Summary
Objectives
Of the 215,000 global deaths from rotavirus estimated in 2013, 41% occur in Asian countries. However, despite a recommendation for global rotavirus vaccination since 2009, only eight countries in Asia have introduced rotavirus vaccine into their national immunization program as of September 2017. To help policy makers assess the potential value of vaccination, we projected the reduction in rotavirus hospitalizations and deaths following a hypothetical national introduction of rotavirus vaccines in all countries in Asia using data on national-level rotavirus mortality, <5 population, rotavirus hospitalizations rates, routine vaccination coverage, and vaccine effectiveness.
Methods
To quantify uncertainty, we generated 1,000 simulations of these inputs.
Results
Our model predicted 710,000 fewer rotavirus hospitalizations, a 49% decrease from the 1,452,000 baseline hospitalizations and 35,000 fewer rotavirus deaths, a 40% decrease from the 88,000 baseline deaths if all 43 Asian countries had introduced rotavirus vaccine. Similar reductions were projected in subanalyses by vaccine introduction status, subregion, and birth cohort size.
Conclusion
Rotavirus vaccines will substantially reduce morbidity and mortality due to rotavirus infections in Asia.
Keywords: rotavirus vaccines, rotavirus, diarrhea, Asia, routine vaccination, mortality
Introduction
Rotavirus is estimated to cause 37% of diarrheal deaths among children <5 years old worldwide [1,2]. The disease burden is particularly severe in Asia; regional hospital-based surveillance detects rotavirus at the highest rate in the world in the Southeast Asian region and India has the largest national number of rotavirus deaths of any country [1]. Of the 215,000 estimated global deaths from rotavirus in 2013, 41% (n=89,000) occurred in Asia [1].
In more than a decade since licensure, rotavirus vaccines have been shown to dramatically reduce the morbidity and mortality of severe rotavirus disease [3]. In addition to two live, oral rotavirus vaccines available internationally (RotaTeq, Merck & Co., West Point, PA, USA and Co and Rotarix, GlaxoSmithKline Biologicals, Rixensart, Belgium) [4,5], there are four rotavirus vaccines manufactured in Asia [6]. Two of these rotavirus vaccines, Rotasiil (Serum Institute of India Pvt. Ltd., Pune, India) and ROTAVAC (Bharat Biotech International Ltd., Hyderabad, India), were recently licensed in India after phase 3 efficacy studies; ROTAVAC was recently approved and Rotasiil is being reviewed for World Health Organization (WHO) pre-qualification [7,8]. Additionally, locally licensed and manufactured vaccines are available in China (Lanzhou lamb, Lanzhou Institute of Biological Products Co. Ltd., Lanzhou, China) and Vietnam (Rotavin, PolyVac, Hanoi, Vietnam) [6,9,10].
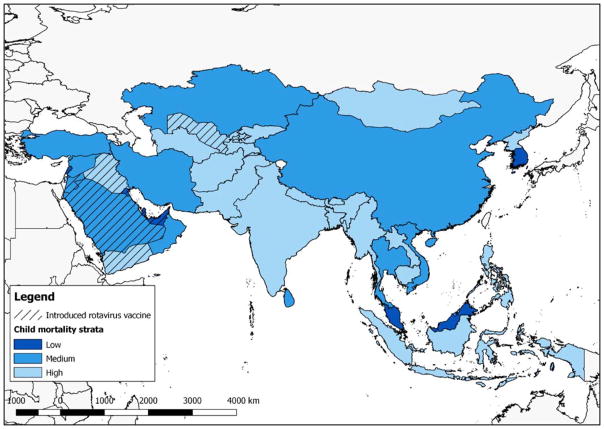
The impact of rotavirus vaccines on morbidity and mortality could be significant in Asia because of the high burden of diarrhea disease and large birth cohorts in several countries, including China, India, Pakistan, Bangladesh and Indonesia. The combine birth cohort of these five countries represents 41% of the global birth cohort. Despite a WHO global recommendation for vaccine use since 2009, of the >80 countries that have introduced a rotavirus vaccine nationally worldwide, only eight are in the region (Figure 1) [11–14]. India and Pakistan began a planned phased introduction of rotavirus vaccines into the routine infant vaccination schedule in some states and districts in 2016, but full national implementation is expected to take several years in both countries. Bangladesh has been approved for rotavirus vaccine use and is expected to introduce the vaccine in 2018.
Figure 1.
Map of countries in Asia as defined by the Millennium Development Indicators, by child mortality strata and rotavirus vaccine introduction status.
To help policy makers assess the potential value of implementing rotavirus vaccination, we projected the reduction in rotavirus hospitalizations and deaths following a hypothetical national introduction of rotavirus vaccines in all countries in Asia.
Methods
We included countries and territories (referred to as “countries”) in this analysis that are in the Eastern Asia, Southern Asia, South-eastern Asia and Western Asia regional groupings defined by the Millennium Development Indicators (Figure 1) [15]. This classification was chosen because WHO regions that include Asian countries overlap with other continents. Countries were further stratified by their 2015 UNICEF <5 year old child mortality rates [16]. The lowest child mortality strata was defined ≤8 deaths per 1,000 live births, which is the 15th percentile among the included countries; medium child mortality as >8–22 deaths per 1,000 live births; and high child mortality as >22 deaths per 1,000 live births (50th percentile among included countries). For sub analyses, we categorized countries by vaccine introduction status, child mortality strata, the Millennium Development Indicator subregions, World Bank income groupings, and Gavi eligibility [12,15–18]. The eight countries that had introduced vaccine nationwide before 2015 were included in the ‘introduced’ group (Bahrain, Iraq, Jordan, Qatar, Saudi Arabia, United Arab Emirates, Uzbekistan, Yemen), and two of these countries (Uzbekistan and Yemen) received Gavi support. Gavi eligibility includes countries currently eligible for Gavi support and countries that received Gavi support for rotavirus vaccine introduction; Gavi provides financial support to countries with a mean per capita gross national income of < $1,580 over the previous 3 years[18].
Literature reviews
Using the PRISMA guidelines, we conducted systematic literature reviews to determine the rate of rotavirus hospitalizations in Asian countries and the vaccine effectiveness of rotavirus vaccines in Asian countries [19]. For hospitalizations rates, we searched PubMed for articles published 1 January 1998– 30 June 2017 whose titles included “rotavirus” and any of the following terms: “burden”, “hospitali*”, “incidence”, “epidemiology”, “surveillance”. We limited results to these years for contemporary data. We limited articles to those that presented a rate of rotavirus diarrhea hospitalization among children <5 years old in an Asian country included in this analysis. In articles that presented more than one rate of rotavirus hospitalizations among children <5 years old, for example, from two different cities or two different countries, all of the published rates were included. We excluded rates from other age groups. Once the literature review was complete, rates were summarized as a median and interquartile range (IQR) by child mortality strata.
For vaccine efficacy and effectiveness (VE), we searched PubMed for articles published 1 January 2006– 30 June 2017 whose titles included “rotavirus” and “vacc*” and “eff*”. Rotarix and RotaTeq were licensed in 2006. We included VE against severe rotavirus disease or hospitalization among children <5 years old for any rotavirus vaccine, except the Lanzhou vaccine because the recommended schedule for administration is substantially different than the other rotavirus vaccines. Articles were included if they presented a pre- or post-licensure VE estimate from at least one Asian country included in this analysis. Once the literature review was complete, rates were summarized as a median and IQR by child mortality strata.
Model construction
Following the methods of Shah et al [20], the expected number of rotavirus hospitalizations was calculated as follows:
Where the <5 population is the national population of children <5 years old in 2015 and hospitalization rate is the strata-specific hospitalization rate calculated from the literature review [16,21].
The number of rotavirus hospitalizations prevented was calculated as follows:
Where expected hospitalizations is the number of expected rotavirus hospitalizations calculated above, VE is the strata-specific VE calculated from the literature review, and coverage is 2016 DTP3 national coverage estimated in the WHO/UNICEF joint reporting form (JRF); if the 2016 estimate was not available, 2015 was used [22]. DTP3 coverage estimates from the Hong Kong Ministry of Health and UNICEF Palestine fact sheet supplemented the JRF data [23,24].
The number of rotavirus deaths prevented was calculated as follows:
Where expected deaths is the number of rotavirus deaths in children <5 year of age in 2013 estimated by WHO [1], VE is the strata-specific VE calculated from the literature review, and coverage is 2016 DTP3 national coverage estimated in the WHO/UNICEF JRF; as above, if the 2016 estimate was not available, 2015 was used [22]. Hong Kong and Palestinian Territories were not included in this portion of the analysis as the expected rotavirus mortality was not available [23,24].
Simulations
To quantify uncertainty, we generated 1,000 simulations of the model inputs. Country-specific rotavirus deaths were randomly selected on a normal distribution from the published point estimate and 95% confidence interval (CI). Hospitalization rate point estimates were randomly selected on a uniform distribution from the hospitalization rate IQR calculated for the appropriate child mortality strata. Similarly, VE point estimates were randomly selected on a uniform distribution from the VE IQR calculated for the appropriate child mortality strata. From the 1,000 simulations, we calculated the median and the point estimate at the 2.5 and 97.5 percentiles as the CIs for the expected and prevented hospitalizations and deaths.
Results
We reviewed 893 articles for hospitalization rates in Asian countries selected for this analysis. Twenty-one articles from 14 countries met our inclusion criteria (Table 1). The median hospitalization rate per 100,000 was 395 (IQR: 200, 542) in low child mortality countries, 277 (IQR: 181, 521) in medium child mortality countries, and 337 (IQR: 240, 633) in high child mortality countries. We reviewed 363 articles for VE in Asian countries. Ten articles from eight countries met our inclusion criteria (Table 2). The median VE was 94% (IQR: 92%, 96%) in low child mortality countries, 64% (IQR: 63%, 81%) in medium child mortality countries, and 49% (IQR: 33%, 55%) in high child mortality countries.
Table 1.
Summary of published rotavirus hospitalization rates among children 0–59 months of age in Asian countries
| Country | Surveillance years | % Rotavirus positive | Hospitalization rate (per 100,000) | Reference |
|---|---|---|---|---|
| Low child mortality | ||||
| Hong Kong | 1987– 1996 | 26 | 200 | [29] |
| Hong Kong | 1997– 2011 | 39 | 542 | [30] |
| Hong Kong | 2001– 2003 | 30 | 810 | [31] |
| Malaysia | 1999–2000 | 50 | 330 | [32,33] |
| Malaysia | 2013 | 45 | 122 | [34] |
| Singapore | 2005– 2008 | Not reported | 459 | [35] |
| Median (IQR) | 395 (200, 542) | |||
|
| ||||
| Medium child mortality | ||||
| China | 2007– 2008 | 34 | 210 | [36] |
| China | 2002 | 20 | 151 | [37] |
| China | 2012–2013 | 30 | 144 | [38] |
| Kazakhstan | 2007–2009 | 30 (28, 32) | 260 | [39] |
| Kyrgyzstan | 2005–2007 | 26 (24, 27) | 362 | [40] |
| Kyrgyzstan | 2005–2009 | 24 (23, 25) | 680 | [39] |
| Turkey | 2007 | 29 | 293 | [41] |
| Vietnam | 1998– 2003 | 55 | 1,500 | [32,42] |
| Median (IQR) | 277 (181, 521) | |||
|
| ||||
| High child mortality | ||||
| Bangladesh | 2000–2006 | 33 | 1,080 | [43] |
| Bhutan | 2010–2012 | 33 | 240 | [44] |
| India | 2000– 2001 | 24 | 337 | [45] |
| India | 2002– 2003 | 27 | 652 | [46,47] |
| India | 2008 | Not reported | 633 | [47] |
| Pakistan | 2005– 2007 | 16 | 230 | [48] |
| Pakistan | 2005– 2007 | 18 | 560 | [48] |
| Philippines | 2005– 2006 | 30 | 281 | [49] |
| Uzbekistan | 2005–2009 | 26 (25, 27) | 210 | [39] |
| Median (IQR) | 337 (240, 633) | |||
Table 2.
Summary of published rotavirus vaccine effectiveness estimates in Asian countries
| Country | Vaccine | Outcome | Vaccine effectiveness (95%CI) 1 | Study Type | Reference |
|---|---|---|---|---|---|
| Low child mortality | |||||
| Hong Kong | Rotarix | Hospitalization | 93 (60, 99) | Clinical Trial | [50] |
| Hong Kong | Rotarix and RotaTeq | Hospitalization | 91 (69, 97) | Observational | [51] |
| Hong Kong, Taiwan, Singapore | Rotarix | Severe diarrhea | 96 (85, 99) | Clinical Trial | [52] |
| Hong Kong, Taiwan, Singapore | Rotarix | Hospitalization | 95 (84, 99) | Clinical Trial | [53] |
| Median (IQR) | 94 (92, 96) | ||||
|
| |||||
| Medium child mortality | |||||
| China | Rotarix | Hospitalization | 81 (44, 95) | Clinical Trial | [54] |
| Lebanon | Rotarix and RotaTeq | Hospitalization | 63 (41, 76) | Observational | [55] |
| Vietnam | RotaTeq | Severe diarrhea | 64 (8, 91) | Clinical Trial | [56] |
| Median (IQR) | 64 (63, 81) | ||||
|
| |||||
| High child mortality | |||||
| Bangladesh | Rotarix | Severe diarrhea | 23 (0, 41) | Clinical Trial | [57] |
| Bangladesh | RotaTeq | Severe diarrhea | 43 (10, 64) | Clinical Trial | [56] |
| India | ROTAVAC | Hospitalization | 55 (40, 66) | Clinical Trial | [58] |
| India | ROTAVAC | Hospitalization | 54 (35, 67) | Clinical Trial | [7] |
| Median (IQR) | 49 (33, 55) | ||||
Full-series vaccine effectiveness
Across the 43 Asian countries included in this analysis, we estimate there were 1,452,257 (95%CI: 1,142,109, 1,776,186) hospitalizations and 88,889 (95%CI: 84,014, 93,478) deaths due to rotavirus annually. With hypothetical universal introduction of rotavirus vaccine, 710,580 (95%CI: 546,045, 889,358) fewer hospitalizations and 35,865 (95%CI: 30,427, 41,370) fewer deaths would occur (Table 3). This represents a 49% (95%CI: 43, 55) and 40% (95%CI: 35, 46) reduction over the expected number of hospitalizations and deaths, respectively. Of the rotavirus hospitalizations prevented, 95% were in countries that had not yet introduced rotavirus vaccine (676,538; 95%CI: 511,378, 855,881) and 54% were in high child mortality countries (383,007; 95%CI: 276,114, 516,655). Of the rotavirus deaths prevented, 96% were from countries that had not yet introduced rotavirus vaccine (34,540; 95%CI: 29,173, 40,010) and 89% were in high child mortality countries (32,080; 95%CI: 26,721, 37,673). Of the projected rotavirus hospitalizations that would be prevented with universal rotavirus vaccine implementation, 44% were in Gavi eligible countries and 81% of projected rotavirus deaths prevented were in Gavi eligible countries.
Table 3.
Estimated expected and prevented numbers of rotavirus hospitalizations and deaths in children <5 years of age in Asian countries with hypothetical introduction of rotavirus vaccine into national immunization programs.
| Rotavirus hospitalizations | Rotavirus deaths | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Expected | Prevented | % Prevented | Expected | Prevented | % Prevented | |||||||
| n | 95%CI | n | 95%CI | % | 95%CI | n | 95%CI | n | 95%CI | % | 95%CI | |
| All countries1 | 1,452,257 | 1142109, 1776186 | 710,580 | 546045, 889358 | 49 | 43, 55 | 88,889 | 84014, 93478 | 35,865 | 30427, 41370 | 40 | 35, 46 |
|
| ||||||||||||
| Introduction status | ||||||||||||
| Introduced2 (n=8) | 73,332 | 56463, 90626 | 33,210 | 25460, 41737 | 46 | 39, 52 | 3,436 | 3238, 3645 | 1,327 | 1119, 1556 | 39 | 33, 45 |
| Not introduced (n=35) | 1,380,365 | 1067841, 1703794 | 676,538 | 511378, 855881 | 49 | 43, 55 | 85,474 | 80591, 90014 | 34,540 | 29173, 40010 | 41 | 35, 46 |
|
| ||||||||||||
| Child mortality strata | ||||||||||||
| Low (n=7) | 22,206 | 15952, 29011 | 20,207 | 14624, 26360 | 91 | 90, 92 | 49 | 46, 52 | 44 | 41, 47 | 90 | 88, 91 |
| Medium (n=16) | 433,218 | 286624, 575540 | 301,210 | 197527, 417547 | 71 | 64, 77 | 5,335 | 5031, 5656 | 3,683 | 3300, 4118 | 69 | 63, 75 |
| High (n=20) | 992,953 | 748982, 1261353 | 383,007 | 276114, 516655 | 39 | 33, 44 | 83,478 | 78615, 88124 | 32,080 | 26721, 37673 | 39 | 33, 45 |
|
| ||||||||||||
| Subregion3 | ||||||||||||
| Central Asia (n=5) | 31,715 | 23105, 39886 | 15,396 | 11117, 20215 | 49 | 43, 55 | 1,380 | 1272, 1490 | 620 | 516, 732 | 45 | 38, 52 |
| Eastern Asia (n=5) | 319,143 | 178741, 456421 | 225,136 | 124677, 335519 | 71 | 63, 79 | 3,444 | 3143, 3767 | 2,392 | 2037, 2799 | 70 | 61, 77 |
| South-Eastern Asia (n=11) | 242,118 | 181975, 299303 | 111,180 | 85083, 141229 | 46 | 40, 52 | 10,767 | 10406, 1154 | 4,556 | 3982, 5125 | 42 | 37, 47 |
| Southern Asia (n=9) | 760,632 | 526894, 1011612 | 302,069 | 202474, 436550 | 40 | 33, 47 | 70,168 | 65235, 74661 | 26,981 | 21753, 32407 | 39 | 32, 46 |
| Western Asia (n=13) | 97,989 | 76699, 118962 | 51,149 | 40336, 63942 | 53 | 47, 58 | 3,133 | 2961, 1428 | 1,231 | 1048, 1428 | 39 | 34, 45 |
|
| ||||||||||||
| Income group4 | ||||||||||||
| High (n=10) | 25,904 | 18517, 33068 | 21,019 | 15145, 26799 | 82 | 76, 86 | 79 | 73, 86 | 58 | 50, 66 | 73 | 66, 80 |
| Upper Middle (n=10) | 408,150 | 263628, 548453 | 278,338 | 177342, 393494 | 69 | 62, 76 | 4,953 | 4631, 5301 | 3,105 | 2723, 3543 | 63 | 57, 69 |
| Lower Middle (n=20) | 971,245 | 726294, 1245855 | 389,167 | 283448, 523905 | 40 | 34, 46 | 78,349 | 73458, 82871 | 30,608 | 25350, 36175 | 39 | 33, 46 |
| Low (n=3) | 42,264 | 30232, 55073 | 15,769 | 10521, 21884 | 38 | 31, 44 | 5,500 | 5050, 5989 | 1,981 | 1529, 2455 | 36 | 28, 44 |
|
| ||||||||||||
| Gavi Eligibility | ||||||||||||
|
| ||||||||||||
| Ineligible (n=31) | 652,031 | 490018, 814420 | 396,348 | 289085, 516507 | 61 | 55, 67 | 13,225 | 12754, 13725 | 6,687 | 6080, 7367 | 51 | 46, 55 |
| Eligible (n=12) | 802,488 | 564372, 1055169 | 310,934 | 209491, 442674 | 39 | 32, 46 | 75,630 | 70735, 80151 | 29,158 | 23767, 34637 | 38 | 32, 45 |
All countries for which model inputs were available; rotavirus mortality information was unavailable for Hong Kong and Palestinian Territories.
National rotavirus vaccine introduction before 2015: Bahrain, Iraq, Jordan, Qatar, Saudi Arabia, United Arab Emirates, Uzbekistan, Yemen
Millennium Development Goals definition
World Bank definition
The largest percent reductions in rotavirus hospitalizations and deaths over the expected numbers were found in the East Asia sub-region, which includes China, Hong Kong, Democratic Republic of Korea, Republic of Korea, and Mongolia. The East Asia region accounted for 22% of rotavirus hospitalizations and 4% of rotavirus deaths in Asia. There were 71% (95%CI: 63%, 79%) fewer hospitalizations and 70% (95%CI: 61%, 77%) fewer deaths. The largest absolute reductions in rotavirus hospitalizations and deaths were in the South Asia sub-region, which includes Afghanistan, Bangladesh, Bhutan, India, Iran, Maldives, Nepal, Pakistan, and Sri Lanka. The South Asia region accounted for 52% of rotavirus hospitalizations and 79% of rotavirus deaths in Asia. There were 302,069 (95%CI: 202,474, 436,550) fewer hospitalizations and 26,981 (95%CI: 21,753, 32,407) fewer deaths. Country-specific point estimates and 95%CIs are presented in Supplementary Table 1.
Discussion
Our model predicted in excess of 710,000 fewer rotavirus hospitalizations and 35,000 fewer rotavirus deaths if all 43 Asian countries introduced rotavirus vaccine, a more than 40% decrease over the baseline estimate of approximately 1,452,000 hospitalizations and 88,000 deaths. Similar to other summary analyses of rotavirus VE, we found a gradient of decreasing VE with increasing national child mortality [25]. Despite estimated VE of just 49% in high child mortality countries, our findings show the largest absolute number of reductions would occur in these 20 countries, underscoring the public health values of even partially effective rotavirus vaccines in high burden settings.
Countries with large birth cohorts may have an out-sized influence on our findings. For example, 30% of all rotavirus hospitalizations prevented were in China represent and over 50% of all rotavirus deaths prevented were in India. These findings underscore the importance of implementing vaccination in these large countries to achieve maximum public health impact. However, when excluding countries with birth cohorts >1,500,000 (that is Bangladesh, China, India, Indonesia, Pakistan, and the Philippines) the percent reductions are slightly higher than that of the region overall, with a 55% reduction in hospitalizations and a 42% reduction in deaths. The country-specific analyses show reductions of at least 32% in diarrhea related morbidity and mortality in all countries.
This analysis has several limitations. The literature identified by the systematic review was not robust enough to calculate hospitalization rates or VE by age group. As rotavirus diarrhea hospitalizations tend to be highest in the youngest children and decrease in older children, the rates presented here cover the entire age group and likely underestimate rotavirus hospitalizations in the youngest children and overestimate hospitalizations in older children. Similarly, there is some evidence that VE decreases in the second year of life in high child mortality settings; the systematic review did not find enough age group-specific VE estimates in Asia to assign VE by age. This may have biased these results, however we cannot speculate on the magnitude or direction. Secondly, we did not account for subnational introductions or the current use of private market vaccination in our baseline hospitalization and death estimates. While this would inflate the estimated number of hospitalizations and deaths, it would not change the percent reductions. In calculating reductions, we used DTP3 and most countries globally to date have introduced a 2-dose vaccine. This may have underestimated coverage and therefore underestimated impact. Our model also does not account for potential indirect effects of introducing rotavirus vaccine. Finally, expected rotavirus mortality estimates were not available for Hong Kong and the Palestinian Territories, therefore they were excluded from the mortality analysis. Due to their size and Hong Kong’s very low child mortality, we do not think this has influenced our overall estimates.
These findings support consideration of rotavirus vaccine to help alleviate diarrheal disease burden. However, there is still a need to examine ways to improve effectiveness of rotavirus vaccines in medium and high child mortality settings to receive even further benefits from vaccination. As countries in Asia prepare for rotavirus vaccine introduction, this analysis supports the importance of high coverage and strengthening of the immunization systems to deliver childhood vaccines on schedule. In some early introducing countries in other regions, initial rotavirus vaccine coverage lagged behind that of routine infant vaccines recommended for concomitant administration, reducing the potential of the vaccines to impact disease morbidity and mortality [26–28]. Although uptake has been slow in Asia so far, rotavirus vaccines are starting to gain traction in the region. Over the next five years, many of these estimated impacts will become real as countries incorporate rotavirus vaccines into their routine immunization programs.
Supplementary Material
Key Issues.
Of the 215,000 estimated global deaths from rotavirus among children <5 years old in 2013, 41% occurred in Asian countries. The Southeast Asian region has the highest rate of rotavirus detection in the world and we estimated more than 1,452,000 annual rotavirus hospitalizations among children <5 years old in Asian countries.
There are four rotavirus vaccines manufactured in Asia: Rotasiil (Serum Institute of India), ROTAVAC (Bharat Biotech), Lanzhou (China) and Rotavin (Vietnam), in addition to two internationally available rotavirus vaccines. Despite this availability, only eight Asian countries have introduced a rotavirus vaccine into their national vaccination program.
Our model predicted more than 710,000 fewer rotavirus hospitalizations and 35,000 fewer rotavirus deaths if all 43 Asian countries introduced rotavirus vaccine, a greater than 40% decrease over the baseline. Similar reductions were projected in sub-analyses by vaccine introduction status, child mortality strata, sub-region, income level, and birth cohort size.
Footnotes
Disclaimer: The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
References
- 1**.Tate JE, Burton AH, Boschi-Pinto C, Parashar UD World Health Organization-Coordinated Global Rotavirus Surveillance N. Global, Regional, and National Estimates of Rotavirus Mortality in Children <5 Years of Age, 2000–2013. Clin Infect Dis. 2016;62(Suppl 2):S96–S105. doi: 10.1093/cid/civ1013. This article synthesizes surveillance data to provide annual regional estimates of rotavirus mortality for countries that have and have not introduced rotavirus vaccine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2**.Rotavirus vaccines. WHO position paper - January 2013. Wkly Epidemiol Rec. 2013;88(5):49–64. The World Health Organization’s recommendations and rationale for introducing rotavirus vaccine are presented in this document. [PubMed] [Google Scholar]
- 3*.Burnett E, Jonesteller CL, Tate JE, Yen C, Parashar UD. Global Impact of Rotavirus Vaccination on Childhood Hospitalizations and Mortality from Diarrhea. J Infect Dis. 2017;215(11):1666–1672. doi: 10.1093/infdis/jix186. This article summarizes published estimates of rotavirus vaccine impact on hospitalizations and deaths among children <5 years of age. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354(1):11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 5.Vesikari T, Matson DO, Dennehy P, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354(1):23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- 6.Kirkwood CD, Ma LF, Carey ME, Steele AD. The rotavirus vaccine development pipeline. Vaccine. 2017 doi: 10.1016/j.vaccine.2017.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhandari N, Rongsen-Chandola T, Bavdekar A, et al. Efficacy of a monovalent human-bovine (116E) rotavirus vaccine in Indian infants: a randomised, double-blind, placebo-controlled trial. Lancet. 2014;383(9935):2136–2143. doi: 10.1016/S0140-6736(13)62630-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isanaka S, Guindo O, Langendorf C, et al. Efficacy of a Low-Cost, Heat-Stable Oral Rotavirus Vaccine in Niger. N Engl J Med. 2017;376(12):1121–1130. doi: 10.1056/NEJMoa1609462. [DOI] [PubMed] [Google Scholar]
- 9.Fu C, He Q, Xu J, et al. Effectiveness of the Lanzhou lamb rotavirus vaccine against gastroenteritis among children. Vaccine. 2012;31(1):154–158. doi: 10.1016/j.vaccine.2012.10.078. [DOI] [PubMed] [Google Scholar]
- 10.Luan le T, Trang NV, Phuong NM, et al. Development and characterization of candidate rotavirus vaccine strains derived from children with diarrhoea in Vietnam. Vaccine. 2009;27(Suppl 5):F130–138. doi: 10.1016/j.vaccine.2009.08.086. [DOI] [PubMed] [Google Scholar]
- 11.Organization WH. Rotavirus vaccines: an update. Weekly epidemiological record. 2009;84(51/52):533–537. [Google Scholar]
- 12.Rotavirus Vaccine Country Introductions: Maps and List. PATH; 2016. http://www.path.org/vaccineresources/details.php?i=2235. [Google Scholar]
- 13.Tharmaphornpilas P, Jiamsiri S, Boonchaiya S, et al. Evaluating the first introduction of rotavirus vaccine in Thailand: Moving from evidence to policy. Vaccine. 2017;35(5):796–801. doi: 10.1016/j.vaccine.2016.12.043. [DOI] [PubMed] [Google Scholar]
- 14.Dayrit E. Philippines Experience. 12th International Rotavirus Symposium; Melbourne, Australia. 2016. [Google Scholar]
- 15.Millennium Development Indicators: World and regional groupings. United Nations Statistics Division: 2014. https://mdgs.un.org/unsd/mdg/Host.aspx?Content=Data/RegionalGroupings.htm. [Google Scholar]
- 16.Levels & Trends in Child Mortality; Report 2015 Estimates Developed by the UN Inter-agency Group for Child Mortality Estimation. UNICEF United Nations Inter-agency Group for Child Mortality Estimation; 2015. http://data.unicef.org/corecode/uploads/document6/uploaded_pdfs/corecode/IGME-report-2015-child-mortality-final_236.pdf. [Google Scholar]
- 17.World Bank Country and Lending Groups. The World Bank; 2016. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups. [Google Scholar]
- 18.Countries eligible for support. Gavi: the Vaccine Alliance; 2016. http://www.gavi.org/support/sustainability/countries-eligible-for-support/ [Google Scholar]
- 19.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 20*.Shah MP, Tate JE, Mwenda JM, Steele AD, Parashar UD. Estimated reductions in hospitalizations and deaths from childhood diarrhea following implementation of rotavirus vaccination in Africa. Expert Rev Vaccines. 2017:1–9. doi: 10.1080/14760584.2017.1371595. Shah et al. analyzed data from African countries to provide estimated benefits of rotavirus vaccine, similar to the analysis presented here. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Annual population by age groups- both sexes. United Nations Population Division; 2015. https://esa.un.org/unpd/wpp/Download/Standard/Population/ [Google Scholar]
- 22.Third dose of diphtheria toxoid, tetanus toxoid, and pertussis vaccine. World Health Organization; 2016. http://apps.who.int/immunization_monitoring/globalsummary/timeseries/tscoveragedtp3.html. [Google Scholar]
- 23.Health Facts of Hong Kong 2017 Edition. The Government of Hong Kong Department of Health; 2017. http://www.dh.gov.hk/english/statistics/statistics_hs/files/Health_Statistics_pamphlet_E.pdf. [Google Scholar]
- 24.At a glance: State of Palestine. UNICEF; 2013. https://www.unicef.org/infobycountry/oPt_statistics.html. [Google Scholar]
- 25*.Jonesteller CL, Burnett E, Yen C, Tate JE, Parashar UD. Effectiveness of Rotavirus Vaccination: A systematic review of the first decade of global post-licensure data, 2006–2016. Clin Infect Dis. 2017 doi: 10.1093/cid/cix369. This article summarizes published estimates of rotavirus vaccine effectiveness against rotavirus diarrhea among children <5 years of age. [DOI] [PubMed] [Google Scholar]
- 26.Elam-Evans LD, Yankey D, Singleton JA, Kolasa M Centers for Disease C, Prevention. National, state, and selected local area vaccination coverage among children aged 19–35 months - United States, 2013. MMWR Morb Mortal Wkly Rep. 2014;63(34):741–748. [PMC free article] [PubMed] [Google Scholar]
- 27.de Oliveira LH, Danovaro-Holliday MC, Matus CR, Andrus JK. Rotavirus vaccine introduction in the Americas: progress and lessons learned. Expert Rev Vaccines. 2008;7(3):345–353. doi: 10.1586/14760584.7.3.345. [DOI] [PubMed] [Google Scholar]
- 28.de Oliveira LH, Danovaro-Holliday MC, Sanwogou NJ, Ruiz-Matus C, Tambini G, Andrus JK. Progress in the introduction of the rotavirus vaccine in Latin America and the Caribbean: four years of accumulated experience. Pediatr Infect Dis J. 2011;30(1 Suppl):S61–66. doi: 10.1097/INF.0b013e3181fefdd6. [DOI] [PubMed] [Google Scholar]
- 29.Chan PK, Tam JS, Nelson EA, et al. Rotavirus infection in Hong Kong: epidemiology and estimates of disease burden. Epidemiol Infect. 1998;120(3):321–325. doi: 10.1017/s0950268898008747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiang GP, Nelson EA, Pang TJ, et al. Rotavirus incidence in hospitalised Hong Kong children: 1 July 1997 to 31 March 2011. Vaccine. 2014;32(15):1700–1706. doi: 10.1016/j.vaccine.2014.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nelson EA, Tam JS, Bresee JS, et al. Estimates of rotavirus disease burden in Hong Kong: hospital-based surveillance. J Infect Dis. 2005;192(Suppl 1):S71–79. doi: 10.1086/431492. [DOI] [PubMed] [Google Scholar]
- 32.Kawai K, O’Brien MA, Goveia MG, Mast TC, El Khoury AC. Burden of rotavirus gastroenteritis and distribution of rotavirus strains in Asia: a systematic review. Vaccine. 2012;30(7):1244–1254. doi: 10.1016/j.vaccine.2011.12.092. [DOI] [PubMed] [Google Scholar]
- 33.Hsu VP, Abdul Rahman HB, Wong SL, et al. Estimates of the burden of rotavirus disease in Malaysia. J Infect Dis. 2005;192(Suppl 1):S80–86. doi: 10.1086/431494. [DOI] [PubMed] [Google Scholar]
- 34.Loganathan T, Ng CW, Lee WS, Jit M. The Hidden Health and Economic Burden of Rotavirus Gastroenteritis in Malaysia: An Estimation Using Multiple Data Sources. Pediatr Infect Dis J. 2016;35(6):601–606. doi: 10.1097/INF.0000000000001129. [DOI] [PubMed] [Google Scholar]
- 35.Phua KB, Tee N, Tan N, et al. A hospital-based surveillance of rotavirus gastroenteritis in children <5 years of age in Singapore. Pediatr Infect Dis J. 2013;32(12):e426–431. doi: 10.1097/INF.0b013e31829f2cb0. [DOI] [PubMed] [Google Scholar]
- 36.Lou JT, Xu XJ, Wu YD, Tao R, Tong MQ. Epidemiology and burden of rotavirus infection among children in Hangzhou, China. J Clin Virol. 2011;50(1):84–87. doi: 10.1016/j.jcv.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Wang XY, Xu ZY, von Seidlein L, et al. Incidence of diarrhea caused by rotavirus infections in rural Zhengding, China: prospective, population-based surveillance. J Infect Dis. 2005;192(Suppl 1):S100–105. doi: 10.1086/431507. [DOI] [PubMed] [Google Scholar]
- 38.Zhang J, Liu H, Jia L, et al. Active, population-based surveillance for rotavirus gastroenteritis in Chinese children: Beijing Municipality and Gansu Province, China. Pediatr Infect Dis J. 2015;34(1):40–46. doi: 10.1097/INF.0000000000000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Latipov R, Utegenova E, Kuatbayeva A, et al. Epidemiology and burden of rotavirus disease in Central Asia. Int J Infect Dis. 2011;15(7):e464–469. doi: 10.1016/j.ijid.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 40.Flem ET, Latipov R, Nurmatov ZS, Xue Y, Kasymbekova KT, Rheingans RD. Costs of diarrheal disease and the cost-effectiveness of a rotavirus vaccination program in kyrgyzstan. J Infect Dis. 2009;200(Suppl 1):S195–202. doi: 10.1086/605040. [DOI] [PubMed] [Google Scholar]
- 41.Hacimustafaoglu M, Celebi S, Agin M, Ozkaya G. Rotavirus epidemiology of children in Bursa, Turkey: a multi-centered hospital-based descriptive study. Turk J Pediatr. 2011;53(6):604–613. [PubMed] [Google Scholar]
- 42.Anh DD, Thiem VD, Fischer TK, et al. The burden of rotavirus diarrhea in Khanh Hoa Province, Vietnam: baseline assessment for a rotavirus vaccine trial. Pediatr Infect Dis J. 2006;25(1):37–40. doi: 10.1097/01.inf.0000195635.05186.52. [DOI] [PubMed] [Google Scholar]
- 43.Zaman K, Yunus M, Faruque AS, et al. Surveillance of rotavirus in a rural diarrhoea treatment centre in Bangladesh, 2000–2006. Vaccine. 2009;27(Suppl 5):F31–34. doi: 10.1016/j.vaccine.2009.08.063. [DOI] [PubMed] [Google Scholar]
- 44.Wangchuk S, Dorji T, Tsheten, et al. A Prospective Hospital-based Surveillance to Estimate Rotavirus Disease Burden in Bhutanese Children under 5 Years of Age. Trop Med Health. 2015;43(1):63–68. doi: 10.2149/tmh.2014-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bahl R, Ray P, Subodh S, et al. Incidence of severe rotavirus diarrhea in New Delhi, India, and G and P types of the infecting rotavirus strains. J Infect Dis. 2005;192(Suppl 1):S114–119. doi: 10.1086/431497. [DOI] [PubMed] [Google Scholar]
- 46.Banerjee I, Ramani S, Primrose B, et al. Comparative study of the epidemiology of rotavirus in children from a community-based birth cohort and a hospital in South India. J Clin Microbiol. 2006;44(7):2468–2474. doi: 10.1128/JCM.01882-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tate JE, Chitambar S, Esposito DH, et al. Disease and economic burden of rotavirus diarrhoea in India. Vaccine. 2009;27(Suppl 5):F18–24. doi: 10.1016/j.vaccine.2009.08.098. [DOI] [PubMed] [Google Scholar]
- 48.Qazi R, Sultana S, Sundar S, et al. Population-based surveillance for severe rotavirus gastroenteritis in children in Karachi, Pakistan. Vaccine. 2009;27(Suppl 5):F25–30. doi: 10.1016/j.vaccine.2009.08.064. [DOI] [PubMed] [Google Scholar]
- 49.Carlos CC, Inobaya MT, Bresee JS, et al. The burden of hospitalizations and clinic visits for rotavirus disease in children aged <5 years in the Philippines. J Infect Dis. 2009;200(Suppl 1):S174–181. doi: 10.1086/605044. [DOI] [PubMed] [Google Scholar]
- 50.Lau YL, Nelson EA, Poon KH, et al. Efficacy, safety and immunogenicity of a human rotavirus vaccine (RIX4414) in Hong Kong children up to three years of age: a randomized, controlled trial. Vaccine. 2013;31(18):2253–2259. doi: 10.1016/j.vaccine.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 51.Yeung KH, Tate JE, Chan CC, et al. Rotavirus vaccine effectiveness in Hong Kong children. Vaccine. 2016;34(41):4935–4942. doi: 10.1016/j.vaccine.2016.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Phua KB, Lim FS, Lau YL, et al. Safety and efficacy of human rotavirus vaccine during the first 2 years of life in Asian infants: randomised, double-blind, controlled study. Vaccine. 2009;27(43):5936–5941. doi: 10.1016/j.vaccine.2009.07.098. [DOI] [PubMed] [Google Scholar]
- 53.Phua KB, Lim FS, Lau YL, et al. Rotavirus vaccine RIX4414 efficacy sustained during the third year of life: a randomized clinical trial in an Asian population. Vaccine. 2012;30(30):4552–4557. doi: 10.1016/j.vaccine.2012.03.030. [DOI] [PubMed] [Google Scholar]
- 54.Li RC, Huang T, Li Y, et al. Human rotavirus vaccine (RIX4414) efficacy in the first two years of life: a randomized, placebo-controlled trial in China. Hum Vaccin Immunother. 2014;10(1):11–18. doi: 10.4161/hv.26319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ali Z, Harastani H, Hammadi M, et al. Rotavirus Genotypes and Vaccine Effectiveness from a Sentinel, Hospital-Based, Surveillance Study for Three Consecutive Rotavirus Seasons in Lebanon. PLoS One. 2016;11(8):e0161345. doi: 10.1371/journal.pone.0161345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zaman K, Dang DA, Victor JC, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376(9741):615–623. doi: 10.1016/S0140-6736(10)60755-6. [DOI] [PubMed] [Google Scholar]
- 57.Zaman K, Sack DA, Neuzil KM, et al. Effectiveness of a live oral human rotavirus vaccine after programmatic introduction in Bangladesh: A cluster-randomized trial. PLoS Med. 2017;14(4):e1002282. doi: 10.1371/journal.pmed.1002282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bhandari N, Rongsen-Chandola T, Bavdekar A, et al. Efficacy of a monovalent human-bovine (116E) rotavirus vaccine in Indian children in the second year of life. Vaccine. 2014;32(Suppl 1):A110–116. doi: 10.1016/j.vaccine.2014.04.079. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.