Abstract
Increased motivation for highly rewarding food is a major contributing factor to obesity. Most of the literature focuses on the mesolimbic nuclei as the core of reward behavior regulation. However, the lateral hypothalamus (LH) is also a key reward-control locus in the brain. Here we hypothesize that manipulating glucagon-like peptide-1 receptor (GLP-1R) activity selectively in the LH can profoundly affect food reward behavior, ultimately leading to obesity. Progressive ratio operant responding for sucrose was examined in male and female rats, following GLP-1R activation and pharmacological or genetic GLP-1R blockade in the LH. Ingestive behavior and metabolic parameters, as well as molecular and efferent targets, of the LH GLP-1R activation were also evaluated. Food motivation was reduced by activation of LH GLP-1R. Conversely, acute pharmacological blockade of LH GLP-1R increased food motivation but only in male rats. GLP-1R activation also induced a robust reduction in food intake and body weight. Chronic knockdown of LH GLP-1R induced by intraparenchymal delivery of an adeno-associated virus-short hairpin RNA construct was sufficient to markedly and persistently elevate ingestive behavior and body weight and ultimately resulted in a doubling of fat mass in males and females. Interestingly, increased food reinforcement was again found only in males. Our data identify the LH GLP-1R as an indispensable element of normal food reinforcement, food intake and body weight regulation. These findings also show, for we believe the first time, that brain GLP-1R manipulation can result in a robust and chronic body weight gain. The broader implications of these findings are that the LH differs between females and males in its ability to control motivated and ingestive behaviors.
Introduction
Obesity and associated metabolic diseases are continuing to increase worldwide while safe and effective obesity treatments remain elusive. As food intake and body weight are controlled by the central nervous system (CNS), understanding the complex CNS mechanisms that regulate energy homeostasis is essential for the discovery of successful antiobesity therapeutics.
The lateral hypothalamus (LH) is historically known as a critical ‘feeding center’ and a powerful coordinator of the drive to eat, drink and move. The key role of this nucleus in feeding control is illustrated perhaps most robustly by a profound aphagia induced by bilateral lesions of the LH.1, 2, 3, 4 Conversely, stimulation of the LH induces eating, even in satiated rats.5 This nucleus is also suggested to be the interface between the homeostatic and hedonic control of feeding behavior. Rats will eagerly press a lever to stimulate their LH, suggesting that LH activation itself is rewarding.6, 7, 8
Food can be a very potent rewarding stimulus. Food consumption and reward are controlled by partly divergent and partly overlapping brain nuclei: areas that respond to hormonal and metabolic signals that are derived from the periphery and elsewhere in the brain.9, 10 The mesolimbic neurocircuitry, especially the ventral tegmental area (VTA) and its dopaminergic projections to the nucleus accumbens (NAc), have garnered much of the attention on neural control of food reward.11, 12, 13 However, the LH is in fact the most potent self-stimulation center in the brain, and given that hungry/food-restricted rats self-stimulate even more than fed rats, an interaction between the metabolic status and reward may require processing by this brain nucleus,7, 14 although it is also possible that stimulation of the median forebrain bundle, which connects the VTA to the NAc and runs through the LH, may contribute to the reinforcing effects of the intracranial self-stimulation studies.15 The LH is innervated by the hindbrain glucagon-like peptide-1 (GLP-1) neurons and GLP-1 receptors (GLP-1R) are expressed in the LH.16 A potential reward impact of this communication is largely unexplored.
GLP-1 is an anorexigenic peptide that is produced both in the peripheral L-cells of the gastrointestinal tract and in the brain, primarily by neurons located in the nucleus of the solitary tract (NTS).16 These neurons project to widespread, but discrete, GLP-1R-expressing brain areas.16 Analogs of GLP-1 are currently approved for treatment of type 2 diabetes and obesity.17 Administration of GLP-1 or the GLP-1R agonist, exendin-4 (Ex4), into the CNS reduces food intake and body weight, actions historically attributed to the hypothalamic and brainstem GLP-1R-expressing targets.18, 19, 20, 21 More recently, central GLP-1 signaling has been linked to food (see Kanoski et al.21 and Skibicka22 for reviews) and drug reinforcement.23, 24, 25 Despite the reliable and potent inhibitory effects of pharmacological activation of GLP-1R on feeding and body weight—paradoxically, pharmacological blockade of central GLP-1R, and especially knockout studies of whole-body or neuronal GLP-1R, produce only minor changes in feeding or body weight.26, 27, 28 Collectively, this phenomenon has led to an ever-increasing view that GLP-1R is only sufficient, but not critical, for body weight control. Here, utilizing a knockdown model of LH GLP-1R, we wanted to challenge that view and propose that central, and specifically LH, GLP-1R activation is indispensable to control of ingestive and food reward behaviors and ultimately body fat mass. We further wanted to examine behavioral, molecular and efferent targets of LH GLP-1R activation. Moreover, conceivable sex differences, as well as potential sources of differential female responses, including estrous cycle, were assessed.
Materials and methods
Animals
Male and female Sprague-Dawley rats (5 weeks of age at arrival, Charles River, Sulzfeld, Germany) were housed in a 12-h light/dark cycle (light on at 0700 hours) in individual cages with ad libitum access to chow (Teklad Global 16% Protein Rodent Diet (2016), Envigo, Huntingdon, UK) and water, unless otherwise stated. All studies were carried out with ethical permissions from the Animal Welfare Committee of the University of Gothenburg, in accordance with legal requirements of the European Community (Decree 86/609/EEC). All efforts were made to minimize suffering.
Drugs
Ex4 and the GLP-1R antagonist exendin-9 (Ex9) were purchased from Tocris (Bristol, UK), dissolved in artificial cerebrospinal fluid (Tocris; used as vehicle) and stored as aliquots at −20 °C.
Brain cannulation
Guide cannulas were implanted into the LH as previously described29 (for details, see Supplementary Information). With the chosen coordinates, our manipulations/injections consistently reached the central, lateral and dorsal LH. Injections may have also reached the zona incerta; however, unlike the LH, this area does not express GLP-1R16 (Supplementary Figure S1).
Operant conditioning
The operant conditioning procedure is used to assess the motivation to obtain a reward, in this case food reward in the form of a sucrose pellet (45 mg TestDiet, Richmond, IN, USA). Training and testing were conducted as described previously30, 31 and in Supplementary Information. All operant response testing was performed under the progressive ratio (PR) schedule.
Effects of pharmacological LH GLP-1R activation or blockade on food intake, body weight, locomotor activity and food-motivated behavior
To test the effects of GLP-1R activation, 11-week-old rats were injected with Ex4 (0.05 or 0.15 μg) or vehicle (artificial cerebrospinal fluid), and operant conditioning response was tested 20 min after injection. Additionally impact of the estrous cycle on LH GLP-1R activation was evaluated in a separate group of 8-week-old females (see Supplementary Information). For experiments examining the effect of GLP-1R blockade, 11-week-old rats were first fasted overnight, then given a chow meal for 20 min and injected with Ex9 (10 μg) immediately after the meal. This design was modified from Hayes et al.32 and used to elicit endogenous GLP-1 release to a controlled meal. Rats were tested in the operant conditioning task 10 min after injection. Food seeking was assessed as the number of head pokes into the feeding chamber during the 60 min operant session. Chow intake was measured 1 and 24 h after the operant testing sessions. Each treatment was counterbalanced where each condition was separated by a 4-day period. Locomotor activity was measured using horizontal infrared beams in the operant chambers (Med-Associates, Georgia, VT, USA).
GLP-1R knockdown
To knockdown the expression of the GLP-1R in the LH, a short hairpin RNA (shRNA) targeting GLP-1R transcripts was used (for details, see Schimdt et al.24). Preliminary in vitro studies demonstrated ~88% knockdown of GLP-1R expression in a rat neuronal cell line transfected with this shRNA.24 To knockdown GLP-1R expression in vivo, this shRNA sequence was cloned and packaged into an adeno-associated virus (AAV) (serotype 1; titer=5.22e12) in collaboration with the Viral Core at the University of Pennsylvania. This construct was previously shown to reduce GLP-1R expression in vivo in the rat VTA by 50%.24 A green fluorescent protein (GFP)-expressing AAV (titer=5.0e12) was used as a control.
To determine the functional significance of endogenous GLP-1R signaling in the LH, rats were surgically implanted with LH-directed guide cannulae as described above. Once rats achieved stable sucrose-motivated behavior on PR (at 9 weeks of age), AAV-expressing GFP (AAV-GFP) or the GLP-1R shRNA (AAV-GLP-1R-shRNA) was infused bilaterally into the LH (0.5 μl per hemisphere during 5 min). Microinjectors were left in place for 10 min after infusion to allow for diffusion away from the injection site. Rats chosen for each treatment group were matched for body weight, food intake and food reinforcement parameters on PR.
Body weight and food intake were measured daily after AAV construct infusion, except for days where fasting or food restriction was applied. Motivation to self-administer sucrose was assessed using a PR schedule 3, 7 and 10 days after AAV injections. On day 7, rats were food restricted overnight to determine whether the contribution of LH GLP-1R signaling to food-motivated behavior is altered by fasting.
Three weeks after viral injections, two experiments were performed that required food restriction: fasting blood glucose measurements and subsequent oral glucose tolerance test (intraperitoneal glucose tolerance test; for details, see Supplementary Information), and a fasting/refeeding experiment.
Four weeks after AAV injection, adipose tissue was weighed and the brains were collected. Brains were dissected and the LH, NAc and NTS were collected to assess GLP-1R expression using quantitative real-time PCR (for details, see Supplementary Information). An LH-containing coronal section (10 μm) was collected from each brain to verify correct placement of AAV injections by visualizing GFP fluorescence (see Figure 4 for a representative placement).
A second smaller group of rats (n=20, 10 males and 10 females, 13-week old) was included after the first study to determine whether there are sex differences in the effects of LH GLP-1R knockdown and to follow food intake and body weight daily without the interruptions for testing that required overnight restriction carried out in the first group of rats. In addition, another group of female rats (n=16) was tested to further evaluate the necessity of LH GLP-1R in younger females whose body weight gain is more rapid compared with the 13-week-old females; these rats were 9 weeks at the time of AAV-shRNA infusion.
Viral tract tracing and in situ hybridization
Injections of cholera toxin subunit B and in situ hybridization were performed as previously described.33 For detailed methods, see Supplementary Information.
Impact of Ex4 or feeding on LH gene expression
Hypothalamic neuronal cells, immortalized from four 8-week-old male mice were used to evaluate the impact of GLP-1R activation on LH neuropeptides in neurons. In order to determine whether the genes found to be altered in the immortalized hypothalamic cell line were also expressed in the LH in vivo and altered by ingestion of fat, chow or sucrose, rats were exposed to respective diets for 1 h per day for 1 week on an every other day schedule. Brains were collected, and the LH was dissected immediately after the last feeding session. Gene expression was then analyzed (see Supplementary Information and Supplementary Table for details). In each feeding group, there was one animal that ate <0.5 g of food; these rats were eliminated from final gene analysis.
Statistical analysis
All the data are presented as mean±s.e.m. Statistical significance was analyzed using t-test or one- or two- way analysis of variance with Holm–Sidak’s multiple comparison tests, when appropriate (GraphPad Software, San Diego, CA, USA). P-values <0.05 were considered statistically significant.
Further information on methods is available in Supplementary Information.
Results
Acute LH-targeted GLP-1R activation is sufficient to reduce food reinforcement and ingestive behavior and affect LH neuropeptide expression
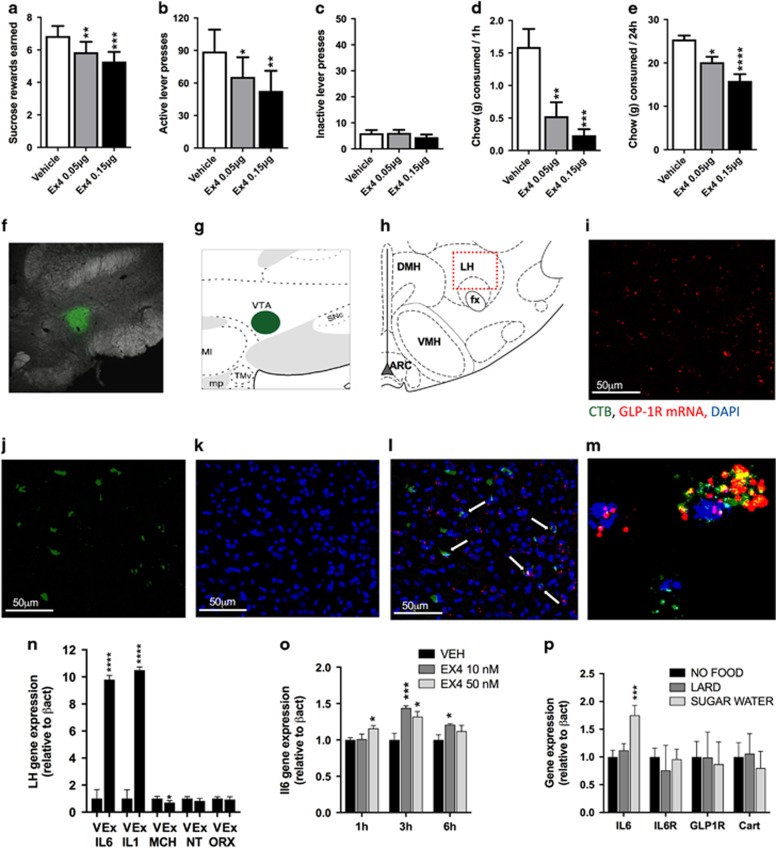
Intra-LH Ex4 microinjection induced a robust reduction in food-motivated behavior, as indicated by the reduced number of sucrose rewards earned (F(2,26)=12.59; P=0.0001; Figure 1a) and reduced number of lever presses emitted for the sucrose rewards (F(2,26)=8.727; P=0.0013; Figure 1b), without changes in inactive lever pressing (F(2,26)=0.7931; P=0.4631; Figure 1c) in male rats. Notably, in situ hybridization combined with viral tract tracing analyses revealed GLP-1R mRNA in ~55.3% of LH cells that project to the VTA, a region that has a well-established role in mediating food reward11, 12, 13 (Figures 1f–m). The suppression of food intake was also very potent (F(2,26)=11.10; P<0.0005; Figure 1d), with Ex4-injected rats consuming only 15% of the amount they ate while injected with vehicle. At 24 h, food intake (F(2,26)=14.49; P<0.0001; Figure 1e) and body weight were still significantly suppressed (Supplementary Figure S2A) by intra-LH Ex4. Both the 0.05 and 0.15 μg doses significantly suppressed reinforcement, intake and body weight relative to intra-LH vehicle injection.
Figure 1.
Behavioral, molecular and neuroanatomical consequences of glucagon-like peptide-1 receptor (GLP-1R) activation in the lateral hypothalamus (LH) in male rats. Activation of GLP-1R in the LH reduces food reinforcement and food intake. Intra-LH GLP-1 analogue, exendin-4 (Ex4), microinjection reduced the amount of sucrose rewards earned (a) and the number of lever presses for the rewards (b) in a progressive ratio schedule, without changing activity at the inactive lever (c) in male rats. Food ingestion is also affected, as illustrated by a potent reduction in 1 (d) and 24 h (e) chow intake. Data are expressed as mean±s.e.m. n=14–15 (male rats). Moreover, GLP-1R-expressing LH neurons were found to project to the mesolimbic ventral tegmental area (VTA), a likely neuroanatomical target area for reinforcement control exerted by LH GLP-1R. Representative images showing GLP-1R on LH neurons that project to the VTA. Cholera toxin subunit B (CTB) conjugated to AlexaFluor 488 was injected into the VTA (f, g). Back-labeled cells were present in the rostral LH region dorsal to the fornix (h) where the majority of the LH GLP-1R-containing neurons are localized. Images of GLP-1R in situ hybridization (red; i), back-labeled CTB-positive neurons from the VTA (green; j) and 4,6-diamidino-2-phenylindole (DAPI) nuclear counterstain (blue; k) at × 20 magnification. A merged image showing co-localization of GLP-1R and CTB in the LH (white arrows; l) and a confocal image taken at × 40 magnification of a double-labeled GLP-1R and CTB-positive neuron with DAPI counterstain (m). Intra-LH Ex4 infusion produced a marked increase in anorexic interleukins 1 (IL1) and 6 (IL6) and also reduced orexigenic LH neuropeptide expression (melanin-concentrated hormone (MCH)) in male rats (n). n=14–22 (male rats). GLP-1R activation with Ex4 increased IL6 expression (o) in hypothalamic neuronal cell culture. In order to determine whether endogenous GLP-1 release also leads to similar changes within the LH, three groups of rats were allowed to eat chow, sugar or lard for 1 h. Lard meal did not alter any of the genes measured; however, a consumption of sucrose water increased LH IL6 expression (p). Data were normalized to the housekeeping gene beta-actin and are expressed as mean±s.e.m.. n=7–9 (male rats). ARC: arcuate nucleus of the hypothalamus; DMH: dorsomedial hypothalamus; VMH: ventromedial hypothalamus; NT: neurotensin; ORX: orexin; IL6R: IL6 receptor; Cart: cocaine- and amphetamine-related peptide. *P<0.05, **P<0.01,***P<0.001, ****P<0.0001 compared with vehicle (artificial cerebrospinal fluid).
In order to determine which of the LH food and reward-regulating neurochemicals are affected by LH-targeted GLP-1R activation, Ex4 was microinjected into the LH of male rats, and gene expression of candidate genes was measured. Marked, 10-fold induction of anorexic interleukins 1 (IL1) and 6 (IL6) was found, along with a reduction in melanin-concentrating hormone (MCH) (Figure 1n). Both ILs were previously shown to mediate anorexic and weight loss effects of GLP-1 analogs.34 We further showed that Ex4 induces IL6 in a hypothalamic neuronal cell culture, indicating that LH neurons could be one potential source of the Ex4-induced ILs (Figure 1o). Interestingly, we found that consumption of a sucrose solution, but not lard or chow meal, leads to LH IL6 induction (Figure 1p).
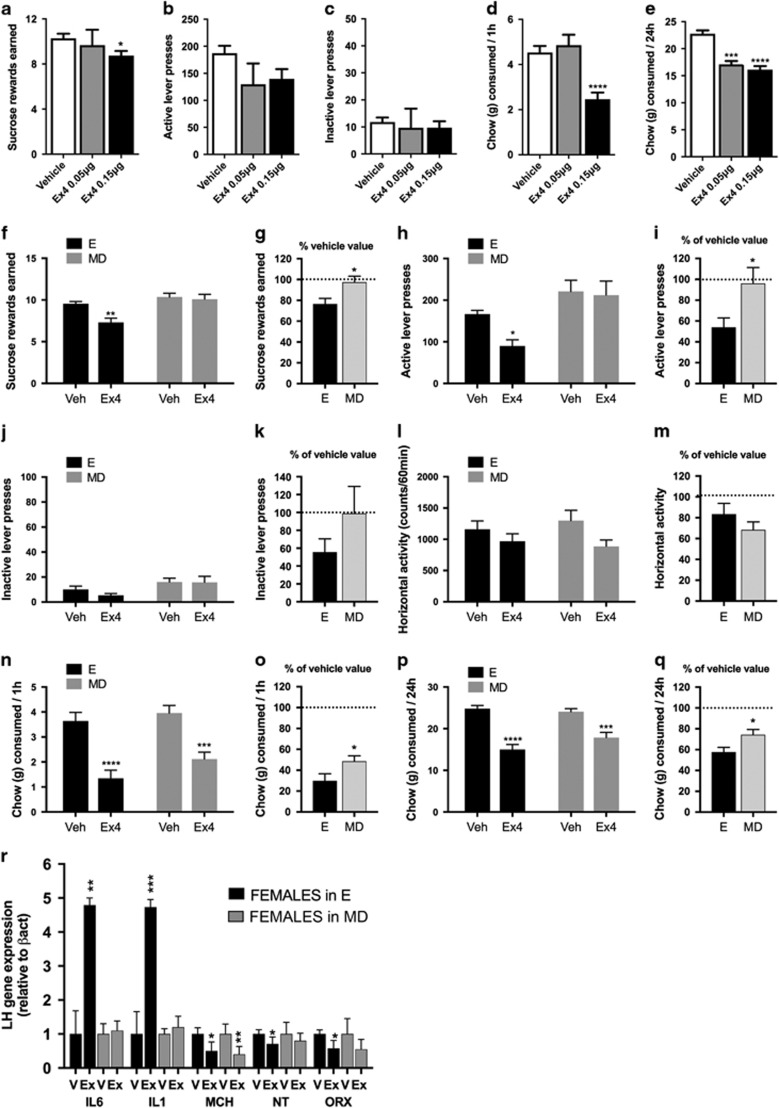
The same treatment produced a slightly less potent reinforcement and intake suppression in female rats, with the amount of sucrose rewards earned suppressed only by the higher Ex4 dose (F(2,74)=2.81; P=0.06; Figure 2a). Active lever pressing did not reach significance (F(2,74)=2.72; P=0.07; Figure 2b). One hour chow intake was also suppressed by the higher dose of Ex4 (F(2,76)=15.43; P=0.07; Figure 2d), yet 24 h chow intake was reduced by both doses (F(2,76)=29.51; P<0.0001; Figure 2e). Changes in 24 h weight gained were detected only for the higher dose (Supplementary Figure S2B). To test whether the estrous cycle stage affects behavioral impact of intra-LH Ex4 treatment, females were tested specifically in the estrus and metestrus/diestrus phases with the higher dose of Ex4 (0.15 μg), which was earlier found to be effective in females. Females in the estrus phase but not in metestrus/diestrus responded with reduced food reinforcement to the LH GLP-1R activation (rewards earned: t19=2.7, P<0.05; active lever: t19=2.3, P<0.05; Figures 2f–i). Although 1 and 24 h chow intake were reduced by the drug in all phases (Figures 2n and p), the drug effect was more potent in females in the estrus phase (1 h: t19=2.2, P<0.05; 24 h: t19=2.5, P<0.05; Figures 2o and q). The effect size seen was similar between males and females in estrus, yet significantly different when males and females in metestrus/diestrus were compared (Supplementary Figure S5). This cycle-dictated divergent behavioral impact of Ex4 was mirrored by Ex4-induced molecular changes in the LH, where a marked induction of anorexic IL1 and IL6 and conversely a reduction of the orexigenic neuropeptide, orexin, was found only in the estrus-phase females (Figure 2r). Reduction of MCH was the only drug-induced change detected in all phases of the cycle. In metestrus/diestrus, females show no changes in ILs compared with the 10-fold induction of each IL1 and IL6 in males (Supplementary Figure S6A). Molecular changes in the LH are similar between males and females in estrus, though males still display a more potent IL induction (Supplementary Figure S6B).
Figure 2.
Behavioral and molecular consequences of glucagon-like peptide-1 receptor (GLP-1R) activation in the lateral hypothalamus (LH), as well as their interactions with the estrous cycle, in female rats. In females only the higher dose of exendin-4 (Ex4) reduced the amount of sucrose rewards earned (a) and resulted in a trend to reduce the number of lever presses for the rewards (b) in a progressive ratio schedule, without changing activity at the inactive lever (c). Food ingestion was also affected in female rats but only by the higher dose of Ex4, as illustrated by a reduction in 1 (d) and 24 h (e) chow intake (n=9–37). LH GLP-1R activation in females has divergent behavioral and molecular impact in different estrus cycle phases. Intra-LH Ex4 reduced the effort to obtain a reward (f–i) in a sucrose-motivated progressive ratio task in females in the estrus phase (E) but not metestrus or diestrus (MD) phase of their cycle. Performance on the inactive lever or locomotor activity during the task was not altered by Ex4 in any of the cycle phases (j–m). Although Ex4 potently reduced ingestive behavior in all females irrespective of the cycle phase, the reduction was enhanced for females in E (n–q). Data are expressed as mean±s.e.m. For each measured parameter, raw values and values calculated as the percentage of vehicle response are presented in order to minimize the impact of baseline differences across the two cycle groups. n=10–11. Behavioral differences were mirrored by similarly divergent gene expression changes induced by intra-LH Ex4 application, where a potent induction of interleukins was only detected in females in E, and a reduction in orexin or neurotensin expression was only present in E (r). A reduced expression of melanin-concentrating hormone (MCH) was the only mRNA change preserved in MD phases. Data were normalized to the housekeeping gene beta-actin and are expressed as mean±s.e.m. n=19: vehicle in E, n=20: Ex4 in E, n=8: vehicle in MD, n=8: Ex4 in MD. *P<0.05, **P<0.01,***P<0.001, ****P<0.0001.
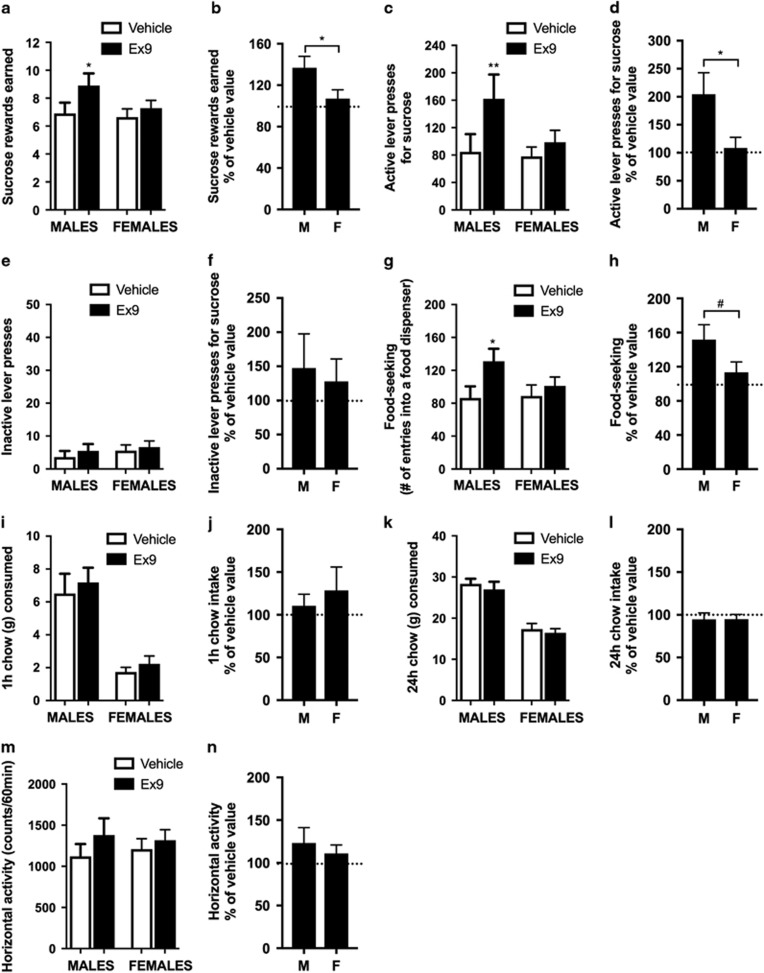
Acute LH-targeted GLP-1R blockade is sufficient to increase food reinforcement but not intake in male rats
Acute pharmacological blockade of LH GLP-1R by bilateral microinjection of Ex9 led to increased food rewards earned (t9=2.739, P<0.05, t13=1.21, P=0.701, t22=2.16, P<0.05 for males, females, and percentage of vehicle comparison between males and females, respectively), lever presses for sucrose (t9=3.59, P=0.005, t13=1.344, P=0.201, t22=2.44, P<0.05) and food-seeking behavior (t9=2.66, P<0.05, t13=0.756, P=0.463, t22=1.85, P=0.07) in male but not in female rats (Figures 3a–h) without changes in horizontal activity (Figures 3m and n). This treatment was, however, not sufficient to increase 1 h (t9=0.96, P=0.36, t13=0.903, P=0.383, t22=0.52, P=0.61) or 24 h food intake (t7=0.91, P=0.39, t13=1.054, P=0.311, t20=0.01, P=0.98) (Figures 3i–l). As previous experiments indicated that LH GLP-1R activation in females is only effective in the estrus phase, an additional group of rats was tested only in the estrus phase. However, even here female rats failed to increase food reinforcement or intake after bilateral LH microinjections of Ex9 (Supplementary Figure S7).
Figure 3.
Acute pharmacological blockade of lateral hypothalamus (LH) glucagon-like peptide-1 receptor (GLP-1R) increases food reinforcement but not intake. Intra-LH microinjection of GLP-1R antagonist, Exendin 9 (Ex9; 10 μg) increased the amount of sucrose rewards earned (a, b) and the number of lever presses for the rewards (c, d) in a progressive ratio schedule, without changing activity at the inactive lever (e, f) in male rats. Similarly food-seeking (number of entries into a food dispenser) was significantly increased by this treatment in male rats (g, h). None of these parameters were altered in female rats. Food ingestion measured as 1 (i, j) and 24 h (k, l) chow intake were unaffected in either sex. Locomotor activity was also not significantly altered (m, n). Data are expressed as mean±s.e.m. n=8–10 (male rats: m) and n=14 (female rats: f). *P<0.05, **P<0.01, #P=0.07.
Chronic LH-targeted GLP-1R silencing leads to increased weight gain, food intake and food reinforcement
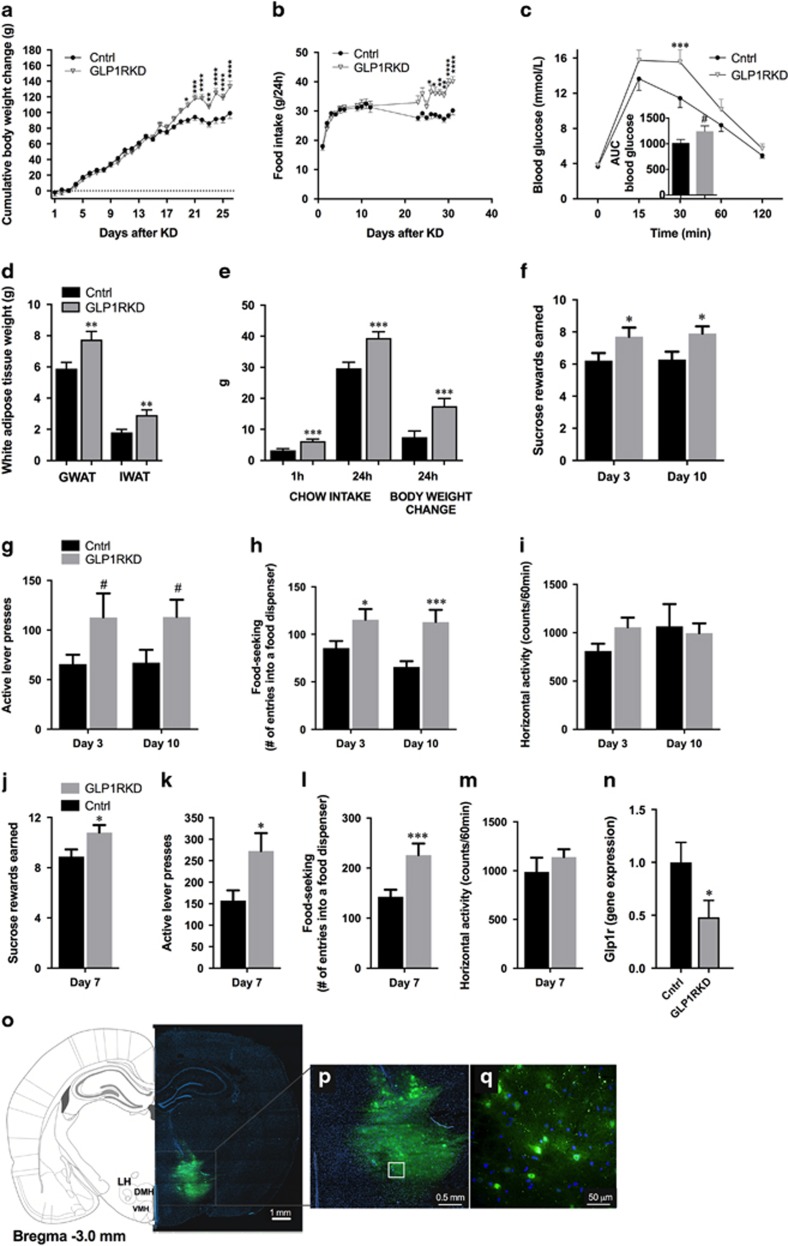
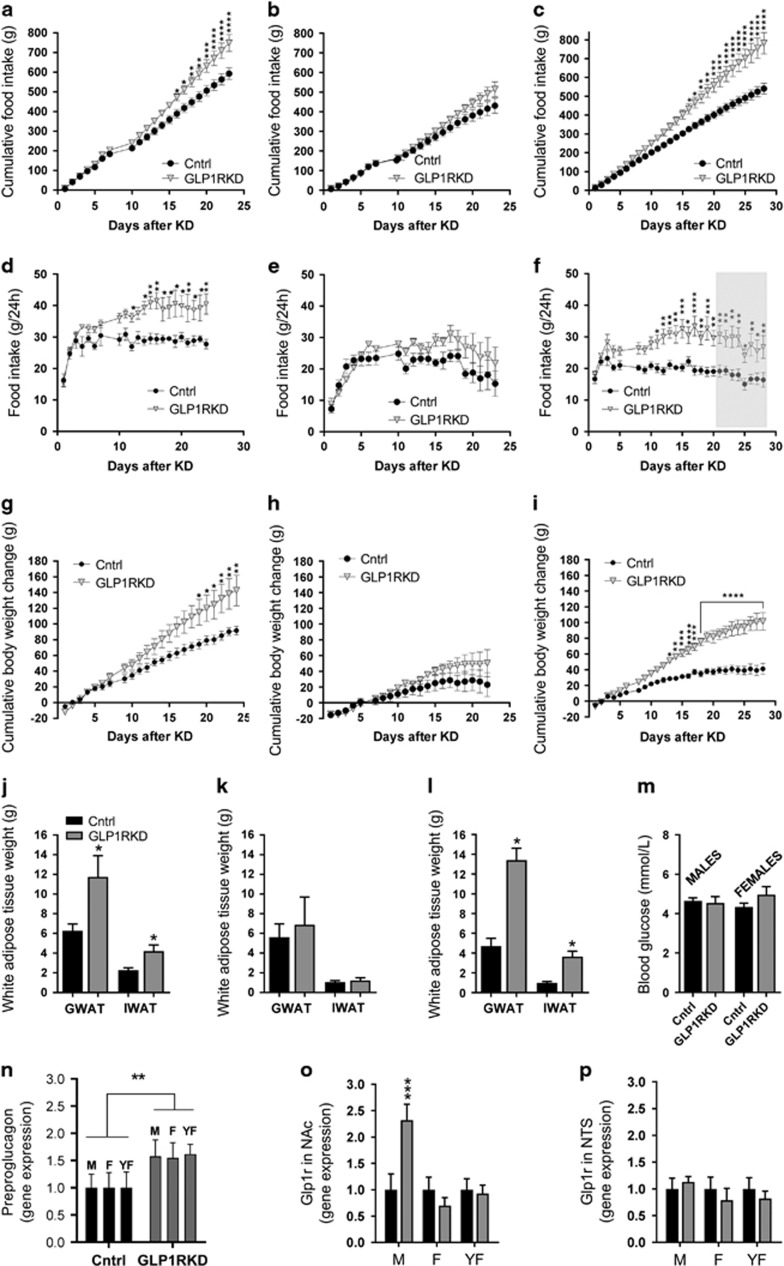
To determine whether endogenous GLP-1R signaling in the LH is necessary for control of body weight, food intake and motivation for food reinforcement, AAV-GFP or AAV-GLP-1R-shRNA were administered bilaterally directly into the LH. This resulted in approximately 50% and 80% reduction (males/younger females and older females, respectively) in the GLP-1R transcript expression in rats infected with AAV-GLP-1R-shRNA when compared with controls (Figure 4n, Supplementary Figures S8A and B). This treatment was sufficient to disturb normal body weight and food intake regulation in 9-week-old male rats, as AAV-GLP-1R-shRNA rats gained significantly more weight (F(23,644)=10.69; P<0.0001, two-way repeated-measures analysis of variance) and ate more chow than control rats (F(18,435)=4.583; P<0.0001) (Figures 4a and b). Elevated food intake and weight gain were detected in the third week after the treatment and the differences persisted until the termination of the study at 4 weeks. At that point, the weight of both fat pads measured, gonadal and inguinal white adipose tissue (GWAT: t27=2.86, P<0.01 and IWAT: t27=2.93, P<0.01), was also significantly higher (Figure 4d). LH GLP-1R knockdown also resulted in a much higher food intake and weight gain upon refeeding (1 h: t27=3.62, P<0.01; 24 h: t27=3.49, P<0.01; weight: t27=3.07, P<0.01; Figure 4e), and a slightly higher blood glucose level during an intraperitoneal glucose tolerance test (area under the curve t33=1.82, P=0.07; Figure 4c).
Figure 4.
Glucagon-like peptide-1 receptor (GLP-1R) in the lateral hypothalamus (LH) are necessary for normal body weight, food intake and food reinforcement control. Decreased GLP-1R expression in the LH promoted weight gain (a) and hyperphagia (b), along with small impairments in glucose tolerance (c). Knockdown of GLP-1R in the LH led to increased fat mass in gonadal and inguinal adipose tissues (GWAT and IWAT, respectively; d). The hyperphagia and increased body weight gain persisted even after food deprivation/refeeding procedure (e). Data are expressed as mean±s.e.m. n=14–15 (male rats). Knockdown of GLP-1R in the LH resulted in an increased amount of sucrose rewards earned (f) and increased number of lever presses for the rewards (g) in a progressive ratio schedule. In addition, food-seeking (number of entries into a food dispenser) behavior was increased (h) without concurrent changes in locomotor activity (i). Similar potentiation of food reinforcement and seeking behaviors was detected in highly motivated (fasted) rats (j–l), again without significant changes in locomotor activity (m). Data are expressed as mean±s.e.m. n=19–20 (9-week-old male rats). Four weeks after adeno-associated virus (AAV) construct injections, GLP-1R expression was reduced by ~50% in the LH of rats treated with AAV-GLP-1R-shRNA (short hairpin RNA) compared with controls (n). Coronal brain section depicting LH-targeted infusions of control and AAV-GLP-1R-shRNA (o). Nuclear stain, DAPI (4,6-diamidino-2-phenylindole), is shown in blue. Panels (p and q) show green fluorescent protein-expressing cells in the LH. *P<0.05, **P<0.01,***P<0.001, ****P<0.0001; #P<0.1. DMH, dorsomedial hypothalamus; KD, knockdown; VMH, ventromedial hypothalamus.
Knockdown of GLP-1R in the LH significantly increased food reinforcement in ad libitum-fed male rats, as indicated by increased number of rewards earned and more lever presses emitted during the PR operant test by rats infused with intra-LH AAV-GLP-1R-shRNA compared with AAV-GFP controls (Figures 4f and g), with the rates of pressing the active lever nearly doubling on both testing occasions. Food seeking was also potently elevated by the treatment (Figure 4h). These changes were not associated with nonspecific changes in locomotor activity (Figure 4i). An identical pattern of effects was detected in fasted rats (Figures 4j–m).
When comparing males and females, in the second experimental group, a significant increase in food intake was detected in male rats already by the end of the second week (cumulative intake: F(20, 160)=9.95 P<0.0001; non-cumulative intake: F(21,168)=4.48 P<0.0001; Figures 5a and d), and body weight gain reached significance 1 week after the onset of hyperphagia (F(22,176)=7.57 P<0.0001; at day 19, Figure 5g). Interestingly, females did not show a consistent hyperphagia or weight gain (Figures 5b, e and h). The weight of the adipose tissue depots, examined 3 weeks after initiation of the knockdown of GLP-1R in the LH, doubled in males (GWAT: t7=2.2, P<0.05 and IWAT: t7=2.6, P<0.05, Figure 5j), but remained unchanged in females (GWAT: t7=0.4, P=0.34 and IWAT: t7=0.6, P=0.3, Figure 5k). Despite the large increase in fat mass and food intake, fasting blood glucose (t7=0.3, P=0.4) levels remained unchanged in male rats. Likewise, female fasting blood glucose levels were unaltered (t6=1.6, P=0.08; Figure 5m). In stark contrast to males, females did not show any changes in food-motivated or food-seeking behaviors (Supplementary Figure S9). Both males and females were adult (13 weeks of age) at the time of AAV infusion, and while males continued to gain weight during the entire 3-week period postinfusion, females at this age reached a weight plateau after the first 2 weeks of the study. To test whether the tapering growth rate in females is masking a potential impact of the GLP-1R knockdown, younger females (week 9, young adult) were tested. Surprisingly, these females displayed a drastically different response from the older females, where food intake (cumulative: F(27,378)=15.18, P<0.0001, non-cumulative: F(25,350)= 3.244, P<0.0001, Figures 5c and f) and body weight (F(26,364)=18.26, P<0.0001, Figure 5i) were massively impacted by the GLP-1R loss in the LH. The striking weight gain was likely due to fat gain, as at the end of the study the adipose tissue of these females nearly tripled (GWAT: t13=5.9, P<0.0001 and IWAT: t14=4.7, P<0.001, Figure 5l). Despite the profound impact of the LH GLP-1R knockdown on chow intake and adipose mass gain, we surprisingly found no effect on food reinforcement (Supplementary Figure S10). In an effort to not miss a potential late-developing effect in these young females or an effect only evident in the estrus phase, we additionally tested both parameters during week 4 and still found no effect (Supplementary Figure S10) of the LH GLP-1R knockdown on food motivation. Based on baseline daily feed efficiency calculations, we did not find any energy expenditure disturbances in either sex (Supplementary Figures S11A–C); however, we did find trends or a significant increase in feed efficiency after an overnight fast and upon re-feeding (Supplementary Figures S11D–F), indicating that LH GLP-1R may be necessary for energy expenditure regulation in some physiological contexts.
Figure 5.
Glucagon-like peptide-1 receptor (GLP-1R) in the lateral hypothalamus (LH) are necessary for normal body weight, body fat and food intake control in males and young females but not older females. Decreased GLP-1R expression in the LH promoted a marked, chronic, hyperphagia and weight gain in male (a, d, g) but not in female (b, e, h) rats (both sexes 13 week old on injection day). In contrast to the 13-week-old females, younger females (9 week old) presented with a massive hyperphagia (c, f) and weight gain after LH GLP-1R KD (i). Likewise, male knockdown rats have double the amount of fat (j) compared with controls, while fat mass in same age females was unaffected by the treatment (k). However, the adipose tissue weight of younger females with LH GLP-1R knockdown was nearly threefold higher compared with control rats (l). Fasted blood glucose levels are not altered by the knockdown in either sex (13 week old; m). Potential compensatory adaptations of the central GLP-1 system were assessed by determining whether the GLP-1 precursor (preproglucagon) expression in the nucleus of the solitary tract (NTS), or GLP-1R expression in the nucleus accumbens (NAc) or in the NTS, were altered in response to the LH GLP-1R knockdown. The expression of preproglucagon was increased in all three knockdown groups tested, thus irrespective of sex, age or weight gain response to the knockdown (n). A compensatory increase in GLP-1R expression was detected in NAc, a nucleus with dense connections to the LH, but interestingly this change was only detected in males (o). This elevation in males seemed to be area specific as GLP-1R expression in the NTS was not altered in males or any of the female groups. Thus chronic loss of LH GLP-1R, induced by an adeno-associated virus (AAV)-shRNA (short hairpin RNA) GLP-1R-mediated knockdown, not only increased food reinforcement behavior but also led to a marked hyperphagia and weight gain, at a level unparalleled to that found by blockade of any other GLP-1R population in rats or mice, despite compensatory changes detected outside of the LH. In conclusion, GLP-1R in the LH is a key component of normal body weight homeostasis and food reward control; LH emerges as one of the most critical sites for the endogenous GLP-1 effect on energy balance in males. Data are expressed as mean±s.e.m. n=10 (male rats, 5 in each treatment group); n=10 (female rats, 5 in each treatment group) for 13-week-old rats and n=16, 8 in each treatment group, for 9-week-old rats. GWAT: gonadal white adipose tissue, IWAT: inguinal (subcutaneous) white adipose tissue mass. M and F, males and females, respectively (13 week old at the time of AAV-shRNA infusion, and 17–18 week old at the time of tissue collection), YF: younger females, 9 week old at the time of infusion, and 15 at the time of tissue collection. Shaded gray area indicates a period of operant testing performed to capture a potential interaction of the knockdown with the estrous cycle. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
To test whether the reduction in GLP-1R in the LH elicits potentially adaptive counter-regulatory responses within the central GLP-1 system, GLP-1R expression was measured in the NAc (core and shell included), where GLP-1 also exerts food reinforcement-suppressing effects.31, 35 GLP-1R expression was increased more than twofold in the NAc of male rats in response to LH GLP-1R loss. Interestingly, this compensatory effect was not seen in any of the female groups (Figure 5o). This counter-regulation seems site-selective, as, for example, the NTS GLP-1R remained unaltered (Figure 5p). Finally, we also tested whether the hindbrain preproglucagon gene, a precursor of GLP-1, was altered in all GLP-1R knockdown groups irrespective of altered physiology or behavior. We found that expression of this gene was increased (Figure 5n), consistent with the brain GLP-1 system attempting to compensate for the loss of the LH node, indicating an interesting, and previously unexplored, capacity for adaptive compensatory plasticity within the brain GLP-1 system.
Discussion
Although much is known about distinct populations of LH neurons, the metabolically relevant inputs to the LH are less clear. Here we show that the LH is a key brain area that mediates weight loss and the reward inhibitory effects of exogenous GLP-1R activation. Importantly, our data also demonstrate that the LH is a crucial site for motivated and ingestive behavior suppression driven by endogenously released GLP-1. With these results, LH emerges as one of the most important sites to date for endogenous GLP-1R activation, especially considering the size and robustness of the hyperphagia, weight gain and hypermotivation when GLP-1R in the LH are blocked and/or reduced. Although local LH GLP-1R activation was sufficient to reduce weight and reinforcement in both sexes, the effect of LH GLP-1R blockade was sex divergent, with the LH-GLP-1R signaling found to be crucial for motivated behavior control in males but disposable in females. Thus the female and male LH differs in its ability to control motivated behavior. The principal finding from these studies is that GLP-1R in the LH is required for normal body weight homeostasis and food reward control. This finding contrasts with conclusions drawn from studies carried out on GLP-1R knockout mice (for example, Finan et al.,26 Scrocchi et al.27 and Sisley et al.28), where little to no necessary contribution of CNS GLP-1R was demonstrated. Thus LH emerges as one of the most critical sites for the endogenous GLP-1 effect on energy balance and reinforcement.
In the context of food reward, the present data suggest that GLP-1, within the LH, inhibits motivation for palatable food. The effect of exogenous local LH Ex4 microinjection was fairly potent with a 30% reduction in the amount of effort the rats chose to expend for food reward. The VTA may be one of the neuroanatomical targets underlying this effect, as we found that more than half of the LH GLP-1R-expressing neurons project to the VTA. However, it is the impact of the endogenous GLP-1 in this area that is most striking, as when the GLP-1 signal is reduced acutely, male rats expend twice as much effort for the sucrose reward, indicating the crucial role of the endogenous GLP-1 in curbing food reward regulation in males. Compared with the LH, a smaller contribution was recently found with acute pharmacological blockade of GLP-1R in the dorsal part of the lateral septum36 and with GLP-1R knockdown in the NTS.37 In the ventral hippocampus, GLP-1R knockdown increases operant responding for food reward in non-restricted male rats (while not influencing chow intake).33 The impact of acute blockade of the GLP-1R in the LH is further supported by the enhanced food motivation detected after GLP-1R knockdown in the LH. It is of interest that the potently elevated food motivation is detected in both ad libitum-fed as well as food-restricted/re-fed male rats. This suggests that the detected food reward deregulation is robust and persists through different physiological/metabolic states.
The most striking result of GLP-1R knockdown in the LH was the persistent and potent hyperphagia: >50% increase in daily chow consumption was detected, starting 2 weeks after the knockdown. This hyperphagia likely contributes to the massive increase in body fat, where the amount of fat detected in both fat depots examined (IWAT and GWAT) doubled in males and tripled in younger females. This is perhaps the most potent collection of effects on reward behavior and metabolism of a single nucleus and one receptor-encoding gene change detected to date. These data demonstrate the important contribution of the LH GLP-1R to homeostatic physiology and behavior. Interestingly, whole-body, or CNS-specific, GLP-1R knockout in a mouse did not result in sizable change in body weight, fat mass or hyperphagia.26, 27, 28 There could be many reasons for this discrepancy; developmental compensation or species difference (mouse vs rat) are two possible explanations. Importantly, the combinations of generally weak effects of the pharmacological blockade of the GLP-1R along with little impact of the GLP-1R knockout led to a long-standing conclusion that, while GLP-1R analogs are an attractive pharmaceutical treatment option, the endogenous GLP-1 system is not necessary or critical for body weight and behavior regulation. Thus current results contradict this view and show that CNS GLP-1R, at least at the level of the LH, is necessary for normal body weight maintenance. Of note, we found that, while acute stimulation of GLP-1R in LH was highly effective at food intake and body weight gain suppression, acute blockade was not sufficient to alter chow intake or body weight. This may suggest that acutely other GLP-1R-expressing sites are able to compensate for the loss of GLP-1R signaling, compensation that proves insufficient when LH GLP-1R is silenced in a chronic manner. Although our data also indicate that, following chronic LH GLP-1R silencing, the brain attempts to compensate for the reduced anorexic signal in the LH by potently increasing GLP-1R expression in the NAc only in males and preproglucagon in the NTS of both sexes. This is clearly not sufficient to rescue the reward and metabolic phenotype in males, although we can hypothesize that simultaneous GLP-1R knockdown in both of these sites may produce an even more pronounced reward phenotype in males. We did not find any compensation in receptors from another GLP-1R-expressing site,37, 38, 39 the NTS, in either sex or age.
Several studies revealed that peripheral hormones, ghrelin or leptin, affect food intake through actions on the LH orexin or neurotensin neurons.40, 41, 42, 43 Leptin and GLP-1 are known to interact, and both have been shown to reduce food intake, body fat, body weight and food reward, although the impact of reduced GLP-1R signaling in the LH observed in the current study is quite distinct from that reported after LH leptin receptor knockdown.44 Leptin receptor knockdown does not change food intake or weight in chow-fed rats, and a high-fat challenge is necessary to bring out the disturbed metabolic phenotype, which is transient. This contrasts with the robust, and long-lasting, weight and intake elevation found here after GLP-1R knockdown. Importantly, leptin receptor knockdown did not yield changes in food-motivated behavior, while potent and lasting upregulation of food motivation was found here after GLP-1R knockdown, at least in males. Collectively, these data suggest that, while there may be some overlap in the cellular and molecular targets of leptin and GLP-1 in the LH, GLP-1 seems to stimulate a broader, at least functionally, fraction of LH cells. Interestingly, the VTA proved to be the key target site for food intake and reward effect of leptin.45, 46, 47 For GLP-1, while many studies suggest that VTA GLP-1R are sufficient to inhibit food intake and motivation,31, 48 no data to date exist to show that they are necessary for these behaviors.
A number of genetically and functionally distinct cell populations reside in the LH,49 although the neuropeptides or neurotransmitters affected by GLP-1R activation remain unknown. Acuna-Goycolea and van den Pol50 reported a possible link between GLP-1 and the LH orexin neurons, where GLP-1 depolarizes orexin neurons and increases their spike frequency ex vivo. In our study, LH GLP-1R activation reduced orexin expression but only in females. Nevertheless, a more potent molecular effect of LH GLP-1R activation was to increase IL1 and IL6 in both males and females and also in a male-derived hypothalamic cell line. These ILs are not only clearly anorexic and weight reducing but are also already clearly indicated as mediators of anorexic and weight-loss effects of GLP-1.34 Interestingly, our data also indicate that IL6 expression levels are increased by food intake, as sucrose meal, but not fat or chow meal, increased the levels of IL6 in the LH. Of note, the hypothalamic cell line was derived from males, thus it is possible, based on our in vivo results, that had this cell line been derived from females a smaller IL6 induction would be expected, as a 5-fold increase was found in the female LH compared with a 10-fold increase in IL6 mRNA in male LH. Expression of MCH, an orexigenic LH neuropeptide, was reduced by LH GLP-1R activation in both males and females, which could contribute to both the reinforcement and adiposity effects of LH GLP-1R activation.
Sex differences were noted in response to GLP-1R activation but especially in the response to GLP-1R blockade or silencing. Females showed a similar but somewhat less potent feeding and intake inhibition after LH-targeting Ex4 microinjections, results consistent with LH GLP-1R expression which was 10-fold higher in adult males compared with females, an age-dependent sex difference. This is somewhat surprising as we have previously shown that females show a more potent feeding and food motivation inhibition after intracerebroventricular Ex4 injections.51 However, our more recent data indicate that the LH is not a neural substrate mediating this enhanced reward response.29 In stark contrast to the effects found in males, the LH does not seem to be a critical site for GLP-1 control of motivated behavior in females, as both the acute pharmacological and chronic genetic GLP-1R inhibition in the LH did not alter food motivation in female rats. Thus female brains may more readily compensate for the reduced LH GLP-1 signal. The molecular signature of LH GLP-1R activation was also partly sex divergent, with ILs and MCH affected in both sexes, while reduced expression of orexin and neurotensin detectable only in females, selectively in their estrus phase. In fact, the estrus phase had a surprisingly potent effect on both molecular and behavioral outcomes of LH GLP-1R activation, which were nearly completely attenuated in females in cycle phases associated with low estrogen signaling.
Our data demonstrate that, contrary to previous findings, GLP-1R in the brain, and specifically in the LH, are indispensable for the regulation of body weight and body fat in males and females. Likewise, they are essential to curb food reward behavior in males. Considering the robust and persistent changes obtained with this single gene manipulation in the LH, this nucleus has a tremendous potential to contribute both to the development of obesity and possibly to identifying strategies to control overeating.
Acknowledgments
This research was funded by the Swedish Research Council (2014–2945 to KPS and 2013–7107 to PR), Novo Nordisk Foundation Excellence project grant (to KPS), Ragnar Söderberg Foundation (to KPS), Harald Jeanssons Stiftelse and Greta Jeanssons Stiftelse (to KPS), Magnus Bergvalls Stiftelse (to KPS), Wallenberg Foundation and Center for Molecular and Translational Medicine (to KPS) and National Institute of Health NIH-DK096139 (to MRH) and NIH-DK104897 (to SEK). We also thank Fredrik Nilsson for his excellent technical assistance.
Footnotes
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
MRH receives funding from Zealand Pharma and Novo Nordisk that was not used in support of these studies. All the other authors declare no conflict of interest.
Supplementary Material
References
- Van den Pol AN. Lateral hypothalamic damage and body weight regulation: role of gender, diet, and lesion placement. Am J Physiol 1982; 242: R265–R274. [DOI] [PubMed] [Google Scholar]
- Anand BK, Brobeck JR. Localization of a "feeding center" in the hypothalamus of the rat. Proc Soc Exp Biol Med 1951; 77: 323–324. [DOI] [PubMed] [Google Scholar]
- Morrison SD, Mayer J. Adipsia and aphagia in rats after lateral subthalamic lesions. Am J Physiol 1957; 191: 248–254. [DOI] [PubMed] [Google Scholar]
- Teitelbaum P, Stellar E. Recovery from the failure to eat produced by hypothalamic lesions. Science 1954; 120: 894–895. [DOI] [PubMed] [Google Scholar]
- Miller NE. Motivational effects of brain stimulation and drugs. Fed Proc 1960; 19: 846–854. [PubMed] [Google Scholar]
- Olds J. Hypothalamic substrates of reward. Physiol Rev 1962; 42: 554–604. [DOI] [PubMed] [Google Scholar]
- Margules DL, Olds J. Identical "feeding" and "rewarding" systems in the lateral hypothalamus of rats. Science 1962; 135: 374–375. [DOI] [PubMed] [Google Scholar]
- Hoebel BG, Teitelbaum P. Hypothalamic control of feeding and self-stimulation. Science 1962; 135: 375–377. [DOI] [PubMed] [Google Scholar]
- Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev 2008; 32: 20–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray S, Tulloch A, Gold MS, Avena NM. Hormonal and neural mechanisms of food reward, eating behaviour and obesity. Nat Rev Endocrinol 2014; 10: 540–552. [DOI] [PubMed] [Google Scholar]
- Wise RA. Rewards wanted: molecular mechanisms of motivation. Discov Med 2004; 4: 180–186. [PubMed] [Google Scholar]
- Wise RA. Dopamine and food reward: back to the elements. Am J Physiol Regul Integr Comp Physiol 2004; 286: R13. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neural mechanisms of drug reinforcement. Ann NY Acad Sci 1992; 654: 171–191. [DOI] [PubMed] [Google Scholar]
- Blundell JE, Herberg LJ. Relative effects of nutritional deficit and deprivation period on rate of electrical self-stimulation of lateral hypothalamus. Nature 1968; 219: 627–628. [DOI] [PubMed] [Google Scholar]
- Gigante ED, Benaliouad F, Zamora-Olivencia V, Wise RA. Optogenetic activation of a lateral hypothalamic-ventral tegmental drive-reward pathway. PLoS ONE 2016; 11: e0158885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol 1999; 403: 261–280. [DOI] [PubMed] [Google Scholar]
- Iepsen EW, Torekov SS, Holst JJ. Liraglutide for type 2 diabetes and obesity: a 2015 update. Expert Rev Cardiovasc Ther 2015; 13: 753–767. [DOI] [PubMed] [Google Scholar]
- Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CM, Meeran K et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 1996; 379: 69–72. [DOI] [PubMed] [Google Scholar]
- Hayes MR. Neuronal and intracellular signaling pathways mediating GLP-1 energy balance and glycemic effects. Physiol Behav 2012; 106: 413–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes MR, Skibicka KP, Grill HJ. Caudal brainstem processing is sufficient for behavioral, sympathetic, and parasympathetic responses driven by peripheral and hindbrain glucagon-like-peptide-1 receptor stimulation. Endocrinology 2008; 149: 4059–4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoski SE, Hayes MR, Skibicka KP. GLP-1 and weight loss: unraveling the diverse neural circuitry. Am J Physiol Regul Integr Comp Physiol 2016; 310: R885–R895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibicka KP. The central GLP-1: implications for food and drug reward. Front Neurosci 2013; 7: 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DL, Erreger K, Galli A, Stanwood GD. GLP-1 analog attenuates cocaine reward. Mol Psychiatry 2013; 18: 961–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, Mietlicki-Baase EG, Ige KY, Maurer JJ, Reiner DJ, Zimmer DJ et al. Glucagon-like peptide-1 receptor activation in the ventral tegmental area decreases the reinforcing efficacy of cocaine. Neuropsychopharmacology 2016; 41: 1917–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen G, Reddy IA, Weikop P, Graham DL, Stanwood GD, Wortwein G et al. The glucagon-like peptide 1 (GLP-1) receptor agonist exendin-4 reduces cocaine self-administration in mice. Physiol Behav 2015; 149: 262–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan B, Yang B, Ottaway N, Stemmer K, Muller TD, Yi CX et al. Targeted estrogen delivery reverses the metabolic syndrome. Nat Med 2012; 18: 1847–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrocchi LA, Hill ME, Saleh J, Perkins B, Drucker DJ. Elimination of glucagon-like peptide 1R signaling does not modify weight gain and islet adaptation in mice with combined disruption of leptin and GLP-1 action. Diabetes 2000; 49: 1552–1560. [DOI] [PubMed] [Google Scholar]
- Sisley S, Gutierrez-Aguilar R, Scott M, D'Alessio DA, Sandoval DA, Seeley RJ. Neuronal GLP1R mediates liraglutide's anorectic but not glucose-lowering effect. J Clin Invest 2014; 124: 2456–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel H, Wolf S, Rabasa C, Rodriguez-Pacheco F, Babaei CS, Stober F et al. GLP-1 and estrogen conjugate acts in the supramammillary nucleus to reduce food-reward and body weight. Neuropharmacology 2016; 110(Pt A): 396–406. [DOI] [PubMed] [Google Scholar]
- la Fleur SE, Vanderschuren LJ, Luijendijk MC, Kloeze BM, Tiesjema B, Adan RA. A reciprocal interaction between food-motivated behavior and diet-induced obesity. Int J Obes (Lond) 2007; 31: 1286–1294. [DOI] [PubMed] [Google Scholar]
- Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. J Neurosci 2012; 32: 4812–4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes MR, Bradley L, Grill HJ. Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology 2009; 150: 2654–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu TM, Noble EE, Liu CM, Cortella AM, Konanur VR, Suarez AN et al. A hippocampus to prefrontal cortex neural pathway inhibits food motivation through glucagon-like peptide-1 signaling. Mol Psychiatry 2017; doi: 10.1038/mp.2017.91 [e-pub ahead of print 2 May 2017]. [DOI] [PMC free article] [PubMed]
- Shirazi R, Palsdottir V, Collander J, Anesten F, Vogel H, Langlet F et al. Glucagon-like peptide 1 receptor induced suppression of food intake, and body weight is mediated by central IL-1 and IL-6. Proc Natl Acad Sci USA 2013; 110: 16199–16204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dossat AM, Diaz R, Gallo L, Panagos A, Kay K, Williams DL. Nucleus accumbens GLP-1 receptors influence meal size and palatability. Am J Physiol Endocrinol Metab 2013; 304: E1314–E1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrill SJ, Jackson CM, Greene HE, Lilly N, Maske CB, Vallejo S et al. Role of lateral septum glucagon-like peptide 1 receptors in food intake. Am J Physiol Regul Integr Comp Physiol 2016; 311: R124–R132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhadeff AL, Mergler BD, Zimmer DJ, Turner CA, Reiner DJ, Schmidt HD et al. Endogenous glucagon-like peptide-1 receptor signaling in the nucleus tractus solitarius is required for food intake control. Neuropsychopharmacology 2016; 42: 1471–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhadeff AL, Grill HJ. Hindbrain nucleus tractus solitarius glucagon-like peptide-1 receptor signaling reduces appetitive and motivational aspects of feeding. Am J Physiol Regul Integr Comp Physiol 2014; 307: R465–R470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard JE, Anderberg RH, Goteson A, Gribble FM, Reimann F, Skibicka KP. Activation of the GLP-1 receptors in the nucleus of the solitary tract reduces food reward behavior and targets the mesolimbic system. PLoS ONE 2015; 10: e0119034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone JJ, McCutcheon JE, Roitman MF. Ghrelin acts as an interface between physiological state and phasic dopamine signaling. J Neurosci 2014; 34: 4905–4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinninger GM, Jo YH, Leshan RL, Louis GW, Yang H, Barrera JG et al. Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metab 2009; 10: 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski PK, Li D, Grace MK, Billington CJ, Kotz CM, Levine AS. Neural basis of orexigenic effects of ghrelin acting within lateral hypothalamus. Peptides 2003; 24: 597–602. [DOI] [PubMed] [Google Scholar]
- Toshinai K, Date Y, Murakami N, Shimada M, Mondal MS, Shimbara T et al. Ghrelin-induced food intake is mediated via the orexin pathway. Endocrinology 2003; 144: 1506–1512. [DOI] [PubMed] [Google Scholar]
- Davis JF, Choi DL, Schurdak JD, Fitzgerald MF, Clegg DJ, Lipton JW et al. Leptin regulates energy balance and motivation through action at distinct neural circuits. Biol Psychiatry 2011; 69: 668–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB et al. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 2006; 51: 801–810. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Corrie LW, Rogers JA, Yamada H. Effects of insulin and leptin in the ventral tegmental area and arcuate hypothalamic nucleus on food intake and brain reward function in female rats. Behav Brain Res 2011; 219: 254–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Plasse G, van Zessen R, Luijendijk MC, Erkan H, Stuber GD, Ramakers GM et al. Modulation of cue-induced firing of ventral tegmental area dopamine neurons by leptin and ghrelin. Int J Obes (Lond) 2015; 39: 1742–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhadeff AL, Rupprecht LE, Hayes MR. GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology 2012; 153: 647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Wise RA. Lateral hypothalamic circuits for feeding and reward. Nat Neurosci 2016; 19: 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acuna-Goycolea C, van den Pol A. Glucagon-like peptide 1 excites hypocretin/orexin neurons by direct and indirect mechanisms: implications for viscera-mediated arousal. J Neurosci 2004; 24: 8141–8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard JE, Anderberg RH, Lopez-Ferreras L, Olandersson K, Skibicka KP. Sex and estrogens alter the action of glucagon-like peptide-1 on reward. Biol Sex Differ 2016; 7: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.