SUMMARY
T cells expressing chimeric antigen receptors (CARs) are promising cancer therapeutic agents, with the prospect of becoming the ultimate smart cancer therapeutics. To expand the capability of CAR T cells, here we present a split, universal, and programmable (SUPRA) CAR system that simultaneously encompasses multiple critical “upgrades”, such as the ability to switch targets without re-engineering the T cells, finely tune T cell activation strength, and sense and logically respond to multiple antigens. These features are useful to combat relapse, mitigate over-activation, and enhance specificity. We test our SUPRA system against two different tumor models to demonstrate its broad utility and humanize its components to minimize potential immunogenicity concerns. Furthermore, we extend the orthogonal SUPRA CAR system to regulate different T cell subsets independently, demonstrating a dually inducible CAR system. Together, these SUPRA CARs illustrate that multiple advanced logic and control features can be implemented into a single, integrated system.
ITI
A chimeric antigen receptor system that can integrate signals from multiple antigens and fine tune T cell activation in a cell type-specific manner holds promises for enhancing the safety and specificity of CAR T cell therapies for cancer treatment.
INTRODUCTION
The transfer of CAR-expressing T cells to patients is a promising approach for cancer immunotherapy (Brentjens et al., 2011; Davila et al., 2014; Grupp et al., 2013; Maude et al., 2014a). Despite these encouraging results, safety and efficacy continue to be major hurdles that hinder CAR T cell therapy development (Brentjens et al., 2013; Kochenderfer et al., 2012; Morgan et al., 2010; Scholler et al., 2012). To improve overall effectiveness and safety of CAR T cell therapy, there is an urgent need for a better system that can finely tune T cell activation, enhance tumor specificity, and independently control different signaling pathways and cell types.
The CAR T cells used in clinical trials typically have a rigid design that is difficult to alter without re-engineering the T cells. Current CAR designs are composed of a fixed antigen-specific single-chain variable fragment (scFv) and intracellular signaling domains (CD3ζ and costimulatory domains). When the constant antigen-specific CAR binds to the target antigen, these invariable signaling domains are activated simultaneously at a predetermined level. Due to the fixed design that limited the controllability of CAR T cell activation level, managing CAR T cell-related toxicities have proven to be challenging (Brentjens et al., 2013; Brudno and Kochenderfer, 2016; Davila et al., 2014).
In addition to constraining the controllability of CAR T cell activity, this fixed CAR design also restricts the antigen specificity and affinity. High-affinity scFvs are often used in the CAR design to ensure high antigen specificity. However, CARs made with high-affinity scFvs have limited capacity in discriminating antigen density, which have led to dangerous reactivity against healthy organs expressing a low level of antigens (Bonifant et al., 2016; Morgan et al., 2010). Using a scFv with lower antigen affinity allowed better antigen density discrimination (Caruso et al., 2015; Liu et al., 2015), but antigen specificity may be compromised. Thus, modulation of CAR components other than scFv affinity may be needed for improving CAR T cell specificity.
Recently, several studies have demonstrated the importance of regulating CD3ζ and the different costimulatory pathways independently to achieve optimal T cell response (Kloss et al., 2013; Lanitis et al., 2013; Zhao et al., 2015). Also, the activation of different costimulatory domains (e.g., CD28 or 4-1BB) is known to have different T cell functions and phenotypes (e.g., T cell differentiation and memory T cell formation) (Kawalekar et al., 2016; Skapenko et al., 2001; Zhu et al., 2007), demonstrating the value of CAR design that allows independent control of different signaling domains.
The composition of the T cell subsets, such as the ratio of CD4+ and CD8+ T cells, has also been shown to be an important parameter for enhancing the antitumor response of CAR T cells (Turtle et al., 2016). Given the fact that our immune system is composed of many different T cell subtypes with distinct effector functions (Golubovskaya and Wu, 2016; Vignali et al., 2008; Vivier et al., 2008), regulating the activity of T cell subtypes independently may be an attractive strategy for optimizing the efficacy of CAR T cell therapy (Sadelain et al., 2017). However, current fixed CAR design limits independent and inducible activation of different signaling domains or different T cell subsets to achieve user-defined diverse T cell response.
New receptor designs have been developed to address some of the deficiencies (e.g., controllability, flexibility, and specificity) in current CAR T cell therapies. For instance, drug-inducible ON and kill switches have been developed to regulate CAR activity (Di Stasi et al., 2011; Wu et al., 2015). Also, to afford greater flexibility in antigen recognition, CARs have been split such that the antigen recognition motif is dissociated from the signaling motif of the CAR. This split CAR configuration uses a universal receptor as the common basis for all interactions, allowing a large panel of antigens to be targeted without re-engineering the immune cells (Cartellieri et al., 2016; Rodgers et al., 2016; Tamada et al., 2012; Urbanska et al., 2012). In addition, to increase tumor specificity, CARs were developed that allow combinatorial antigen sensing (Kloss et al., 2013; Lanitis et al., 2013; Roybal et al., 2016) or target two tumor specific antigens that can reduce tumor antigen escape rate (Grada et al., 2013; Zah et al., 2016). All of these features are arguably vital to ensure a safe and effective CAR T therapy. However, none of these advanced CARs has incorporated all of these features into one system. Additionally, the signaling pathways and cell types that can be activated are also fixed, thus limiting the diverse immune responses that can be achieved.
To enhance the specificity, safety, and programmability of CARs, we develop a split, universal, and programmable (SUPRA) CAR system composed of a universal receptor expressed on T cells and a tumor-targeting scFv adaptor molecule (Figure 1A). The activity of SUPRA CARs can be finely regulated via multiple mechanisms to limit overactivation. SUPRA CARs can also logically respond to multiple antigens for improving tumor specificity. We show the SUPRA CAR system is effective against two different tumor models, demonstrating the broad clinical potential of this system. In addition, we show that SUPRA components can be humanized to reduce potential immunogenicity. Furthermore, we use orthogonal SUPRA CARs to inducibly regulate multiple signaling pathways or different human T cell subtypes to increase the range of the immune responses that can be achieved. Together, the SUPRA CAR system is a feature-rich system with inducible and logical control capabilities that can improve the safety and efficacy of current cellular cancer immunotherapy.
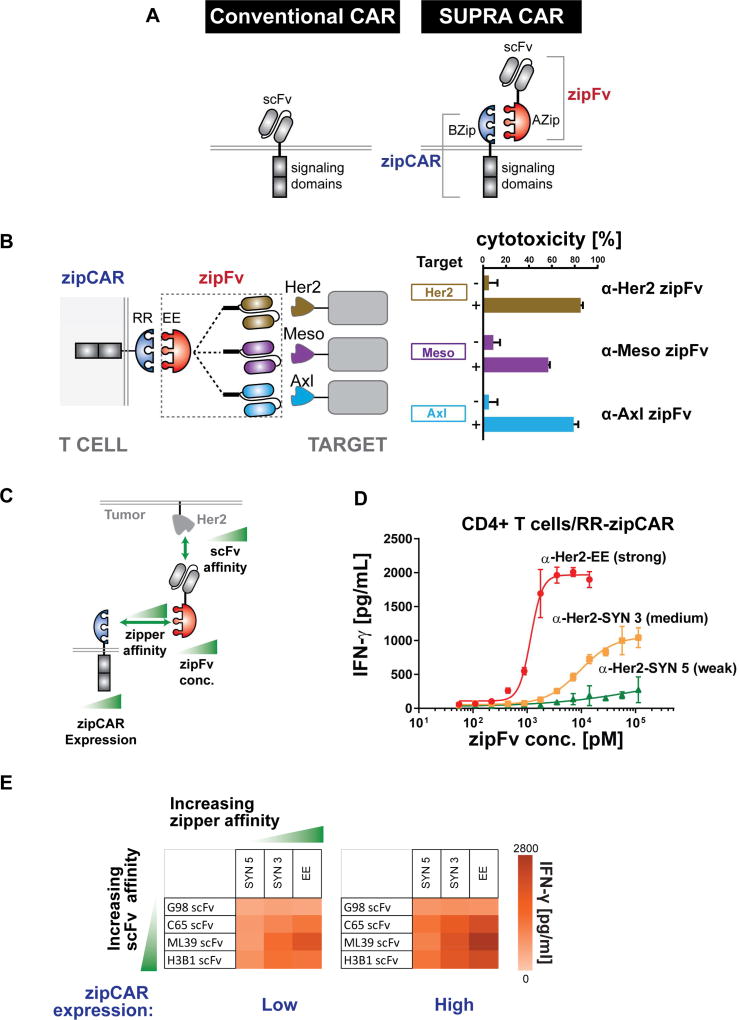
Figure 1. Design and characterization of the SUPRA CAR system.
(A) Comparison between the conventional CAR and SUPRA CAR design. A SUPRA CAR system is composed of a zipCAR and zipFv. A zipCAR has a leucine zipper as the extracellular portion of the CAR and zipFv has a scFv fused to a cognate leucine zipper that can bind to the leucine zipper on the zipCAR.
(B) A SUPRA CAR system targeting multiple tumor antigens using different zipFvs. K562 cells expressing Her2, Mesothelin, or Axl were co-cultured in vitro with RR zipCAR expressing CD8+ human primary T cells (n=3, mean ± SD).
(C) Variables explored for characterization of the SUPRA CAR system: (1) the affinity between leucine zipper pairs, (2) the affinity between tumor antigen and scFv, (3) the concentration of zipFv, and (4) the expression level of zipCAR.
(D) Effect of concentration of three zipFvs with leucine zippers (SYN 3, SYN5, and EE) that have different affinities to RR zipCAR on the IFN-γ production by primary CD4+ T cells (n=3, mean ± SD).
(E) Effect of zipper affinity, scFv-tumor affinity, and zipCAR expression level on the IFN-γ production by primary CD4+ T cells expressing RR zipCAR (n=3, mean).
RESULTS
Design and characterization of the SUPRA CAR system
The SUPRA CAR is a two-component receptor system composed of a universal receptor (zipCAR) expressed on T cells and a tumor-targeting scFv adaptor (zipFv) (Figure 1A). The zipCAR universal receptor is generated from the fusion of intracellular signaling domains and a leucine zipper as the extracellular domain. The zipFv adaptor molecule is generated from the fusion of a cognate leucine zipper and a scFv. The scFv of the zipFv binds to the tumor antigen, and the leucine zipper binds and activates the zipCAR on the T cells (Figures S1A and S2). Unlike the conventional fixed CAR design, the SUPRA CAR modular design allows targeting of multiple antigens without further genetic manipulations of a patient’s immune cells (Figure 1B, left). To test the ability of the SUPRA CAR system targeting multiple antigens with the same batch of T cells expressing the zipCAR, we first engineered human primary CD8+ T cells to express an RR zipCAR (RR leucine zipper with CD28, 4-1BB co-stimulatory and a CD3ζ signaling domain, Figure S1A). Next, we designed three different zipFvs to target three common tumor antigens (α-Her2, α-Axl, and α-Mesothelin, Figure S1A) by fusing the corresponding scFvs to an EE leucine zipper, which binds to the RR zipCAR on T cells. The engineered CD8+ T cells were co-cultured in vitro with K562 myelogenous leukemia cells that express Her2, Axl, or Mesothelin tumor antigens. The CD8+ zipCAR T cells killed the corresponding tumor cells when the matching zipFvs were added (Figure 1B, right).
A unique feature of the split CAR design is that it has multiple tunable variables, such as (1) the affinity between leucine zipper pairs, (2) the affinity between tumor antigen and scFv, (3) the concentration of zipFv, and (4) the expression level of zipCAR, that can be used to modulate the T cell response (Figure 1C). We first characterized the effect of zipFv concentration and zipper affinity on T cell activation. We generated three zipFvs with the same α-Her2 scFv, but fused to leucine zippers (SYN5, SYN 3, and EE) that have different affinity to the RR zipCAR (Reinke et al., 2010; Thompson et al., 2012). The amount of zipFv required to activate T cells to half-maximal IFN-γ secretion and cytotoxicity inversely correlated with the affinity of leucine zipper pairs where α-Her2-EE zipFv showed the lowest EC50 and α-Her2-SYN5 zipFv showed the highest EC50 value (Figures 1D and S1C). Also, the maximum level of IFN-γ secretion or killing efficiency correlated with the affinity of leucine zipper pairs.
We next investigated the effect of scFv–tumor antigen affinity, leucine zipper affinity, and zipCAR expression levels on the IFN-γ secretion and cancer-killing efficiency by the SUPRA CAR T cells (Figure 1E and S1D). We created 12 different zipFvs (three different leucine zippers with different affinity and four scFvs against Her2 (G98, C65, ML39, and H3B1) with Kd ranging from 3.2 × 10−7 to 1.2 × 10−10 M) (Chmielewski et al., 2004). We also generated two batches of T cells with high or low RR zipCAR expression level using fluorescence-activated cell sorting (Figure S1B). Cells expressing higher levels of zipCAR exhibited greater cytokine secretion when activated (Figure 1E). The affinity of scFv to Her2 correlated weakly with cytokine secretion or target cell lysis (Chmielewski et al., 2004). The affinity between leucine zippers, however, correlated well with cellular activation regarding cancer cell killing efficiency and cytokine secretion. As high affinity scFv CARs often over-activate and show severe toxicities in clinical trials (Bonifant et al., 2016; Brudno and Kochenderfer, 2016), the SUPRA platform can mitigate these toxicities by controlling other factors (e.g., zipFv concentration, affinity between leucine zipper pairs) to regulate T cell activation level. Together, these results demonstrate the tunable and modular nature of the SUPRA CAR design.
Competitive zipFvs for tuning SUPRA CAR activity
As many patients treated with CAR T cell therapy face cytokine release syndrome which can be life threatening (Maude et al., 2014b; Morgan et al., 2010), it is important to prevent CAR T cell activity when necessary. Thus, we explored the possibility of inhibiting the SUPRA CAR T cell activation through the addition of a competitive zipFv that can bind to the other zipFv, thus preventing zipCAR from being activated (Figure 2A, left). To test this approach, we screened several competitive zipFvs with different affinities for the EE zipFv (strong, medium and weak) (Figures S3B and S3C). Human CD8+ T cells were transduced with RR zipCAR and co-cultured with Her2+ K562 cells. Then EE zipFv (22.5nM, red) was subsequently added to activate the T cells. Without the competitive zipFv, the EE zipFv alone could activate T cells to destroy Her2+ cancer cells (Figure 2A, right). However, when the competitive zipFv (SYN4, SYN 47, or SYN 13) was also introduced (90nM, green), it bound to the EE zipFv and prevented the EE zipFv from activating the zipCAR T cells. By utilizing this competitive approach, we were able to inhibit primary CD8+ T cell activation in vitro with the strong competitive zipFv (SYN4). Furthermore, we were able to tune the activation levels with weaker binding zippers (SYN 47 and SYN 13). To understand inhibition dynamics, we varied the amount of competitive zipFv and timing of its addition. Increasing amount of competitive zipFv or delaying competitive zipFv addition did not affect inhibition strength greatly (Figure S3D).
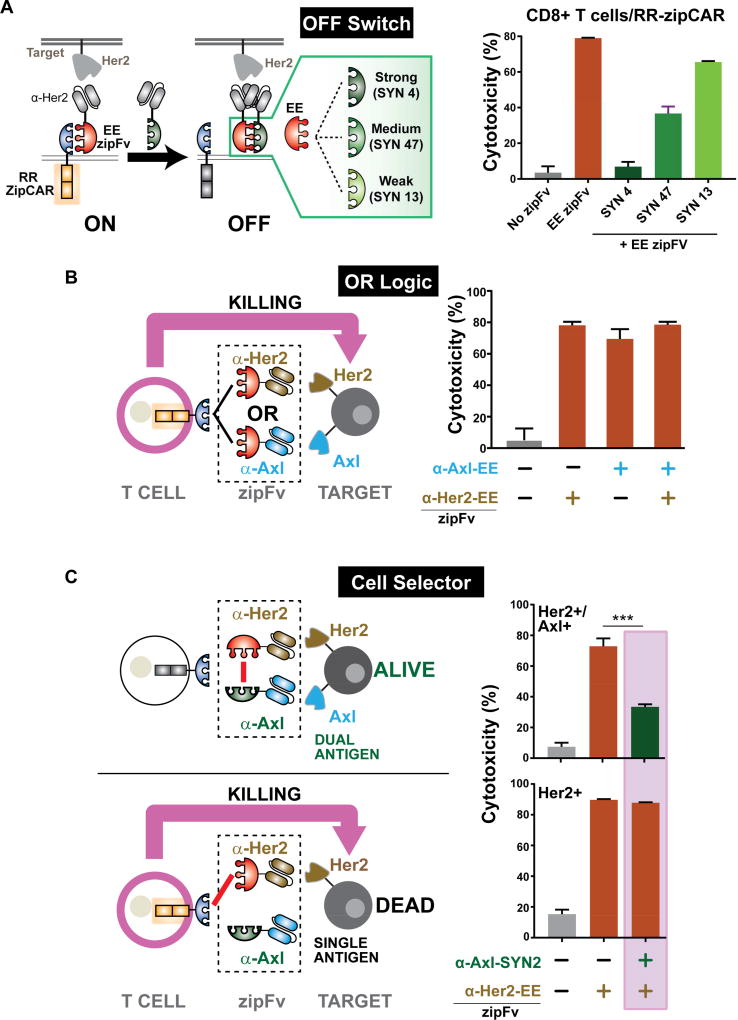
Figure 2. Utilizing SUPRA CAR for OFF switch function and combinatorial antigens targeting.
(A) (Left) A Schematic diagram of the SUPRA CAR system with an OFF switch zipFv. Three competitive leucine zippers that can bind to the EE leucine zipper with different affinities are used to tune the T cell activation level. (Right) A cytotoxicity plot demonstrating the effect of competitive zipFvs (n=3, mean ± SD).
(B) A cytotoxicity plot of “OR” gate implementation of the SUPRA CAR system. Her2+/Axl+ K562 tumor cells were co-cultured with RR zipCAR expressing CD8+ T cells with different zipFv combinations (n=3, mean ± SD).
(C) Using the SUPRA CAR system as cell selector. Cells either expressed Her2 or Her2 and Axl. Axl acted as a “safe marker” that can inhibit SUPRA CAR T cell activity (n=3, mean ± SD, statistical significance was determined by Student’s t test, *** = p ≤0.001).
Logical operation with the SUPRA CAR system
Antigen escape is a major challenge for targeted cancer therapies (Scott et al., 2012), including adoptive T cell therapies (Hegde et al., 2013; Perna and Sadelain, 2016). As such, bispecific receptors that can be triggered by CD19 and Her2 or CD19 and CD20, respectively, have been developed to combat antigen escape (Grada et al., 2013; Zah et al., 2016). Also, CD22 and CD123 CARs have been recently developed to increase tumor specificity (Haso et al., 2013; Ruella et al., 2016). However, as illustrated in a recent clinical trial with CD22 CAR T cells for patients relapsed from CD19 CAR T therapy, tumors can still evade detection by the engineered T cells by losing or down-regulating both antigens (Fry et al., 2018). In such cases, T cells would need to be re-engineered to target another antigen. Using the SUPRA CAR platform, however, different antigens can be easily targeted without further genetic manipulation. To test if SUPRA CAR could be used to target either one of the two antigen on the cell surface, we co-cultured Her2/Axl+ K562 cancer cells with RR zipCAR expressing CD8+ T cells. Then, different zipFv combinations were added to the cell mixture (α-Axl zipFv, α-Her2 zipFv, or both) (Figure 2B). As expected, the addition of zipFv targeting either Her2, Axl or both led to high killing efficiency, illustrating the potential of programming the SUPRA CAR system to combat antigen escape.
Another limitation of targeted tumor therapy is the difficulty in identifying a single tumor-specific antigen, which affects both tumor specificity and toxicity. Receptor systems that can perform combinatorial antigen detection have been developed to enhance the specificity of CAR T cell therapy (Kloss et al., 2013; Roybal et al., 2016). However, these receptor systems have a fixed antigen specificity design. Here, we investigated if the SUPRA CAR system can also be used to increase tumor specificity through combinatorial antigen sensing (Figure 2C). In particular, we used the SUPRA CAR system to target cells that express Her2 only and spare cells that express both Her2 and Axl, where Axl served as a “safety marker”. To achieve our design, we developed an α-Axl-SYN2 zipFv (green) that binds to α-Her2-EE zipFv (red) through a complementary zipper on each zipFv; this prevents the α-Her2-EE zipFv from binding to the zipCAR, thus protecting the Her2+/Axl+ cells. In contrast, since α-Axl-SYN2 zipFv cannot bind to Her2-only cells, the α-Her2-EE zipFv will not be blocked from activating the zipCAR.
To demonstrate such Her2 but NOT Axl logical operation, we first co-cultured RR zipCAR expressing CD8+ T cells with Her2+/Axl+ cells and zipFvs (Figure 2C, left). As expected, the addition of the α-Her2-EE zipFv alone achieved high tumor killing efficiency. However, when the α-Her2-EE zipFv was added after the α-Axl-SYN2 zipFv, the two zipFvs bound to each other and prevented the activation of the zipCAR, which led to a significant reduction in cytotoxicity (Figure 2C, right). As a control, we designed an α-Axl-SYN13 zipFv that did not bind strongly to the α-Her2-EE zipFv and showed no inhibition of T cell activity (Figure S3E). When the same SUPRA CAR system was challenged equally with cells expressing only Her2, the presence of α-Her2-EE zipFv alone again showed a high cytotoxicity rate (Figure 2C, right). Moreover, the addition of α-Axl zipFvs did not affect the killing efficiency. For both dual and single antigen target cells, α-Axl-SYN2 zipFv that binds strongly to α-Her2-EE zipFv or α-Axl-SYN13 zipFv that does not bind strongly to α-Her2-EE zipFv was first added to the cell mixture. After unbound α-Axl zipFvs were washed away, α-Her2-EE zipFv was added to stimulate CD8+ T cell activity (refer to STAR Method section for further detail). As expected, RR zipCAR engineered CD8+ T cells showed high activity toward Her2+ cancer cells, regardless of zipFv combinations, thus demonstrating the potential of the SUPRA CAR system to increase tumor specificity and reduce the toxicity of CAR T cell therapy.
Tumor clearance in a xenograft tumor model
After characterizing the SUPRA CAR system in vitro, we next tested whether SUPRA CARs can be used to reduce the tumor burden in a mouse xenograft model. For this experiment, we injected SK-BR-3 breast cancer cells (Her2 positive) intraperitoneally into immunocompromised NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) mice. After two weeks to allow for tumor establishment, we injected primary CD8+ human T cells expressing RR zipCAR or conventional Her2 CAR into the mice. α-Her2-EE zipFv was subsequently injected every 2 days at 5mg/kg for 2 weeks. Tumor growth was monitored by in vivo imaging (IVIS) of the luciferase signal from SK-BR-3 cancer cells in each mouse over the course of 41 days. RR zipCAR with α-Her2-EE zipFv showed robust tumor burden clearance, comparable to the conventional Her2 CAR. However, T cells expressing RR zipCAR alone without zipFvs were not able to reduce tumor burden (Figure 3A).
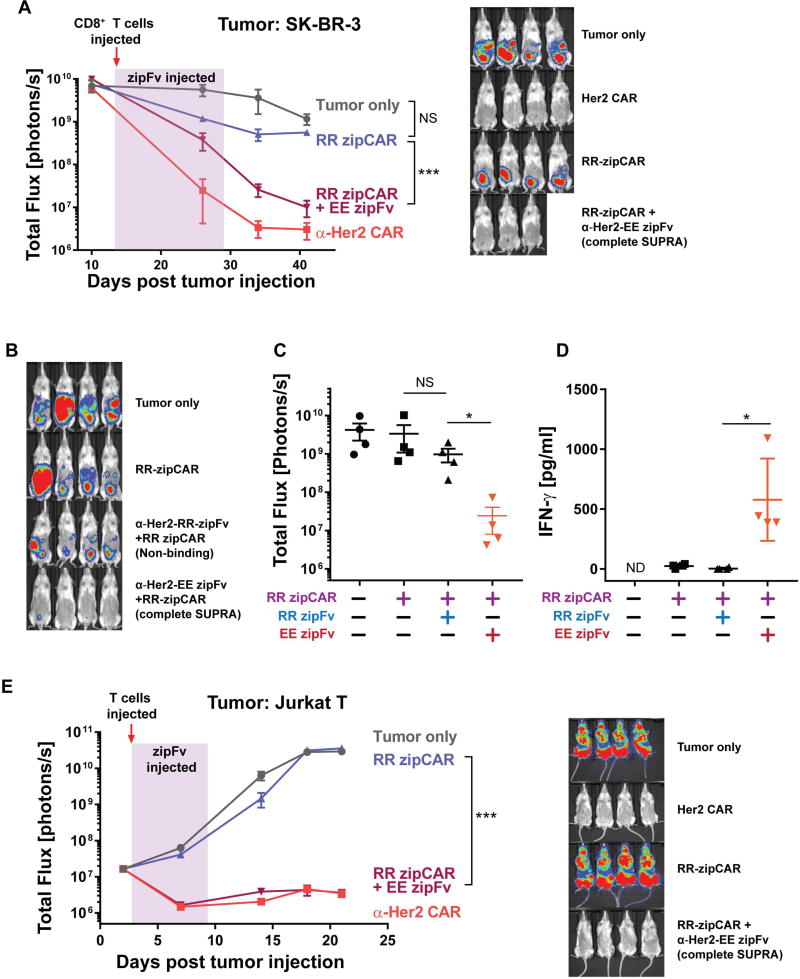
Figure 3. In vivo activity of SUPRA CAR in SK-BR-3 and Jurkat xenograft models.
(A) (Left) The tumor burden was quantified as the total flux (photons/sec) from the luciferase activity of each mouse using IVIS imaging. Compared to RR zipCAR or tumor only control groups, the RR zipCAR + EE zipFv group showed significantly reduced tumor burden, comparable to conventional Her2 CAR (red arrow indicates injection of engineered CD8+ T cells and highlighted region indicates injection of zipFv (every 2 days at 5mg/kg for 2 weeks)). (Right) Representative IVIS images of groups treated with (1) no T cells, (2) conventional Her2 CAR, (3) RR zipCAR, and (4) RR zipCAR with EE zipFv at day 41 (n=4, mean ± SEM).
(B) Representative IVIS images of groups treated with (1) no T cells, (2) RR zipCAR, (3) α-Her2 RR zipFv with RR zipCAR (non-binding), and (4) α-Her2 EE zipFv with RR zipCAR (complete SUPRA) (day 57, Figure S4B).
(C) Tumor burden as total flux (photons per sec) of each mouse shown in Figure 3B (n=4, mean ± SEM).
(D) In vivo IFN-γ cytokine level after 24 hours of initial CD8+ T cells and zipFv injection (n=4, mean ± SD).
(E) (Left) Jurkat tumor cells were injected intravenously on day 0 to immune-compromised NSG mice. At day 3, primary human CD8+ T cells expressing RR zipCAR were injected once (red arrow) with α-Her2-EE zipFv which was dosed every day for 6 days at 3mg/kg (highlighted). The tumor burden was quantified as the total flux (photons/sec) from the luciferase activity of each mouse using IVIS imaging. (Right) Representative IVIS images of groups treated with (1) no T cells, (2) conventional Her2 CAR, (3) RR zipCAR, and (4) RR zipCAR with EE zipFv at day 21 (n=4, mean ± SEM, statistical significance was determined by Student’s t test, *= p ≤0.05, ***= p ≤0.001).
To verify that the decrease in tumor burden was due to binding between zipCAR and zipFv, we also tested an α-Her2-RR zipFv, which does not bind to the RR zipCAR (Figures 3B and S4A). We set up a similar tumor model with SK-BR-3 breast cancer cells and injected RR zipCAR expressing CD8+ T cells at day 38 (Figures S4A and S4B). Both zipFvs (α-Her2-RR or α-Her2-EE) were dosed every two days for two weeks at 8mg/kg. Representative IVIS images and quantified luminescence from each mouse at day 57 demonstrated a decrease in tumor burden only when the α-Her2-EE zipFv that binds to zipCAR was injected (Figures 3B and 3C). We also measured the cytokine release in vivo to verify that cytokine production is specific to the binding between zipFv and zipCAR. As expected, the injection of α-Her2-RR zipFv did not increase IFN-γ level in vivo. However, the administration of the α-Her2-EE zipFv showed a significant increase in IFN-γ after 24 hours of CD8+ T cells and zipFv injection (Figure 3D).
Intraperitoneal xenograft tumor models are frequently used to evaluate immunotherapy against human ovarian cancers (Geller et al., 2013; Lee et al., 2002; Lengyel et al., 2014; Shaw et al., 2004), but demonstrating the efficacy of the SUPRA system with a blood tumor model could further validate the applicability of this system against different tumors (e.g., T cell cancers). As such, we also tested the SUPRA system against a blood tumor model. We injected modified Jurkat T cancer cells (engineered to be Her2 positive) intravenously into immunocompromised NSG mice. After three days, we injected primary CD8+ human T cells expressing RR zipCAR or conventional Her2 CAR into the mice. α-Her2-EE zipFv was subsequently injected every day at 3mg/kg for six days. Tumor growth was monitored by in vivo imaging (IVIS) of the luciferase signal from Jurkat cancer cells in each mouse over the course of 21 days. RR zipCAR with α-Her2-EE zipFv showed robust tumor burden clearance, comparable to the conventional Her2 CAR. However, T cells expressing RR zipCAR alone without zipFvs were not able to reduce tumor burden (Figure 3E). Moreover, long-term survival rate was observed for the group that received both RR zipCAR with α-Her2-EE zipFv (Figure S5A). We further characterized our SUPRA CAR system in this blood tumor model by modulating T cell numbers (from 10×106 per mice to 2.5×106 per mice) and lowering zipFv dose to 1mg/kg (Figures S5B and S5C). At these reduced T cell numbers and zipFv doses, robust reduction of tumor burden was still observed. The observed robust activity of the SUPRA system against different xenograft tumor models demonstrates the potential of the SUPRA CAR system to combat many different cancers.
Characterization of the humanized SUPRA CAR system in vitro and in vivo
To mitigate the potential immunogenicity against synthetic leucine zippers, we created a new zipCAR and zipFv pair using zipper domains derived from human FOS and JUN transcription factors, respectively (Karin et al., 1997; Reinke et al., 2010). Human primary CD8+ T cells were engineered to express a FOS zipCAR. An α-Her2-JUN zipFv was used to activate the FOS zipCAR (Figure S6A). FOS zipCAR and RR zipCAR engineered CD8+ T cells have comparable in vivo killing efficiencies against Jurkat T cells that express Her2 (Figure S6A). In addition, FOS zipCAR can be activated only when α-Her2-JUN zipFv and Her2 expressing tumor cells are present as measured by CD69 expression and IFN-γ secretion (Figures S6B and S6C). FOS zipCAR with α-Her2-JUN zipFv can efficiently eliminate leukemia in vivo as demonstrated in a blood tumor model (Figure S6D). Together, these results illustrate that the SUPRA components can be humanized to reduce potential immunogenicity and they are as effective as the ones derived from synthetic zippers.
Controlling SUPRA CAR activity in vivo
Strategies that enable controlled cytokine production by CAR T cells in vivo are critical to preventing cytokine release syndrome (Maude et al., 2014b; Morgan et al., 2010). As such, we explored in vivo cytokine production by SUPRA CAR T cells in a zipFv dose-dependent manner. We first injected SK-BR-3 breast cancer cells and allowed the tumor to be established (Figure S4C). After verifying tumor establishment, RR-zipCAR expressing CD8+ T cells were injected and mice were dosed with 8, 4, 2, or 0.5 mg/kg of the α-Her2-EE zipFv every other day for 2 weeks (Figure S4C). Again, the tumor burden was monitored by in vivo imaging (IVIS) of the luciferase signal from SK-BR-3 cancer cells in each mouse. Increasing zipFv dose beyond 2mg/kg (e.g., 4mg/kg or 8mg/kg) resulted in faster and more efficient killing of cancer cells. There were no significant differences between groups that received 0.5mg/kg and 2mg/kg or between groups that received 4mg/kg and 8mg/kg (Figure S4D). However, zipFv dosage correlated with cytokine release in vivo in a step-wise fashion, which demonstrates the possibility of using the SUPRA CAR to finely regulate cytokine release in vivo (Figure 4A).
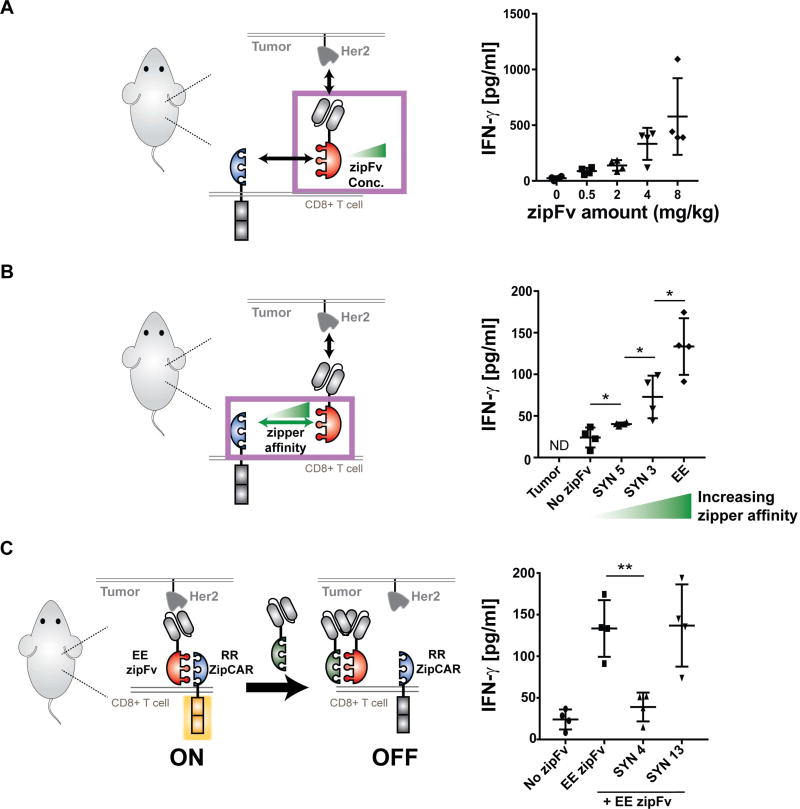
Figure 4. In vivo control of cytokine production by the SUPRA CAR.
(A) In vivo IFN-γ cytokine level at 24 hr. In vivo cytokine level increased in a dose-dependent manner (n=4, mean ± SD).
(B) In vivo IFN-γ cytokine level at 24hr, demonstrating a leucine zipper affinity-dependent increase of in vivo IFN-γ cytokine (n=4, mean ± SD).
(C) In vivo IFN-γ cytokine level demonstrating the effect of competitive zipFv (n=4, mean ± SD, the statistical significance was determined by Student’s t test, * = p ≤0.05, *** = p ≤0.001).
We also examined if in vivo cytokine production could be modulated with different leucine zippers (SYN3, SYN5, EE) or α-Her scFv (G98, ML39, and H3B1) affinity on the zipFv (4mg/kg) (Figures 4B and S4E). Indeed, in vivo IFN-γ release correlated with the affinity between leucine zippers (Figure 4B). The cytokine release also increased as the scFv changed from low (G98, Kd = 3.2 × 10−7) to medium-high affinity (ML39, H3B1, Kd < 1 × 10−8). However, as shown in the in vitro results, zipFvs with medium-high binding domains affinity (ML39, H3B1, Kd < 1 × 10−8) did not increase IFN-γ secretion (Figure S4E).
Lastly, we investigated if the SUPRA CAR could be inhibited in vivo with a competitive zipFv to reduce cytokine production (Figure 4C). As expected, the group that only received α-Her2-EE zipFv (4mg/kg) secreted a high level of IFN-γ. Moreover, when a competitive zipFv (8mg/kg) that can bind to the activating zipFv was added (SYN4), the IFN-γ level reduced significantly, similar to that of the no-zipFv control. However, addition of a control zipFv, which does not bind to the activating EE zipFv, did not decrease cytokine release in vivo (SYN13). We did not observe a significant effect of competitive zipFvs on the anti-tumor cytotoxicity in vivo. However, in vivo cytokine production results demonstrate that the SUPRA CAR platform affords multiple approaches to control cytokine production, providing the tools needed to manage severe cytokine release syndrome and other potential toxicities that arise from conventional CAR T cell therapies.
Controlling different signaling domains using orthogonal SUPRA CARs
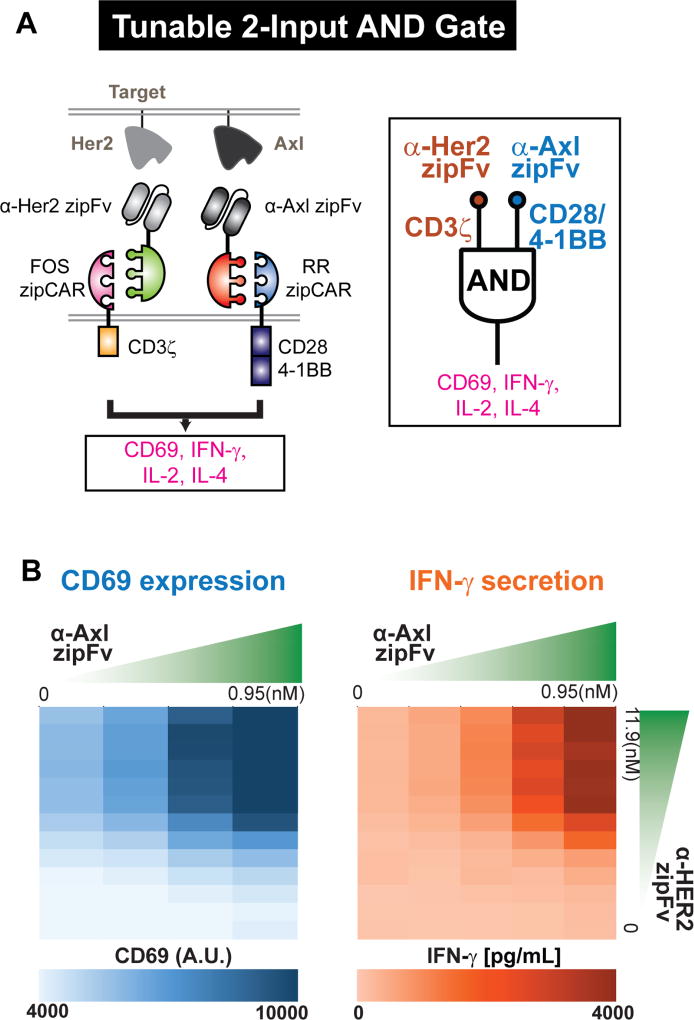
Currently, only a small number of signaling domains are being utilized in CAR T cell therapy to regulate T cell responses. Moreover, we lack independent control of different signaling domains as they are activated simultaneously. However, the repertoire of co-signaling domains – both co-stimulatory and co-inhibitory domains – are highly diverse, and it is the activation of different co-signaling domains that sculpts ultimate T cell function (Chen and Flies, 2013; Greenwald et al., 2005; Zhu et al., 2011). One of the key attributes of the SUPRA CAR design is that multiple orthogonal SUPRA CARs can be designed to control distinct signaling pathways in the same cell, which provides highly customizable tuning of T cell signaling and response. To identify orthogonal SUPRA CARs, Jurkat T cells expressing different zipCARs were co-cultured with Her2+ K562 target cells and different zipFvs. (Figures S7A and S7B). From the screen, we identified several pairs of orthogonal SUPRA CARs (Figure S7C). We then engineered two orthogonal SUPRA CARs to regulate separate signaling pathways in primary CD4+ T cells – FOS zipCAR (binds to α-Her2-SYN9 zipFv) that contains only a CD3ζ domain and RR zipCAR (binds to α-Axl-EE zipFv) that contains CD28 and 4-1BB co-stimulatory domains (Figure 5A). We chose these signaling domains because they have been used previously for demonstrating combinatorial antigen detection (“AND” logic) in vivo (Kloss et al., 2013). To trigger each zipCAR independently, we co-cultured engineered CD4+ T cells and K562 target cells that express Her2 and Axl. We then added two different zipFvs at varying concentrations and measured CD69 expression, which can be triggered by CD28 activation (Vandenberghe et al., 1993). Triggering CD3ζ alone increased CD69 expression. Activating the CD28/4-1BB and CD3ζ domain, however, led to a further increase in CD69 expression (Figures 5B and S7F). To confirm this dual antigen sensing functionality, we also measured different cytokine (IFN-γ, IL-2, and IL-4) levels that are known to be regulated by CD28 and 4-1BB signaling (Howland et al., 2000; Kane et al., 2001; Lucas et al., 1995). For all cytokines tested, we observed similar synergistic AND logic effects of these two receptors (Figures 5B, S7D, S7E, and S7F). Surprisingly, the effect of the CD28 and 4-1BB co-stimulatory signaling was much higher when we increased the α-Her2-SYN9 zipFv concentration (as CD3ζ signaling strength increased) (Figure S7F).
Figure 5. Controlling different signaling domains with orthogonal SUPRA CARs.
(A) Design of orthogonal SUPRA CARs that control either CD3ζ or CD28/4-1BB signaling domains. The RR zipCAR and FOS zipCAR contain CD28/4-1BB co-stimulatory and CD3ζ signaling domain, respectively. α-Her2 zipFv binds to the FOS zipCAR and activates the CD3ζ domain, whereas the α-Axl zipFv binds to the RR zipCAR and activates CD28/4-1BB co-stimulatory domains. CD69 expression, IFN-γ, IL-2, and IL-4 secretion were measured as outputs.
(B) Amount of each zipFv was varied to define signaling strength from each receptor. CD69 expression (left) and IFN-γ secretion (right) were measured (n=3, data are represented as mean).
Controlling different cell types using orthogonal SUPRA CARs
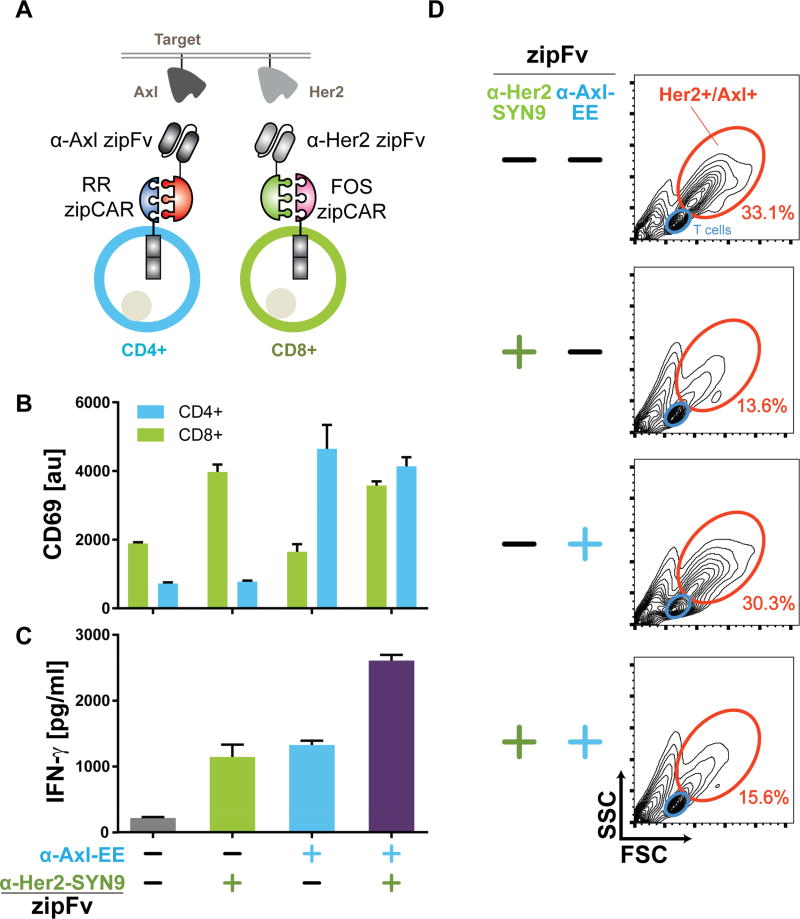
We recognized that orthogonal SUPRA CARs can also be used to control different T cell subtypes, such as CD4+ and CD8+ T cells (Figure 6A). CD4+ T cells are helper T cells that secrete a variety of cytokines and regulate the immune responses such as activation, growth, and memory formation of CD8+ T cells (Luckheeram et al., 2012). CD8+ T cells are cytotoxic T cells that can directly kill cancer cells. As the use of both CD4+ and CD8+ T cells has been shown to enhance the antitumor response of CAR T cells (Turtle et al., 2016), independent control of both cell types to induce different T cell reponses could further improve the effectiveness of CAR T cell therapy. To demonstrate orthogonal control of both T cell types, we introduced an RR zipCAR into CD4+ T cells (binds to α-Axl-EE zipFv) and an orthogonal FOS zipCAR (binds to the α-Her2-SYN9 zipFv) into CD8+ T cells. All zipCARs here contain CD3ζ, CD28, and 4-1BB signaling domains. To trigger each cell type independently, we co-cultured both engineered CD4+ and CD8+ T cells with K562 cells that express Axl and Her2 (Figure 6A). CD69 expression level was upregulated in CD4+ or CD8+ T cells only in response to the addition of α-Axl zipFv or α-Her2 zipFv, respectively. When both zipFvs were added simultaneously, the CD69 level was upregulated for both cell types (Figure 6B). Furthermore, activating both cell types simultaneously achieved IFN-γ secretion levels similar to the sum of cytokine secretion levels from two subsets of T cells activated individually (Figure 6C). Finally, 24-hour co-culture of CD4+ and CD8+ T cells with Axl+/Her2+ K562 cells led to cytotoxicity against tumor cells (as measured by K562 population percentage through flow cytometry) when CD8+ T cells were activated, but minimally for CD4+ T cells (Hombach et al., 2006) (Figure 6D).
Figure 6. Controlling different cell types with orthogonal SUPRA CARs.
(A) Design of orthogonal SUPRA CARs that control either CD4+ or CD8+ human primary T cells. The RR zipCAR and FOS zipCAR control CD4+ and CD8+ T cells activity, respectively. α-Axl zipFv binds to RR zipCAR and activates CD4+ T cells. α-Her2 zipFv binds to the FOS zipCAR and activates CD8+ T cells.
(B) The CD69 expression and (C) IFN-γ measurements showing independent control of CD4+ and CD8+ T cells with orthogonal SUPRA CARs (n=3, mean ± SD).
(D) Forward- and side-scatter FACS plots of the cell mixture after 24 hours co-culture of T cells (both CD4+ and CD8+, blue) with Her2+/Axl+ K562 tumor cells (orange). Tumor cells are killed efficiently when CD8+ cytotoxic T cells were activated by the α-HER2 zipFv (representative of three biological replicates).
DISCUSSION
SUPRA CAR: The Swiss Army knife of CAR
There is a great need for a flexible platform that can control T cell activation with improved precision and tunability to make CAR T cell therapy safer and more effective. Here we have developed a split CAR system with enhanced flexibility, specificity, and controllability. We demonstrated that our SUPRA CAR system can target different antigens without having to re-engineer the T cells. The activity of the SUPRA CAR system can also be flexibly modulated through multiple mechanisms. In addition, the SUPRA CAR can be easily designed for combinatorial logic antigen detection and regulate different signaling pathways in the same cell as well as different cell types independently. Together, the SUPRA CAR represents a feature-rich receptor system for adoptive T cell therapy.
While several split systems have been introduced with biomolecule-labelled antibodies (Kudo et al., 2014; Ma et al., 2016; Rodgers et al., 2016; Tamada et al., 2012; Urbanska et al., 2012) to redirect the specificity of CARs, none of these systems have demonstrated the same level of flexibility and functionality as our SUPRA CAR system. Furthermore, some of the systems require extensive and non-intuitive optimization of the receptor design. The modular design approach of our SUPRA CAR platform, however, allows convenient redirection of target specificity and adjustment to T cell activity. Several structural parameters, such as the location of the scFv binding to the antigen, scFv affinity, and extracellular spacing (Hudecek et al., 2015; Rodgers et al., 2016; Zah et al., 2016) are important to the CAR signaling. Given that the leucine zipper design constrains some of the structure parameters (e.g. the extracellular spacing) of the zipCAR, further investigation would be needed to determine the effect of these parameters on the SUPRA CAR system activity.
We were able to generate zipFvs targeting three different targets without having to individually optimize each zipFv. Moreover, we showed that SUPRA CAR components can be humanized by using leucine zipper domains derived from human transcription factors to reduce potential immunogenicity. Because these endogenous leucine zippers are localized inside the cells, we do not expect crosstalk to occur between humanized SUPRA CAR components and human transcription factors. In addition, although not investigated here, we anticipate that each zipFv will have a short pharmacokinetic half-life in vivo, similar to an scFv (Gould et al., 2005), which could allow increased temporal control, and we expect that its small size will lead to increased tumor penetration (Carter, 2001). However, the short serum half-life could also be problematic as the SUPRA system requires zipFvs to maintain in vivo T cell activity. Nonetheless, SUPRA CAR T cells were able to clear tumor burden in vivo with a zipFv dose comparable to other FDA approved protein drugs. Moreover, several well-developed protein engineering approaches are available (e.g., PEGylation, Fc/Albumin fusion, Glyco-engineering) to increase half-life of scFv, which can be employed to modulate zipFv stability in vivo if necessary (Kimchi-Sarfaty et al., 2013; Kontermann, 2011; Strohl, 2015; Szlachcic et al., 2011).
SUPRA CAR could enhance the safety of T cell therapy
Controlling cytokine secretion is critical to limiting cytokine release syndrome in patients. We were able to titrate cytokine secretion and cytotoxicity with different zipFv doses and configurations, demonstrating the tunabilty of the SUPRA system in vivo. Furthermore, we showed through the use of competitive leucine zippers for OFF switch function, that one can significantly reduce cytokine secretion both in vitro and in vivo. Moreover, this competition-based strategy can improve target specificity by directing the competitive zipFv toward a surface marker for “normal” cells, thus safeguarding them from being targeted by the zipCAR. We anticipate that to efficiently block the accessibility of zipCAR, the expression level of the cancer cell marker and the normal cell marker will need to be comparable.
Toward the synthesis of a prosthetic immune system
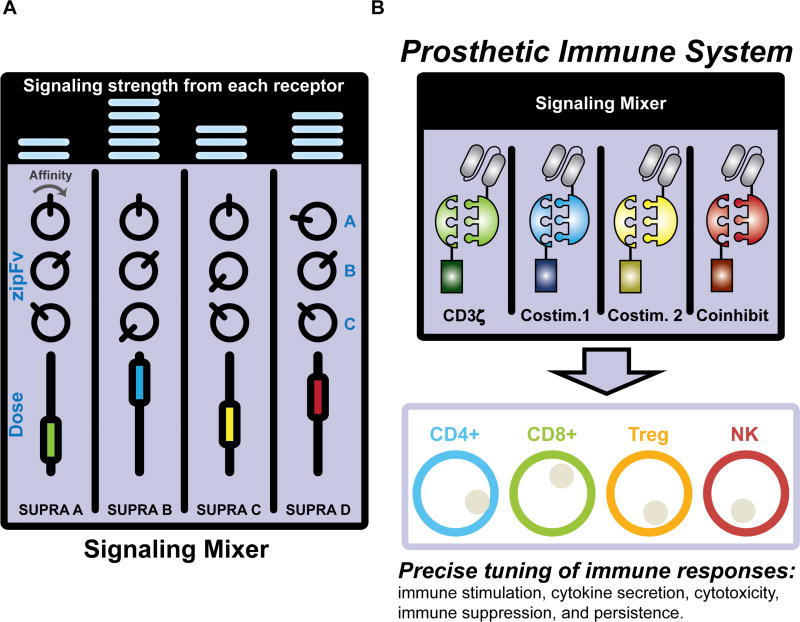
A central feature of the SUPRA CAR system is the availability of orthogonal zippers with varying affinity (Reinke et al., 2010), thus providing a valuable resource for engineering facile and complex control of T cell signaling. Most CARs have been designed as a single receptor controlling all the necessary signaling domains at the same time at a preset (but undefined) level. In contrast, we have shown that multiple orthogonal SUPRA CARs can be utilized to control different signaling domains (e.g., CD3ζ, CD28, 4-1BB), which enable independent and tunable control of different signaling pathways. Moreover, each CAR can be paired with zipFvs that target different tumor antigens or with a different affinity of the leucine zipper, thus allowing regulatable combinatorial antigen sensing, a useful feature that has not been achieved in other CAR or receptor systems (Figure 7A). Furthermore, previous designs for combinatorial antigen sensing using two CARs often require precise control of the expression level of each receptor or careful choice of scFv affinity to achieve an optimal balance of signaling strength (Kloss et al., 2013). While useful and novel, such designs lack the flexibility to combat tumor relapse due to antigen escape, which will require extensive design of a different dual CAR system. However, with the SUPRA system, the zipFv composition can be simply changed and the signaling strength from each receptor can be easily tuned by the amount of zipFv, rather than re-engineering T cells, to meet the challenge of antigen escape.
Figure 7. Engineering a prosthetic immune system with SUPRA CARs.
(A) The SUPRA CAR system enables the flexible and advanced control of signaling in T cells, reminiscent of an audio mixing console for controlling audio signals. The affinity and dosage of each zipFv are like knobs and dials on the mixing console, which can be varied to achieve user-defined T cell activation levels. Different orthogonal pairs of SUPRA CARs are like different channels, which can be utilized to control different signaling pathways in same cells. (B) The SUPRA CAR system can also be implemented in other cell types, thus setting the foundation for creating a prosthetic immune system.
In addition to regulating different signaling pathways, multiple orthogonal SUPRA CAR can also be used to control different subsets of immune cells. Current CAR-based therapy is typically implemented in a cell mixture (e.g., peripheral blood mononuclear cells) or single subsets of immune cell types (e.g., CD4+, CD8+, Treg, or NK cells (Glienke et al., 2015; Klingemann, 2014; MacDonald et al., 2016; Yoon et al., 2017)). Even when two defined T cell subsets (CD4+ and CD8+) were utilized, they were not regulated independently. Given that our immune system is composed of many different cell types with unique effector functions and behaviors in vivo, a platform that enables independent control of different cell types could greatly increase the range of responses that can be achieved by the engineered cells (Figure 7B). As a proof of concept, we engineered two different T cells subtypes (CD4+ and CD8+) and demonstrated that two orthogonal SUPRA receptors can independently regulate two cell types. While it is possible to engineer two cell types with conventional fixed CARs that target different antigens, our SUPRA system enables the first orthogonal inducible control of two cell types simultaneously. We anticipate that the SUPRA system can also be applied to regulatory T cells and NK cells along with CD4+ and CD8+ T cells to form a prosthetic immune system that allows unparalleled control for cell-based immunotherapy (Figure 7B).
STAR★METHODS
Detailed methods are provided in the online version of this paper and include the following:
KEY RESOURCES TABLE
CONTACT FOR REAGENT AND RESOURCE SHARING
- EXPERIMENTAL MODEL AND SUBJECT DETAILS
-
◦Source of Primary Human T cells
-
◦Animal Model Details
-
◦
- METHOD DETAILS
-
◦zipCAR Receptor Construct Design
-
◦zipFv Construct Design
-
◦Expression and Purification of zipFv
-
◦Western Blot and SDS-PAGE Gel Electrophoresis
-
◦Primary Human T cells Isolation and Culture
-
◦Lentiviral Transduction of Human T cells
-
◦Cancer Cell Lines
-
◦Cytokine Release Assays
-
◦Luciferase Cytotoxic T Lymphocyte Assay
-
◦Xenograft Mouse Models
-
◦
QUANTIFICATION AND STATISTICAL ANALYSIS
CONTACT FOR REAGENT AND RESOURCE SHAING
Reagent requests should be directed and will be fulfilled by lead author Wilson Wong (wilwong@bu.edu).
EXPERIEMENTAL MODEL AND SUBJECT DETAILS
Source of Primary Human T Cells
Anonymous healthy donor blood was obtained from the Blood Donor Center at Boston Children’s Hospital (Boston, MA) as approved by the University Institutional Review Board. Primary CD4+ and CD8+ T cells were isolated from anonymous donor blood by negative selection (described in METHOD DETAILS).
Animal Model Details
All experimental procedures were conducted at the Boston University Medical School Animal Science Center (BUASC) under a protocol approved by the Boston University Institutional Animal Care and Use Committee. Female immunocompromised NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) mice, 4-6 week of age, were purchased from Jackson Laboratories (#005557). Mice used in the experiment were healthy and immunocompromised. These animals were not involved in previous procedures and drug or test naïve. These mice were housed in sterile cages at BUASC with 12hr light/dark cycle. Health and immune status of mice were monitor daily by BUASC husbandry staff. Mice were euthanized during experiment if they showed hunched abnormal posture, impaired mobility, rough coat, or paralysis.
METHOD DETAILS
zipCAR Receptor Construct Design
zipCARs were designed by fusing different leucine zippers (Reinke et al., 2010; Thompson et al., 2012) to the hinge region of the human CD8α chain and transmembrane and cytoplasmic regions of the human CD28, 4-1BB, and CD3z signaling endodomains. They were under SFFV promoter for all primary T cell experiments and under CAG promoter for all Jurkat cell experiments. All zipCARs contain myc tag to verify surface expression. Furthermore, zipCARs used in primary T cell experiments were fused to mCherry after CD3z chain to visualize expression. zipCARs used in Jurkat experiments were cloned into the piggyback vector (System Bioscience Inc.), which has been modified by replacing CMV promoter to CAG promoter.
zipFv Construct Design
The general design of zipFv is as follows. scFv (α-HER2, α-Axl or α-MESO) is linked by a 35-aa glycine/serine linker to leucine zipper. Constructs were cloned into pSecTag2A vectors (Thermo Fisher) for transient expression. These vectors contain the CMV promoter, murine Ig-k-chain leader sequence, C-terminal c-myc epitope, and a 6× His tag for purification.
Expression and Purification of zipFv
For transient expression of protein, Freestyle 293-F cells (Thermo Scientific #R79007) were transfected with pSecTag2A plasmid according to the supplier’s protocol. After 4 days of culture, cells were pelleted by centrifugation at 300 × g for 5 minutes, and supernatant protein expression was confirmed by Coomassie gel stain (Thermo Scientific #24592) and western blot (abcam #ab62928). Proteins derived from transient transfection were purified as follows. Supernatant was passed through columns containing ProBond nickel chelating resin (Thermo Scientific #R80101). Then, each column was washed four times with native purification buffer (50 mM NaH2PO4 and 0.5 M NaCl pH 8.0) plus 20 mM imidazole (Sigma Aldrich # I5513), and eluted three times with native purification buffer plus 250mM imidazole concentrations. Eluted proteins were concentrated to ~2ml and dialyzed into 1× PBS (Thermo Scientific #AM9625). After dialysis, protein was verified by western blot and SDS-PAGE gel electrophoresis and protein concentration was quantified by the Pierce BCA Protein Assay Kit (Thermo Scientific # 23227).
Western Blot and SDS-PAGE Gel Electrophoresis
SDS-PAGE gel electrophoresis was perform via standard protocol. Briefly, protein samples were mixed with NuPAGE LDS sample buffer (Thermo Scientific #NP0008) and NuPAGE reducing agent (Thermo Scientific). Samples were heated at 90°C for 20 minutes and run in NuPAGE MOPS SDS running buffer (Thermo Scientific #NP0001). SDS-PAGE gel was stained with GelCode Blue stain (Thermo Scientific #24590) and images were taken using Gel Doc EZ imager (Biorad). Western blot was performed using iBlot2 gel transfer device (Thermo Scientific #NP0009), following the manufacturers protocol. For detection, α-C-Myc-HRP antibody (Abcam #ab62928) was used.
Primary Human T cells Isolation and Culture
Normal whole peripheral blood was obtained from Boston Children’s hospital, as approved by the University Institutional Review Board (IRB) approved consent forms and protocols. Primary human CD4+ and CD8+ T cells were isolated from anonymous healthy donor blood by negative selection (STEMCELL Technologies #15062 and #15063). T cells were cultured in human T cell medium consisting of X-Vivo 15 (Lonza), 5% Human AB serum (Valley Biomedical #HP1022), 10 mM N-acetyl L-Cysteine (Sigma-Aldrich #A9165), 55µM 2-mercaptoethanol (Thermo Scientific #31350010) supplemented with 50 units/mL IL-2 (NCI BRB Preclinical Repository). T cells were cryopreserved in 90% heat-inactivated FBS and 10% DMSO.
Lentiviral Transduction of Human T cells
Replication-incomplete lentivirus was packaged via transfection of HEK 293 FT cells (Invitrogen) with a pHR transgene expression vector and the viral packaging plasmids: pMD2.G encoding for VSV-G pseudotyping coat protein (Addgene #12259), pDelta 8.74 (Addgene#22036), and pAdv (Promega). One day after transfection, viral supernatant was harvested every day for 3 days and replenished with pre-warmed Ultraculture media (Lonza #12-725F) with 2mM L-glutamine, 100U/ml penicillin, 100ug/mL streptomycin, 1mM sodium pyruvate, and 5mM sodium butyrate. Then, harvested virus was purified through ultracentrifugation or Lentivirus concentrator (Takara #631232). Primary T cells were thawed 2 days before ultracentrifugation and cultured in T cell medium described above. One day before ultracentrifugation, T cells were stimulated with Human T-activator CD3/CD28 Dynabeads (Thermo Scientific #11132D) at a 1:3 cell:bead ratio and cultured for 24 hr. After viral supernatant purification, rectronectin (Clontech #T100B) was used to transduce cells. Briefly, non-TC treated 6-well plates were coated with rectronectin following the supplier’s protocol. Then, concentrated viral supernatant was added to each well and spun for 90 min at 1200×g. After centrifugation, viral supernatant was removed and 4ml of human T cells at 250k/ml in T cell growth media supplemented with 100U/ml of IL-2 was added to well. Cells were spun at 1200×g for 60 min and moved to an incubator at 37 °C.
Cancer Cell Lines
The cancer cell lines used were K562 myelogenous leukemia cells (obtained from UCSF), Jurkat T cells (obtained from UCSF), and SK-BR-3 (ATCC #HTB-30). K562 and Jurkat cells were cultured in RPMI-1640(Lonza#12-702Q) with 5% (v/v) heat-inactivated FBS, 2mM L-glutamine, 100U/ml penicillin and 100ug/mL streptomycin. SK-BR-3 cells were cultured in DMEM(Corning #10-013) supplemented with 10% (v/v) heat-inactivated FBS, 2mM L-glutamine, 100U/ml penicillin and 100ug/mL streptomycin. Jurkat, K562, and SK-BR-3 were electroporated or transfected with PiggyBac Transposon system (System biosciences) to stably express zipCAR or surface antigens: Mesothelin, AXL, and/or HER-2. Two days after transfection, antibiotic (Puromycin (Thermo Scientific #A1113803), Zeocin (Thermo Scientific # R25005), or Hygromycin B (Thermo Fisher #10687010)) was added to the medium to select for cells that express the transgenes. Cell lines were routinely verified by their morphology surface antigens expression using microscope and flow cytometry respectively.
Cytokine Release Assays
Cytokine release assays were carried out using IFN-γ or IL-2 ELISA Kit (BD Biosciences #555142, #555190). Primary T cells expressing zipCAR were incubated with K562 target cells (10 × 104 cells/well) at an E:T ratio of 2:1 with corresponding zipFvs (amount of zipFvs were titrated to give maximum response). After 24 hr, supernatant was harvested and followed supplier’s protocol to determine IFN-γ or IL-2 level. In order to determine in vivo cytokine release level, murine blood was drawn submandibularly after 24hr of initial injection of engineered CD8+ T cells and zipFv. Blood plasma was harvested by centrifuging collected blood for 10 minutes at 3000 × g. In vivo IFN-γ release was measured by Luminex Magpix at BUMC (Boston University Medical Campus) core facility.
Luciferase Cytotoxic T Lymphocyte Assay
Cytotoxicity assays were carried out using bioluminescence previously described (Fu et al., 2010). Briefly, CAR-T cells were incubated with zipFv and target cells (K562 cells) that were engineered to express luciferase at varying effector to target ratio (e.g., E:T=8:1, 4:1, 2:1, or 1:1) for 4hr at 37 °C. Initially, target cells were seeded at 75,000 or 100,000 cells per well (96-well plate) and zipFv at varying concentrations were added (amount of zipFvs were titrated to give maximum response). Then, engineered T cells were added (unless otherwise noted, T cells used in the experiment were not sorted based on the SUPRA CAR expression level). After ~4hr incubation, culture medium was removed to leave 50ul per well, then 50ul of prepared luciferase reagent (Promega #E2610) was added to each well of the 96-well plate (Corning #3904). Measurements were performed with the SpectraMax M5 (Molecular Devices). Target cell cytotoxicity was calculated using the following formula: Cytotoxicity = 100 × [(Total Target cell luminescence − luminescence of remaining cells after lysis)/(Total Target cell luminescence)]. For in vitro cytotoxicity assay shown in Figure 2C, single or dual expressing target cells was first added into each well. In both of the cases, both cells were equally treated by adding α-Axl zipFv. After ~20 minutes of incubation to allow α-Axl zipFv to bind to target cells, cells were spun down and washed to remove unbound α-Axl zipFv. Subsequently, α-Her2 zipFv and SUPRA CAR T cells were added to the washed target cells sequentially. After ~4hr incubation, luminescence from each well was measured as described above.
Xenograft Mouse Models
Female NSG mice, 4–6 weeks of age, were purchased from Jackson Laboratories (#005557) and maintained in the BUMC Animal Science Center (ASC). All protocols were approved by the Institutional Animal Care and Use Committee at BUMC. In order to carry out the intraperitoneal xenograft models, NSG mice were initially injected with 7.5 × 106 luciferized SK-BR-3 intraperitoneally. After 2 weeks or after tumor burden reached ~1010 luminescence (photons/sec), 35 × 106-CD8+ CAR-T cells were infused intraperitoneally along with zipFv. zipFvs were added every two days for 2 weeks (total of 8 times) at specified doses. For blood tumor model, NSG mice were initially injected with 5 × 106 luciferized Jurkat tumor cells intravenously. After 3 or 5 days, 2.5 to 10 × 106-CD8+ CAR-T cells were infused intravenously along with zipFv. zipFvs were added every day for 6 or 9 days at specified doses. Tumor burden was measured by IVIS Spectrum (Xenogen) and was quantified as total flux (photons per sec) in the region of interest. Images were acquired within 30 minutes following intraperitoneal injection of 150mg/kg of D-luciferin (PerkinElmer #122799).
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical significance was determined by student’s T test (two tailed) unless otherwise noted. All curve fitting was performed with Prism 7 (Graphpad) and p values are reported (not significant = p > 0.05, * = p ≤ 0.05, ** = p ≤ 0.01, *** ≤ 0.001). All error bars are represented either SEM or SD.
Supplementary Material
(A) Schematic of zipCAR and zipFv construct designs. zipCAR composes of leucine zipper on extracellular domain and intracellular signaling domains which contain two costimulatory signaling domains (CD28 and 4-1BB), and CD3ζ. zipCAR is fused to mCherry for visualization.
(B) (Left) FACS histogram that shows different CAR expression level of CD4+ T cells used in Figure 1E (Right) Quantified fluorescence value. WT is represented as gray, high CAR-expressing cells as red and low CAR-expressing cells as blue (n=1).
(C) Effect of concentration of three zipFvs with leucine zippers (SYN3, SYN5, and EE) that have different affinity to RR zipCAR on cytotoxicity (n=3, data are represented as mean ± SD).
(D) Effect of zipper affinity and scFv affinity on cytotoxicity (n=3, data are represented as mean).
(A) (Left) Schematic of SUPRA CAR (EE-RR pair) and a-Her2 CAR. (Right) Forward- and side-scatter FACS plots of the cell mixture after 24 hours co-culture of T cells (blue) with Her2+ K562 tumor cells (orange) (representative of three biological replicates).
(B, C) The CD69 expression and IFN-γ measurement after 24hr of co-culturing with RR zipCAR/α-Her2 CAR and Her2+ K562 target cells (n=3, data are represented as mean ± SD).
(D, E) Denaturing SDS-PAGE and western blot images of the different zipFvs used in the paper
(F) Table of expected protein mass (Da) of different zipFvs
(A) Leucine zippers with different affinities to EE leucine zipper.
(B) EE zipCAR expressing Jurkat T cells were co-cultured with Her2 expressing K562. Then, different zipFvs (α-Her2-SYN2, α-Her2-SYN4, α-Her2-SYN47, or α-Her2-SYN13) were added. GFP expression was measured after 24 hours to quantify the NFAT promoter activity.
(C) Normalized NFAT promoter activity measured by GFP expression of different zipFvs (n=2, data are represented as mean ± SD).
(D) RR zipCAR expressing CD8+ T cells were co-cultured with Her2 expressing K562. α-Her2-EE zipFv (22.5nM) was added to activate T cells. Then, different amount of competitive zipFv (90nM, 45nM, and 22.5nM) was added at a different time after EE zipFv was added (n=3, data are represented as mean).
(E) Cell selector with zipFv (α-Axl-SYN13) that does not bind strongly to α-Her2-EE zipFv (n=3, data are represented as mean ± SD).
(A) Diagram showing xenograft study described in Figure 3B. Two different zipFvs were used; α-Her2-EE zipFv that binds to RR zipCAR strongly and α-Her2-RR zipFv that does not bind to RR zipCAR strongly.
(B) SK-BR-3 breast cancer cells were injected intraperitoneally at day 0 and at day 26 (black arrow) to immune compromised NSG mice. After verifying tumor establishment, RR zipCAR expressing CD8+ T cells (red arrow) were injected along with α-Her2 EE zipFv or α-Her2 RR zipFv (dosed every 2 days for 2 weeks at 8mg/kg, highlighted). Tumor burden was quantified as total flux (photons/sec) of luciferase activity from each mouse using IVIS imaging (n= 4, data are represented as mean ± SEM, statistical significance was determined by two-tailed student’s t test, * = p ≤0.05).
(C) SK-BR-3 breast cancer cells were injected intraperitoneally at day 0 and at day 26 (black arrow) to NSG mice. Primary human CD8+ T cells expressing the RR zipCAR were injected (red arrow) with the α-Her2-EE zipFv (injected every 2 days for 2 weeks at varying concentrations, highlighted). Tumor burden was quantified as total flux (photons/sec) from luciferase activity of each mouse using IVIS imaging (n=4, data are represented as mean ± SEM).
(D) Tumor burden as total flux (photons per sec) of each mouse shown in Figure S4C at day 57 (n=4, data are represented as mean ± SEM)
(E) In vivo IFN-γ release as scFv affinity changes from low (G98) to medium-high affinity (ML39, H3B1) (n=4, data are represented as mean ± SD, statistical significance was determined by two-tailed student’s t test, *** = p ≤0.001).
(A) Kaplan-Meier survival curves of various groups shown in Figure 3E
(B) Effect of different T cell dose on tumor burden. (Left) The tumor burden was quantified as the total flux (photons/sec) from the luciferase activity of each mouse using IVIS imaging (red arrow indicates injection of T cells (day 5) and zipFv was dosed daily for 9 days at 4mg/kg). (Right) Representative IVIS images of different groups at day 20 (n=4, data are represented as mean ± SEM).
(C) Effect of zipFv dose on tumor burden. (Left) The tumor burden was quantified as the total flux (photons/sec) from the luciferase activity of each mouse using IVIS imaging (red arrow indicates injection of T cells (day 5) and zipFv was dosed daily for 9 days). (Right) Representative IVIS images of different groups at day 20 (n=4, data are represented as mean ± SEM).
(A) Schematic of Humanized SUPRA CAR (FOS-JUN pair) and RR-EE SUPRA CAR. FACS plots of the cell mixture after 24 hours co-culture of SUPRA CAR T cells with Her2+ Jurkat tumor cells. Jurkat tumor cells express blue fluoresce protein (BFP) (representative of three biological replicates).
(B, C) The CD69 expression and IFN-γ measurements after 24hr of co-culturing with FOS/RR zipCAR and Her2+ Jurkat target cells (n=3, data are represented as mean ± SD).
(D) (Left) The tumor burden was quantified as the total flux (photons/sec) from the luciferase activity of each mouse using IVIS imaging (red arrow indicates injection of T cells (day 5) and zipFv was dosed daily for 9 days at 4mg/kg). (Right) Representative IVIS images of groups treated with (1) no T cells, (2) RR zipCAR (10×106 T cells per mouse), (3) FOS zipCAR (10×106 T cells per mouse), (4) RR zipCAR with α-Her2 EE zipFv (4mg/kg), and (5) FOS zipCAR with α-Her2 JUN zipFv (4mg/kg) at day 20 (n=4, data are represented as mean ± SEM).
(A) Jurkat T cells expressing different zipCARs were co-cultured with Her2 expressing K562 target cells and different zipFvs. GFP expression was used to measure the interaction between two leucine zippers.
(B) To identify several pairs of orthogonal SUPRA CARs, we chose 18 zipCARs and 30 zipFvs and quantified NFAT promoter activity (n= 2, data are represented as mean).
(C) Sets of orthogonal SUPRA CARs (n= 2, data are represented as mean).
(D, E) Quantified IL-2 and IL-4 production when dual orthogonal CARs were activated with varying concentration of two zipFvs (n=2 for IL-2 and n=3 for IL-4, data are represented as mean, Related to Figure 5A).
(F) Comparison of quantified CD69 expression, IFN-g, IL-2, and IL-4 production when varying concentrations of two zipFvs were added as shown in Figures 5B, S7D, and S7E.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| APC Mouse Anti-Human CD69 Clone L78 | BD Biosciences | Cat#340560 |
| FITC Mouse Anti-Human CD4 Clone M-T477 | BD Biosciences | Cat#556615 |
| FITC Mouse Anti-Human CD4 Clone RPA-T4 | BD Biosciences | Cat# 555346 |
| Pacific Blue™ Mouse Anti-Human CD8 | BD Biosciences | Cat#558207 |
| Dynabeads™ Human T-Activator CD3/CD28 | Thermo Scientific | Cat#11132D |
| Anti-c-Myc antibody (HRP) | ABCAM | ab62928 |
| Cell Culture Reagents | ||
| FreeStyle™ 293 Expression Medium | Thermo Scientific | Cat#12338026 |
| RPMI-1640 | Lonza | Cat#12-702Q |
| Human AB serum | Valley Medical | Cat#HP1022 |
| 2-Mercaptoethanol (50 mM) | Thermo Scientific | Cat#31350010 |
| Ultraculture | Lonza | Cat# 12-725F |
| DMEM | Corning | Cat# 10-013 |
| X-VIVO 15 | Lonza | Cat# 04-380Q |
| Puromycin | Thermo Scientific | Cat# A1113803 |
| Zeocin | Thermo Scientific | Cat# R25005 |
| Hygromycin B | Thermo Scientific | Cat# 10687010 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| 293fectin™ Transfection Reagent | Thermo Scientific | Cat#12347019 |
| XenoLight D-Luciferin - K+ Salt Bioluminescent Substrate | Perkin Elmer | Cat#122799 |
| N-Acetyl-L-cysteine | Sigma-Aldrich | Cat#A9165 |
| Lenti-X™ Concentrator | Clontech | Cat# 631232 |
| RetroNectin® Recombinant Human Fibronectin Fragment | Clontech | Cat# T100B |
| Imidazole | Sigma-Aldrich | Cat# I5513 |
| ProBond™ Nickel-Chelating Resin | Thermo Scientific | Cat#R80101 |
| Phosphate-Buffered Saline (10×) pH 7.4 | Thermo Scientific | Cat#AM9625 |
| NuPAGE™ LDS Sample Buffer (4×) | Thermo Scientific | Cat#NP0008 |
| NuPAGE™ MOPS SDS Running Buffer (20×) | Thermo Scientific | Cat#NP0001 |
| GelCode Blue Stain Reagent | Thermo Scientific | Cat#24592 |
| NuPAGE™ Sample Reducing Agent (10×) | Thermo Scientific | Cat#NP0009 |
| Lenti-X™ Concentrator | Takara | Cat#631232 |
| Critical Commercial Assays | ||
| RosetteSep™ Human CD4+ T Cell Enrichment Cocktail | STEMCELL Technologies | Cat# 15062 |
| RosetteSep™ Human CD8+ T Cell Enrichment Cocktail | STEMCELL Technologies | Cat# 15063 |
| Human IL-2 ELISA Set | BD Biosciences | Cat# 555190 |
| Human IFN-γ ELISA Set | BD Biosciences | Cat#555142 |
| Bright-Glo™ Luciferase Assay System | Promega | Cat#E2610 |
| Pierce™ BCA Protein Assay Kit | Thermo Scientific | Cat# 23227 |
| XenoLight D-Luciferin - K+ Salt Bioluminescent Substrate | PerkinElmer | Cat#122799 |
| GelCode™ Blue Stain Reagent | Thermo Scientific | Cat#24590 |
| Experimental Models: Cell Lines | ||
| FreeStyle 293-F | Thermo Scientific | #R79007 |
| K562 cells | Art Weiss (UCSF) | N/A |
| K562 cells HER2+Luc+ | This paper | N/A |
| K562 cells HER2+Axl+Luc+ | This paper | N/A |
| Jurkat NFAT-GFP | Art Weiss (UCSF) | N/A |
| SK-BR-3 | ATCC | ATCC® HTB-30™ |
| Experimental Models: Organisms/Strains | ||
| NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (female 4–6 weeks) | Jackson Laboratory | Cat#005557 |
| Recombinant DNA | ||
| pHR-SFFV vector | Addgene | ID#79121 |
| pHR-RR zipCAR-mCherry | This paper | N/A |
| pHR-SYNZIP CARs-mCherry backbone | This paper | N/A |
| CMV-zipFvs-6× HIS backbone | This paper | N/A |
| pHR-anti-Her2 CAR-mCherry | This paper | N/A |
| PB-pCAG-MCS-EF1a-Puro-mCherry vector | This paper | N/A |
| PB-pCAG-MCS-EF1a-zeocine-BFP vector | This paper | N/A |
| PB-pCAG-MCS-EF1a-Hygromycin-GFP vector | This paper | N/A |
| Software and Algorithms | ||
| Graph Pad Prism 7 | Graph Pad | N/A |
| FlowJo V10 | TreeStar | N/A |
| Living Image | Perkin Elmer | N/A |
A split, universal, and programmable (SURPA) CAR system for T cell therapy
SURPA CAR can fine tune T cell activation strength to mitigate toxicity
SUPRA CAR can sense and logically respond to multiple antigens to combat relapse
SURPA CAR can inducibly control cell type-specific signaling
Acknowledgments
J.H.C acknowledges funding from the Kwanjeong Educational Foundation. J.J.C. acknowledges funding from the Paul G. Allen Frontiers Group and the Wyss Institute. W.W. acknowledges funding from the Boston University Ignition Award, NIH Director’s New Innovator Award (1DP2CA186574), NSF Expedition in Computing (1522074), NSF CAREER (162457), and NSF BBSRC (1614642). We thank Teresa Wiese for cloning zipCAR constructs used for Jurkat T cell experiments, Devin R. Burrill for initial guidance on protein purifications, and Jeong Min Park for protein purifications during her summer research at Wong lab. We also thank Wong lab members for suggestions on the manuscript; Dr. Jennifer Cappione, Dr. Anna Belkina, Mr. Brian Tilton, and Dr. Patrick Autissier from the BU flow cytometry core for flow cytometry assistance; Dr. Thomas Balon from the BU animal facility for IVIS imaging assistance; Mr. Matthew Au from BU Analytical Instrumentation Core for helping with Luminex analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
J.H.C. designed and generated genetic constructs, performed experiments, and generated figures. J.H.C., J.J.C. and W.W.W analyzed data. W.W.W conceived the project. J.H.C., J.J.C. and W.W. prepared the manuscript. All authors commented on and approved the paper.
DECLARATION OF INTERESTS
A patent application has been filed based on this work (J.H.C and W.W.W). W.W.W. and J.J.C are scientific co-founders and shareholders of Senti Biosciences.
References
- Bonifant CL, Jackson HJ, Brentjens RJ, Curran KJ. Toxicity and management in CAR T-cell therapy. Mol. Ther. Oncolytics. 2016;3:16011. doi: 10.1038/mto.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brentjens RJ, Rivière I, Park JH, Davila ML, Wang X, Stefanski J, Taylor C, Yeh R, Bartido S, Borquez-Ojeda O, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–4828. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, Bartido S, Stefanski J, Taylor C, Olszewska M, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Transl. Med. 2013;5:177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127:3321–3330. doi: 10.1182/blood-2016-04-703751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartellieri M, Feldmann A, Koristka S, Arndt C, Loff S, Ehninger A, von Bonin M, Bejestani EP, Ehninger G, Bachmann MP. Switching CAR T cells on and off: a novel modular platform for retargeting of T cells to AML blasts. Blood Cancer J. 2016;6:e458. doi: 10.1038/bcj.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P. Improving the efficacy of antibody-based cancer therapies. Nat. Rev. Cancer. 2001;1:118–129. doi: 10.1038/35101072. [DOI] [PubMed] [Google Scholar]
- Caruso HG, Hurton LV, Najjar A, Rushworth D, Ang S, Olivares S, Mi T, Switzer K, Singh H, Huls H, et al. Tuning sensitivity of CAR to EGFR density limits recognition of normal tissue while maintaining potent antitumor activity. Cancer Res. 2015;75:3505–3518. doi: 10.1158/0008-5472.CAN-15-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and coinhibition. Nat. Rev. Immunol. 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmielewski M, Hombach A, Heuser C, Adams GP, Abken H. T cell activation by antibody-like immunoreceptors: increase in affinity of the single-chain fragment domain above threshold does not increase T cell activation against antigen-positive target cells but decreases selectivity. J. Immunol. 2004;173:7647–7653. doi: 10.4049/jimmunol.173.12.7647. [DOI] [PubMed] [Google Scholar]
- Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, Chung SS, Stefanski J, Borquez-Ojeda O, Olszewska M, et al. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med. 2014;6:224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry TJ, Shah NN, Orentas RJ, Stetler-Stevenson M, Yuan CM, Ramakrishna S, Wolters P, Martin S, Delbrook C, Yates B, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat. Med. 2018;24:20–28. doi: 10.1038/nm.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Tao L, Rivera A, Williamson S, Song X, Ahmed N, Zhang X. A simple and sensitive method for measuring tumor-specific T cell cytotoxicity. PLoS One. 2010;5:e11867. doi: 10.1371/journal.pone.0011867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller MA, Knorr DA, Hermanson DA, Pribyl L, Bendzick L, Mccullar V, Miller JS, Kaufman DS. Intraperitoneal delivery of human natural killer cells for treatment of ovarian cancer in a mouse xenograft model. Cytotherapy. 2013;15:1297–1306. doi: 10.1016/j.jcyt.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glienke W, Esser R, Priesner C, Suerth JD, Schambach A, Wels WS, Grez M, Kloess S, Arseniev L, Koehl U. Advantages and applications of CAR-expressing natural killer cells. Front. Pharmacol. 2015;6:1–7. doi: 10.3389/fphar.2015.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould LH, Sui J, Foellmer H, Oliphant T, Wang T, Ledizet M, Murakami A, Noonan K, Lambeth C, Kar K, et al. Protective and Therapeutic Capacity of Human Single-Chain Fv-Fc Fusion Proteins against West Nile Virus. J. Virol. 2005;79:14606–14613. doi: 10.1128/JVI.79.23.14606-14613.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grada Z, Hegde M, Byrd T, Shaffer DR, Ghazi A, Brawley VS, Corder A, Schönfeld K, Koch J, Dotti G, et al. TanCAR: A Novel Bispecific Chimeric Antigen Receptor for Cancer Immunotherapy. Mol. Ther. Nucleic Acids. 2013;2:e105. doi: 10.1038/mtna.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu. Rev. Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- Grupp Sa, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haso W, Lee DW, Shah NN, Stetler-stevenson M, Yuan CM, Pastan IH, Dimitrov DS, Morgan RA, Fitzgerald DJ, Barrett DM, et al. Anti-CD22 – chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia. Blood. 2013;121:1165–1175. doi: 10.1182/blood-2012-06-438002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde M, Corder A, Chow KK, Mukherjee M, Ashoori A, Kew Y, Zhang YJ, Baskin DS, Merchant FA, Brawley VS, et al. Combinational Targeting Offsets Antigen Escape and Enhances Effector Functions of Adoptively Transferred T Cells in Glioblastoma. Mol. Ther. 2013;21:2087–2101. doi: 10.1038/mt.2013.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombach A, Kohler H, Rappl G, Abken H. Human CD4+ T Cells Lyse Target Cells via Granzyme/Perforin upon Circumvention of MHC Class II Restriction by an Antibody-Like Immunoreceptor. J. Immunol. 2006;177:5668–5675. doi: 10.4049/jimmunol.177.8.5668. [DOI] [PubMed] [Google Scholar]
- Howland KC, Ausubel LJ, London CA, Abbas AK. The Roles of CD28 and CD40 Ligand in T Cell Activation and Tolerance. J. Immunol. 2000;164:4465–4470. doi: 10.4049/jimmunol.164.9.4465. [DOI] [PubMed] [Google Scholar]
- Hudecek M, Sommermeyer D, Kosasih PL, Silva-Benedict A, Liu L, Rader C, Jensen MC, Riddell SR. The Nonsignaling Extracellular Spacer Domain of Chimeric Antigen Receptors Is Decisive for In Vivo Antitumor Activity. Cancer Immunol. Res. 2015;3:125–135. doi: 10.1158/2326-6066.CIR-14-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane LP, Andres PG, Howland KC, Abbas AK, Weiss A. Akt provides the CD28 costimulatory signal for up-regulation of IL-2 and IFN-gamma but not TH2 cytokines. Nat. Immunol. 2001;2:37–44. doi: 10.1038/83144. [DOI] [PubMed] [Google Scholar]
- Karin M, Liu Zg, Zandi E. AP-1 function and regulation. Curr. Opin. Cell Biol. 1997;9:240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- Kawalekar OU, O’Connor RS, Fraietta JA, Guo L, McGettigan SE, Posey AD, Patel PR, Guedan S, Scholler J, Keith B, et al. Distinct Signaling of Coreceptors Regulates Specific Metabolism Pathways and Impacts Memory Development in CAR T Cells. Immunity. 2016;44:380–390. doi: 10.1016/j.immuni.2016.01.021. [DOI] [PubMed] [Google Scholar]
- Kimchi-Sarfaty C, Schiller T, Hamasaki-Katagiri N, Khan MA, Yanover C, Sauna ZE. Building better drugs: Developing and regulating engineered therapeutic proteins. Trends Pharmacol. Sci. 2013;34:534–548. doi: 10.1016/j.tips.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Klingemann H. Are natural killer cells superior CAR drivers? Oncoimmunology. 2014;3:e28147. doi: 10.4161/onci.28147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloss CC, Condomines M, Cartellieri M, Bachmann M, Sadelain M. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat. Biotechnol. 2013;31:71–75. doi: 10.1038/nbt.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, Stetler-Stevenson M, Phan GQ, Hughes MS, Sherry RM, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontermann RE. Strategies for extended serum half-life of protein therapeutics. Curr. Opin. Biotechnol. 2011;22:868–876. doi: 10.1016/j.copbio.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Kudo K, Imai C, Lorenzini P, Kamiya T, Kono K, Davidoff AM, Chng WJ, Campana D. T lymphocytes expressing a CD16 signaling receptor exert antibody-dependent cancer cell killing. Cancer Res. 2014;74:93–102. doi: 10.1158/0008-5472.CAN-13-1365. [DOI] [PubMed] [Google Scholar]
- Lanitis E, Poussin M, Klattenhoff AW, Song D, Sandaltzopoulos R, June CH, Powell DJ. Chimeric antigen receptor T Cells with dissociated signaling domains exhibit focused antitumor activity with reduced potential for toxicity in vivo. Cancer Immunol. Res. 2013;1:43–53. doi: 10.1158/2326-6066.CIR-13-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MJ, Cho SS, You JR, Lee Y, Kang BD, Choi JS, Park JW, Suh YL, Kim JA, Kim DK, et al. Intraperitoneal gene delivery mediated by a novel cationic liposome in a peritoneal disseminated ovarian cancer model. Gene Ther. 2002;9:859–866. doi: 10.1038/sj.gt.3301704. [DOI] [PubMed] [Google Scholar]
- Lengyel E, Burdette JE, Kenny HA, Matei D, Pilrose J, Haluska P, Nephew KP, Hales DB, Stack MS. Epithelial ovarian cancer experimental models. Oncogene. 2014;33:3619–3633. doi: 10.1038/onc.2013.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Jiang S, Fang C, Yang S, Olalere D, Pequignot EC, Cogdill AP, Li N, Ramones M, Granda B, et al. Affinity-tuned ErbB2 or EGFR chimeric antigen receptor T cells exhibit an increased therapeutic index against tumors in mice. Cancer Res. 2015;75:3596–3607. doi: 10.1158/0008-5472.CAN-15-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas PJ, Lucas PJ, Negishi I, Negishi I, Nakayama K, Nakayama K, Fields LE, Fields LE, Loh DY, Loh DY. Naive CD28-deficient T cells can initiate but not sustain an in vitro antigen-specific immune response. J. Immunol. 1995;154:5757–5768. [PubMed] [Google Scholar]
- Luckheeram RV, Zhou R, Verma AD, Xia B. CD4+T cells: differentiation and functions. Clin. Dev. Immunol. 2012;2012:925135. doi: 10.1155/2012/925135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JSY, Kim JY, Kazane SA, Choi S-H, Yun HY, Kim MS, Rodgers DT, Pugh HM, Singer O, Sun SB, et al. Versatile strategy for controlling the specificity and activity of engineered T cells. Proc. Natl. Acad. Sci. U. S. A. 2016;113:E450–8. doi: 10.1073/pnas.1524193113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald KG, Hoeppli RE, Huang Q, Gillies J, Luciani DS, Orban PC, Broady R, Levings MK. Alloantigen-specific regulatory T cells generated with a chimeric antigen receptor. J. Clin. Invest. 2016;126:1413–1424. doi: 10.1172/JCI82771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maude SL, Frey N, Shaw Pa, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, et al. Chimeric Antigen Receptor T Cells for Sustained Remissions in Leukemia. N. Engl. J. Med. 2014a;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maude SL, Barrett D, Teachey DT, Grupp SA. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J. 2014b;20:119–122. doi: 10.1097/PPO.0000000000000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan Ra, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg Sa. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perna F, Sadelain M. Myeloid leukemia switch as immune escape from CD19 chimeric antigen receptor (CAR) therapy. Transl. Cancer Res. 2016;5:S221–S225. doi: 10.21037/tcr.2016.08.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke AW, Grant RA, Keating AE. A synthetic coiled-coil interactome provides heterospecific modules for molecular engineering. J. Am. Chem. Soc. 2010;132:6025–6031. doi: 10.1021/ja907617a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers DT, Mazagova M, Hampton EN, Cao Y, Ramadoss NS, Hardy IR, Schulman A, Du J, Wang F, Singer O, et al. Switch-mediated activation and retargeting of CAR-T cells for B-cell malignancies. Proc. Natl. Acad. Sci. U. S. A. 2016;113:E459–68. doi: 10.1073/pnas.1524155113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roybal KT, Rupp LJ, Morsut L, Walker WJ, McNally KA, Park JS, Lim WA. Precision Tumor Recognition by T Cells With Combinatorial Antigen-Sensing Circuits. Cell. 2016;164:770–779. doi: 10.1016/j.cell.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruella M, Barrett DM, Kenderian SS, Shestova O, Hofmann TJ, Perazzelli J, Klichinsky M, Aikawa V, Nazimuddin F, Kozlowski M, et al. Dual CD19 and CD123 targeting prevents antigen-loss relapses after CD19-directed immunotherapies. J. Clin. Invest. 2016;126:3814–3826. doi: 10.1172/JCI87366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadelain M, Rivière I, Riddell S. Therapeutic T cell engineering. Nature. 2017;545:423–431. doi: 10.1038/nature22395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholler J, Brady TL, Binder-Scholl G, Hwang W-TW-T, Plesa G, Hege KM, Vogel AN, Kalos M, Riley JL, Deeks SG, et al. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci. Transl. Med. 2012;4:132ra53. doi: 10.1126/scitranslmed.3003761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat. Rev. Cancer. 2012;12:278–287. doi: 10.1038/nrc3236. [DOI] [PubMed] [Google Scholar]
- Shaw TJ, Senterman MK, Dawson K, Crane CA, Vanderhyden BC. Characterization of intraperitoneal, orthotopic, and metastatic xenograft models of human ovarian cancer. Mol. Ther. 2004;10:1032–1042. doi: 10.1016/j.ymthe.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Skapenko a, Lipsky PE, Kraetsch HG, Kalden JR, Schulze-Koops H. Antigen-independent Th2 cell differentiation by stimulation of CD28: regulation via IL-4 gene expression and mitogen-activated protein kinase activation. J. Immunol. 2001;166:4283–4292. doi: 10.4049/jimmunol.166.7.4283. [DOI] [PubMed] [Google Scholar]
- Di Stasi A, Tey S-K, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C, Straathof K, Liu E, Durett AG, Grilley B, et al. Inducible Apoptosis as a Safety Switch for Adoptive Cell Therapy. N. Engl. J. Med. 2011;365:1673–1683. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohl WR. Fusion Proteins for Half-Life Extension of Biologics as a Strategy to Make Biobetters. BioDrugs. 2015;29:215–239. doi: 10.1007/s40259-015-0133-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szlachcic A, Zakrzewska M, Otlewski J. Longer action means better drug: Tuning up protein therapeutics. Biotechnol. Adv. 2011;29:436–441. doi: 10.1016/j.biotechadv.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Tamada K, Geng D, Sakoda Y, Bansal N, Srivastava R, Li Z, Davila E. Redirecting gene-modified T cells toward various cancer types using tagged antibodies. Clin. Cancer Res. 2012;18:6436–6445. doi: 10.1158/1078-0432.CCR-12-1449. [DOI] [PubMed] [Google Scholar]
- Thompson KE, Bashor CJ, Lim WA, Keating AE. SYNZIP Protein Interaction Toolbox: in Vitro and in Vivo Specifications of Heterospecific Coiled-Coil Interaction Domains. ACS Synth. Biol. 2012;1:118–129. doi: 10.1021/sb200015u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turtle CJ, Hanafi L, Berger C, Gooley TA, Cherian S, Hudecek M, Sommermeyer D, Melville K, Pender B, Budiarto TM, et al. CD19 CAR – T cells of defined CD4 + : CD8 + composition in adult B cell ALL patients. J Clin Invest. 2016;1:1–16. doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanska K, Lanitis E, Poussin M, Lynn RC, Gavin BP, Kelderman S, Yu J, Scholler N, Powell DJ. A universal strategy for adoptive immunotherapy of cancer through use of a novel T-cell antigen receptor. Cancer Res. 2012;72:1844–1852. doi: 10.1158/0008-5472.CAN-11-3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe P, Verwilghen J, Van Vaeck F, Ceuppens JL. Ligation of the CD5 or CD28 molecules on resting human T cells induces expression of the early activation antigen CD69 by a calcium- and tyrosine kinase-dependent mechanism. Immunology. 1993;78:210–217. [PMC free article] [PubMed] [Google Scholar]
- Wu C, Roybal KT, Puchner EM, Onuffer J, Lim WA. Remote control of therapeutic T cells through a small molecule-gated chimeric receptor. Science. 2015;350:aab4077. doi: 10.1126/science.aab4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J, Schmidt A, Zhang A-H, Königs C, Kim YC, Scott DW. FVIII-specific human chimeric antigen receptor T-regulatory cells suppress T- and B-cell responses to FVIII. Blood. 2017;129:238–245. doi: 10.1182/blood-2016-07-727834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zah E, Lin M-Y, Silva-Benedict A, Jensen MC, Chen YY. T cells expressing CD19/CD20 bi-specific chimeric antigen receptors prevent antigen escape by malignant B cells. Cancer Immunol. Res. 2016;4:498–508. doi: 10.1158/2326-6066.CIR-15-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Condomines M, van der Stegen SJC, Perna F, Kloss CC, Gunset G, Plotkin J, Sadelain M. Structural Design of Engineered Costimulation Determines Tumor Rejection Kinetics and Persistence of CAR T Cells. Cancer Cell. 2015;28:415–428. doi: 10.1016/j.ccell.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Zhu G, Luo L, Flies AS, Chen L. CD137 stimulation delivers an antigen-independent growth signal for T lymphocytes with memory phenotype. Blood. 2007;109:4882–4889. doi: 10.1182/blood-2006-10-043463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Yao S, Chen L. Cell Surface Signaling Molecules in the Control of Immune Responses: A Tide Model. Immunity. 2011;34:466–478. doi: 10.1016/j.immuni.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Schematic of zipCAR and zipFv construct designs. zipCAR composes of leucine zipper on extracellular domain and intracellular signaling domains which contain two costimulatory signaling domains (CD28 and 4-1BB), and CD3ζ. zipCAR is fused to mCherry for visualization.
(B) (Left) FACS histogram that shows different CAR expression level of CD4+ T cells used in Figure 1E (Right) Quantified fluorescence value. WT is represented as gray, high CAR-expressing cells as red and low CAR-expressing cells as blue (n=1).
(C) Effect of concentration of three zipFvs with leucine zippers (SYN3, SYN5, and EE) that have different affinity to RR zipCAR on cytotoxicity (n=3, data are represented as mean ± SD).
(D) Effect of zipper affinity and scFv affinity on cytotoxicity (n=3, data are represented as mean).
(A) (Left) Schematic of SUPRA CAR (EE-RR pair) and a-Her2 CAR. (Right) Forward- and side-scatter FACS plots of the cell mixture after 24 hours co-culture of T cells (blue) with Her2+ K562 tumor cells (orange) (representative of three biological replicates).
(B, C) The CD69 expression and IFN-γ measurement after 24hr of co-culturing with RR zipCAR/α-Her2 CAR and Her2+ K562 target cells (n=3, data are represented as mean ± SD).
(D, E) Denaturing SDS-PAGE and western blot images of the different zipFvs used in the paper
(F) Table of expected protein mass (Da) of different zipFvs
(A) Leucine zippers with different affinities to EE leucine zipper.
(B) EE zipCAR expressing Jurkat T cells were co-cultured with Her2 expressing K562. Then, different zipFvs (α-Her2-SYN2, α-Her2-SYN4, α-Her2-SYN47, or α-Her2-SYN13) were added. GFP expression was measured after 24 hours to quantify the NFAT promoter activity.
(C) Normalized NFAT promoter activity measured by GFP expression of different zipFvs (n=2, data are represented as mean ± SD).
(D) RR zipCAR expressing CD8+ T cells were co-cultured with Her2 expressing K562. α-Her2-EE zipFv (22.5nM) was added to activate T cells. Then, different amount of competitive zipFv (90nM, 45nM, and 22.5nM) was added at a different time after EE zipFv was added (n=3, data are represented as mean).
(E) Cell selector with zipFv (α-Axl-SYN13) that does not bind strongly to α-Her2-EE zipFv (n=3, data are represented as mean ± SD).
(A) Diagram showing xenograft study described in Figure 3B. Two different zipFvs were used; α-Her2-EE zipFv that binds to RR zipCAR strongly and α-Her2-RR zipFv that does not bind to RR zipCAR strongly.
(B) SK-BR-3 breast cancer cells were injected intraperitoneally at day 0 and at day 26 (black arrow) to immune compromised NSG mice. After verifying tumor establishment, RR zipCAR expressing CD8+ T cells (red arrow) were injected along with α-Her2 EE zipFv or α-Her2 RR zipFv (dosed every 2 days for 2 weeks at 8mg/kg, highlighted). Tumor burden was quantified as total flux (photons/sec) of luciferase activity from each mouse using IVIS imaging (n= 4, data are represented as mean ± SEM, statistical significance was determined by two-tailed student’s t test, * = p ≤0.05).
(C) SK-BR-3 breast cancer cells were injected intraperitoneally at day 0 and at day 26 (black arrow) to NSG mice. Primary human CD8+ T cells expressing the RR zipCAR were injected (red arrow) with the α-Her2-EE zipFv (injected every 2 days for 2 weeks at varying concentrations, highlighted). Tumor burden was quantified as total flux (photons/sec) from luciferase activity of each mouse using IVIS imaging (n=4, data are represented as mean ± SEM).
(D) Tumor burden as total flux (photons per sec) of each mouse shown in Figure S4C at day 57 (n=4, data are represented as mean ± SEM)
(E) In vivo IFN-γ release as scFv affinity changes from low (G98) to medium-high affinity (ML39, H3B1) (n=4, data are represented as mean ± SD, statistical significance was determined by two-tailed student’s t test, *** = p ≤0.001).
(A) Kaplan-Meier survival curves of various groups shown in Figure 3E
(B) Effect of different T cell dose on tumor burden. (Left) The tumor burden was quantified as the total flux (photons/sec) from the luciferase activity of each mouse using IVIS imaging (red arrow indicates injection of T cells (day 5) and zipFv was dosed daily for 9 days at 4mg/kg). (Right) Representative IVIS images of different groups at day 20 (n=4, data are represented as mean ± SEM).
(C) Effect of zipFv dose on tumor burden. (Left) The tumor burden was quantified as the total flux (photons/sec) from the luciferase activity of each mouse using IVIS imaging (red arrow indicates injection of T cells (day 5) and zipFv was dosed daily for 9 days). (Right) Representative IVIS images of different groups at day 20 (n=4, data are represented as mean ± SEM).
(A) Schematic of Humanized SUPRA CAR (FOS-JUN pair) and RR-EE SUPRA CAR. FACS plots of the cell mixture after 24 hours co-culture of SUPRA CAR T cells with Her2+ Jurkat tumor cells. Jurkat tumor cells express blue fluoresce protein (BFP) (representative of three biological replicates).
(B, C) The CD69 expression and IFN-γ measurements after 24hr of co-culturing with FOS/RR zipCAR and Her2+ Jurkat target cells (n=3, data are represented as mean ± SD).
(D) (Left) The tumor burden was quantified as the total flux (photons/sec) from the luciferase activity of each mouse using IVIS imaging (red arrow indicates injection of T cells (day 5) and zipFv was dosed daily for 9 days at 4mg/kg). (Right) Representative IVIS images of groups treated with (1) no T cells, (2) RR zipCAR (10×106 T cells per mouse), (3) FOS zipCAR (10×106 T cells per mouse), (4) RR zipCAR with α-Her2 EE zipFv (4mg/kg), and (5) FOS zipCAR with α-Her2 JUN zipFv (4mg/kg) at day 20 (n=4, data are represented as mean ± SEM).
(A) Jurkat T cells expressing different zipCARs were co-cultured with Her2 expressing K562 target cells and different zipFvs. GFP expression was used to measure the interaction between two leucine zippers.
(B) To identify several pairs of orthogonal SUPRA CARs, we chose 18 zipCARs and 30 zipFvs and quantified NFAT promoter activity (n= 2, data are represented as mean).
(C) Sets of orthogonal SUPRA CARs (n= 2, data are represented as mean).
(D, E) Quantified IL-2 and IL-4 production when dual orthogonal CARs were activated with varying concentration of two zipFvs (n=2 for IL-2 and n=3 for IL-4, data are represented as mean, Related to Figure 5A).
(F) Comparison of quantified CD69 expression, IFN-g, IL-2, and IL-4 production when varying concentrations of two zipFvs were added as shown in Figures 5B, S7D, and S7E.