Abstract
Existing implantable neurotechnologies for understanding the brain and treating neurological diseases have intrinsic properties that have limited their capability to achieve chronically-stable brain interfaces with single-neuron spatiotemporal resolution. These limitations reflect what has been dichotomy between the structure and mechanical properties of living brain tissue and non-living neural probes. To bridge the gap between neural and electronic networks, we have introduced the new concept of mesh electronics probes designed with structural and mechanical properties such that the implant begins to ‘look and behave’ like neural tissue. Syringe-implanted mesh electronics have led to the realization of probes that are neuro-attractive and free of the chronic immune response, as well as capable of stable long-term mapping and modulation of brain activity at the single-neuron level. This review provides a historical overview of a 10-year development of mesh electronics by highlighting the tissue-like design, syringe-assisted delivery, seamless neural tissue integration, and single-neuron level chronic recording stability of mesh electronics. We also offer insights on unique near-term opportunities and future directions for neuroscience and neurology that now are available or expected for mesh electronics neurotechnologies.
Conception of mesh electronics: A historical overview
Limitations of neurotechnologies for probing the brain
Our understanding of the brain has for more than century been advanced by technological breakthroughs [1]. Existing neurotechnologies allow for interrogation and manipulation of the brain activity at different spatiotemporal scales, and are leading to an increasingly better understanding of the brain. Nevertheless, current neurotechnologies remain limited in their capability to cover large spatiotemporal range relevant to understanding the brain; that is, from the spatial scale of individual synapses/neurons with millisecond time resolution to that of neural networks comprising different brain regions evolving over months to years. Functional magnetic resonance imaging can map the longitudinal activity of the entire brain, although is unable to achieve spatiotemporal resolution necessary to follow individual neurons underlying observed activity [2]. Alternatively, implanted electrodes can achieve single-neuron level electrophysiology, although with limited chronic recording stability [3,4]. Optical electrophysiology offers high-resolution and relatively large-volume mapping and manipulation of brain activity but has limitations in terms of photon penetration in tissue [5].
The gap between living and non-living systems
Our hypothesis is centered on the observation that brain probes have not been designed to look or behave like the brain tissue, and thus blurring the distinction between the living biological system – the brain – and the non-living electronic system – the probe – will provide new capabilities for addressing fundamental questions in neuroscience and treating neurological/neurodegenerative diseases. Stated in another way, we have worked under the premise that by matching the structural and mechanical properties of the electronic and biological systems, which are traditionally viewed as distinct entities, it should be possible to achieve seamless integration.
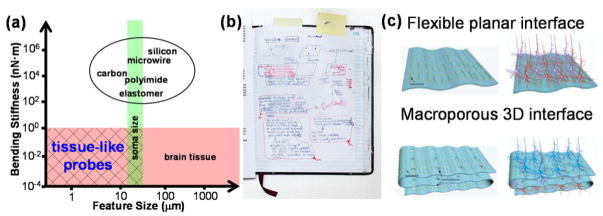
The challenges in meeting these constraints are summarized as follows. First, the brain feature sizes scale from tens of nanometers for synapses connecting individual neurons to tens of centimeters for long-range projections integrating distinct brain regions [6]. In comparison, the overall sizes of silicon microelectrode arrays are almost always >4 times larger than a single neuron regardless of channel numbers [7], and microwire-based brain probes become significantly larger than neuron somata with increasing channel numbers, despite subcellular feature size for single-channel carbon electrodes [8,9]. This mismatch in size (Figure 1a, x axis) may contribute to chronic immune response and obscure the natural three-dimensional (3D) connectivity and circuit activity where the probe is implanted [10,11].
Figure 1.
Challenges of state-of-the-art neural probes and original conception of mesh electronics. (a) Comparison of feature size and bending stiffness between existing neural probes [7–9,12,15–17] and a 20~100 μm thick slice of brain tissue. Ideal brain probes should have critical feature sizes and bending stiffness values similar to or smaller than those of the brain tissue to afford ‘tissue-like probes’. (b) Original page from Lieber’s notebook in 2008 showing key idea leading to free-standing 3D neuron-like nanoelectronic devices to interface with neurons in a biological manner. (c) Schematic drawings presented in 2007/2008 talks and NIH proposal illustrating the key concept leading to mesh electronics with porosity that enables interpenetration and integration of neural networks with electronics structure [24].
Second, brain tissue is very soft with a Young’s modulus of 0.1–16 kPa, resulting in a bending stiffness of 10−4-10−1 nN·m per unit width of brain slices [12,13]. In striking contrast, brain probes are much more rigid, with bending stiffness values of 103~105 nN·m (Figure 1a, y axis) [9,14–18]. The large mismatch in bending stiffness results in relative shear motion, glial scar formation and neuron depletion at the probe-brain interfaces, leading to degradation of recording and stimulation capabilities over extended time periods [11,19].
Third, brain tissue comprises organized 3D networks of neurons and non-neuronal cells, such as astrocytes and microglia, which impacts two major points for probe design: (1) The probe structure should not disrupt the 3D connectivity where each neuron is innervated by as many as 10,000 presynaptic endings [6]. (2) The probe should not disrupt the endogenous distribution of cells given their cooperative importance in defining the functional evolution of neural networks [20]. Since solid brain probes exclude a volume of tissue and disrupt the endogenous cell distribution, one should ask whether there are fundamentally new probe concepts that could overcome these limitations.
Seamlessly bridging the brain and electronics: mesh electronics comes of age
Our approach for overcoming limitations of conventional probes and enabling seamless integration of electronics with tissue originated at least decade ago with the convergence of ideas from two directions focused on interfacing nanoelectronics with biological systems. First and building on our studies of nanowire field-effect transistors (FETs) [21–23], which show readily measured changes in electrical conductivity as the adjacent environment varies, one of us (C.M.L.) suggested implementing these subcellular-size detectors as free-standing 3D neuron-like devices that could interface to live cells via ‘artificial synapses’ (Figure 1b). Second and recognizing the importance of promoting interpenetration of device arrays with 3D neural networks, C.M.L. proposed macroporous flexible scaffolds to create pathways for cell projections and other cells to ‘cross’ the device detector plane (Figure 1c) [24]. Together these ideas have driven the realization of mesh electronics with feature sizes similar to neuron somata, mechanical properties akin to brain tissue and mostly free volume that have led to the exceptional properties and opportunities discussed below.
Realization of mesh electronics: Synapse-like nanosensors and macroporous electronic scaffolds
Nanosensors for subcellular resolution recording
A key idea involved in the initial development of mesh electronics was incorporating subcellular-sized sensors to make artificial synapses with neurites and/or enable minimally-invasive intracellular recording [23,25,26]. To this end we first developed nanowire FETs as general biological nanosensors [21,22] and detected propagating action potentials from neurons cultured on arrays of nanowire FETs that formed synapse-like junctions with neurites [23].
Recognizing the limitation of planar electronics for interfacing 3D biological systems also led us to develop the first 3D nanoscale FET cellular probes, in which active FET detectors at the tips of acute-angle kinked nanowires enabled intracellular recording with a point-like detector [27,28]. Compared to other approaches for extracellular and intracellular recording using chip-based organic transistors, nanostraws and nanopillars [18,29–31], the 3D nanoscale FETs allowed localized recording through synapse-like junctions that also could be readily incorporated into macroporous scaffolds as free-standing elements.
Innervated synthetic neural tissue
Initially, our efforts leading to implanted mesh electronics probes for brain science focused on fabrication of active 3D scaffold with addressable nanoelectronic devices followed by cell culture to create innervated tissues. For example, innervated synthetic neural tissue, where rat hippocampal neurons were cultured within a 3D mesh electronics scaffold, led to interpenetrating neural and electronic networks [32]. From a functional perspective, this work further demonstrated recording of highly local field potentials (LFPs) due to postsynaptic signal propagation, owing to the 103 – 106 smaller footprint of the integrated nanowire FET sensors versus organic transistors and passive metal electrodes [17,18].
Seamless 3D integration of electronics has been implemented in several types of synthetic tissues (e.g., cardiac and vascular) using nanowire devices capable of detecting chemical signals, mechanical strain and extracellular potentials [32,33], as well as simultaneous electrical stimulation and recording that allows for bidirectional flow of information [34]. For example, innervated 3D synthetic cardiac tissue incorporating both nanowire FET detectors and low-impedance stimulation electrodes within the 3D mesh electronics scaffold affords simultaneous mapping and regulation of action potential propagation in 3D with subcellular spatial resolution and submillisecond temporal resolution [34]. This work has obvious implications for closed-loop cardiac electrophysiology and pacing using implanted mesh electronics, for example, to stimulate tissue foci or the whole ventricle [35] when the ultraflexible mesh electronics is placed conformally on the heart surface.
Mesh electronics for brain science
To move from an in vitro scaffold for synthetic tissue to implantable neural probes for in vivo electrophysiology, four key issues must be addressed. First, a minimally-invasive delivery method that affords precise targeting of brain regions needs to be developed for implantation of mesh electronics. Second, the interface between mesh electronics and neural tissue must be characterized to quantify any chronic immune response and probe-tissue interactions. Third, a method to make input/output (I/O) connections is required for in vivo electrophysiology. Last, it is critical to evaluate the single-neuron level chronic recording/stimulation stability afforded by tissue-mimicking mesh electronics. Below these four key areas are discussed.
Unique delivery of ultra-flexible mesh probes
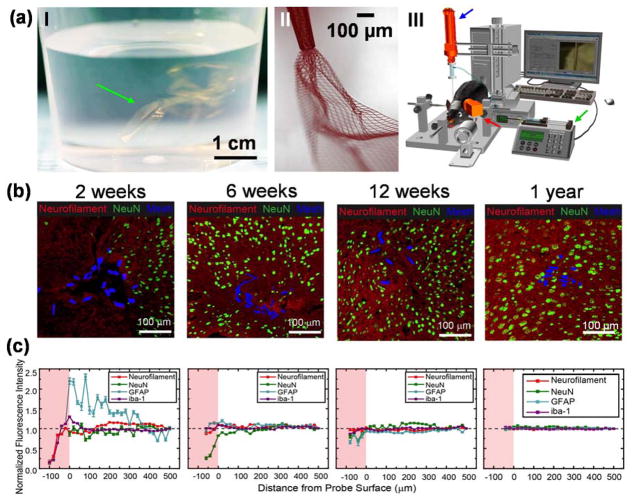
Mesh electronics probes are designed to have element sizes smaller than soma, bending stiffness values similar to brain tissue, and unit openings >100 times larger than soma. They incorporate arrays of recording/stimulation electrodes with positions defined during fabrication to target specific brain regions, and individually addressed by metal interconnects encapsulated within longitudinal polymer elements, which then terminated at I/O connection pads. The ultra-flexibility of submicron-thick mesh structure is readily apparent in aqueous solution where the ‘mesh probes’ literally suspend much like colloids (Figure 2a, I) [14,36]. Unlike conventional rigid probes that are directly inserted into the brain at the cost of long-term immune response and chronic recording instability [4,37–39], the ultra-flexibility of mesh electronics opens up a simple solution commonly used in biology for delivery of biomolecules and cells – direct syringe injection through a needle.
Figure 2.
Syringe delivery of mesh electronics into the brain to yield neuron interpenetration without a chronic immune response. (a) Unique structural and mechanical properties of mesh electronics allow for syringe delivery into the brain, highlighting a photograph of multiple mesh electronics probes (green arrow) floating in an aqueous saline solution similar to colloidal particles (I), a bright-field microscope image showing partially ejected mesh electronics with significant expansion in solution (II), and a schematic of controlled stereotaxic injection (III) that allows precisely targeted delivery of mesh electronics using a motorized translational stage for controlling needle withdrawal (blue arrow), a syringe pump for controlling the injection rate (green arrow), and a camera for visualizing the mesh during injection (red arrow) [14,36]. (b) Time-dependent immunohistochemical staining images of horizontal brain slices at 2 weeks (hippocampus), 6 weeks (cortex), 12 weeks (cortex) and 1 year (cortex) post injection. In all images of panel (b), red, green and blue colors correspond to neuron axons (Neurofilament antibody), neuron nuclei (NeuN antibody) and mesh elements. (c) Normalized fluorescence intensities plotted versus distance from the mesh/brain tissue interface at different time points; the intensities were normalized versus background far from the probe (black dashed horizontal lines). The pink shaded regions indicate the interior of mesh electronics [40].
Once suspended in aqueous solution, a centimeter-scale mesh electronics probe can be drawn into a syringe needle, and then injected under positive pressure into tissue or solution (Figure 2a, II) [14]. The same flexibility also poses a challenge in precise targeting due to potential crumpling during injection, which could yield ill-defined electrode positions. To solve this challenge, a semi-automated controlled injection method was developed by balancing the injection rate and needle withdrawal using a standard rodent stereotaxic frame (Figure 2a, III), resulting in reproducible fully extended mesh structures in targeted brain regions after implantation [36].
Implanted mesh electronics do not exhibit chronic immune response
Cross-sectional immunohistology studies were used to evaluate the chronic response of neural tissue following mesh implantation [40,41]. Conventional rigid and nonporous silicon, tungsten and carbon probes typically produce glial scarring and neuron depletion at the probe-tissue interface due to mechanical mismatch between the implanted probes and brain tissue [4,9,11,19,38,39,42,43]. Mesh electronics, by contrast, was designed to “look and behave” like neural tissue both structurally and mechanically to overcome these long-standing issues with conventional probes.
Indeed, immunohistology studies at times up to a year post-implantation [14,40,41,44] demonstrate that the distribution of neuron somata, axons, astrocytes and microglia at the mesh-tissue interface is nearly the same as natural tissue baseline by 4–6 weeks, and maintains this natural distribution to at least a year (Figure 2b&c). Despite the slight elevation of astrocyte and microglia signals at early times, there is no evidence for chronic proliferation of astrocytes and microglia or depletion of neurons; instead, time-dependent penetration of axonal projections and somata into the interior of mesh electronics has been found during the first 12 weeks post-injection. These unprecedented results highlight the seamless neural interface without chronic gliosis and natural distribution of both neurons and non-neuronal cells achieved with mesh electronics, thus raising expectations for stable recording of neural activity critical for advancing fundamental studies and long-term therapeutic implants.
Facile I/O connections for electrophysiology
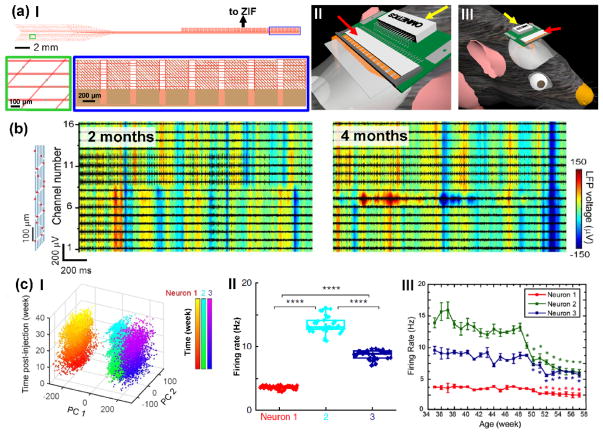
A critical challenge associated with translating mesh electronics from ex-vivo tissue scaffolds to implantable brain probes involved developing reliable methods for multi-channel I/O connection to standard measurement electronics, since syringe-injection through fine needles makes it topologically impossible to pre-bond I/O pads to connectors. To address this challenge, we have developed computer-controlled conductive ink printing and plug-and-play I/O interfacing methods [36,45]. The plug-and-play I/O interface features an ultra-flexible mesh region with recording/stimulation electrodes to be implanted into the brain tissue, a stem region that routes all interconnect lines, and an I/O region where regular pads are oriented perpendicular to parallel interconnects for plugging into standard zero insertion force (ZIF) interface connectors (Figure 3a, I).
Figure 3.
Electrical I/O connection and long-term stable recording at the single-neuron level using mesh electronics. (a) Quantitative and scalable high throughput I/O connection by a plug-and-play interface: structural design of the plug-and-play mesh electronics (I), insertion of I/O pads of plug-and-play mesh electronics into a ZIF connector (II), and compact headstage comprising mesh electronics inserted into the ZIF connector (red arrows) on a PCB that provides an interface to a standard Omnetics connector (yellow arrows) for recording (III) [45]. (b) 16-channel multiplexed recording of LFP (background heat map) and single-unit firing (foreground black traces) from the same mouse brain at 2 and 4 months post injection. The relative positions of all 16 recording electrodes are marked by red dots in the schematic (leftmost panel), and span the somatosensory cortex to hippocampus. (c) Chronic tracking of same individual neurons by time-dependent PCA (I) and firing rate analysis (II) that allows for study of brain aging on the single-neuron level by tracking firing rate evolution of the same three individual neurons from 35 to 57 weeks of age (III) [40].
This new design is attractive for general users since it enables “by hand” plug-and-play connection to a ZIF connector after injection (Figure 3a, II). The ZIF connector is mounted on a printed circuit board (PCB) with a standard Omnetics connector, thus resulting in a compact head-stage for acute and chronic multiplexed recording/stimulation studies (Figure 3a, III) [45]. In addition, this compact head-stage can be readily expanded to include multiple ZIF connectors that allow plug-and-play connection and multiplexed recording from multiple mesh probes implanted in different brain regions.
Stable chronic recording and stimulation at the single-neuron level
The above sections set the stage for chronic multiplexed recording and stimulation studies, which have demonstrated single-neuron level brain mapping of the same neurons and local circuits on a year timescale in mice [40]. Several key results from these studies are summarized below. First, 16-channel multiplexed recordings at 2 and 4 months post-injection of mesh electronics yielded stable modulation of LFPs and consistent amplitudes of single-unit spikes across this two-month period (Figure 3b). Statistical analysis of recording data from multiple mice revealed 85% of channels with identifiable single-unit spikes and on average 2–3 neurons per electrode [46]. In addition, multiplexed data recorded over 6–8 months in different mice showed similar single-unit and LFP stability, despite a gradual increase in single-unit amplitude at early times reflecting the tissue healing process. Moreover, recent studies highlight that further pushing mesh designs towards more neural-network-like and optimizing injection/implantation protocols can reduce and even eliminate the early time amplitude changes (unpublished).
In addition, detailed analyses of recordings revealed stable chronic mapping of multiple neurons and their encompassing neural circuits at the single-neuron level, as evidenced by consistent principal component analysis (PCA, Figure 3c, I), highly similar average spike waveforms, largely unchanged inter-spike interval (ISI) histograms (Figure 3c, II) and stable phase locking to hippocampal theta oscillations across 8 months. The long-term recording stability offers an unprecedented opportunity to carry out longitudinal brain aging studies with single-neuron spatiotemporal resolution. For example, chronic tracking of firing dynamics of the same single neurons showed consistent decline in firing rate (Figure 3c, III) and increase in spike peak-to-trough time for mice aged >48 weeks, providing a new insight into brain aging by revealing the distinct single-neuron changes over extended timescales.
Last, the capabilities of mesh electronics can be readily expanded by incorporating stimulation electrodes to afford simultaneous chronic stimulation and recording at the single-neuron level, where the stimulus-induced artifact in recording can be easily removed owing to its predictable characteristics [47]. Time-dependent studies of post-stimulus spike incidence and latency confirmed stable single-neuron responses to chronic electrical stimulation, highlighting the potential for using multi-functional mesh probes for chronic neuron/circuit modulation and recording studies.
Outlook: mesh electronics for neuroscience and neurology
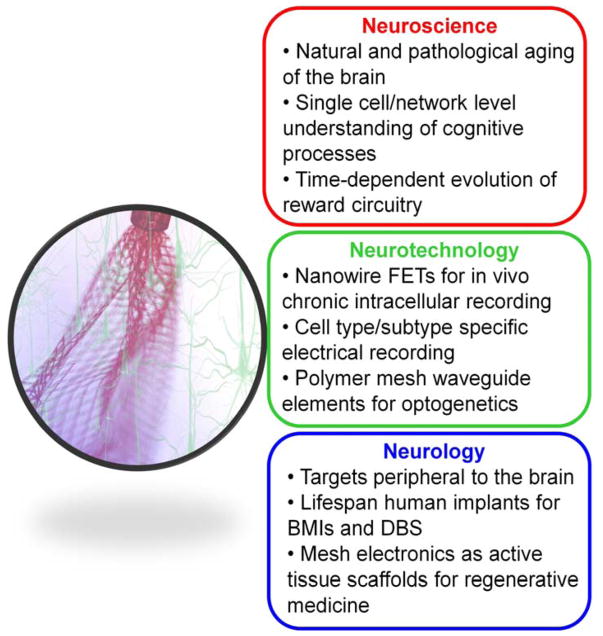
The unique capabilities of syringe-injectable mesh electronics as tissue-like and seamlessly integrating brain probes suggest a number of exciting directions. Below we highlight three general areas from the perspectives of neuroscience opportunities, neurotechnology development and neurological applications (Figure 4).
Figure 4.
Outlook and three basic areas of opportunity for mesh electronics neural probes, including neuroscience opportunities, neurotechnology developments, and neurology applications.
Neuroscience opportunities
The unique single-neuron level, long-term recording and stimulation capability of mesh electronics could provide previously unavailable data crucial for understanding many important brain functions and cognitive processes that span orders of magnitude in their relevant time and length scales. For example, conventional low-resolution longitudinal studies [48] and higher-resolution cross-sectional studies [49] are incapable of studying brain aging, cognitive learning and memory and reward circuitry evolution [50,51] by tracking underlying electrophysiological changes at the individual neuron level over months to years in multiple interconnected brain regions. The long-term stability of mesh electronics now makes possible studies of brain circuit evolution over these heretofore missing spatiotemporal scales, and thus could provide single-neuron/neural circuit level insight into the neurological basis of these important brain functions and cognitive processes.
Neurotechnology development
There is great opportunity for further development of mesh electronics paradigm. For example, owing to the active detector areas that are much smaller than conventional passive electrodes, nanowire FETs are potential candidates for incorporation into mesh electronics to provide highly-localized detection of both extracellular and intracellular field/action potentials in vivo [26,52–54]. Additionally, the ultra-flexibility of mesh electronics and the natural cell distribution post-implantation suggest that functionalization of recording/stimulation devices with targeting molecules for in vivo neuron-subtype-specific electrophysiology. Moreover, mesh electronics provides a platform for incorporating polymer optical waveguides as a chronically-stable deep-tissue light source for optogenetics, eliminating degradation of the fiber/optrode performance over time due to chronic gliosis that is usually observed for existing rigid optogenetic probes [55].
Neurological applications
Last, we believe mesh electronics offers important opportunities for neurology and clinical translation. First, minimally-invasive syringe injection allows for delivery of mesh probes into virtually any soft tissue in vivo, including the retina, spinal cord and neuromuscular junctions, resulting in injectable neuroprostheses to restore vision and motor functions in models of retinal and muscular dystrophy [42,56]. Second, the chronically-stable and seamless integration afforded by mesh electronics suggests an ideal platform and even a lifespan implant for long-term deep-brain stimulation (DBS) in Parkinsonian patients without chronic gliosis and brain-machine interfaces (BMIs) with single-unit activity based decoding for neuroprosthetic control [57,58]. Third, by understanding and manipulating the extracellular matrix-like properties of mesh electronics to favor migration and development of neural progenitor cells [59], while simultaneously monitoring/modulating neural activity, we envision mesh electronics to serve as an active therapeutic for repairing injured brain regions.
Conclusions
Our goal to bridge the gap between the structure and mechanical properties of neural and electronic networks a decade ago has now led to the realization of mesh electronics that ‘look’ and ‘behave’ like neural tissue, evidenced by the lack of chronic immune response, seamless 3D integration with neural tissue, and unprecedented stable long-term multiplexed mapping and modulation of local neural circuits at the single-neuron level. Together, these advances open up exciting opportunities for studies in neuroscience, neurology and further development of the mesh electronics paradigm. Finally, we quote from ‘Imagined Worlds’ authored by theoretical physicist and mathematician Freeman Dyson [60]: “New directions in science are launched by new tools much more often than by new concepts.” Given the unique advantages offered by mesh electronics as discussed in this review, we are excited to be equipped with a new and general tool that will launch new directions and discoveries at the research frontiers of neuroscience and neurology.
Acknowledgments
We thank Theodore J. Zwang for helpful discussions. This work was funded by the Air Force Office of Scientific Research (FA9550-14-1-0136), a Harvard University Physical Sciences and Engineering Accelerator award, the National Institute on Drug Abuse of the National Institutes of Health (1R21DA043985-01) and a National Institutes of Health Director’s Pioneer Award (1DP1EB025835-01). G.H. is supported by a Pathway to Independence Award (Parent K99/R00) from the National Institute on Aging of the National Institutes of Health (1K99AG056636-01).
Footnotes
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Yuste R. From the neuron doctrine to neural networks. Nat Rev Neurosci. 2015;16:487–497. doi: 10.1038/nrn3962. [DOI] [PubMed] [Google Scholar]
- 2.Poldrack RA, Farah MJ. Progress and challenges in probing the human brain. Nature. 2015;526:371–379. doi: 10.1038/nature15692. [DOI] [PubMed] [Google Scholar]
- 3.Harris KD, Quiroga RQ, Freeman J, Smith SL. Improving data quality in neuronal population recordings. Nat Neurosci. 2016;19:1165–1174. doi: 10.1038/nn.4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4•.Polikov VS, Tresco PA, Reichert WM. Response of brain tissue to chronically implanted neural electrodes. J Neurosci Methods. 2005;148:1–18. doi: 10.1016/j.jneumeth.2005.08.015. This review article details the biochemical features and time-dependent evolution of the acute and chronic reactions of the brain tissue in response to implantation of conventional neural probes. [DOI] [PubMed] [Google Scholar]
- 5.Lin MZ, Schnitzer MJ. Genetically encoded indicators of neuronal activity. Nat Neurosci. 2016;19:1142–1153. doi: 10.1038/nn.4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kandel ER, Schwartz JH, Jessell TM, Siegelbaum SA, Hudspeth AJ. Principles of neural science. McGraw-hill; New York: 2013. [Google Scholar]
- 7.Shobe JL, Claar LD, Parhami S, Bakhurin KI, Masmanidis SC. Brain activity mapping at multiple scales with silicon microprobes containing 1,024 electrodes. J Neurophysiol. 2015;114:2043–2052. doi: 10.1152/jn.00464.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwarz DA, Lebedev MA, Hanson TL, Dimitrov DF, Lehew G, Meloy J, Rajangam S, Subramanian V, Ifft PJ, Li Z, et al. Chronic, wireless recordings of large-scale brain activity in freely moving rhesus monkeys. Nat Methods. 2014;11:670–676. doi: 10.1038/nmeth.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kozai TDY, Langhals NB, Patel PR, Deng XP, Zhang HN, Smith KL, Lahann J, Kotov NA, Kipke DR. Ultrasmall implantable composite microelectrodes with bioactive surfaces for chronic neural interfaces. Nat Mater. 2012;11:1065–1073. doi: 10.1038/nmat3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kipke DR, Shain W, Buzsaki G, Fetz E, Henderson JM, Hetke JF, Schalk G. Advanced neurotechnologies for chronic neural interfaces: new horizons and clinical opportunities. J Neurosci. 2008;28:11830–11838. doi: 10.1523/JNEUROSCI.3879-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biran R, Martin DC, Tresco PA. Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays. Exp Neurol. 2005;195:115–126. doi: 10.1016/j.expneurol.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 12•.Tyler WJ. OPINION The mechanobiology of brain function. Nat Rev Neurosci. 2012;13:867–878. doi: 10.1038/nrn3383. This article provides a good overview on the mechanical properties of the brain tissue with insight on the importance of integration of mechanobiology for studying brain functions. [DOI] [PubMed] [Google Scholar]
- 13.Steif PS. Mechanics of materials. Upper Saddle River, NJ: Pearson; 2012. [Google Scholar]
- 14••.Liu J, Fu TM, Cheng ZG, Hong GS, Zhou T, Jin LH, Duvvuri M, Jiang Z, Kruskal P, Xie C, et al. Syringe-injectable electronics. Nat Nanotechnol. 2015;10:629–636. doi: 10.1038/nnano.2015.115. This paper is the first demonstration of the syringe-injectable mesh electronics concept showing minimally invasive delivery of centimeter-scale electronics through 100-μm diameter needles into rodent brains. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rousche PJ, Pellinen DS, Pivin DP, Williams JC, Vetter RJ, Kipke DR. Flexible polyimide-based intracortical electrode arrays with bioactive capability. IEEE Trans Biomed Eng. 2001;48:361–371. doi: 10.1109/10.914800. [DOI] [PubMed] [Google Scholar]
- 16.Minev IR, Musienko P, Hirsch A, Barraud Q, Wenger N, Moraud EM, Gandar J, Capogrosso M, Milekovic T, Asboth L, et al. Electronic dura mater for long-term multimodal neural interfaces. Science. 2015;347:159–163. doi: 10.1126/science.1260318. [DOI] [PubMed] [Google Scholar]
- 17.Viventi J, Kim DH, Vigeland L, Frechette ES, Blanco JA, Kim YS, Avrin AE, Tiruvadi VR, Hwang SW, Vanleer AC, et al. Flexible, foldable, actively multiplexed, high-density electrode array for mapping brain activity in vivo. Nat Neurosci. 2011;14:1599–1605. doi: 10.1038/nn.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khodagholy D, Gelinas JN, Thesen T, Doyle W, Devinsky O, Malliaras GG, Buzsaki G. NeuroGrid: recording action potentials from the surface of the brain. Nat Neurosci. 2015;18:310–315. doi: 10.1038/nn.3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19••.Chen R, Canales A, Anikeeva P. Neural recording and modulation technologies. Nature Reviews Materials. 2017;2:16093. doi: 10.1038/natrevmats.2016.93. This review summarizes recent materials-driven progresses in developing neural probes and neurotechnologies for recording and modulation of neural activities at unprecedented spatiotemporal scales. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eroglu C, Barres BA. Regulation of synaptic connectivity by glia. Nature. 2010;468:223–231. doi: 10.1038/nature09612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21•.Cui Y, Wei QQ, Park HK, Lieber CM. Nanowire nanosensors for highly sensitive and selective detection of biological and chemical species. Science. 2001;293:1289–1292. doi: 10.1126/science.1062711. This study provides the first demonstration of sensing biological and chemical species in aqueous solutions with silicon nanowire FETs. [DOI] [PubMed] [Google Scholar]
- 22.Zheng GF, Patolsky F, Cui Y, Wang WU, Lieber CM. Multiplexed electrical detection of cancer markers with nanowire sensor arrays. Nat Biotechnol. 2005;23:1294–1301. doi: 10.1038/nbt1138. [DOI] [PubMed] [Google Scholar]
- 23•.Patolsky F, Timko BP, Yu GH, Fang Y, Greytak AB, Zheng GF, Lieber CM. Detection, stimulation, and inhibition of neuronal signals with high-density nanowire transistor arrays. Science. 2006;313:1100–1104. doi: 10.1126/science.1128640. This work demonstrates the formation of ‘artificial synapses’ by allowing neurites of cultured neurons to cross individual nanowire FETs to afford highly localized extracellular recording of neural activity. [DOI] [PubMed] [Google Scholar]
- 24.Lieber CM. Nanotechnology and the Life Sciences. Nano/Bio Interface Center (NBIC) Award for Research Excellence in Nanotechnology Speech, University of Pennsylvania; Oct, 2007. [Google Scholar]
- 25•.Kruskal PB, Jiang Z, Gao T, Lieber CM. Beyond the Patch Clamp: Nanotechnologies for Intracellular Recording. Neuron. 2015;86:21–24. doi: 10.1016/j.neuron.2015.01.004. This NeuroView article offers insights on next-generation intracellular neural recording methods enabled by advances in nanoscience and nanotechnology. [DOI] [PubMed] [Google Scholar]
- 26•.Lee JH, Zhang AQ, You SS, Lieber CM. Spontaneous Internalization of Cell Penetrating Peptide-Modified Nanowires into Primary Neurons. Nano Lett. 2016;16:1509–1513. doi: 10.1021/acs.nanolett.6b00020. This work demonstrates spontaneous internalization of silicon nanowires into primary neurons via a general cell penetrating peptide modification method. [DOI] [PubMed] [Google Scholar]
- 27•.Tian BZ, Cohen-Karni T, Qing Q, Duan XJ, Xie P, Lieber CM. Three-Dimensional, Flexible Nanoscale Field-Effect Transistors as Localized Bioprobes. Science. 2010;329:830–834. doi: 10.1126/science.1192033. This work demonstrates the internalization of kinked nanowire FETs for intracellular recording of cultured cardiomyocytes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qing Q, Jiang Z, Xu L, Gao RX, Mai LQ, Lieber CM. Free-standing kinked nanowire transistor probes for targeted intracellular recording in three dimensions. Nat Nanotechnol. 2014;9:142–147. doi: 10.1038/nnano.2013.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benfenati V, Toffanin S, Bonetti S, Turatti G, Pistone A, Chiappalone M, Sagnella A, Stefani A, Generali G, Ruani G, et al. A transparent organic transistor structure for bidirectional stimulation and recording of primary neurons. Nat Mater. 2013;12:672–680. doi: 10.1038/nmat3630. [DOI] [PubMed] [Google Scholar]
- 30.Cao Y, Hjort M, Chen H, Birey F, Leal-Ortiz SA, Han CM, Santiago JG, Pasca SP, Wu JC, Melosh NA. Nondestructive nanostraw intracellular sampling for longitudinal cell monitoring. Proc Natl Acad Sci USA. 2017;114:E1866–E1874. doi: 10.1073/pnas.1615375114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie C, Lin Z, Hanson L, Cui Y, Cui B. Intracellular recording of action potentials by nanopillar electroporation. Nat Nanotechnol. 2012;7:185–190. doi: 10.1038/nnano.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32••.Tian BZ, Liu J, Dvir T, Jin LH, Tsui JH, Qing Q, Suo ZG, Langer R, Kohane DS, Lieber CM. Macroporous nanowire nanoelectronic scaffolds for synthetic tissues. Nat Mater. 2012;11:986–994. doi: 10.1038/nmat3404. This paper is the first demonstration of seamless 3D integration of mesh electronics as an active nanoelectronic scaffold for synthetic tissues. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J, Xie C, Dai XC, Jin LH, Zhou W, Lieber CM. Multifunctional three-dimensional macroporous nanoelectronic networks for smart materials. Proc Natl Acad Sci USA. 2013;110:6694–6699. doi: 10.1073/pnas.1305209110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34••.Dai XC, Zhou W, Gao T, Liu J, Lieber CM. Three-dimensional mapping and regulation of action potential propagation in nanoelectronics-innervated tissues. Nat Nanotechnol. 2016;11:776–782. doi: 10.1038/nnano.2016.96. This paper demonstrates 3D synthetic cardiac tissues comprising mesh electronics with recording and stimulation devices for bidirectional closed-loop interface with the seamlessly innervated cardiac tissue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park J, Choi S, Janardhan AH, Lee SY, Raut S, Soares J, Shin K, Yang SX, Lee C, Kang KW, et al. Electromechanical cardioplasty using a wrapped elasto-conductive epicardial mesh. Sci Transl Med. 2016;8:344ra86. doi: 10.1126/scitranslmed.aad8568. [DOI] [PubMed] [Google Scholar]
- 36•.Hong GS, Fu TM, Zhou T, Schuhmann TG, Huang JL, Lieber CM. Syringe Injectable Electronics: Precise Targeted Delivery with Quantitative Input/Output Connectivity. Nano Lett. 2015;15:6979–6984. doi: 10.1021/acs.nanolett.5b02987. This paper describes methods for precise injection of mesh electronics in vivo and I/O connection of multiplexed mesh electronics for post-injection electrophysiological recording. [DOI] [PubMed] [Google Scholar]
- 37.Perge JA, Homer ML, Malik WQ, Cash S, Eskandar E, Friehs G, Donoghue JP, Hochberg LR. Intra-day signal instabilities affect decoding performance in an intracortical neural interface system. J Neural Eng. 2013;10:036004. doi: 10.1088/1741-2560/10/3/036004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bensmaia SJ, Miller LE. Restoring sensorimotor function through intracortical interfaces: progress and looming challenges. Nat Rev Neurosci. 2014;15:313–325. doi: 10.1038/nrn3724. [DOI] [PubMed] [Google Scholar]
- 39.Prasad A, Xue QS, Sankar V, Nishida T, Shaw G, Streit WJ, Sanchez JC. Comprehensive characterization and failure modes of tungsten microwire arrays in chronic neural implants. J Neural Eng. 2012;9:056015. doi: 10.1088/1741-2560/9/5/056015. [DOI] [PubMed] [Google Scholar]
- 40••.Fu TM, Hong GS, Zhou T, Schuhmann TG, Viveros RD, Lieber CM. Stable long-term chronic brain mapping at the single-neuron level. Nat Methods. 2016;13:875–882. doi: 10.1038/nmeth.3969. This work demonstrates stable chronic interrogation and manipulation of neural activity at the single-neuron level on a year time scale in mice using syringe-implanted mesh electronics. [DOI] [PubMed] [Google Scholar]
- 41••.Zhou T, Hong G, Fu TM, Yang X, Schuhmann TG, Viveros RD, Lieber CM. Syringe-injectable mesh electronics integrate seamlessly with minimal chronic immune response in the brain. Proc Natl Acad Sci USA. 2017;114:5894–5899. doi: 10.1073/pnas.1705509114. This paper summarizes time-dependent immunohistological studies of the mesh electronics - brain tissue interface, demonstrating a natural distribution of neuronal and glial cells at the interface and seamless integration of mesh electronics with the endogenous neural network. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42•.Lacour SP, Courtine G, Guck J. Materials and technologies for soft implantable neuroprostheses. Nature Reviews Materials. 2016;1:16063. This review emphasizes the importance of minimizing the physical and mechanical mismatch between the implantable probes and the neural tissue for the development of prostheses. [Google Scholar]
- 43•.Rivnay J, Wang HL, Fenno L, Deisseroth K, Malliaras GG. Next-generation probes, particles, and proteins for neural interfacing. Sci Adv. 2017;3:e1601649. doi: 10.1126/sciadv.1601649. This comprehensive review highlights the latest bidirectional neural interfacing techniques. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44•.Xie C, Liu J, Fu TM, Dai XC, Zhou W, Lieber CM. Three-dimensional macroporous nanoelectronic networks as minimally invasive brain probes. Nat Mater. 2015;14:1286–1292. doi: 10.1038/nmat4427. This paper describes another method of delivering mesh electronics into live rodent brain by rapid intracortical insertion of a frozen probe. [DOI] [PubMed] [Google Scholar]
- 45•.Schuhmann TG, Yao J, Hong G, Fu T-M, Lieber CM. Syringe-Injectable Electronics with a Plug-and-Play Input/Output Interface. Nano Lett. 2017;17:5836–5842. doi: 10.1021/acs.nanolett.7b03081. This work demonstrates a facile parallel plug-and-play interfacing approach for rapid and user-friendly I/O connection of highly multiplexed mesh electronics. [DOI] [PubMed] [Google Scholar]
- 46.Fu T-M, Hong GS, Viveros RD, Zhou T, Lieber CM. Highly-scalable multi-channel mesh electronics for stable chronic brain electrophysiology. Proc Natl Acad Sci. 2017;114:E10046–E10055. doi: 10.1073/pnas.1717695114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heffer LF, Fallon JB. A novel stimulus artifact removal technique for high-rate electrical stimulation. J Neurosci Methods. 2008;170:277–284. doi: 10.1016/j.jneumeth.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grady C. BRAIN AGEING The cognitive neuroscience of ageing. Nat Rev Neurosci. 2012;13:491–505. doi: 10.1038/nrn3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang M, Gamo NJ, Yang Y, Jin LE, Wang XJ, Laubach M, Mazer JA, Lee D, Arnsten AFT. Neuronal basis of age-related working memory decline. Nature. 2011;476:210–213. doi: 10.1038/nature10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moser EI, Roudi Y, Witter MP, Kentros C, Bonhoeffer T, Moser MB. Grid cells and cortical representation. Nat Rev Neurosci. 2014;15:466–481. doi: 10.1038/nrn3766. [DOI] [PubMed] [Google Scholar]
- 51.Luthi A, Luscher C. Pathological circuit function underlying addiction and anxiety disorders. Nat Neurosci. 2014;17:1635–1643. doi: 10.1038/nn.3849. [DOI] [PubMed] [Google Scholar]
- 52••.Zhang A, Zheng G, Lieber C. Nanowires: Building blocks for nanoscience and nanotechnology. Springer; 2016. This book provides a comprehensive review of all aspects of nanowires with a focus on the applications of nanowire sensors to interface biological systems. [Google Scholar]
- 53••.Zhang AQ, Lieber CM. Nano-Bioelectronics. Chem Rev. 2016;116:215–257. doi: 10.1021/acs.chemrev.5b00608. This is a comprehensive review that covers rapidly progressing frontiers of the nano-bioelectronics, with an emphasis on advances in electrophysiology enabled by nanoelectronic devices and mesh electronics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duan XJ, Gao RX, Xie P, Cohen-Karni T, Qing Q, Choe HS, Tian BZ, Jiang XC, Lieber CM. Intracellular recordings of action potentials by an extracellular nanoscale field-effect transistor. Nat Nanotechnol. 2012;7:174–179. doi: 10.1038/nnano.2011.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yazdan-Shahmorad A, Diaz-Botia C, Hanson TL, Kharazia V, Ledochowitsch P, Maharbiz MM, Sabes PN. A Large-Scale Interface for Optogenetic Stimulation and Recording in Nonhuman Primates. Neuron. 2016;89:927–939. doi: 10.1016/j.neuron.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 56.Lorach H, Goetz G, Smith R, Lei X, Mandel Y, Kamins T, Mathieson K, Huie P, Harris J, Sher A, et al. Photovoltaic restoration of sight with high visual acuity. Nat Med. 2015;21:476–482. doi: 10.1038/nm.3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kringelbach ML, Jenkinson N, Owen SLF, Aziz TZ. Translational principles of deep brain stimulation. Nat Rev Neurosci. 2007;8:623–635. doi: 10.1038/nrn2196. [DOI] [PubMed] [Google Scholar]
- 58.Hochberg LR, Bacher D, Jarosiewicz B, Masse NY, Simeral JD, Vogel J, Haddadin S, Liu J, Cash SS, van der Smagt P, et al. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature. 2012;485:372–375. doi: 10.1038/nature11076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ghashghaei HT, Lai C, Anton ES. Neuronal migration in the adult brain: are we there yet? Nat Rev Neurosci. 2007;8:141–151. doi: 10.1038/nrn2074. [DOI] [PubMed] [Google Scholar]
- 60.Dyson FJ. Imagined worlds. Cambridge, Mass: Harvard University Press; 1997. [Google Scholar]