Abstract
Evidence of executive dysfunction in autism spectrum disorders (ASD) across development remains mixed and establishing its role is critical for guiding diagnosis and intervention. The primary objectives of this meta-analysis is to analyse executive function (EF) performance in ASD, the fractionation across EF subdomains, the clinical utility of EF measures and the influence of multiple moderators (for example, age, gender, diagnosis, measure characteristics). The Embase, Medline and PsychINFO databases were searched to identify peer-reviewed studies published since the inclusion of Autism in DSM-III (1980) up to end of June 2016 that compared EF in ASD with neurotypical controls. A random-effects model was used and moderators were tested using subgroup analysis. The primary outcome measure was Hedges’ g effect size for EF and moderator factors. Clinical sensitivity was determined by the overlap percentage statistic (OL%). Results were reported according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. A total of 235 studies comprising 14 081 participants were included (N, ASD=6816, Control=7265). A moderate overall effect size for reduced EF (Hedges’ g=0.48, 95% confidence interval (CI) 0.43–0.53) was found with similar effect sizes across each domain. The majority of moderator comparisons were not significant although the overall effect of executive dysfunction has gradually reduced since the introduction of ASD. Only a small number of EF measures achieved clinical sensitivity. This study confirms a broad executive dysfunction in ASD that is relatively stable across development. The fractionation of executive dysfunction into individual subdomains was not supported, nor was diagnostic sensitivity. Development of feasible EF measures focussing on clinical sensitivity for diagnosis and treatment studies should be a priority.
Introduction
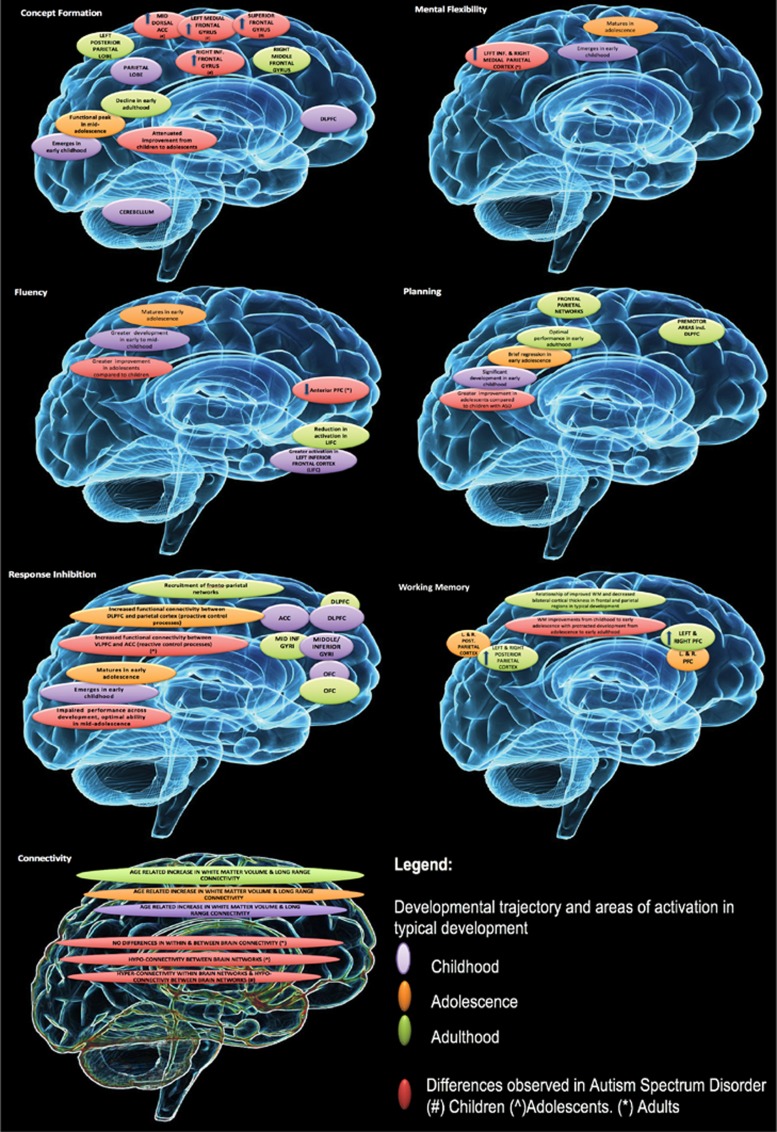
Autism spectrum disorder (ASD) is a neurodevelopmental condition defined by deficits in social communication and interaction and restricted and repetitive patterns of behaviour.1 Although genetic and neurobiological factors contribute to the ASD phenotype, neurocognitive functions also play an important role in the core behaviours of ASD. Executive function (EF) has long been of interest given its proposed role in contributing to specific impairments in ASD in the areas of theory of mind2 and social cognition, social impairment,3 restricted and repetitive behaviour patterns4 as well as broader impacts on quality of life.5 EF encompasses a broad range of purposeful higher-order neuropsychological domains, including goal-directed behaviour, abstract reasoning, decision making and social regulation.6 It is generally accepted that EF difficulties have an important role in ASD, described as poor regional coordination and integration of prefrontal executive processes that integrate with other emotion and social circuits.7 In ASD, brain abnormalities have been observed in cortical volume and thickness in both frontal and other cortical brain regions.8 Aberrant functional network connectivity influencing EF have also been reported between prefrontal and other cortical and subcortical areas9 that may be influenced by different EF subdomains. A summary of key EF domains, associated brain areas and related ASD phenotype is presented in Supplementary Table 1. Figure 1 illustrates developmental changes in EF and observed impairment in ASD.
Figure 1.
Developmental changes in executive function and associated impairment in autism spectrum disorders (ASD).
Despite extensive research, however, including a number of meta-analyses10, 11 and reviews,12, 13 the role of EF in ASD remains unclear. Individual research studies place different emphasis on the EF constructs of interest and few studies evaluate EF across most of the accepted domains of interest. The identification of a cognitive profile of executive dysfunction could provide a better understanding of the neural circuitry underpinning ASD, and may assist with clinical utility, diagnosis and treatment. Previous published ASD meta-analyses and systematic reviews of EF focus on one or two specific subdomains and thus an overall framework of the executive dysfunction profile in ASD has not been established.
Other factors may contribute to these mixed findings. Studies inconsistently control for potential moderators of the relationship between EF and ASD and the observed high interindividual cognitive variability within the spectrum.14 Moderators considered in ASD studies include variables that affect sample selection or task characteristics and may influence the observed relationship between executive dysfunction and ASD. Selection of the ASD and comparison samples varies between studies depending on the ASD classification(s) of interest (see Supplementary Table 2) and choice of a clinical or typical comparison group (or a combination). Matching criteria between ASD and comparison groups also vary and may be based on a range of variables including cognitive measures (different IQ indices), age (chronological/mental age) and gender. Sample characteristics including age and gender may moderate EF performance given that EF domains may follow a differential developmental trajectory in the typically developing brain15, 16 and those with ASD.17 However, many studies do not examine developmental trajectories in ASD and also utilise mixed age cohorts,18, 19 making outcomes on EF performance difficult to interpret. Finally, task characteristics may vary between studies on a range of variables including assessment type (psychometric tests vs experimental tasks), features of presented stimulus (verbal vs visuospatial), presentation format (computerised vs traditional) and the response type required from the participant (verbal or motor response). Yet, these potential moderators have not been systematically studied.20
The observed interindividual cognitive variability within the spectrum and differences in EF performance that may be differentially modulated by distinct EF domains (and associated brain areas) and/or different mediating factors may translate to the need for a more individualised approach for diagnostic measures and clinical interventions. Thus, research is needed to explore the clinical utility of group-based EF measures (based on standardised psychometric tests and experimental tasks) in discriminating between ASD and comparison typical populations.21
The objectives of the study were: (1) to examine evidence for executive dysfunction in ASD including the individual contribution of EF subdomains; (2) to assess the influence of moderating variables based on sample or task characteristics; and (3) to review the clinical sensitivity of individual EF measures. We hypothesised that overall EF will be impaired in ASD, individual EF subdomains will make a differential contribution to executive dysfunction and this will be correlated with improved clinical sensitivity in associated behavioural and informant EF measures. An exploratory approach was taken for reviewing moderator impact and no specific hypotheses were made.
Materials and methods
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses)22 and MOOSE (Meta-analyses Of Observational Studies in Epidemiology)23 guidelines (refer Supplementary Materials) were followed in conducting this study.
Study selection
Included in the meta-analysis were studies published in peer-reviewed journals in the English language with an a priori aim to assess EF in ASD. The selected publication date was between 1980 (first inclusion of Autism diagnosis in the DSM-III (The Diagnostic and Statistical Manual of Mental Disorders, Third Edition)24 and end of June 2016. The majority of selected studies utilised a cross-sectional design. For data extracted from clinical trials or longitudinal study designs, only the baseline data were included in the meta-analysis. Eligible studies included participants with a diagnosis of ASD based on DSM or International Classification of Diseases (ICD) classifications) and/or a diagnosis of ASD based on structured and validated diagnostic instruments (Autism Diagnostic Observations Schedule (ADOS) and/or the Autism Diagnostic Interview (ADI)). Given that the search period ranged from 1980 to 2016, the diagnostic criteria for the selected studies varied depending on the edition of DSM and ICD publications. Studies with participants <6 years of age were excluded from the meta-analysis to account for the qualitative differences in the types of assessment instruments used in younger aged groups.25 Eligible studies evaluated one or more of six key EF domains (Concept Formation/Set Shifting, Mental Flexibility/Set Switching, Fluency, Planning, Response Inhibition and Working Memory; refer to Supplementary Table 1). These EF domains were selected as they have been widely investigated in the ASD literature.
Search strategy and study variables
The literature search was conducted on the computerised databases of Medline, Embase and PsycINFO using search criteria based on EF domains and measures of interest. The first author (EAD) screened search results for initial eligibility based on title and abstract. Full-text versions of the potentially eligible studies were then assessed and included if satisfying the selection criteria. Coding of individual outcomes into EF domains was done by the first author based on accepted neuropsychological categorisation6, 26 and verified by a second independent reviewer (JEP). Reported outcomes were extracted as mean and s.d., F-test value or t-test value for each group at a single time point. In order to avoid selective data extraction, when studies included more than one measure of EF (either within the same domain or for more than one domain), all relevant outcomes were extracted. This was based on the assumption that within assessment measures are at least moderately correlated, and to avoid selective data reporting.
Moderator analysis
Age
A stratified approach (based on the mean age reported in the study plus or minus 1 s.d.) was utilised to categorise each study in one of the following age categories: ‘Children<12’, ‘Youth >12<18’, ‘Adults>18’, ‘Mixed age<18 years’ and ‘Mixed age’.
Gender
A comparison between studies that included female or male participants only.
Diagnostic group
Participants were grouped based on their study classification (Autism Diagnosis, Asperger or ASD combined (including a combination of two or more of the above classifications).
Control type
A comparison between studies utilising neurotypical controls vs sibling controls.
Diagnostic tool
Studies were classified based on the assessment tool(s) utilised for the diagnosis. These may have included one or more of the following: DSM, ICD, ADOS and ADI.
Sample matching criteria
A comparison between studies that used one or more matching criteria for sample selection.
IQ differences
A comparison based on whether a significant IQ difference was observed between the study groups.
Assessment tool format
A comparison between computer vs traditional administration of assessments.
Stimulus processing mode
A comparison based on the presentation features of test stimuli, verbal vs nonverbal.
Response mode
A comparison based on the response mode required from the participants, verbal vs motor.
Study appraisal and risk of bias in individual studies
Quality review was based on the Quality Assessment Tool27 and was completed by two independent assessors (see Acknowledgements), not involved in any other aspects of the study. To assess risk of publication bias, funnel plots for overall outcomes as well as for each cognitive domain were inspected for asymmetry and formally assessed using Egger’s regression test.
Data analysis
Data analysis was performed on Comprehensive Meta-analysis (CMA) version 3 (Biostat, Englewood, NJ, USA) using the random-effects model. The unit of analysis was standardised mean difference (calculated as Hedges’ g) on each measure between ASD and healthy controls. When more than one control group was reported in the study, the control groups were combined following established statistical procedures.28 A positive effect size indicated that the control group performed better on the EF measure compared with the ASD group.
The data analysis was planned a priori and was completed in three stages. The initial analysis combined all EF outcomes to assess the overall EF effect size in ASD. The second analysis examined subgroup comparison of the individual EF domains. In the final step, subgroup analyses were conducted to examine between study variability and moderator impact for overall EF and individual EF domains and ‘Year of Publication’ was assessed as a covariate in meta-regression analyses.
Hedges’ g effect sizes ⩽0.30, >0.30 and <0.60 and ⩾0.60 are described as small, moderate or large following the same convention applied to Cohen’s d effect sizes. Heterogeneity across studies was assessed using the I2 statistic with 95% confidence intervals (CIs). The I2 values of 25, 50 and 75% define small, moderate and large heterogeneity. Between-subgroup heterogeneity was tested using Cochrane’s Q-statistic.
Clinical sensitivity was determined by the overlap percentage statistic (OL%) based on Cohen’s29 idealised distributions. This can be converted to a percentage representing the degree that the performance of the ASD group overlaps with the control group; OL<15% represents clinical marker criteria.
Results
The literature search resulted in 235 studies (see Supplementary Table 2) that satisfied the selection criteria with a total of 14 081 participants (ASD: N=6816, Control: N=7265).
Overall effect of EF
The overall effect of EF was large and statistically significant (k=235, g=0.60, 95% CI 0.53–0.67, P<0.001). True heterogeneity across studies was large (I2=75.5%). The forest plot revealed that studies including results based on self- or carer-reported ratings had higher effect sizes with the majority of results ranging from large to very large (0.64<g<5.60) compared with studies with psychometric tests and/or experimental tasks. Egger’s regression test was significant (Egger’s intercept=1.5, P<0.001), but a trim-and-fill analysis did not result in the imputation of any studies.
A statistical comparison of ‘Assessment Tool Type’ revealed significant differences between the different tool types (psychometric test vs experimental task vs questionnaire) with the questionnaire format having the largest effect size (g=1.84, 95% CI 1.48–2.20, P<0.001). Comparably, true heterogeneity was highest for the questionnaire format (I2=89.6) compared with experimental tasks (I2=56.7) and psychometric tests (I2=50.4). It is of note that most studies including self/informant reports used the Behavioural Rating Inventory of Executive Function (BRIEF).30 A sensitivity analysis revealed that by excluding questionnaire outcomes, the revised effect size was moderate (g=0.49, 95% CI 0.44–0.55, P<0.001). Homogeneity was similarly reduced to I2=54.2%.
Given the above results the questionnaire data were excluded from the remainder of the meta-analysis and results are reported based on dependent measures assessed by psychometric tests and/or experimental tasks only.
The forest plot revealed two conspicuous outliers with Hedges’ g values >5.31, 32 Following the removal of these two outliers there was a marginal reduction in effect size ((k=221, g=0.48, 95% CI 0.43–0.53, P<0.001) and heterogeneity was also comparably reduced (I2=46.2%). The funnel plot suggested evidence of small study effect (Egger’s intercept=1.21, P<0.001). A trim-and-fill analysis however did not result in imputation of any studies and the overall effect size remained the same.
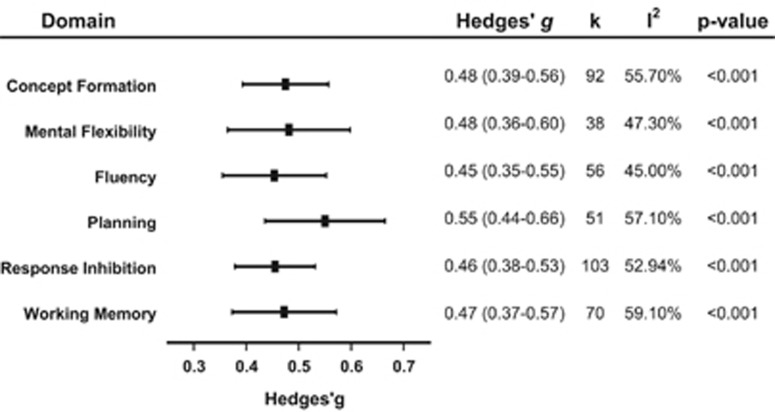
EF domain-specific effects
Small to moderate effect sizes were observed for each of the EF domains of interest (see Figure 2 and Supplementary Table 3). The subgroup analysis between EF domains was not significant (P>0.05).
Figure 2.
Subgroup analysis of executive function domains.
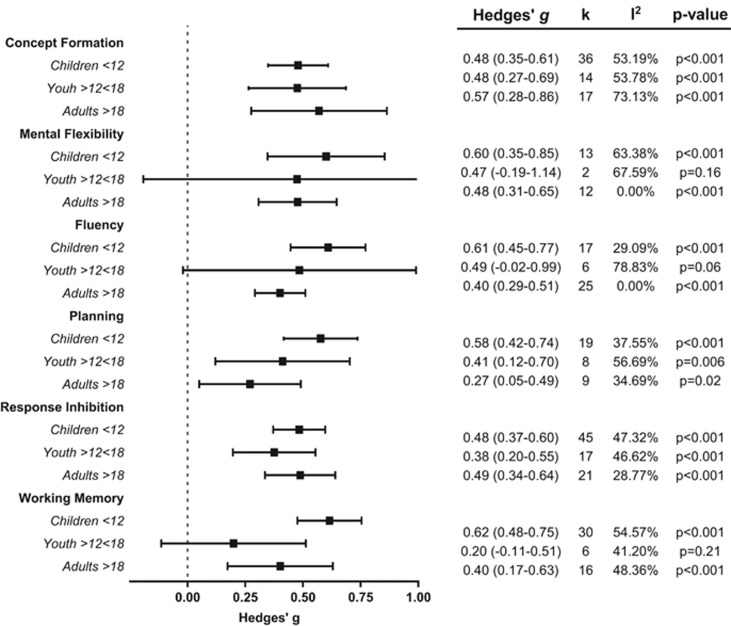
Moderator analysis
Figure 3 summarises the EF subdomain analysis by age. A detailed summary of subgroup analysis for moderator effects is presented in Supplementary Table 3.
Figure 3.
Subgroup analysis of executive function domains by age.
All within-subgroup analyses on moderator effects were significant with effect sizes ranging from small–moderate to large, but the majority of the between-subgroup analyses assessing moderator impact were not significant. Significant between-group effects were observed for the subgroup age comparison for the Working Memory domain. This was driven by lack of significant difference between ASD and controls for the Youth age grouping. The subgroup comparison between computer and traditional assessment format was also significant with presentation of tasks by computer having an attenuating effect on overall EF and this effect was also observed for the EF domains of Concept Formation and Response Inhibition. Year of Publication was statistically significant in moderating effect on overall EF and also for the subdomains of Concept Formation, Fluency and Planning.
Clinical specificity and sensitivity
Only a very limited number of measures achieved the criterion of clinical sensitivity as defined by Cohen’s ‘idealised population distribution’ (see Supplementary Table 4). The majority of the measures reaching clinical sensitivity were based on the BRIEF30 questionnaire.
Discussion
The meta-analysis extracted all EF data since ASD was introduced as a psychiatric diagnosis and showed consistent evidence of an overall moderate effect size of executive dysfunction in ASD. Individuals with a diagnosis of ASD performed on average significantly worse on EF in comparison with neurotypical controls. However, contrary to our prediction that individual EF subdomains would be differentially impaired, no significant differences in effect sizes were observed between these. Moderate effect sizes were observed for all of the established individual EF subdomains of interest. These findings suggest that there is relative equivalence of EF impairments in ASD across the constructs that were examined. This was further supported in this study by the largely homogeneous impact of most moderators on EF outcomes.
These findings are also consistent with the largely linear trajectory observed in the development of EF in ASD (see Supplementary Table 1) and recent trends in ASD research focussing on aberrant brain connectivity in predicting cognitive deficits and symptom severity in ASD.33, 34 A global impairment due to either under- or overconnectivity between brain networks broadly contributing to EF, as opposed to discrete anatomical deficits, could account for the lack of differences between subdomains of EF. The age comparison was only significant for the working memory domain. The age effect for working memory may relate to the developmental trajectory reported for some EF in neurotypical populations where performance may decline around puberty because of synapse reorganisation.35 Thus, the lack of significant difference in working memory observed in adolescents may reflect the underlying neural changes observed in this developmental period that may contribute to a decrease in EF performance in typically developing individuals. Differences therefore in this subdomain between the two groups may be least pronounced in this age range.
The generally smaller effect sizes on EF observed for the adult ASD group support other research that, either due to developmental maturity and/or increased use of compensatory strategies, adults with ASD perform better in EF than younger age groups, whereas residual executive dysfunction still persists. In addition, a smaller effect size between ASD and controls was observed when only one of the ADOS or ADI was used for diagnosis. Given the variability across ASD and recommendations for multifactorial assessment, use of a single diagnostic tool may result in a less severe cohort meeting much broader ASD criteria.
It was of interest to also note the small but significant moderating impact of Year of Publication on overall EF and for the domains of Concept Formation, Fluency and Planning that may reflect the broadening of the Autism Spectrum criteria over successive editions of the DSM since the diagnosis was first made in DSM-III.
It was hypothesised that the different diagnostic classifications of ASD may introduce variability between studies of EF, because of potential heterogeneity in cognitive function reflecting different classifications. However, our results failed to find differences in effect sizes between different diagnostic groups. This lends support to the recent focus on individual variability within the spectrum rather than between classification groups guiding EF outcomes.36
Similarly, an evaluation of the potential differences between different matching criteria did not reach significance, although the largest effect size was observed for matching based on chronological age. Differences in EF are likely to be more pronounced between experimental and comparison groups within the same age range when no other moderators such as IQ or mental age are taken into account.
Our findings on the clinical utility of EF measures show that the majority of EF measures did not achieve clinical utility in differentiating between ASD and typical controls, with mostly informant-based measures based on the BRIEF30 achieving absolute clinical marker criteria. This lends further support to the proposition that measures with ecological validity (that is, based on more representative environmental situations) may be more appropriate especially in clinical practice. Informant measures such as the BRIEF may offer greater clinical utility, but further investigations are needed to consider whether outcomes represent higher validity or might be influenced by demand or reporter characteristics. Based on the results of this study, however, the superior ecological validity of informant measures21 supports their use for circuitry-based models (Research Domain Criteria)37 and clinical staging models38 (matching developmental stage of impairment with clinical intervention and risk factors39) and for diagnostic and intervention frameworks. In addition, laboratory-based EF neuropsychological tests should be chosen based on feasibility and ease of use, given the relative equivalence of performance across domains. Taken together, these findings suggest that the focus of diagnostic and intervention measures needs to shift to a more ecologically and clinically valid framework while taking into account the likely individual differences within the spectrum.
A number of limitations may have influenced the findings of this study. The self- or informant-reported questionnaires were excluded from the majority of analyses given the significant differences in effect sizes compared against psychometric tests and experimental tasks. In addition, only accuracy-based measures were included in the analysis with all reaction time variables excluded given the direction and specification of the reaction time variable to EF can be unclear. Furthermore, we did not explore the impact that intraindividual variability within the spectrum may have on the observed findings. Finally, although we attempted to consider a comprehensive number of moderators, there remain a number of factors that may influence EF in ASD. These may relate to task characteristics (for example, task complexity, open-ended vs structured task format20) or participant characteristic including symptom severity, emotional states (for example, depression/anxiety) or comorbidities (for example, attention deficit hyperactivity disorder). Anxiety in particular has been noted to have a strong association with poor EF performance in ASD populations.40
Conclusion and further directions
In this meta-analysis we conducted an evaluation of the role of EF in ASD, including an assessment of a large range of potential moderators. Our findings, across a large number of research participants with ASD, suggest there is an overall effect of executive dysfunction and this applies evenly across individual domains, where moderate effect sizes were observed. Predictions of a differential profile of executive dysfunction based on subdomain performance were not supported and our review of moderators mostly returned null results. Taken together, these findings suggest that ASD populations are impaired in EF, but this reflects an overall and not fractionated impairment in EF performance and may be best accounted by the observed aberrant long-range and local over- and underconnectivity between brain networks in ASD. Further work is needed to identify feasible and sensitive EF general markers for use in diagnosis and clinical trials. Given the stability of EF performance in ASD across neurodevelopment, early intervention may provide the best opportunity to alter trajectories over the lifetime to improve outcomes for people with ASD.
Acknowledgments
We thank Karen Gould and Magdalena Durrant for completing the quality review of the studies and Benedikt Langenbach with assistance with data collection. We acknowledge NHMRC postgraduate scholarship to EAD (GNT1056587), a NHMRC Dementia Research Development Fellowship (ID 1108520) to AL, an NHMRC Australian Fellowship (APP 511921) to IH and a NHMRC career development fellowship (APP1061922) and project grant (1043664) to AJG. JEP is supported by NeuroSleep, NHMRC Centre of Research Excellence.
Footnotes
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
IH is a Commissioner in Australia’s new National Mental Health Commission from 2012. He was a director of headspace: the national youth mental health foundation until January 2012. He was previously the chief executive officer (till 2003) and clinical adviser (till 2006) of beyondblue, an Australian National Depression Initiative. He is the Co-Director, Health and Policy at the Brain and Mind Centre that operates two early-intervention youth services under contract to headspace. He has led a range of community-based and pharmaceutical industry-supported depression awareness and education and training programs. He has led projects for health professionals and the community supported by governmental, community agency and pharmaceutical industry partners (Wyeth, Eli Lily, Servier, Pfizer, AstraZeneca) for the identification and management of depression and anxiety. He has received honoraria for presentations of his own work at educational seminars supported by a number of non-government organisations and the pharmaceutical industry (including Servier, Pfizer, AstraZeneca and Eli Lilly). He is a member of the Medical Advisory Panel for Medibank Private and also a Board Member of Psychosis Australia Trust. He leads an investigator-initiated study of the effects of agomelatine on circadian parameters (supported in part by Servier) and has participated in a multicentre clinical trial of the effects of agomelatine on sleep architecture in depression and a Servier-supported study of major depression and sleep disturbance in primary care settings. The other authors declare no conflict of interest.
Supplementary Material
References
- American Psychiatric AssociationDiagnostic and Statistical Manual of Mental Disorders, 5th edn. Washington DC, 2013.
- Pellicano E. Links between theory of mind and executive function in young children with autism: clues to developmental primacy. Dev Psychol 2007; 43: 974–990. [DOI] [PubMed] [Google Scholar]
- Leung RC, Vogan VM, Powell TL, Anagnostou E, Taylor MJ. The role of executive functions in social impairment in autism spectrum disorder. Child Neuropsychol 2016; 22: 336–344. [DOI] [PubMed] [Google Scholar]
- Mostert-Kerckhoffs MA, Staal WG, Houben RH, Jonge MV. Stop and change: inhibition and flexibility skills are related to repetitive behavior in children and young adults with autism spectrum disorders. J Autism Dev Disord 2015; 45: 3148–3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries M, Geurts H. Influence of autism traits and executive functioning on quality of life in children with an autism spectrum disorder. J Autism Dev Disord 2015; 45: 2734–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Bigler ED, Tranel D. Neuropsychological Assessment. Oxford University Press: New York, 2012. [Google Scholar]
- Maximo JO, Cadena EJ, Kana RK. The implications of brain connectivity in the neuropsychology of autism. Neuropsychol Rev 2014; 24: 16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libero LE, DeRamus TP, Lahti AC, Deshpande G, Kana RK. Multimodal neuroimaging based classification of autism spectrum disorder using anatomical, neurochemical, and white mattter correlates. Cortex 2015; 66: 46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomi JS, Uddin LQ. Developmental changes in large-scale network connectivity in autism. Neuroimage Clin 2015; 7: 732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurts HM, van den Bergh SF, Ruzzano L. Prepotent response inhibition and interference control in autism spectrum disorders: two meta-analyses. Autism Res 2014; 7: 407–420. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit hyperactivity disorder: a meta-analytic review. Biol Psychiatry 2006; 57: 1336–1346. [DOI] [PubMed] [Google Scholar]
- Kercood S, Grskovic JA, Banda D, Begeske J. Working memory and autism: a review of literature. Res Autism Spectr Disord 2014; 8: 1316–1332. [Google Scholar]
- Barendse EM, Hendriks MP, Jansen JF, Backes WH, Hofman PA, Thoonen G et al. Working memory deficits in high-functioning adolescents with autism spectrum disorders: neuropsychological and neuroimaging correlates. J Neurodev Disord 2013; 5: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czermainski FR, Bosa CA, Mina CS, Meimes MA, Miranda MC, Carim D et al. Performance of children/adolescents with autism spectrum disorders in executive function: study of case series. Psychol Neurosci 2015; 8: 305–320. [Google Scholar]
- Anderson P. Assessment and development of executive function (EF) during childhood. Child Neuropsychol 2002; 8: 71–82. [DOI] [PubMed] [Google Scholar]
- Jurado MB, Rosselli M. The elusive nature of executive functions: a review of our current understanding. Neuropsychol Rev 2007; 17: 213–233. [DOI] [PubMed] [Google Scholar]
- O'Hearn K, Asato M, Ordaz S, Luna B. Neurodevelopment and executive function in autism. Dev Psychopathol 2008; 20: 1103–1132. [DOI] [PubMed] [Google Scholar]
- Griebling J, Minshew NJ, Bodner K, Libove R, Bansal R, Konasale P et al. Dorsolateral prefrontal cortex magnetic resonance imaging measurements and cognitive performance in autism. J Child Neurol 2010; 25: 856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Gadea ML, Chennu S, Bekinschtein TA, Rattazzi A, Beraudi A, Tripicchio P et al. Predictive coding in autism spectrum disorder and attention deficit hyperactivity disorder. J Neurophysiol 2015; 114: 2625–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eylen L, Boets B, Steyaert J, Wagemans J, Noens I. Executive functioning in autism spectrum disorders: influence of task and sample characteristics and relation to symptom severity. Eur Child Adolesc Psychiatry 2015; 24: 1399–3417. [DOI] [PubMed] [Google Scholar]
- Leung RC, Zakzanis KK. Brief report: cognitive flexibility in autism spectrum disorders: a quantitative review. J Autism Dev Disord 2014; 44: 2628–2645. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting System for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLOS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroup DF, Berlin DA, Morton SC, Ilkin I, Williamson GD, Rennie D et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA 2000; 283: 2008–2012. [DOI] [PubMed] [Google Scholar]
- American Psychiatric AssociationDiagnostic and Statistical Manual of Mental Disorders, 3rd edn. Washington DC, 1980.
- Espy KA. Using developmental, cognitive and neuroscience approaches to understand executive control in young children. Dev Neuropsychol 2004; 26: 379–384. [DOI] [PubMed] [Google Scholar]
- Miyaki A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex "frontal lobe" tasks: a latent variable analysis. Cogn Psychol 2000; 41: 49–100. [DOI] [PubMed] [Google Scholar]
- Armijo-Olivo S, Stiles CR, Hagen NA, Biondo PD, Cummings GG. Assessment of study quality for systematic reviews: a comparison of the Cochrane Collaboration Risk of Bias Tool and the Effective Public Health Practice Project Quality Assessment Tool: methodological research. J Eval Clin Pract 2012; 18: 12–18. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistencies in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Erlbaum Associates: Hillsdale, NJ, 1988. [Google Scholar]
- Gioia G, Isquith PK, Guy SC, Kenworthy L. Behavior rating inventory of executive function. Child Neuropsychol 2000; 6: 235–238. [DOI] [PubMed] [Google Scholar]
- Scott FJ, Baron-Cohen S. Imagining real and unreal things: evidence of a dissociation in autism. J Cogn Neurosci 1996; 8: 371–382. [DOI] [PubMed] [Google Scholar]
- Kushki A, Drumm E, Pla Mobarak M, Tanel N, Dupuis A, Chau T et al. Investigating the autonomic nervous system response to anxiety in children with autism spectrum disorders. PLoS ONE 2013; 8: e59730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissers EM, Cohen MX, Geurts HM. Brain connectivity and high functioning autism: a promising path of research that needs refined models, methodological convergence, and stronger behavioural links. Neurosci Biobehav Rev 2012; 36: 604–625. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Abe O, Nippashi Y, Yamasue H. Comparison of white matter integrity between auism spectrum disorder subjects and typically developing individuals: a meta-analysis of diffusion tensor imaging tractography studies. Mol Autism 2013; 4: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S-J, Choudhury S. Development of the adolescent brain: implications for executive function and social cognition. J Child Psychol Psychiatry 2006; 47: 296–312. [DOI] [PubMed] [Google Scholar]
- Geurts HM, Sinzig J, Booth R, Happe F. Neuropsychological heterogeneity in executive functioning in autism spectrum disorders. Int J Dev Disabil 2014; 60: 155–162. [Google Scholar]
- Foss-Feig JH, McPartland JC, Anticevic A, Wolf J. Re-conceptualizing ASD within a dimensional framework: positive, negative, and cognitive feature clusters. J Autism Dev Disord 2016; 46: 342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGorry PD. Staging in neuropsychiatry: a heuristic model for understanding prevention and treatment. Neurotox Res 2010; 18: 244–255. [DOI] [PubMed] [Google Scholar]
- McGorry P, Keshavan M, Goldstone S, Amminger P, Allott K, Berk M et al. Biomarkers and clinical staging in psychiatry. World Psychiatry 2014; 13: 211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollocks MJ, Jones CR, Pickles A, Baird G, Happe F, Charman T et al. The association between social cognition and executive functioning and symptoms of anxiety and depression in adolescents with autism spectrum disorders. Autism Res 2014; 7: 216–228. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.