Abstract
Clinical cases have reported increased peripheral blood flow following continuous epidural spinal cord stimulation (SCS), and that reduced muscle sympathetic nerve activity (MSNA) may be a potential mechanism. However, no studies in humans have directly examined the effects of acute SCS (<60 min) on vascular conductance and MSNA. In study 1, we tested the hypothesis that acute SCS (<60 min) of the thoracic spine would lead to increased common femoral vascular conductance, but not brachial vascular conductance, in 11 patients that previously underwent surgical SCS implantation for management of neuropathic pain. Throughout 60 min of SCS, common femoral artery conductance was elevated and significantly different from brachial artery conductance (15min: Δ26±37% vs. Δ−2±19%; 30min: Δ28±45% vs. Δ0±26%; 45min: Δ48±43% vs. Δ2±21%; 60min: Δ36±61% vs. Δ1±24%; 15min post SCS: Δ51±64% vs. Δ6±33% ml/min, P=0.013). A similar examination in a patient with cervical SCS revealed minimal changes in vascular conductance. In study 2, we further examined whether acute SCS reduces peroneal MSNA in a subset of SCS patients (n=5). MSNA burst incidence in response to acute SCS gradually declined and was significantly reduced at 45- and 60-min of SCS (15min: Δ−1±12%, 30min: Δ−14±12%, 45min: Δ−19±16%, 60min: Δ−24±18%, 15min post SCS: Δ−11±7% bursts/100heartbeats, P=0.015). These data demonstrate that acute SCS rapidly increases femoral vascular conductance and reduces peroneal MSNA. The gradual reduction in peroneal MSNA observed during acute SCS suggests that neural mechanisms in addition to attenuated MSNA may be involved in the acute rise in femoral vascular conductance.
Keywords: MSNA, spinal cord stimulation, peripheral blood flow
Introduction
Electrical stimulation of neurons within the dorsal columns of the spinal cord (spinal cord stimulation, SCS) is a neuromodulation therapy for increasing blood flow to the extremities. In patients with peripheral artery disease (PAD), continuous SCS leads to an increase in circulation and a subsequent reduction in ischemic pain (Linderoth & Foreman, 1999), and promotes healing of ischemic ulcers likely through improved microcirculation (Cook, Oygar, Baggenstos, Pacheco, & Kleriga, 1976; Jacobs et al., 1988). SCS acts on multiple pathways in the central nervous system, and is therefore used to treat a variety of other conditions such as neuropathic pain (e.g., chronic back and/or lower extremity pain) (Epstein & Palmieri, 2012; Manchikanti et al., 2013) and angina pectoris and ventricular arrhythmias (Mannheimer, Augustinsson, Carlsson, Manhem, & Wilhelmsson, 1988; Odenstedt et al., 2011). However, despite the long history of SCS that spans nearly six decades, studies have yet to fully elucidate the specific mechanisms by which SCS provides benefit in humans.
What we know about the effects of SCS on peripheral blood flow stem largely from outcomes of months or years of patient treatment. It is unclear whether acute SCS treatment has immediate effects on lower limb blood flow. Evidence supports an increase in cerebral blood flow following acute (<60 min) cervical SCS (Hautvast et al., 1997; Meglio et al., 1991). In regard to lower limb blood flow, early studies inferring from arterial pulse pressure at the toe reported that acute (<60 min) SCS increased arterial dilation (Dooley & Kasprak, 1976). Some evidence suggest that continuous SCS (Jacobs et al., 1988), but not acute SCS (Kemler, Barendse, van Kleef, & Egbrink, 2000), increases cutaneous blood flow, although studies were performed in patients with history of sympathectomy and/or vascular surgery. To our knowledge, no studies in humans have directly examined the acute effects of SCS (<60 min) on leg vascular conductance.
Reduction in muscle sympathetic nerve activity (MSNA) has been considered a potential mechanism of action by which SCS improves circulation (Linderoth & Foreman, 1999). The theory that SCS reduces MSNA originated from clinical observations of pain relief (Augustinsson, Carlsson, Holm, & Jivegard, 1985) and normalization in measures of peripheral blood flow following SCS in patients with peripheral vascular disease (Broseta et al., 1986; Horsch, Schulte, & Hess, 2004). Increase in limb blood flow following SCS appears to mimic the effects of sympathetic block or sympathectomy (Augustinsson et al., 1985; Linderoth, Gunasekera, & Meyerson, 1991). However, animal models of SCS have demonstrated that extremity blood flow can increase independent of sympathetic inhibition depending on the stimulation parameters used (Croom, Foreman, Chandler, & Barron, 1997). To date, no studies have directly examined MSNA responses to SCS in humans. Moreover, studies of circulatory improvement with SCS often include patients with sympathectomy, which makes interpretation of the effects of SCS on sympathetic activity difficult. Thus, whether acute SCS-mediated increases in blood flow in the lower limbs can be associated with reduced MSNA remains unclear.
In the present study, we tested the hypothesis that acute SCS (60 min) significantly increases leg vascular conductance. Patients studied herein previously received surgical SCS implantation to treat neuropathic conditions (e.g., chronic back pain). Therefore, SCS lead placement and configurations were optimized to induce paresthesiae and provide neuropathic pain relief, and perhaps not ideal for influencing leg blood flow. Nonetheless, we performed these studies to provide proof of concept that acute SCS increases leg blood flow in addition to the neuropathic pain relief that these patients typically experience with SCS. Because SCS implants were at the level of the thoracic spine, we hypothesized that acute SCS would increase femoral vascular conductance, but not brachial vascular conductance. One patient with cervical SCS was also studied to observe whether femoral vascular conductance can be increased with acute cervical SCS. In addition, to examine whether increased femoral vascular conductance following acute SCS can be associated with decreased MSNA, we performed a second study in a subset of the SCS patients in which MSNA was measured in response to acute SCS.
Methods
Ethical approval
Studies conformed to the standards set by the latest revision of the Declaration of Helsinki, and all procedures were approved by the Institutional Review Board at the University of Iowa (Project#: 201605777). This study was not registered in a database. Each participant received a verbal and written explanation of the study objectives, measurement techniques, and risks and benefits associated with the investigation prior to providing written informed consent.
Subjects
Twelve patients (54 ± 12 yrs, 5 men/7 women) that previously underwent SCS implantation (2.5 ± 1.3 years) to treat neuropathic pain originating in the lower back and/or limbs were recruited through the Department of Neurosurgery at the University of Iowa Hospitals and Clinics. Subject characteristics are presented in Table 1. Eleven of the 12 patients had thoracic SCS implants, and 1 patient (patient 11) had cervical SCS implants. With each patient’s condition in mind, patients were studied and evaluated in protocol 1 (vascular conductance responses to 60 min of SCS), and based on the level of ease in which protocol 1 was performed, patients returned to participate in protocol 2 (MSNA responses to 60 min of SCS). Patients were excluded from this study if previously diagnosed or suffered from peripheral vascular disease, heart disease, renal failure, type 1 diabetes, cancer, autonomic dysfunction, have had history of sympathectomy or any form of heart surgery. Six patients had history of hypertension, 5 of which were currently taking anti-hypertensive medication (Table 1).
Table 1.
Subject characteristics
| Patient | Age (years) |
Sex (M/W) |
SBP/DBP (mmHg) |
Medications |
|---|---|---|---|---|
| Patient 1 | 42 | W | 109/62 | Cyclobenzaprine, Tramadol |
| Patient 2 | 75 | M | 133/68 | Pregabalin, Atenolol, Tamsulosin |
| Patient 3 | 43 | W | 125/83 | HCTZ, Cyclobenzaprine, Acetaminophen/Hydrocodone |
| Patient 4 | 36 | W | 129/75 | - |
| Patient 5 | 64 | W | 163/71 | Enalapril, Gabapentin, Levetiracetam, Oxycodone, Tizanidine |
| Patient 6 | 49 | W | 113/63 | Metoprolol, Simvastatin, Gabapentin, Topiramate |
| Patient 7 | 55 | W | 107/69 | Methadone |
| Patient 8 | 61 | M | 148/81 | Carvedilol, Losartan, Gabapentin, Hydromorphone |
| Patient 9 | 52 | M | 124/72 | Lisinopril, Gabapentin, Glipizide, Pioglitazone, Atorvastatin, Tramadol |
| Patient 10 | 44 | M | 106/65 | Oxycodone, Prednisone |
| Patient 11 | 66 | M | 110/78 | Pregabalin, Pramipexole, Baclofen, Triamterene, Simvastatin, Tramadol |
| Patient 12 | 57 | W | 96/59 | Tizanidine, Atorvastatin, Estradiol |
SBP, systolic blood pressure; DBP, diastolic blood pressure. (−) no daily medications.
Epidural dorsal column stimulation
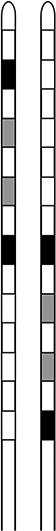
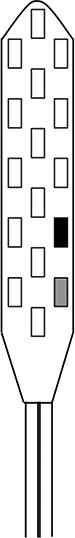
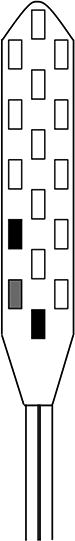
The spinal cord stimulator (SCS) consists of a lead wire with up to 16 electrodes implanted posterior of the dorsal column of the spinal cord, and a pulse generator with a rechargeable battery to deliver electrical current (Epstein & Palmieri, 2012; Holland et al., 2016). The SCS device is controlled via a hand-held wireless remote, which has the capability to turn the device on and off and adjust programs that determine the level of stimulation. Individual patient electrode implant locations and SCS programming configurations are summarized in Table 2.
Table 2.
Spinal cord stimulator programming configurations
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | |
|---|---|---|---|---|---|---|
| Lead type | Percutaneous | Percutaneous | 5-6-5 Paddle | Percutaneous | 5-6-5 Paddle | Percutaneous |
| Lead location | T6–T8 | Left: T8–T9 | T8–T9 | T6–T8 | T7–T9 | T9–T10 |
| Right: T9–T10 | ||||||
| Voltage (V) | 2.8 | Left: 4 | 9.1 | Left: 5.8 | 5.85 | 2.6 |
| Right: 2.5 | Right: 4.5 | |||||
| Pulse width (µs) | 450 | Left: 450 | 610 | Left: 630 | 450 | 450 |
| Right: 210 | Right: 450 | |||||
| Frequency (Hz) | 130 | Left: 60 | 20 | Left: 120 | 25 | 50 |
| Right: 60 | Right: 120 | |||||
| Contact polarity |
|

|
|
|

|
|

|
| Patient 7 | Patient 8 | Patient 9 | Patient 10 | Patient 11 | Patient 12 | |
|---|---|---|---|---|---|---|
| Lead type | 2×8 Paddle | 2×8 Paddle | 5-6-5 Paddle | Percutaneous | Percutaneous | Percutaneous |
| Lead location | T8–T9 | T9–T11 | T9–T10 | Left: T7–T8 | C2–C5 | T7–T9 |
| Right: T7–T8 | ||||||
| Voltage (V) | Left: 5 | 7.2 | 4.3 | Left: 1.5 | 1.6 | 6 |
| Right: 3.3 | Right: 1.5 | |||||
| Pulse width (µs) | 40 | 450 | 450 | Left: 1000 | 450 | 90 |
| Right: 450 | ||||||
| Frequency (Hz) | 390 | 50 | 8 | Left: 60 | 60 | 360 |
| Right: 60 | ||||||
| Contact polarity |
|

|

|
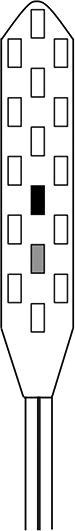
|

|

|

|
Experimental Measurements
Vascular conductance
Common femoral artery blood velocity and diameter were determined with a 12-MHz linear-array Doppler probe (Logiq7, GE Healthcare, Inc.) on the right common femoral artery approximately 2–3 cm proximal of the bifurcation. Common femoral and brachial artery blood velocity was measured continuously for 2 min at baseline and at each time point during SCS (15, 30, 45, and 60 min), and at 15 min post SCS, with a probe insonation angle of 60 degrees. Blood flow was calculated as the product of mean blood velocity (cm/s) and cross-sectional area (cm2) and multiplied by 60 (milliliters per minute). Brachial artery blood flow on the right arm at heart level was measured approximately 2–3 cm proximal of the antecubital fossa at the same time points as femoral artery blood flow. The order of femoral and brachial artery blood flow measures for each patient was randomized. Femoral and brachial artery blood flow was divided by mean blood pressure (automated sphygmomanometer on the left arm) to derive vascular conductance.
Muscle sympathetic nerve activity
Multi-unit MSNA was recorded using standard microneurographic techniques as have been previously described (Holwerda, Restaino, et al., 2016; Holwerda, Vianna, et al., 2016; Vallbo, Hagbarth, Torebjork, & Wallin, 1979). Briefly, a tungsten microelectrode was placed into the peroneal nerve near the left fibular head. Signals were amplified, filtered (bandwidth 0.7–2.0 kHz), rectified and integrated (0.1 s time constant) to obtain mean voltage neurograms (Nerve Traffic Analyzer 662c-3; University of Iowa Bioengineering, Iowa City, IA). MSNA data was acquired at a frequency of 1,000 Hz using a Powerlab data acquisition system (ADInstruments, Colorado Springs, CO) and analyzed using LabChart version 8.1.5 (ADInstruments). MSNA was identified by the presence of spontaneous bursts with characteristic pulse synchronicity and by its responsiveness to end-expiratory breath holds, but not to arousal or skin stimulation.
Experimental protocols
Study 1: Femoral and brachial vascular conductance responses to acute SCS
SCS study patients arrived at the University of Iowa Clinical Research Unit following an overnight fast (n=12). Study patients were instructed to deactivate their SCS unit and abstain from medications for 48 hours prior to the study visit, and to abstain from caffeine the morning of the study. Previous studies of acute SCS used a 24-hour washout of SCS (Kemler et al., 2000); however, in the present study, deactivation of SCS was extended to 48 hours prior to the study visit to maximize washout of electrical stimulation, but without overburdening patients by limiting their pain management strategy. Study visits were initiated between 7:00am and 9:00am, and were performed in a quiet, dimly lit, temperature-controlled room (21–22° C). After a 15-min rest period, patients were instrumented for heart rate (HR, determined from lead II of the 3-lead ECG), blood pressure (BP, arm cuff), and femoral and brachial artery blood flow. Following baseline measures of femoral and brachial artery blood flow, the SCS unit was activated via remote control at the individual patient’s clinically prescribed level of stimulation (see Table 1), and femoral and brachial artery blood flow was measured every 15 min during SCS (60 min) and 15-min post SCS. The SCS unit can be sensed by patients when functioning (typically described as paresthesiae or sensation of vibration), therefore a sham condition in the study protocol was precluded. Visual analogue score of neuropathic pain on a scale of 0–10 was reported by the patient at each time point.
Study 2: Influence of acute SCS on muscle sympathetic nerve activity
Within 1–3 weeks of Study 1, SCS patients returned to the University of Iowa Clinical Research Unit following an overnight fast (n=5). The remaining 7 patients from study 1 are not included in study 2 because their conditions precluded them from resting comfortably and still for stable 60-min peroneal MSNA recordings. Study patients were instructed to deactivate their SCS unit and abstain from medications for 48 hours prior to the study visit, and to abstain from caffeine the morning of the study. Patients were instrumented for HR, BP, and MSNA after a 15-min rest period. Following a 10-min baseline, the SCS unit was activated and remained active for 60 min at the individual patient’s prescribed level of stimulation. MSNA, HR and BP were measured for a 5-min period every 15 min during SCS, and 15-min post SCS. Post-SCS recordings of Visual analogue score of neuropathic pain on a scale of 0–10 was reported by the patient at each time point.
Data Analysis
Femoral and brachial vascular conductance and MSNA were calculated as percent change from baseline. Absolute values of femoral vascular conductance and MSNA are also presented. Since patient 11 had cervical SCS, femoral and brachial vascular conductance and femoral blood flow are reported separately for this patient. MSNA was quantified as burst incidence (bursts/100heartbeats), burst frequency (bursts/min), and total activity (burst frequency multiplied by mean burst amplitude, arbitrary units/min). MSNA burst amplitude was calculated by attributing the value of 100 to the maximum burst height during the baseline recording, which was determined from the average of the 3 largest bursts, and expressing all other burst amplitudes as a percentage of the maximum burst height as previously described (Fairfax, Padilla, Vianna, Davis, & Fadel, 2013; Holwerda, Restaino, et al., 2016; Vranish et al., 2018). MSNA for patient 11 is presented individually (Figure 2) and within the group mean (Figure 1). Changes in blood flow, HR, BP, MSNA and neuropathic pain ratings during SCS were examined using repeated measures ANOVA. Comparison of changes in femoral and brachial artery blood flow during SCS was examined using two-way repeated measures ANOVA. Where there were missing data after 60 min of SCS (15 min post-SCS) for measures for blood flow (n=3) and MSNA (n=2), the estimate of these values was calculated as the marginal sums of squares (Type III, adjusted sums of squares) using a general linear model approach. Fisher LSD post-hoc analysis was used where significant main effects were observed. Statistical analyses were performed using SigmaPlot version 13.0 (Systat Software, Inc.). Data are presented as mean ± SD.
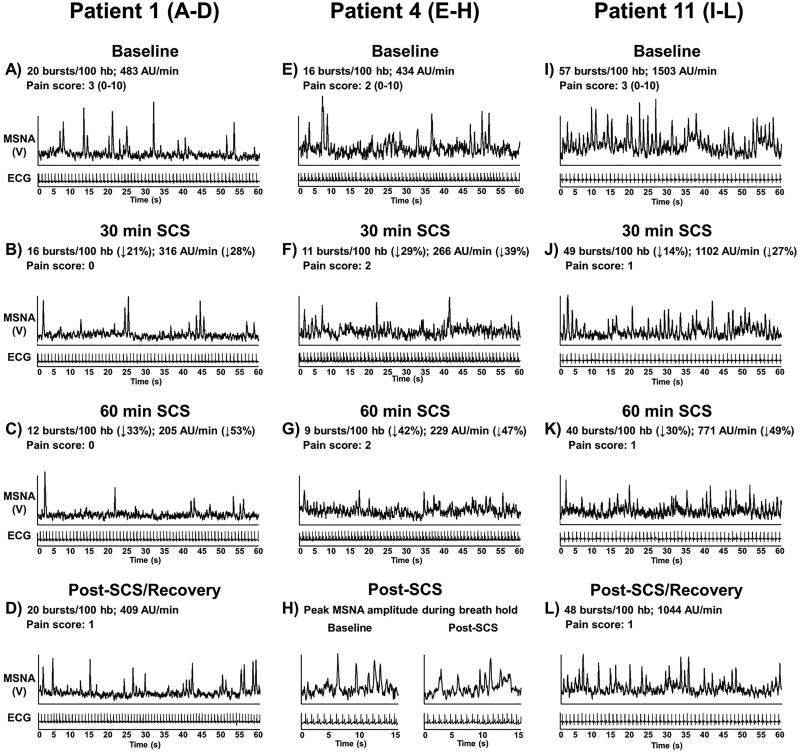
Figure 2.
Individual muscle sympathetic nerve activity (MSNA) recordings in 3 spinal cord stimulation (SCS) patients at baseline, during SCS, and 15 min post SCS. MSNA recording was not able to be collected 15 min post SCS in patient 4 to observe recovery of MSNA burst incidence and amplitude; however, peak MSNA burst amplitude in response to end-expiratory breath hold was recorded to display that quality of the MSNA signal during SCS was maintained (Panel H).
Figure 1.
Study 1: Percent change in femoral and brachial artery conductance (Panel A) and individual values for absolute femoral artery blood flow (Panel B) during spinal cord stimulation (SCS) and 15-min post SCS. Patient 11 with cervical SCS not included in group means, therefore SCS: n=11 and 15-min post SCS: n=8. Study 2: Percent change in muscle sympathetic nerve activity (MSNA) burst incidence (Panel C) and individual values for MSNA burst incidence (Panel D) during SCS (n=5) and 15-min post SCS (n=3) in study #2. * Significantly different from baseline, # significantly different from brachial artery, † significantly different from 15 min.
Results
Study 1
Vascular conductance responses to acute SCS
The increase in femoral vascular conductance was statistically significant throughout 60 min of SCS and 15 min post SCS (P=0.02), and significantly different from brachial vascular conductance at 30-, 45- and 60-min of SCS and 15 min post SCS (Artery × time point interaction: P=0.010) (Figure 1A). Despite the absence of stimulation at 15 min post SCS, femoral vascular conductance remained elevated compared to brachial vascular conductance. Femoral artery blood flow was elevated and significantly increased at 45-min of SCS and 15-min post SCS (P=0.005) (Figure 1B). No changes in end-diastolic femoral artery diameter were detected during SCS (BL: 7.9 ± 1.9, 15min: 7.9 ± 1.9, 30min: 7.8 ± 2, 45min: 8 ± 1.8, 60min: 8.1 ± 1.8 mm, P=0.215). Blood flow after 60 min of SCS (15 min post-SCS) was not able to be collected on patients 2, 4 and 5 because of the inability to remain rested in the supine position. Femoral and brachial artery conductance and femoral artery blood flow for patient 11 are not included in group means in Figure 1 because patient 11 had cervical and not thoracic SCS lead placement. Patient 11 demonstrated a minimal change in femoral artery blood flow (15min: Δ10%, 30min: Δ9%, 45min: Δ−12%, 60min: Δ−4%, 15min post: Δ14% ml•min−1) and no immediate change in brachial artery blood flow (15min: Δ9%, 30min: Δ−3%, 45min: Δ−12%, 60min: Δ26%, 15min post: Δ7% ml•min−1) during SCS. Self-reported neuropathic pain for patient 11 was low at baseline and during SCS (scale 0–10, BL: 2, 15min: 0, 30min: 1, 45min: 0, 60min: 0, 15min post: 2).
Cardiovascular responses to acute SCS
No changes in HR were observed throughout SCS (BL: 60 ± 6, 15 min: 59 ± 7, 30 min: 60 ± 8, 45 min: 60 ± 8, 60 min: 61 ± 8, 15 min post: 60 ± 6 bpm, P=0.778). Although there were no significant changes in systolic BP (P=0.086) or diastolic BP (P=0.133) during SCS, there was a small but significant increase in mean BP (BL: 87 ± 10, 15min: 90 ± 11, 30min: 92 ± 11, 45min: 90 ± 12, 60min: 91 ± 12, 15min post: 87 ± 13 mmHg, P=0.012). The increase in mean BP was consistent among patients. As expected, self-reported neuropathic pain was reduced following SCS and remained reduced throughout SCS (scale 0–10, BL: 3.5 ± 3.3, 15min: 1.7 ± 2.2, 30min: 1.6 ± 1.9, 45min: 1.6 ± 2.1, 60min: 1.6 ± 2.1, 15min post: 2.2 ± 3.0, P=0.003).
Study 2
Influence of acute SCS on muscle sympathetic nerve activity
MSNA during SCS gradually declined and became significantly reduced at 45- and 60-min of SCS compared with baseline and 15 min (P=0.015) (Figure 1C). Similar results were observed for the change in MSNA burst frequency (15 min: Δ−1 ± 13%, 30 min: Δ−13 ± 10%, 45 min: Δ−17 ± 14%, 60 min: Δ−21 ± 15%, 15 min post: Δ−4 ± 12% bursts/min, P=0.027). Absolute values of MSNA burst incidence were significantly reduced compared to baseline at 45- and 60-min of SCS, and remained attenuated 15 min post-SCS (Figure 1D). MSNA recording in patient 11 (cervical SCS) is included in the group mean data in Figure 1C, and also reported individually in Figure 1D (patient with baseline MSNA burst incidence of 57 bursts/100hb) and in Figure 2. Figure 2 shows individual MSNA recordings (burst incidence and total activity) during SCS from 3 patients. Interestingly, patient 11 (cervical SCS) demonstrated a considerable decrease in peroneal MSNA in response to SCS, although no major changes in vascular conductance were observed during study visit 1 in this patient. MSNA recording did not continue after 60 min of SCS (15 min post-SCS) in patients 4 because of the inability to remain in the fixed position required to maintain the MSNA recording. However, an end-expiratory breath hold was performed by patient 4 immediately following the cessation of SCS, and the peak MSNA amplitude response was comparable to the response at baseline (Figure 2H), confirming that the reduction in MSNA during SCS was not a result of deterioration in the MSNA signal. Recording of MSNA after 60 min of SCS (15 min post-SCS) was also not able to be collected in patient 2 because of the inability to remain in the fixed position. When comparing changes in MSNA during SCS in study 2 to changes in the same patients’ femoral artery conductance during study 1, there was no apparent relation because MSNA was gradually reduced while femoral artery conductance rapidly increased.
Cardiovascular responses to acute SCS
Similar to Study 1, no change in HR was observed during SCS (BL: 63 ± 9, 15 min: 64 ± 10, 30 min: 64 ± 10, 45 min: 64 ± 9, 60 min: 65 ± 9, 15 min post: 67 ± 12 bpm, P=0.197). Also, no significant changes in systolic BP (P=0.773) and diastolic BP (P=0.598) were observed. Mean BP during SCS was not significantly different compared to baseline (BL: 90 ± 7, 15 min: 91 ± 11, 30 min: 92 ± 10, 45 min: 91 ± 11, 60 min: 93 ± 11, 15 min post: 86 ± 14 mmHg, P=0.197), although the pattern tended to be similar to Study 1. Neuropathic pain appeared reduced within the first 15 min and remain unchanged thereafter, although not reaching statistical significance (scale 0–10, BL: 2.2 ± 1.3, 15min: 0.8 ± 0.8, 30min: 0.6 ± 0.9, 45min: 0.8 ± 1.1, 60min: 0.6 ± 0.9, 15min post: 1.8 ± 1.9, P=0.098).
Discussion
The major novel findings of the present study are two-fold. First, acute SCS significantly increases femoral vascular conductance in patients treated for neuropathic pain, while no change in brachial vascular conductance was observed. In fact, femoral vascular conductance was significantly elevated within 15 minutes of SCS, and the increase was sustained throughout SCS for 60 minutes. Second, as observed in a subset of the SCS patients, acute SCS significantly reduced MSNA, but the reduction in MSNA was gradual and reached significance at 45 minutes of SCS. These findings demonstrate that acute SCS rapidly increases femoral vascular conductance and reduces peroneal MSNA.
The influence of SCS on peripheral blood flow has been documented in several clinical studies spanning months or years of SCS treatment in patients with peripheral vascular disease. However, no studies have examined the effects of acute SCS treatment on femoral vascular conductance, and most studies that have examined SCS have been limited to patients with history of sympathectomy and/or vascular surgery. We rationalized that acute SCS in the thoracic region of the spinal cord in patients without history of sympathectomy and/or vascular surgery would lead to increased femoral vascular conductance, but not brachial vascular conductance. As hypothesized, significant increases in vascular conductance were observed in the femoral artery following SCS, whereas no change in vascular conductance was observed in the brachial artery. Interestingly, the typical vertebral level of SCS for the purpose of increasing limb blood flow in cases of peripheral arterial disease is slightly lower (~T9-L1) (Broseta et al., 1986) compared with the range of stimulation in present study (~T6–T10), although there is some overlap. The findings of the present study raise speculation as to whether SCS may serve multiple purposes including both neuropathic pain relief and increased lower limb blood flow. That is, SCS acts on multiple pathways in the central nervous system that independently cause neuropathic pain relief and increased lower limb blood flow. Indeed, a recent case report demonstrated simultaneous relief of neuropathic pain and Willis-Eckbom disease (restless legs syndrome), which may be associated with reduced lower limb blood flow (Salminen, Rimpila, & Polo, 2014), with SCS leads spanning the T10–T11 vertebral bodies (Holland et al., 2016). SCS-mediated vasodilation is hypothesized to occur via reduction of efferent sympathetic nerve activity in the periphery, whereas the mechanism of action by which SCS effectively treats neuropathic conditions such as chronic back pain involves the blockade of afferent nociceptive information ascending the spinal cord (Epstein & Palmieri, 2012; Manchikanti et al., 2013).
Experimental animal studies have provided evidence that SCS leads to vasodilation via reduction in sympathetic activity (Linderoth et al., 1991; Linderoth, Herregodts, & Meyerson, 1994). It is proposed that SCS activation of afferent sensory fibers in the dorsal columns stimulate GABAergic interneurons which reduces activity of sympathetic preganglionic neurons and peripheral release of norepinephrine (Foreman & Linderoth, 2012). The present study is the first to directly examine efferent sympathetic activity to muscle of the lower limbs in patients with SCS. The results support and extend findings from experimental animal studies that demonstrate increased peripheral blood flow via SCS modulation of sympathetic activity. Our findings are also consistent with human studies that have examined the influence of SCS on circulating norepinephrine during atrial pacing-induced myocardial stress (Norrsell et al., 1997). Results of these studies demonstrated a blunted increase in total body norepinephrine spillover during application of SCS, indicating that SCS decreased efferent sympathetic activity. However, the aforementioned study also demonstrated no change in cardiac sympathetic activity during SCS, which is consistent with a more recent study by Naar and colleagues (2017) (Naar et al., 2017). These findings support a preferential reduction in sympathetic nerve activity to the periphery during SCS.
Interestingly, femoral vascular conductance was significantly elevated within 15 min of SCS while MSNA gradually decreased, suggesting that other neural mechanisms in addition to reduced MSNA may be contributing to elevated vascular conductance. Studies of SCS in a rodent model have demonstrated that SCS in the dorsal columns causes antidromic activation of small myelinated sensory fibers, leading to peripheral release of cutaneous vasodilators such as calcitonin gene-related peptide (CGRP) (Croom et al., 1997; Tanaka, Barron, Chandler, Linderoth, & Foreman, 2003). However, stimulation frequency higher than typically used in the clinic is likely required to activate small myelinated sensory fibers and cause release of CGRP (Gao et al., 2010). Consistent with this, studies of acute SCS (<60 min) in patients with complex regional pain syndrome failed to demonstrate an increase in skin microcirculation (Kemler et al., 2000). Thus, it is unlikely that activation of small myelinated sensory fibers played a major role in SCS-mediated increases in femoral vascular conductance. SCS-mediated vasodilation in other vascular beds such as in the splanchnic circulation should also be considered. Reduction in abdominal pain following SCS in a case of mesenteric ischemia has previously been reported, suggesting SCS-mediated vasodilation in the splanchnic circulation (Ceballos, Cabezudo, Bovaira, Fenollosa, & Moro, 2000). Future studies are needed to examine vasodilatory responses to acute SCS in other vascular beds such as the mesentery (e.g., ultrasonography of the superior mesenteric artery), and whether vasodilation in other vascular beds contributes to changes in femoral vascular conductance.
Despite increased vascular conductance, a small but significant increase in mean BP during SCS was observed, and appeared to return toward baseline after SCS. The reason(s) behind this increase in BP are unclear. In response to increased femoral vascular conductance, and presumably an initial reduction in BP, baroreflex-mediated increases in sympathetic activity would have been expected, although assessment of increased sympathetic activity is limited because MSNA in the leg was at least partially blocked by SCS. However, no changes in HR were observed during SCS, suggesting that the increase in BP was likely not mediated by the baroreflex. An important consideration is the influence of SCS on neural control of the bladder. Bladder activation can occur with SCS by activating preganglionic parasympathetic fibers to the detrusor muscle (Rijkhoff, Wijkstra, van Kerrebroeck, & Debruyne, 1997), which has previously been a point of focus in paraplegic animal and human studies (Friedman, Nashold, & Senechal, 1972; Grimes & Nashold, 1974). Although SCS lead placement was not ideal for bladder activation, the need to empty the bladder during SCS was frequently reported by patients in the present study. Thus, urinary retention and related discomfort, which was not covered by neuropathic pain self-reports collected during the study, is considered a potential factor explaining the small rise in BP during SCS.
Since patients studied herein were receiving SCS for neuropathic pain management, whether reduced MSNA and increased femoral vascular conductance during SCS can be explained by reduction in neuropathic pain warrants discussion. Pain relief associated with deep brain stimulation in patients with neuropathic pain can influence sympathetic firing pattern (Sverrisdottir et al., 2014). In healthy individuals, MSNA responses to tonic muscle pain differ; some individuals demonstrate a sustained increase in MSNA while others demonstrate a reduction (Fazalbhoy, Birznieks, & Macefield, 2012). In the present study, the reduction in self-reported neuropathic pain immediately followed SCS (15 min), while MSNA followed a pattern of a progressive decline, suggesting that neuropathic pain and changes in MSNA were dissociated. When considering individual responses, patient 12, for example, demonstrated 72% increase in femoral vascular conductance at 15 min while self-reported neuropathic pain at baseline was “0.” Also, self-reported neuropathic pain for patient 2 was “0” at baseline while an increase in femoral vascular conductance of 35% and a reduction in MSNA of -16% at 45 min of SCS was observed. Overall, patients did not report high levels of pain at baseline. In fact, most patients reported that normal use of SCS for neuropathic pain is in the standing position and not while lying supine. Thus, changes in neuropathic pain during SCS did not appear to explain changes in MSNA or femoral vascular conductance.
Numerous aspects of SCS influence the individualized effectiveness, including the vertebral level of the stimulation, the utilized active contacts of the electrode array, and the polarity of the active contacts which shapes the delivered current. Initial optimization of SCS settings involves interaction between the trained SCS programmer and the patient reporting areas of pain relief. As seen in Table 2, SCS parameters are highly variable between individuals. The variable SCS configurations in the present study likely play a role in the level of MSNA inhibition and changes in vascular conductance. For example, patients 2 and 10 with percutaneous leads spanning T7–T10 and a similar SCS programming configuration demonstrated moderate increases in femoral vascular conductance during SCS in Study 1 and moderate reductions in MSNA during SCS in Study 2. In comparison, patients 1 and 4 had percutaneous leads placed T6–T8 with different programming configurations; patient 1 demonstrated a peak increase in femoral vascular conductance of 71% during SCS, while patient 4 demonstrated minimal changes in femoral vascular conductance. However, in Study 2, both patients 1 and 4 demonstrated a reduction in MSNA (Figure 2). These findings highlight the complexity by which SCS modulates neural activity. Importantly, in this patient population, SCS lead placement and configurations were for optimal paresthesiae and pain relief, and may not have been ideal for increased leg blood flow or MSNA inhibition. In some studies of SCS for pain relief, lead type and position, and specific patient factors such as sex and age, did not show a significant effect on optimal parameter settings (Gordon, Zou, Kim, & Gharibo, 2007; Kumar, Toth, Nath, & Laing, 1998). The impact of such factors on MSNA inhibition and vascular conductance are less clear, particularly when considering variables such as dorsal cerebrospinal fluid layer thickness, lead migration over time, and variation in position such as supine vs. standing.
While an increase in femoral vascular conductance was observed with thoracic SCS, no major change in femoral vascular conductance was observed in patient 11 that had cervical SCS. Previous studies have demonstrated increases in cerebral blood flow following acute cervical SCS (Hautvast et al., 1997; Hosobuchi, 1985; Meglio et al., 1991). Cervical SCS is also employed to manage unstable angina (Gonzalez-Darder, Canela, & Gonzalez-Martinez, 1991; Hautvast, DeJongste, Staal, van Gilst, & Lie, 1998), cluster headache (Wolter, Kiemen, & Kaube, 2011), and upper limb neuropathic pain such as the case for patient 11 in the present study. To our knowledge, no studies have demonstrated increased limb blood flow (arm or leg) in response to cervical SCS. Despite no major changes in limb blood flow in the present study, patient 11 did demonstrate a considerable reduction in peroneal MSNA following SCS during study visit 2. These data highlight our overall observation that increased femoral vascular conductance following SCS was not directly proportional to reduced peroneal MSNA as hypothesized, and that additional neural mechanisms are likely involved.
Several limiting factors should be acknowledged in present study. Although our study findings demonstrate proof of concept that SCS increases femoral conductance and reduces MSNA in humans, the study design lacked a placebo condition to compare with SCS. Paresthesiae or the sensation of vibration that occurs with typical SCS parameters prevented a true placebo condition. However, future investigations might also include time controls with no SCS. Secondly, the number of observations was limited, particularly for measures of MSNA (blood flow: n=12, MSNA: n=5). Although self-reported neuropathic pain during the study was only moderate, the ability to rest comfortably and still for stable 60-min peroneal MSNA recordings was limited for this patient population.
In summary, we demonstrate for the first time in humans that acute SCS significantly increases femoral vascular conductance. In addition, peroneal MSNA was significantly attenuated in response to acute SCS. However, in contrast to the rapid rise in femoral vascular conductance, peroneal MSNA in response to acute SCS demonstrated a progressive reduction and did not appear to be directly associated with the change in femoral vascular conductance. Neural mechanisms in addition to attenuated MSNA may be involved in the initial SCS-mediated increases in femoral vascular conductance.
New Findings.
What is the central question of this research?
Does acute spinal cord stimulation increase vascular conductance and decrease muscle sympathetic nerve activity in the lower limbs of humans?
What is the main finding and its importance?
Acute spinal cord stimulation lead to a rapid rise in femoral vascular conductance while peroneal muscle sympathetic nerve activity demonstrated a delayed reduction that was not associated with the initial increase in femoral vascular conductance. These findings suggest that neural mechanisms in addition to attenuated MSNA may be involved in the initial rise in femoral vascular conductance during acute spinal cord stimulation.
Acknowledgments
Grants
This work was supported in part by the Iowa Cardiovascular Interdisciplinary Research Fellowship (T32HL007121) (S.W.H). American Heart Association grants 17POST33440101 (S.W.H) and 13SDG143400012 (G.L.P). NIH P01 HL014388-48 (F.M.A., G.L.P., J.G.F.) and NIH U54TR001356 (University of Iowa).
We would like to acknowledge the University of Iowa Institute for Clinical and Translational Science Clinical Research Unit staff for assistance during studies.
Footnotes
Disclosures
No conflicts of interest, financial or otherwise, are declared by the authors.
Author Contributions
Author contributions: S.W.H., M.T.H., C.G.R., G.L.P. conception and design of research; S.W.H., M.T.H., G.L.P. performed experiments; S.W.H. analyzed data and prepared figures; S.W.H., M.T.H., G.L.P. interpreted results of experiments; S.W.H drafted manuscript; S.W.H., M.T.H., G.L.P. edited and revised the manuscript; S.W.H., M.T.H., C.G.R., G.L.P. approved the final version of the manuscript.
References
- Augustinsson LE, Carlsson CA, Holm J, Jivegard L. Epidural electrical stimulation in severe limb ischemia. Pain relief, increased blood flow, and a possible limb-saving effect. Ann Surg. 1985;202(1):104–110. doi: 10.1097/00000658-198507000-00017. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/3874610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broseta J, Barbera J, de Vera JA, Barcia-Salorio JL, Garcia-March G, Gonzalez-Darder J, Joanes V. Spinal cord stimulation in peripheral arterial disease. A cooperative study. J Neurosurg. 1986;64(1):71–80. doi: 10.3171/jns.1986.64.1.0071. [DOI] [PubMed] [Google Scholar]
- Ceballos A, Cabezudo L, Bovaira M, Fenollosa P, Moro B. Spinal cord stimulation: a possible therapeutic alternative for chronic mesenteric ischaemia. Pain. 2000;87(1):99–101. doi: 10.1016/S0304-3959(00)00266-9. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10863050. [DOI] [PubMed] [Google Scholar]
- Cook AW, Oygar A, Baggenstos P, Pacheco S, Kleriga E. Vascular disease of extremities. Electric stimulation of spinal cord and posterior roots. N Y State J Med. 1976;76(3):366–368. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1062688. [PubMed] [Google Scholar]
- Croom JE, Foreman RD, Chandler MJ, Barron KW. Cutaneous vasodilation during dorsal column stimulation is mediated by dorsal roots and CGRP. Am J Physiol. 1997;272(2 Pt 2):H950–957. doi: 10.1152/ajpheart.1997.272.2.H950. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9124459. [DOI] [PubMed] [Google Scholar]
- Dooley DM, Kasprak M. Modification of blood flow to the extremities by electrical stimulation of the nervous system. South Med J. 1976;69(10):1309–1311. doi: 10.1097/00007611-197610000-00017. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/1086511. [DOI] [PubMed] [Google Scholar]
- Epstein LJ, Palmieri M. Managing chronic pain with spinal cord stimulation. Mt Sinai J Med. 2012;79(1):123–132. doi: 10.1002/msj.21289. [DOI] [PubMed] [Google Scholar]
- Fairfax ST, Padilla J, Vianna LC, Davis MJ, Fadel PJ. Spontaneous bursts of muscle sympathetic nerve activity decrease leg vascular conductance in resting humans. Am J Physiol Heart Circ Physiol. 2013;304(5):H759–766. doi: 10.1152/ajpheart.00842.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazalbhoy A, Birznieks I, Macefield VG. Individual differences in the cardiovascular responses to tonic muscle pain: parallel increases or decreases in muscle sympathetic nerve activity, blood pressure and heart rate. Exp Physiol. 2012;97(10):1084–1092. doi: 10.1113/expphysiol.2012.066191. [DOI] [PubMed] [Google Scholar]
- Foreman RD, Linderoth B. Neural mechanisms of spinal cord stimulation. Int Rev Neurobiol. 2012;107:87–119. doi: 10.1016/B978-0-12-404706-8.00006-1. [DOI] [PubMed] [Google Scholar]
- Friedman H, Nashold BS, Jr, Senechal P. Spinal cord stimulation and bladder function in normal and paraplegic animals. J Neurosurg. 1972;36(4):430–437. doi: 10.3171/jns.1972.36.4.0430. [DOI] [PubMed] [Google Scholar]
- Gao J, Wu M, Li L, Qin C, Farber JP, Linderoth B, Foreman RD. Effects of spinal cord stimulation with "standard clinical" and higher frequencies on peripheral blood flow in rats. Brain Res. 2010;1313:53–61. doi: 10.1016/j.brainres.2009.11.072. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Darder JM, Canela P, Gonzalez-Martinez V. High cervical spinal cord stimulation for unstable angina pectoris. Stereotact Funct Neurosurg. 1991;56(1):20–27. doi: 10.1159/000099389. [DOI] [PubMed] [Google Scholar]
- Gordon AT, Zou SP, Kim Y, Gharibo C. Challenges to setting spinal cord stimulator parameters during intraoperative testing: factors affecting coverage of low back and leg pain. Neuromodulation. 2007;10(2):133–141. doi: 10.1111/j.1525-1403.2007.00101.x. [DOI] [PubMed] [Google Scholar]
- Grimes JH, Nashold BS. Clinical application of electronic bladder stimulation in paraplegics. Br J Urol. 1974;46(6):653–657. doi: 10.1111/j.1464-410x.1974.tb08900.x. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/4451832. [DOI] [PubMed] [Google Scholar]
- Hautvast RW, DeJongste MJ, Staal MJ, van Gilst WH, Lie KI. Spinal cord stimulation in chronic intractable angina pectoris: a randomized, controlled efficacy study. Am Heart J. 1998;136(6):1114–1120. doi: 10.1016/s0002-8703(98)70171-1. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9842028. [DOI] [PubMed] [Google Scholar]
- Hautvast RW, Ter Horst GJ, DeJong BM, DeJongste MJ, Blanksma PK, Paans AM, Korf J. Relative changes in regional cerebral blood flow during spinal cord stimulation in patients with refractory angina pectoris. Eur J Neurosci. 1997;9(6):1178–1183. doi: 10.1111/j.1460-9568.1997.tb01472.x. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9215701. [DOI] [PubMed] [Google Scholar]
- Holland MT, Rettenmaier LA, Flouty OE, Thomsen TR, Jerath NU, Reddy CG. Epidural Spinal Cord Stimulation: A Novel Therapy in the Treatment of Restless Legs Syndrome. World Neurosurg. 2016;92:582, e515–588. doi: 10.1016/j.wneu.2016.05.077. [DOI] [PubMed] [Google Scholar]
- Holwerda SW, Restaino RM, Manrique C, Lastra G, Fisher JP, Fadel PJ. Augmented pressor and sympathetic responses to skeletal muscle metaboreflex activation in type 2 diabetes patients. Am J Physiol Heart Circ Physiol. 2016;310(2):H300–309. doi: 10.1152/ajpheart.00636.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holwerda SW, Vianna LC, Restaino RM, Chaudhary K, Young CN, Fadel PJ. Arterial baroreflex control of sympathetic nerve activity and heart rate in patients with type 2 diabetes. Am J Physiol Heart Circ Physiol. 2016;311(5):H1170–H1179. doi: 10.1152/ajpheart.00384.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsch S, Schulte S, Hess S. Spinal cord stimulation in the treatment of peripheral vascular disease: results of a single-center study of 258 patients. Angiology. 2004;55(2):111–118. doi: 10.1177/000331970405500201. [DOI] [PubMed] [Google Scholar]
- Hosobuchi Y. Electrical stimulation of the cervical spinal cord increases cerebral blood flow in humans. Appl Neurophysiol. 1985;48(1–6):372–376. doi: 10.1159/000101161. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/3879799. [DOI] [PubMed] [Google Scholar]
- Jacobs MJ, Jorning PJ, Joshi SR, Kitslaar PJ, Slaaf DW, Reneman RS. Epidural spinal cord electrical stimulation improves microvascular blood flow in severe limb ischemia. Ann Surg. 1988;207(2):179–183. doi: 10.1097/00000658-198802000-00011. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/3257679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemler MA, Barendse GA, van Kleef M, Egbrink MG. Pain relief in complex regional pain syndrome due to spinal cord stimulation does not depend on vasodilation. Anesthesiology. 2000;92(6):1653–1660. doi: 10.1097/00000542-200006000-00024. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/10839916. [DOI] [PubMed] [Google Scholar]
- Kumar K, Toth C, Nath RK, Laing P. Epidural spinal cord stimulation for treatment of chronic pain--some predictors of success. A 15-year experience. Surg Neurol. 1998;50(2):110–120. doi: 10.1016/s0090-3019(98)00012-3. discussion 120-111. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9701116. [DOI] [PubMed] [Google Scholar]
- Linderoth B, Foreman RD. Physiology of spinal cord stimulation: review and update. Neuromodulation. 1999;2(3):150–164. doi: 10.1046/j.1525-1403.1999.00150.x. [DOI] [PubMed] [Google Scholar]
- Linderoth B, Gunasekera L, Meyerson BA. Effects of sympathectomy on skin and muscle microcirculation during dorsal column stimulation: animal studies. Neurosurgery. 1991;29(6):874–879. doi: 10.1097/00006123-199112000-00012. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/1758600. [DOI] [PubMed] [Google Scholar]
- Linderoth B, Herregodts P, Meyerson BA. Sympathetic mediation of peripheral vasodilation induced by spinal cord stimulation: animal studies of the role of cholinergic and adrenergic receptor subtypes. Neurosurgery. 1994;35(4):711–719. doi: 10.1227/00006123-199410000-00018. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/7808615. [DOI] [PubMed] [Google Scholar]
- Manchikanti L, Abdi S, Atluri S, Benyamin RM, Boswell MV, Buenaventura RM, Hirsch JA. An update of comprehensive evidence-based guidelines for interventional techniques in chronic spinal pain. Part II: guidance and recommendations. Pain Physician. 2013;16(2 Suppl):S49–283. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23615883. [PubMed] [Google Scholar]
- Mannheimer C, Augustinsson LE, Carlsson CA, Manhem K, Wilhelmsson C. Epidural spinal electrical stimulation in severe angina pectoris. Br Heart J. 1988;59(1):56–61. doi: 10.1136/hrt.59.1.56. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/3257701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meglio M, Cioni B, Visocchi M, Nobili F, Rodriguez G, Rosadini G, Sandric S. Spinal cord stimulation and cerebral haemodynamics. Acta Neurochir (Wien) 1991;111(1–2):43–48. doi: 10.1007/BF01402512. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/1927623. [DOI] [PubMed] [Google Scholar]
- Naar J, Jaye D, Linde C, Neuzil P, Doskar P, Malek F, Stahlberg M. Effects of Spinal Cord Stimulation on Cardiac Sympathetic Nerve Activity in Patients with Heart Failure. Pacing Clin Electrophysiol. 2017;40(5):504–513. doi: 10.1111/pace.13050. [DOI] [PubMed] [Google Scholar]
- Norrsell H, Eliasson T, Mannheimer C, Augustinsson LE, Bergh CH, Andersson B, Friberg P. Effects of pacing-induced myocardial stress and spinal cord stimulation on whole body and cardiac norepinephrine spillover. Eur Heart J. 1997;18(12):1890–1896. doi: 10.1093/oxfordjournals.eurheartj.a015197. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9447316. [DOI] [PubMed] [Google Scholar]
- Odenstedt J, Linderoth B, Bergfeldt L, Ekre O, Grip L, Mannheimer C, Andrell P. Spinal cord stimulation effects on myocardial ischemia, infarct size, ventricular arrhythmia, and noninvasive electrophysiology in a porcine ischemia-reperfusion model. Heart Rhythm. 2011;8(6):892–898. doi: 10.1016/j.hrthm.2011.01.029. [DOI] [PubMed] [Google Scholar]
- Rijkhoff NJ, Wijkstra H, van Kerrebroeck PE, Debruyne FM. Urinary bladder control by electrical stimulation: review of electrical stimulation techniques in spinal cord injury. Neurourol Urodyn. 1997;16(1):39–53. doi: 10.1002/(sici)1520-6777(1997)16:1<39::aid-nau6>3.0.co;2-f. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9021789. [DOI] [PubMed] [Google Scholar]
- Salminen AV, Rimpila V, Polo O. Peripheral hypoxia in restless legs syndrome (Willis-Ekbom disease) Neurology. 2014;82(21):1856–1861. doi: 10.1212/WNL.0000000000000454. [DOI] [PubMed] [Google Scholar]
- Sverrisdottir YB, Green AL, Aziz TZ, Bahuri NF, Hyam J, Basnayake SD, Paterson DJ. Differentiated baroreflex modulation of sympathetic nerve activity during deep brain stimulation in humans. Hypertension. 2014;63(5):1000–1010. doi: 10.1161/HYPERTENSIONAHA.113.02970. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Barron KW, Chandler MJ, Linderoth B, Foreman RD. Role of primary afferents in spinal cord stimulation-induced vasodilation: characterization of fiber types. Brain Res. 2003;959(2):191–198. doi: 10.1016/s0006-8993(02)03740-x. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12493606. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Hagbarth KE, Torebjork HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev. 1979;59(4):919–957. doi: 10.1152/physrev.1979.59.4.919. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/227005. [DOI] [PubMed] [Google Scholar]
- Vranish JR, Holwerda SW, Young BE, Credeur DP, Patik JC, Barbosa TC, Fadel PJ. Exaggerated Vasoconstriction to Spontaneous Bursts of Muscle Sympathetic Nerve Activity in Healthy Young Black Men. Hypertension. 2018;71(1):192–198. doi: 10.1161/HYPERTENSIONAHA.117.10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolter T, Kiemen A, Kaube H. High cervical spinal cord stimulation for chronic cluster headache. Cephalalgia. 2011;31(11):1170–1180. doi: 10.1177/0333102411412627. [DOI] [PubMed] [Google Scholar]