Abstract
Nuclear magnetic resonance spectroscopy (NMR) is a powerful technique for characterizing the structural and dynamic properties of intrinsically disordered proteins and protein regions (IDPs & IDRs). However, the application of NMR to IDPs has been limited by poor chemical shift dispersion in two-dimensional (2D) 1H-15N heteronuclear correlation spectra. Among the various detection schemes available for heteronuclear correlation spectroscopy, 13C direct-detection has become a mainstay for investigations of IDPs owing to the favorable chemical shift dispersion in 2D 13C′-15N correlation spectra. Recent advances in cryoprobe technology have enhanced the sensitivity for direct detection of both 13C and 15N resonances at high magnetic field strengths, thus prompting the development of 15N direct-detect experiments to complement established 13C-detection experiments. However, the application of 15N-detection has not been widely explored for IDPs. Here we compare 1H, 13C, and 15N detection schemes for a variety of 2D heteronuclear correlation spectra and evaluate their performance on the basis of resolution, chemical shift dispersion, and sensitivity. We performed experiments with a variety of disordered systems ranging in size and complexity; from a small IDR (99 amino acids), to a large low complexity IDR (185 amino acids), and finally a ~73 kDa folded homopentameric protein that also contains disordered regions (133 amino acids/monomer). We conclude that, while requiring high sample concentration and long acquisition times, 15N-detection often offers enhanced resolution over other detection schemes in studies of disordered protein regions with low complexity sequences.
Keywords: Intrinsically disordered proteins, IDP, NMR, 13C-Detection, 15N-Detection
Graphical Abstract
1. Introduction
NMR is a powerful technique for studies of the structural and dynamic properties of biomolecules with atomic resolution in solution. Of particular interest are NMR studies of intrinsically disordered proteins, and disordered protein regions (IDPs and IDRs), because, due to their dynamic features, x-ray crystallography is often not possible or relevant. However, the study of IDPs and IDRs is often limited by poor chemical shift dispersion in heteronuclear correlation spectra [1]. For IDPs and IDRs (in the following, we use the term IDPs to mean both IDPs and IDRs), the absence of highly populated secondary and tertiary structure, extensive dynamics and conformational averaging, and low amino acid sequence complexity, homogenize the chemical environments of amino acid residues, leading to limited chemical shift dispersion and severe resonance overlap. These resolution challenges become more pronounced as the length of the polypeptide increases, thus placing practical limitations on the size of IDPs/IDRs for which full backbone resonance assignments can be made using traditional, 1H-detection-based, multi-dimensional protein NMR techniques.
The extensive conformational flexibility that underlies poor NMR spectral dispersion is, however, associated with favorable magnetization relaxation properties, which provides opportunities to achieve ultra-high spectral resolution for IDPs. The NMR linewidth, or full-width at half-height (ΔFWHH), of the Lorentzian lineshape is an important factor affecting the resolution and the signal-to-noise (S/N) ratio of NMR spectra. The value of ΔFWHH is proportional to the transverse relaxation rate R2 (ΔFWHH = 1/πT2 = R2/π); however, additional factors contribute to observed resonance linewidths, including conformational exchange, magnetic field (B0) inhomogeneity, sample heterogeneities, and temperature gradients. The latter sources of resonance broadening can be minimized by using modern NMR spectrometers and probes and through preparation of monodisperse protein samples; given this, the intrinsic relaxation properties of the detected nuclei strongly influence the resolution and sensitivity that can be achieved in NMR spectra. Among the spin ½ nuclei present in proteins, those with small values of the gyromagnetic ratio (γ; e.g., 13C and 15N) exhibit intrinsically lower sensitivity, as given by the relation S0 ∞ γeγ d 3/2, where γe and γd correspond to the γ values for the excitation and detection nuclei, respectively. However, this can be compensated by slower relaxation rates relative to those for 1H nuclei due to reduced dipole-dipole relaxation associated with low-γ nuclei [2]. These properties were originally exploited in a series of “proton-less” 13C direct-detection heteronuclear correlation experiments [3], which have subsequently been widely applied in studies of IDPs. In addition, however, direct detection of 15N resonances for backbone amide groups (15NH) has re-emerged as a means to enhance resolution and sensitivity for systems that experience rapid amide group 1H relaxation, including high molecular weight proteins [4]. However, the application of 15N detection to IDPs has not been widely explored. To assess the benefits and limitations of 13C and 15N detection for IDPs, we analyzed 1HN, 13C′, and 15NH resonance line shapes and compared detection schemes for the most commonly used 2D correlation experiments, including 2D 1H-15N and 13C-15N correlation experiments. As test cases, we investigated the central, Arf binding IDR of the E3 ubiquitin-protein ligase Hdm2 (Hdm2-ABD; residues 210 to 304) [5], a 182 amino acid, low complexity region of Surfeit locus protein 6 (Surf6-N; residues 1 to 182) [6], and a 130 amino acid region of Nucleophosmin (NPM1) that contains the pentamerization domain (residues 13–119) and two flanking IDRs (N130; residues 1 to 130) [7].
2. Materials and Methods
2.1. Protein Expression and purification
Hdm2-ABD
The Arf binding domain of Hdm2 (residues 210–304) with an N-terminal polyhistidine tag was expressed in Escherichia coli (E. coli) BL21 (λDE3) from the pET28a expression vector (Novagen) as described previously [5]. 13C/15N-labeled Hdm2-ABD was expressed using MOPS-based minimal media [8] containing [13C] D-glucose and 15NH4Cl (Cambridge Isotope Laboratories). Cultured cells were harvested by centrifugation and lysed in 25 mM Tris HCl (pH 8.0), 500 mM NaCl, 5 mM β-mercaptoethanol (BME), and protease inhibitor cocktail (Sigma) by sonication. Lysates were clarified by centrifugation and Urea was added to the clarified extract to a concentration of 3 M. His-tagged Hdm2-ABD was purified by Ni2+-NTA affinity chromatography and eluted with buffer containing 6 M urea and 0.5 M Imidazole. Fractions containing Hdm2-ABD were dialyzed against 25 mM Tris HCl (pH 8.0), 150 mM NaCl, 5 mM BME and treated with thrombin to cleave the His tag. Cleaved Hdm2-ABD was buffer exchanged by dialysis into 25 mM sodium phosphate (pH 7.0), 50 mM NaCl and further purified using anion-exchange chromatography (Q Sepharose; Amersham Pharmacia Biotech, Inc.) using a linear gradient of 0.05′1 M NaCl over 0.1 L. NMR experiments were performed at a protein concentration of 1 mM in 25 mM Sodium Phosphate pH 6.0, 10 mM NaCl, 0.03 % NaN3, and 10% D2O.
Surf6-N
Surf6-N (residues 1–182 of human Surf6) was cloned into a pET28a expression vector encoding an N-terminal polyhistidine tag and a tobacco etch virus protease (TEV) cleavage site. E. coli Rosetta2 (DE3) cells were grown at 37 oC in MOPS based minimal media containing [13C] D-glucose and 15NH4Cl (Cambridge Isotope Laboratories) to an optical density at 600 nm of 1.0 and expression was induced by the addition of 0.4 mM Isopropyl β-D-1-thiogalactopyranoside (IPTG) followed by incubation at 37 oC for an additional 3 hours. Cultured cells were harvested by centrifugation. Surf6-N was purified from inclusion bodies using the following procedure. Cells were lysed in 25 mM Tris HCl (pH 8.0), 500 mM NaCl, 5 mM β-mercaptoethanol (BME), 0.1% Triton-1000, and protease inhibitor cocktail (Sigma). The crude lysates were clarified by centrifugation and the supernatant was removed. The insoluble fraction was resuspended in buffer containing 8M GuHCl and disrupted by sonication. Following an additional round of centrifugation, Surf6-N was purified by Ni2+-NTA affinity chromatography and eluted with buffer containing 6 M urea and 0.5 M Imidazole. Fractions containing Surf6-N were dialyzed against 25 mM Tris HCl (pH 8.0), 500 mM NaCl, 5 mM BME to remove the urea and treated with TEV protease to cleave the His tag. Cleaved Surf6-N was mixed with 6M urea buffer and passed over Ni2+ resin to remove the His tag and TEV protease. The column flow-through fraction was then concentrated by ultracentrifugation using a 10 kDa MWCO filter (Amicron) and further purified by HPLC using an H2O/CH3CN/0.1% trifluoroacetic acid solvent system. NMR experiments were performed at a protein concentration of 1 mM in 25 mM Sodium Phosphate pH 7.0, 500 mM NaCl, 5 mM DTT, 0.03 % NaN3, and 10% D2O.
N130
N130 (human NPM1 residues 1–130) was expressed in E. coli as described previously [7]. N130 was purified via Ni2+-NTA affinity chromatography, followed by proteolytic removal of the polyhistidine tag by TEV protease followed by HPLC using an H2O/CH3CN/0.1% trifluoroacetic acid solvent system. NMR experiments were performed at a protein concentration of 400 μM in 10 mM Tris HCl pH 7.0, 150 mM NaCl, 2 mM DTT, 0.03 % NaN3, and 10% D2O.
NMR Spectroscopy
All experiments were collected at 298 K on a Bruker AVANCE NEO spectrometer operating at a proton frequency of 800 MHz equipped with a TXO cryoprobe optimized for 13C. For 1D spectra, processing (including Fourier transform and phase correction) and peak integration was performed by global spectral deconvolution using MestReNova software (Mestrelab Research). 2D Spectra were acquired with acquisition times corresponding to >3 X T2 and >1 X T2 in the F2 and F1 dimensions, respectively. 2D spectra were processed using data points corresponding to 3.14 X T2 and 1 X T2 in the F2 and F1 dimensions, respectively, and each dimension was zero-filled to the next power of 2. Apodization was performed by applying a cosine function (Shifted-Sine Bell; SSB = 2) and forward linear prediction was applied to complex data using 32 coefficients. 2D spectra were processed in Topspin 4.0 (Bruker). For 2D spectra, peak picking and integration by Gaussian fitting was performed using the program Sparky (UCSF).
3. Results
3.1. Choosing the Optimal Nucleus for Direct Detection; Analysis of Lineshapes in 1D NMR spectra for a Prototypical IDR, Hdm2-ABD
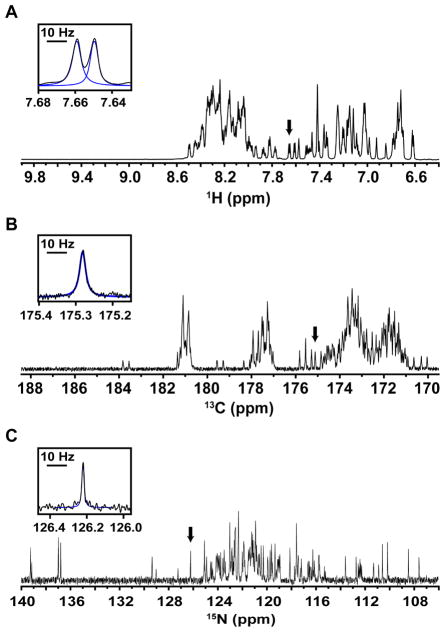
Backbone atoms in IDPs generally experience rapid local motions (e.g., local correlation times of <6 ns] [9]) that are uncorrelated with those in distal protein regions, giving rise to large transverse relaxation times (T2 values) and sharp resonances. This is exemplified in 1D spectra for 13C/15N–labeled Hdm2-ABD; for example, ΔVFWHH values for 1HN resonances were 4.7 ± 0.6 Hz (Fig. 1A, Fig. S1, Table S1), those for 13C′ resonances were 5.4 ± 1.5 Hz (Fig. 1B, Fig. S2, Table S1), and, finally, those for 15NH resonances were 1.2 ± 0.3 Hz (Fig. 1C, Fig. S3, Table S1), by far the sharpest. The ΔvFWHH values are consistent with theoretical predictions for 1HN, 13C′, and 15NH transverse relaxation rates for these nuclei in a uniformly 13C/15N-labelled protein at 800 MHz [2] and highlight the potential of 15N-detection for yielding narrow linewidths and optimal spectral resolution for IDPs.
Figure 1.
1D NMR lineshape analysis for 13C/15N Hdm2-ABD. (A) The amide region of a 1D 1H spectrum. (B) The carbonyl region of a 1D 13C spectrum. (C) A 1D 15N spectrum showing amide nitrogen resonances. Insets in each panel provide a detailed view of well resolved resonances, as indicated by the black arrows, along with the fits from global spectral deconvolution (blue; see Methods). The black bar corresponds to a width of 10 Hz. It should be noted that the carbonyl region was chosen here to assess the obtainable resolution of 13C′ direct detection. 13C′ linewidths are influenced by magnetic field strength due to chemical shift anisotropy and should not be used to generally assess all 13C linewidths.
3.2. Choosing the Optimal Heteronuclear Correlation Scheme; Comparison of 2D Spectra for Disordered Proteins of Varying Size and Sequence Complexity
We next evaluated the resolution and sensitivity associated with different detection schemes for recording 2D 1H-15N and 13C-15N correlation spectra for several IDRs. These two types of 2D spectra were chosen for analysis here because they are the basis for commonly used multi-dimensional NMR experiments used for establishing backbone resonance assignments. For 2D 1H-15N correlation spectra, the 1H-detected 1H-15N HSQC [10] (Fig. S4) and 15N-detected 1H-15N INEPT (Fig. S5) experiments were used. For 2D 13C-15N correlation spectra, the 13C-detected CON-IPAP experiment [3] (Fig. S6) and 15N-detected CON experiment [11] (Fig. S7) were used.
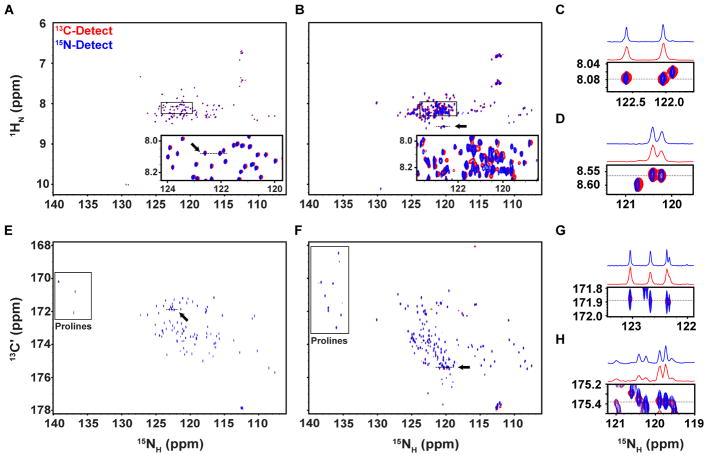
The results for 2D 1H-15N correlation experiments with Hdm2-ABD, recorded for similar times (~5 hours for each) showed that most non-proline resonances (96 expected resonances) were resolved using either detection scheme, although the average S/N ratio for the 1H-detected spectrum was >16-fold greater than for the 15N-detected spectrum (Fig. 2A,C; Table 1). The poor spectral dispersion associated with IDPs, especially in the 1H dimension of 2D 1H-15N correlation spectra, can limit spectral resolution. However, given the relatively narrow 15NH resonances for Hdm2-ABD (~1 Hz), spectral resolution for the 1H-deteced spectrum was sufficient to resolve 88 out of 96 possible resonances. With the acquisition and apodization parameters used for the 1H-detected 2D spectrum, the resonance linewidths were ~15 Hz in the 1H dimension and ~6 Hz in the 15N dimension. When directly detecting 15N magnetization, 91 of the 96 possible resonances were resolved owing to the enhancement in 15NH linewidths, which were ~3 Hz using our standardized acquisition scheme. These results demonstrate that for small, well behaved IDRs like Hdm2-ABD, the majority of resonances may be resolved using 2D 1H-15N correlation experiments, with 15N-detection offering superior resolution although with dramatically reduced sensitivity.
Figure 2.
Comparison of 2D heteronuclear correlation spectra for 13C/15N Hdm2-ABD and 13C/15N Surf6-N. (A) Overlaid 1H- (red) and 15N-detected (blue) 2D 1H-15N correlation spectra for Hdm2-ABD. The boxed region is shown enlarged with contours in the 1H-detected spectrum scaled by 0.5 for clarity (A, inset) (B) Overlaid 1H- (red) and 15N-detected (blue) 2D 1H-15N correlation spectra for Surf6-N. The boxed region is shown enlarged with contours in the 1H-detected spectrum scaled by 0.5 for clarity. Chemical shift perturbations were observed due to sample heating during decoupling (B, inset) (C) Zoomed view of a region from panel A, as indicated by the black arrow, with 1D projections along 8.08 ppm in the 1H dimension (dotted line) highlighting the differences in 15N linewidths. (D) Zoomed view of a region from panel B (black arrow), with 1D projections along 8.57 ppm in the 1H dimension (dotted line) (E) Overlaid 13C- (red) and 15N-detected (blue) 2D 13C’-15N correlation spectra for Hdm2-ABD. (F) Overlaid 13C- (red) and 15N-detected (blue) 2D 13C’-15N correlation spectra for Surf6-N. In panels E and F, proline resonances are denoted by the boxed regions. (G) Zoomed view of a region from panel E (black arrow), with 1D projections along 171.9 ppm in the 13C dimension (dotted line). (H) Zoomed view of a region from panel F (black arrow), with 1D projections along 175.4 ppm in the 13C dimension (dotted line). Axes for 1H and 13 C-detected spectra have been rotated for clarity.
Table 1.
Acquisition parameters and observed spectral features for 2D heteronuclear correlation experiments performed on 13C/15N Hdm2-ABD.
| Experiment | 1H,15N-HSQC | N_HNINEPT | 13C-CON | 15N-CON |
|---|---|---|---|---|
| Points (F2 x F1) | 4096 x 1500 | 5120 x 548 | 1024 x 1500 | 5120 x 136 |
| Acquisition time (ms) | 225 x 264 | 828 x 68 | 177 x 264 | 886 x 24 |
| Scans | 8 | 16 | 8 | 124 |
| Recycle Delay (s) | 1 | 1 | 1 | 1 |
| Exp Time (hrs:min) | 4:36 | 4:46 | 9:17 | 9:13 |
| <FWHH 1HN> (Hz) | 15.1 ± 0.9 (n = 33) | 15.3 ± 1.0 (n = 41) | -- | -- |
| <FWHH 13C′> (Hz) | -- | -- | 4.9 ± 2.1 (n = 41) | 21.7 ± 1.8 (n = 35) |
| <FWHH 15NH> (Hz) | 6.1 ± 1.3 (n = 33) | 2.9 ± 1.1 (n = 41) | 10.2 ± 1.0 (n = 41) | 2.8 ± 0.8 (n = 35) |
| Signal-to-noise | 3878 | 240 | 169 | 42 |
| # peaks (observed/possible) | 88/96 | 91/96 | 96/99 | 98/99 |
In contrast to Hdm2-ABD, Surf6-N is a 182 residue-long IDR that exhibits low sequence complexity and self-association that leads to broadening of some resonances. Consequently, the average ΔFWHH values from 1D NMR lineshape analysis were larger than those measured for Hdm2-ABD, with values of 15.4 ± 1.8 Hz, 6.18 ± 1.2 Hz, and 3.4 ± 2.4 Hz for 1HN, 13C′, and 15NH resonances, respectively (Fig. S8, Table S2). Note that, due to extensive resonance overlap, 1HN line widths were determined using a very high resolution 2D 1H-15N HSQC spectrum (Fig. S8 A,D). Furthermore, the 1H-detected 2D 1H-15N HSQC spectrum (that used 3.14 X T2 data points in the 1H dimension) exhibited extensive resonance overlap (Fig. 2B,D). Resonance linewidths were ~26 Hz in the 1HN dimension and ~10 Hz in the 15NH dimension, permitting resolution of 142 of the 177 possible resonances. Using 15N-detection, the resonance linewidths were ~31 Hz in the 1HN dimension and ~7 Hz in the 15NH dimension, yielding an additional 13 well resolved resonances (Table 2). However, it should be noted that chemical shift perturbations were observed for several resonances as a result of sample heating (Fig. 2B, inset, Fig. S9), presumably due to the high decoupling pulse power applied during detection that was exacerbated by the high salt concentration in the sample buffer used (500 mM NaCl). This demonstrates that for a large IDR, spectral crowding can be partially overcome through the enhanced resolution provided by 15N-detection. In this case, probably due to the high salt concentration [12] or the differential effects of transient self-association on 1HN and 15NH line widths, the signal-to-noise advantage of detecting 1H was only ~6.5 (versus ~16 for the corresponding spectra for Hdm2-ABD) (Table 1). This example illustrates the potential of 15N-detection to improve resolution in 2D1H-15N correlation spectra of challenging IDRs such as Surf6-N.
Table 2.
Acquisition parameters and observed spectral features for 2D heteronuclear correlation experiments performed on 13C/15N Surf6-N.
| Experiment | 1H,15N-HSQC | N_HNINEPT | 13C-CON | 15N-CON |
|---|---|---|---|---|
| Points (F2 x F1) | 2048 x 510 | 2048 x 154 | 1024 x 510 | 2048 x 104 |
| Acquisition time (ms) | 113 x 92 | 369 x 21 | 236 x 92 | 369 x 24 |
| Scans | 16 | 48 | 32 | 296 |
| Recycle Delay (s) | 1 | 1 | 1 | 1 |
| Exp Time (hrs:min) | 2:40 | 2:54 | 12:22 | 12:25 |
| <FWHH 1HN> (Hz) | 26.1 ± 2.0 (n = 35) | 30.7 ± 2.1 (n = 31) | -- | -- |
| <FWHH 13C′> (Hz) | -- | -- | 7.5 ± 0.8 (n = 34) | 20.9 ± 0.8 (n = 34) |
| <FWHH 15NH> (Hz) | 9.7 ± 1.5 (n = 35) | 7.3 ± 1.6 (n = 31) | 9.7 ± 1.5 (n = 34) | 5.8 ± 1.0 (n = 34) |
| Signal-to-noise | 923 | 143 | 142 | 80 |
| # peaks (observed/possible) | 142/177 | 155/177 | 161/185 | 162/185 |
For IDPs, 2D 13C-15N correlation spectra provide enhanced chemical shift dispersion and tolerance to small variations in pH (in comparison with 2D 1H-15N correlation spectra) and, importantly, resonances for proline residues [13]. For Hdm2-ABD, 2D 13C- 15N correlation experiments recorded for similar times (~9 hours for each) show that almost all resonances were resolved using either detection scheme, although the average S/N ratio for the 13C-detected spectrum was ~4-fold greater than that for the 15N-detected spectrum (Fig. 2E,G). For the 13C-detected spectrum, the average resonance linewidths were ~5 Hz in the 13C’ dimension and ~10 Hz in the 15NH dimension, yielding 96 of the 99 possible resonances. For the 15N-detected spectrum, average resonance linewidths were ~3 Hz in the 15NH dimension and ~22 Hz in the indirect 13C’ dimension. Despite the limited resolution enforced by constant-time editing in the indirect 13C dimension, the 15N-detected 13C-15N correlation spectrum provided the largest number of resolved resonances (98 of 99).
For the 13C-detected 13C-15N correlation spectrum for Surf6-N, the average resonance linewidths were ~8 Hz in the 13C’ dimension and ~10 Hz in the 15NH dimension, yielding 161 of the 185 possible resonances. For the 15N-detected spectrum, average resonance linewidths were ~6 Hz in the 15NH dimension and ~21 Hz in the indirect 13C’ dimension, yielding 162 of 185 possible resonances. Furthermore, all the resonances that were observed in the 13C-detect 13C-15N correlation spectrum were also observed in the 15N-detected spectrum, with the exception of two resonances, presumably due to the lower sensitivity of 15N detection. Therefore, in this case, where 15NH and 13C’ linewidths are more similar than with Hdm2-ABD, 15N-detection provides only a slight resolution advantage and a less than 2-fold reduced S/N ratio (Table 2).
Taken together, our results show that for two IDRs that are characterized by rapid backbone conformational fluctuations and correspondingly slow transverse relaxation, 15NH linewidths were narrower than 1HN and 13C′, thus allowing 15N-detection of 2D correlation spectra to afford the highest resolution at the expense of lower S/N. The S/N differential for 1H- versus 15N- detected 2D 1H-15N correlation spectra is much greater than for 13C- versus 15N- detected 2D 13C-15N correlation spectra. Therefore, 15N-detection may be reserved for cases where the highest possible resolution in 2D 1H-15N correlation spectra is needed and the protein of interest can be prepared at high concentration (> 1 mM). In contrast, 15N-detection may be optimally used to record 2D 13C-15N correlation spectra when 15NH line widths are significantly narrower than those for 13C’ nuclei, in which case the resolution advantages of 15N-detection can be manifested. Estimates of 13C’ and 15NH line widths can be made from directly-detected 1D spectra and used to decide which detection strategy, 13C versus 15N, is likely to yield the highest resolution 2D 13C-15N correlation spectra. Based upon our results, we conclude that 15N-detection should be considered for the analysis of IDPs that exhibit poor resonance dispersion due to large size and/or low sequence complexity.
3.3. Comparison of 1H- and 15N-detected TROSY-enhanced 2D 1H-15N Correlation Spectra for N130, a High Molecular Weight Folded Protein with Disordered Regions
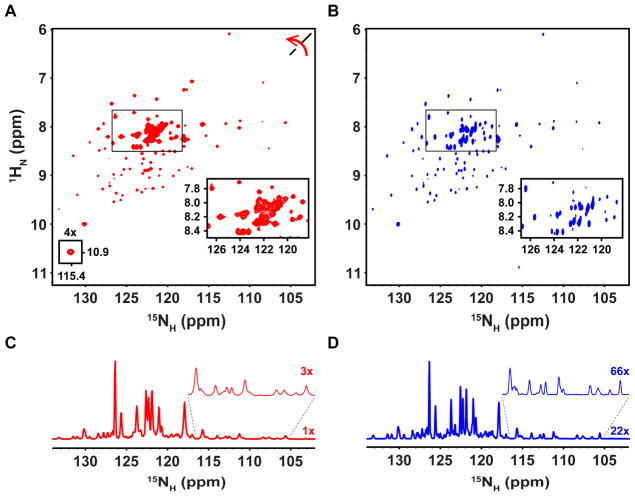
The development of transverse relaxation-optimized spectroscopy (TROSY) [14] dramatically extended the size limits of detection of 2D 1H-15N correlation spectra for large molecular weight proteins. Additionally, the BEST-TROSY strategy that affords improved sensitivity and resolution was exploited in a set of multi-dimensional experiments to obtain sequential assignments for large IDPs [15]. Historically, TROSY has been utilized for 1H-detection experiments; however, the long T2 values of 15NH resonances have prompted the recent development of 15N-detected TROSY experiments [4, 12]. Therefore, we tested 1H- and 15N-detection to record TROSY-enhanced 2D 1H-15N correlation spectra (Fig. S10 and S11, respectively) for the N-terminal oligomerization domain of Nucleophosmin (NPM1; termed N130), a 73.4 kDa homopentamer (residues 13–119, with a Tc value of 53.6 ± 0.9 ns) which also has two short IDRs (residues 1–13 and 120–130, with Tc values of 2.10 ± 0.05 ns for the latter segment) [16]. The first increments of 1H- and 15N-detected 1H-15N TROSY-enhanced correlation spectra were analyzed to estimate the T2 values of resonances in the pentamerization domain (Fig. S12, Table S3); the average ΔFWHH values of TROSY-enhanced 1HN and 15NH resonances were 22.7 ± 7.0 Hz and 7.6 ± 0.8 Hz, respectively. These values were used to establish acquisition parameters for the two 2D spectra according to our standard scheme (see Methods). For the 1H-detected TROSY-enhanced 2D 1H-15N correlation spectrum, the average resonance linewidths were ~38 Hz and ~23 Hz in the 1HN and 15NH dimensions, respectively, for residues within the pentamerization domain and ~31 Hz and ~17 Hz, respectively for IDR residues (Fig. 3A,C; Table 3). The corresponding 15N-detected 2D spectrum yielded slightly higher resolution albeit with lower sensitivity (~3-fold less). For example, the average resonance linewidths were ~43 Hz and ~15 Hz in the 1HN and 15NH dimensions, respectively, for residues within the pentamerization domain and ~39 Hz and ~12 Hz, respectively for IDR residues (Fig. 3B,D; Table 3). These results illustrate the benefits of 15N detection for realizing the resonance narrowing effects of TROSY, especially for 15NH resonances of the large pentamerization domain. In addition, 15NH resonances for residues in the IDRs were narrower in the 15N-detected 2D spectrum, illustrating the resolution advantage associated with 15N detection for a large protein comprised of both a folded domain and IDRs.
Figure 3.
Comparison of 1H- and 15N-detected TROSY-enhanced 2D 1H-15N correlation spectra for 2H/15N N130. (A) 1H-detected TROSY-enhanced 2D 1H-15N correlation spectrum of N130, where the axes have been rotated for clarity. The small inset reveals a weak resonance (contoured at 4X). (B) 15N-detected TROSY-enhanced 2D 1H-15N correlation spectrum of N130. The boxed regions in (A) and (B) are enlarged in the insets to show spectral regions containing resonances for IDR residues. (C) 1D projection in of 15N dimension of the 1H-detected spectrum in (A) showing 15NH linewidths. The expanded region of the spectrum, which contains pentamerization domain resonances, is scaled 3x for clarity. (D) 1D projection in the 15N dimension of the 15N-detected spectrum in (B) showing 15NH linewidths at a scale of 22x. The expanded region of the spectrum containing pentamerization domain resonances is shown at a scale of 66x for clarity.
Table 3.
Acquisition parameters and observed spectral features for 2D heteronuclear correlation experiments performed on 2H/15N N130.
| Experiment | 1H-TROSY | 15N-TROSY |
|---|---|---|
| Points (F2 x F1) | 1024 x 218 | 1024 x 136 |
| Acquisition time (ms) | 45 x 42 | 198 x 14 |
| Scans | 64 | 96 |
| Recycle Delay (s) | 1 | 1 |
| Exp Time (hrs:min) | 4:18 | 4:31 |
| <FWHH 1HN> pentamer (Hz) | 37.8 ± 3.2 (n = 20) | 43.2 ± 3.9 (n = 20) |
| <FWHH 1HN> IDR (Hz) | 31.4 ± 1.9 (n = 8) | 39.1 ± 2.2 (n = 8) |
| <FWHH 15NH> pentamer (Hz) | 22.9 ± 2.6 (n = 20) | 15.2 ± 2.4 (n = 20) |
| <FWHH 15NH> IDR (Hz) | 17.4 ± 2.6 (n = 8) | 11.6 ± 1.7 (n = 8) |
| Signal-to-noise | 115 | 40 |
| # peaks (observed/possible) | 124/124 | 124/124 |
4. Discussion
NMR studies of IDPs at atomic resolution are often challenging due to poor resonance dispersion, especially for backbone amide protons. Further, the general lack of secondary and tertiary structure leads to very similar chemical shift values for sidechain carbons of amino acids of the same type, often limiting the establishment of sequential resonance assignments. The latter factor is exacerbated by the low complexity that is associated with the sequences of many IDPs and IDRs. The advent of cryogenic NMR probes offering high-sensitivity detection of resonances for 13C nuclei led to the development of a new generation of multi-dimensional NMR experiments based upon correlation of resonances for 13C’ and 15NH rather than 1H and 15NH [3]. Because 2D 13C-15N correlation spectra offer improved spectral dispersion for IDPs, and because resonances of often abundant proline residues are detected, derivative multidimensional (≥3D) heteronuclear correlation experiments provided advantages for establishing sequential resonance assignments for IDPs [13, 17–20]. However, those based on sequential linkage of sidechain carbon resonances still suffered from amino acid-type chemical shift degeneracy, as discussed above. To overcome this problem, Showalter and co-workers developed a set of 13C-deteted 3D heteronuclear correlation experiments that allowed resonance assignments to be established on the basis of the chemical shifts of sequential 15NH nuclei [13, 17]. However, the inclusion of two indirect 15N dimensions in these experiments was non-ideal with regard to achieving the highest possible resolution due to the time demands of extensive sampling in the indirect 15N dimensions. Recently, cryogenic NMR probes have become available that offer high sensitivity detection of 15NH resonances in addition to those of 13C′, offering the opportunity to explore the possible advantages of direct 15N detection of spectra of IDPs and IDRs for improved resolution. Here, we compared spectral resolution and sensitivity for 2D 1H-15N and 13C-15N correlation spectra recorded through direct detection of each of the two correlated nuclei for two protein regions that are entirely disordered (Hdm2-ABD and Surf6-N) and 2D 1H-15N correlation spectra for a ~73 kDa folded, pentameric protein with two short IDRs (N130).
Our results for Hdm2-ABD showed that 15NH resonances were by far the narrowest (1.2 ± 0.3 Hz ) amongst those also for 1HN and 13C′ nuclei (4.7 ± 0.6 Hz and 5.4 ± 1.5 Hz, respectively), and that this resolution advantage was manifested in improved resolution in 15N-detected 2D 1H-15N (Fig. 2A,C) and 13C-15N correlation spectra (Fig. 2E,G). However, direct detection of 15N resonances was accompanied by significantly reduced S/N ratios in comparison with the corresponding 1H- and 13C-detected 2D spectra. However, with a ~1 mM protein sample, the two 15N-detected 2D correlation spectra were recorded in reasonable time periods (2D 1H-15N spectrum, ~4 ¾ hours; and 13C- 15N spectrum, 9 ¼ hours; Table 1). These measurement times were influenced by the design of our data sampling scheme, which involved linear sampling in the direct dimensions for >3 x T2 and in the indirect dimensions for >1 x T2 (except for the 15N-detected 2D 13C-15N correlation experiment, which was limited by the need for a constant evolution time for the 13C indirect dimension). This scheme was used to afford a level of resolution in both the directly and indirectly detected dimensions that was scaled to the relaxation properties of the relevant nuclei. This allowed for very high resolution in the directly detected dimensions and moderate resolution in the indirectly detected dimensions and also balanced considerations regarding sensitivity and total acquisition time.
Hdm2-ABD is a relatively small and well-behaved IDR and, despite its extensive disorder [21], yielded well resolved 2D 1H-15N and 13C-15N correlation spectra that did not provide the opportunity to illustrate the full potential of 15N-detection for improved resolution. However, the Surf6-N IDR presented greater resolution challenges due to its larger size (185 versus 99 residues for Surf6-N and Hdm2-ABD, respectively), low sequence complexity (17.7% Ala, 15.5% Lys, 12.6% Glu and 11.0% Arg residues), and tendency for self-association (unpublished results, E. Gibbs and R. Kriwacki). In this case, 15N-detection of the 2D 1H-15N correlation spectrum, which was recorded in ~3 hours, exhibited 155 resolved resonances versus 142 in the corresponding 1H-detected spectrum (Fig. 2B, Table 1). In contrast, nearly identical numbers of resonances were resolved in the 13C- and 15N-detected 2D 13C’-15N correlation spectra for Surf6-N (161 and 162 resonances, respectively). This reflects the limitation on the maximal resolution that can be achieved in the 15N-detected experiment used here due to constant time evolution in the indirect 13C′ dimension (the ΔFWHH value for 13C′ was ~21 Hz in comparison with ~8 Hz in the 13C-detected experiment; Table 1). Despite this limitation, these two spectra illustrate the advantage associated with 2D 13C-15N versus 1H-15N correlation spectra in resolving resonances for a challenging, moderately sized IDR.
We further evaluated the performance of 1H- and 15N-detection of 2D 1H-15N correlation spectra for N130, which is comprised of a highly stable, ~70 kDa pentamerization domain flanked by short N- and C-terminal IDRs [7]. As expected [2, 14], 15NH linewidths for resonances of residues in both the pentamerization domain and IDRs were much narrower than the corresponding 1HN resonances, and this was manifested in enhanced resolution in the 15N-detected 2D spectrum. However, because N130 is a symmetric pentamer, it exhibits only 124 resonances for non-proline residues and does not present the degree of resonance overlap that would be associated with a single polypeptide chain protein comprised of a similar size folded domain and IDRs. In this latter case, 15N-detection is likely to be required to achieve the highest possible spectral resolution in 2D 1H-15N correlation spectra, as discussed by Wagner and co-workers [2, 4].
In summary, recent technical developments enable direct detection of 1H, 13C, and 15N nuclei for isotope-labeled disordered proteins in reasonable time periods given that high concentration (~1 mM) can be achieved. This allows optimization of data acquisition to address the poor spectral dispersion associated with NMR studies of IDPs. Our data for several IDRs shows that the 15NH resonance of amide groups is usually narrower than amide 1HN resonances and 13C’ resonances. Consequently, direct detection of 15NH resonances will afford 2D 1H-15N and 13C-15N correlation spectra with the highest possible resolution. However, this resolution advantage must be balanced against the sensitivity disadvantage associated with the low γ value of 15N versus that for 1H and 13C. Because linewidths for 1HN, 13C’, and 15NH resonances in IDPs can vary widely, we recommend assessing these linewidths for each protein and to choose a detection scheme accordingly. The amide 15NH resonance linewidth will usually be much narrower than the corresponding 1HN linewidth, favoring 15N detection for high resolution when high protein concentration can be achieved. Alternatively, for proteins at lower concentrations (significantly less than 1 mM), 1H detection of the 2D 1H-15N correlation spectrum with extended sampling in the indirect 15N dimension may give the best compromise between resolution and sensitivity. In contrast to 2D 1H-15N correlation spectra, 2D 13C-15N correlation spectra allow detection of resonances for proline residues, and also afford improved spectral dispersion for IDPs due to the broader range of chemical shift values for 13C’ and 15NH resonances versus 15HN resonances. Our results showed that the linewidths of 13C’ resonances were 2-fold or less large than those for 15NH resonances, reducing the resolution benefits associated with direct 15N detection. Nonetheless, direct 15N detection was associated with slightly improved resolution in 2D 13C-15N correlation spectra for Hdm2-ABD and Surf6-N (Tables 1 and 2) and these advantages may be more dramatically manifested in studies of larger IDPs displaying highly crowded 2D 13C-15N correlation spectra. In our experiments, we employed linear data sampling in the indirect dimensions of 2D correlation spectra and advocate optimizing this to match the relaxation behavior of the sampled nuclei. The use of non-uniform sampling in the indirect dimensions and data processing using other than Fourier transformation [22–24] are likely to further extend the resolution benefits of direct 15N detection and also improve sensitivity, and should be utilized in the future. For 15N-detected 2D 13C-15N correlation spectra, in particular, resolution enhancements may be afforded by using D2O buffer [2] and greater sensitivity may be achieved using magnetization transfer pathways starting from H-alpha in analogy to 13C-detected 13C- 15 N correlation spectra described previously [18]. In conclusion, as had been demonstrated previously by others [3, 13], 2D 13C-15N correlation spectra afford superior spectral dispersion and resolution for IDPs and the advantages of this 2D fingerprint can be enhanced through 15N detection when high protein concentration can be achieved.
Supplementary Material
Highlights.
NMR detection schemes can be tailored to the unique linewidth features of IDPs.
15N-detection often offers enhanced resolution over 1H- or 13C-detection schemes.
High resolution 15N-detection is balanced by intrinsically lower S/N.
Acknowledgments
The authors thank Mr. Cheon-Gil Park and Dr. Diana Mitrea for assistance with protein preparation; Drs. Grace Royappa, Youlin Xia, and Aaron Phillips for assistance with NMR spectroscopy; and Dr. Haribabu Arthanari (Harvard Medical School) for assistance with NMR spectroscopy and fruitful discussion. This work was supported by NIH R01CA092035 and 1R01GM115634 (to R.W.K.); National Cancer Institute Cancer Center Support Grant P30CA21765 (to SJCRH); and ALSAC (to SJCRH). E.G. is a recipient of an NIH Research Supplement to Promote Diversity in Health-related Research (R01GM115634-02S1).
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
A supplemental document containing Figures S1–S12 and Tables S1–S3 is provided.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gibbs EB, Cook EC, Showalter SA. Application of NMR to studies of intrinsically disordered proteins. Arch Biochem Biophys. 2017;628:57–70. doi: 10.1016/j.abb.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Takeuchi K, Gal M, Shimada I, Wagner G. Low-gamma Nuclei Detection Experiments for Biomolecular NMR. Rsc Biomol Sci. 2012;25:25–52. [Google Scholar]
- 3.Bermel W, Bertini I, Felli IC, Piccioli M, Pierattelli R. C-13-detected protonless NMR spectroscopy of proteins in solution. Prog Nucl Mag Res Sp. 2006;48:25–45. [Google Scholar]
- 4.Takeuchi K, Arthanari H, Shimada I, Wagner G. Nitrogen detected TROSY at high field yields high resolution and sensitivity for protein NMR. J Biomol Nmr. 2015;63:323–331. doi: 10.1007/s10858-015-9991-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bothner B, Lewis WS, DiGiammarino EL, Weber JD, Bothner SJ, Kriwacki RW. Defining the molecular basis of Arf and Hdm2 interactions. J Mol Biol. 2001;314:263–277. doi: 10.1006/jmbi.2001.5110. [DOI] [PubMed] [Google Scholar]
- 6.Magoulas C, Zatsepina OV, Jordan PWH, Jordan EG, Fried M. The SURF-6 protein is a component of the nucleolar matrix and has a high binding capacity for nucleic acids in vitro. Eur J Cell Biol. 1998;75:174–183. doi: 10.1016/S0171-9335(98)80059-9. [DOI] [PubMed] [Google Scholar]
- 7.Mitrea DM, Grace CR, Buljan M, Yun MK, Pytel NJ, Satumba J, Nourse A, Park CG, Babu MM, White SW, Kriwacki RW. Structural polymorphism in the N-terminal oligomerization domain of NPM1. P Natl Acad Sci USA. 2014;111:4466–4471. doi: 10.1073/pnas.1321007111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neidhardt FC, Bloch PL, Smith DF. Culture Medium for Enterobacteria. J Bacteriol. 1974;119:736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen MR, Ruigrok RWH, Blackledge M. Describing intrinsically disordered proteins at atomic resolution by NMR. Curr Opin Struc Biol. 2013;23:426–435. doi: 10.1016/j.sbi.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Palmer AG, Cavanagh J, Wright PE, Rance M. Sensitivity Improvement in Proton-Detected 2-Dimensional Heteronuclear Correlation Nmr-Spectroscopy. J Magn Reson. 1991;93:151–170. [Google Scholar]
- 11.Takeuchi K, Heffron G, Sun ZYJ, Frueh DP, Wagner G. Nitrogen-detected CAN and CON experiments as alternative experiments for main chain NMR resonance assignments. J Biomol Nmr. 2010;47:271–282. doi: 10.1007/s10858-010-9430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takeuchi K, Arthanari H, Imai MK, Wagner G, Shimada I. Nitrogen-detected TROSY yields comparable sensitivity to proton-detected TROSY for non-deuterated, large proteins under physiological salt conditions. J Biomol Nmr. 2016;64:143–151. doi: 10.1007/s10858-016-0015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bastidas M, Gibbs EB, Sahu D, Showalter SA. A primer for carbon-detected NMR applications to intrinsically disordered proteins in solution. Concept Magn Reson A. 2015;44:54–66. [Google Scholar]
- 14.Pervushin K, Riek R, Wider G, Wuthrich K. Attenuated T-2 relaxation by mutual cancellation of dipole-dipole coupling and chemical shift anisotropy indicates an avenue to NMR structures of very large biological macromolecules in solution. P Natl Acad Sci USA. 1997;94:12366–12371. doi: 10.1073/pnas.94.23.12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solyom Z, Schwarten M, Geist L, Konrat R, Willbold D, Brutscher B. BEST-TROSY experiments for time-efficient sequential resonance assignment of large disordered proteins. J Biomol Nmr. 2013;55:311–321. doi: 10.1007/s10858-013-9715-0. [DOI] [PubMed] [Google Scholar]
- 16.Mitrea DM, Cika JA, Guy CS, Ban D, Banerjee PR, Stanley CB, Nourse A, Deniz AA, Kriwacki RW. Nucleophosmin integrates within the nucleolus via multimodal interactions with proteins displaying R-rich linear motifs and rRNA. Elife. 2016;5 doi: 10.7554/eLife.13571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sahu D, Bastidas M, Showalter SA. Generating NMR chemical shift assignments of intrinsically disordered proteins using carbon-detected NMR methods. Anal Biochem. 2014;449:17–25. doi: 10.1016/j.ab.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bermel W, Bertini I, Felli IC, Pierattelli R. Speeding Up C-13 Direct Detection Biomolecular NMR Spectroscopy. J Am Chem Soc. 2009;131:15339–15345. doi: 10.1021/ja9058525. [DOI] [PubMed] [Google Scholar]
- 19.Bermel W, Bertini I, Felli IC, Gonnelli L, Kozminski W, Piai A, Pierattelli R, Stanek J. Speeding up sequence specific assignment of IDPs. J Biomol Nmr. 2012;53:293–301. doi: 10.1007/s10858-012-9639-0. [DOI] [PubMed] [Google Scholar]
- 20.Novacek J, Haba NY, Chill JH, Zidek L, Sklenar V. 4D Non-uniformly sampled HCBCACON and (1) J(NC alpha)-selective HCBCANCO experiments for the sequential assignment and chemical shift analysis of intrinsically disordered proteins. J Biomol Nmr. 2012;53:139–148. doi: 10.1007/s10858-012-9631-8. [DOI] [PubMed] [Google Scholar]
- 21.Sivakolundu SG, Nourse A, Moshiach S, Bothner B, Ashley C, Satumba J, Lahti J, Kriwacki RW. Intrinsically Unstructured Domains of Arf and Hdm2 Form Bimolecular Oligomeric Structures In Vitro and In Vivo. J Mol Biol. 2008;384:240–254. doi: 10.1016/j.jmb.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Novacek J, Zidek L, Sklenar V. Toward optimal-resolution NMR of intrinsically disordered proteins. J Magn Reson. 2014;241:41–52. doi: 10.1016/j.jmr.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Mobli M, Hoch JC. Nonuniform sampling and non-Fourier signal processing methods in multidimensional NMR. Prog Nucl Mag Res Sp. 2014;83:21–41. doi: 10.1016/j.pnmrs.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hyberts SG, Arthanari H, Robson SA, Wagner G. Perspectives in magnetic resonance: NMR in the post-FFT era. J Magn Reson. 2014;241:60–73. doi: 10.1016/j.jmr.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.