Abstract
During cytokinesis, the mitotic spindle communicates with the cell cortex to position a cleavage furrow that will cut through the cell in the plane defined by the metaphase plate. We investigated the molecular basis of this communication in Xenopus laevis eggs, where the signal has to travel ~400 μm in ~30 min to reach the cortex from the first anaphase spindle. At anaphase onset, huge microtubule asters grow out from the poles of the spindle and meet at the plane previously defined by the metaphase plate. This disc-shaped boundary plane recruits the chromosome passenger complex (CPC) and centralspindlin to antiparallel microtubule bundles. It grows out to the cell cortex as the asters expand, where it induces the furrow. CPC and centralspindlin were not recruited to boundaries between asters from different spindles, suggesting a role of chromatin in triggering the CPC-positive state. Recruitment of CPC to aster boundaries was reconstituted in an extract system, and we observed that recruitment was stimulated by proximity to chromatin. Finally, we discuss models for molecular processes involved in initiation and growth of the CPC-positive disc that communicates the position of the metaphase plate to the cortex over hundreds of micrometers in frog eggs.
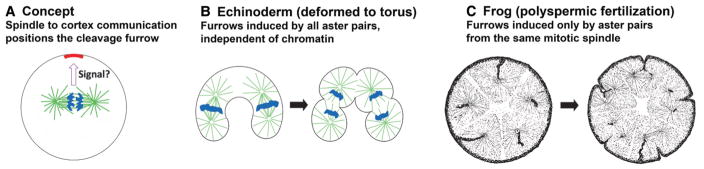
During cytokinesis in animal cells an actomyosin-powered cleavage furrow cleaves the cell into two daughters. The furrow is precisely positioned in space and time to ensure that each daughter is approximately equal in volume and contains an intact, single genome. It has long been recognized that the cleavage furrow initiates after anaphase onset and tends to cut through the plane defined by the metaphase plate. Our focus is on the positioning of the furrow. Rappaport (1971, 1986) used elegant micromanipulation experiments in echinoderm eggs to show that the anaphase spindle transmits a spatially restricted signal to the egg cortex, which is uniformly receptive to furrow induction during a defined cell cycle window (Fig. 1A). Similar signaling occurs in somatic cells, and spindle-to-cortex communication during cytokinesis is perhaps the most famous example of spatial communication between two parts of the same cell. Rappaport showed that the signal propagates outward from the anaphase spindle at a rate of 6–7 μm/min and involves the concerted action of the pair of microtubule asters that emanate from the poles of the spindle, which we will call sister asters. He and others considered models where the aster pair promotes contractility at the equator (“equatorial stimulation”) or inhibits it at the poles (“polar relaxation”). Part of Rappaport’s evidence supporting that the signal is transmitted by a pair of asters came from a famous micromanipulation experiment where asters from two different spindles were brought into proximity during anaphase, whereupon they induced an ectopic furrow (Fig. 1B). This experiment also showed that chromatin is not required for spindle-to-cortex communication in some systems.
Figure 1.
Spindle-to-cortex communication in different systems. (A) In most animal cells the spindle sends a signal to localize the cleavage furrow, which then cuts through the cell in the plane defined by the earlier metaphase plate. (B) In mechanically deformed echinoderm eggs, aster pairs trigger furrows whether they grow from the same or different spindles. This shows that chromatin is not essential to general the furrow signal. (C) In polyspermic frog eggs, only aster pairs from the same spindle trigger furrows. These images are drawn from sections of fixed frog eggs after polyspermic fertilization. The left egg was fixed before first mitosis illustrating five sperm asters. Black trails mark the path of the sperm as it moves into the cytoplasm after entering the egg. The right egg was fixed during first cleavage. It illustrates cleavage with five spindles, where each furrow cuts through the sites previously occupied by a metaphase plate. Note the lack of furrows between spindles, unlike the Echinoderm system. (B, Redrawn from Rappaport and Conrad 1963, with estimated chromatin localization added in blue; C, modified from Brachet 1910.)
Here, we will consider spindle-to-cortex communication in frog eggs and focus entirely on equatorial stimulation, because there is currently little evidence for polar relaxation in frog eggs. Given the molecular conservation of cell division proteins in general, there is every reason to expect that frog eggs use the same molecules and mechanisms to signal from the spindle to the cortex as echinoderm eggs and somatic cells. However, their emergent behavior may differ in interesting ways. For example, classic observations in polyspermic frog eggs showed that furrows are only induced between asters that emanate from the same spindle and are not induced between spindles (Fig. 1C; Brachet 1910; Herlant 1911). This suggests a role for chromatin in spindle-to-cortex communication, which we explore below.
Frog eggs are also huge. The Xenopus eggs we study are ~1.2 mm in diameter, more than 10 times the size of the Rappaport’s echinoderm eggs. The distance between the first metaphase spindle and the nearest cortex is ~500 μm in Xenopus laevis, and the time interval between anaphase onset and ingression of the first furrow is ~30 min. These large spatial and temporal scales likely require special adaptation of the conserved mechanisms that mediate spindle-to-cortex communication, and might make it easier to observe the relevant molecules in action. Xenopus eggs are opaque because of distributed yolk platelets and lipid droplets, which makes live imaging of the intact egg difficult. To get around this problem, we either image fixed and stained eggs in a high refractive index clearing solvent or used a cell-free extract system that reconstitutes the microtubule and actin organization characteristic of cytokinesis (Nguyen et al. 2014; Field et al. 2017).
ASTER GROWTH AND RECRUITMENT OF FURROW-STIMULATING COMPLEXES
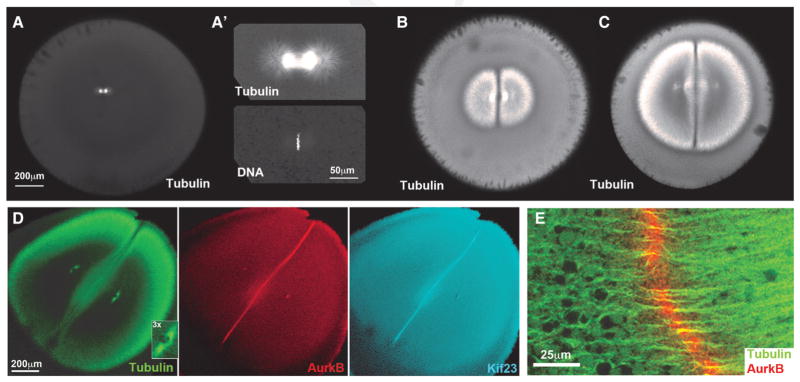
The first metaphase spindle is much smaller than the egg in X. laevis (Fig. 2A, A′). Its position and orientation are determined by dynein-dependent forces actin on the sperm aster before nuclear envelope breakdown (Wühr et al. 2010). At anaphase onset, a pair of huge sister asters grow progressively outward from the poles of the spindle, reaching the cortex ~30 min later (Fig. 2B, C). The microtubules in these asters appear “bushy,” and their density at the aster periphery remains approximately constant as the aster grows (Fig. 2B–D). Egg extract experiments suggest this morphology is due to the aster being built of a network of short microtubules mostly nucleated away from the centrosome (Ishihara et al. 2016). Furrowing initiates when the asters touch the cortex, consistent with spindle-to-cortex communication occurring via aster growth. Treatment of eggs with nocodazole, which inhibits microtubule polymerization, completely blocks furrowing (Haraetal. 1980).
Figure 2.
Aster growth and recruitment of furrow-stimulating complexes in Xenopus laevis eggs. Images are confocal sections viewed with the animal–vegetal axis in the microscope z-axis. The first cleavage furrow cuts through the animal pole, parallel to the z-axis in these images. (A, A′) Egg fixed ~60 min after fertilization, at metaphase of first mitosis. Note the spindle is small compared to the egg. (B, C) Eggs fixed ~70 and ~80 min after fertilization, between first anaphase and first cleavage. Note aster pairs grow out from the poles of the spindle and meet at the midplane. Each aster is dome-shaped, with a sharp plane of lower microtubule density where they meet. In cross section this geometry appears as two D-shaped asters arranged back-to-back with a line between them. (D) Egg fixed at cleavage initiation, ~90 min after fertilization. The asters are just touching the cortex at the animal pole. AurkB is a subunit of the CPC; Kif23 is a subunit of centralspindlin. Both furrow-stimulating complexes are enriched at a disc between the asters that marks the future path of the cleavage furrow. Note the centrosomes are already positioned for the second round of mitosis and cleavage that will occur orthogonal to the first (3× inset in tubulin panel). (E) Higher-magnification view of the boundary region between asters. Note the CPC localizes to the center of presumably antiparallel microtubule bundles. (A–C, Modified, with permission, from Mitchison et al. 2012; D, modified, with permission, from Field et al. 2015; E, TJ Mitchison and CM Field, unpubl. data.)
A characteristic feature of sister aster pairs in fixed Xenopus eggs is the presence of a sharp boundary between the asters where the microtubule density is lower (Fig. 2B–D). The boundary region contains microtubule bundles that we interpret as antiparallel overlaps (Fig. 2E). It defines a plane at the center of the egg that extends to the cortex, and predicts the path that the furrow will later follow as it ingresses. Because sister asters grow symmetrically from the poles of the spindle, the boundary plane between them coincides with the plane previously occupied by the metaphase plate. Therefore, radial growth of the boundary plane between asters provides a structural mechanism for propagating information on the localization of the metaphase plate to the cortex. As the asters grow, the boundary between them remains at the midplane of the egg while the centrosomes and nuclei at their centers move apart, so they are approximately centered in between the midplane and the cortex by the time the furrow ingresses (Wühr et al. 2010; Mitchison et al. 2012). This centering movement ensures that the furrow will cut between the separating nuclei and also positions the centrosomes for the next round of mitosis and cleavage. Prepositioning of centrosomes for the next round of cleavage is evident in the tubulin image, Figure 2D (3× inset), where the two centrosomes within each aster have moved to the center of the half-cell and split apart parallel to the boundary between the asters. After first cleavage, each centrosome pairs will establish a second mitotic spindle on this axis, which will cause the second furrows to cleave orthogonal to the first. We hypothesized that this prepositioning of centrosomes was accomplished by dynein pulling on astral microtubules (Wühr et al. 2010).
To understand how the boundary between sister asters instructs cleavage furrow assembly, we localized conserved cytokinesis proteins that are known to have this function in other system. Two conserved microtubule binding complexes, chromosome passenger complex (CPC) and centralspindlin, promote furrow assembly in somatic cells. CPC is a 1:1:1:1 complex of AURKB, INCENP, borealin/dasra/CDCA8, and survivin/BIRC5 (Ruchaud et al., 2007). In Xenopus eggs, CDCA8 is replaced by the egg-specific ortholog Dasra2/CDCA9 (Sampath et al., 2004). The AURKB subunit of the CPC is a multifunctional kinase that promotes autophosphorylation and autoactivation of the CPC when it clusters on chromatin or microtubules. CPC is transported to microtubule plus ends by MKLP2/Kif20A, where it is thought to activate other cytokinesis proteins including centralspindlin (Gruneberg et al. 2004). Centralspindlin is a 2:2 complex of RACGAP1 and MKLP1/KIF23. It is transported to plus ends by its intrinsic kinesin activity and functions to activate RhoA and contractility (White and Glotzer 2012; Mishima 2016). Both CPC and centralspindlin accumulate on antiparallel microtubule bundles at the boundary between sister asters in frog eggs, defining a disc between the asters that will trigger the furrow when it touches the cortex (Fig. 2D, E). We believe that this CPC-centralspindlin-positive disc between the asters, which initiates at anaphase and then grows with the asters, mediates spindle-to-cortex communication in frog eggs.
ONLY ASTER PAIRS FROM THE SAME SPINDLE RECRUIT CPC AND CENTRALSPINDLIN
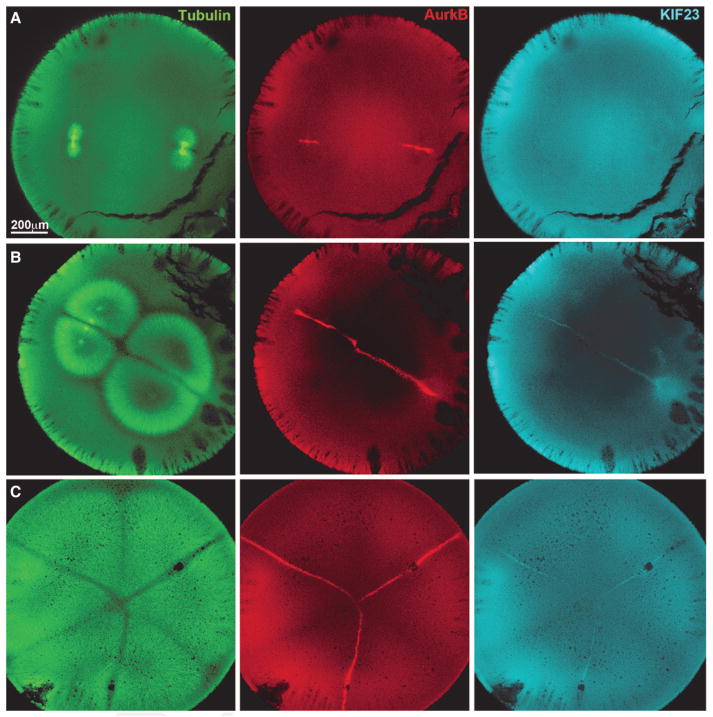
In polyspermic frog eggs, only aster pairs from the same spindle triggered furrows (Fig. 1C). We repeated this classic experiment and stained for CPC and centralspindlin. Figure 3 shows polyspermic eggs fixed at different times between first anaphase and first cleavage. CPC and centralspindlin were recruited to the boundary plane between asters from the same spindle but not to planes where asters from different spindle met. Recruitment of CPC is better visualized, in part because it is more abundant in eggs (Field et al. 2015). These images also provide a clear view of the growth of the furrow-simulating plane between sister asters, starting as a small disc making the plane previously occupied by the metaphase plate, then growing outward normal to the axis of chromosome separation. These observations provide a molecular explanation for the classic observation that only aster pairs from the same spindle induce furrows in frog eggs, but they prompt the question of why only these asters pairs recruit furrow-simulating complexes to their shared boundary. This could be due to the presence of chromatin at anaphase, timing differences in when the asters meet, or other factors. This selectivity is not absolute and can be overridden by experimental perturbation. Artificially boosting CPC activation with an injected antibody, reducing the distance between asters by highly polyspermic fertilization, or stabilizing microtubules with paclitaxel all triggered indiscriminant recruitment of CPC to aster boundaries independent of their origin (Field et al. 2015). Evidently, the requirement that the two spindles originate from the same spindle to induce furrows only holds under normal conditions.
Figure 3.
Furrow-stimulating complexes in polyspermic Xenopus laevis eggs. Eggs fixed at successive times between first anaphase and first cleavage, with confocal sections oriented as in Figure 2. Cleavage has just initiated at the animal pole in C. The eggs in A and B were fertilized with two sperm and in C with four, but asters are only visible for three. Note recruitment of CPC and centralspindlin to a subset of boundaries between asters in B and C. In B, the aster morphology shows that the CPC-positive boundaries are between asters from the same spindle. In C, we infer the same is true given classic evidence that furrows cut through spindles in frog eggs and not between them (Fig. 1C). (Modified, with permission, from Field et al. 2015.)
The CPC was discovered by Earnshaw and Cooke (1991), and named to reflect its localization to chromatin in metaphase and midzone microtubule bundles in anaphase–telophase. They proposed that the CPC was transported to the metaphase plate by chromosomes, where it later instructs the furrow. This model was questioned by experiments showing that the CPC can be recruited to microtubule bundles between spindles in somatic cells (Savoian et al. 1999) in a somatic cell version of Figure 1B. The apparent chromatin requirement for CPC localization in polyspermic Xenopus eggs (Fig. 3) triggered our interest in testing if proximity to chromatin could stimulate formation of aster boundaries with furrow-inducing potential in an egg extract system.
RECRUITMENT OF CPC AND CENTRALSPINDLIN TO ASTER BOUNDARIES IN EGG EXTRACT
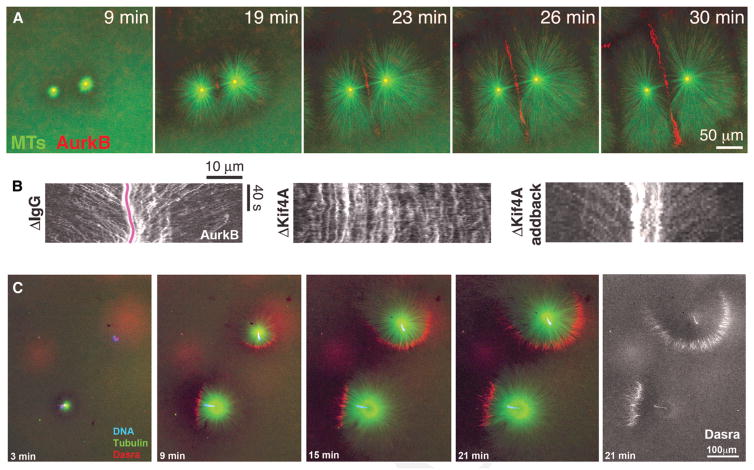
We turned to an actin-intact egg extract system to probe how the boundary region between asters forms, grows, accumulates CPC and centralspindlin, and signals to the cortex. Figure 4A shows a typical experiment where the extract was squashed to a ~20-μm deep layer between two passivated coverslips, illustrating aster growth and recruitment of CPC to a boundary between asters. Centralspindlin localized in a similar manner (Nguyen et al. 2014). CPC recruitment to aster boundaries was stimulated by low concentrations of paclitaxel or inhibition of the catastrophe factor MCAK, suggesting that microtubule stabilization plays a role in CPC recruitment (Field et al. 2015). To probe how CPC is recruited we used total internal reflection fluorescence (TIRF) microscopy on reactions similar to Figure 4A. We observed individual CPC aggregates moving toward microtubule plus ends and accumulating at the aster boundary. Figure 4B shows kymograph analysis of the movement, in which the red line in the first panel traces the center of the boundary region. Note multiple CPC aggregates moving inward. This movement required two plus end–directed kinesins, Kif20A and Kif4. When Kif20Awas depleted, we observed no CPC recruitment to microtubules, consistent with a central role of this kinesin, which is also called MKLP2, in CPC recruitment (Nguyen et al. 2014). This observation is consistent with a Kif20A/MKLP2 requirement for CPC localization to midzones in somatic cells (Gruneberg et al. 2004). Removal of Kif4A, which is involved in microtubule length regulation in midzones (Hu et al. 2011), still allowed CPC accumulation as boundaries between asters but appeared to block the active transport we could visualize (Fig. 4B). In more complex experiments designed to reconstitute signaling to the cortex, we layered extract over a supported lipid bilayer and observed recruitment of RhoA.GTP to the bilayer in proximity to CPC recruited to boundaries between asters, showing that the extract system can fully reconstitute spindle-to-cortex signaling (Nguyen et al. 2014).
Figure 4.
Recruitment of CPC to aster boundaries in egg extract. Actin-intact extract from unfertilized eggs was supplemented with imaging probes, converted to interphase with a calcium transient, and squashed between passivated coverslips. (A) Widefield imaging of asters nucleated by anti-AurkA coated beads. CPC was visualized with anti-AurkB. Note recruitment of CPC to antiparallel bundles at the boundary between asters. (B) Total internal reflection fluorescence (TIRF) imaging of CPC movement in reactions similar to A. Movies were recorded at CPC-positive boundaries between asters, and kymograph analysis was used to visualize movement of CPC aggregates. The pink line denotes the center of the boundary region. Diagonal streaks represent movement of CPC aggregates toward plus ends at ~15 μm/min. CPC recruitment to microtubules depended on an egg ortholog of Kif20Awe called Kif20AE, and movement depended on Kif4A as shown by this depletion-add back experiment. (C) Widefield imaging of asters nucleated by sperm centrosomes. CPC was imaged with GFP-DasraB. Note formation of polarized monopolar asters with CPC recruited to a crescent at the periphery. The CPC-positive crescent always formed on the chromatin-proximal side of asters and usually expanded radially as the asters grew. (A, B, Modified, with permission, from Nguyen et al. 2014; C, modified, with permission, from Field et al. 2015.)
Chromatin was not required for assembly of CPC- and centralspindlin-positive zones between asters in the extract system (Fig. 4A). However, in the absence of chromatin there was a delay between aster–aster contact and CPC recruitment, and only a subset of aster boundaries recruited CPC. CPC recruitment to zones between asters was greatly enhanced by artificially stabilizing microtubules or activating the CPC (Field et al. 2015). These observations suggested chromatin might promote CPC recruitment to aster boundaries if it was present.
To test if chromosomes could stimulate CPC recruitment, as we suspect occurs in eggs, we nucleated asters from permeabilized sperm nuclei with attached centrosomes. We followed the growth of isolated sperm and noted that, as they grew, they usually accumulated CPC on a crescent-shaped region of the aster periphery (Fig. 4C). When this CPC-positive crescent formed it always did so on the side of the aster closest to the sperm chromatin. The chromatin is visible as a blue dot in the color panels and is shown more clearly by the Dasra probe alone in the last panel. CPC was recruited, in a chromatin-polarized manner, to unipolar microtubules bundles at the aster periphery in this experiment. Polarized recruitment of CPC to the periphery of monopolar asters was observed previously during monopolar cytokinesis in tissue culture cells (Canman et al. 2003; Hu et al. 2008). In that system, CPC and centralspindlin are recruited during anaphase to the side of a monopolar microtubule array that is closest to chromatin, and a partial furrow is later induced on the CPC-positive side.
In the extract monopolar system, CPC was first recruited to the aster periphery around the time when the aster periphery was growing past chromatin (Fig. 4C, 9 min). As asters grew out further, CPC-positive crescents persisted and tended to expand radially (see the upper aster in Fig. 4C). By the end of the experiment, the CPC-positive crescent at the aster periphery was >100 μm from the nearest chromatin, spanned more than half that aster circumference, and contained much more CPC that was present when it initially formed close to chromatin. These observations do not support a simple chromosome passenger model, because the amount of CPC present at the aster periphery at late times is much larger than that which could have been initially recruited from chromatin. CPC recruitment to aster boundaries far from chromatin in frog eggs also seems inconsistent with a chromosome passenger model. Rather, we prefer a model where proximity to chromatin triggers initial formation of a CPC-positive crescent, and, once formed, this crescent persists, grows and recruits more CPC, all independent of chromatin.
DISCUSSION
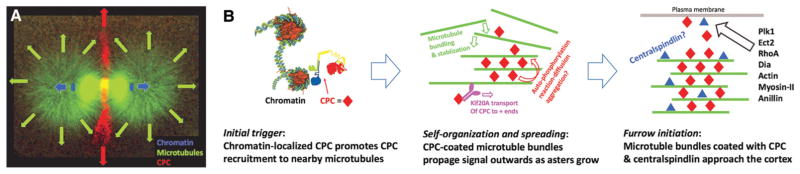
Putting together our observations in eggs and egg extract, we propose the model shown in Figure 5. Figure 5A illustrates an early stage in propagation of a spatial signal from the anaphase spindle toward the cortex, where directional arrows and estimated location of chromatin are superimposed on a late-anaphase spindle taken from the earliest time point in Figure 3. The arrows indicate chromatin movement (blue), aster growth (green), and growth of the CPC-positive disc of microtubule bundles (red). This disc expands normal to the axis of chromosome movement, and thus transmits information on the position of the metaphase plate outward toward the cortex. Figure 5B illustrates working models for the molecular subprocesses involved in generation, growth, and action of the CPC-positive disc. It initiates in the anaphase spindles at antiparallel microtubule close to chromatin, in an update of Earnshaw’s classic chromosome passenger model. CPC is recruited to metaphase chromatin in Xenopus eggs by binding of its BIRC5/survivin subunit to histone H3 T3 phosphosites that are generated by haspin kinase (Kelly et al. 2010). We hypothesize that haspin-dependent recruitment of CPC to chromatin is important for the chromatin-to-microtubule handoff during anaphase. Once initiated, the CPC-positive disc grows laterally as the asters grow radially. Reactions involved in growth of the disc likely include lateral recruitment of microtubules from the growing asters by bundle formation, CPC recruitment from solution both directly and via KIF20A-mediated microtubule transport, CPC autoactivation by proximity-driven autophosphorylation, and perhaps direct CPC aggregation. When the disc of CPC-coated microtubule bundles reaches the plasma membrane, it triggers furrow assembly by some combination of CPC and centralspindlin stimulation of conserved furrow assembly pathways.
Figure 5.
Working model for spindle-to-cortex communication in frog eggs. (A) Late-anaphase spindle morphology, shortly after initiating spindle-to-cortex communication. The spindle image is taken from the earliest time point in Figure 3. Arrows indicate directions of movement/growth. Note that the furrow-stimulating CPC-positive disc (red arrows) grows outward on a plane orthogonal to the axis of chromosome separation (blue arrows). (B) Models for the molecular events involved in triggering formation of the CPC-positive disc near chromatin (left), growing it outward (middle), and signaling to the cortex (right).
The model in Figure 5 provides a preliminary structural and molecular basis for Rappaport’s spindle-to-cortex communication in frog eggs. The signal consists of a disc-shaped array of CPC and centralspindlin-coated antiparallel microtubule bundles that grow out from the spindle to the cortex by a combination of aster growth and CPC-powered self-organization. In frog eggs, aster boundaries that lack the initial trigger from chromatin do not acquire aster-inducing potential (Figs. 1C, 3), though they do in echinoderm eggs (Fig. 1B). The model in Figure 5 model makes testable predictions—for example, that we could remove chromatin once the self-organizing CPC-positive disc is established. It poses many unanswered questions at the molecular level—for example, how chromatin triggers the initial assembly of the CPC-positive disc, and how the disc grows by recruiting more microtubules and CPC.
Our focus in investigating the Rappaport signal from spindle to cortex has been on the role of the CPC, whereas others have focused on centralspindlin (Mishima 2016). The CPC is more abundant than centralspindlin in frog eggs (~100 nM vs. ~25 nM) (Wühr et al. 2014), and in our experiments appears to enrich more on microtubules. The CPC plays a major role in organizing microtubule bundles between asters in egg extracts, whereas depleting the Kif23 subunit of centralspindlin had little effect (Nguyen et al. 2014). However, in other systems centralspindlin plays a central role in stimulating RhoA activity at the cortex and inducing furrows. Both complexes are clearly important for spindle-to-cortex communication, although their precise functions differ, as may their relative importance in different systems. Spindle-to-cortex communication has to propagate over an unusually large distance in frog eggs, which might explain their greater reliance on CPC. In particular, the propensity of CPC to auto-activate and aggregate may help the signal travel hundreds of microns. An interesting future question is how the CPC auto-recruitment system is tuned in frog eggs (but not echinoderm eggs) such that the CPC-positive state spreads robustly away from chromatin along antiparallel microtubule bundles, yet does not emerge spontaneously among similar bundles when they are formed between asters that were not part of the same spindle.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grant no. GM39565. Microscopy was supported at Harvard Medical School by the Nikon Imaging Center and at the Marine Biological Laboratory by Nikon Inc. We thank the National Xenopus Resource (NXR) for Xenopus animals and care.
References
- Brachet A. La polyspermie experimental comme moyen d’analyse de la fecondacion. Arch Entwicklungsmech Org. 1910;30:261–303. [Google Scholar]
- Canman JC, Cameron LA, Maddox PS, Straight A, Tirnauer JS, Mitchison TJ, Fang G, Kapoor TM, Salmon ED. Determining the position of the cell division plane. Nature. 2003;424:1074–1078. doi: 10.1038/nature01860. [DOI] [PubMed] [Google Scholar]
- Earnshaw WC, Cooke CA. Analysis of the distribution of the INCENPs throughout mitosis reveals the existence of a pathway of structural changes in the chromosomes during metaphase and early events in cleavage furrow formation. J Cell Sci. 1991;98:443–461. doi: 10.1242/jcs.98.4.443. [DOI] [PubMed] [Google Scholar]
- Field CM, Groen AC, Nguyen PA, Mitchison TJ. Spindle-to-cortex communication in cleaving, polyspermic Xenopus eggs. Mol Biol Cell. 2015;26:3628–3640. doi: 10.1091/mbc.E15-04-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field CM, Pelletier JF, Mitchison TJ. Xenopus extract approaches to studying microtubule organization and signaling in cytokinesis. Methods Cell Biol. 2017;137:395–435. doi: 10.1016/bs.mcb.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruneberg U, Neef R, Honda R, Nigg EA, Barr FA. Relocation of Aurora B from centromeres to the central spindle at the metaphase to anaphase transition requires MKlp2. J Cell Biol. 2004;166:167–172. doi: 10.1083/jcb.200403084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K, Tydeman P, Kirschner M. A cytoplasmic clock with the same period as the division cycle in Xenopus eggs. Proc Natl Acad Sci. 1980;77:462–466. doi: 10.1073/pnas.77.1.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlant M. Recherches sur les oeufs di-et-trispermiques de grenouille. Archs Biol. 1911;26:103–328. [Google Scholar]
- Hu C-K, Coughlin M, Field CM, Mitchison TJ. Cell polarization during monopolar cytokinesis. J Cell Biol. 2008;181:195–202. doi: 10.1083/jcb.200711105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C-K, Coughlin M, Field CM, Mitchison TJ. KIF4 regulates midzone length during cytokinesis. Curr Biol. 2011;21:815–824. doi: 10.1016/j.cub.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara K, Korolev KS, Mitchison TJ. Physical basis of large microtubule aster growth. Elife. 2016;5:e19145. doi: 10.7554/eLife.19145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AE, Ghenoiu C, Xue JZ, Zierhut C, Kimura H, Funabiki H. Survivin reads phosphorylated histone H3 threonine 3 to activate the mitotic kinase Aurora B. Science. 2010;330:235–239. doi: 10.1126/science.1189505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima M. Centralspindlin in Rappaport’s cleavage signaling. Semin Cell Dev Biol. 2016;53:45–56. doi: 10.1016/j.semcdb.2016.03.006. [DOI] [PubMed] [Google Scholar]
- Mitchison T, Wühr M, Nguyen P, Ishihara K, Groen A, Field CM. Growth, interaction, and positioning of microtubule asters in extremely large vertebrate embryo cells. Cytoskeleton. 2012;69:738–750. doi: 10.1002/cm.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen PA, Groen AC, Loose M, Ishihara K, Wühr M, Field CM, Mitchison TJ. Spatial organization of cytokinesis signaling reconstituted in a cell-free system. Science. 2014;346:244–247. doi: 10.1126/science.1256773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport R. Cytokinesis in animal cells. Int Rev Cytol. 1971;31:169–213. doi: 10.1016/s0074-7696(08)60059-5. [DOI] [PubMed] [Google Scholar]
- Rappaport R. Establishment of the mechanism of cytokinesis in animal cells. Int Rev Cytol. 1986;105:245–281. doi: 10.1016/s0074-7696(08)61065-7. [DOI] [PubMed] [Google Scholar]
- Rappaport R, Conrad GW. An experimental analysis of unilateral cleavage in invertebrate eggs. J Exp Zool. 1963;153:99–112. doi: 10.1002/jez.1401530203. [DOI] [PubMed] [Google Scholar]
- Ruchaud S, Carmena M, Earnshaw WC. Chromosomal passengers: Conducting cell division. Nat Rev Mol Cell Biol. 2007;8:798–812. doi: 10.1038/nrm2257. [DOI] [PubMed] [Google Scholar]
- Sampath SC, Ohi R, Leismann O, Salic A, Pozniakovski A, Funabiki H. The chromosomal passenger complex is required for chromatin-induced microtubule stabilization and spindle assembly. Cell. 2004;118:187–202. doi: 10.1016/j.cell.2004.06.026. [DOI] [PubMed] [Google Scholar]
- Savoian MS, Earnshaw WC, Khodjakov A, Rieder CL. Cleavage furrows formed between centrosomes lacking an intervening spindle and chromosomes contain microtubule bundles, INCENP, and CHO1 but not CENP-E. Mol Biol Cell. 1999;10:297–311. doi: 10.1091/mbc.10.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White EA, Glotzer M. Centralspindlin: At the heart of cytokinesis. Cytoskeleton. 2012;69:882–892. doi: 10.1002/cm.21065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wühr M, Tan ES, Parker SK, Detrich HW, III, Mitchison TJ. A model for cleavage plane determination in early amphibian and fish embryos. Curr Biol. 2010;20:2040–2045. doi: 10.1016/j.cub.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wühr M, Freeman RM, Presler M, Horb ME, Peshkin L, Gygi SP, Kirschner MW. Deep proteomics of the Xenopus laevis egg using an mRNA-derived reference database. Curr Biol. 2014;24:1467–1475. doi: 10.1016/j.cub.2014.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]