Abstract
Purpose
Ongoing cancer cachexia trials evaluate sarcopenia by skeletal muscle index (SMI) at the L3 vertebrae level, commonly used as a standard. Routine chest CT institutional protocols widely differ in including L3. We investigated whether SMI at L1 assessment, rather than L3, would be reliable and more practicable for NSCLC.
Methods
NSCLC patients with routine CT chest had SMI measurements performed at L1 using Slice-O-Matic software. Accuracy of including L1 level, imaging quality and ability to detect sarcopenia was collected and correlation of L1 SMI with BMI was performed.
Results
37 patients with NSCLC (73 CT assessments) were enlisted at three institutions. Characteristics: 47% female; medians: age 59, KPS 80%; BMI 25.49, weight 72.97 kg, SMI 59.24. Sarcopenia was detected in 14.7% of patients; 20% had sarcopenic obesity. Of the 73 CTs, 94.5% included L1 (95% CI 86.6%–98.5%). Three images (4%) were difficult to evaluate. Inclusion of L1 was similar among the three participating institutions (90.4% to 96.7% inclusion). BMI correlation with SMI was weak (r = 0.329).
Conclusions
SMI assessment at L1 is achievable in patients with NSCLC receiving routine chest CT, with 96% having acceptable quality evaluations. Similar to results previously reported at L3, BMI showed poor correlation and low sensitivity to detect muscle mass loss. The use of CT at L1 is reliable and presents the opportunity for easier patient evaluation of sarcopenia in patients with lung cancer without the need for additional testing or radiation exposure.
Keywords: Lung cancer, sarcopenia, cachexia, skeletal muscle mass, chest CT, L1
Introduction
Cancer anorexia-cachexia syndrome, including skeletal muscle wasting, is a key aspect affecting survival and quality of life of cancer patients [1]. Definitions for these terms vary and at times can be controversial [2]. Nonetheless, it has been widely accepted that sarcopenia, defined as loss of skeletal muscle mass, is a major component of cancer cachexia [3]. Sarcopenia is an independent predictor of poor quality of life, increased chemotherapy toxicity, lower response to treatment, shorter time to tumor progression, prolonged length of stay and post-operative complications and decreased quality of life and survival in numerous different malignancies with ample supportive evidence [4–8]. As is the case with other cancers, sarcopenia in lung cancer has been shown to be associated with similarly poor outcomes [9–13]. This study was initiated to document a practical approach to assess early sarcopenia in non-small cell lung cancer (NSCLC) for daily management of patients and/or for use in research settings.
Over the past decade, several methods to measure skeletal muscle mass in cancer have been investigated, as listed in Table 1 [14–17]. All of these methods are more accurate than routine anthropometric measurements such as weight or body mass index (BMI, which is based solely on weight and height) [18,19]. Whole body MRI is highly accurate, but is impractical in most clinical settings. CT scanning is now well established for measuring muscle mass and is more precise than the others non-MRI methods listed in Table 1. The use of CT for measuring muscle mass offers an excellent opportunity, as CT imaging is routinely utilized in cancer care [20]. Prior studies in healthy volunteers demonstrated that the area determined from a single abdominal slice on MRI correlated closely with total body skeletal muscle (r=0.924) and adipose tissue (r= 0.889). The closest correlation was at the L3 intervertebral space [21,22]. Previously, a strong correlation between MRI and CT imaging had been established using cadaveric thighs (r= 0.999) [23].
Table 1.
Methods to measure skeletal muscle mass
| Tool | Accuracy (SD in kgs) | Advantages | Disadvantages |
|---|---|---|---|
| Bioelectrical impedance (BIA) | 9.3 | Safe; inexpensive; portable; expedient; no radiation | Relies on population-specific regressions not patient centered; not available at most cancer centers; skeletal muscle quality not evaluated; limited by BMI>34k/m2 that may overestimate muscle mass |
| Ultrasound (US) | NA | Safe; portable; expedient; low-cost; accessible; reliable for adipose tissue | No standardization technique (anatomical site, position or compression); patient habitus/hydration limitations and muscle contraction/relaxation state; operator dependent; provides muscle qualitative rather than quantitative measurements; no studies on cancer patients |
| Dual X ray absorptiometry (DXA) | 3 | Low-cost; can add limb muscle to trunk evaluation; low-radiation; better precision and accuracy | Low radiation exposure; cannot discriminate adipose and lean tissue; two-dimensional; influenced by tissue thickness and hydration; skeletal muscle quality not evaluated |
| Computed Tomography (CT) | <1.2 | Clinically routinely accessible; accurately discriminates quantitative and qualitative muscle-fat body composition; high precision and reproducible predicting total LBM, CV=0.13–1.6% | Radiation exposure; dependent on a ‘slice’ at L3 availability; cannot accommodate large individuals in the scanner; presumed strong correlation to MRI not evaluated |
| Magnetic Resonance Imaging (MRI) | <1 | Gold Standard; safe; excellent image quality; L3 level correlates with whole body scan (r: 0.924) | Costly and time consuming; not routinely used; cannot accommodate large individuals in the scanner |
Given the demonstration of usefulness of CT scanning as an accurate measure of skeletal muscle mass, several clinical trials have used this method. Based on the MRI results, all of these trials have focused on imaging at L3. While it is understandable that L3 was selected, this level can be problematic for use in patients with lung cancer. At many institutions a chest CT does not always include the third lumbar vertebrae or interspace. In a recent trial in which the majority of patients had lung cancer, only 65% had useful scans for the evaluation of skeletal muscle mass at the L3 level [24]. Future enrollment of lung cancer patients in trials studying sarcopenia, or clinical assessment of patients, would require additional imaging in addition to the chest CT in many patients to assess skeletal muscle index (SMI) at L3. This would increase costs, radiation exposure, and inconvenience. The aforementioned study by Shen et. al., that indicated the closest correlation of total body skeletal mass with L3 level, also showed an excellent correlation at the L1 level (r = 0.903) for skeletal muscle mass measurement, in normal volunteers [21,22]. A study with small cell lung cancer patients confirmed a strong correlation between L1 and L3 muscle mass index (r=0.8551) [25] and on a similar methodology with head and neck cancer patients, C3 level assessment strongly predicted L3 muscle (r=0.785) [26]. In this study, the objective was to establish that in patients with NSCLC, the L1 level would be included in most chest CT scans and would prove evaluable for patients with advanced cancer. Conditions such as muscle edema, abdominal ascites, and whole body anasarca, can potentially interfere with accurate measurement at any potential lumbar vertebrae level. If it can be established that routine chest CTs in this population can be evaluated for skeletal muscle mass at the L1 level, then such measurement could be practical both for routine patient assessment and in research studies investigating methods to prevent, ameliorate and reverse sarcopenia.
Methods
Patients and study design
All patients included in this trial were required to have non-small cell lung cancer and were about to start a new chemotherapy. Patients’ treating oncologists chose the specific cytotoxic chemotherapy regimen. These patients were also part of a prospective, randomized multicenter clinical trial which incorporated the use of a well validated health related quality of life measure, the LCSS [27], which was administered using electronic media every 3 weeks [28]. All patients had stage IIIB or IV biopsy-proven NSCLC, had a Karnofsky performance status of KPS ≥ 60 or Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0–2, and a life expectancy greater than three months. Enrollees could be receiving first-line, second-line, or third-line chemotherapy (NCT01924416).
Radiological evaluation
Initial CT scans were performed prior to chemotherapy, as a baseline. Repeat CT scans were performed at the discretion of the treating physician. The SMI was determined from these CT scans. The determination of the adequacy of inclusion of the L1 level on the CT was made using a Picture Archiving and Communication System (PACS) workstation with utilization of sagittal reformats when available in assisting precise localization. Contiguous 5-mm Chest CT slices were reviewed. To allow for occasional anatomical variations, if twelve rib pairs were not present (whether greater than or less than twelve), the level below T12 was chosen as the level of interest. The most central slice within the vertebral level was selected and exported into the Slice-O-Matic software® (Tomovision, Magog, Quebec Canada) [29]. This software views and measures the area of interest, using appropriate Hounsfield unit ranges as previously established for skeletal muscle [−29 to150] [20,29]. The abdominal wall, intercostal, and paraspinal muscles, as well as the visualized diaphragmatic crura were tagged. Data pertaining to the selected tissue including the surface area was expressed in squared centimeters (cm2) by the software. CT chest scan with L1 inclusion and software analysis were performed by trained researchers; with further evaluation and approval by a board certified clinical radiologist.
Outcome measurements
The anthropometric measurements (height and weight) were determined to the closest centimeter and 100 grams. Body mass index (BMI) was calculated and defined for adults. Body surface area was calculated by Dubois formula. Skeletal muscle mass (SMM) assessment is presented as an index - the skeletal muscle index (SMI, measured in cm2/m2) - obtained by the skeletal muscle mass cross sectional area (cm2) at L1 and BSA (m2). Previously reported cut-off values of SMI (<38.5 cm2/m2 for women and <52.4 cm2/m2 for men) were used to define sarcopenia [20].
Statistical analysis
Descriptive statistics were summarized by frequency percentages for categorical variables. When normally distributed, data were presented by mean, median, standard deviation and range for continuous variables, with confidence intervals. Correlations were expressed using the Pearson correlation coefficient (r). The coefficient of determination (r2) was used to calculate the relationship between BMI and SMI.
Results
Patient characteristics
Participants were enrolled from three institutions (Jacobi Medical Center, Montefiore Medical Center, and University of Virginia Medical Center). This analysis evaluated 37 patients who received 73 CT scans including baseline and follow up studies (32 second and 4 third scans after baseline performed at the discretion of the treating physician). Patient demographic and anthropomorphic characteristics are summarized in Table 2. The population was typical for those presenting with advanced lung cancer, with 77% having stage IV disease, a mean age of 62 and with 47% women. The mean Karnofsky PS was 80% and the mean BMI was at the top of the normal range, at 25. The mean weight was 73 kg; only 3% of patients had a low BMI (BMI<18.5), and 15% were characterized as obese with BMI>30.
Table 2.
Patient demographic and anthropomorphic characteristics
| Demographics | Value (Range) | SD |
|---|---|---|
| Age (median years) | 59 (42–84) | 11.08 |
| Female/Male (%) | 45/55 | NA |
| Stage III/IV (%) | 23/77 | NA |
| KPS (%) | 80 (50–100) | 13.65 |
| Weight (Kgs) | 72.97 (45.6–112.9) | 13.38 |
| BMI (kg/m2) | 25.49 (18.3–41.3) | 4.26 |
| SMI (cm2/m2) | 59.24 (38.1–91.1) | 12.26 |
Adequacy of imaging at L1 for SMI (skeletal muscle index) evaluation
Of the 73 CT scans, 94.5% included L1 (95% CI = 86.6% – 98.5%). Inclusion of L1 differed only slightly among the 3 institutions ranging from 90.4% to 96.7% of CT scans. Additionally, 3 images (4%) were difficult to evaluate due to the following reasons: one patient was too obese for proper imaging, one had poor quality scans and one patient had marked effusions making it difficult to distinguish muscle accurately with the imaging and software.
Detection of Sarcopenia and relationship to BMI and Weight
As seen in Figure 1, sarcopenia was found in 14.7% of all patients; in 34.6% of males (the skeletal muscle index or SMI < 52.4 cm2/m2) and in 5.8% of females (SMI < 38. cm2/m2) at baseline. In contrast, as seen in Figure 2, there was a weak correlation between BMI and SMI of r= 0.329 at L1 level (with a low coefficient of determination, r2=0.108). Weight and SMI were not well correlated (r=0.389, r2=0.151).
Figure 1. Skeletal Muscle Index at L1 level Computed Tomography Images.
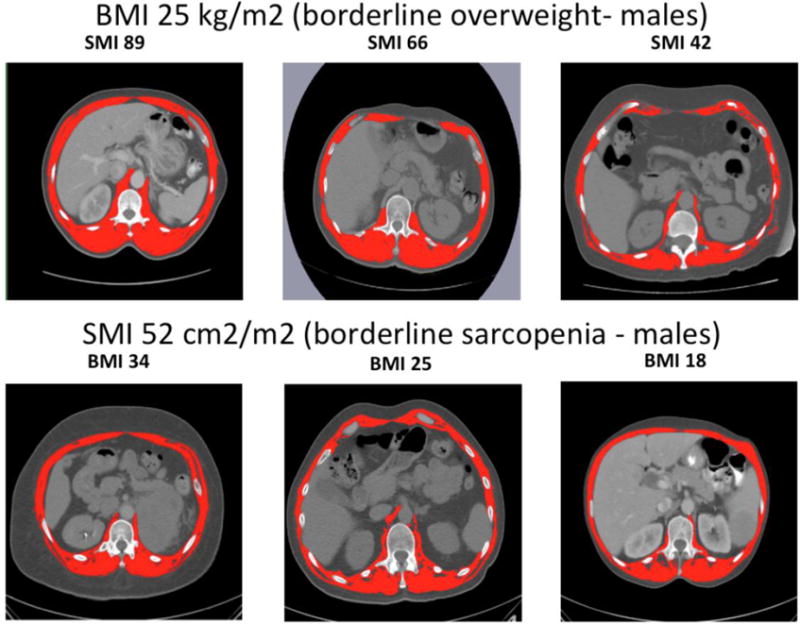
Upper row: Difference between patients with BMI 25 kg/m2 (borderline overweight) and different levels of SMI as determined by CT scan. Lower row: Difference between patients with SMI 52 cm2/m2 (borderline sarcopenia - males) and different levels of BMI. Muscle mass tagged in red.
Figure 2.
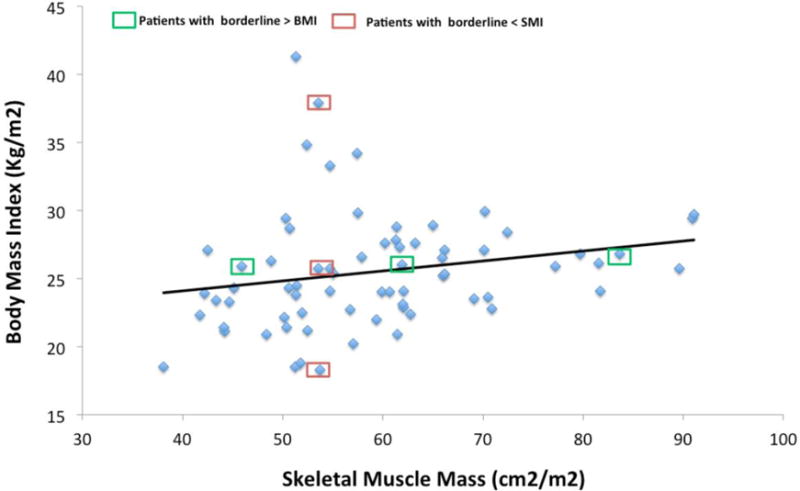
Correlation of Skeletal Muscle Index at L1 level with Body Mass Index
Discussion
In this study, Skeletal Muscle Index (SMI) evaluation in routine chest CT scans in patients with lung cancer was practicable at L1 level. This was demonstrated by the fact that 93% of scans from three different institutions had acceptable quality for evaluation. L1 inclusion varied from 90% – 97% given the different radiologic protocols employed at various institutions. These results are superior when compared to prior lung cancer studies reporting L3 level CT image inclusion ranging from 65% to 84% in four prior studies (Prado et al 84% N=275 [30], and Stene et al 84% N=54 [10], Martin et al 70% N=2115 [18], Sun L et al 65% N=140 [24]). Two studies reported unacceptable images in 3.6% and 18% of included patients [10,30], while the current study found the frequency of unacceptable images in 4% due to conditions that were likely to be present at the L3 level as well.
Wasting of skeletal muscle is common in patients with NSCLC, even in those with normal or high body weight or normal BMI, and is associated with poor outcomes, as its severity muscle wasting increases, mortality rates increase as well. In a recent study in patients with NSCLC, sarcopenia was found on 61% of men and in 31% of women at L3 level (N414) [31], independently, our evaluation showed 34% of men had sarcopenia whereas women had a lower of 6% at L1 level. This major results difference is likely due to continuing evolving cut-points. As previously reported, Martin, et. al. proposes a novel and detailed definition of sarcopenia established by a retrospective stratification including cut off values of weight loss >8% and BMI ≥25 cm2/m2 [18]. This would then define SMIs at different levels (<53 cm2/m2 for males and <41 cm2/m2 for females). This newer SMI definition would have included more of the current patients as having sarcopenia. Other authors have adjusted their definitions for sarcopenia according to their populations’ skeletal muscle mass and target outcomes [32, 33]. Most recently, Kim EY et. al. estimated a specific L1M1 cut-points for a Korean weight and BMI baseline population to an even lower level for males (<46 cm2/m2) and for females (<29 cm2/m2), with sensitivity, specificity and accuracy of 98.2, 100 and 98.9 % respectively [25]. Determination of specific cut-points for the L1 level continues to evolve and may be tailored to specific populations in the future.
Selecting the optimum method for assessing body composition using anthropometry depends on the purpose (i.e. evaluating obesity or undernutrition) and requires practitioners to have a good understanding of both practical and theoretical limitations [19]. BMI is not adequate for estimation of muscle mass at either the L3 or the L1 level, as shown in this study with a weak correlation of r= 0.33. This is similar to prior studies at the L3 levels showing the following correlation in various malignancies: a) in NSCLC (r=0.35, in 441 patients) by Baracos et al [31]; b) in SCLC r=0.597 (n=149) by Kim et al [11]; c) in Lung and GI cancers r=0.35 (n=1400) by Martin et al [18]; d) in lymphomas r=0.31 (n=46) by Sarkozy et al [34]; and e) in colorectal cancer r=0.42 9 (n=684) by Prado et al [30]. The coefficient of determination (r2=0.11) showed that only 11% of the total variation in BMI could be explained by the linear relationship between BMI and SMI while the other 89% of the total variation in BMI remains unexplained. Skeletal muscle mass loss and function may precede overt cachexia, demonstrating the importance of evaluating sarcopenia, rather than weight loss alone. CT images may reveal otherwise occult muscle depletion [9], including reported sarcopenic obesity in 15% of Lung cancer patients [30]. The current study found a 22% rate of sarcopenic obesity in this lung cancer population. On the other hand, an increase in muscle mass during chemotherapy, has been shown to be associated with improved survival [10] but without a correlation with an increase in BMI.
Advanced lung cancer continues to lead in cancer death for both men and women in the U.S. [35], often presenting with muscle loss, impaired physical activity, and diminished quality of life. The early recognition of sarcopenia, often manifesting before decline in weight or BMI, supports evaluation of muscle mass through already obtained CT scans. Such evaluation can reveal sarcopenia earlier than performance status determination as well [36]. The use of CT at L1 is reliable and presents the opportunity for easy patient evaluation of SMI without the need for additional testing or radiation exposure. Utilization of assessment of muscle mass at L1 enhances identification of patients with sarcopenia, and should permit easier evaluation for patients entered into cancer cachexia clinical trials [37], such as those investigating selective androgen receptors [38], gherlin agonist hormones [39], and other new approaches [40–42].
Acknowledgments
-Source of funding in part from NIH / NCI 1 R01 CA157409.
Footnotes
Conflict of interest
Authors indicated no potential conflict of interest.
Contributors
Conception and Design RJG, ARB, JNG
Data collection ARB, JNG, KS, CC, LMWM, RDG, JG
Data analysis and Interpretation ARB, JNG, RJG
Manuscript writing ARB
Radiological Supervision BG
Final editing and approval ARB, RJG, PJH, LMWM, RDG, RJG
References
- 1.Prado CM, Cushen SJ, Orsso CE, Ryan AM. Sarcopenia and cachexia in the era of obesity: clinical and nutritional impact. Proc Nutr Soc. 2016 May;75(2):188–98. doi: 10.1017/S0029665115004279. 2016. [DOI] [PubMed] [Google Scholar]
- 2.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011 May;12(5):489–95. doi: 10.1016/S1470-2045(10)70218-7. (2011) [DOI] [PubMed] [Google Scholar]
- 3.Morley JE, Anker SD, von Haehling S. Prevalence, incidence, and clinical impact of sarcopenia: facts, numbers, and epidemiology-update 2014. J Cachexia Sarcopenia Muscle. 2014;5:253–259. doi: 10.1007/s13539-014-0161-y. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Veasey Rodrigues H, Baracos VE, Wheler JJ, Parsons HA, Hong DS, Naing A, Fu S, Falchoock G, Tsimberidou AM, Piha-Paul S, Chisholm G, Kurzrock R. Body composition and survival in the early clinical trials setting. Eur J Cancer. 2013 Jul 15; doi: 10.1016/j.ejca.2013.06.026. (2013) [DOI] [PubMed] [Google Scholar]
- 5.Gibson DJ, Burden ST, Strauss BJ, Todd C, Lal S. The role of computed tomography in evaluating body composition and the influence of reduced muscle mass on clinical outcome in abdominal malignancy: a systematic review. Eur J Clin Nutr. 2015 Oct;69(10):1079–86. doi: 10.1038/ejcn.2015.32. (2015) [DOI] [PubMed] [Google Scholar]
- 6.Levolger S, van Vugt JL, de Bruin RW, IJzermans JN. Systematic review of sarcopenia in patients operated on for gastrointestinal and hepatopancreatobiliary malignancies. Br J Surg. 2015 Nov;102(12):1448–58. doi: 10.1002/bjs.9893. (2015) [DOI] [PubMed] [Google Scholar]
- 7.Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur J Cancer. 2016 Feb 13;57:58–67. doi: 10.1016/j.ejca.2015.12.030. (2016) [DOI] [PubMed] [Google Scholar]
- 8.Kazemi-Bajestani SM, Mazurak VC, Baracos V. Computed tomography-defined muscle and fat wasting are associated with cancer clinical outcomes. Semin Cell Dev Biol. 2016 Jun;54:2–10. doi: 10.1016/j.semcdb.2015.09.001. (2016) [DOI] [PubMed] [Google Scholar]
- 9.Collins J, Noble S, Chester J, Coles B, Byrne A. The assessment and impact of sarcopenia in lung cancer: a systematic literature review. BMJ Open. 2014 Jan 2;4(1):e003697. doi: 10.1136/bmjopen-2013-003697. (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stene GB, Helbostad JL, Amundsen T, Sørhaug S, Hjelde H, Kaasa S, Grønberg BH. Changes in skeletal muscle mass during palliative chemotherapy in patients with advanced lung cancer. Acta Oncol. 2015 Mar;54(3):340–8. doi: 10.3109/0284186X.2014.953259. (2015) [DOI] [PubMed] [Google Scholar]
- 11.Kim EY, Kim YS, Park I, Ahn HK, Cho EK, Jeong YM. Prognostic Significance of CT-Determined Sarcopenia in Patients with Small-Cell Lung Cancer. J Thorac Oncol. 2015 Dec;10(12):1795–9. doi: 10.1097/JTO.0000000000000690. (2015) [DOI] [PubMed] [Google Scholar]
- 12.Suzuki Y, Okamoto T, Fujishita T, Katsura M, Akamine T, Takamori S, Morodomi Y, Tagawa T, Shoji F, Maehara Y. Clinical implications of sarcopenia in patients undergoing complete resection for early non-small cell lung cancer. Lung Cancer. 2016 Nov;101:92–97. doi: 10.1016/j.lungcan.2016.08.007. (2016) [DOI] [PubMed] [Google Scholar]
- 13.Nattenmüller J, Wochner R, Muley T, Steins M, Hummler S, Teucher B, Wiskemann J, Kauczor HU, Wielpütz MO, Heussel CP. Prognostic Impact of CT-Quantified Muscle and Fat Distribution before and after First-Line-Chemotherapy in Lung Cancer Patients. PLoS One. 2017 Jan 20;12(1):e0169136. doi: 10.1371/journal.pone.0169136. (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lustgarten MS, Fielding RA. Assessment of analytical methods used to measure changes in body composition in the elderly and recommendations for their use in phase II clinical trials (2011) J Nutr Health Aging. 2011;15:368–375. doi: 10.1007/s12603-011-0049-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prado CM, Heymsfield SB. Lean tissue imaging: a new era for nutritional assessment and intervention. JPEN J Parenter Enteral Nutr. 2014 Nov;38(8):940–53. doi: 10.1177/0148607114550189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yip C, Dinkel C, Mahajan A, Siddique M, Cook GJ, Goh V. Imaging body composition in cancer patients: visceral obesity, sarcopenia and sarcopenic obesity may impact on clinical outcome. Insights Imaging. 2015 Aug;6(4):489–97. doi: 10.1007/s13244-015-0414-0. (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boutin RD, Yao L, Canter RJ, Lenchik L. Sarcopenia: Current Concepts and Imaging Implications. AJR Am J Roentgenol. 2015 Sep;205(3):W255–66. doi: 10.2214/AJR.15.14635. 2015. [DOI] [PubMed] [Google Scholar]
- 18.Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, Murphy R, Ghosh S, Sawyer MB, Baracos VE. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539–1547. doi: 10.1200/JCO.2012.45.2722. 2013. [DOI] [PubMed] [Google Scholar]
- 19.Madden AM, Smith S. Body composition and morphological assessment of nutritional status in adults: a review of anthropometric variables. J Hum Nutr Diet. 2016 Feb;29(1):7–25. doi: 10.1111/jhn.12278. 2016. [DOI] [PubMed] [Google Scholar]
- 20.Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008 Oct;33(5):997–1006. doi: 10.1139/H08-075. 2008. [DOI] [PubMed] [Google Scholar]
- 21.Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, Heymsfield SB, Heshka S. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol. 2004 Dec;97(6):2333–8. doi: 10.1152/japplphysiol.00744.2004. (2004) [DOI] [PubMed] [Google Scholar]
- 22.Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, Heymsfield SB, Heshka S. Visceral adipose tissue: relations between single-slice areas and total volume. Am J Clin Nutr. 2004 Aug;80(2):271–8. doi: 10.1093/ajcn/80.2.271. (2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol. 1998 Jul;85(1):115–122. doi: 10.1152/jappl.1998.85.1.115. 1985. 1998. [DOI] [PubMed] [Google Scholar]
- 24.Sun L, Quan XQ, Yu S. An Epidemiological Survey of Cachexia in Advanced Cancer Patients and Analysis on Its Diagnostic and Treatment Status. Nutr Cancer. 2015;67(7):1056–62. doi: 10.1080/01635581.2015.1073753. 2015. [DOI] [PubMed] [Google Scholar]
- 25.Kim EY, Kim YS, Park I, Ahn HK, Cho EK, Jeong YM, Kim JH. Evaluation of sarcopenia in small-cell lung cancer patients by routine chest CT. Support Care Cancer. 2016 Nov;24(11):4721–6. doi: 10.1007/s00520-016-3321-0. (2016) [DOI] [PubMed] [Google Scholar]
- 26.Swartz JE, Pothen AJ, Wegner I, Smid EJ, Swart KM, de Bree R, Leenen LP, Grolman W. Feasibility of using head and neck CT imaging to assess skeletal muscle mass in head and neck cancer patients. Oral Oncol. 2016 Nov;62:28–33. doi: 10.1016/j.oraloncology.2016.09.006. (2016) [DOI] [PubMed] [Google Scholar]
- 27.Gralla RJ, Edelman MJ, Detterbeck FC, Jahan TM, Loesch DM, Limentani SA, Govindan R, Peng G, Monberg MJ, Obasaju CK, Socinski MA. Assessing quality of life following neoadjuvant therapy for early stage non-small cell lung cancer (NSCLC): results from a prospective analysis using the Lung Cancer Symptom Scale (LCSS) Support Care Cancer. 2009 Mar;17(3):307–13. doi: 10.1007/s00520-008-0489-y. (2009) [DOI] [PubMed] [Google Scholar]
- 28.Hollen PJ, Gralla RJ, Stewart JA, Meharchand JM, Wierzbicki R, Leighl N. Can a computerized format replace a paper form in PRO and HRQL evaluation? Psychometric testing of the computer-assisted LCSS instrument (eLCSS-QL) Support Care Cancer. Jan;21(1):165–72. doi: 10.1007/s00520-012-1507-7. (20130. (2013) [DOI] [PubMed] [Google Scholar]
- 29.Irving BA, Weltman JY, Brock DW, Davis CK, Gaesser GA, Weltman A. NIH Image and Slice-O-Matic computed tomography imaging software to quantify soft tissue. Obesity (Silver Spring) 2007 Feb;15(2):370–6. doi: 10.1038/oby.2007.573. (2007) [DOI] [PubMed] [Google Scholar]
- 30.Prado CM, Liefers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, Baracos VE. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629–635. doi: 10.1016/S1470-2045(08)70153-0. 2008. [DOI] [PubMed] [Google Scholar]
- 31.Baracos VE, Reiman T, Mourtzakis M, Gioulbasanis I, Antoun S. Body composition in patients with non-small cell lung cancer: a contemporary view of cancer cachexia with the use of computed tomography image analysis. Am J Clin Nutr. 2010 Apr;91(4):1133S–1137S. doi: 10.3945/ajcn.2010.28608C. (2010) [DOI] [PubMed] [Google Scholar]
- 32.Fujiwara N, Nakagawa H, Kudo Y, Tateishi R, Taguri M, Watadani T, Nakagomi R, Kondo M, Nakatsuka T, Minami T, Sato M, Uchino K, Enooku K, Kondo Y, Asaoka Y, Tanaka Y, Ohtomo K, Shiina S, Koike K. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol. 2015;63:131–40. doi: 10.1016/j.jhep.2015.02.031. 2015. [DOI] [PubMed] [Google Scholar]
- 33.van Dijk DP, Bakens MJ, Coolsen MM, Rensen SS, van Dam RM, Bours MJ, Weijenberg MP, Dejong CH, Olde Damink SW. Low skeletal muscle radiation attenuation and visceral adiposity are associated with overall survival and surgical site infections in patients with pancreatic cancer. J Cachexia Sarcopenia Muscle. 2016 Oct 26; doi: 10.1002/jcsm.12155. (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarkozy C, Camus V, Tilly H, Salles G, Jardin F. Body mass index and other anthropometric parameters in patients with diffuse large B-cell lymphoma: physiopathological significance and predictive value in the immunochemotherapy era. Leuk Lymphoma. 2015 Jul;56(7):1959–68. doi: 10.3109/10428194.2014.979412. (2015) [DOI] [PubMed] [Google Scholar]
- 35.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017 Jan;67(1):7–30. doi: 10.3322/caac.21387. (2017) [DOI] [PubMed] [Google Scholar]
- 36.Bruggeman AR, Kamal AH, LeBlanc TW, Ma JD, Baracos VE, Roeland EJ. Cancer Cachexia: Beyond Weight Loss. J Oncol Pract. 2016 Nov;12(11):1163–1171. doi: 10.1200/JOP.2016.016832. (2016) [DOI] [PubMed] [Google Scholar]
- 37.Roeland EJ, Ma JD, Nelson SH, Seibert T, Heavey S, Revta C, Gallivan A, Baracos VE. Weight loss versus muscle loss: re-evaluating inclusion criteria for future cancer cachexia interventional trials. Support Care Cancer. 2017 Feb;25(2):365–369. doi: 10.1007/s00520-016-3402-0. (2017) [DOI] [PubMed] [Google Scholar]
- 38.Crawford J, Prado CM, Johnston MA, Gralla RJ, Taylor RP, Hancock ML, Dalton JT. Study Design and Rationale for the Phase 3 Clinical Development Program of Enobosarm, a Selective Androgen Receptor Modulator, for the Prevention and Treatment of Muscle Wasting in Cancer Patients (POWER Trials) Curr Oncol Rep. 2016 Jun;18(6):37. doi: 10.1007/s11912-016-0522-0. (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Temel JS, Abernethy AP, Currow DC, Friend J, Duus EM, Yan Y, Fearon KC. Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double-blind, phase 3 trials. Lancet Oncol. 2016 Apr;17(4):519–31. doi: 10.1016/S1470-2045(15)00558-6. (2016) [DOI] [PubMed] [Google Scholar]
- 40.Prado CM, Sawyer MB, Ghosh S, Lieffers JR, Esfandiari N, Antoun S, Baracos VE. Central tenet of cancer cachexia therapy: do patients with advanced cancer have exploitable anabolic potential? Am J Clin Nutr. 2013;98:1012–19. doi: 10.3945/ajcn.113.060228. 2013. [DOI] [PubMed] [Google Scholar]
- 41.Argilés JM, Busquets S, Stemmler B, López-Soriano FJ. Cachexia and sarcopenia: mechanisms and potential targets for intervention. Curr Opin Pharmacol. 2015 Jun;22:100–6. doi: 10.1016/j.coph.2015.04.003. (2015) [DOI] [PubMed] [Google Scholar]
- 42.Tarricone R, Ricca G, Nyanzi-Wakholi B, Medina-Lara A. Impact of cancer anorexia-cachexia syndrome on health-related quality of life and resource utilisation: A systematic review. Crit Rev Oncol Hematol. Dec 22;:pii. doi: 10.1016/j.critrevonc.2015.12.008. (2015) S1040-8428(15)30097-4. [DOI] [PubMed] [Google Scholar]


