Abstract
Objectives
Since approval of IV acetaminophen (IV APAP), its use has become quite common without strong positive evidence. Our goal was to determine the effect of IV APAP on hospital length of stay (LOS) via mediation of opioid-related side effects in pediatric patients.
Methods
Following IRB approval, 114 adolescents undergoing posterior spinal fusion were prospectively recruited and managed postoperatively with patient controlled analgesia and adjuvant therapy. Patients were divided into 2 groups based on the use of IV APAP: Control (n=70) and Treatment (n=44). Association of IV APAP use with opioid outcomes was analyzed using inverse probability of treatment weighing (IPTW) adjusted propensity scores to balance the two groups for all significant covariates except postoperative opioid consumption. Mediation analysis was carried out for LOS with IV APAP as the independent variable and morphine consumption as the mediator.
Results
Oral intake was delayed by ~1 day (p < 0.001) and LOS was 0.6 days longer in the control group (p=0.044). After IPTW, time to oral intake remained significantly longer in the control group (p=0.014). The mediation model with IPTW revealed a significant negative association between IV APAP and morphine consumption (p < 0.001), which significantly increased LOS (p<0.003). IV APAP had a significant opioid-sparing effect associated with shorter LOS.
Discussion
IV APAP hastens oral intake and is associated with decreased LOS in an adolescent surgery population likely through decreased opioid consumption. Through addition of IV APAP in this population, LOS may be decreased, an important implication in the setting of escalating health care costs.
Keywords: IV acetaminophen, opioid-sparing, length of stay, pediatric surgery, posterior spinal fusion, mediation
Introduction
Practice guidelines by the American Society of Anesthesiologists Task Force on Acute Pain Management recommend the use of multimodal therapy consisting of an around-the-clock regimen of acetaminophen and/or non-steroidal anti-inflammatory drugs (NSAIDs) [1]. Since the United States Food and Drug Administration approved the drug in 2010, intravenous acetaminophen (IV APAP) is commonly used at many institutions to optimize pain management, including in pediatric settings [2]. The key advantage of IV APAP is that it results in greater central nervous system penetration with corresponding superior analgesic efficacy in the surgical setting as compared to the oral version. However, routine use of IV APAP may be questionable in the absence of evidence of clear positive effects on clinical outcomes, especially given its rapid increase in cost over time.
Despite the drive to use alternative therapies, the mainstay of perioperative pain management for invasive surgeries remains opioids. In addition to its many side effects and addiction potential, opioid therapy is limited by several adverse effects on the gastrointestinal system, including emesis and constipation, which can delay recovery and discharge from the hospital [3], resulting in significant increases in healthcare costs [4]. To better understand the economic implications of using IV acetaminophen, we chose the length of hospital stay (LOS) as the primary outcome measure to assess the utility of IV acetaminophen in postoperative pain management [5]. We selected posterior spinal fusion patients as our population as this type of surgery represents one of the populations with the most rapidly increasing health care costs [6, 7].
We hypothesized that the perioperative use of IV acetaminophen will reduce opioid-related side effects including vomiting, constipation and delayed oral intake resulting in earlier hospital discharge through its effect on decreasing postoperative IV opioid consumption. To test these hypotheses, we analyzed data from a large prospective study composed of otherwise healthy adolescents undergoing posterior spinal fusion.
Materials and Methods
Following Institutional Review Board (IRB) approval at Cincinnati Children’s Hospital Medical Center, patients aged 10 to 18 years of American Society of Anesthesiologist (ASA) physical status 1 or 2, with a diagnosis of idiopathic scoliosis and/or kyphosis, undergoing elective posterior spinal fusion were recruited as part of a larger pharmacogenomics study from October 2009 to August 2014. Patients were excluded if they were pregnant or breastfeeding; if they had used opioids in the past 6 months or had a history of chronic pain, liver or renal disease; or had a history of developmental delay. Of the 247 eligible patients, data was prospectively obtained from 114 adolescents (Figure 1); all patients arrived to the hospital the morning of surgery, received general anesthesia and post-operative pain control following standard protocols and were admitted to the post-surgical floor post-operatively. Written informed consent was obtained from parents and assent was obtained from children before enrollment.
Figure 1.
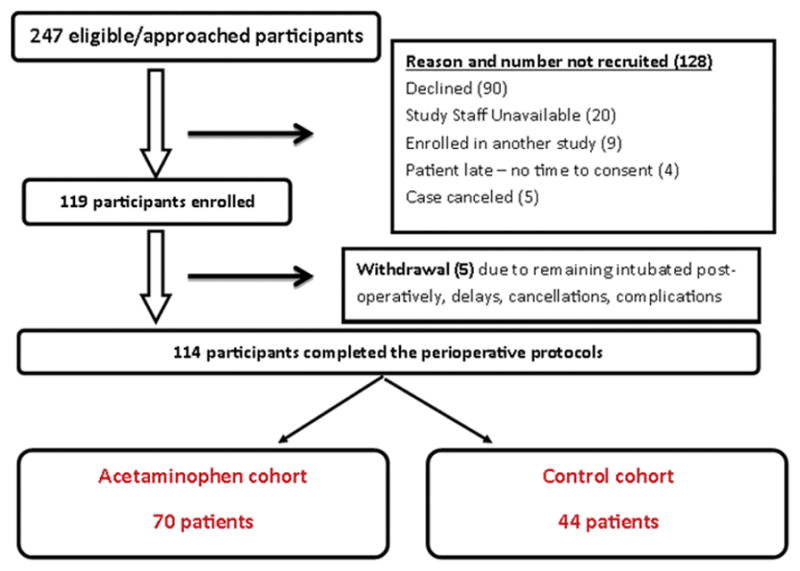
Enrollment flow chart of patients in the study
All patients received midazolam 2 mg IV prior to the induction of anesthesia and diazepam 0.05 mg/kg during emergence. General anesthesia was maintained using a propofol and remifentanil Total Intravenous Anesthesia (TIVA) titrated to maintain an appropriate depth of anesthesia per electroencephalogram (EEG) as monitored via neuromonitoring. Postoperatively, patients were followed by the perioperative pain service and managed primarily with a morphine or hydromorphone Patient Controlled Analgesia (PCA). PCA settings were standardized for weight and adjustments were made per the discretion of the perioperative pain service based upon patients’ analgesic needs. Patients were started with morphine 20 mcg/kg/demand dose every 7 minutes with or without a continuous infusion of 10 mcg/kg/hr. If the patient was allergic to morphine or had any other contraindication to this drug, they were given an equivalent dose of hydromorphone via the PCA (5 mcg/kg/demand every 7 minutes ± 3 mcg/kg/hr infusion). Patients were routinely monitored using continuous pulse oximetry (oxygen saturation/SpO2), electrocardiograph (EKG) and impedance plethysmography (respiratory rate/RR) while on the PCA. When appropriately tolerating an oral diet, usually by postoperative (POD) day 2, patients were started on oral oxycodone and the PCA was discontinued. Our standard pain protocol also includes diazepam 0.05 mg/kg every 4 hours as needed for muscle spasms, ketorolac 0.5 mg/kg every 6 hours for 2 days (maximum dose 30 mg), and methocarbamol 15 mg/kg every 8 hours for 3 days. Patients in both groups received these standard medications. Surgical orders included Senna 30 mg chewable tablets at bedtime every day, polyethylene glycol 3350 packet 17gm two times daily and the use of bisacodyl suppository as needed on POD 1 and 2, followed by a standing order to administer the suppository on POD 3 if the patient had not yet had a bowel movement. Pain scores were recorded by the nursing staff every 4 to 6 hours on the floor using the numerical rating scale (NRS), an 11-point scale that allows a patient to describe pain from 0 (no pain) to 10 (worst pain of one’s life). The patients in the IV APAP group received 15 mg/kg IV acetaminophen every 6 hours around the clock for a total of 12 doses.
Data collection
Demographic data was collected preoperatively and included patient age, weight, gender, race, degree of scoliotic curve and any underlying comorbidities, including existence of pain. Intraoperative data collected included: duration of surgery (anesthesia time), number of vertebral levels fused, doses of propofol and remifentanil administered and pain medications administered, including both opioids and benzodiazepines. Postoperatively, data collection included: opioid consumption (via PCA and IV) and non-opioid analgesic use (acetaminophen and ketorolac). Hydromorphone doses were converted to morphine equi-analgesic doses using a 1:5 :: morphine: hydromorphone conversion ratio. We recorded pain scores (Numerical Rating Scale 0–10), occurrence of opioid-related respiratory depression (RD) over 48 hours (defined as occurrence of respiratory rate < 8 breaths per minute for greater than 3 minutes or oxygen saturation < 92%) and emesis (occurrence of at least one emesis). In addition, intake/output charts provided information about the initial postoperative day on which 50% of meals were tolerated (Oral (PO) intake) as well as the postoperative days on which a suppository or enema was administered (surrogate for constipation). LOS was calculated by subtracting the procedure date from the discharge date. Discharge summaries were used to identify any pertinent postoperative complications such as infection and pneumonia.
Patients were divided into two groups based on the use of IV acetaminophen: Control (no IV APAP) and Treatment (IV APAP). The primary outcome was LOS in days (measured as a continuous variable). Secondary outcomes included a) Vomiting, b) RD, c) Day of PO intake and d) Need for a suppository or enema on or after POD 3 to achieve a bowel movement.
Statistical analysis
Descriptive statistics were calculated for all variables (mean, SD for continuous variables and frequency, percentage for categorical variables). Demographics, intraoperative, and postoperative measures were compared between treatment and control groups using two sample t-tests or Wilcoxon sum rank tests as appropriate for continuous variables and chi-square or fisher’s exact test for categorical variables. If significant differences were found between the two groups, propensity scores [8, 9] were calculated and inverse probability of treatment weighting (IPTW) [10] using the propensity score was used to balance the two groups for all covariates except for postoperative opioid consumption, the assumed mediator. Unlike in randomized controlled trials, in observational studies, there are often systematic differences in baseline characteristics between the treatment and control groups. Thus, outcomes cannot be compared directly between groups to obtain an unbiased estimate of the effect of treatment between the two groups without minimizing the effects of confounding. The propensity score is the probability of receiving a treatment based on a set of observed baseline characteristics. Conditional on the propensity score, subjects in different treatment groups will have a similar distribution of measured baseline covariates, which reduces or removes the effect of confounding when analyzing an observational study. Therefore, a propensity score analysis attempts to decrease the effect of confounding variables on group differences on the outcome of interest. In particular, IPTW uses weights based on the propensity score to balance the distribution of measured baseline covariates between the groups. In this study, the dependent variable in the propensity score model was the assignment to the IV APAP group. The independent variables were: age (years), weight (kg), duration of surgery, mean postoperative pain scores on POD 1 and POD 2 using the numerical rate scale, ketorolac administration, sex, race, number of vertebral levels fused, respiratory rate of POD 1 and incidence of vomiting on POD 1. The amount of oxycodone given per kg was not included in analysis due to the high correspondence of oxycodone consumption with starting to tolerate oral intake, and LOS (earlier the PO intake, and longer the LOS, the subjects used more oxycodone; this value was, however, balanced between the two groups after propensity scoring. The propensity score was estimated using a multivariable logistic regression model with all of the independent variables included. After IPTW, balance on covariates between the two groups was assessed and mediation analysis were carried out as follows for LOS outcome with IV APAP as the independent variable and morphine consumption as the mediator [11–14]: 1) Linear regression with IV APAP as the independent variable and outcome (LOS) as the dependent variable (total effect), 2) Linear regression with IV APAP as the independent variable and morphine consumption as the dependent variable (essential), 3) Linear regression with IV APAP and morphine as the independent variable and outcome (LOS), as the dependent variable, and 4) Mediation was tested using Sobel tests [15, 16]. All the above linear models were conducted with weighting by the inverse probability of treatment. For all the above models, distribution and Q-Q plots of the residuals were examined for model assumptions.
All missing data were assumed to be missing at random (MAR) and we employed a list-wise deletion method to handle MAR observations as they were minimal.
Results
Our study identified 247 eligible patients; 119 were enrolled and 114 were included in the analysis (Figure 1). The treatment group was composed of 70 patients and the control group was composed of 44 patients. Table 1 displays the patient demographics. Of note, the two groups did not differ with respect to age, sex, race or weight. The control group included patients who had surgery between October 1, 2009 and January 31, 2014 whereas the treatment group included patients who had surgery between March 1, 2011 and July 31, 2014. The groups differed by the duration of anesthesia and remifentanil doses used, with the control group having a significantly longer anesthesia time and receiving a significantly greater amount of remifentanil (p < 0.001 and p = 0.024, respectively, Table 1). The two groups, however, did not differ on the number of vertebral levels fused or degree of scoliotic curvature (Table 1). Ketorolac use was significantly higher in the IV APAP group (p < 0.001, Table 1). Of note, 87.5% of patients reported having no pain preoperatively. Of the 12.5% that reported pain, the mean pain score was 2.8 ± 1.3 with a range of 1 to 5 on the NRS. There was no difference between the treatment and control groups with respect to baseline pain.
Table 1.
Demographics, surgical, scoliosis and peri-operative factors in the cohort and comparison among treatment and control groups
| Variable | Cohort (n=114) | Treatment Group (n=70) | Control Group (n=44) | p-value |
|---|---|---|---|---|
| Age (years, mean ± SD) | 14.37±1.97 | 14.26± 2.04 | 14.55 ± 1.85 | 0.8097 |
| Weight (kg, mean ± SD) | 56.73±15.18 | 55.77 ± 15.18 | 58.25 ± 15.23 | 0.3638 |
| Female | 78 (68.42%) | 49 (70%) | 29 (65.91%) | 0.647 |
| Race (white) | 96 (84.21%) | 61 (87.14%) | 35 (79.55%) | 0.279 |
| Anesthesia time (min, mean ± SD) | 303.42±75.16 | 286.0 ± 70.30 | 332.5 ± 74.76 | <0.001* |
| Vertebral levels involved (mean ± SD) | 11.50±2.13 | 11.67±1.99 | 11.25±2.32 | 0.1627 |
| Scoliosis curve (degree, mean ± SD) | 57.37±13.39 | 58.98±14.84 | 55.07±10.76 | 0.1496 |
| Remifentanil (mcg/kg, mean ± SD) | 1.30±0.44 | 1.20±0.44 | 1.41± 0.42 | 0.0063* |
| Ketorolac use (yes) | 52 (45.61%) | 44 (62.87%) | 8 (18.18%) | <0.001* |
Univariate comparison of groups for postoperative opioid consumption and outcomes
Pain scores on POD 1 and 2 were also comparable between the 2 groups. The morphine equivalents used were significantly different on both POD 1 and POD 2 with the IV APAP group using significantly less morphine equivalents in the immediate postoperative period as compared to those patients that did not receive IV APAP (p < 0.001 for both PODs, Table 2). The LOS was significantly longer in the control group as compared to the treatment group by about 0.6 days (p = 0.044, Table 2). The opioid-related side effects of RD and emesis were not different between the groups; however, PO intake was delayed by approximately 1 day in the control group (p < 0.001) and the need for suppository at or beyond POD 3 was higher in the control group as compared to the treatment group although this did not reach statistical significance. Patients who were not taking adequate PO were maintained on intravenous fluids.
Table 2.
Univariate analysis of post-operative opioid consumption and opioid effects in the cohort, treatment and control groups, and weighting by the inverse probability of treatment for opioid outcomes
| Variable | Cohort (n=114) | Treatment Group (n=70) | Control Group (n=44) | p-value | p-value after IPTW |
|---|---|---|---|---|---|
| NRS POD 1 (mean ± SD) | 4.74±1.83 | 4.71 ± 1.79 | 4.81 ± 1.92 | 0.774 | - |
| NRS POD 2 (mean ± SD) | 5.52±11.98 | 4.31 ± 1.54 | 4.48 ± 1.55 (n=43) | 0.578 | - |
| Morphine Equivalents POD 1 (mg/kg, mean ± SD) | 1.06±0.45 | 0.94 ± 0.41 | 1.25 ± 0.46 | <0.001* | - |
| Morphine Equivalents POD 2 (mg/kg, mean ± SD) | 0.75±0.35 | 0.62 ± 0.28 | 0.88 ± 0.37 (n=43) | <0.001* | |
| Respiratory depression POD 1 (yes) | 26 (22.81%) | 17 (24.29%) | 9 (20.45%) | 0.635 | - |
| Vomiting POD 1 (yes) | 40 (35.09%) | 26 (37.14%) | 14 (31.82%) | 0.562 | - |
| Suppository POD # (mean ± SD) | 3.57±2.88 | 3.51±3.47 | 3.69±0.92 | 0.797 | - |
| Opioid outcomes adjusted using IPTW adjusted propensity scores | |||||
| Length of Hospital Stay (days, mean ± SD) | 4.76±1.40 | 4.54±1.44 | 5.10±1.29 | 0.044* | 0.157 |
| POD # for PO intake (mean ± SD) | 2.30±1.26 | 1.98±1.70 | 2.86±2.43 | <0.001* | 0.0137* |
| Suppository after POD 3 (yes) | 28 (24.56%) | 11 (16.92%, n=65) | 17 (22.97%, n=74) | 0.086 | 0.0557 |
NRS – Numerical rating scale for pain (11-point scale allowing raters to define pain on a 0 (no pain) to 10 (worst pain of your life) scale)
Propensity scores
Since the use of IV ketorolac, surgical duration and remifentanil doses were significantly different between the IV APAP and control groups, propensity scores were estimated using logistic regression with treatment group as the outcome and other baseline covariates (including demographics, intraoperative and postoperative measures, except postoperative opioid consumption, the assumed mediator) as predictors. The IV ketorolac, surgical duration and remifentanil dose covariates were balanced after IPTW (p-value < .05 for comparison for these covariates between the two groups and standardized differences < .20) allowing us to appropriately compare the control and treatment groups based upon the use of IV APAP. After IPTW, there was no association between the groups and LOS and need for suppository. However, the no APAP group was found to have a longer time to PO intake when compared to the IV APAP group (p=0.0137, Table 2).
Mediation models
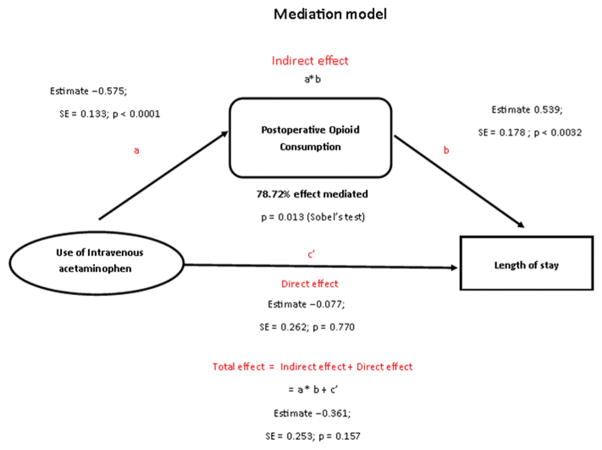
The mediation models with IPTW revealed that there was a significant negative association between the use of IV APAP and morphine consumption (p<0.0001). The model for IV APAP+morphine predicting LOS showed that while controlling for IV APAP, morphine consumption was positively associated with increased LOS (p=0.0032). While the total effect of IV APAP on LOS (sum of direct and indirect) was not statistically significant, the Sobel test showed that the mediation effect of morphine consumption on the association between IV APAP and LOS was statistically significant (p=0.013); this finding indicated that IV APAP’s effect on LOS is through its decrease in postoperative morphine consumption. This indirect effect was found to explain 78.72% of the total effect of IV APAP on LOS (Figure 2).
Figure 2.
Schematic representation of the mediation model and the results of mediation. This diagram demonstrates that postoperative morphine consumption mediates the association between IV APAP on LOS (P=0.013, Sobel’s test) and that the percent of mediation is 78.72%.
Discussion
We conducted a prospective, observational study in adolescent, otherwise healthy patients undergoing posterior spinal fusion receiving standardized anesthesia and postoperative pain management via PCA. We evaluated the effect of IV APAP use over 3 days on opioid outcomes and length of hospital stay using propensity-score adjusted mediation modeling with IV opioid consumption as the mediator. This is the first study to show that use of IV APAP significantly prevents delay of PO intake, has a marginal effect on need for suppository on or after POD 3 and is associated with LOS after spinal fusion, an effect mediated by decreased IV opioid consumption.
Although medications like IV APAP and NSAIDs are known to be opioid-sparing, the effects on opioid outcomes reported by prior studies are controversial. A meta-analysis published in 2015 included 7 studies with over 540 participants; this review showed an overall reduction in 24-hour opioid consumption, lower pain score at 1 and 2 hours and a lower incidence of postoperative vomiting in the group of patients receiving IV APAP[17]. A similar review from 2013 looking specifically at orthopedic surgery patients found that although IV paracetamol was a safe and effective adjunct to opioid use after orthopedic surgery, there was no data to show whether or not this drug reduced opioid-related adverse effects[18]. In ambulatory eye, ear, nose, or throat (EENT) patients, a retrospective study demonstrated a decrease in postoperative pain following surgery but no difference in PACU LOS [19]. Use of IV APAP was found to decrease opioid consumption after hysterectomy and bariatric surgery [20, 21]; however, there was a trend towards increased opioid use after knee arthroplasty [22]. The data remains inconclusive in cardiac surgery patients: A meta-analysis revealed minimal clinical benefits of IV APAP [23] whereas a single-center randomized trial demonstrated a reduction in mean pain scores without a reduction in opioid-related adverse effects with the addition of IV APAP [24]. A retrospective study evaluating use of IV APAP after spine surgery showed a reduction in postoperative opioid use but no decrease in antiemetic or laxative use [25]. These studies clearly demonstrate mixed results when considering the potential benefit of IV APAP in post-surgical patients. Although not the aim of our study, we sought to explore the opioid dose as a mediator of side effects in our analysis.
The Healthcare Cost and Utilization Project identified the ten procedures with the most rapidly increasing hospital costs between 2004 and 2007. The largest increase in costs was for hospital stays involving spinal fusion (93.6%) [6], a surgery associated with considerable hospital expenditures; in 2007, these costs were estimated at $511.2 million [7]. Despite technological improvements, hospital stays following surgery in North America continue to average between 4.2 and 9.3 days [5]. Analysis of data from a publicly available, searchable national database of insurance billing records for patients with orthopedic diagnoses, identified an average LOS of 5.6 days in 955 patients following posterior spinal fusion [26]. In our study, subjects in the group who received IV APAP had an average LOS of 4.54 days, approximately 1 day shorter than the average in this national database. Since hospital stay costs is a large contributor to overall surgery costs (22%) [27] and about 38,000 spinal fusions are performed each year, any reduction in LOS would translate to a significant potential healthcare cost savings in our country.
To test our hypothesis, we used the novel technique of propensity scores in mediation analysis [28]. Mediation analysis is a key statistical tool used to identify the processes by which a treatment affects an outcome [29]. A particular challenge of mediation analysis is that mediator values are only observed under the treatment condition to which each individual is assigned. Propensity scores help circumvent this issue by matching individuals in one treatment group with an observed mediator value to individuals in another treatment group who look as if they would have had that value of the mediator had they been in the other treatment group [28]. In our study, we were interested in investigating not just whether IV APAP treatment had an overall effect on the primary outcome variable but also how IV APAP achieved its effect on LOS. Therefore, we identified other factors that may have confounded the IV APAP – the morphine requirement association and the morphine requirement – LOS association – and used propensity scores to achieve balance on baseline covariates and surgical and postoperative factors between the treatment and control groups. Further, we used IPTW to support our findings. After IPTW, we saw no association between the groups and LOS, supporting our findings in the mediation model suggesting that the total effect between the two groups of IV APAP is not statistically significant but rather that morphine mediates the association between IV APAP and LOS. Similar results using other methods was found in a study of patients undergoing orthopedic surgery. The authors in this study found that constipation, emesis and confusion from the use of opioids were associated with an increased LOS [3]. In fact, LOS was increased by 15%, 40% and 82%, respectively, depending on whether the subject had 2 adverse effects (p = 0.02), 3 adverse effects (p < 0.001), or 4 adverse effects (p < 0.001) compared to patients with no adverse effects [3]. Our study goes one step beyond as it utilizes mediation of the observed effects. In contrast, our findings are inconsistent with a study that evaluated LOS in abdominal surgery patients in which the authors found no difference in postoperative LOS with the addition of IV APAP; these authors, however, did note a reduction in opioid consumption in the first 24 hours following surgery[30]. Clearly, further randomized controlled trials are needed to best understand the role of IV APAP in various surgical populations.
The basis for the efficacy of IV APAP comes from pharmacokinetic studies which have consistently shown that mean plasma concentrations are significantly higher with IV rather than oral administration of APAP as it avoids first-pass hepatic metabolism [31]. Within the IV group, 100% of patients achieved plasma concentrations above proposed analgesic level (66 μmol/L) [32–34]. Despite this theoretical improvement in efficacy, decision-making regarding the use of IV APAP must take into account other important considerations such as convenience and cost [35].
Although our study included a large patient population, one of the limitations of our study is the lack of randomization as this study was conducted as a prospective, observational study. In addition, there were inherent differences between the two groups, specifically with respect to duration of surgery, dose of remifentanil received, and ketorolac administration. Despite the lack of randomization and baseline differences between the two groups, the use of propensity scores helped increase the similarity of the treatment and comparison groups with respect to relevant factors for the mediation model lending credence to our findings. However, propensity scores can only balance measured covariates between two groups and biases may still exist due to unmeasured covariates. In addition, because of the wide time span of the study, there could have been some variability in postoperative management of these patients; however, at our institution, postoperative pain protocols are standardized based on the surgery performed and there was a large overlap between the two groups after 2010 minimizing the differential effects of management practices over time. We also could not extend the study to earlier than 2010 given that IV APAP was not approved in this country prior to this year. Another drawback of our study is that we did not obtain liver function tests. In our institution, appropriate doses of IV APAP are restricted to a 3-day course which is not anticipated to lead to liver toxicity. Therefore, we did not feel there was utility in checking liver enzymes. However, there are about 27 reports of adverse outcomes reported from IV APAP use in pediatric patients, mostly associated with errors in dosing [36]. Another limitation of our study is that there may be other factors that may have affected LOS that we did not consider in this study as the focus was to assess how IV APAP affects LOS through its adverse effects. However, by inclusion criteria, none of the participants had preexisting systemic comorbidities, and none had immediate postoperative complications that might have affected their postoperative course. Lastly, our study results may have been impacted by the number of covariates included in our models relative to the analyzed sample size.
In summary, our study found that the use of IV APAP hastens PO intake and is associated with a reduction in the length of hospital stay after posterior spinal fusion likely through a decrease in IV opioid consumption. We present evidence for a strategy that can result in decreased LOS, an important outcome, given recent trends indicating a transition from traditional “fee-for-service” models to “bundled payments” whereby health care providers are encouraged to deliver care more efficiently while improving quality, cost and outcomes [37]. Our results spur the need for a cost-effectiveness analysis to further inform pain management practices after major surgeries. To date, the only evidence supporting cost-effectiveness of IV APAP use comes from a study of single dose IV APAP for outpatient pediatric tonsillectomy [38]. Future studies are clearly needed to further elucidate the potential benefits of IV APAP. Based on our findings, we recommend the use of IV APAP every 6 hours in weight and age appropriate doses for children undergoing posterior spinal fusion.
Supplementary Material
Footnotes
Disclosure of Funding: The work was supported by the Eunice Kennedy Shriver National institute of child health and human development, National institutes of Health [5K23HD082782] to [VC].
References
- 1.Apfelbaum JLAM, Connis RT, Gan TJ, Nickinovich DG, Caplan RA, Carr DB, Ginsberg B, Green CR, Lema MJ, Rice LJ. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116:248–73. doi: 10.1097/ALN.0b013e31823c1030. [DOI] [PubMed] [Google Scholar]
- 2.Mathiesen O, Dahl B, Thomsen BA, Kitter B, Sonne N, Dahl JB, Kehlet H. A comprehensive multimodal pain treatment reduces opioid consumption after multilevel spine surgery. Eur Spine J. 2013;22:2089–96. doi: 10.1007/s00586-013-2826-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pizzi LT, Toner R, Foley K, Thomson E, Chow W, Kim M, Couto J, Royo M, Viscusi E. Relationship between potential opioid-related adverse effects and hospital length of stay in patients receiving opioids after orthopedic surgery. Pharmacotherapy. 2012;32:502–14. doi: 10.1002/j.1875-9114.2012.01101.x. [DOI] [PubMed] [Google Scholar]
- 4.Oderda GM, Said Q, Evans RS, Stoddard GJ, Lloyd J, Jackson K, Rublee D, Samore MH. Opioid-related adverse drug events in surgical hospitalizations: impact on costs and length of stay. Ann Pharmacother. 2007;41:400–6. doi: 10.1345/aph.1H386. [DOI] [PubMed] [Google Scholar]
- 5.Fletcher ND, Shourbaji N, Mitchell PM, Oswald TS, Devito DP, Bruce RW. Clinical and economic implications of early discharge following posterior spinal fusion for adolescent idiopathic scoliosis. J Child Orthop. 2014;8:257–63. doi: 10.1007/s11832-014-0587-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russo CA, Merrill CT, Friedman B. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD): 2006. Procedures with the Most Rapidly Increasing Hospital Costs, 2000–2004: Statistical Brief #28. [Google Scholar]
- 7.USBJI. Spinal deformity and related conditions. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2011. [Google Scholar]
- 8.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Austin PC. A Tutorial and Case Study in Propensity Score Analysis: An Application to Estimating the Effect of In-Hospital Smoking Cessation Counseling on Mortality. Multivariate Behav Res. 2011;46:119–151. doi: 10.1080/00273171.2011.540480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661–79. doi: 10.1002/sim.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–82. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 12.Judd CM, Kenny DA, McClelland GH. Estimating and testing mediation and moderation in within-subject designs. Psychol Methods. 2001;6:115–34. doi: 10.1037/1082-989x.6.2.115. [DOI] [PubMed] [Google Scholar]
- 13.VanderWeele TJ. Explanation in causal inference: developments in mediation and interaction. Int J Epidemiol. 2016;45:1904–1908. doi: 10.1093/ije/dyw277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.James LR, Brett JM. Mediators, Moderators and Tests for Mediation. Journal of Applied Psychology. 1984;69:15. [Google Scholar]
- 15.Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. Sociological Methdology. 1982;13:23. [Google Scholar]
- 16.Sobel ME. Some New Results on Indirect Effects and Their Standard Errors in Covariance Structure. Sociological Methdology. 1986;16:28. [Google Scholar]
- 17.Doleman B, Read D, Lund JN, Williams JP. Preventive Acetaminophen Reduces Postoperative Opioid Consumption, Vomiting, and Pain Scores After Surgery: Systematic Review and Meta-Analysis. Reg Anesth Pain Med. 2015;40:706–12. doi: 10.1097/AAP.0000000000000311. [DOI] [PubMed] [Google Scholar]
- 18.Jebaraj B, Maitra S, Baidya DK, Khanna P. Intravenous paracetamol reduces postoperative opioid consumption after orthopedic surgery: a systematic review of clinical trials. Pain Res Treat. 2013;2013:402510. doi: 10.1155/2013/402510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khobrani MA, Camamo JM, Patanwala AE. Effect of Intravenous Acetaminophen on Post-Anesthesia Care Unit Length of Stay, Opioid Consumption, Pain, and Analgesic Drug Costs After Ambulatory Surgery. P T. 2017;42:125–139. [PMC free article] [PubMed] [Google Scholar]
- 20.Herring BO, Ader S, Maldonado A, Hawkins C, Kearson M, Camejo M. Impact of intravenous acetaminophen on reducing opioid use after hysterectomy. Pharmacotherapy. 2014;34(Suppl 1):27S–33S. doi: 10.1002/phar.1513. [DOI] [PubMed] [Google Scholar]
- 21.Saurabh S, Smith JK, Pedersen M, Jose P, Nau P, Samuel I. Scheduled intravenous acetaminophen reduces postoperative narcotic analgesic demand and requirement after laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2015;11:424–30. doi: 10.1016/j.soard.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 22.Kelly JS, Opsha Y, Costello J, Schiller D, Hola ET. Opioid use in knee arthroplasty after receiving intravenous acetaminophen. Pharmacotherapy. 2014;34(Suppl 1):22S–26S. doi: 10.1002/phar.1518. [DOI] [PubMed] [Google Scholar]
- 23.Douzjian DJ, Kulik A. Old Drug, New Route: A Systematic Review of Intravenous Acetaminophen After Adult Cardiac Surgery. J Cardiothorac Vasc Anesth. 2016 doi: 10.1053/j.jvca.2016.03.134. [DOI] [PubMed] [Google Scholar]
- 24.Mamoun NF, Lin P, Zimmerman NM, Mascha EJ, Mick SL, Insler SR, Sessler DI, Duncan AE. Intravenous acetaminophen analgesia after cardiac surgery: A randomized, blinded, controlled superiority trial. J Thorac Cardiovasc Surg. 2016;152:881–889. e1. doi: 10.1016/j.jtcvs.2016.04.078. [DOI] [PubMed] [Google Scholar]
- 25.Smith AN, Hoefling VC. A retrospective analysis of intravenous acetaminophen use in spinal surgery patients. Pharm Pract (Granada) 2014;12:417. doi: 10.4321/s1886-36552014000300004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daffner SD, Beimesch CF, Wang JC. Geographic and demographic variability of cost and surgical treatment of idiopathic scoliosis. Spine (Phila Pa 1976) 2010;35:1165–9. doi: 10.1097/BRS.0b013e3181d88e78. [DOI] [PubMed] [Google Scholar]
- 27.Kamerlink JR, Quirno M, Auerbach JD, Milby AH, Windsor L, Dean L, Dryer JW, Errico TJ, Lonner BS. Hospital cost analysis of adolescent idiopathic scoliosis correction surgery in 125 consecutive cases. J Bone Joint Surg Am. 2010;92:1097–104. doi: 10.2106/JBJS.I.00879. [DOI] [PubMed] [Google Scholar]
- 28.Jo B, Stuart EA, Mackinnon DP, Vinokur AD. The Use of Propensity Scores in Mediation Analysis. Multivariate Behav Res. 2011;46:425–452. doi: 10.1080/00273171.2011.576624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jo B. Causal inference in randomized experiments with mediational processes. Psychol Methods. 2008;13:314–36. doi: 10.1037/a0014207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madere TC, Mendez JB, Nordmeyer ST, Heidel RE, Hamilton LA. Evaluation of Intravenous Acetaminophen on Length of Stay in Abdominal Surgery Patients. Hosp Pharm. 2016;51:230–236. doi: 10.1310/hpj5103-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jahr JS, Lee VK. Intravenous acetaminophen. Anesthesiol Clin. 2010;28:619–45. doi: 10.1016/j.anclin.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 32.van der Westhuizen J, Kuo PY, Reed PW, Holder K. Randomised controlled trial comparing oral and intravenous paracetamol (acetaminophen) plasma levels when given as preoperative analgesia. Anaesth Intensive Care. 2011;39:242–6. doi: 10.1177/0310057X1103900214. [DOI] [PubMed] [Google Scholar]
- 33.Singla NK, Parulan C, Samson R, Hutchinson J, Bushnell R, Beja EG, Ang R, Royal MA. Plasma and cerebrospinal fluid pharmacokinetic parameters after single-dose administration of intravenous, oral, or rectal acetaminophen. Pain Pract. 2012;12:523–32. doi: 10.1111/j.1533-2500.2012.00556.x. [DOI] [PubMed] [Google Scholar]
- 34.Brett CN, Barnett SG, Pearson J. Postoperative plasma paracetamol levels following oral or intravenous paracetamol administration: a double-blind randomised controlled trial. Anaesth Intensive Care. 2012;40:166–71. doi: 10.1177/0310057X1204000121. [DOI] [PubMed] [Google Scholar]
- 35.Jibril F, Sharaby S, Mohamed A, Wilby KJ. Intravenous versus Oral Acetaminophen for Pain: Systematic Review of Current Evidence to Support Clinical Decision-Making. Can J Hosp Pharm. 2015;68:238–47. doi: 10.4212/cjhp.v68i3.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Royal M. [Accessed July 7th, 2016];Pediatric Safety Review of Ofirmev (acetaminophen) injection [Web Page] Available at http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/pediatricadvisorycommittee/ucm319370.pdf.
- 37.Sood N, Huckfeldt PJ, Escarce JJ, Grabowski DC, Newhouse JP. Medicare’s bundled payment pilot for acute and postacute care: analysis and recommendations on where to begin. Health Aff (Millwood) 2011;30:1708–17. doi: 10.1377/hlthaff.2010.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Subramanyam R, Varughese A, Kurth CD, Eckman MH. Cost-effectiveness of intravenous acetaminophen for pediatric tonsillectomy. Paediatr Anaesth. 2014;24:467–75. doi: 10.1111/pan.12359. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.