biol Psychiatry 2010; 67: 471–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norbury R, Godlewska B, Cowen PJ. When less is more: a functional magnetic resonance imaging study of verbal working memory in remitted depressed patients. Psychol Med 2014; 44: 1197–1203. [DOI] [PubMed] [Google Scholar]
- Baune BT, Renger L. Pharmacological and non-pharmacological interventions to improve cognitive dysfunction and functional ability in clinical depression—a systematic review. Psychiatry Res 2014; 219: 25–50. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Asin KE, Artigas F. Vortioxetine a novel antidepressant with multimodal activity: review of preclinical and clinical data. Pharmacol Ther 2015; 145C: 43–57. [DOI] [PubMed] [Google Scholar]
- Jensen JB, du Jardin KG, Song D, Budac D, Smagin G, Sanchez C et al. Vortioxetine, but not escitalopram or duloxetine, reverses memory impairment induced by central 5-HT depletion in rats: evidence for direct 5-HT receptor modulation. Eur Neuropsychopharmacol J Eur Coll Neuropsychopharmacol 2014; 24: 148–159. [DOI] [PubMed] [Google Scholar]
- Alvarez E, Perez V, Dragheim M, Loft H, Artigas F. A double-blind, randomized, placebo-controlled, active reference study of Lu AA21004 in patients with major depressive disorder. Int J Neuropsychopharmacol Off Sci J Coll Int Neuropsychopharmacol CINP 2012; 15: 589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulenger J-P, Loft H, Olsen CK. Efficacy and safety of vortioxetine (Lu AA21004), 15 and 20mg/day: a randomized, double-blind, placebo-controlled, duloxetine-referenced study in the acute treatment of adult patients with major depressive disorder. Int Clin Psychopharmacol 2014; 29: 138–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henigsberg N, Mahableshwarkar AR, Jacobsen P, Chen Y, Thase ME. A randomized, double-blind, placebo-controlled 8-week trial of the efficacy and tolerability of multiple doses of Lu AA21004 in adults with major depressive disorder. J Clin Psychiatry 2012; 73: 953–959. [DOI] [PubMed] [Google Scholar]
- Mahableshwarkar AR, Zajecka J, Jacobson W, Chen Y, Keefe RS. A randomized, placebo-controlled, active-reference, double-blind, flexible-dose study of the efficacy of vortioxetine on cognitive function in major depressive disorder. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol 2015; 40: 2025–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona C, Hansen T, Olsen CK. A randomized, double-blind, placebo-controlled, duloxetine-referenced, fixed-dose study comparing the efficacy and safety of Lu AA21004 in elderly patients with major depressive disorder. Int Clin Psychopharmacol 2012; 27: 215–223. [DOI] [PubMed] [Google Scholar]
- McIntyre RS, Lophaven S, Olsen CK. A randomized, double-blind, placebo-controlled study of vortioxetine on cognitive function in depressed adults. Int J Neuropsychopharmacol Off Sci J Coll Int Neuropsychopharmacol CINP 2014; 17: 1557–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M. Structured Clinical Interview for the DSM-IV. New York State Psychiatric Institute: New York, NY, USA, 2002. [Google Scholar]
- Nelson HE. National Adult Reading Test. Test Manual. NFER-Nelson: Windosr, UK, 1982.
- Spielberger CD, Gorsuch RL, Lushene RD. Manual for the State-Trait Anxiety Inventory (STAI). Consulting Psychologists Press: Palo Alto, CA, USA, 1983. [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Soc Psychol 1988; 54: 1063–1070. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Assess 1996; 67: 588–597. [DOI] [PubMed] [Google Scholar]
- Sulivan JJL, Edgley K, Dehoux EA. A survey of multiple sclerosis Part 1: perceived cognitive problems and compensatory strategy use. Can J Rehabil 1990; 4: 99–105. [Google Scholar]
- Lezak MD. Neuropsychological Assessment, 2nd edn. Oxford Univeristy Press: New York, NY, USA, 1983. [Google Scholar]
- Rey A. L ’examen clinique en psychologie [Clinical Tests in Psychology]. Presses Universitaires de France: Paris, France, 1964. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale, 3rd edn. Harcourt Assessmnet: San Antonio, TX, USA, 1997. [Google Scholar]
- Svendsen AM, Kessing LV, Munkholm K, Vinberg M, Miskowiak KW. Is there an association between subjective and objective measures of cognitive function in patients with affective disorders? Nord J Psychiatry 2012; 66: 248–253. [DOI] [PubMed] [Google Scholar]
- Mørk A, Montezinho LP, Miller S, Trippodi-Murphy C, Plath N, Li Y et al. Vortioxetine (Lu AA21004), a novel multimodal antidepressant, enhances memory in rats. Pharmacol Biochem Behav 2013; 105: 41–50. [DOI] [PubMed] [Google Scholar]
- Walsh ND, Williams SCR, Brammer MJ, Bullmore ET, Kim J, Suckling J et al. A longitudinal functional magnetic resonance imaging study of verbal working memory in depression after antidepressant therapy. Biol Psychiatry 2007; 62: 1236–1243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.