Abstract
Four new homoisoflavonoids, 7-O-methyl-8-demethoxy-3′-hydroxy-3,9-dihydropunctatin (4), 6-hydroxy-8-demethoxy-4′-O-methyl-3,9-dihydropunctatin (8), 7,4′-O-dimethyl-8-demethoxy-3,3′-dihydroxy-3,9-dihydropunctatin (13), and 7-O-methyl-3-hyroxy-3,9-dihydropunctatin (14) were identified from a chloroform extract of the bulbs of Bellevalia flexuosa, along with 13 known analogues. The structures were determined by analysis of HRMS and NMR data, while ECD spectroscopy enabled the assignment of the absolute configurations of the new compounds 4, 8, 13 and 16. The cytotoxic activities of the isolated compounds (1–17) were evaluated using a panel of human cancer cell lines. Compounds 2 and 7 were the most potent against the MDA-MB-435 (melanoma) cancer cell line with IC50 values of 1.6 and 2.0 µM, respectively, and were essentially equipotent against the OVCAR3 (ovarian) cancer cell line with IC50 values of 9.5 and 10.8 µM, respectively. However, compound 7, with an IC50 value of 3.6 µM, was the most potent against the MDA-MB-231 (breast) cancer cell line.
Keywords: Homoisoflavonoids, Bellevalia, Bulbs, Absolute configuration, Cytotoxicity, Human cancer cell lines
Graphical abstract
1. Introduction
Homoisoflavonoids are a rare subclass of flavonoids possessing an extra carbon atom in their skeleton [1–5]. Biosynthetically, chalcones are thought to be the precursors of homoisoflavonoids [3]. Currently, about 250 natural homoisoflavonoids have been reported in the literature, with the majority being isolated from several genera of Asparagaceae and Fabaceae [1–4, 6, 7]. Based on their structures, homoisoflavonoids have been classified into five groups: 3-benzyl-4-chromanones, 3-benzyl-3-hydroxy-4-chromanones, 3-benzylidene-4-chromanones (E or Z), 3-benzylchrom-2-en-4-ones, and the scillascillins [4]. Homoisoflavonoids have attracted attention because of their various biological activities, including antioxidant, anti-inflammatory, antimutagenic, antimicrobial, antiallergic and antihistaminic, anti-diabetic, cytotoxic, and anti-angiogenic effects, as well as protein tyrosine kinase inhibition activity [3, 4].
Although Jordan is a small country of about 96,188 km2, it has a remarkable diversity of wildlife, which could be attributed to its unique location at the intersection of three continents, encapsulating four bio-geographical zones: Mediterranean, Irano-Turanian, Saharo-Arabian, and Tropical (Sudanian penetration) [8–10]. Within these different zones, thirteen vegetation types are identified, reflecting Jordan’s diverse landscape, climate, and geology [8, 11]. More than 2,600 vascular plant species belonging to 113 family and 810 genera are reported to grow in the wild, of which 100 are endemic and more than 70 species are considered extinct [8]. Hence, studies of Jordan’s wild plants have been initiated [12–14] as a potential source of drug leads.
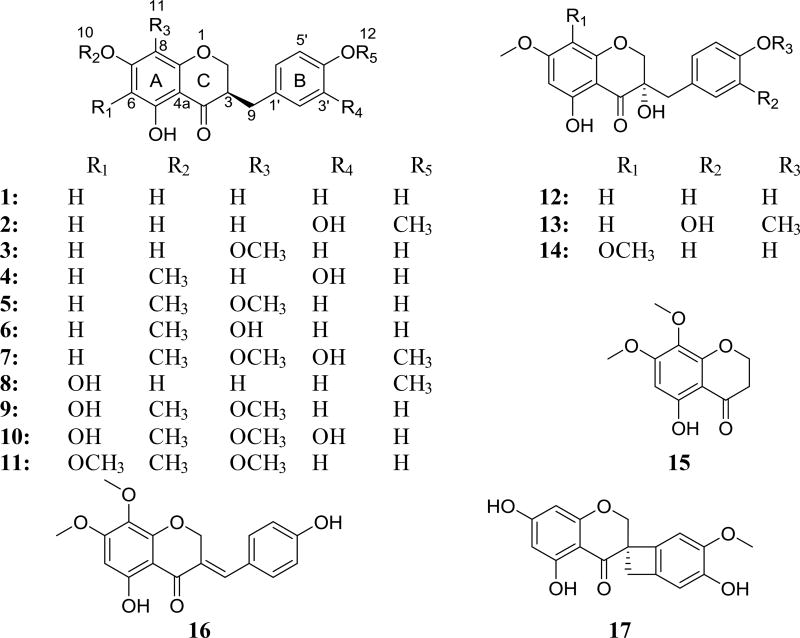
In the current study, the bulbs of Bellevalia flexuosa Boiss. (Asparagaceae) have been explored. B. flexuosa, which is known as “Common Roman Squill”, and in Jordan as “Drooping Onion” [15], is one of eleven species that are reported by Al-Eisawi to grow in Jordan in the wild [16]. Although no medical use is reported for this plant species by the local people of Jordan, a closely related species (B. eigii) was investigated recently, yielding a set of homoisoflavoinids [6]. B. flexuosa is a perennial herb with underground bulbs that is found flowering from February to March. It flourishes in mountains and waste grounds [16]. Chemical investigation of the chloroform extract of the bulbs of B. flexuosa resulted in the isolation and identification of seventeen homoisoflavonoids; of which thirteen were known (1–3, 5–7, 9–12, and 15–17) and four were new analogues: 7-O-methyl-8-demethoxy-3′-hydroxy-3,9-dihydropunctatin (4), 6-hydroxy-8-demethoxy-4′-O-methyl-3,9-dihydropunctatin (8), 7,4′-O-dimethyl-8-demethoxy-3,3′-dihydroxy-3,9-dihydropunctatin (13), and 7-O-methyl-3-hyroxy-3,9-dihydropunctatin (14). ECD spectroscopy was used to assign the absolute configurations of the new compounds 4, 8, 13, and 14. Homoisoflavonoids were reported to have a broad range of biological activities, including cytotoxic effects [3]. Therefore, the isolated compounds (1–17) were tested for their cytotoxicity using three human cancer cell lines, namely MDA-MB-435 (melanoma), MDA-MB-231 (breast), and OVCAR3 (ovarian).
2. Experimental
2.1. General experimental procedures
Optical rotations, UV data, and ECD spectra were obtained using a Rudolph Research Autopol III polarimeter (Rudolph Research Analytical), a Varian Cary 100 Bio UV–vis spectrophotometer (Varian Inc.), and an Olis DSM 17 ECD spectrophotometer (Olis, Inc.). NMR data were collected using either a JEOL ECA-500 NMR spectrometer operating at 500 MHz for 1H and 125 MHz for 13C (JEOL Ltd.) or an Agilent 700 MHz NMR spectrometer (Agilent Technologies), equipped with a cryoprobe, operating at 700 MHz for 1H and 175 MHz for 13C. Residual solvent signals were utilized for referencing. HRMS data were acquired using a Thermo QExactive Plus mass spectrometer equipped with an electrospray ionization source (Thermo Fisher Scientific). Gemini–NX C18 analytical (5 µm; 250 × 4.6 mm) and preparative (5 µm; 250 × 21.2 mm) columns (both from Phenomenex) along with Atlantis T3 C18 analytical (5 µm; 250 × 4.6 mm) and semipreparative (5 µm; 250 × 10.0 mm) columns (both from Waters Corp.) were used on a Varian Prostar HPLC system equipped with ProStar 210 pumps and a Prostar 335 photodiode array detector (PDA), with data collected and analyzed using Galaxie Chromatography Workstation software (version 1.9.3.2, Varian Inc.). Flash chromatography was performed on a Teledyne ISCO CombiFlash Rf 200 using Silica Gold columns (both from Teledyne Isco) and monitored by UV and evaporative light-scattering detectors. All other reagents and solvents were obtained from Fisher Scientific and were used without further purification.
2.2. Plant material
Bulbs of B. flexuosa were collected by Mohammad Bashabshah during flowering stage from the campus of the Jordan University of Science and Technology (JUST), Irbid, Jordan in March/April 2016. The plant material was identified by Mohammad Al-Gharaibeh, Plant Taxonomist, Faculty of Agriculture, JUST. A voucher specimen (PHS-122) was deposited in the herbarium of the Faculty of Pharmacy, JUST. The bulbs were cleaned of mud, sliced into small pieces, and air dried at rt in a well-ventilated area.
2.3. Extraction and isolation
Air-dried bulbs of B. flexuosa were ground to a powder using a laboratory mill. The powdered bulbs (850 g) were extracted exhaustively with CHCl3 by soaking at rt. The solvent was evaporated under reduced pressure to yield the CHCl3 extract (3.1 g), which was reconstituted in a 500 mL mixture of 5:4:1 H2O:CHCl3:MeOH. The mixture was stirred for 30 min and left to separate in a separatory funnel. The organic layer was collected and evaporated to dryness under reduced pressure. The dried organic extract was reconstituted in 200 mL of 1:1 MeOH:CH3CN and 200 mL of hexanes and transferred into a separatory funnel. The biphasic solution was shaken vigorously. The MeOH/MeCN layer was evaporated to dryness under vacuum. The dried MeOH/CH3CN layer (2.1 g) was dissolved in CHCl3 and mixed with Celite 545. Normal-phase flash chromatography was run using a gradient solvent system of hexanes-CHCl3-MeOH, at a flow rate of 35 mL/min, and column volumes of 41.3 over a total run time of 30.7 min, to yield seven fractions. Fraction 3 (267.9 mg) was subjected to preparative HPLC over a Gemini column using a gradient system of 50:50 to 60:40 of MeOH-H2O (0.1% formic acid) over 30 min at a flow rate of 20 mL/min to yield 8 subfractions, of which subfraction 3 was identified as compound 15 (2.1 mg). Subfraction 7 (1.9 mg) was subjected to semipreparative HPLC purification over Atlantis T3 column using a gradient solvent system of 40:60 to 50:50 CH3CN-H2O (0.1% formic acid) over 15 min at a flow rate of 4.6 mL/min to yield compounds 5 (0.7 mg) and 13 (0.4 mg). Subfraction 8 (8.3 mg) was subjected to semipreparative HPLC using Atlantis T3 column and a gradient solvent system of 40:60 to 60:40 CH3CN-H2O (0.1% formic acid) over 20 min at a flow rate of 4.6 mL/min to yield compounds 7 (5.4 mg) and 13 (0.3 mg).
Fraction 4 (544.7 mg) was subjected to preparative HPLC over a Gemini column using a gradient system of 60:40 to 70:30 of MeOH-H2O (0.1% formic acid) over 20 min at a flow rate of 21.2 mL/min to yield compound 5 (335.6 mg).
Fraction 5 (116.0 mg) was subjected to preparative HPLC over a Gemini column using a gradient system of 50:50 to 70:30 of MeOH-H2O (0.1% formic acid) over 20 min at a flow rate of 21.2 mL/min to yield seven sub-fractions. Further HPLC purification of subfractions 3 (3.4 mg) and 4 (3.2 mg) using semipreparative HPLC (Atlantis T3 column) and a gradient solvent systems of 40:60 to 50:50 CH3CN-H2O (0.1% formic acid) over 15 min at a flow rate of 4.6 mL/min yielded compounds 17 (1.3 mg) and 2 (2.1 mg) from subfractions 3 and 4, respectively. Subfractions 6 (2.2 mg) and 7 (14.3 mg) were purified using semipreparative HPLC (Atlantis T3 column) and a gradient solvent system of 40:60 to 60:40 CH3CN-H2O (0.1% formic acid) over 20 min at a flow rate of 4.6 mL/min to yield compounds 4 (0.9 mg) and 16 (10.8 mg) from subfractions 6 and 7, respectively.
Fraction 6 (379.3 mg) was purified using preparative HPLC (Gemini column) and a gradient solvent system of 50:50 to 55:45 of MeOH-H2O (0.1% formic acid) over 20 min, hold for 5 min and then increasing to 60:40 over 10 min at a flow rate of 21.2 mL/min to yield 5 subtractions. Subfraction 1 (13.2 mg) was further purified using semipreparative (Atlantis T3 column) and a gradient solvent system of 30:70 to 40:60 CH3CN-H2O (0.1% formic acid) over 15 min at a flow rate of 4.6 mL/min to yield compound 8 (2.3 mg) and another subfraction that was further purified using semipreaprative HPLC method over Atlantis T3 column and an isocratic solvent system of 28:72 CH3CN-H2O (0.1% formic acid) for 50 min at a flow rate of 4.6 mL/min to yield compounds 9 (2.3 mg) and 14 (1.9 mg). Subfractions 2 (15.6 mg) and 3 (39.0 mg) were purified using semipreparative HPLC (Atlantis T3 column) and a gradient solvent systems of 40:60 to 60:50 CH3CN-H2O (0.1% formic acid) over 15 min at a flow rate of 4.6 mL/min to yield compound 6 (9.1 mg) from subfraction 2 and compounds 3 (1.4 mg), 6 (1.9 mg), and 10 (19.2 mg) from subfraction 3. Subfractions 4 (3.4 mg) and 5 (3.8 mg) were purified using semipreparative HPLC (Atlantis T3 column) and gradient solvent systems of 40:60 to 50:50 CH3CN-H2O (0.1% formic acid) over 15 min at a flow rate of 4.6 mL/min to yield compounds 1 (0.5 mg) and 10 (0.5 mg) from fraction 4 and compounds 2 (0.9 mg), 5 (2.3 mg), 11 (0.9 mg), and 12 (1.5 mg) from subfraction 5.
2.3.1. 7-O-Methyl-8-demethoxy-3′-hydroxy-3,9-dihydropunctatin (4)
Yellowish oil; ]; UV (MeOH) λmax (log ε) 334 (3.23), 288 (3.98), 220 (3.94) nm; ECD (c 0.9 × 10−4 M, MeOH) λ (Δε) 244 (+0.68) nm, 269 (+0.96) nm, 295 (−2.07), 320 (+0.18) nm (Fig. 3); HRESIMS m/z 317.1016 [M + H]+ (calcd for C17H17O6, 317.1020).
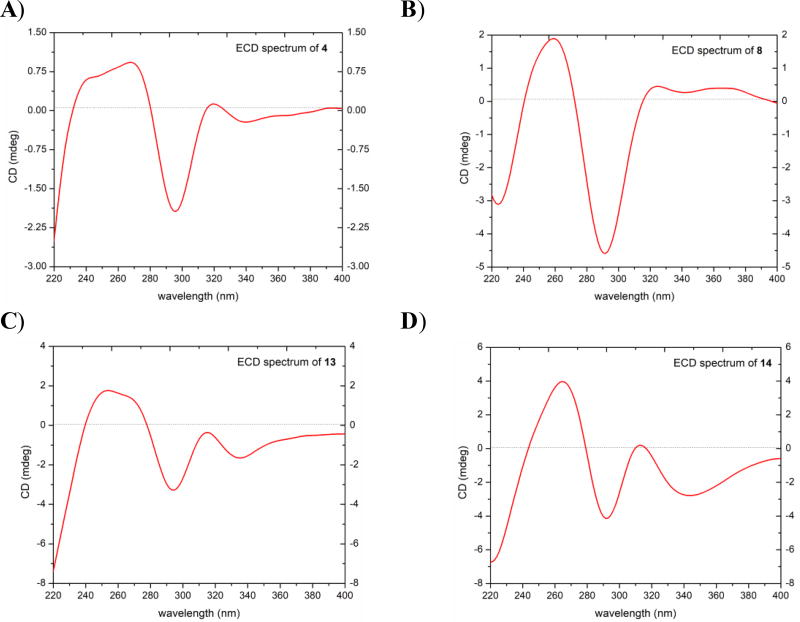
Fig. 3.
ECD spectra for compounds A) 4, B) 8, C) 13, and D) 14 [0.09 mM, MeOH, cell length 2 cm].
2.3.2. 6-Hydroxy-8-demethoxy-4′-O-methyl-3,9-dihydropunctatin (8)
Light yellow amorphous powder; ]; UV (MeOH) λmax (log ε) 360 (3.32), 291 (4.05), 242 (3.94), 229 (3.99) nm; ECD (c 0.9 × 10−4 M, MeOH) λ (Δε) 260 (+2.02) nm, 293 (−4.79), 323 (+0.50) nm (Fig. 3); HRESIMS m/z 317.1016 [M + H]+ (calcd for C17H17O6, 317.1020).
2.3.3. 7,4′-O-dimethyl-8-demethoxy-3,3′-dihydroxy-3,9-dihydropunctatin (13)
White amorphous powder; ]; UV (MeOH) λmax (log ε) 334 (3.35), 288 (4.08), 218 (4.10) nm; ECD (c 0.9 × 10−4 M, MeOH) λ (Δε) 253 (+1.80) nm, 295 (−3.52), 320 (−0.53) nm (Fig. 3); HRESIMS m/z 347.1122 [M + H]+ (calcd for C18H19O7, 347.1125).
2.3.4. 7-O-methyl-3-hyroxy-3,9-dihydropunctatin (14)
Light yellow amorphous powder; ]; UV (MeOH) λmax (log ε) 348 (3.58), 292 (4.15), 224 (4.15) nm; ECD (c 0.9 × 10−4 M, MeOH) λ (Δε) 265 (+4.16) nm, 292 (−4.53), 313 (+0.42) nm (Fig. 3); HRESIMS m/z 347.1124 [M + H]+ (calcd for C18H19O7, 347.1125).
2.4. Cytotoxicity assay
Compounds (1–17) were tested for cytotoxicity against human melanoma cancer cells MDA-MB-435 [17], human breast cancer cells MDA-MB-231, and human ovarian cancer cells OVCAR3 as described previously [18, 19]. Briefly, the cell lines were propagated at 37 °C in 5% CO2 in RPMI 1640 medium, supplemented with fetal bovine serum (10%), penicillin (100 units/mL), and streptomycin (100 µg/mL). Cells in log phase growth were harvested by trypsinization followed by two washings to remove all traces of enzyme. A total of 5,000 cells were seeded per well of a 96-well clear, flat-bottom plate (Microtest 96®, Falcon) and incubated overnight (37 °C in 5% CO2). Samples dissolved in DMSO were then diluted and added to the appropriate wells. The cells were incubated in the presence of test substance for 72 h at 37 °C and evaluated for viability with a commercial absorbance assay (CellTiter 96® AQueous One Solution Cell Proliferation Assay, Promega Corp, Madison, WI) that measured viable cells. IC50 values are expressed in µM relative to the solvent (DMSO) control. Taxol was used as a positive control.
3. Results and discussion
Air-dried bulbs of B. flexuosa were extracted with CHCl3, and the resulting crude extract was reconstituted in a mixture of 5:4:1 H2O:CHCl3:MeOH. The dried organic extract was reconstituted in 1:1 MeOH/CH3CN and then defatted using hexanes. The resulting dried MeOH/CH3CN extract was fractionated using normal phase flash chromatography. HPLC methods, preparative and semipreprative, were used for purifications of the fractions leading to the isolation of seventeen homoisoflavonoids (1–17) (Fig. 1).
Fig. 1.
Structures of compounds 1–17.
The structures of thirteen known homoisoflavonoids analogues (1–3, 5–7, 9–12, and 15–17) were established by comparison of NMR (1D/2D), HRMS, and ECD data with literature values and were identified as: 4′,5,7-trihydroxyhomoisoflavanone (1) [6], 3′-hydroxy-3,9-dihydroeucomin (2) [6], 3,9-dihydropunctatin (3) [20], 7-O-methyl-3,9-dihydropunctatin (5) [6], 8-O-demethyl-7-O-methyl-3,9-dihydropunctatin (6) [6], 7,4′-di-O-methyl-3′-hydroxy-3,9-dihydropunctatin (7) [6], 6-hydroxy-7-O-methyl-3,9-dihydropunctatin (9) [6], 7-O-methyl-3′-hydroxypunctatin (10), 3-(4-hydroxybenzyl)-5-hydroxy-6,7,8-trimethoxychroman-4-one (11) [21], 7-O-methyl-8-demethoxy-3-hydroxy-3,9-dihydropunctatin (12) [6], 5-hydroxy-7,8-dimethoxychroman-4-one (15) [6], 7-O-methylpunctatin (16) [6], and isomuscomosin (17) [6], (Figs. S1–S3, S5–S7, S9–S12, S15–S17, Supplementary Data)
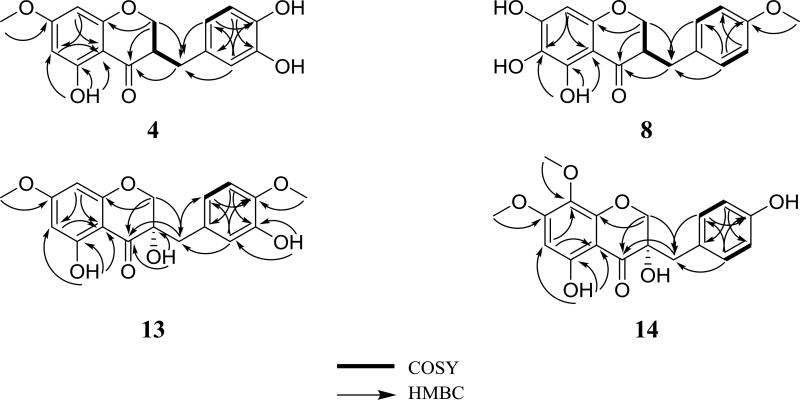
Compound 4 (0.93 mg) was isolated as a yellowish oil with a molecular formula of C17H16O6 (10 degrees of unsaturation) as determined by HRESIMS (m/z 317.1016 [M + H]+, calcd. 317.1020), which was further supported by 1H, 13C, and edited HSQC NMR data (Tables 1 and 2, Fig. S4, Supplementary Data). Compound 4 showed characteristic NMR signals indicative of a 3-benzylchroman-4-one homoisoflavonoid analogue with very high structural similarity to 2, both sharing the same molecular formula. HMBC data analysis indicated the structure of 4 to be different from that of 2 by the methylation position. HMBC correlations from H3-7´ (δH 3.88, s) to C-4′ (δC 145.5) in 2 and from H3-10 (δH 3.81, s) to C-7 (δC 168.0) in 4 enabled the assignment of 2 as a 4′-O-methyl analogue and 4 as a 7-O-methyl analogue. In the latter, HMBC correlations from H-8 (δH 5.97, d, J = 2.3) to C-4a (δC 102.7), C-6 (δC 95.1), C-7 (δC 168.0), and C-8a (δC 163.0); from H-6 (δH 6.06, d, J = 2.3) to C-5 (δC 164.6), C-7, C-8 (δC 94.0), and C-4a, from 5-OH (δH 12.11, s) to C-4a, C-5, and C-6 established the substitution pattern of ring A. In addition, HMBC correlations from H-2′ (δH 6.75, d, J = 1.3) to C-9 (δC 32.3), C-4′ (δC 142.4), and C-6′ (δC 121.8); from H-5′ (δH 6.81, d, J = 7.9) to C-1′ (δC 131.0) and C-3′ (δC 143.8); from H-6′ (δH 6.66, dd, J = 7.9, 1.3) to C-9, C-2′ (δC 116.3), and C-4′ established the substitution pattern of ring C. Further examination of the 2D NMR data established the structure of 4 (Fig. 2) as 7-O-methyl-8-demethoxy-3′-hydroxy-3,9-dihydropunctatin. The absolute configurations of 3-benzylchroman-4-one-type homoisoflavonoids are assigned using electronic circular dichroism (ECD) spectroscopy [22], in which a negative Cotton effect in the 287–295 nm region of the ECD curves is indicative of a 3R configuration [22]. Compound 4 showed a negative Cotton effect at 295 nm (Δε = −2.07) in the ECD spectrum, supporting an R-configuration at C-3 (Fig. 3).
Table 1.
1H NMR Data for compounds 4, 8, 13, and 14 (500 MHz in CDCl3)a.
| position | 4 | 8 | 13 | 14 |
|---|---|---|---|---|
| 2 | 4.12, dd (11.4, 7.1) | 4.08, dd (11.4, 7.1) | 4.06, d (11.2) | 4.09, d (11.2) |
| 4.28, dd (11.4, 4.1) | 4.25, dd (11.4, 4.1) | 4.23, d (11.2) | 4.36, d (11.2) | |
| 3 | 2.80, m | 2.78, m | ||
| 6 | 6.06, d (2.3) | 6.11, d (2.3) | 6.16, s | |
| 8 | 5.97, d (2.3) | 6.05, s | 6.05, d (2.3) | |
| 9 | 2.65, dd (13.9, 10.4) | 2.69, dd (13.8, 10.6) | 2.89, d (13.8) | 2.94, d (14.1) |
| 3.12, dd (13.9, 4.5) | 3.16, dd (13.8, 4.5) | 2.93, d (13.8) | 2.98, d (14.1) | |
| 10 | 3.81, s | 3.85, s | 3.91, s | |
| 11 | 3.88, s | 3.83, s | ||
| 12 | 3.91, s | |||
| 2′ | 6.75, d (1.3) | 7.08, d (8.5) | 6.82, d (2.1) | 7.04, d (8.5) |
| 3′ | 6.78, d (8.5) | 6.75, d (8.5) | ||
| 5′ | 6.81, d (7.9) | 6.76, d (8.1) | ||
| 6′ | 6.66, dd (7.9, 1.3) | 6.65, dd (8.1, 2.1) | ||
| 3-OH | 3.36, s | |||
| 5-OH | 12.11, s | 11.78, s | 11.25, s | 11.21, s |
| 6-OH | 5.03, br. s | |||
| 7-OH | 4.81, br. s | |||
| 3′-OH | 5.20 or 5.33b, br. s | 5.58, s | ||
| 4′-OH | 5.20 or 5.33b, br. s | 4.83, br. s |
δ in ppm, mult (J in Hz);
Could be swapped
Table 2.
13C NMR Data for 4, 8, 14 (125 MHz), and for 13 (175 MHz) in CDCl3.
| position | 4 | 8 | 13 | 14 |
|---|---|---|---|---|
| 2 | 69.1 | 69.4 | 71.9 | 72.5 |
| 3 | 46.9 | 47.2 | 72.3 | 72.2 |
| 4 | 198.0 | 198.8 | 198.3 | 198.6 |
| 4a | 102.7 | 102.5 | 100.6 | 100.4 |
| 5 | 164.6 | 148.2 | 164.1 | 160.0 |
| 6 | 95.1 | 156.1 | 95.5 | 93.7 |
| 7 | 168.0 | 127.4 | 168.8 | 162.3 |
| 8 | 94.0 | 91.1 | 94.6 | 129.9 |
| 8a | 163.0 | 154.8 | 162.9 | 153.5 |
| 9 | 32.3 | 32.1 | 41.0 | 40.9 |
| 10 | 55.8 | 56.0 | 56.6 | |
| 11 | 56.0 | 61.7 | ||
| 12 | 56.5 | |||
| 1′ | 131.0 | 130.1 | 127.3 | 126.2 |
| 2′ | 116.3 | 130.5 | 116.8 | 131.9 |
| 3′ | 143.8 | 115.7 | 145.4 | 115.4 |
| 4′ | 142.4 | 154.5 | 145.9 | 155.0 |
| 5′ | 115.7 | 110.5 | ||
| 6′ | 121.8 | 122.2 |
Fig. 2.
Key COSY and HMBC correlations of 4, 8, 13, and 14.
HRESIMS and NMR data of compound 8 (2.3 mg), which was obtained as a yellow amorphous powder, revealed its molecular formula as C17H16O6 (m/z 317.1016 [M + H]+, calcd 317.1020) (Tables 1 and 2, Fig. S8, Supplementary Data). As in compound 4, the NMR data of 8 showed characteristic signals indicative of a 3-benzylchroman-4-one-type homoisoflavonoid with structural similarity to 3 as they shared the same molecular formula. However, the 8-methoxy group of ring A (δH/δC 3.95/61.7 for H3-11/C-11) in 3 was replaced by an aromatic proton singlet (δH/δC 6.05/91.1 for H-8/C-8) in 8. In addition, the aromatic proton singlet (δH/δC 6.13/96.0 for H-6/C-6) in 3 was replaced by an exchangeable proton singlet (δH 5.03 for 6-OH) in 8. Moreover, the exchangeable proton singlet in 3 (δH 4.75 for 4′-OH) was replaced by a methyl group (δH/δC 3.91/56.5 for H3-7´/C-7´). The structure of 8 was inferred by further analysis of HMBC data. HMBC correlation from H-8 to C-4 (198.8), C-4a (102.5), C-6 (156.1), and C-8a (154.8) established the substitution pattern of ring A, while the HMBC correlation from H3-7´ (δH 3.91, s) to C-4′ (δC 154.5) confirmed the methylation position in 8. Further analysis of the 2D NMR data established the structure of 8 to which the trivial name 6-hydroxy-8-demethoxy-4′-O-methyl-3,9-dihydropunctatin was assigned (Fig. 2). A negative Cotton effect at 293 nm in the ECD spectrum of compound 8 (Δε = −4.79) indicated an R-configuration at C-3 (Fig. 3).
Compound 13 (0.66 mg) was obtained as a white amorphous powder. HRESIMS (m/z 347.1122 [M + H]+, calcd 347.1125) NMR data indicated a molecular formula of C18H18O7 (Tables 1 and 2, Fig. S13, Supplementary Data). The NMR data indicated compound 13 as a 3-benzyl-3-hydroxychroman-4-one homoisoflavonoid analogue. The compound showed high structural similarity to 12. However, compound 13 showed a methoxy group, as indicated by 1H and 13C NMR data (δH/δC 3.88/56.0) and a hydroxyl group at δH 5.58 (3'-OH) consistent with the 30 amu difference in the HRMS data of 13 relative to 12. The aromatic A2B2 system of ring B in 12 was replaced by an ABM spin system in 13 (δH 6.82, d, J = 2.1; 6.76, d, J = 8.1; and 6.65, dd, J = 8.1, 2.1, for H-2´, H-5´, and H-6´, respectively), indicating the presence of a 1,3,4-trisubstituted benzene ring. An HMBC correlation from the 4′-OCH3 protons to C-4′ (δC 145.9) confirmed its connectivity. The structure of compound 13 was deduced by further inspection of the 2D NMR data, including COSY and HMBC spectra (Fig. 2). Compound 13 was given the trivial name 7,4′-O-dimethyl-8-demethoxy-3,3′-dihydroxy-3,9-dihydropunctatin. A negative Cotton effect (Δε = −3.52) at 297 nm of the ECD spectrum of 13 indicated an S-configuration at C-3 (Fig. 3) [22].
The HRESIMS (m/z 347.1124 [M + H]+, calcd 347.1125) and NMR data of compound 14 (2.38 mg), which was obtained as a light yellow amorphous powder, indicated a molecular formula of C18H18O7 (Tables 1 and 2, Fig. S14, Supplementary Data). The NMR data of 14 showed distinctive peaks indicative of a 3-benzyl-3-hydroxychroman-4-one homoisoflavonoid analogue. The compound showed high structural similarity to 12. However, compound 14 had an extra methoxy group, as indicated by 1H and 13C NMR data (δH/δC 3.83/61.7), consistent with the 30 amu difference in the HRMS data of 14 relative to 12, replacing the aromatic doublet (δH 6.05, d, J = 2.1 for H-8) in 12. HMBC correlations from H-6 to C-4 (198.6), C-4a (100.4), C-5 (160.0), C-7 (162.3), and C-8 (129.9) confirmed the substitution pattern of ring A. The structure of compound 14 was deduced by further inspection of the 2D NMR data, including COSY and HMBC spectra (Fig. 2). Compound 14 was given the trivial name 7-O-methyl-3-hyroxy-3,9-dihydropunctatin. A negative cotton effect (Δε = −4.53) at 292 nm in the ECD spectrum of 14 was indicative of an S-configuration at C-3 (Fig. 3) [22].
Compounds (1–17) were evaluated for their cytotoxic activities against the MDA-MB-435 (melanoma), MDA-MB-231 (breast), and OVCAR3 (ovarian) cancer cell lines. Compounds 2 and 7 were the most potent on the three cancer cell lines with IC50 values of 1.6, 14.2, 9.5 and 2.0, 3.6, and 10.8 µM, respectively (Table 4). Compound 2 was reported to be active against colon cancer (HT-29 cell line ED50 = 2.78 µM) and breast cancer (MDA-MB-435 cell line ED50 = 1.33 µM). Compounds 2 and 7 were reported previously by our group to be active against breast cancer (MDA-MB-435 cell line) with IC50 values of 1.0 and 1.1 µM, respectively [6]. Moreover, compound 7 showed moderate activity when tested against colon cancer (HT-29 cell line IC50 = 17.3 µM) [6]. The cytotoxicity data of the new and known analogues isolated in the current work expanded our understanding of the structure-activity relationships of this unique class of flavonoids. The substitution pattern of ring B affects the activity significantly. Compound 1, with a 4′-OH group, was inactive. However, compound 4, with 3′,4′-dihydroxy substituents, showed IC50 values of 14.3, 17.5, and 24.8 µM against the MDA-MB-435, MDA-MB-231, and OVCAR3 cancer cell lines, respectively. Moreover, reducing the polarity of the ring B substituents results in improved cytotoxic activity. For example, the activity of compound 2, with a 4′-methoxy substituent, increased by ~9- and 3-folds the activity against MDA-MB-435 and OVCAR3 cancer cell lines in comparison with compound 4 via IC50 values of 1.6 and 9.5 µM, respectively. Similarly, although compound 10 was inactive, compound 7, with a 4′-methoxy substituent, showed IC50 values of 2.0, 3.6, and 10.8 µM against the MDA-MB-435, MDA-MB-231, and OVCAR3 cancer cell lines, respectively. The same pattern can be noticed when comparing the cytotoxic activities of compounds 12 and 13. Compound 12 with a 4′-OH substituent was inactive, however compound 13, with 3′-hydroxy and 4′-methoxy substituents, showed activity against the MDA-MB-435 cancer cell line with IC50 value of 14.4 µM. In addition, introducing an OH group at C-3 increases the activity significantly, although compound 5 was inactive, compound 14, with a 3-OH substituent, showed IC50 value of 15.2 µM against the MDA-MB-435 cancer cell line.
Table 4.
Cytotoxic activities of compounds 2, 4, 7, 13, and 14.
| compounda | IC50 values in µMb | ||
|---|---|---|---|
| MDA-MB-435 | Ovcar3 | MDA-MB-231 | |
| 2 | 1.6 | 14.2 | 9.5 |
| 4 | 14.3 | 17.5 | 24.8 |
| 7 | 2.0 | 3.6 | 10.8 |
| 13 | 14.4 | >25 | >25 |
| 14 | 15.2 | >25 | >25 |
| Taxolc | 0.0001 | 0.0015 | 0.17 |
Compounds 1, 3, 5, 6, 8–12, 15–17 were inactive, IC50 values >25 µM.
IC50 is the concentration to inhibit 50% of growth with a 72 h incubation.
Positive control.
Supplementary Material
Acknowledgments
This research was supported, in part, by the Deanship of Research, Jordan University of Science and Technology, Irbid, Jordan (Grant No. 284/2017) and via program project grant P01 CA125066 from the National Cancer Institute/National Institutes of Health, Bethesda, MD, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors whose names are listed immediately below certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Appendix A. Supplementary data
Supplementary data to this article can be found online at
References
- 1.Abegaz BM, Mutanyatta-Comar J, Nindi M. Naturally occurring homoisoflavonoids: Phytochemistry, biological activities and synthesis. Nat. Prod. Commun. 2007;2:475–498. [Google Scholar]
- 2.Heller W, Tamm C. Homoisoflavanones and biogenetically related compounds. In: Cadby PA, Cooke RG, Edwards JM, Heller W, Jefford CW, Lederer E, Lefrancier P, Dev S, Tamm C, Herz W, Grisebach H, Kirby GW, editors. Fortschritte der Chemie organischer Naturstoffe / Progress in the Chemistry of Organic Natural Products. Springer; Vienna, Vienna: 1981. pp. 105–152. [Google Scholar]
- 3.Lin L-G, Liu Q-Y, Ye Y. Naturally occurring homoisoflavonoids and their pharmacological activities. Planta Med. 2014;80:1053–1066. doi: 10.1055/s-0034-1383026. [DOI] [PubMed] [Google Scholar]
- 4.Mulholland DA, Schwikkard SL, Crouch NR. The chemistry and biologica l activity of the Hyacinthaceae. Nat. Prod. Rep. 2013;30:1165–1210. doi: 10.1039/c3np70008a. [DOI] [PubMed] [Google Scholar]
- 5.Calvo MI. Three new homoisoflavanones from the bulbs of Ledebouria floribunda. Fitoterapia. 2009;80:394–398. doi: 10.1016/j.fitote.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Alali F, El-Elimat T, Albataineh H, Al-Balas Q, Al-Gharaibeh M, Falkinham JO, 3rd, Chen WL, Swanson SM, Oberlies NH. Cytotoxic homoisoflavones from the Bulbs of Bellevalia eigii. J. Nat. Prod. 2015;78:1708–1715. doi: 10.1021/acs.jnatprod.5b00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou X, Yuping Z, Zhao H, Liang J, Zhang Y, Shi S. Antioxidant homoisoflavonoids from Polygonatum odoratum. Food Chem. 2015;186:63–68. doi: 10.1016/j.foodchem.2015.02.058. [DOI] [PubMed] [Google Scholar]
- 8.Jordanian Fifth National Report under the Convention on Biological Diversity. Ministry of Environment; Amman, Jordan: 2014. [Google Scholar]
- 9.Feinbrun-Dothan N. Flora Palestina. The Israel Academy of Sciences and Humanities; Jeruselum: 1986. [Google Scholar]
- 10.Al-Eisawi DM. Field Guide to wild Flowers of Jordan and Neighbouring Contries. Amman, Jordan: Jordan Press, Foundation Al-Rai; 1998. pp. 1–2. [Google Scholar]
- 11.Al-Eisawi D. Vegetation of Jordan. UNESCO, Cairo office; 1996. [Google Scholar]
- 12.Alali FQ, Amrine CSM, El-Elimat T, Alkofahi A, Tawaha K, Gharaibah M, Swanson SM, Falkinham JO, III, Cabeza M, Sánchez A, Figueroa M, Oberlies NH. Bioactive withanolides from Withania obtusifolia. Phytochem. Lett. 2014;9:96–101. [Google Scholar]
- 13.Alali FQ, El-Elimat T, Li C, Qandil A, Alkofahi A, Tawaha K, Burgess JP, Nakanishi Y, Kroll DJ, Navarro HA, Falkinham JO, Wani MC, Oberlies NH. New colchicinoids from a native Jordanian meadow saffron, Colchicum brachyphyllum: Isolation of the first naturally occurring dextrorotatory colchicinoid. J. Nat. Prod. 2005;68:173–178. doi: 10.1021/np0496587. [DOI] [PubMed] [Google Scholar]
- 14.Alali FQ, Gharaibeh A, Ghawanmeh A, Tawaha K, Oberlies NH. Colchicinoids from Colchicum crocifolium Boiss.: a case study in dereplication strategies for (−)-Colchicine and related analogues using LC-MS and LC-PDA techniques. Phytochem. Anal. 2008;19:385–394. doi: 10.1002/pca.1060. [DOI] [PubMed] [Google Scholar]
- 15.Taifour H, El-Oqlah A. Jordan Plant Red List. Royal Botanic Garden; Amman, Jordan: 2014. [Google Scholar]
- 16.Al-Eisawi DM. Field Guide to Wild Flowers of Jordan and Neighbouring Countries. Jordan Press Foundation; Al Rai, Amman: 1998. [Google Scholar]
- 17.Rae J, Creighton C, Meck J, Haddad B, Johnson M. MDA-MB-435 cells are derived from M14 melanoma cells–a loss for breast cancer, but a boon for melanoma research. Breast Cancer Res. Treat. 2007;104:13–19. doi: 10.1007/s10549-006-9392-8. [DOI] [PubMed] [Google Scholar]
- 18.El-Elimat T, Figueroa M, Raja HA, Swanson SM, Falkinham JO, Lucas DM, Grever MR, Wani MC, Pearce CJ, Oberlies NH. Sorbicillinoid Analogues with Cytotoxic and Selective Anti-Aspergillus Activities from Scytalidium album. J. Antibiot. 2015;68:191–196. doi: 10.1038/ja.2014.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Elimat T, Raja HA, Falkinham JO, Day CS, Oberlies NH. Greensporones: Resorcylic Acid Lactones from an Aquatic Halenospora sp. J. Nat. Prod. 2014;77:2088–2098. doi: 10.1021/np500497r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adinolfi M, Barone G, Belardini M, Lanzetta R, Laonigro G, Parrilli M. Homoisoflavanones from Muscari comosum bulbs. Phytochemistry. 1985;24:2423–2426. [Google Scholar]
- 21.Amschler G, Frahm AW, Hatzelmann A, Kilian U, Müller-Doblies D, Müller-Doblies U. Constituents of Veltheimia viridifolia; I. Homoisoflavanones of the bulbs. Planta Med. 1996;62:534–539. doi: 10.1055/s-2006-957964. [DOI] [PubMed] [Google Scholar]
- 22.Adinolfi M, Barone G, Corsaro MM, Mangoni L, Lanzetta R, Parrilli M. Absolute configuration of homoisoflavanones from Muscari species. Tetrahedron. 1988;44:4981–4988. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.