Abstract
Background
Conditioned stimuli (CS) that predict reward delivery acquire the ability to induce phasic dopamine release in the nucleus accumbens. This dopamine release may facilitate conditioned approach behavior, which often manifests as approach to the site of reward delivery (called ‘goal-tracking’) or to the CS itself (called ‘sign-tracking’). Previous research has linked sign-tracking in particular to impulsivity and drug self-administration, and addictive drugs may promote the expression of sign-tracking. Ethanol acutely promotes phasic release of dopamine in the accumbens, but it is unknown whether an alcoholic reward alters dopamine release to a CS. We hypothesized that Pavlovian conditioning with an alcoholic reward would increase dopamine release triggered by the CS and subsequent sign-tracking behavior. Moreover, we predicted that chronic intermittent ethanol (CIE) exposure would promote sign-tracking while acute administration of naltrexone would reduce it.
Methods
Rats received 14 doses of ethanol (3-5 g/kg, IG) or water followed by 6 days of Pavlovian conditioning training. Rewards were a chocolate solution with or without 10% (w/v) alcohol. We used fast-scan cyclic voltammetry to measure phasic dopamine release in the nucleus accumbens core in response to the CS and the rewards. We also determined the effect of naltrexone (1 mg/kg, SC) on conditioned approach.
Results
Both CIE and alcoholic reward, individually but not together, associated with greater dopamine to the CS than control conditions. However, this increase in dopamine release was not linked to greater sign-tracking, as both CIE and alcoholic reward shifted conditioned approach from sign-tracking to goal-tracking behavior. However, they both also increased sensitivity to naltrexone, which reduced goal-tracking behavior.
Conclusions
While a history of ethanol exposure or alcoholic reward enhanced dopamine release to a CS, they did not promote sign-tracking under the current conditions. These findings are consistent with the interpretation that ethanol can stimulate conditioned approach, but indicate that the conditioned response may manifest as goal-tracking.
Keywords: Alcohol, Pavlovian, naltrexone, extinction, dopamine
Introduction
In alcohol and other substance use disorders, excessive attention is allocated to drug-associated stimuli (e.g., Townshend and Duka, 2001). This attentional bias is thought to arise from Pavlovian learning, whereby alcohol cues come to activate alcohol seeking and consumption (Robinson and Berridge, 2003, Stacy and Wiers, 2010, Field and Cox, 2008). Attentional bias in heavy drinkers can predict relapse risk (e.g., Cox et al., 2002), suggesting that enhanced salience of alcohol-associated cues contributes to relapse, and supporting the need to understand the neurobiological response to these cues.
In animal models, Pavlovian conditioning can be established by the presentation of a neutral stimulus that immediately precedes an appetitive, unconditioned stimulus (US), such as sucrose or alcohol. As the neutral stimulus gains predictive value, it becomes a conditioned stimulus (CS) and will typically trigger a conditioned approach response. When the CS is a stimulus that can be approached, such as an illuminated lever, rodents often develop a conditioned response to approach the CS; this behavior is called sign-tracking. An alternative conditioned response to CS onset is to approach the location where the US will be delivered, a behavior called goal-tracking. Dopamine release in the nucleus accumbens (NAc) core can track the process of Pavlovian conditioning. Specifically, while dopamine release accompanies rewarding US delivery early in training, it shifts to accompany the predictive CS later in training (Day et al., 2007), and this shift is more pronounced in animals exhibiting sign-tracking behavior (Flagel et al., 2011). The propensity to show sign-tracking behavior has been considered to be an ‘addictive phenotype’ (Tomie and Sharma, 2013), as rats bred to exhibit high reactivity to a novel environment also exhibit both high impulsivity and sign-tracking behavior (Flagel et al., 2010), and sign-tracking rats self-administer more cocaine infusions than goal-tracking rats (Tunstall and Kearns, 2015). In addition, several studies have reported that a history of stimulant or alcohol exposure can promote sign-tracking behavior (Robinson et al., 2015, Simon et al., 2009, Wyvell and Berridge, 2001, McClory and Spear, 2014, Doremus-Fitzwater and Spear, 2011, Srey et al., 2015, Madayag et al., 2017, Villaruel and Chaudhri, 2016).
We hypothesized that both a history of ethanol exposure and an alcoholic US would promote conditioned approach, and specifically sign-tracking behavior, by inducing greater dopamine release to the CS. Moreover, we predicted that the μ-opioid antagonist naltrexone (NTX) would reduce conditioned approach in rats with a history of ethanol exposure or alcoholic US, consistent with NTX's ability to modulate ethanol-induced dopamine release (Gonzales and Weiss, 1998). In order to test these hypotheses, rats underwent a binge-like chronic intermittent ethanol (CIE) or control (CON) exposure, followed by Pavlovian conditioning in which a CS was associated with either alcoholic or nonalcoholic rewards. We used a binge ethanol regimen to model exposure that would occur via intermittent binge drinking, but would not produce alcohol dependence. Next, we used fast-scan cyclic voltammetry (FSCV) to measure phasic dopamine release in the NAc core in response to the CS and to the alcoholic and nonalcoholic rewards. The NAc core is well known to support CS-evoked dopamine transients (Flagel et al., 2011, Flagel et al., 2007) as compared to other striatal subregions (Brown et al., 2011). Finally, we measured the effect of NTX on conditioned approach.
Materials and Methods
Animals
Adult, male Sprague–Dawley rats (303±5 g at start of study) were purchased (Charles River; Raleigh, NC) and housed in a controlled environment (21 ± 1°C) with 12h light/dark cycles and ad libitum access to food and water during the entire study. Animals were pair-housed during ethanol or control exposure, and then individually housed during Pavlovian training and dopamine measurements. The Institutional Animal Care and Use Committee of University of North Carolina at Chapel Hill approved all procedures.
Ethanol and control exposure
The experimental timeline is shown in Figure 1. Rats were run in cohorts of 4 and were assigned to exposure groups in a balanced order so that each cohort included rats from two exposure groups. Exposure groups were CIE, Water controls, or No Exposure controls; cage-mates were assigned to the same exposure group. Next, rats in the CIE group received ethanol (20% w/v) and rats in the Water group received equivalent volumes of water on Mondays, Tuesdays, Thursdays and Fridays by IG gavage as follows: 3g/kg on day 1, 4g/kg on day 2 and 5g/kg on the next twelve administration days, for a total of 14 doses. This administration models intermittent binge drinking, producing blood ethanol concentrations (BEC) of > 100 mg/dl. Tail blood samples were collected from the Water and CIE rats 30 min after the first and final 5g/kg doses. The No Exposure rats were weighed but did not undergo any treatment or blood sampling.
Figure 1.
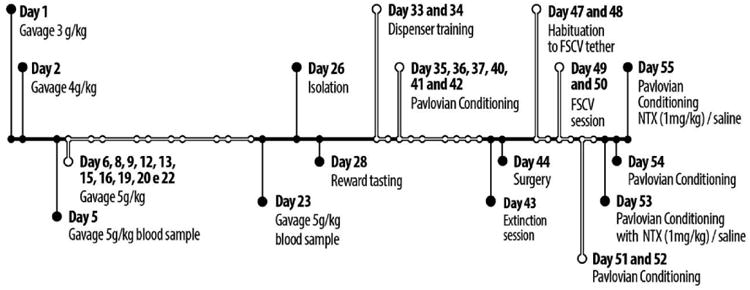
Day-by-day timeline of the study. Note that day 42 of the study is Day 6 of Pavlovian conditioning training and day 43 of the study is Day 7 of training (the extinction session).
Behavioral training
Two days after the exposure period, pair-housed rats were isolate-housed, with one rat assigned to receive alcoholic reward and the other to receive nonalcoholic reward. The reward consisted of chocolate Ensure® with or without 10% ethanol (w/v), denoted as ALC and ENS, respectively. Five days before behavioral training began, rats were given access to 30 ml of Ensure® in their home cage to allow familiarization with the fluid. Next, rats were given 2 days of dispenser training followed by 6 days of Pavlovian conditioning and 1 day of extinction training, as described below. Behavior training was performed in a chamber (Med Associates, St Albans, VT) with two levers on either side of one wall, each with a cue light above. There were two fluid cups in between the levers and a house light on the opposite wall. Each session started with the chamber darkened; after 5 minutes the house light illuminated. For dispenser training, 25 reward (US) deliveries were made into the left fluid cup on a variable interval schedule that averaged 90 s (VI90). For the conditioning sessions, 25 CS-US pairs were presented on a VI90 schedule. When a trial started, the house light turned off, the right cue light illuminated and the right lever extended; together, these events made up the CS. After 8 s, the house light turned on, the cue light extinguished, and the lever retracted. Simultaneously, 0.1 ml of reward was dispensed into the left fluid cup (US). Fluid consumption was verified after each session by inspection of the fluid cup. As extinction has been shown to alter goal-tracking more than sign-tracking (Ahrens et al., 2016), we also measured behavior during an extinction session (Day 7), when the CS was delivered on the VI90 schedule but no US was delivered. Day 6 (Pavlovian conditioning session) and Day 7 (extinction session) experiments were video-recorded for subsequent analysis.
Surgery
Rats were anesthetized with isoflurane (5% induction, 2% maintenance) and secured in a stereotaxic frame on a heated pad. Anterior–posterior (AP), medial–lateral (ML), and dorsal–ventral (DV) positions refer to bregma and coordinates were obtained from a rat brain atlas (Paxinos and Watson 1998). A guide cannula (Bioanalytical Systems, West Lafayette, IN) was implanted above the NAc (AP +1.7 mm, ML +1.7 mm, DV -2.5 mm). An Ag/AgCl reference electrode was implanted in the contralateral cortex. A bipolar stimulating electrode (Plastics One, Roanoke, VA, polished tips, 1 mm apart) was implanted in the VTA (AP -5.2 mm, ML +1.4 mm, DV -8.5 mm). Rats received ibuprofen (15 mg/kg, PO) immediately after surgery and for the following two days.
Electrochemical dopamine measurements
For FSCV data collection, rats were in a behavioral chamber identical to those used during behavioral training, except that it was positioned in a Faraday cage and contained a tether connecting the electrodes to an electrical swivel. For the first session after surgery, rats received a regular conditioning session without the tether; for the second session, rats were tethered; and during the third or fourth session, FSCV data were collected. Carbon-fiber (T650, Thornel/Cytec Industries Inc., Woodland Park, NJ, USA) electrodes were sealed in glass with 60–120 μm fiber exposed. The electrodes were soaked for >10 min in isopropyl alcohol cleaned with activated carbon before use (Bath et al., 2000). The carbon-fiber electrode was lowered in increments of approximately 75 μm into the NAc core via a manipulator inserted into the guide cannula. Voltammetric parameters, electrical stimulation parameters, and data acquisition were controlled by a computer using LabVIEW instrumentation software (National Instruments, Austin, TX) as previously described (Robinson et al., 2011, Shnitko and Robinson, 2015). Voltammetric recordings were made at the carbon-fiber microelectrodes by applying a ramping, triangle-waveform potential (-0.4V to +1.3V to -0.4V, 400 V/s). With the microelectrode positioned just dorsal to the NAc core, rats were left undisturbed for 15-20 min while the triangle-waveform was applied at 60 Hz to condition the electrode. Next, the triangle-waveform application was reduced to 10 Hz, and assessments were made for spontaneous and electrically-evoked dopamine transients. Electrical stimulation (16–24 biphasic pulses, 40–60 Hz, 120 μA, 2 ms/phase) was delivered to the VTA. The carbon-fiber electrode was lowered in 75-μm increments until dopamine transients were detected, at which point the electrode was secured in place and the behavioral session was initiated. At the end of the session, the carbon-fiber electrode was removed and returned to isopropyl alcohol until calibrated in a flow-cell with 0.5 and 1 μM DA in TRIS buffer (Robinson et al., 2009). The average calibration factor for dopamine was 0.082 ± 0.002 μM/nA.
Naltrexone experiments
Following FSCV recordings, rats were no longer tethered and underwent 5 additional Pavlovian sessions. On the 3rd and 5th sessions, naltrexone (1 mg/kg, SC) or saline was administered in a balanced order 30 minutes before the start of the session. As the half-life of naltrexone is less than 5 hours (Akala et al., 2008, Yoburn et al., 1986), 48 hours was sufficient for wash-out of the drug.
Blood ethanol concentration assessment
BEC was determined from tail blood samples analyzed with an AM1 Analox Alcohol Analyzer (Analox Instruments, MA). During CIE, blood samples were collected 30 min after the first and final 5 g/kg doses. Blood samples were also collected after a Pavlovian conditioning session in the final week of the experiment. Plasma was separated by centrifugation and stored at −80°C until analysis. Ethanol concentrations in samples were compared to a 100 mg/dl ethanol standard.
Histology
At the end of all experiments, rats were euthanized with urethane (50% w/w in saline, >1.5 g/kg) and perfused through the heart with saline followed by 10% formalin. Brains were extracted, frozen and sectioned. Forty-μm sections were mounted on slides and stained with thionin to visualize damage due to the guide cannula and glass electrode.
Behavior analysis
Behavior was analyzed for 5 sessions: Day 6 (Pavlovian conditioning session), Day 7 (extinction session), FSCV recording, and NTX/saline days. To assess behavior during conditioning sessions, sessions were videotaped and analyzed by researchers blind to the rat's experimental group as previously described (Palmatier et al., 2014) by using Noldus Observer behavioral analysis software (Wageningen, Netherlands). We designated an area along the chamber wall surrounding the CS lever/light (13.7 cm height × 7.7 cm width) and another area surrounding the US cup (9.25 cm height × 7.0 cm width) and determined from the video record when the rat's head moved in and out of the CS and US areas (see Figure S1 in Supplemental Materials for picture). A 16-s interval was scored around each CS presentation (8 s prior to CS onset and the 8 s of CS presentation) to characterize time spent at the CS or US location. First, the latency from the onset of the cue to entrance in either area was scored. If a rat did not enter the CS or US area during the CS presentation, the latency for entrance to that area was scored as 8 s (i.e., the CS duration), and if the rat was already in the area it was scored as 0 s. We also calculated the duration of time spent within an area (e.g., simple sum of time spent inside each square for multiple entrances) and the number of entries into an area. These scores were divided into “pre-CS” (time before the CS) and “CS” (during CS presentation), and the “pre-CS” score was subtracted from the “CS” score to yield an elevation score (i.e., the change in behavior induced by the CS presentation, which is the conditioned response). Measures related to the CS zone were considered sign-tracking conditioned responses, while behaviors directed to the US zone were considered goal-tracking conditioned responses. Note: although many studies used lever presses as a proxy for approach to the CS, we observed that most rats in this study approached the lever and light without actually depressing the lever. Thus, we used approach to the CS zone to quantify sign-tracking as described above, which had the advantage of allowing calculation of an elevation score to better determine conditioned approach to the CS.
Electrochemical data analysis
Dopamine transients from the FSCV recording were statistically identified by using principle component analysis as previously described (Shnitko and Robinson, 2015). Data around each CS presentation were extracted into 25-s windows: 5 s before the CS, 8 s of CS presentation, and 12 s after the CS. These data snippets were digitally filtered with a 2 kHz, fourth-order Bessel low-pass filter and the color plot data of each 25 s around the CS presentation underwent two passes of two-dimensional smoothing before background subtraction. Trials within a session were averaged for each rat to enhance signal-to-noise and then analyzed with principle component analysis (Keithley et al., 2009, Heien et al., 2004) by using training sets of 5 dopamine and 5 basic pH cyclic voltammograms obtained in vivo and triggered by electrical stimulation of the VTA. The analysis yielded an extracted concentration-versus-time trace of estimated dopamine concentrations, which were analyzed for the change in dopamine concentration, or Δ[DA], during the CS presentation and US delivery. Specifically, we selected a window around each event (CS, US) that included 0.5 s before through 3 s after the event onset. We determined the minimum and maximum concentrations of dopamine within the target window. Note that the maximum was only selected from the part of the target window after event onset, and the minimum was typically immediately before event onset. The dependent variable Δ[DA] was calculated as the difference between these values.
Statistical analysis
We planned to combine the two control groups (Water and No Exposure) in the event that they did not differ behaviorally. Thus, we compared behavior (CS/US entry elevation scores, CS/US duration elevations scores and CS/US latency) on the 6th day of Pavlovian conditioning as well as Δ[DA] after the CS and US presentation. Water and No Exposure groups trained to drink ENS were analyzed separately from those trained to drink ALC, and they were compared on each measure by using t-tests, not corrected for multiple comparisons, to inflate our ability to detect a difference. As there were no significant differences between the two control groups on any of these measures (Table S1 in Supplemental Materials), we combined them into a single control group labeled CON.
Data are represented as mean ± SEM unless otherwise noted and were analyzed in SigmaPlot for Windows 11 (Systat Software Inc., San Jose, CA) and SPSS (PASW-SPSS Statistics 18, Chicago, IL). Behavioral parameters (CS/US entry elevation scores, CS/US duration elevation scores and CS/US latency) and Δ[DA] were analyzed by using 2-way ANOVA with exposure (CON, CIE) and reward (ENS, ALC) as factors. Significant interactions of exposure by reward were followed by Holm-Sidak post-hoc comparisons. If data assumptions of normal distribution and equal variance were not met, then data were transformed before analysis by rank. Data from the NTX/saline sessions were analyzed by using 3-way ANOVA with exposure (CON, CIE), reward (ENS, ALC) and drug (NTX, saline) as factors. If Mauchly's Test of Sphericity was not met, then the degrees of freedom were adjusted with the Greenhouse-Geisser method. Effect sizes for 2-way ANOVA were calculated as η2 and those for 3-way ANOVA were calculated as partial η2, due to the within-subject design (Cohen, 1988). Effect sizes were considered small, medium and large if they corresponded to classical or partial η2 of at least 0.0099, 0.0588 and 0.1379, respectively, based on values of f of; as described by Cohen (1988).
Results
Animal numbers and blood ethanol concentrations
Only rats that underwent successful FSCV recordings, including recording sites within the NAc core, were included in the final data set, resulting in the following group numbers: CON-ENS n=7; CON-ALC n=10; CIE-ENS n=6; CIE-ALC n=6. On the first and final days of 5 g/kg ethanol administration in CIE rats, BEC were 125±14 and 151±12 mg/dl, respectively, indicating that binge-like BEC (>80 mg/dl) were reached during CIE exposure. BEC detected in ALC groups after a Pavlovian conditioning session were 22±6 mg/dl in CON-ALC rats and 27±8 mg/dl in CIE-ALC rats, roughly consistent with BEC achieved after 1-2 drinks over 30 minutes in adult humans.
Pavlovian conditioned approach
Behavioral data for the Pavlovian conditioning session on Day 6 are shown in Table 1; behavioral data were missing from one CON-ENS rat on Day 6 due to technical difficulties with the video. We first assessed conditioned approach to the CS or the US zones on Day 6, the final day of training. The first measure was the latency of the rats to approach either the CS or US zone. We found no effects of reward type or alcohol exposure on US latency, but the 2-way ANOVA revealed a significant main effect of alcoholic reward to slow CS latency (F1,24=8.43, η2=0.26, p<0.01). Likewise, alcoholic reward was associated with a lower likelihood of approaching the CS before approaching the US (percent sign-tracking trials: main effect of reward, F1,24=10.02, η2=0.27, p<0.01), and this effect was larger in CIE-exposed rats, although not significantly (reward by exposure interaction, F1,24=3.38, η2=0.09, p=0.079). When we examined US zone entries elevation, there was a significant interaction between reward and exposure (2-way ANOVA, F1,24=4.94, η2=0.16, p<0.05), with no main effects. Post-hoc comparisons found that CIE-ALC rats made twice as many conditioned US entries than CON-ALC rats (t=2.23, p<0.05). There were no significant main effects or interacting effects of exposure or reward on other behavioral measures on Day 6. Thus, contrary to our hypothesis, alcoholic reward reduced sign-tracking and CIE exposure increased goal-tracking on Day 6 of Pavlovian conditioning.
Table 1.
Measures of approach to CS zone (sign-tracking) and US zone (goal-tracking) on the final Pavlovian training day (Day 6), the extinction day (Day 7), and the electrochemical recording day (FSCV).
| CS Zone | US Zone | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Behavior | Session | CON-ENS | CON-ALC | CIE-ENS | CIE-ALC | CON-ENS | CON-ALC | CIE-ENS | CIE-ALC |
| Latency (s) to enter zone | Day 6 | 2.31 ± 0.51 a | 4.02 ± 0.52 a | 2.32 ± 0.80 a | 4.43 ± 0.77 a | 2.09 ± 0.25 | 2.96 ± 0.65 | 2.80 ± 0.72 | 1.55 ± 0.22 |
| Day 7 | 2.71 ± 0.48 a | 3.97 ± 0.53 a | 3.23 ± 0.78 a | 4.44 ± 0.48 a | 4.40 ± 0.49 | 5.52 ± 0.58 | 4.60 ± 0.36 | 3.79 ± 0.29 | |
| FSCV | 4.39 ± 0.77 a | 7.14 ± 0.28 a | 4.84 ± 1.21 a | 7.54 ± 0.17 a | 2.33 ± 0.70 | 2.36 ± 0.59 | 3.38 ± 0.60 | 2.27 ± 0.64 | |
|
| |||||||||
| Zone entries, elevation | Day 6 | 0.73 ± 0.14 | 0.47 ± 0.07 | 0.84 ± 0.24 | 0.59 ± 0.16 | 1.07 ± 0.17 | 0.67 ± 0.15 | 0.79 ± 0.25 | 1.23 ± 0.15 b |
| Day 7 | 0.99 ± 0.11 a | 0.63 ± 0.09 a | 0.89 ± 0.65 a | 0.77 ± 0.12 a | 0.87 ± 0.16 | 0.49 ± 0.14 | 0.65 ± 0.15 | 0.85 ± 0.11 | |
| FSCV | 0.41 ± 0.17 a | 0.12 ± 0.05 a | 0.28 ± 0.10 a | 0.10 ± 0.04 a | 0.56 ± 0.23 a | 0.17 ± 0.10 a | 0.73 ± 0.13 a | 0.42 ± 0.11 a | |
|
| |||||||||
| Time (s) spent in zone | Day 6 | -0.33 ± 0.31 | 0.48 ± 0.51 | 1.16 ± 0.64 | -0.12 ± 0.19 | 3.00 ± 0.28 | 2.38 ± 0.73 | 2.30 ± 0.84 | 3.28 ± 0.43 |
| Day 7 | 2.14 ± 0.50 a | 1.46 ± 0.37 a | 2.01 ± 0.81 a | 0.84 ± 0.19 a | 0.81 ± 0.28 | 0.53 ± 0.35 | 0.57 ± 0.35 | 0.36 ± 0.29 | |
| FSCV | 0.92 ± 0.74 | 0.32 ± 0.16 | 0.35 ± 0.24 | 0.10 ± 0.07 | 2.42 ± 0.67 | 0.92 ± 0.50 | 2.11 ± 0.52 | 1.97 ± 0.42 | |
|
| |||||||||
| Percent sign-tracking trials, based on latency | Day 6 | 53 ± 9 a | 44 ± 6 a | 71 ± 4 a | 34 ± 9 a | ||||
| Day 7 | 54 ± 7 a | 48 ± 5 a | 51 ± 8 a | 29 ± 5 a | |||||
| FSCV | 44 ± 9 a | 10 ± 3 a | 36 ± 12 a | 5 ± 2 a | |||||
Collapsed across exposure, ALC significantly different from ENS, p<0.05
Significantly different from CON-ALC, p<0.05
We also assessed behavior during a single extinction session (Day 7), when the CS was delivered but the US reward was withheld, a condition that generally reduces goal-tracking but not sign-tracking (Ahrens et al., 2016, Beckmann and Chow, 2015). Under this behavioral challenge, rats trained on alcoholic reward showed significantly less sign-tracking behavior than those trained on nonalcoholic reward (Table 1). Specifically, rats trained with alcoholic reward were slower to enter the CS zone (main effect of reward, F1,25=4.42, η2=0.15, p<0.05), made fewer entries into the CS zone (main effect of reward, F1,25=4.62, η2=0.14, p<0.05) and spent less time in the CS zone (CS duration elevation, main effect of reward, F1,25=4.32, η2=0.14, p<0.05) than rats trained on nonalcoholic reward. Moreover, rats trained on the nonalcoholic reward were more likely to approach the CS zone before the US zone than rats trained on alcoholic reward (percent sign-tracking trials: main effect of reward, F1,25=5.33, η2=0.16, p<0.05). There were no significant main effects or interactions of exposure or reward on goal-tracking behavioral measures on Day 7. Thus, similar to Day 6, rats trained with the alcoholic reward displayed less sign-tracking behavior during extinction than rats trained on the nonalcoholic reward, although effect sizes of reward on these variables were somewhat smaller when the US was withheld than on Day 6.
On the FSCV recording day, we simultaneously assessed both behavioral parameters and dopamine release during the Pavlovian conditioning session. Note that behavior from two CON-ALC and two CON-ENS rats was unavailable due to technical difficulties with the video. We observed that the rats were noticeably less active when tethered for FSCV; specifically, the rats tended to stay near the cup rather than move around the chamber, resulting in fewer zone entries. Nevertheless, group differences in conditioned approach were qualitatively similar to Days 6 and 7 (Table 1): the main observation was that alcoholic reward reduced CS-directed approach. Specifically, 2-way ANOVAs of exposure by reward revealed that rats trained on alcoholic reward were slower to approach the CS zone (main effect of reward, F1,21=10.27, η2=0.32, p<0.01), were less likely to approach the CS zone first (F1,21=12.41, η2=0.36, p<0.01), and exhibited fewer CS entries (F1,21=6.75, η2=0.24, p<0.05) than rats trained on nonalcoholic reward. In addition, rats receiving alcoholic reward also made fewer conditioned entries into the US Zone (F1,21=6.45, η2=0.20, p<0.05), although there were no differences in the duration or latency to enter the US zone.
Dopamine measurements
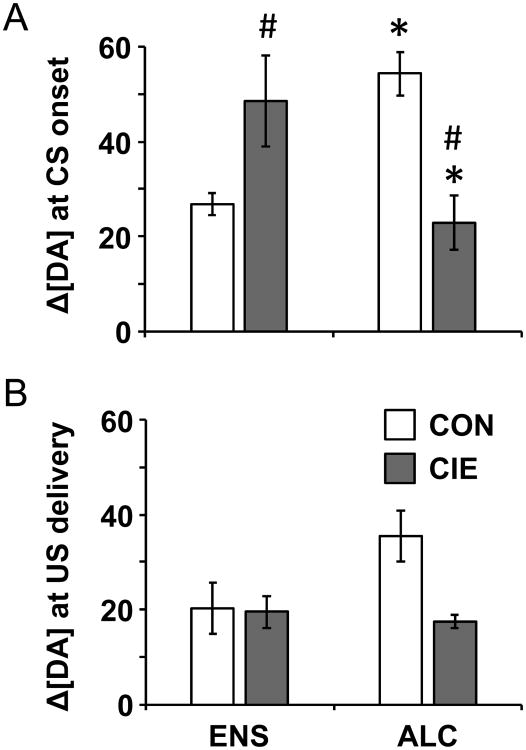
Against this behavioral background, we analyzed dopamine release as change in dopamine concentration, or Δ[DA], at CS onset and at US delivery. Overall, we found differences in Δ[DA] between groups at CS onset but not at US delivery (Figure 2). A 2-way ANOVA of Δ[DA] yielded a significant interaction between exposure and reward (F1,25=34.66, η2=0.53, p<0.001), with no significant main effects. Post-hoc comparisons showed that in CON rats, those expecting alcoholic reward had greater Δ[DA] to the CS onset as compared to those trained on nonalcoholic reward (t=5.06, p<0.001). However, the opposite relationship was observed in CIE rats: CIE-exposed rats expecting alcoholic reward showed smaller Δ[DA] to the CS onset as compared to those trained on nonalcoholic reward (t=3.41, p<0.01). This inverse relationship also played out when comparing groups with the same reward: in rats trained on non-alcoholic reward, a history of CIE boosted Δ[DA] to the CS onset (t=3.22, p<0.01), while in those trained on alcoholic reward, a history of CIE blunted Δ[DA] (t=5.19, p<0.001). Thus, the largest Δ[DA] to CS onset was observed in rats with either previous or current exposure to ethanol (CON-ALC and CIE-ENS rats), but having both previous and current exposure to ethanol (CIE-ALC) reduced the Δ[DA] back to the levels of naïve rats (CON-ENS). Individual examples of electrochemical signals from each group at the CS onset are shown in Figure 3, as well as composite concentration-versus-time traces for each group. As depicted in Figure 4, histology revealed that all FSCV data were recorded in the NAc core.
Figure 2.
Both history of alcohol exposure and alcoholic reward affect dopamine transients at the onset of the CS, but not at delivery of the US reward. Bars represent mean ± SEM of the change in dopamine concentration, Δ[DA], occurring within 3 s of the onset of the CS (A) or the delivery of the US reward (B). * Within treatment group, ALC significantly different from ENS. # Within reward group, CIE significantly different from CON. CON, control exposure; CIE, chronic intermittent ethanol; ENS, nonalcoholic reward; ALC, alcoholic reward.
Figure 3.
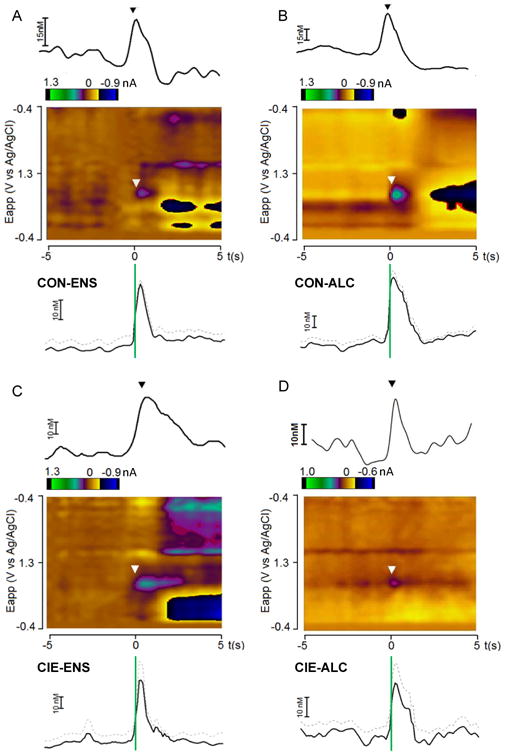
Individual examples of electrochemical signals at the CS onset as well as group-averaged traces of dopamine concentration over time: (A) CON-ENS, (B) CON-ALC, (C) CIE-ENS, and (D) CIE-ALC. The top of each panel shows a concentration-time trace and a color plot from an individual rat (all trials averaged), with arrows indicating the CS onset. The trace depicts concentration across the 10-s window at the peak oxidation potential of dopamine (∼0.65 V versus the Ag/AgCl reference electrode). The color plot indicates the current (color) over time (x-axis) at each applied potential (y-axis) for the same rat data as the trace above. Below the individual example, the average concentration across the 10-s window for the group is depicted as mean (filled line) and SEM (dashed gray line), and CS onset is represented by the green vertical line. Note the different concentration scale bars across groups. CON, control exposure; CIE, chronic intermittent ethanol; ENS, nonalcoholic reward; ALC, alcoholic reward.
Figure 4.
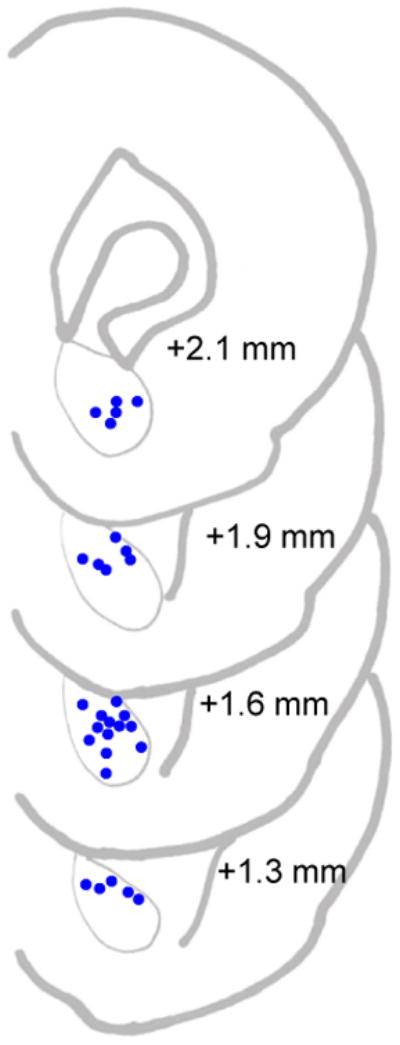
Site of recording electrodes (circles) in the nucleus accumbens core of rats. Coordinates of coronal slices are approximate locations anterior to bregma (in mm), based on the Paxinos and Watson (1998) rat brain atlas.
We next analyzed dopamine release at the start of US delivery (Figure 2B), which coincided with the end of the CS. Dopamine release at US delivery tended to be smaller than release at the CS onset, and neither chronic nor current ethanol exposure significantly affected the Δ[DA] at US delivery. A 2-way ANOVA of Δ[DA] at US delivery yielded no significant main effects or interaction of exposure by reward (each F<3.1, each η2<0.095, each p>0.09). One caveat is that FSCV is best suited to measure changes in dopamine, and the smaller dopamine signals at US onset may be in addition to elevated dopamine concentrations triggered by the CS. Individual examples of electrochemical signals from each group at the US delivery as well as composite concentration-versus-time traces for each group are shown in Figure S2 in Supplemental Materials.
Naltrexone and Pavlovian conditioned approach
During the following week, we measured behavior (untethered) during Pavlovian conditioning sessions to determine whether NTX altered Pavlovian sign-tracking or goal-tracking responses (Table 2). One CON-ALC rat did not complete this phase of the experiment due to health issues. We found that CIE history, and to a lesser extent alcoholic reward, increased sensitivity to NTX as measured by reduced conditioned responses. As observed in earlier phases of the study, rats trained with alcoholic reward exhibited slower approach to the CS zone (main effect of reward, F1,25=6.29, partial η2=0.20, p<0.05), and this effect was not moderated by NTX. Also as before, rats trained with alcoholic reward were more likely to initially approach the US rather than the CS zone (main effect of reward, F1,25=7.52, partial η2=0.23, p<0.05). On this measure, the 3-way ANOVA also yielded a significant exposure by drug interaction (F1,25=5.38, partial η2=0.18, p<0.05), and post-hoc comparisons found that in CIE rats, NTX significantly reduced the probability to approach the CS zone first (collapsed across reward, t=2.32, p<0.05). Duration of time spent in the CS zone and number of entries were not significantly affected by drug, alcohol exposure, or reward type.
Table 2.
Measures of approach to CS zone (sign-tracking) and US zone (goal-tracking) (mean ± SEM) after administration of 1 mg/kg naltrexone (NTX) or saline (SAL), using a within-subject design (counterbalanced order).
| CS Zone | US Zone | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Behavior | Session | CON-ENS | CON-ALC | CIE-ENS | CIE-ALC | CON-ENS | CON-ALC | CIE-ENS | CIE-ALC |
| Latency (s) to enter zone | SAL | 2.49 ± 0.53 | 5.20 ± 0.80 a | 2.90 ± 0.79 | 4.46 ± 0.89 a | 2.54 ± 0.59 | 2.57 ± 0.44 | 1.76 ± 0.18 | 1.59 ± 0.23 |
| NTX | 2.26 ± 0.39 | 4.60 ± 0.93 a | 3.60 ± 1.10 | 4.97 ± 0.80 a | 2.35 ± 0.63 | 2.50 ± 0.51 | 1.84 ± 0.45 b | 3.33 ± 0.70 b | |
|
| |||||||||
| Zone entries, elevation | SAL | 0.53 ± 0.11 | 0.34 ± 0.09 | 0.31 ± 0.16 | 0.32 ± 0.12 | 070 ± 0.19 | 0.52 ± 0.13 | 0.81 ± 0.09 c | 0.81 ± 0.14 c |
| NTX | 0.46 ± 0.13 | 0.34 ± 0.10 | 0.31 ± 0.16 | 0.48 ± 0.23 | 0.60 ± 0.13 | 0.46 ± 0.06 | 0.83 ± 0.13 c | 0.86 ± 0.21 c | |
|
| |||||||||
| Time (s) spent in zone | SAL | 1.21 ± 0.90 | 0.67 ± 0.55 | -0.04 ± 0.48 | -0.17 ± 0.18 | 1.39 ± 0.71 | 2.25 ± 0.49 | 3.50 ± 0.22 d | 3.50 ± 0.25 d |
| NTX | 0.58 ± 0.91 | 1.12 ± 0.89 | 0.05 ± 0.67 | 0.31 ± 0.30 | 2.92 ± 0.69 | 1.29 ± 0.76 e | 2.31 ± 0.63 b | 1.88 ± 0.48 e,b | |
|
| |||||||||
| Percent sign-tracking trials, based on latency | SAL | 43 ± 7% | 22 ± 7% a | 52 ± 8% | 37 ± 10% a | ||||
| NTX | 52 ± 8% | 21 ± 6% a | 41 ± 12% b | 26 ± 6% a,b | |||||
Collapsed across exposure and drug, ALC significantly different from ENS, p<0.05
Collapsed across reward, NTX significantly different from SAL, p<0.05
Collapsed across reward and drug, CIE significantly different from CON, p<0.05
Collapsed across reward, CIE significantly different from CON, p<0.05
Collapsed across exposure, NTX significantly different from SAL, p<0.05
NTX more effectively reduced measures of goal-tracking approach, especially in rats with a history of CIE. NTX slowed the latency to approach the US (exposure by drug interaction, F1,25=4.80, partial η2=0.16, p<0.05) in CIE but not CON rats (collapsed across reward, t=2.38, p<0.05). When examining the time spent in the US zone, the 3-way ANOVA yielded an exposure by drug interaction (F1,25=9.03, partial η2=0.27, p<0.01) as well as a reward by drug interaction (F1,25=6.69, partial η2=0.21, p<0.05). Post-hoc comparisons found that duration of time spent in the US zone was longer in CIE-exposed rats under saline conditions (collapsed across reward, t=2.54, p<0.05). Moreover, NTX reduced time in the US zone in CIE-exposed rats (collapsed across reward, t=2.82, p<0.01) and in rats trained on ALC reward (collapsed across exposure, t=2.81, p<0.01). Finally, rats with CIE exposure exhibited more US zone entries (main effect of exposure, F1,25=4.44, partial η2=0.15, p<0.05), but this was not moderated by NTX. Thus, both history of CIE and alcoholic reward made rats sensitive to NTX on measures of goal-tracking.
Discussion
Here, we tested the hypothesis that exposure to alcohol, either prior to training or in the reward, would promote both dopamine release to a predictive CS and sign-tracking in a Pavlovian conditioning paradigm. In support, we found that rats that either trained with alcoholic reward or had a history of alcohol exposure showed enhanced dopamine release to a CS as compared to naïve rats or those having both alcohol exposure and alcoholic reward. However, the behavioral findings did not support our hypothesis – alcoholic reward reliably reduced sign-tracking, even under extinction conditions that typically favor sign-tracking behavior. Moreover, previous alcohol exposure promoted some measures of goal-tracking, and goal-tracking was preferentially reduced in these rats by naltrexone. These findings suggest that alcohol's effects on dopamine release to a Pavlovian cue can dissociate from its effects on conditioned approach triggered by that cue, at least in early stages of training.
We observed dopamine transients to CS and US presentations, consistent with the role of dopamine to encode CS-US associations and reward prediction errors (Schultz, 2016). Prior studies have demonstrated that as the predictive nature of the CS develops, dopamine release to the CS emerges in the NAc core, but not the shell (Brown et al., 2011), even within the first training session (Day et al., 2007). Moreover, Flagel et al. (2011) found that over 6 days of Pavlovian training, dopamine release to the CS and US started out similarly in rats that were eventually sign-trackers and those that were eventually goal-trackers. However, while dopamine transients to the CS and US were largely maintained across 6 days of training in goal-tracking rats, transients to the US became smaller in sign-tracking rats. Here, we detected dopamine transients in all rats, and either a history of ethanol exposure or alcoholic reward (but not both) was associated with larger dopamine transients to the CS. It is possible that the alcoholic reward pharmacologically boosted dopamine release, as both tonic and phasic dopamine release increase after acute alcohol administration (e.g., Robinson et al., 2009). However, data on the effect of CIE on dopamine release are mixed. Dopamine measurements after adult CIE have mostly been made within 24 hours after exposure (i.e., in withdrawal) rather than days or weeks later, as in the present study. In the hours after exposure, phasic dopamine release is known to be reduced by CIE, at least in part via faster clearance by the dopamine transporter (e.g., Karkhanis et al., 2016). In one report, vapor CIE in adult mice did not alter optically-evoked dopamine release in NAc 72 hours after exposure, although uptake was still enhanced (Melchior and Jones, 2017). In contrast, in studies that measured dopamine function days or weeks after CIE, alcohol exposure occurred during adolescence. Badanich et al. (2007) found that a daily, low-dose ethanol regimen for 20 days during adolescence resulted in increased extracellular dopamine concentrations in the NAc of rats 15 days later, as compared to saline-exposed rats. Moreover, Spoelder et al. (2015) reported that dopamine transients to a CS predicting a nonalcoholic cue were larger in rats exposed to alcohol during adolescence versus control rats. If similar neuroadaptations occurred after binge-like CIE in the present study, that would explain why dopamine transients to the CS were enhanced in rats trained on nonalcoholic reward. On the other hand, why would CIE enhance dopamine to a CS predicting a nonalcoholic reward but not to a CS predicting an alcoholic reward? The adult CIE literature presents a mechanism by which the combination of CIE and alcoholic reward might reduce phasic dopamine release. Specifically, CIE in adulthood upregulates kappa opioid receptor function (Karkhanis et al., 2016, Melchior and Jones, 2017), and this change reverses the typical effect of acute ethanol: while a 2 g/kg alcohol challenge enhanced dopamine concentrations in the NAc of control mice, the same challenge reduced extracellular dopamine in CIE-exposed mice (Karkhanis et al., 2016). Importantly, in CIE-exposed mice, the kappa opioid antagonist nor-BNI restored the typical increase in dopamine to alcohol challenge. However, whether similar regulation occurred in the present study is unknown and requires additional experiments.
While some studies have linked greater dopamine release to a CS to greater levels of sign-tracking (Flagel et al., 2011, Spoelder et al., 2015), we did not observe that association. To assess effects of alcoholic reward on conditioned approach, we trained rats for 6 days prior to surgery, a timeframe that is typical in the literature (e.g., Flagel et al., 2009). We assessed behavior on Day 6, Day 7 (extinction) and in the two weeks following surgery (FSCV and NXT days), and we consistently observed that alcoholic reward reduced sign-tracking. Similarly, Srey et al. (2015) found that in rats exposed to alcohol in the home cage then trained in Pavlovian approach with an alcoholic reward, initial sessions produced predominately goal-tracking behavior. However, in that study, sign-tracking emerged in the group average after 8-10 sessions. The same researchers later analyzed those data along with other studies from the lab and determined that while the majority of rats maintained a goal-tracking, sign-tracking, or intermediate phenotype throughout training, a subset of rats shifted from goal-tracking to sign-tracking (Villaruel and Chaudhri, 2016). These data suggest that if we had trained rats for additional sessions, we might have observed the emergence of sign-tracking in some rats consuming alcoholic reward. Nevertheless, within the timeframe of the present study, the effect of alcoholic reward to promote goal-tracking relative to sign-tracking was consistent across days.
Another possibility is that the alcoholic US has a different value or palatability than the nonalcoholic US, and that affected the behavioral or neurochemical data. This possibility was not tested, as rats received only one of the reward solutions throughout the study. Notably, all rats consumed the US solutions without being food or water restricted, suggesting that both solutions were palatable and that no apparent aversion to alcohol was induced by the gavage exposure procedure. Previous studies have compared the effect of reward magnitude (e.g., the number of pellets or concentration of sucrose) on Pavlovian conditioning, and while some found that magnitude did not alter conditioned responses (Tabbara et al., 2016, van den Bos et al., 2004), other reports found it did (Palmatier et al., 2013), and all of these studies used food- or water-restricted rats. Food restriction itself may enhance the rewarding properties of the US, but when Andersen et al. tested its effects on the development of sign-tracking and goal-tracking, they found that food restriction modestly promoted both types of conditioned responses (Anderson et al., 2013). This finding suggests that differences in US reward per se might change the quantity but not quality of the conditioned response. On the other hand, it is possible that the shift toward goal-tracking that we observed in rats given an alcoholic US is due to the alcohol itself. For example, Versaggi et al. reported that a low dose of alcohol given immediately before five Pavlovian conditioning sessions for a food cue reduced sign-tracking and elevated goal-tracking (Versaggi et al., 2016). This supports the possibility that the pharmacological effect of the alcohol in the US, as opposed to a difference in palatability or reward value, could produce many of the behavioral effects we observed.
The CIE regimen used here was relatively brief and mild compared to regimens designed to produce dependence, which are typically vapor or forced diet exposure. The present CIE modeled binge drinking, producing 125-150 mg/dl BEC. The observation that NTX reduced goal-tracking conditioned approach in the CIE groups suggest that this binge CIE was effective to produce some persistent neurobiological adaptations. However, CIE did not promote sign-tracking. In contrast, other studies have found that CIE during adolescence either promotes sign-tracking, reduces goal-tracking, or both in Pavlovian studies using nonalcoholic rewards (Madayag et al., 2017, McClory and Spear, 2014, Spoelder et al., 2015). For example, Spoelder et al. (2015) tested rats in adulthood after they repeatedly consumed alcohol during adolescence. The alcohol-exposed and control groups began to differentiate around day 13-15, when alcohol-exposed rats began to exhibit more sign-tracking. This study also observed dopamine transients on the first day of Pavlovian training to both CS and US presentation, with higher dopamine in alcohol-exposed rats than in controls, although this difference was gone by day 5 of training. However, after extinction and reinstatement of Pavlovian conditioned approach, DA release to the CS was again higher in alcohol-exposed rats. Overall, this profile of dopamine release is consistent with our finding that dopamine release was higher to the CS predicting nonalcoholic reward in CIE-exposed rats than in control-exposed rats. But what about conditioned behavior? While CIE during adolescence promoted sign-tracking and/or reduced goal-tracking many weeks after the final exposure (Madayag et al., 2017, McClory and Spear, 2014, Spoelder et al., 2015), there are discrepancies among these studies as to how many sessions were required for differences to emerge, again suggesting that the present study may not have trained the rats long enough for conditioned approach behavior to fully differentiate. On the other hand, McClory and Spear (2014) included a group of rats given alcohol exposure during adulthood, and this group did not show differences from control groups in conditioned approach to a CS predicting a nonalcoholic reward.
NTX is one of the primary pharmacotherapies for alcohol use disorder and is thought to work by reducing alcohol reward and craving (O'Malley et al., 2002, Gianoulakis et al., 1996, Ripley and Stephens, 2011). In the present study, NTX reduced goal-tracking and, to a lesser extent, sign-tracking in rats with a history of CIE. Moreover, both alcohol exposure and alcoholic reward conferred sensitivity to NTX. This is consistent with NTX reducing alcohol reward, as well as with CIE promoting neuroadaptations in opioid signaling pathways (Herz, 1997). Indeed, reward devaluation has been reported to reduce goal-tracking conditioned responses (Morrison et al., 2015). As we did not measure dopamine release during NTX administration, it is unknown whether NTX diminished the dopamine transients triggered by the CS. However, NTX can reduce ethanol-evoked dopamine release (Gonzales and Weiss, 1998), and reductions in the dopamine signals associated with CS and US presentation would presumably reduce any incentive value of the CS due to a reduction in reward prediction. Thus, future studies can investigate this and other pharmacologic approaches to reduce alcohol reward and associated learned behaviors that are thought to maintain attentional biases contributing to alcohol use disorder (Field and Cox, 2008).
Supplementary Material
Acknowledgments
The authors thank Dr. Margaret Broadwater, Dawnya Zitzman and Megan Villegas for valuable technical assistance. This research was funded by the National Institutes of Health (P60 AA011605 Component 3) and by the University of North Carolina Bowles Center for Alcohol Studies. A.M.F. was supported by a Doctoral Program Abroad Fellowship (CAPES) – Coordination of Improvement of Higher Education Personnel (Brazil). Analysis of behavioral data for this project partially fulfilled honors thesis requirements for S.R.V. in the UNC Department of Biology.
Footnotes
Conflict of Interest: The authors have no conflicts of interest to declare.
References
- Ahrens AM, Singer BF, Fitzpatrick CJ, Morrow JD, Robinson TE. Rats that sign-track are resistant to Pavlovian but not instrumental extinction. Behav Brain Res. 2016;296:418–30. doi: 10.1016/j.bbr.2015.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akala EO, Wang H, Adedoyin A. Disposition of naltrexone after intravenous bolus administration in Wistar rats, low-alcohol-drinking rats and high-alcohol-drinking rats. Neuropsychobiology. 2008;58:81–90. doi: 10.1159/000159776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RI, Bush PC, Spear LP. Environmental manipulations alter age differences in attribution of incentive salience to reward-paired cues. Behavioural brain research. 2013;257:83–9. doi: 10.1016/j.bbr.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badanich KA, Maldonado AM, Kirstein CL. Chronic ethanol exposure during adolescence increases basal dopamine in the nucleus accumbens septi during adulthood. Alcohol Clin Exp Res. 2007;31:895–900. doi: 10.1111/j.1530-0277.2007.00370.x. [DOI] [PubMed] [Google Scholar]
- Bath BD, Michael DJ, Trafton BJ, Joseph JD, Runnels PL, Wightman RM. Subsecond adsorption and desorption of dopamine at carbon-fiber microelectrodes. Anal Chem. 2000;72:5994–6002. doi: 10.1021/ac000849y. [DOI] [PubMed] [Google Scholar]
- Beckmann JS, Chow JJ. Isolating the incentive salience of reward-associated stimuli: value, choice, and persistence. Learn Mem. 2015;22:116–27. doi: 10.1101/lm.037382.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown HD, Mccutcheon JE, Cone JJ, Ragozzino ME, Roitman MF. Primary food reward and reward-predictive stimuli evoke different patterns of phasic dopamine signaling throughout the striatum. The European journal of neuroscience. 2011;34:1997–2006. doi: 10.1111/j.1460-9568.2011.07914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlabaum Associates; 1988. [Google Scholar]
- Cox WM, Hogan LM, Kristian MR, Race JH. Alcohol attentional bias as a predictor of alcohol abusers' treatment outcome. Drug Alcohol Depend. 2002;68:237–43. doi: 10.1016/s0376-8716(02)00219-3. [DOI] [PubMed] [Google Scholar]
- Day JJ, Roitman MF, Wightman RM, Carelli RM. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat Neurosci. 2007;10:1020–1028. doi: 10.1038/nn1923. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Spear LP. Amphetamine-induced incentive sensitization of sign-tracking behavior in adolescent and adult female rats. Behav Neurosci. 2011;125:661–7. doi: 10.1037/a0023763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Cox WM. Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug Alcohol Depend. 2008;97:1–20. doi: 10.1016/j.drugalcdep.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to reward-related cues: Implications for addiction. Neuropharmacology. 2009;56(1):139–48. doi: 10.1016/j.neuropharm.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PE, Akil H. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53–7. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Robinson TE, Clark JJ, Clinton SM, Watson SJ, Seeman P, Phillips PE, Akil H. An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: implications for addiction. Neuropsychopharmacology. 2010;35:388–400. doi: 10.1038/npp.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Watson SJ, Robinson TE, Akil H. Individual differences in the propensity to approach signals vs goals promote different adaptations in the dopamine system of rats. Psychopharmacology (Berl) 2007;191:599–607. doi: 10.1007/s00213-006-0535-8. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C, De Waele JP, Thavundayil J. Implication of the endogenous opioid system in excessive ethanol consumption. Alcohol. 1996;13:19–23. doi: 10.1016/0741-8329(95)02035-7. [DOI] [PubMed] [Google Scholar]
- Gonzales RA, Weiss F. Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J Neurosci. 1998;18:10663–10671. doi: 10.1523/JNEUROSCI.18-24-10663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heien ML, Johnson MA, Wightman RM. Resolving neurotransmitters detected by fast-scan cyclic voltammetry. Anal Chem. 2004;76:5697–704. doi: 10.1021/ac0491509. [DOI] [PubMed] [Google Scholar]
- Herz A. Endogenous opioid systems and alcohol addiction. Psychopharmacology (Berl) 1997;129:99–111. doi: 10.1007/s002130050169. [DOI] [PubMed] [Google Scholar]
- Karkhanis AN, Huggins KN, Rose JH, Jones SR. Switch from excitatory to inhibitory actions of ethanol on dopamine levels after chronic exposure: Role of kappa opioid receptors. Neuropharmacology. 2016;110:190–197. doi: 10.1016/j.neuropharm.2016.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keithley RB, Heien ML, Wightman RM. Multivariate concentration determination using principal component regression with residual analysis. Trends Analyt Chem. 2009;28:1127–1136. doi: 10.1016/j.trac.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madayag AC, Stringfield SJ, Reissner KJ, Boettiger CA, Robinson DL. Sex and Adolescent Ethanol Exposure Influence Pavlovian Conditioned Approach. Alcohol Clin Exp Res. 2017;41:846–856. doi: 10.1111/acer.13354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcclory AJ, Spear LP. Effects of ethanol exposure during adolescence or in adulthood on Pavlovian conditioned approach in Sprague-Dawley rats. Alcohol. 2014;48:755–63. doi: 10.1016/j.alcohol.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior JR, Jones SR. Chronic ethanol exposure increases inhibition of optically targeted phasic dopamine release in the nucleus accumbens core and medial shell ex vivo. Mol Cell Neurosci. 2017;85:93–104. doi: 10.1016/j.mcn.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SE, Bamkole MA, Nicola SM. Sign Tracking, but Not Goal Tracking, is Resistant to Outcome Devaluation. Front Neurosci. 2015;9:468. doi: 10.3389/fnins.2015.00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology (Berl) 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Kellicut MR, Brianna Sheppard A, Brown RW, Robinson DL. The incentive amplifying effects of nicotine are reduced by selective and non-selective dopamine antagonists in rats. Pharmacol Biochem Behav. 2014;126:50–62. doi: 10.1016/j.pbb.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, Marks KR, Jones SA, Freeman KS, Wissman KM, Sheppard AB. The effect of nicotine on sign-tracking and goal-tracking in a Pavlovian conditioned approach paradigm in rats. Psychopharmacology. 2013;226:247–59. doi: 10.1007/s00213-012-2892-9. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Ripley TL, Stephens DN. Critical thoughts on current rodent models for evaluating potential treatments of alcohol addiction and withdrawal. British journal of pharmacology. 2011;164:1335–56. doi: 10.1111/j.1476-5381.2011.01406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DL, Howard EC, Mcconnell S, Gonzales RA, Wightman RM. Disparity between tonic and phasic ethanol-induced dopamine increases in the nucleus accumbens of rats. Alcohol Clin Exp Res. 2009;33:1187–96. doi: 10.1111/j.1530-0277.2009.00942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DL, Zitzman DL, Smith KJ, Spear LP. Fast dopamine release events in the nucleus accumbens of early adolescent rats. Neuroscience. 2011;176:296–307. doi: 10.1016/j.neuroscience.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MJ, Anselme P, Suchomel K, Berridge KC. Amphetamine-induced sensitization and reward uncertainty similarly enhance incentive salience for conditioned cues. Behavioral neuroscience. 2015;129:502–11. doi: 10.1037/bne0000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Schultz W. Dopamine reward prediction-error signalling: a two-component response. Nature reviews Neuroscience. 2016 doi: 10.1038/nrn.2015.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shnitko TA, Robinson DL. Regional variation in phasic dopamine release during alcohol and sucrose self-administration in rats. ACS chemical neuroscience. 2015;6:147–54. doi: 10.1021/cn500251j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Mendez IA, Setlow B. Effects of prior amphetamine exposure on approach strategy in appetitive Pavlovian conditioning in rats. Psychopharmacology. 2009;202:699–709. doi: 10.1007/s00213-008-1353-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoelder M, Tsutsui KT, Lesscher HM, Vanderschuren LJ, Clark JJ. Adolescent Alcohol Exposure Amplifies the Incentive Value of Reward-Predictive Cues Through Potentiation of Phasic Dopamine Signaling. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2015;40:2873–85. doi: 10.1038/npp.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srey CS, Maddux JM, Chaudhri N. The attribution of incentive salience to Pavlovian alcohol cues: a shift from goal-tracking to sign-tracking. Frontiers in behavioral neuroscience. 2015;9:54. doi: 10.3389/fnbeh.2015.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacy AW, Wiers RW. Implicit cognition and addiction: a tool for explaining paradoxical behavior. Annu Rev Clin Psychol. 2010;6:551–75. doi: 10.1146/annurev.clinpsy.121208.131444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabbara RI, Maddux JM, Beharry PF, Iannuzzi J, Chaudhri N. Effects of sucrose concentration and water deprivation on Pavlovian conditioning and responding for conditioned reinforcement. Behav Neurosci. 2016;130:231–42. doi: 10.1037/bne0000138. [DOI] [PubMed] [Google Scholar]
- Tomie A, Sharma N. Pavlovian sign-tracking model of alcohol abuse. Current drug abuse reviews. 2013;6:201–19. doi: 10.2174/18744737113069990023. [DOI] [PubMed] [Google Scholar]
- Townshend JM, Duka T. Attentional bias associated with alcohol cues: differences between heavy and occasional social drinkers. Psychopharmacology (Berl) 2001;157:67–74. doi: 10.1007/s002130100764. [DOI] [PubMed] [Google Scholar]
- Tunstall BJ, Kearns DN. Sign-tracking predicts increased choice of cocaine over food in rats. Behavioural brain research. 2015;281:222–8. doi: 10.1016/j.bbr.2014.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Bos R, Van Der Harst J, Vijftigschild N, Spruijt B, Van Luijtelaar G, Maes R. On the relationship between anticipatory behaviour in a Pavlovian paradigm and Pavlovian-to-Instrumental Transfer in rats (Rattus norvegicus) Behav Brain Res. 2004;153:397–408. doi: 10.1016/j.bbr.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Versaggi CL, King CP, Meyer PJ. The tendency to sign-track predicts cue-induced reinstatement during nicotine self-administration, and is enhanced by nicotine but not ethanol. Psychopharmacology (Berl) 2016;233:2985–97. doi: 10.1007/s00213-016-4341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villaruel FR, Chaudhri N. Individual Differences in the Attribution of Incentive Salience to a Pavlovian Alcohol Cue. Front Behav Neurosci. 2016;10:238. doi: 10.3389/fnbeh.2016.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Incentive sensitization by previous amphetamine exposure: increased cue-triggered “wanting” for sucrose reward. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:7831–40. doi: 10.1523/JNEUROSCI.21-19-07831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoburn BC, Cohen AH, Inturrisi CE. Pharmacokinetics and pharmacodynamics of subcutaneous naltrexone pellets in the rat. J Pharmacol Exp Ther. 1986;237:126–30. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.