Summary
Background
Platelets are dynamic effector cells with functions that span hemostatic, thrombotic, and inflammatory continuums. Phosphoinositide-dependent protein kinase 1 (PDK1) regulates protease-activated receptor 4 (PAR4)-induced platelet activation and thrombus formation through glycogen synthase kinase 3 beta (GSK3β). However, whether PDK1 also signals through the Adenosine diphosphate (ADP) receptor and its functional importance in vivo remains unknown.
Objective
To establish the mechanism of PDK1 in ADP-induced platelet activation and thrombosis.
Methods
We assessed the role of PDK1 on 2MeSADP-induced platelet activation by measuring aggregation, thromboxane generation, and phosphorylation events in the presence of BX-795, which inhibits PDK1 or by using platelet specific PDK1 knockout mice using western blot analysis. PDK1 function in thrombus formation was assessed using an in vivo pulmonary embolism model.
Results
PDK1 inhibition with BX-795 reduced 2MeSADP-induced aggregation of human and murine platelets by abolishing thromboxane generation. Similar results were observed in pdk1−/− mice. PDK1 was also necessary for the phosphorylation of Mitogen-activated protein kinase kinase 1/2 (MEK1/2), Extracellular signal-regulated kinase 1/2 (ERK1/2), and Cytosolic phospholipase A2 (cPLA2), indicating that PDK1 regulates an upstream kinase in the MAPK pathway. We next determined that this upstream kinase is t Raf-1, a serine/threonine kinase necessary for the phosphorylation of MEK1/2, as pharmacological inhibition and genetic ablation of PDK1 were sufficient to prevent Raf1 phosphorylation. Furthermore, in vivo inhibition or genetic ablation of PDK1 protected mice from collagen/epinephrine-induced pulmonary embolism.
Conclusion
PDK1 governs thromboxane generation and thrombosis in platelets that are stimulated with 2MeSADP by regulating activation of MAPK pathway
Keywords: Platelet activation, Thrombosis, Purinergic Receptor P2Y12, 3-Phosphoinositide-Dependent Kinase 1, Thromboxane A2
Introduction
Platelets are involved in many processes ranging from fighting microbial infections and triggering inflammation to promoting tumor angiogenesis and metastasis [1–9]. Nevertheless, one of the primary physiological functions of platelets is maintaining homeostasis of the circulatory system by forming hemostatic thrombi that prevent blood loss and maintain vascular integrity [10–13]. When there is vascular damage, exposure of the extracellular matrix recruits and activates platelets, thereby leading to aggregation and formation of a fibrin-rich hemostatic plug at the injured site. Platelet activation is associated with platelet shape change, secretion of granule contents [Adenosine diphosphate (ADP) and serotonin], and activation of the fibrinogen receptor, resulting in platelet aggregation [14–17]. Activated platelets also generate the important lipid mediator thromboxane A2 (TxA2), which serves to recruit other platelets to the site of injury and reinforces the platelet plug [16, 18–20]. Thromboxane generation induced by 2MeSADP is coordinated by P2Y purinoceptor 1 (P2Y1), P2Y purinoceptor 12 (P2Y12), and integrin αIIbβ3. Inhibition of either ERK1/2 or Protein tyrosine kinase 2 (Pyk2) activation results in abolished thromboxane generation in ADP stimulated platelets. 2MeSADP-induced ERK1/2 phosphorylation occurs independently of outside-in signaling via MEK1/2, Src family kinases (SFK) and PI3K.
Thromboxane is generated from its precursor arachidonic acid through cyclooxygenase pathways, which act as a positive feedback mediator to amplify the initial platelet functional responses and reinforce the stability of the hemostatic plug. Previous studies have shown that ADP-induced thromboxane generation depends on both inside-out and outside-in pathways [16, 21]. In particular, ERK1/2 activation during inside-out signaling through P2Y receptors plays a critical role in TxA2 generation, as ERK1/2 is important for cPLA2 phosphorylation and its activation [22–24]. Inhibition of Phosphoinositide 3-kinaseβ (PI3Kβ) abolishes ERK1/2 activation when platelets are stimulated with ADP [24]. However, the mechanism through which the PI3K pathway regulates the phosphorylation and activation of ERK1/2 has not been identified.
PDK1, a member of the protein A, G and C (AGC) family of proteins, is a Serine/Threonine protein kinase that phosphorylates and activate other protein kinases from the AGC family, all of which mediate cellular responses[25–27]. Chen and colleagues demonstrated that aggregation of PDK1-deficient platelets was reduced in response to thrombin and U46619 [28]. For thrombin signaling, this involved αIIbβ3-mediated outside-in signaling. They further identified that activation of PDK1 with thrombin inhibits GSK3β (a major substrate of PDK1), thereby enhancing thrombin-induced platelet functional responses and thrombus formation. In other studies, Dangelmaier et al demonstrated that inhibition of PDK1 by BX-795, a well-characterized and specific inhibitor of PDK1, impaired platelet aggregation and Protein Kinase B (Akt) T308 phosphorylation[29].
However, thrombin and ADP activate via distinct signaling pathways. These published data suggested to us an unknown component to the PDK1-ADP-thromboxane signaling pathway and prompted us to investigate the thromboxane related mechanisms whereby PDK1 regulates ADP-induced platelet activation and thrombosis. The mechanism by which PDK1 controls ADP-induced platelet activation has not been examined and its role in TxB2 generation is not known. These knowledge gaps are important to fill, especially as PDK1 has recently gained attention as a potential drug target for thrombosis and cancer[30–38] and a deeper understanding of PDK1’s mechanism may shed light on clinical outcomes where PDK1 inhibitors are being used.
In the present study, we demonstrate that PDK1, which is activated downstream of the PI3K pathway, plays a crucial role in 2MeSADP-induced platelet aggregation and thromboxane generation by regulating the MAPK pathway. We show that pharmacological blockade or genetic deletion of PDK1 in platelets abolishes 2MeSADP-induced thromboxane generation and activation of critical MAPK pathway proteins (e.g. Raf1, MEK1/2 and ERK1/2). Moreover, inhibition or deletion of PDK1 reduces death from pulmonary embolism in vivo. Therefore, we conclude that crosstalk between the MAPK and PI3K pathways via PDK1 is crucial for 2MeSADP induced platelet activation, thromboxane generation and thrombosis.
Materials and Methods
Reagents
2MeSADP (Cat.no-1624) obtained from Tocris (Minneapolis, MN), epinephrine, SB-216763 and Apyrase (type VII) were obtained from Sigma (St. Louis, MO). BX-795 was obtained from Selleckchem (Houston, TX). MSC 2032964A and Whatman protein nitrocellulose transfer membrane was obtained from Fisher Scientific (Pittsburg, PA), LI-COR Odyssey blocking buffer was purchased from LI-COR Biosciences (Lincoln, NE). phospho-Raf1 (S338), MEK1/2 (S217/221), ERK1/2 (T202/Y204), cPLA2 (S505), PDK1 (S241), GSK3β (S9), Akt (T308), Akt (Ser473), total ERK1/2, PDK1 and GSK3β antibodies were from Cell Signaling Technology (Beverly, MA). Phospho-MBP Total MEK1/2 and beta-actin antibodies were obtained from Santa Cruz Biotechnologies (Santa Cruz, CA).
Mice
Platelet specific PDK1 knock-out mice platelet samples were provided by Dr. Oliver Borst from University of Tübingen.
Human Platelet isolation
Washed non-aspirinated human platelets were prepared as previously described[39]. The platelet count was adjusted to 2–2.5 × 108/ml. Approval was obtained from the institutional review board for these studies. Informed consent was provided prior to blood donation.
Murine platelet isolation
Washed murine platelets were prepared as previously described[39]. Platelet counts were determined using a Hemavet 950FS blood cell counter (Drew Scientific Inc., Dallas, TX, USA). The platelet count was adjusted to 2×108 cells/ml for all the studies.
Aggregation
Aggregation was measured using a lumi-aggregometer (Chrono-Log, Havertown, PA) at 37°C under stirred conditions. Washed platelets (0.5 ml) were stimulated with different agonists and the change in light transmission measured. In select experiments, platelets were pre-incubated with different inhibitors before agonist stimulation.
Western blotting
Protein samples for platelets treated with or without the inhibitors, followed by stimulation with 2MeSADP were prepared and western blots were performed to analyzed phosphorylation events, as previously described. [40]
Measurement of Thromboxane A2 Generation
Washed platelets were prepared at a concentration of 2 × 108 platelets/ml. Stimulations were performed for 3.5 min and the reaction was stopped by snap freezing. Levels of TxB2 were determined in duplicate using a Correlate-EIA thromboxane B2 enzyme immunoassay kit (Assay Designs, Inc., Ann Arbor, MI), according to the manufacturer’s instructions.
MEK1/2 Kinase Assay
Washed human platelets (1×109 cells/ml) were stimulated with the 2MeSADP in the presence and absence of BX795. The reaction was stopped by addition of equal volume of cold 2x NP40 lysis buffer (2x Lysis Buffer: 50 mM HEPES, pH7.4, 100 mM NaCl, 2 mM EGTA, 2% NP40 plus Halt protease and phosphatase inhibitors) and the samples were rocked at 4°C for 30 minutes. Samples were centrifuged at 12000g at 4°C for 10 minutes. Supernatants were transferred to clean tubes and 5 μg anti-MEK1/2 added. Samples were rocked for an hour at 4°C, 50 μl washed TRUBLOT anti-mouse IgG IP beads (Rockland) added and rocked for an additional hour at 4°C. Beads were washed three times with 1X lysis buffer and once with Kinase buffer (50 mM MOPS (pH 7.4), 5 mM MgCl2, 5 mM MnCl2, and 1 mM DTT). The Kinase reaction was started by adding Kinase buffer (50 μl) containing 10 μl (20 μg) MAP kinase substrate cocktail, 10 μl of kinase buffer solution, 10 μl of Mg2+/ATP and incubated for 20–30 minutes in 30°C shaking incubator. Reactions were terminated by addition of 20 mM EDTA. Beads were pelleted by centrifugation, 30 μl supernatant mixed with 10μl 4X sample buffer for measurement of Myline basic protein (MBP) phosphorylation. All samples were boiled for 10 minutes. The samples were run on a 12 % SDS-PAGE. The non-Radioactive MEK1/2 activation assay kit (EMD Millipore) was used for this experiment.
Pulmonary embolism model
Thromboembolism experiments were performed as described previously using anesthetized pdk1fl/f and pdk1−/− mice [41]. In additional studies, wild-type mice were treated with BX-795 (10 μg/kg) or its vehicle (DMSO 1%). A mixture of collagen (0.4 mg/kg; Chronolog) or 2MeSADP (20 mg/kg; Sigma-Aldrich) and epinephrine (30 mg/kg; Sigma-Aldrich) in 100 μL of PBS was administered through retro-orbital injection or vena cava, respectively. Time of cessation of respiration (time needed to the onset of respiratory arrest that lasted at least 2–3 minutes) was recorded. Two minutes after the onset of respiratory arrest, but while the heart was still beating, 0.5 mL of Evans blue solution (1% in saline; Sigma-Aldrich) was injected into the heart.
Statistics
Each experiment was repeated at least 3 times. Results are expressed as mean ± SEM with number of observations (n). Data was analyzed using Prism. Significant differences were determined using Student’s t-test. Differences were considered significant at a two-tailed p<0.05.
Results
PDK1, but not GSK3β inhibition regulates 2MeSADP-induced platelet aggregation and thromboxane generation in human and mouse platelets
Previous studies with a PDK1 inhibitor and knockout murine platelets have shown that PDK1 plays an important role in platelet activation and thrombus formation [28, 29]. However, the mechanism through which PDK1 regulates 2MeSADP-induced platelet activation has not been elucidated. The functional role of PDK1 in human and murine platelets stimulated with 2MeSADP was investigated by pre-treating platelets with BX-795, a selective PDK1 inhibitor. Pre-treatment of human and murine platelets with BX-795 abolished secondary aggregation induced by 2MeSADP (Figure 1A), with an effect size similar to platelets treated with aspirin or indomethacin. These results indicate that PDK1 regulates thromboxane generation. Prior work indicates that PDK1 regulates thrombin-induced platelet activation through the enzyme GSK3β [28]. Thus, we evaluated whether inhibition of GSK3β with SB-216763 (a GSK3β specific inhibitor) rescues the defect of 2MeSADP-induced platelet aggregation we observed in BX-795 treated platelets. However, as shown in the Figure 1B, the inhibition in 2MeSADP-induced platelet aggregation was not rescued by GSK3β inhibition in either human or murine platelets. Previous studies have shown that thromboxane plays a key role in secondary aggregation when platelets are stimulated with 2MeSADP [16]. Therefore, we evaluated the effect of PDK1 inhibition on thromboxane generation when platelets were stimulated with 2MeSADP in the presence or absence of BX-795, SB-216763, or both. Figure 1C shows that the PDK1 inhibitor BX-795 almost completely abolishes 2MeSADP-induced thromboxane generation while the GSK3β inhibitor did not rescue the thromboxane generation. These data indicate that PDK1 is essential, and GSK3β is dispensable, for 2MeSADP-induced platelet secondary aggregation and thromboxane generation.
Figure 1. GSK3β Inhibition does not rescue 2MeSADP-induced platelet aggregation and thromboxane generation in human and murine platelets.
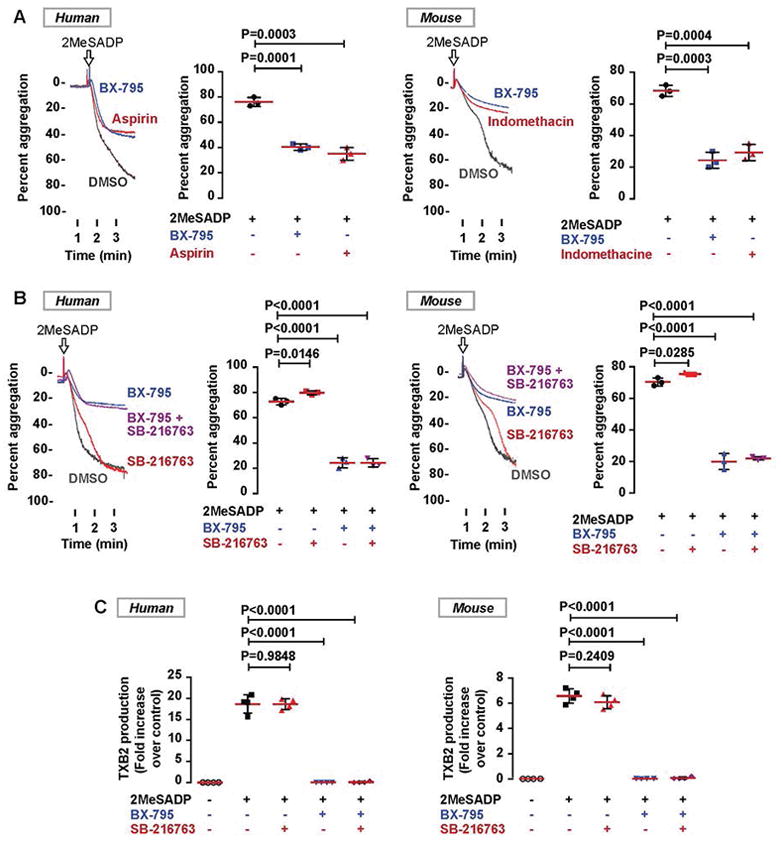
A) Washed human and murine (e.g. mouse) platelets were pre-treated with the DMSO (vehicle control), BX-795 (1 μM), aspirin (10 μM) or indomethacin (10 μM) at 37°C for 5 min followed by stimulation with 2MeSADP (50 nM) under stirred conditions. Platelet aggregation was measured by aggregometry. The tracings are representative of data from at least three independent experiments. B) Washed human and murine platelets were pre-treated with DMSO (vehicle control), the PDK1 inhibitor BX-795 (1 μM) or the GSK3β inhibitor SB-216763 (5 μM) or both for 5 min followed by stimulation with 2MeSADP (50 nM) under stirred conditions for 3.5 min at 37°C. Platelet aggregation was measured by aggregometry. The tracings are representative of data from at least three independent experiments. C) Thromboxane generation was measured as described in the experimental section. Graphs represent mean ± SD from at least three independent experiments.
PDK1 regulates the MAPK pathway in platelets stimulated with 2MeSADP
In platelets, the MAPK pathway is known to play an important role in thromboxane generation [22]. Nevertheless, cross-talk between the PI3K and MAPK pathways has never been studied in platelets. As ERK1/2 regulates thromboxane generation by phosphorylating cPLA2 [16], we first evaluated the effect of PDK1 inhibition on ERK1/2 and cPLA2 phosphorylation. We measured Akt (T308) phosphorylation in platelets treated with BX-795 as a positive control. As shown in Figure 2A, phosphorylation of ERK1/2 and cPLA2 was abolished in the presence of BX-795, which suggests that PDK1 regulates thromboxane generation by inhibiting the MAPK pathway.
Figure 2. PDK1 regulates the MAPK signaling pathway through phosphorylation of Raf1.
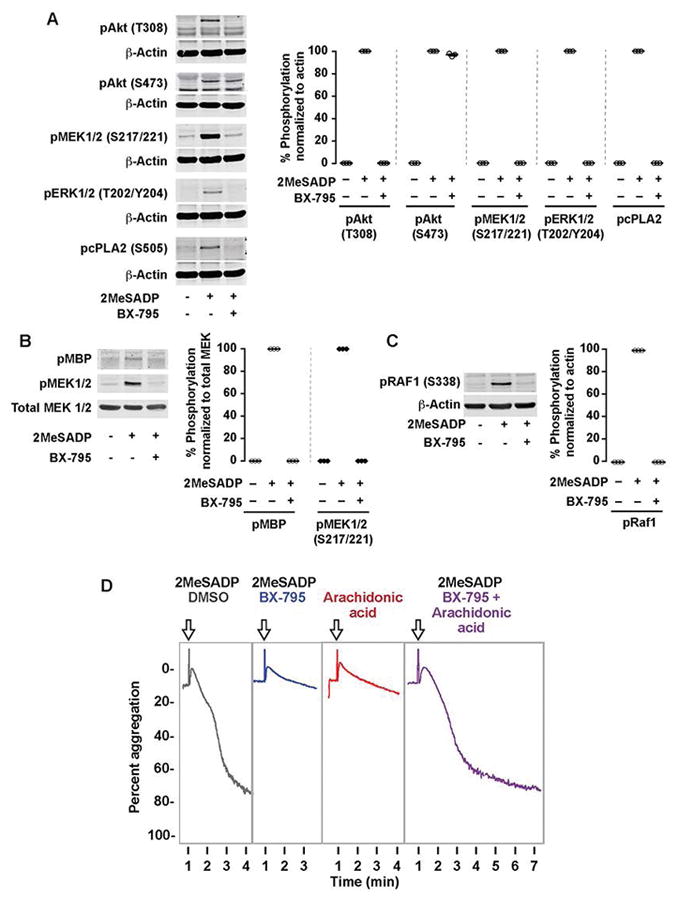
(A) Washed human platelets were left alone or pre-treated with the PDK1 inhibitor BX-795 (1 μM) for 5 min followed by stimulation with 2MeSADP (50 nM) under stirred conditions for 5 minutes. Western blots were then probed for phospho Akt (Thr308), Akt (Ser473), GSK3β (Ser9), MEK1/2 (Ser217/221), ERK1/2 (Thr202/Tyr204) and cPLA2 (Ser505). The western blot shown is representative of three independent experiments. (B) Washed human platelets were pre-treated with BX-795 (1 μM) for 5 min followed by stimulation 2MeSADP (50 nM) under stirred conditions for 5 minutes. Platelets were then lysed in NP40 lysis buffer and immunoprecipitated for MEK1/2. A MEK1/2 kinase activity assay was performed on the platelet lysates to measure phosphorylation of the MEK1/2 substrate myelin basic protein (pMBP). The Western blot shown is representative of three experiments. C) Washed human platelets were left alone or pre-treated with the PDK1 inhibitor BX-795 (1 μM) for 5 min followed by stimulation with 2MeSADP (50 nM) under stirred conditions for 5 minutes. Western blots were then probed for phospho Raf1 (Ser338), and total beta-actin. The western blot shown is representative of three independent experiments. D) Washed human platelets were pre-treated with the DMSO (vehicle control), BX-795 (1 μM, 37°C 5 min) followed by stimulation with 2MeSADP (50 nM) (Black and Blue), arachidonic acid (10 μM) (Red), or both (Purple) while stirring Platelet aggregation was measured by aggregometry. Tracings representative of n≥3 independent experiments.
PDK1 regulates MEK1/2 phosphorylation by controlling Raf1 activation
MEK1/2 is a master kinase that is known to activate ERK1/2 in many cells including platelets [22, 23]. Saori et al has shown that inhibition of PDK1 activity by gene silencing or SNP mutation suppressed MEK1/2 activation in cell lines [42–44]. However, the effect of PDK1 on MEK1/2 activity has not been studied in platelets. To evaluate the effect of PDK1 on MEK1/2 activation, we treated platelets with BX795 and studied its effect on MEK1/2 Ser221 phosphorylation. As shown in Figure 2A, 2MeSADP-induced MEK1/2 phosphorylation in platelets was abolished in the presence of BX-795.
We also evaluated the kinase activity of MEK1/2 from platelets pretreated with BX-795 that were stimulated with 2MeSADP by using an in vitro kinase assay. MEK1/2 that was immunoprecipitated from BX-795-treated and 2MeSADP-activated platelets did not phosphorylate the known and canonical MEK1/2 substrate MBP (myelin basic protein) in vitro, as shown in Figure 2B. These results further demonstrate that PDK1 plays a crucial role in MEK1/2 activation and establishes that PDK1 plays an important role in thromboxane generation when platelets are stimulated with 2MeSADP. These results further establishes that PI3K/PDK1 pathway has an important role in 2MeSADP-induced platelet activation and thromboxane generation.
We further studied the mechanism, through which MEK1/2 activation is regulated, by studying Raf1, a proto-oncogene, serine threonine kinase. Studies in other cells have shown that Raf1 regulates MEK1/2 activation [45], so we studied the effect of PDK1 inhibition on Raf1 by measuring the phosphorylation at Ser338 position which is known to be important for its activity[46]. As shown in Figure 2C, inhibition of PDK1 abolished Raf1 phosphorylation and its activity. These results further demonstrate that PDK1 plays a crucial role in activation of the Raf1/MEK1/2/ERK1/2 pathway and establishes that the PI3K/PDK1 pathway regulates 2MeSADP-induced platelet activation and thromboxane generation.
Addition of arachidonic acid restored the aggregation defect in BX-795 treated platelets
Activation of cPLA2 upon platelet stimulation releases arachidonic acid from phospholipids in the membrane. This arachidonic acid then produces thromboxane A2 that is required for ADP-induced platelet aggregation. As we found that PDK1 regulates thromboxane generation via cPLA2 phosphorylation and activation (Figures 1–2), we hypothesized that addition of arachidonic acid to BX-795 treated platelets would rescue aggregation defects. We stimulated platelet 2MeSADP and with or without arachidonic acid in the presence of BX-795. As shown in Figure 2D, stimulation of platelets with both 2MeSADP and arachidonic acid (purple) fully restored normal platelet aggregation. These results indicate that PDK1 regulates thromboxane generation, thereby controlling platelet aggregation.
Apoptosis signal-regulating kinase 1 (ASK1), Akt and GSK3β does not regulate MAPK pathway downstream of ADP receptors
Recent studies have shown that ASK1 plays an important role in regulating the activation of p38 and thromboxane generation, when platelets are stimulated with thrombin, convulxin, or collagen [47]. However, the role of ASK1 in 2MeSADP induced thromboxane generation has not been evaluated. As PDK1 and ASK1 regulate signaling by direct interaction and phosphorylation [48], we evaluated the role of ASK1 by using MSC 2032964A, an ASK1 specific inhibitor. Platelet aggregation, thromboxane generation, and cPLA2 phosphorylation were not abolished when platelets were stimulated with 2MeSADP in the presence of MSC 2032964A (Figure 3A & B). Consistent with a previous ASK1 knockout study [47], ERK1/2 phosphorylation was not inhibited upon treatment with an ASK1 inhibitor, but ERK1/2 phosphorylation was inhibited when platelets were treated with BX-795 or both BX-795 and MSC 2032964A (Figure 3C & D). Using SB-203580, a p38 inhibitor, we found that both p38 (P38 Mitogen-activated protein kinase) and ERK1/2 are required for activation of cPLA2 when platelets are stimulated with 2MeSADP (Figure 4A). Inhibiting ASK1 decreased p38 kinase activation at lower, but not higher, doses of 2MeSADP (Figure 4B), suggesting that at higher agonist concentrations p38 is regulated by another kinase than ASK1. These results indicate that PDK1, but not ASK1, predominantly regulates 2MeSADP induced platelet aggregation and thromboxane generation.
Figure 3. PDK1, but not ASK1, regulates 2MeSADP induced secondary aggregation and thromboxane generation in platelets.
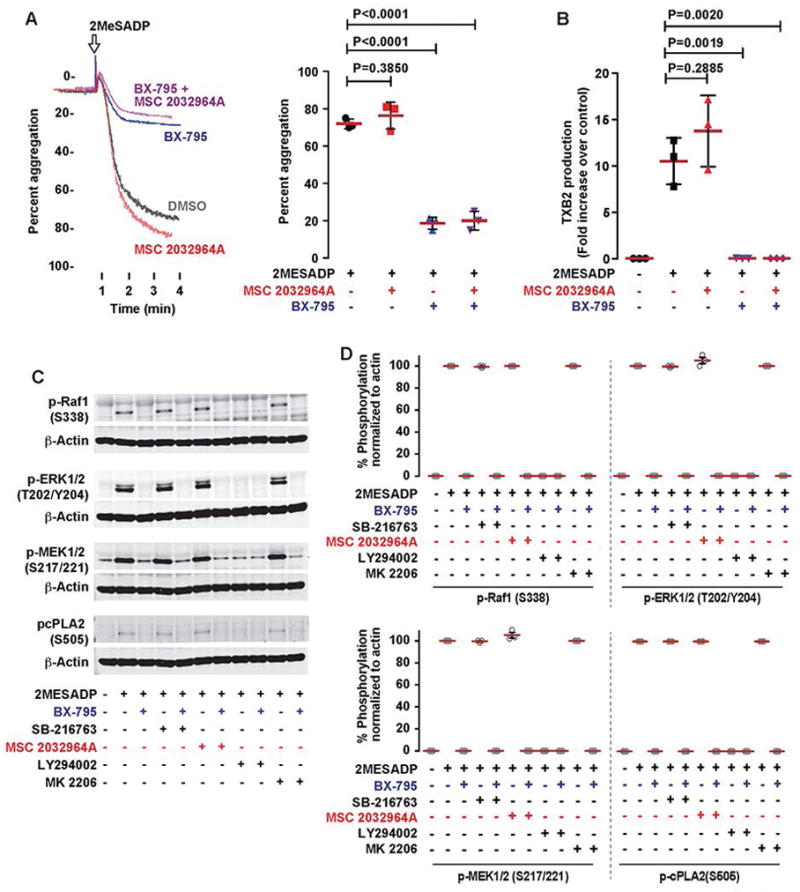
(A) Washed human platelets were pre-treated with the ASK1 inhibitor DMSO (vehicle control), MSC 2032964A (5 μM), BX-795 (1 μM), or both at 37°C for 5 min followed by stimulation with 2MeSADP (50 nM) under stirred conditions. Platelet aggregation was measured by aggregometry. The shown tracings are representative of at least three independent experiments. (B) Washed human platelets were pre-treated with DMSO (vehicle control), MSC 2032964A (5 μM), BX-795 (1 μM), or both for 5 min followed by stimulation with 2MeSADP (50 nM) under stirred conditions for 3.5 min at 37°C. Thromboxane generation was measured as described in the experimental section. Graphs represent mean ± SEM from at least 3 different experiments (*P<0.05). (C) Washed human platelets were incubated BX-795 (1 μM), SB-216763 (5 μM), MSC 2032964A (5 μM), LY294002 (25 μM), MK2206 (1 μM), or DMSO (vehicle control) followed by stimulation with 2MeSADP (50 nM) under stirred conditions for 5 minutes. Platelet proteins were separated using SDS-PAGE and probed for phospho Raf1 (Ser338), MEK1/2 (Ser212/221), ERK1/2 (Thr202/Tyr204), and cPLA2 (S505). Respective proteins are used as loading control for all western blots. The results shown are representative of data from at least three independent experiments.
Figure 4. Role of p38 Kinase in 2MeSADP induced platelet activation.

(A) Washed human platelets were incubated BX-795 (1 μM), SB-203580 (5 μM), or DMSO (vehicle control) followed by stimulation with 2MeSADP (2.5 nM) under stirred conditions for 5 minutes. Platelet proteins were separated using SDS-PAGE and probed for phosphorylation of p38 (Thr180/Tyr182), ERK1/2 (Thr202/Tyr204), and cPLA2 (S505). (B) Washed human platelets were incubated with SB-203580 (5 μM) followed by stimulation with 2MeSADP at various concentrations under stirred conditions for 5 minutes. Platelet proteins were separated using SDS-PAGE and probed for phosphorylation of p38 (Thr180/Tyr182). Actin is loading control. Representative of n≥3 independent experiments. (C, D) Proposed models for ADP-induced activation of cPLA2 and p38.
The genetic ablation of PDK1 decreases 2MeSADP-induced aggregation, thromboxane generation, and thrombus formation in vivo
We evaluated the role of PDK1 in 2MeSADP-induced platelet activation by using platelet specific PDK1 deficient mice (pdk1−/−), comparing the results with studies using the PDK1 pharmacological inhibitor. Consistent with our inhibitor studies (Figure 1), PDK1 deficient platelets stimulated with 2MeSADP demonstrated significantly decreased aggregation and the complete absence of thromboxane generation (Figure 5A & B). Furthermore, Raf1, MEK1/2, ERK1/2, Akt (T308), and cPLA2 phosphorylation were also abolished in PDK1 deficient murine platelets stimulated 2MeSADP (Figure 5C). These results support our pharmacologic studies demonstrating that PDK1 controls 2MeSADP-induced platelet aggregation by regulating thromboxane generation.
Figure 5. Genetic ablation of PDK1 reduces 2MeSADP induced platelet aggregation, and abolished MAPK pathway mediated thromboxane generation.
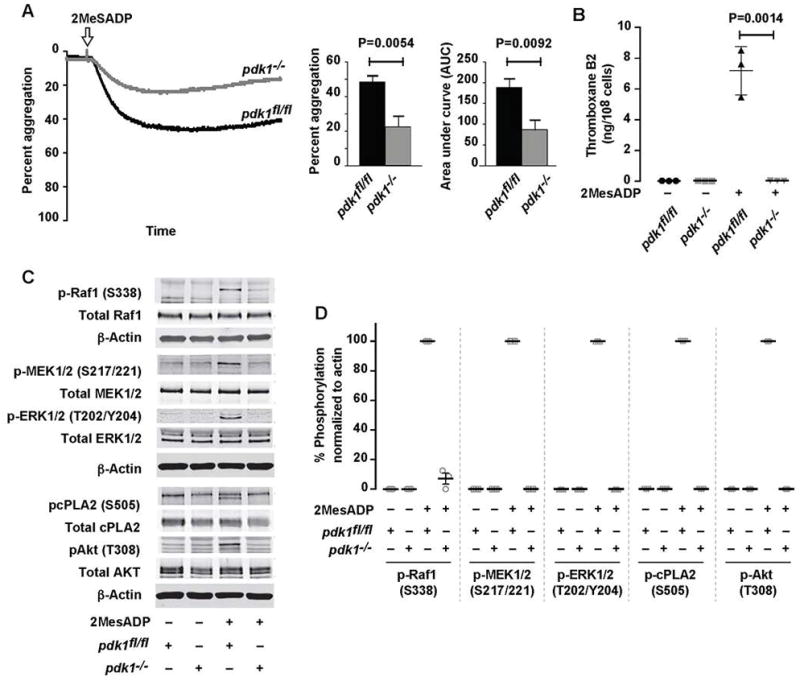
(A) Washed platelets from pdk1fl/fl and pdk1−/− mice were stimulated with 2MeSADP (50 nM) under stirred conditions at 37°C for 5 min. Aggregation was measured using light transmission aggregometry. Light transmission and area under the curve (mean ± SEM) are calculated from six independent experiments. (B) Washed platelets from pdk1fl/fl and pdk1−/− mice were stimulated with 2MeSADP (50 nM) under stirred conditions for 5 min at 37°C. Thromboxane generation was measured using ELISA. Graphs represent mean ± SD from at least three different experiments (P=0.0014). (C) Washed platelets from pdk1fl/fl and pdk1−/− mice were stimulated with 2MeSADP (50 nM) under stirred conditions for 3 minutes. Platelet proteins were separated by SDS-PAGE, immunoblotted and probed for phospho Raf1 (Ser338), MEK1/2 (Ser217/221), ERK1/2 (Thr202/Tyr204), cPLA2 (Ser505) and Akt (Thr308). The Western blot shown is a representative of three independent experiments.
Finally, we evaluated the effect of PDK1 on thrombosis in vivo. We treated mice with BX-795 in vivo for 5-days, which was necessary to fully inhibit PDK1 activity, based on initial experiments ((Figures 6A–B) and similar studies [49]. Inhibition of PDK1 with BX-795 significantly improved survival from ADP/epinephrine-induced thromboembolism (Figure 6C). We confirmed this in a second thrombosis model, where inhibition of PDK1 with BX-795 significantly improved survival from collagen/epinephrine induced pulmonary thromboembolism, as platelet aggregation induced by low doses of collagen depends on thromboxane (Figure 6D). Consistent with studies pharmacologically inhibiting PDK1, the genetic ablation of PDK1 also significantly rescued mortality from pulmonary thromboembolism (Figure 6E). Thus, the genetic ablation or pharmacologic inhibition of PDK1 reduces platelet-dependent thrombosis mortality in vivo.
Figure 6. Pharmacologic inhibition or genetic ablation of PDK1 delays pulmonary thromboembolism induced mortality.

(A) Wildtype mice were treated with BX-795 (10 μg/ml) or vehicle (DMSO) by intraperitionial injection once a day for 0, 3, or 5 days. Phosphorylation of Akt (T308) in isolated platelets stimulated with 2MeSADP ex vivo was measured to determine the duration of BX-795 treatment necessary to completely block phosphorylation of AKT. (B) Wildtype mice were treated with different concentrations of BX-795 (or vehicle) by intraperitoneal injection once a day for 5 days at various concentrations. Phosphorylation of Akt (T308) in isolated platelets stimulated with 2MeSADP ex vivo was measured. For Panels A and B, actin is a loading control and the immunoblot is representative of n=3 independent experiments. (C, D) Wildtype mice were treated with BX795 (10 μg/ml) or vehicle for 5 days. Pulmonary thromboembolism was induced by injecting (C) 2MeSADP (20mg/kg) plus epinephrine (30mg/kg) or (D) collagen (0.4 mg/kg) plus epinephrine (30mg/kg). N=7 mice/group for Panels C and D. Representative histological sections from lungs of mice injected with ADP are shown above. (E) pdk1fl/fl or pdk1−/− mice after induction of pulmonary thromboembolism (n=6/group). Representative histological sections from pdk1fl/fl or pdk1−/−lungs are shown to the right.
Discussion
PDK1 is a Ser/Thr protein kinase that can phosphorylate and activate other protein kinases from the AGC family. PDK1 plays important roles in mediating cellular responses such as cell growth, apoptosis, and angiogenesis[50–55]. Dysregulated activation of PI3K/PDK1/Akt is common in cancer and different inhibitors are being developed to block the downstream effectors of this pathway[38, 50, 56–58]. In our prior studies, we extensively characterized the PDK1 inhibitor BX-795, including its effects on aggregation at different doses and to various agonists such as collagen and AYPGKF in dose-dependent fashion[29]. ATP secretion was similarly reduced.
PDK1 also mediates thrombin and collagen mediated platelet aggregation, secretion, thromboxane generation[28, 29, 59]. However, the mechanism whereby PDK1 mediates ADP-induced platelet activation has never been elucidated. In our study, we used both pharmacological and genetic approaches to identify the mechanism through which PDK1 regulates ADP-induced platelet activation. The results from our study show a novel crosstalk between the PI3K and MAPK pathways that regulates ADP-triggered platelet activation and thromboxane generation via PDK1.
PDK1 has two well-studied downstream effectors: Akt and GSK3β. In resting platelets, activated GSK3β binds to its substrate to inhibit platelet activation. However, when platelets are stimulated, PDK1 is activated and subsequent phosphorylation of GSK3β at Ser9 inhibits its activity. Recent studies from Chen et al showed that PDK1 inhibition blocks thrombin induced platelet aggregation and that this defect could be rescued by treating cells with the GSK3β inhibitor SB-216763 [28]. In contrast, while our studies also identified that PDK1 inhibition blocks ADP induced platelet aggregation, SB-216763 did not rescue the aggregation defect when platelets were stimulated with 2MeSADP (Figure 1B). These results demonstrate that PDK1 regulates ADP-induced platelet activation through a novel GSK3β independent mechanism. Studies from Dangelmaier et al have shown that platelets treated with BX-795, a PDK1 specific inhibitor, affected 2MeSADP induced platelet activation and thromboxane generation [29]. Our study shows that treatment of platelets with BX-795 inhibited 2MeSADP mediated secondary platelet aggregation similar to platelets treated with aspirin or indomethacin (Figure 1A). It is known that 2MeSADP mediated secondary platelet aggregation is thromboxane dependent. Therefore, we evaluated the novel mechanism through which PDK1 regulates 2MeSADP-induced platelet thromboxane generation. Our results (Figure 1C & 4) show that pharmacologic inhibition or genetic deletion of PDK1 abolishes thromboxane generation.
Thromboxane generation induced by 2MeSADP is coordinated by P2Y1, P2Y12 and integrin αIIbβ3 [16]. Studies from Kim et al and others have also shown that inhibition of either ERK1/2 or Pyk2 activation results in abolished thromboxane generation in ADP stimulated platelets[21, 60, 61]. Previous studies have shown that 2MeSADP-induced ERK1/2 phosphorylation occurs independently of outside-in signaling, but rather MEK1/2, SFK and PI3K proteins regulate ERK1/2 activation[21, 24, 60]. Inhibition of MEK1/2 and PI3K using pharmacological inhibitors prevents 2MeSADP-mediated secondary platelet aggregation by abolishing thromboxane generation, similar to PDK1 inhibition [24]. Taken together, the analysis of these signaling pathways indicates that PDK1 activation downstream of the PI3K pathway regulates the MAPK pathway (Figure 3C & D) and 2MeSADP-induced thromboxane generation and thrombosis. Previous studies in transfected cell lines show that PDK1 is critical for MEK1/2 activation [43]. However, the functional importance of a crosstalk between the PI3K pathway and the MAPK pathway has never been studied in platelets until now. Garcia et al showed that intracellular calcium (Ca2+) released downstream of P2Y1, and PI3K activation downstream of P2Y12 play a crucial role in the activation of ERK2 when cells are stimulated with 2MeSADP [16]. Nevertheless, the mechanism through which PI3K pathway regulates ERK1/2 activation and thromboxane generation is not studied. In our study, we found that inhibition or deletion of PDK1 (a Ser/Thr protein kinase activated downstream of PI3K pathway) resulted in abolished ERK2 and cPLA2 phosphorylation (Figure 2A). These results suggest that PDK1 regulates the MAPK pathway and also thromboxane generation. Our study also evaluated the effect of PDK1 on the MEK1/2 kinases activity, as it is an important upstream kinase for ERK activation, using a MEK1/2 kinase assay. As shown in Figure 2B, MEK1/2 activity is abolished in BX795 treated platelets stimulated with 2MeSADP. These results provide further evidence that the PI3K and MAPK pathways are linked by PDK1.
In other cells, Raf1 is an important protein kinase that is crucial for phosphorylation of MEK1/2 [62]. Raf1 is expressed in platelets and upon stimulation its activity is regulated by intracellular calcium and Protein Kinase C (PKC) upon stimulation [63, 64]. Interestingly, studies in 2MeSADP-stimulated platelets have shown that inhibition of PKC does not inhibit ERK1/2 phosphorylation and thromboxane generation [65]. This data suggest that Raf1 is regulated by a new Serine/Threonine protein kinase other than PKC located downstream of ADP receptors. We evaluated the effect of PDK1 and/or PKC inhibition on Raf1 phosphorylation in platelets stimulated with 2MeSADP. Our results show that Raf1 phosphorylation is abolished in platelets in which PDK1, but not PKC, is inhibited (Figure 2C).
Naik et al demonstrated that ASK1 regulate thromboxane generation and thrombosis when platelets are stimulated with thrombin, collagen or convulxin by abolishing p38 and cPLA2 phosphorylation [47]. However, the relative effect of ASK1 deletion on 2MeSADP-induced thromboxane generation is not studied. In other cells, PDK1 and ASK1 has been shown to directly interact and phosphorylate each other and act as negative regulators of their respective kinases [48]. We evaluated the effect of PDK1 and ASK1 inhibition on 2MeSADP induced platelet aggregation, thromboxane generation and cPLA2 phosphorylation. Figure 3A & B shows that inhibition of ASK1 (MSC 2032964A) did not abolish 2MeSADP-induced secondary aggregation, thromboxane generation or cPLA2 phosphorylation, but these are abolished when platelets are treated with BX-795. We used ERK1/2 phosphorylation as a negative control to determine the specificity of the inhibitor (data not shown), as this protein has been shown to be regulated by ASK1 [47].
We also evaluated the role of p38 in 2MeSADP induced platelet activation and especially cPLA2 activation. Our data (Figure 4) indicate that both p38 and ERK1/2 are required for ADP-induced activation of cPLA2. At higher concentrations of 2MeSADP (50nM), ASK1 inhibition did not fully abolish p38 kinase activation (Figure 4B), suggesting that under these conditions, ASK1-independent pathways may regulate p38 activation.
Pharmacological inhibitors, particularly at higher concentrations may have off-target effects. Akt and GSK3β regulate platelet functions and are downstream effectors of PDK1. Thus, we employed specific inhibitors for Akt and GSK3β and evaluated their effects on the MAPK pathway. As shown in Figure 3C & D, Akt or GSK3β inhibitors have no effect on activation of the MAPK pathway. However, when platelets were treated with the PDK1 inhibitor, BX-795, in combination with Akt or GSK3β inhibitors, phosphorylation of Raf1/MEK/ERK were abolished. These results show that PDK1, but not Akt or GSK3β, regulates MAPK pathway activation. We further confirmed the role of PDK1 on MAPK pathway by using platelet specific PDK1 knockout mice. Our results from Figure 5 show that PDK1 deletion has critical effects on MAPK pathway activation similar to what we uncovered with BX-795. These results from our study show the not only the specificity of the PDK1 inhibitor but also the role of this important serine/threonine kinase protein in MAPK pathway activation and platelet activation with ADP. Moreover, our findings demonstrate that this pathway is conserved between humans and mice.
Münzer et al and others have shown that inhibition of PDK1 protects mice from stroke and thrombosis [59]. This study also demonstrates that collagen mediated adhesion and aggregation was affected by PDK1 inhibition. As thromboxane plays a crucial role in platelet activation when stimulated with low dose of collagen [66, 67], we evaluated the effect of PDK1 inhibition on collagen and epinephrine induced pulmonary embolism in mice treated with BX-795 or PDK1 knockout. Our results show that PDK1 inhibition or platelet specific genetic deletion of PDK1 is protective against thrombosis using several different models (Figure 6). Coupling pharmacologic inhibitor studies with in vivo models where PDK1 has been genetically ablated is a strength of our studies. Moreover, we used a specific and well-studied inhibitor of PDK1, BX-795, at concentrations that minimize off-target effects (Figures 6A, 6B). Nevertheless, BX-795 may affect other cells and tissues and this point should be considered in future studies investigating BX-795 or similar inhibitors targeting PDK1.
In conclusion, PDK1 regulates 2MeSADP-induced platelet activation and thromboxane generation via the MAPK pathway (Figure 7). PDK1, but not PKCs, phosphorylate and activate Raf1 in MAPK pathway when platelets are stimulated with 2MeSADP. Furthermore, PDK1 inhibition protects mice from collagen/epinephrine induced pulmonary embolism. Our study provides clear evidence for a novel crosstalk between PI3K and MAPK pathways, regulating platelet function via PDK1.
Figure 7. Proposed model illustrating PDK1 regulation of the MAPK signaling pathway in platelets stimulated with 2MeSADP.
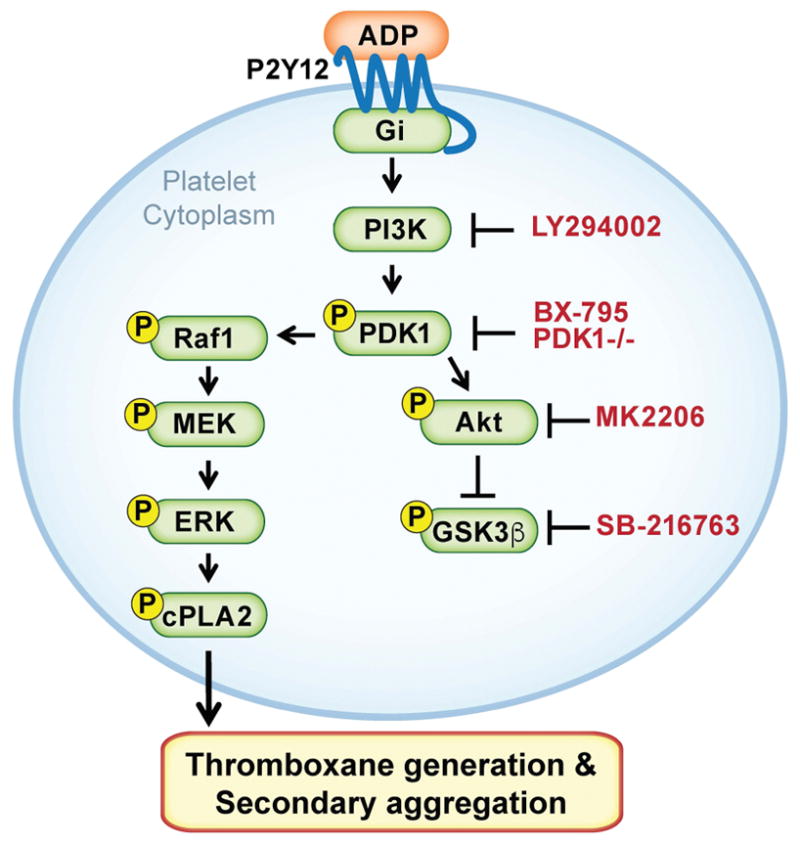
We propose that upon activation of P2Y12 in platelets, PDK1 becomes activated and then phosphorylates Raf1. Phosphorylated Raf1, in turn, regulates activation of MEK1/2, ERK1/2 and cPLA2 proteins, which are necessary for 2MeSADP-induced thromboxane generation and platelet aggregation.
Essentials.
Phosphoinositide 3-kinase and Mitogen-activated protein kinase pathways crosstalk via PDK1.
PDK1 is required for adenosine diphosphate-induced platelet activation and thromboxane generation.
PDK1 regulates RAF proto-oncogene Ser/Thr kinase (Raf1) activation in the MAPK pathway.
Genetic ablation of PDK1 protects against platelet-dependent thrombosis in vivo.
Acknowledgments
This work was supported by the NHLBI and NIA (HL112311 and HL126547 to M. T. Rondina and A. S. Weyrich and AG048022 to M. T. Rondina). This material is the result of work supported with resources and the use of facilities at the George E. Wahlen VA Medical Center, Salt Lake City, Utah. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government. We thank O. Borst, University of Tübingen, Germany for providing PDK1 knockout mice samples for the experiments. We also thank S. P. Kunapuli (HL118593), Temple University, USA for critically reviewing the manuscript.
Footnotes
Addendum
B. K. Manne designed and performed experiments, analyzed and interpreted data, and wrote manuscript. P. Münzer performed experiments, and analyzed and interpreted data. R. Badolia performed experiments. B. Walker-Allgaier performed experiments, and analyzed and interpreted data. R. Campbell analyzed and interpreted data. E. Middleton analyzed and interpreted data. A. S. Weyrich provided reagents and reviewed the manuscript. S. P. Kunapuli performed experiments and reviewed the manuscript. O. Borst provided reagents, performed experiments and reviewed the manuscript. M. T. Rondina designed experiments, analyzed and interpreted data.
Disclosure of Conflict of Interests
The authors state that they have no conflict of interest.
References
- 1.Donati MB. Cancer and thrombosis. Haemostasis. 1994;24:128–31. doi: 10.1159/000217092. [DOI] [PubMed] [Google Scholar]
- 2.Mannaioni PF, Di Bello MG, Masini E. Platelets and inflammation: role of platelet-derived growth factor, adhesion molecules and histamine. Inflamm Res. 1997;46:4–18. doi: 10.1007/PL00000158. [DOI] [PubMed] [Google Scholar]
- 3.Goldschmidt-Clermont PJ, Kandzari DE, Sketch MH, Jr, Phillips HR. Inflammation, platelets, and glycoprotein IIb/IIIa inhibitors. J Invasive Cardiol. 2002;14(Suppl E):18E–25E. quiz 6E. [PubMed] [Google Scholar]
- 4.Gear AR, Camerini D. Platelet chemokines and chemokine receptors: linking hemostasis, inflammation, and host defense. Microcirculation. 2003;10:335–50. doi: 10.1038/sj.mn.7800198. [DOI] [PubMed] [Google Scholar]
- 5.Weyrich AS, Lindemann S, Zimmerman GA. The evolving role of platelets in inflammation. J Thromb Haemost. 2003;1:1897–905. doi: 10.1046/j.1538-7836.2003.00304.x. [DOI] [PubMed] [Google Scholar]
- 6.Projahn D, Koenen RR. Platelets: key players in vascular inflammation. J Leukoc Biol. 2012;92:1167–75. doi: 10.1189/jlb.0312151. [DOI] [PubMed] [Google Scholar]
- 7.Jenne CN, Urrutia R, Kubes P. Platelets: bridging hemostasis, inflammation, and immunity. Int J Lab Hematol. 2013;35:254–61. doi: 10.1111/ijlh.12084. [DOI] [PubMed] [Google Scholar]
- 8.Dovizio M, Alberti S, Guillem-Llobat P, Patrignani P. Role of platelets in inflammation and cancer: novel therapeutic strategies. Basic Clin Pharmacol Toxicol. 2014;114:118–27. doi: 10.1111/bcpt.12156. [DOI] [PubMed] [Google Scholar]
- 9.Honn KV, Tang DG, Crissman JD. Platelets and cancer metastasis: a causal relationship? Cancer Metastasis Rev. 1992;11:325–51. doi: 10.1007/BF01307186. [DOI] [PubMed] [Google Scholar]
- 10.Mustard JF. Hemostasis and thrombosis. Semin Hematol. 1968;5:91–106. [PubMed] [Google Scholar]
- 11.Koupenova M, Kehrel BE, Corkrey HA, Freedman JE. Thrombosis and platelets: an update. Eur Heart J. 2016 doi: 10.1093/eurheartj/ehw550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meade TW. Thrombosis and cardiovascular disease. Ann Epidemiol. 1992;2:353–64. doi: 10.1016/1047-2797(92)90084-4. [DOI] [PubMed] [Google Scholar]
- 13.Andrews RK, Gardiner EE, Shen Y, Berndt MC. Platelet interactions in thrombosis. IUBMB Life. 2004;56:13–8. doi: 10.1080/15216540310001649831. [DOI] [PubMed] [Google Scholar]
- 14.Puri RN, Colman RW. ADP-induced platelet activation. Crit Rev Biochem Mol Biol. 1997;32:437–502. doi: 10.3109/10409239709082000. [DOI] [PubMed] [Google Scholar]
- 15.Jin J, Kunapuli SP. Coactivation of two different G protein-coupled receptors is essential for ADP-induced platelet aggregation. Proc Natl Acad Sci U S A. 1998;95:8070–4. doi: 10.1073/pnas.95.14.8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin J, Quinton TM, Zhang J, Rittenhouse SE, Kunapuli SP. Adenosine diphosphate (ADP)-induced thromboxane A(2) generation in human platelets requires coordinated signaling through integrin alpha(IIb)beta(3) and ADP receptors. Blood. 2002;99:193–8. doi: 10.1182/blood.v99.1.193. [DOI] [PubMed] [Google Scholar]
- 17.Murugappa S, Kunapuli SP. The role of ADP receptors in platelet function. Front Biosci. 2006;11:1977–86. doi: 10.2741/1939. [DOI] [PubMed] [Google Scholar]
- 18.De Caterina R, Giannessi D, Gazzetti P, Bernini W. Thromboxane-B2 generation during ex-vivo platelet aggregation. J Nucl Med Allied Sci. 1984;28:185–96. [PubMed] [Google Scholar]
- 19.Lands WE, Culp BR, Hirai A, Gorman R. Relationship of thromboxane generation to the aggregation of platelets from humans: effects of eicosapentaenoic acid. Prostaglandins. 1985;30:819–25. doi: 10.1016/0090-6980(85)90010-3. [DOI] [PubMed] [Google Scholar]
- 20.Kyrle PA, Eichler HG, Jager U, Lechner K. Inhibition of prostacyclin and thromboxane A2 generation by low-dose aspirin at the site of plug formation in man in vivo. Circulation. 1987;75:1025–9. doi: 10.1161/01.cir.75.5.1025. [DOI] [PubMed] [Google Scholar]
- 21.Kim S, Cipolla L, Guidetti G, Okigaki M, Jin J, Torti M, Kunapuli SP. Distinct role of Pyk2 in mediating thromboxane generation downstream of both G12/13 and integrin alphaIIbbeta3 in platelets. J Biol Chem. 2013;288:18194–203. doi: 10.1074/jbc.M113.461087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia A, Quinton TM, Dorsam RT, Kunapuli SP. Src family kinase-mediated and Erk-mediated thromboxane A2 generation are essential for VWF/GPIb-induced fibrinogen receptor activation in human platelets. Blood. 2005;106:3410–4. doi: 10.1182/blood-2005-05-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shankar H, Garcia A, Prabhakar J, Kim S, Kunapuli SP. P2Y12 receptor-mediated potentiation of thrombin-induced thromboxane A2 generation in platelets occurs through regulation of Erk1/2 activation. J Thromb Haemost. 2006;4:638–47. doi: 10.1111/j.1538-7836.2006.01789.x. [DOI] [PubMed] [Google Scholar]
- 24.Garcia A, Kim S, Bhavaraju K, Schoenwaelder SM, Kunapuli SP. Role of phosphoinositide 3-kinase beta in platelet aggregation and thromboxane A2 generation mediated by Gi signalling pathways. Biochem J. 2010;429:369–77. doi: 10.1042/BJ20100166. [DOI] [PubMed] [Google Scholar]
- 25.Gagliardi PA, di Blasio L, Puliafito A, Seano G, Sessa R, Chianale F, Leung T, Bussolino F, Primo L. PDK1-mediated activation of MRCKalpha regulates directional cell migration and lamellipodia retraction. J Cell Biol. 2014;206:415–34. doi: 10.1083/jcb.201312090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zurashvili T, Cordon-Barris L, Ruiz-Babot G, Zhou X, Lizcano JM, Gomez N, Gimenez-Llort L, Bayascas JR. Interaction of PDK1 with phosphoinositides is essential for neuronal differentiation but dispensable for neuronal survival. Mol Cell Biol. 2013;33:1027–40. doi: 10.1128/MCB.01052-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weber DS, Taniyama Y, Rocic P, Seshiah PN, Dechert MA, Gerthoffer WT, Griendling KK. Phosphoinositide-dependent kinase 1 and p21-activated protein kinase mediate reactive oxygen species-dependent regulation of platelet-derived growth factor-induced smooth muscle cell migration. Circ Res. 2004;94:1219–26. doi: 10.1161/01.RES.0000126848.54740.4A. [DOI] [PubMed] [Google Scholar]
- 28.Chen X, Zhang Y, Wang Y, Li D, Zhang L, Wang K, Luo X, Yang Z, Wu Y, Liu J. PDK1 regulates platelet activation and arterial thrombosis. Blood. 2013;121:3718–26. doi: 10.1182/blood-2012-10-461897. [DOI] [PubMed] [Google Scholar]
- 29.Dangelmaier C, Manne BK, Liverani E, Jin J, Bray P, Kunapuli SP. PDK1 selectively phosphorylates Thr(308) on Akt and contributes to human platelet functional responses. Thromb Haemost. 2014;111:508–17. doi: 10.1160/TH13-06-0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maurer M, Su T, Saal LH, Koujak S, Hopkins BD, Barkley CR, Wu J, Nandula S, Dutta B, Xie Y, Chin YR, Kim DI, Ferris JS, Gruvberger-Saal SK, Laakso M, Wang X, Memeo L, Rojtman A, Matos T, Yu JS, Cordon-Cardo C, Isola J, Terry MB, Toker A, Mills GB, Zhao JJ, Murty VV, Hibshoosh H, Parsons R. 3-Phosphoinositide-dependent kinase 1 potentiates upstream lesions on the phosphatidylinositol 3-kinase pathway in breast carcinoma. Cancer Res. 2009;69:6299–306. doi: 10.1158/0008-5472.CAN-09-0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mora A, Komander D, van Aalten DM, Alessi DR. PDK1, the master regulator of AGC kinase signal transduction. Semin Cell Dev Biol. 2004;15:161–70. doi: 10.1016/j.semcdb.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 32.Bayascas JR, Leslie NR, Parsons R, Fleming S, Alessi DR. Hypomorphic mutation of PDK1 suppresses tumorigenesis in PTEN(+/−) mice. Curr Biol. 2005;15:1839–46. doi: 10.1016/j.cub.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 33.Feldman RI, Wu JM, Polokoff MA, Kochanny MJ, Dinter H, Zhu D, Biroc SL, Alicke B, Bryant J, Yuan S, Buckman BO, Lentz D, Ferrer M, Whitlow M, Adler M, Finster S, Chang Z, Arnaiz DO. Novel small molecule inhibitors of 3-phosphoinositide-dependent kinase-1. J Biol Chem. 2005;280:19867–74. doi: 10.1074/jbc.M501367200. [DOI] [PubMed] [Google Scholar]
- 34.Garcia-Echeverria C, Sellers WR. Drug discovery approaches targeting the PI3K/Akt pathway in cancer. Oncogene. 2008;27:5511–26. doi: 10.1038/onc.2008.246. [DOI] [PubMed] [Google Scholar]
- 35.Najafov A, Sommer EM, Axten JM, Deyoung MP, Alessi DR. Characterization of GSK2334470, a novel and highly specific inhibitor of PDK1. Biochem J. 2011;433:357–69. doi: 10.1042/BJ20101732. [DOI] [PubMed] [Google Scholar]
- 36.Gagliardi PA, di Blasio L, Orso F, Seano G, Sessa R, Taverna D, Bussolino F, Primo L. 3-phosphoinositide-dependent kinase 1 controls breast tumor growth in a kinase-dependent but Akt-independent manner. Neoplasia. 2012;14:719–31. doi: 10.1593/neo.12856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Velpula KK, Tsung AJ. PDK1: a new therapeutic target for glioblastoma? CNS Oncol. 2014;3:177–9. doi: 10.2217/cns.14.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou J, Song S, He S, Zhu X, Zhang Y, Yi B, Zhang B, Qin G, Li D. MicroRNA-375 targets PDK1 in pancreatic carcinoma and suppresses cell growth through the Akt signaling pathway. Int J Mol Med. 2014;33:950–6. doi: 10.3892/ijmm.2014.1638. [DOI] [PubMed] [Google Scholar]
- 39.Manne BK, Getz TM, Hughes CE, Alshehri O, Dangelmaier C, Naik UP, Watson SP, Kunapuli SP. Fucoidan is a novel platelet agonist for the C-type lectin-like receptor 2 (CLEC-2) J Biol Chem. 288:7717–26. doi: 10.1074/jbc.M112.424473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manne BK, Badolia R, Dangelmaier C, Eble JA, Ellmeier W, Kahn M, Kunapuli SP. Distinct pathways regulate Syk protein activation downstream of immune tyrosine activation motif (ITAM) and hemITAM receptors in platelets. J Biol Chem. 2015;290:11557–68. doi: 10.1074/jbc.M114.629527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rowley JW, Chappaz S, Corduan A, Chong MM, Campbell R, Khoury A, Manne BK, Wurtzel JG, Michael JV, Goldfinger LE, Mumaw MM, Nieman MT, Kile BT, Provost P, Weyrich AS. Dicer1-mediated miRNA processing shapes the mRNA profile and function of murine platelets. Blood. 2016;127:1743–51. doi: 10.1182/blood-2015-07-661371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bradley EW, Ruan MM, Vrable A, Oursler MJ. Pathway crosstalk between Ras/Raf and PI3K in promotion of M-CSF-induced MEK/ERK-mediated osteoclast survival. J Cell Biochem. 2008;104:1439–51. doi: 10.1002/jcb.21719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sato S, Fujita N, Tsuruo T. Involvement of 3-phosphoinositide-dependent protein kinase-1 in the MEK/MAPK signal transduction pathway. J Biol Chem. 2004;279:33759–67. doi: 10.1074/jbc.M402055200. [DOI] [PubMed] [Google Scholar]
- 44.Montiel M, de la Blanca EP, Jimenez E. P2Y receptors activate MAPK/ERK through a pathway involving PI3K/PDK1/PKC-zeta in human vein endothelial cells. Cell Physiol Biochem. 2006;18:123–34. doi: 10.1159/000095180. [DOI] [PubMed] [Google Scholar]
- 45.Pearson G, Bumeister R, Henry DO, Cobb MH, White MA. Uncoupling Raf1 from MEK1/2 impairs only a subset of cellular responses to Raf activation. J Biol Chem. 2000;275:37303–6. doi: 10.1074/jbc.C000570200. [DOI] [PubMed] [Google Scholar]
- 46.Chiloeches A, Mason CS, Marais R. S338 phosphorylation of Raf-1 is independent of phosphatidylinositol 3-kinase and Pak3. Mol Cell Biol. 2001;21:2423–34. doi: 10.1128/MCB.21.7.2423-2434.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Naik MU, Patel P, Derstine R, Turaga R, Chen X, Golla K, Neeves KB, Ichijo H, Naik UP. Ask1 regulates murine platelet granule secretion, thromboxane A2 generation, and thrombus formation. Blood. 2017;129:1197–209. doi: 10.1182/blood-2016-07-729780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seong HA, Jung H, Ichijo H, Ha H. Reciprocal negative regulation of PDK1 and ASK1 signaling by direct interaction and phosphorylation. J Biol Chem. 2010;285:2397–414. doi: 10.1074/jbc.M109.064295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mazharian A, Ghevaert C, Zhang L, Massberg S, Watson SP. Dasatinib enhances megakaryocyte differentiation but inhibits platelet formation. Blood. 2011;117:5198–206. doi: 10.1182/blood-2010-12-326850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu T, Yin H. PDK1 promotes tumor cell proliferation and migration by enhancing the Warburg effect in non-small cell lung cancer. Oncol Rep. 2017;37:193–200. doi: 10.3892/or.2016.5253. [DOI] [PubMed] [Google Scholar]
- 51.Zhang L, Lei J, Fang ZL, Xiong JP. MiR-128b is down-regulated in gastric cancer and negatively regulates tumour cell viability by targeting PDK1/Akt/NF-kappaB axis. J Biosci. 2016;41:77–85. doi: 10.1007/s12038-016-9586-0. [DOI] [PubMed] [Google Scholar]
- 52.Makris C, Voisin L, Giasson E, Tudan C, Kaplan DR, Meloche S. The Rb-family protein p107 inhibits translation by a PDK1-dependent mechanism. Oncogene. 2002;21:7891–6. doi: 10.1038/sj.onc.1205964. [DOI] [PubMed] [Google Scholar]
- 53.Xie Z, Yuan H, Yin Y, Zeng X, Bai R, Glazer RI. 3-phosphoinositide-dependent protein kinase-1 (PDK1) promotes invasion and activation of matrix metalloproteinases. BMC Cancer. 2006;6:77. doi: 10.1186/1471-2407-6-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakamura K, Sakaue H, Nishizawa A, Matsuki Y, Gomi H, Watanabe E, Hiramatsua R, Tamamori-Adachi M, Kitajima S, Noda T, Ogawa W, Kasuga M. PDK1 regulates cell proliferation and cell cycle progression through control of cyclin D1 and p27Kip1 expression. J Biol Chem. 2008;283:17702–11. doi: 10.1074/jbc.M802589200. [DOI] [PubMed] [Google Scholar]
- 55.Zaru R, Matthews SP, Edgar AJ, Prescott AR, Gomez-Nicola D, Hanauer A, Watts C. The PDK1-Rsk Signaling Pathway Controls Langerhans Cell Proliferation and Patterning. J Immunol. 2015;195:4264–72. doi: 10.4049/jimmunol.1501520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zheng N, Ding X, Sun A, Jahan R. PDK1 Activity Regulates Proliferation, Invasion and Growth of Hemangiomas. Cell Physiol Biochem. 2015;36:1903–10. doi: 10.1159/000430159. [DOI] [PubMed] [Google Scholar]
- 57.Wada M, Horinaka M, Yasuda S, Masuzawa M, Sakai T, Katoh N. PDK1 is a potential therapeutic target against angiosarcoma cells. J Dermatol Sci. 2015;78:44–50. doi: 10.1016/j.jdermsci.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 58.Raimondi C, Falasca M. Targeting PDK1 in cancer. Curr Med Chem. 2011;18:2763–9. doi: 10.2174/092986711796011238. [DOI] [PubMed] [Google Scholar]
- 59.Munzer P, Walker-Allgaier B, Geue S, Geuss E, Hron G, Rath D, Eissler D, Winter S, Schaeffeler E, Meinert M, Schaller M, Greinacher A, Schwab M, Geisler T, Kleinschnitz C, Lang F, Gawaz M, Borst O. PDK1 Determines Collagen-Dependent Platelet Ca2+ Signaling and Is Critical to Development of Ischemic Stroke In Vivo. Arterioscler Thromb Vasc Biol. 2016;36:1507–16. doi: 10.1161/ATVBAHA.115.307105. [DOI] [PubMed] [Google Scholar]
- 60.Garcia A, Shankar H, Murugappan S, Kim S, Kunapuli SP. Regulation and functional consequences of ADP receptor-mediated ERK2 activation in platelets. Biochem J. 2007;404:299–308. doi: 10.1042/BJ20061584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Canobbio I, Cipolla L, Consonni A, Momi S, Guidetti G, Oliviero B, Falasca M, Okigaki M, Balduini C, Gresele P, Torti M. Impaired thrombin-induced platelet activation and thrombus formation in mice lacking the Ca(2+)-dependent tyrosine kinase Pyk2. Blood. 2013;121:648–57. doi: 10.1182/blood-2012-06-438762. [DOI] [PubMed] [Google Scholar]
- 62.Schonwasser DC, Marais RM, Marshall CJ, Parker PJ. Activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway by conventional, novel, and atypical protein kinase C isotypes. Mol Cell Biol. 1998;18:790–8. doi: 10.1128/mcb.18.2.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kawauchi K, Lazarus AH, Sanghera JS, Man GL, Pelech SL, Delovitch TL. Regulation of BCR- and PKC/Ca(2+)-mediated activation of the Raf1/MEK/MAPK pathway by protein-tyrosine kinase and -tyrosine phosphatase activities. Mol Immunol. 1996;33:287–96. doi: 10.1016/0161-5890(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 64.Wu X, Simpson J, Hong JH, Kim KH, Thavarajah NK, Backx PH, Neel BG, Araki T. MEK-ERK pathway modulation ameliorates disease phenotypes in a mouse model of Noonan syndrome associated with the Raf1(L613V) mutation. J Clin Invest. 2011;121:1009–25. doi: 10.1172/JCI44929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bynagari-Settipalli YS, Lakhani P, Jin J, Bhavaraju K, Rico MC, Kim S, Woulfe D, Kunapuli SP. Protein kinase C isoform epsilon negatively regulates ADP-induced calcium mobilization and thromboxane generation in platelets. Arterioscler Thromb Vasc Biol. 2012;32:1211–9. doi: 10.1161/ATVBAHA.111.242388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moog S, Mangin P, Lenain N, Strassel C, Ravanat C, Schuhler S, Freund M, Santer M, Kahn M, Nieswandt B, Gachet C, Cazenave JP, Lanza F. Platelet glycoprotein V binds to collagen and participates in platelet adhesion and aggregation. Blood. 2001;98:1038–46. doi: 10.1182/blood.v98.4.1038. [DOI] [PubMed] [Google Scholar]
- 67.Jang JY, Kim TS, Cai J, Kim J, Kim Y, Shin K, Kim KS, Lee SP, Kang MH, Choi EK, Rhee MH, Kim YB. Perilla oil improves blood flow through inhibition of platelet aggregation and thrombus formation. Lab Anim Res. 2014;30:21–7. doi: 10.5625/lar.2014.30.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]


