Abstract
Objective
The human corneal epithelium expresses both the insulin-like growth factor type 1 receptor (IGF-1R) and the IGF-1R/insulin receptor (INSR) hybrid. Despite the previous identification of IGF-1 in human tear fluid, little is known regarding the regulation of IGF-1 in tear fluid and its role in corneal epithelial homeostasis. In the present study, we investigated the impact of biological parameters on the concentration of human tear levels of IGF-1.
Design
Tear levels of IGF-1 were measured in 41 healthy, human volunteers without any reported symptoms of dry eye. All volunteers underwent standard biomicroscopic examination of the cornea and tear film. In a subgroup of volunteers, corneal staining with sodium fluorescein, tear film break up time and tear production using a Schirmer’s test strip were measured to assess clinical signs of dry eye. Tears were collected from the inferior tear meniscus using glass microcapillary tubes and IGF-1 levels were measured using a solid phase sandwich ELISA.
Results
Tear levels of IGF-1 were highest in young adults and significantly decreased in older adults (P=0.003). There were no differences in tear IGF-1 between males and females (P=0.628). Tear IGF-levels were correlated with tear film break up time (R=0.738) and tear production (R=0.826).
Conclusions
These data indicate that there is a progressive decline in tear IGF-1 due to aging that is associated with clinical signs of dry eye. This effect is likely due to age-related changes in the lacrimal gland.
Keywords: tears, IGF-1, dry eye, aging
INTRODUCTION
Dry eye disease is thought to affect millions of adults in the United States with an estimated prevalence ranging from 5 – 50%.[34] Severe dry eye is associated with significant morbidity and negatively impacts quality of life. Common risk factors for dry eye disease include systemic disease, female sex, and advanced age; however, multiple factors including medications, contact lens wear and ocular surgery can also contribute to dry eye.[23, 24, 30, 34, 36] For a complete review on risk factors for dry eye disease, the reader is directed to two recent reviews on pathogenicity and iatrogenic causes of dry eye.[3, 11] Dry eye disease can be classified as aqueous deficient dry eye defined by insufficient tear production from the lacrimal gland, and evaporative dry eye, due to alterations in the lipid composition of the tear film. At the proteomic level, the healthy tear film is composed of a few high abundant tear proteins, including lysozyme, lactoferrin and immunoglobulin, which exist in mg/ml concentrations.[9, 31, 37, 41] These highly abundant proteins constitute more than 80% of the tear film proteome.[10] This is followed by moderately abundant proteins, which approximate concentrations in the ug/ml range and are easily identified through mass spectrometry. Finally, low abundant proteins range from ng/ml to pg/ml concentrations and are often masked by more abundant proteins, requiring sensitive assays such as enzyme linked immunoassays (ELISAs) for individual identification and quantification.
Insulin-like growth factor-1 (IGF-1) is a pleiotropic growth factor with known roles in proliferation, migration and survival of epithelial cells.[5, 17, 45, 46] While both the IGF-1 receptor (IGF-1R) and the IGF-1R/insulin receptor (INSR) hybrid (Hybrid-R) are abundantly expressed in the corneal epithelium, the role of IGF-1 in maintaining normal corneal epithelial homeostasis is unclear.[26, 32, 45] Our in vitro studies indicate that IGF-1, and not insulin, activate IGF-1R and Hybrid-R to regulate cell proliferation. In a prior study, we reported the presence of IGF-1 and its principal binding protein, IGF-binding protein 3 (IGFBP-3), in healthy and diabetic human tears.[44] We further showed that the increased ratio between IGFBP-3 and IGF-1 in diabetic tears is sufficient to inhibit IGF-1 activation of the IGF-1R.
Studies characterizing changes in the IGF-1 axis indicate that measured levels of IGF-1 in serum are influenced by both age and sex.[12, 14, 39] The impact of biological variables on tear concentration of IGF-1 is unknown. Given the multitude of factors that can affect measurement of low abundant growth factors in tears, the purpose of this study was to extend our prior findings and investigate the impact of parameters such as age, sex, and dry eye on tear concentration of IGF-1, a low abundant growth factor present in tear fluid.
MATERIALS AND METHODS
This study conformed to the tenets outlined in the Declaration of Helsinki. All procedures were approved by the Institutional Review Board at the University of Texas Southwestern Medical Center and all patients signed an informed consent prior to participating in the study. A total of 41 patients were recruited for this study. For inclusion, all patients were non-contact lens wearers without any ocular disease or any history of ocular surgery. All patients denied having a diagnosis or any symptoms or complaints of dry eye and none required the use of artificial tears or any other therapy for dry eye. Five µl of tears were collected from the right and then left eye. For this single visit study, all tears were collected between 8 and 10 AM. All volunteers underwent tear collection as the first clinical test. Visualization of a normal tear meniscus height was required for tear collection and inclusion in the study. After allowing sufficient time for tear film recovery, subjects completed a slit lamp examination of the cornea and tear film, including basic dry eye measures of tear film break up time, tear production and corneal staining, as detailed below.
Tear Collection
Using a Haag-Streit slit lamp biomicroscope with low illumination, minimally stimulated basal tear fluid was collected from the inferior temporal tear meniscus using 1 or 2 µl glass microcapillary tubes (Sigma, St. Louis, MO), with care to avoid touching the eye to minimize reflex tearing. A total of 10 µl were collected and pooled from both eyes. Samples were immediately placed on ice and stored at −80C until use.
Assessment of cornea and tear film
Health of the cornea and ocular surface was assessed by a single clinical examiner (DMR). Following a brief biomicroscopic examination to rule out any existing corneal pathology, the ocular surface was evaluated for corneal staining using fluorescein, and measures of tear film break up time (TFBUT) and basal tear production were obtained. For the determination of tear film break up time, two µl of 2.0% non-preserved fluorescein (Greenpark Pharmacy, Houston, TX) were instilled onto the superior bulbar conjunctiva. After 3 normal blinks, the duration between the last blink and the first dark spot was timed using a stopwatch. A total of 3 readings were recorded with 30 second rest periods in between and values were averaged. Immediately following, the cornea was evaluated for staining. Corneal staining was graded using a modified-NEI approach that consisted of assessment of corneal staining in five regions using a scale of 0–3 in 0.1 increments.[1, 18, 29] For all fluorescein-based measurements, a Wratten #12 filter was used. As the last clinical test, basal tear production was assessed using a Shirmer’s tear test 1 with anesthesia.[20, 42] The Schirmer’s strip was placed in the lower fornix near the lateral canthus and the subject was instructed to close their eyes. The length of the wetted area after five minutes was measured.[19] All tests were performed for both eyes and the values for the right and left eyes were averaged to achieve a final measurement.
ELISA Assay
Tear levels of IGF-1 were determined from pooled samples collected from the right and left eyes of each patient using a human IGF-I Quantikine ELISA Kit (R&D Systems, Minneapolis, MN), a solid phase sandwich ELISA. For analysis of each sample, 10 µl of tears were diluted in assay buffer to bring the resulting sample volume up to 50 µl. The ELISA was performed according to manufacturer instructions. Final concentrations were measured using a Bio-Rad iMark microplate absorbance reader (Bio-Rad, Hercules, CA) at 540 nm with wavelength correction and calculated from a standard curve of known concentrations of human recombinant IGF-1.
Bicinchoninic Assay
In the subgroup of patients undergoing dry eye testing, an additional 6 µl was collected to allow for measurement of total tear protein. Total protein was measured using a bicinchoninic assay (BCA, Thermo-Fisher Scientific, Richardson, TX) according to manufacturer instructions. Concentration was measured using a Bio-Rad iMARk microplate absorbance reader at 562 nm.
Statistical Analysis
Statistical analysis was performed using Sigma Plot 11.0 (Systat Software, Inc., San Jose, CA). All data are expressed as mean ± standard deviation. A One-way ANOVA was used to test for differences in tear IGF-1 between age ranges. A Mann-Whitney Rank Sum test was used to assess differences in tear IGF-1 levels between sexes. A Pearson’s correlation coefficient was used to test for correlations between variables including tear IGF-1 level and age and between tear IGF-1 and clinical measures of dry eye. A Shapiro-Wilk test was done to test for a normal distribution in age and dry eye measures. A log transformation was performed to achieve a normal distribution for TFBUT. Statistical significance was set at p<0.05.
RESULTS
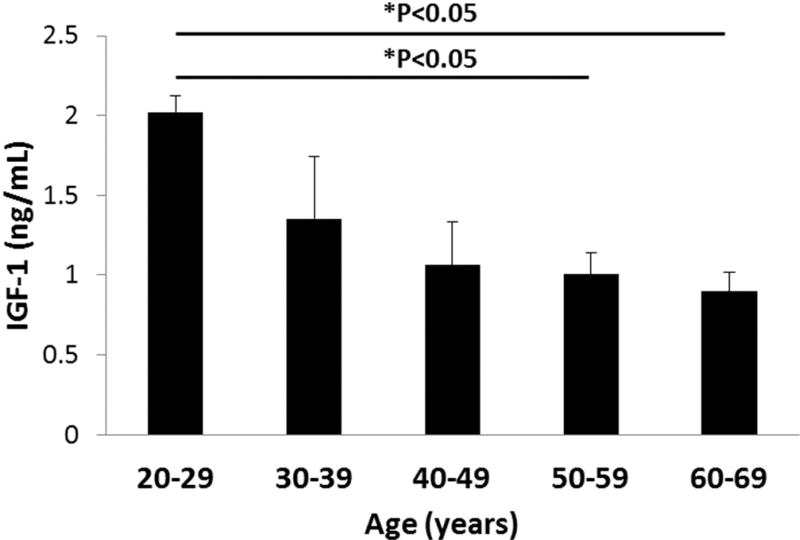
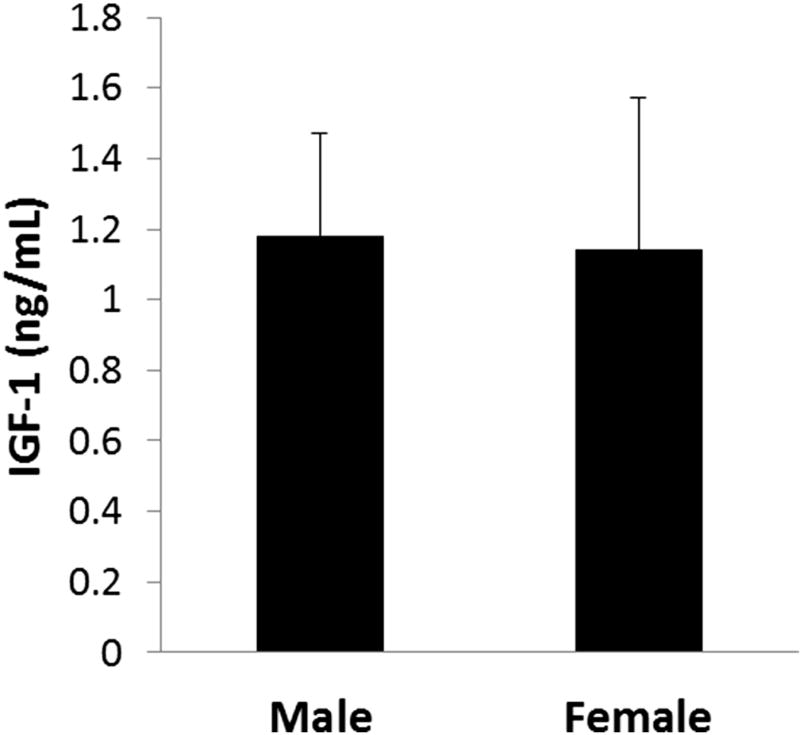
As summarized in Table 1, in the total cohort, 22 patients were female and 19 were male. The mean age was 45.8 ± 10.8 years. Mean tear IGF-1 concentration was 1.16 ± 0.36 ng/ml. When stratified by age, tear IGF-1 was highest in participants aged 20–29 (2.02 ± 0.10 ng/ml) and decreased with each successive decade (Fig. 1). Tear IGF-1 was significantly decreased by 50% in participants aged 50–59 and remained decreased for patients aged 60–69 (P=0.003, One-way ANOVA, Dunn’s post hoc multiple comparisons test). Correlation analysis showed a strong negative correlation between age and tear IGF-1 concentration (R=−0.756, P=0.00009, Pearson’s correlation coefficient). There was no significant difference in tear IGF-1 concentration between females and males, 1.14 ± 0.29 ng/ml and 1.18 ± 0.43, respectively (P=0.628, Mann-Whitney Rank Sum Test, Fig. 2).
Table 1.
Patient demographics
| Age | Sex | |
|---|---|---|
| Study cohort | 45.8 ± 10.8 years (range: 25 – 66) | M = 19 (46.3%) |
| F = 22 (53.7%) | ||
|
| ||
| Subset with ocular surface testing | 35.7 ± 7.4 years (range: 26 – 51) | M = 2 (22.2%) |
| F = 7 (77.8%) | ||
Figure 1.
Tear levels of IGF-1 are decreased as a function of age. Tear levels of IGF-1 were significantly decreased in the 5th and 6th decades compared to the youngest group (*P<0.05, One-way ANOVA, Dunn’s multiple comparison test).
Figure 2.
Tear levels of IGF-1 were unaffected by sex or time of day. There was no difference in tear IGF-1 levels between males and females (P=0.628, Mann-Whitney Rank Sum test).
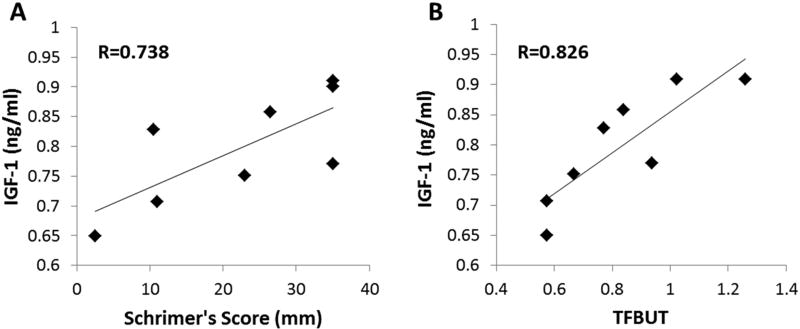
In the subgroup tested for dry eye, 7 patients were female and 2 were male. The mean age in this smaller cohort was 35.7 ± 7.4 years. Mean tear protein concentration in this group was 8.8 ± 2.1 mg/ml, which is consistent with prior reports. None of the patients presented with clinically significant staining (defined as > 3.0/15.0 or 20% using the NEI industry scale). The mean Schirmer’s score for subjects was normal (20.7 ± 13.0 mm) and the mean TFBUT time was 7.4 ± 4.6 seconds. Schirmer’s score was correlated with tear IGF-1 concentration (R=0.738, P=0.0365, Pearson’s correlation coefficient, Fig. 3A). Likewise, TFBUT was also correlated with tear IGF-1 concentration (R=0.826, P=0.0219, Pearson’s correlation coefficient, Fig. 3B).
Figure 3.
Tear IGF-1 levels were correlated to dry eye status. (A) There was a strong correlation between Schirmer’s score and tear IGF-1 (R=0.738, P=0.0365, Pearson’s correlation coefficient). (B) Likewise, there was also a strong correlation between tear film break up time and tear IGF-1 (R=0.826, P=0.0219, Pearson’s correlation coefficient).
DISCUSSION
This is the first study to show an age-related decrease in human tear IGF-1 concentration. The significant 50% decrease in tear IGF-1 that was present in the later decades compared to younger adults confirms an age-related response. This is similar to the age-related decline in serum IGF-1 that has been previously reported.[15, 16] While the liver is the primary source of circulating IGF-1, in the tear film we hypothesize that the age-related decrease in tear IGF-1 may be due to age-related changes in the lacrimal gland. While it is possible that ocular surface epithelia and meibomian glands may contribute in part to tear levels of IGF-1, our hypothesis that this change is due to decreased lacrimal input is supported by the high correlation between tear IGF-1 levels and tear production. This age-related decrease in tear-derived growth factors such as IGF-1 may explain why the topical administration of IGF-1 helps to facilitate epithelial healing in a subset of patients with persistent epithelial defects that are refractory to conventional therapies.[25, 27]
In our prior study, we measured levels of tear IGF-1 in non-diabetic and well-controlled diabetic adults to be 1.2 ng/ml.[44] While we were able to measure a significant increase in the IGF-binding protein IGFBP-3, we did not detect a difference in tear IGF-1 levels between diabetic and non-diabetics participants. Importantly, in the present study, we again found tear IGF-1 levels to be 1.16 ng/ml. This is in good agreement with our prior data and provides further evidence that IGF-1 is present in human tears at very low levels.
The driving stimulus for the production of specific tear proteins is not well characterized. During corneal epithelial wounding, mRNA for epidermal growth factor, keratinocyte growth factor, and hepatocyte growth factor are upregulated in the lacrimal gland.[40] Similarly, others have reported an increase in tear levels of nerve growth factor following wounding in both the wounded and contralateral eyes.[43] Following a meal, serum glucose levels have been shown to influence tear insulin concentration when compared to the fasted state.[6] While fasting can impact serum levels of IGF-1 to some extent, we did not include this parameter in our analysis.[38] Other factors that may impact IGF-1 levels include the density and distribution of IGF-1R and Hybrid receptors across the ocular surface epithelia, as binding of the growth factor to the membrane-bound receptor results in internalization and receptor degradation.
Various methods have been reported for collecting tears, including elution of protein from Schirmer’s strips, eye washing, the inclusion of absorbent materials, and the use of glass microcapillary tubes, as used in the present study.[2, 8, 13, 21, 22, 35] Differences between collection techniques have been shown to influence the number and type of proteins collected.[13, 35] Studies of the tear film proteome using samples obtained from Schirmer’s strips have consistently been shown to yield a higher number of proteins than those in involving capillary tubes.[7, 33, 47] In addition, while proteins collected by microcapillary tube methods have been shown to consist primarily of secreted proteins, proteins eluted from Schirmer’s strips contain both secretory and intracellular proteins.[13] This latter difference is due in part to contact of the test strip with the lower eyelid and significant contamination from dermal keratinocytes and damaged conjunctival epithelia. Similarly, the presence of the test strip can elicit significant amounts of reflex tearing. While microcapillary tubes may reduce the risk of epithelial cell contamination, as seen in the present study, a skilled examiner is required to reduce microtrauma to the ocular surface from the glass tube that can also stimulate reflex tearing. This can be particularly cumbersome in older patients that have a reduced tear volume and may not be possible in patients with severe dry eye disease.
Limitations to the current study include the low concentration of IGF-1 in human tears and the small sample volume collected. The normal volume of the inferior tear meniscus is approximately 3 – 5 µl.[4] Thus, sample volumes greater than this are comprised of high levels of stimulated or reflex tears. This limited our collection to 5 µl of tear sample from both eyes and subsequent pooling for each patient. While we did not assess reflex tearing in this study, our finding of 8.8 µg/µl total protein is consistent with the literature reporting an average of 6 – 11 µg/µl in tear fluid.[28]
Conclusions
In summary, the findings reported herein indicate that the IGF-axis is shifted as a consequence of normal aging. This is likely due to the development of age-related dry eye and reduced secretion of IGF-1 from the lacrimal gland. A larger cohort stratified by participants with mild to severe dry eye symptoms is needed to further elucidate the impact of changes in the IGF-1 axis on normal corneal epithelial homeostasis and in the pathobiology of dry eye disease.
HIGHLIGHTS.
Tear concentration of IGF-1 is significantly decreased during aging.
Tear concentration of IGF-1 is highly correlated with clinical symptoms of dry eye.
Ocular surface damage from age-related dry eye may be due, in part, to a reduction in tear IGF-1.
Acknowledgments
This study was funded by NIH/NEI grants R01 EY024546 (DMR), R21 EY024433 (DMR), a core grant for vision research P30 EY020799, and an unrestricted grant from Research to Prevent Blindness, New York, NY. The funders had no role in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest: none.
References
- 1.Bailey IL, Bullimore MA, Raasch TW, Taylor HR. Clinical grading and the effects of scaling. Invest Ophthalmol Vis Sci. 1991;32:422–432. [PubMed] [Google Scholar]
- 2.Bjerrum KB, Prause JU. Collection and concentration of tear proteins studied by SDS gel electrophoresis. Presentation of a new method with special reference to dry eye patients. Graefes Arch Clin Exp Ophthalmol. 1994;232:402–405. doi: 10.1007/BF00186580. [DOI] [PubMed] [Google Scholar]
- 3.Bron AJ, de Paiva CS, Chauhan SK, Bonini S, Gabison EE, Jain S, Knop E, Markoulli M, Ogawa Y, Perez V, Uchino Y, Yokoi N, Zoukhri D, Sullivan DA. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15:438–510. [Google Scholar]
- 4.Chen F, Shen M, Chen W, Wang J, Li M, Yuan Y, Lu F. Tear meniscus volume in dry eye after punctal occlusion. Invest Ophthalmol Vis Sci. 2010;51:1965–1969. doi: 10.1167/iovs.09-4349. [DOI] [PubMed] [Google Scholar]
- 5.Chitnis MM, Yuen JS, Protheroe AS, Pollak M, Macaulay VM. The type 1 insulin-like growth factor receptor pathway. Clin Cancer Res. 2008;14:6364–6370. doi: 10.1158/1078-0432.CCR-07-4879. [DOI] [PubMed] [Google Scholar]
- 6.Cunha DA, Carneiro EM, Alves MC, Jorge AG, de Sousa SM, Boschero AC, Saad MJ, Velloso LA, Rocha EM. Insulin secretion by rat lacrimal glands: effects of systemic and local variables. Am J Physiol Endocrinol Mebal. 2005;289:E768–E775. doi: 10.1152/ajpendo.00469.2004. [DOI] [PubMed] [Google Scholar]
- 7.de Souza GA, Godoy LM, Mann M. Identification of 491 proteins in the tear fluid proteome reveals a large number of proteases and protease inhibitors. Genome Biol. 2006;7:R72. doi: 10.1186/gb-2006-7-8-r72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esmaeelpour M, Cai JW, Boulton PM, Murphy PJ. Tear sample collection using cellulose acetate absorbent filters. Ophthalmic Physiol Opt. 2008;28:577–583. doi: 10.1111/j.1475-1313.2008.00603.x. [DOI] [PubMed] [Google Scholar]
- 9.Flanagan JL, Willcox MD. Role of lactoferrin in the tear film. Biochimie. 2009;91:35–43. doi: 10.1016/j.biochi.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Fullard RJ, Tucker D. Tear protein composition and the effects of stimulus. Adv Exp Med Biol. 1994;350:309–314. doi: 10.1007/978-1-4615-2417-5_52. [DOI] [PubMed] [Google Scholar]
- 11.Gomes JAP, Azar DT, Baudouin C, Efron N, Hirayama M, Horwath-Winter J, Kim T, Mehta JS, Messmer EM, Pepose JS, Sangwan VS, Weiner AL, Wilson SE, Wolffsohn JS. TFOS DEWS II iatrogenic report. Ocul Surf. 2017;15:511–538. doi: 10.1016/j.jtos.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Gomez JM, Maravall FJ, Gomez N, Navarro MA, Casamitjana R, Soler J. Interactions between serum leptin, the insulin-like growth factor-I system, and sex, age, anthropometric and body composition variables in a healthy population randomly selected. Clinical endocrinology. 2003;58:213–219. doi: 10.1046/j.1365-2265.2003.01698.x. [DOI] [PubMed] [Google Scholar]
- 13.Green-Church KB, Nichols KK, Kleinholz NM, Zhang L, Nichols JJ. Investigation of the human tear film proteome using multiple proteomic approaches. Mol Vis. 2008;14:456–470. [PMC free article] [PubMed] [Google Scholar]
- 14.Kucera R, Topolcan O, Pecen L, Kinkorova J, Svobodova S, Windrichova J, Fuchsova R. Reference values of IGF1, IGFBP3 and IGF1/IGFBP3 ratio in adult population in the Czech Republic. Clinica chimica acta; international journal of clinical chemistry. 2015;444:271–277. doi: 10.1016/j.cca.2015.02.036. [DOI] [PubMed] [Google Scholar]
- 15.Lamberts SW, van den Beld AW, van der Lely AJ. The endocrinology of aging. Science. 1997;278:419–424. doi: 10.1126/science.278.5337.419. [DOI] [PubMed] [Google Scholar]
- 16.Laron Z. The GH-IGF1 axis and longevity. The paradigm of IGF1 deficiency, Hormones. 2008;7:24–27. doi: 10.14310/horm.2002.1111034. [DOI] [PubMed] [Google Scholar]
- 17.Lee HK, Lee JH, Kim M, Kariya Y, Miyazaki K, Kim EK. Insulin-like growth factor-1 induces migration and expression of laminin-5 in cultured human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2006;47:873–882. doi: 10.1167/iovs.05-0826. [DOI] [PubMed] [Google Scholar]
- 18.Lemp MA. Report of the National Eye Institute/Industry workshop on Clinical Trials in Dry Eyes. The CLAO journal : official publication of the Contact Lens Association of Ophthalmologists, Inc. 1995;21:221–232. [PubMed] [Google Scholar]
- 19.Li N, Deng XG, He MF. Comparison of the Schirmer I test with and without topical anesthesia for diagnosing dry eye. International journal of ophthalmology. 2012;5:478–481. doi: 10.3980/j.issn.2222-3959.2012.04.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li N, Deng XG, He MF. Comparison of the Schrimer I test with and without topical anesthesia for diagnosing dry eye. Int J Ophthalmol. 2012;5:475–481. doi: 10.3980/j.issn.2222-3959.2012.04.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez-Cisternas J, Castillo-Diaz J, Traipe-Castro L, Lopez-Solis RO. Use of polyurethane minisponges to collect human tear fluid. Cornea. 2006;25:312–318. doi: 10.1097/01.ico.0000183531.25201.0d. [DOI] [PubMed] [Google Scholar]
- 22.Markouli M, Papas E, Petznick A, Holden B. Validation of the flush method as an alternative to basal or reflex tear collection. Curr Eye Res. 2011;36:198–207. doi: 10.3109/02713683.2010.542867. [DOI] [PubMed] [Google Scholar]
- 23.Moss SE, Klein R, Klein BE. Prevalence of and risk factors for dry eye syndrome. Archives of ophthalmology (Chicago, Ill. : 1960) 2000;118:1264–1268. doi: 10.1001/archopht.118.9.1264. [DOI] [PubMed] [Google Scholar]
- 24.Moss SE, Klein R, Klein BE. Long-term incidence of dry eye in an older population. Optometry and vision science : official publication of the American Academy of Optometry. 2008;85:668–674. doi: 10.1097/OPX.0b013e318181a947. [DOI] [PubMed] [Google Scholar]
- 25.Nagano T, Nakamura M, Nakata K, Yamaguchi T, Takase K, Okahara A, Ikuse T, Nishida T. Effects of substance P and IGF-1 in corneal epithelial barrier function and wound healing in a rat model of neurotrophic keratopathy. Invest Ophthalmol Vis Sci. 2003;44:3810–3815. doi: 10.1167/iovs.03-0189. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura M, Chikama TI, Nishida T. Characterization of insulin-like growth factor-1 receptors in rabbit corneal epithelial cells. Exp Eye Res. 2000;70:199–204. doi: 10.1006/exer.1999.0775. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura M, Kawahara M, Nakata K, Nishida T. Restoration of corneal epithelial barrier function and wound healing by substance P and IGF-1 in rats with capsaicin-induced neurotrophic keratopathy. Invest Ophthalmol Vis Sci. 2003;44:2937–2940. doi: 10.1167/iovs.02-0868. [DOI] [PubMed] [Google Scholar]
- 28.Ng V, Cho P, To C. Tear proteins of normal young Hong Kong Chinese. Graefes Arch Clin Exp Ophthalmol. 2000;238:738–745. doi: 10.1007/s004170000140. [DOI] [PubMed] [Google Scholar]
- 29.Paugh JR, Marsden HJ, Edrington TB, Deland PN, Simmons PA, Vehige JG. A pre-application drop containing carboxymethylcellulose can reduce multipurpose solution-induced corneal staining. Optom Vis Sci. 2007;84:65–71. doi: 10.1097/01.opx.0000254044.94356.23. [DOI] [PubMed] [Google Scholar]
- 30.Paulsen AJ, Cruickshanks KJ, Fischer ME, Huang GH, Klein BE, Klein R, Dalton DS. Dry eye in the beaver dam offspring study: prevalence, risk factors, and health-related quality of life. Am J Ophthalmol. 2014;157:799–806. doi: 10.1016/j.ajo.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perumal N, Funke S, Pfeiffer N, Grus FH. Proteomics analysis of human tears from aqueous-deficient and evaporative dry eye patients. Scientific reports. 2016;6:29629. doi: 10.1038/srep29629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robertson DM, Zhu M, Wu YC. Cellular distribution of the IGF-1R in corneal epithelial cells. Exp Eye Res. 2012;94:179–186. doi: 10.1016/j.exer.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Srinivasan S, Thangavelu M, Zhang L, Green KB, Nichols KK. iTRAQ quantitative proteomics in the analysis of tears in dry eye patients. Invest Ophthalmol Vis Sci. 2012;53:5052–5059. doi: 10.1167/iovs.11-9022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stapleton F, Alves M, Bunya VY, Jalbert I, Lekhanont K, Malet F, Na K-S, Schaumberg D, Uchino M, Vehof J, Viso E, Vitale S, Jones L. TFOS DEWS II Epidemiology Report. Ocul Surf. 2017;15:334–365. doi: 10.1016/j.jtos.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 35.Stuchell RN, Feldman JJ, Farris RL, Mandel ID. The effect of collection technique on tear composition. Invest Ophthalmol Vis Sci. 1984;25:374–377. [PubMed] [Google Scholar]
- 36.Sullivan DA, Rocha EM, Aragona P, Clayton JA, Ding J, Golebiowski B, Hampel U, McDermott AM, Schaumberg DA, Srinivasan S, Versura P, Willcox MDP. TFOS DEWS II Sex, Gender, and Hormones Report. Ocul Surf. 2017;15:284–333. doi: 10.1016/j.jtos.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 37.Tan KO, Sack RA, Holden BAS. H.A.,Temporal sequence of changes in tear film composition during sleep. Curr Eye Res. 1993;12:1001–1007. doi: 10.3109/02713689309029226. [DOI] [PubMed] [Google Scholar]
- 38.Thissen JP, Ketelslegers JM, Underwood LE. Nutritional regulation of the insulin-like growth factors. Endocr Rev. 1994;15:80–101. doi: 10.1210/edrv-15-1-80. [DOI] [PubMed] [Google Scholar]
- 39.Tiryakioglu O, Kadiolgu P, Canerolgu NU, Hatemi H. Age dependency of serum insulin - like growth factor (IGF)-1 in healthy Turkish adolescents and adults. Indian journal of medical sciences. 2003;57:543–548. [PubMed] [Google Scholar]
- 40.Wilson SE, Liang Q, Kim WJ. Lacrimal gland HGF, KGF, and EGF mRNA levels increase after corneal epithelial wounding. Invest Ophthalmol Vis Sci. 1999;40:2185–2190. [PubMed] [Google Scholar]
- 41.Wizert A, Iskander DR, Cwiklik L. Interaction of lysozyme with a tear film lipid layer model: A molecular dynamics simulation study. Biochimica et biophysica acta. 2017;1859:2289–2296. doi: 10.1016/j.bbamem.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 42.Wolffsohn JS, Arita R, Chalmers R, Djalilian A, Dogru M, Dumbleton K, Gupta PK, Karpecki P, Lazreg S, Pult H, Sullivan BD, Tomlinson A, Tong L, Villani E, Yoon KC, Jones L, Craig JP. TFOS DEWS II Diagnostic Methodology report. Ocul Surf. 2017;15 doi: 10.1016/j.jtos.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 43.Woo HM, Bentley E, Campbell SF, Marfurt CF, Murphy CJ. Nerve growth factor and corneal wound healing in dogs. Exp Eye Res. 2005;80:633–642. doi: 10.1016/j.exer.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 44.Wu YC, Buckner BR, Zhu M, Cavanagh HD, Robertson DM. Elevated IGFBP3 levels in diabetic tears: a negative regulator of IGF-1 signaling in the corneal epithelium. Ocul Surf. 2012;12:100–107. doi: 10.1016/j.jtos.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu YC, Zhu M, Robertson DM. Novel nuclear localization and potential function of insulin-like growth factor-1 receptor/insulin receptor hybrid in corneal epithelial cells. PLoS One. 2012;7:e42483. doi: 10.1371/journal.pone.0042483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yanai R, Yamada N, Inui M, Nishida T. Correlation of proliferative and anti-apoptotic effects of HGF, insulin, IGF-, IGF-2, and EGF in SV40-transformed human corneal epithelial cells. Exp Eye Res. 2006;83:76–83. doi: 10.1016/j.exer.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 47.Zhou L, Zhao SZ, Koh SK, Chen L, Vaz C, Tanavde V, Li XR, Beuerman RW. In-depth analysis of the human tear proteome. J Proteomics. 2012;75:3877–3885. doi: 10.1016/j.jprot.2012.04.053. [DOI] [PubMed] [Google Scholar]