Abstract
Objectives
Evaluate whether insulin resistance (IR) predicts the risk of oral inflammation, assessed as the number of sites with bleeding on probing (BOP) and number of teeth with probing pocket depths (PPD) ≥ 4 mm and BOP.
Methods
Data on 870 overweight/obese diabetes free adults, aged 40-65 years from the San Juan Overweight Adults Longitudinal Study over a three-year period, was analyzed. Baseline IR, assessed using the Homeostasis Model Assessment of IR (HOMA-IR) index, was divided into tertiles. BOP was assessed at buccal and lingual sites, and PPD at six sites per tooth. Negative binomial regression was used to estimate the risk ratios (RRs) for oral inflammation adjusted for baseline age, gender, smoking status, alcohol intake, education, physical activity, waist circumference, mean plaque index, and baseline number of sites with BOP, or number of teeth with PPD≥4 mm and BOP. The potential impact of tertiles of serum TNF-α and adiponectin on the IR-oral inflammation association was also assessed in a subsample of 597 participants.
Results
Participants in the highest HOMA-IR tertile at baseline, had significantly higher numbers of sites with BOP [RR=1.19, 95% confidence interval (CI): 1.03-1.36] and number of teeth with PPD≥4 mm and BOP (RR=1.39, 95% CI: 1.09 - 1.78) at follow-up, compared with individuals in the lower two HOMA-IR tertiles. Neither TNF-α nor adiponectin confounded the associations.
Conclusion
IR significantly predicts gingival/periodontal inflammation in this population.
Keywords: insulin resistance, obesity, type 2 diabetes, inflammation, gingivitis, periodontitis, risk ratio, prospective studies
INTRODUCTION
The number of individuals with diabetes worldwide has quadrupled from 108 million in 1980 to 422 million in 2014,1 which is largely due to the increase in overweight and obesity. In 2014, over 1.9 billion adults were overweight and over 600 million were obese.2 Obesity, especially abdominal obesity, has been associated with insulin resistance (IR), a precursor of type 2 diabetes,3 and with periodontitis.4
Periodontitis is thought to be the sixth complication of diabetes.5 Periodontitis is a polymicrobial chronic oral inflammatory disorder resulting in the destruction of the tissues supporting the teeth.6 Nearly 8-10% of the dentate US adult population aged 30-90 years has severe periodontitis.7 Chronic periodontitis is clinically characterized by the existence of gingival erythema and edema, bleeding on probing (BOP), periodontal pockets, and destruction of the tissue supporting the teeth.8 The tissue damage due to periodontitis seems to follow an intermittent time course, and may happen in sporadic “bursts”.9, 10 The disease may remain stable for a while, but thereafter develops briskly, usually at the level of the individual tooth site.11 The tissue destruction may be low (despite poor oral hygiene status) in some individuals, but it can also be high and generalized leading to tooth loss in susceptible individuals such as those with diabetes.12 Diabetes can cause oral vascular complications leading to periodontitis.13 Several systematic reviews and meta-analyses suggest that obesity is a risk factor for periodontitis.14–17 However, the biological mechanisms have not yet been fully understood since research in this area has been limited. IR may contribute to the core metabolic abnormalities in obesity or diabetes. A few reports in the literature, mostly from cross-sectional studies and/or small sample sizes, have indicated an association between IR and periodontitis,18–21 but it is important to understand the biological mechanisms by which IR may affect the progression of periodontal tissue destruction. We assessed the longitudinal associations between IR and oral inflammation as determined by the number of tooth sites with BOP and the number of teeth with probing pocket depth (PPD) ≥ 4 mm and BOP. We used the number of sites with BOP since this indicates the presence of both reversible plaque-induced gingivitis and irreversible periodontitis whereas the number of teeth with both deep PPD≥4mm and BOP reflects more specifically the number of teeth with possible periodontitis only.22–24
MATERIALS AND METHODS
Study population
The San Juan Overweight Adults Longitudinal Study (SOALS)25–27 recruited overweight [body mass index (BMI) between 25.0 kg/m2 and 29.9 kg/m2] or obese (BMI ≥ 30 kg/m2 28) Puerto Rican adults aged 40 to 65 years, free of self-reported or diagnosed diabetes, and followed them from baseline which started on April 1st 201, for around three years, with the completion of the last follow-up visit on May 25, 2016. The main goals of SOALS were to assess the bi-directional association between the preclinical phase of diabetes and periodontitis. Baseline exclusion criteria of SOALS included self-reported or physician-diagnosis of type 1 or type 2 diabetes and history of insulin use or oral anti-diabetic agents. Other exclusion criteria included: 1) less than four natural teeth; 2) braces or orthodontic appliances which might impair periodontal measurement; and 3) health conditions which could increase the risk of systemic complications from the periodontal examination, including congenital heart murmurs, heart valve disease, congenital heart disease, endocarditis, stroke, rheumatic fever, and bleeding disorders. Participants were also excluded if they had a provisional diabetes diagnosis defined as either a fasting serum glucose ≥ 126 mg/L, a two-hour oral glucose tolerance ≥ 200 mg/L, or a glycated hemoglobin ≥ 6.5% at baseline visit. Further detailed exclusion criteria of the SOALS study are published elsewhere.25–27
A total of 1,451 participants came to the baseline visit, of whom 245 were excluded (see Figure 1). Of the excluded individuals, 100 were either ineligible, unable or unwilling to complete the study procedures, and 145 had a provisional diabetes diagnosis. The remaining 1,206 eligible participants underwent both the glucose testing and periodontal measurements. A total of 255 participants were lost to follow-up (68 refused to continue the study, 140 were lost or did not complete follow-up, 6 died of conditions unrelated to diabetes, and 41 moved out of Puerto Rico). Of the remaining 951 participants, 62 had incomplete outcome measures and 19 had incomplete information on the other key variables, resulting in a sample of 870 (72%) participants. A subsample of 597 participants had both baseline and follow-up measures of serum Tumor Necrosis Factor (TNF)-α and adiponectin levels. The University of Puerto Rico Institutional Review Board approved the study, and all participants signed the informed consent prior to study procedures.
Figure 1.
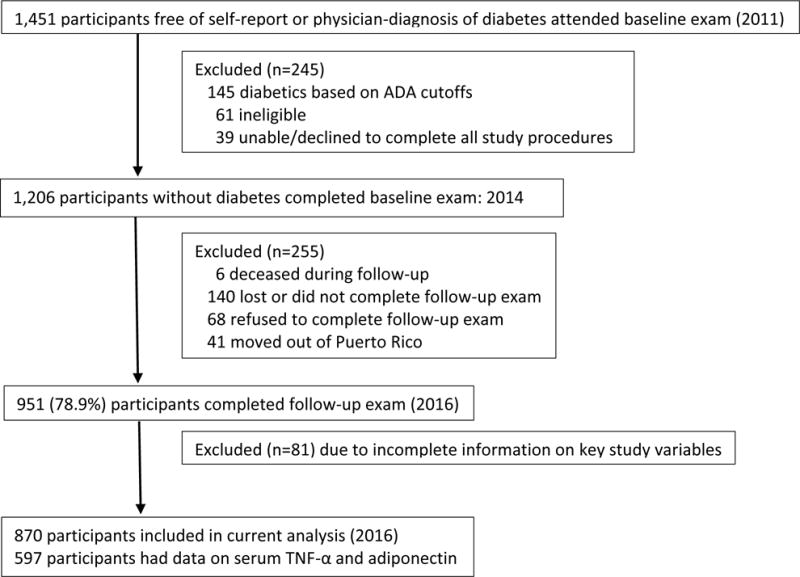
Flow Diagram of the San Juan Adults Longitudinal Study, 2011-2016
Main exposure: IR
Morning fasting blood samples were drawn using a standard protocol and silicone coated sterile vacutainer blood collection tubes.‖ Serum glucose levels in a minimum volume of 200 μL were measured using an enzymatic colorimetric assay.¶ The coefficient of variation (CV) within the laboratory was 1.7%. An aliquot of approximately 2 mL was sent to a reference laboratory within four hours to measure serum insulin level using an Immunoenzymometric assay.# The intra- and inter-assay CVs for the measurement of insulin were 1.49% and 4.42%, respectively.29 Baseline IR was computed as fasting insulin μU/L × (fasting glucose mmol/L)/22.530 and categorized into tertiles.31
Outcome measurements
The oral examination included measures of PPD and gingival BOP. The dental examination was performed by one of three trained and calibrated dental examiners. We used a modified version of the Oral Health Component of National Health and Nutrition Examination Survey (NHANES) protocol32 as we assessed six sites per tooth for all teeth except the third molars, using an identical protocol at the baseline and follow-up visits. The periodontal probe was gently inserted to the base of the sulcus or pocket. PPD was measured in millimeters, with the number rounded down if the reading fell between two probe markings.32 We considered BOP to be present at the buccal or lingual site of each tooth if the probed site bled within approximately 20 seconds after probing.24 Two case definitions for gingival/periodontal outcomes were used: number of sites with BOP and number of teeth with PPD≥4 mm and BOP.
Before the conduct of the study, the dental examiners and recorders underwent training on dental examination and recording. Three dental examiners were trained and calibrated by the NHANES reference examiner, Dr. Bruce Dye. Results from the calibration sessions between the dental examiners and the gold standard showed a 96% agreement within 1 mm of attachment loss. The intra- and inter- examiner variability measures for BOP were not available in the study as it is generally difficult to measure its reproducibility.33–35 Findings from the study by Muller and colleagues indicated the involvement of the time factor in the inaccuracy of the repeated measurement of bleeding tendency.35
Covariates
Face-to-face interviews were conducted to obtain information on socio-demographic characteristics, including age, gender, years of education), health behaviors, smoking status (never, former, and current), alcohol consumption (abstainer, former drinker, and current drinker), and physical activity (compliant with the World Health Organization recommendation of engaging in 75 minutes of vigorous or 150 minutes of moderate physical activity per week, not compliant with recommendation).36
We also collected medical history of chronic health conditions, such as dyslipidemia, coronary artery disease, angina, diabetes, and hypertension as well as reports of medications used for these conditions, including lipid lowering agents, anti-hypertensive drugs, and anti-inflammatory medications. Blood pressure was measured three times within 1-2 minute intervals (rounded up to the nearest 2 mm Hg). Waist circumference (WC), assessed as an average of two repeated measures, was classified as high if the WC was ≥88 cm in females and ≥102 cm in males.37 Weight and height were measured twice to the nearest 0.5 kg and 0.1 cm, respectively, and BMI was computed as weight in kilograms divided by height in meters squared (kg/m2). Triglycerides and high density lipoprotein cholesterol (HDL-C) levels were determined from the baseline blood samples using commercially available methods. Low density lipoprotein cholesterol (LDL-C) was estimated using the Friedewald equation. The level of TNF-α was measured using multiplex assays.** The inter- and intra-assay CVs for TNF-α were 5.73% and 5.77%, respectively, and the minimum sensitivity was 1.30pg/ml. Adiponectin levels were measured using radioimmunoassay.†† The inter- and intra-assay CVs were 9.07% and 6.04%, respectively, and the minimum sensitivity was 0.50μg/ml.
Oral hygiene status was evaluated by the Silness and Loe Plaque Index38 through visual assessment of the presence of bacterial plaque after passing a periodontal probe around the tooth surface of six pre-selected Ramfjord teeth.39 Each of the buccal, lingual, mesial, and distal surfaces of these six teeth was scored from 0 to 3 according to dental plaque abundance: 0: no visible plaque; 1: presence of a film of plaque adhering to the free gingival margin and adjacent area of the tooth; 2: presence of moderate accumulation of visible plaque; and 3: presence of abundant plaque. Moreover, number of missing teeth was recorded during the dental examination, and was used as a covariate.
Statistical analysis
Baseline socio-demographic characteristics and outcome variables were described across HOMA-IR tertiles. To account for over-dispersion in the distribution of the outcomes, separate negative binominal regression models40 were used to assess the associations between the baseline HOMA-IR level and each of the outcomes (sites with BOP; teeth with PPD≥4 mm, and BOP) measured at the three-year follow-up examination. Thus, the risk ratio (RR) was used instead of the incidence rate ratio (IRR) to assess the risk of having gingival/periodontal inflammation at the follow-up examination. Risk ratios (RRs) were estimated with 95% confidence intervals (CI). Potential confounding factors were entered one at a time into each model and were retained in the model if they changed the effect estimates for the exposure by at least 10% or if they were predictive of the outcome based on the literature. Potential confounding factors reported in the literature included baseline measures of age, gender, years of education, smoking status, alcohol consumption, physical activity, missing teeth, BMI, lipid profile (i.e., triglycerides, HDL-C, and LDL-C,), BMI or WC, blood pressure, and PI. Self-reported use of lipid lowering agents or anti-inflammatory medications was also considered as potential confounders. The models were additionally adjusted for the baseline number of sites with BOP and the baseline number of teeth with PPD≥4 mm and BOP.41 We also evaluated whether each of the baseline biomarkers changed the estimate of the association between IR and periodontal inflammation by adding each biomarker separately in the full model (n= 597).
Since the outcomes are continuous and there are no optimal cutoffs to define the presence or absence of these outcomes at baseline, we performed a secondary sensitivity analysis, which assessed whether the associations between HOMA-IR and oral inflammation remained significant after excluding participants with elevated oral inflammation (e.g., in the upper quartile of sites with BOP or teeth with PPD≥4 mm and BOP at the baseline visit). We also conducted analysis using the log transformation of HOMA-IR since its distribution was skewed. All statistical analyses were carried out using a standard statistical software.‡‡
RESULTS
Table 1 summarizes the study population characteristics across baseline HOMA-IR tertiles. Participants in the highest HOMA-IR tertile (n=290) were more likely to be males, more educated, and had higher BMI or WC, fasting glucose or glycated hemoglobin (HbA1c), triglycerides, TNF-α, and blood pressure, and lower HDL-C and adiponectin levels than those in the lower two tertiles. Participants in the upper two HOMA-IR tertiles had higher fasting glucose, triglycerides, and LDL-C levels; were more likely to report taking lipid lowering agents or anti-inflammatory drugs and do less physical activity; and were less current drinkers than those in the lowest HOMA-IR tertile.
Table 1.
Baseline characteristics by tertiles of HOMA-IR index of Study participants (N= 870)
| CHARACTERISTICS | HOMA-IR
|
||
|---|---|---|---|
| 1st tertile (n= 290) | 2nd tertile (n=290) | 3rd tertile (n=290) | |
| Mean ± SD or N (%) | Mean ± SD or N (%) | Mean ± SD or N (%) | |
|
| |||
| Age (years) | 50.3 ± 6.8 | 50.7 ± 6.9 | 50.4 ± 6.6 |
| Male (%) | 71 (24.5) | 68 (23.5) | 93 (32.1) |
| Smoking (%) | |||
| Never | 172 (59.3) | 194 (66.9) | 193 (66.6) |
| Former | 54 (18.6) | 45 (15.5) | 52 (17.9) |
| Current | 64 (22.1) | 51 (17.6) | 54 (15.5) |
| Alcohol consumption (%) | |||
| Abstainer | 116 (40.0) | 132 (45.5) | 129 (44.5) |
| Former drinker | 36 (12.4) | 31 (10.7) | 33 (11.4) |
| Current drinker | 138 (47.6) | 127 (43.8) | 128 (44.1) |
| Educational level (%) | |||
| Less than high school | 30 (10.3) | 34 (11.7) | 23 (7.9) |
| High school graduate | 127 (43.8) | 132 (45.5) | 117 (40.3) |
| More than high school | 133 (45.9) | 124 (42.8) | 150 (51.7) |
| Physical activity (%)* | 176 (60.7) | 158 (54.5) | 152 (52.4) |
| High waist circumference (%) † | 72 (75.2) | 36 (87.6) | 16 (94.5) |
| Body mass index (BMI) | 30.6 ± 4.7 | 32.7 ± 5.5 | 36.4 ± 6.8 |
| Blood pressure (plus treatment) ‡ | |||
| Normal | 91 (31.4) | 74 (25.5) | 32 (11.0) |
| Pre-hypertension | 79 (27.2) | 91 (31.4) | 96 (33.1) |
| Hypertension | 120 (41.4) | 125 (43.1) | 162 (55.9) |
| Fasting serum glucose (mg/dL) | 87.6 ± 7.4 | 93.0 ± 7.8 | 96.7 ± 8.7 |
| HbA1c (%) | 5.6 (0.3) | 5.7 (0.3) | 5.8 (0.3) |
| LDL-C (mg/dL) | 120.9 ± 31.0 | 124.1 ± 31.8 | 124.3 ± 35.4 |
| Triglycerides (mg/dL) | 120.0 ± 59.3 | 147.0 ± 71.4 | 169.5 ± 95.1 |
| HDL-C (mg/dL) | 52.7 ± 13.1 | 47.4 ± 11.4 | 44.1 ± 11.2 |
| Lipid lowering agents use§ | 26 (9.2) | 39 (13.7) | 46 (16.1) |
| Anti-inflammatory drugs use§ | 23 (7.9) | 24 (8.3) | 31 (10.7) |
| Plaque index | 0.8 ± 0.6 | 0.8 ± 0.6 | 0.8 ± 0.6 |
| TNF-α (top tertile %)‖ | 48 (25.3) | 66 (33.2) | 84 (40.4) |
| Adiponectin (top tertile %)‖ | 90 (47.4) | 63 (31.7) | 46 (22.1) |
Compliance with WHO guidelines on physical activity: Engaging in 75 minutes of vigorous or 150 minutes of moderate physical activity per week;
High WC: females with WC ≥ 88 cm, males with WC ≥102 cm;
Blood pressure defined by actual measures, self-report of diagnosis of hypertension, or self-report of current use of anti-hypertensive medication
Self-report of current use of the medication (yes, no, missing value)
Measures of TNF-α and adiponectin were based on a total of 597 participants (HOMA-IR 1st tertile n= 190; 2nd tertile n= 199, and 3rd tertile n= 208)
At both baseline and follow-up visits (Table 2), participants in the highest HOMA-IR tertile had a higher number of sites with BOP (median: 11.5 vs. 7 for 1st tertile or 8 for 2nd tertile at baseline; 8 vs. 6 for 1st tertile or 7 for 2nd tertile at follow-up) and number of teeth with PPD ≥ 4 mm and BOP (2 vs. 1 for both 1st and 2nd tertiles at baseline; 1 vs. 0 for both 1st and 2nd tertiles at follow-up). These numbers were mostly lower at follow-up visit in each HOMA-IR category. For instance, the median number of sites with BOP in the 3rd HOMA-IR tertile was 11.5 at baseline, and it was 8 at follow-up visit. Similarly, the median number of teeth with PPD≥4 mm and BOP was 2 at baseline and 1 at follow-up in the high 3rd HOMA-IR tertile).
Table 2.
Baseline and follow-up distribution of number of sites with BOP, and number of teeth with PPD ≥ 4 mm and BOP by baseline HOMA-IR tertiles (N=870)
| HOMA-IR | ||||
|---|---|---|---|---|
|
| ||||
| OUTCOME | TIME POINTS | 1st tertile (n=290) | 2nd tertile (n=290) | 3rd tertile (n=290) |
| Sites with BOP | Baseline | 11.9±11.9 [8 (3, 17)]* | 11.9±12.3 [7 (3, 16)] | 13.9 ± 11.8 [11.5 (4, 22)] |
| Follow-up | 10.6±11.6 [6 [2, 16)] | 9.6±9.9 [7 (2, 13)] | 11.8 ± 11.4 [8 (3,18)] | |
| Teeth with PD ≥ 4 mm and BOP | Baseline | 3.4±5.3 [1 (0, 5)] | 3.0±5.0 [1 (0, 4)] | 4.0 ± 5.6 [2 (0, 5)] |
| Follow-up | 3.0±5.2 [0 (0, 4)] | 2.4±4.4 [0 (0, 3)] | 3.3 ± 4.9 [1 (0, 4)] | |
Mean ± SD [Median (25th, 75th percentiles)]
Since the distribution of the outcome variables in the 1st and 2nd HOMA-IR tertiles was quite comparable, we grouped these two tertiles into one group and redefined HOMA-IR as low (i.e. 1st and 2nd tertiles combined) vs. high (3rd tertile). The crude RRs for the associations of high HOMA-IR with the number of sites with BOP and number of teeth with PPD≥4 mm and BOP were 1.17 (95% CI: 1.00–1.36, p=0.05) and 1.21 (95% CI: 0.93-1.57, p=0.16), respectively (Table 3). After adjusting for age, gender, smoking status, educational level, alcohol consumption, physical activity, WC, mean plaque index, and baseline number of sites with BOP (or baseline number of teeth with PPD≥4 mm and BOP for the model with the outcome number of teeth with PPD ≥ 4 mm and BOP), individuals with high HOMA-IR had a significant increased number of sites with BOP (RR=1.19, 95% CI: 1.03-1.36, p=0.02) and a significant increased number of teeth with PPD≥4 mm and BOP (RR=1.39, 95% CI: 1.09-1.78, p=0.01). Given that our study population consisted of overweight/obese Hispanic adults, the estimates were further adjusted for WC (Table 3). To rule out the potential confounding effect of glucose level on the associations, the models were further adjusted for the baseline level of HbA1c. However, the estimates did not change (number of sites with BOP: RR=1.19, 95% CI: 1.03-1.36, p=0.02; number of teeth with PPD≥4 mm and BOP: RR=1.39, 95% CI: 1.09–1.78, p=0.01).
Table 3.
Risk ratio (RR) of the associations between baseline HOMA-IR and increased number of sites with BOP or number of teeth with PPD ≥ 4mm and BOP at follow-up, N= 870
| Models (HOMA-IR 3rd vs. 1, 2 tertiles) | ||||
|---|---|---|---|---|
| Outcomes | Unadjusted | Multivariable Analysis* | ||
| IRR (95%CI) | P value | IRR (95%CI) | P value | |
| Sites with BOP† | 1.17 (1.00 – 1.36) | 0.05 | 1.19 (1.03 – 1.36) | 0.02 |
| Teeth with PPD≥ 4 mm and BOP‡ | 1.21 (0.93 – 1.57) | 0.16 | 1.39 (1.09 – 1.78) | 0.01 |
Adjusted for baseline age (years); male gender; smoking status (never, former, current); alcohol consumption (abstainer, former, current); educational level (< high school, high school, some college, college degree or higher); physical activity; WC; mean plaque index.
Additionally adjusted for baseline number of sites with BOP or
Additionally adjusted for baseline number of teeth with PPD ≥ 4mm and BOP.
Addition of biomarkers into the full models slightly changed the estimates of the association between HOMA-IR and number of teeth with PPD ≥ 4 mm and BOP (Table 4). However, the estimates remained significant (adding TNF-α: RR=1.46, 95% CI: 1.09-1.95, p=0.01; adding adiponectin: RR=1.44, 95% CI: 1.07-1.94, p=0.02).
Table 4.
Risk ratio (RR) of the associations between HOMA-IR and number of sites with BOP or number of teeth with PPD ≥ 4mm and BOP with serum levels TNF-α and adiponectin in the models.
| Unadjusted | Multivariable Analysis* | ||||||
|---|---|---|---|---|---|---|---|
| IRR (95%CI) | P value | IRR (95%CI) | P value | ||||
| Outcome at follow-up visit | Models (HOMA-IR 3rd vs. 1, 2 tertiles) | N | |||||
| Sites with BOP† | HOMA-IR | 870 | 1.17 (1.00 – 1.36) | 0.05 | 1.19 (1.03 – 1.36) | 0.02 | |
| Adding TNF-α (tertiles) | 579 | 1.17 (0.99 – 1.37) | 0.06 | ||||
| Adding Adiponectin (tertiles) | 579 | 1.16 (0.98 – 1.36) | 0.08 | ||||
| Teeth with PPD≥ 4 mm and BOP‡ | HOMA-IR | 870 | 1.21 (0.93 – 1.57) | 0.16 | 1.39 (1.09 – 1.78) | 0.01 | |
| Adding TNF-α (tertile) | 579 | 1.46 (1.09 – 1.95) | 0.01 | ||||
| Adding Adiponectin (tertile) | 579 | 1.44 (1.07 – 1.94) | 0.02 | ||||
Adjusted for baseline age (years); male gender; smoking status (never, former, current); alcohol consumption (abstainer, former, current); educational level (< high school, high school, some college, college degree or higher); physical activity; WC; mean plaque index.
Additionally adjusted for baseline number of sites with BOP or
Additionally adjusted for baseline number of teeth with PPD ≥ 4mm and BOP.
In sensitivity analyses, after excluding adults classified in the upper quartile of baseline number sites with BOP or number of teeth with PPD≥4 mm and BOP, the RRs increased to 1.25 (95% CI: 1.04-1.49, p= 0.02) for number of sites with BOP and 1.60 (95% CI: 1.15-2.22, p=0.005) for number of teeth with PPD≥4 mm and BOP at follow-up (data not shown). The use of log-transformed HOMA-IR showed a significant linear association with number of sites with BOP (RR=1.12, 95% CI: 1.00-1.24; p=0.05) but not with number of teeth with PPD≥4 mm and BOP (RR=1.15, 95% CI: 0.96-1.39, p=0.13) (data not shown). Addition of baseline number of missing teeth, BMI, lipid profile, or the use of lipid lowering agents that may have anti-inflammatory properties or use of anti-inflammatory medications did not significantly change the estimates (data not shown).
DISCUSSION
Our results showed that baseline IR is an independent predictor of gingival/periodontal inflammation after three years of follow-up. Overweight/obese individuals in the upper tertile of HOMA-IR had a 19% increase in the number of sites with BOP and a 39% increase in the number of teeth with PPD≥4 mm and BOP.
Our findings are similar to an earlier longitudinal study by Timonen and colleagues, which indicated a 70% increase in PPD≥4 mm during a four-year follow-up of 157 non-smoking adults without diabetes.31 To the best of our knowledge, our study represents the largest longitudinal study assessing the association between IR and periodontal measures, and the first longitudinal study assessing the association between IR and active gingival/periodontal inflammation. In addition, only 157 out of 393 (~40%) eligible participants in the study by Timonen and colleagues had completed data at the baseline to be analyzed in their longitudinal study whereas we had a retention rate of approximately 72%. We found statistically-significant RRs only in the highest tertile of HOMA-IR, suggesting a threshold effect. Participants with high HOMA-IR had an increased number of sites with BOP and teeth with deep pocket depth and BOP compared to those in the lowest HOMA-IR tertile (Table 2). However, these numbers decreased three years later (i.e. from baseline to follow-up visits) which might indicate either disease stability or improvement (Table 2). This could be related to participants’ health awareness from the health information flyers we provided at the exit interview. The mean HOMA-IR slightly increased from baseline to follow-up visits (baseline: 2.4 ± 1.7 vs. follow-up: 2.9 ± 2.4, data not shown), but the associations between HOMA-IR and gingival/periodontal inflammation at the follow-up visit remained statistically significant (Tables 3 and 4). Hence, independent of oral disease stability or improvement, the associations between IR and oral inflammation remained significant. We included overweight or obese Hispanic adults, who are at higher risk for IR, and this may limit the generalizability of our results, although we may still expect the association to be similar in other populations. Atypical levels of lipids, fatty acids, and cytokines may stimulate monocytes thereby increasing the secretion of inflammatory cytokines and leading to an increased IR.42,43 According to this concept, one of the mechanisms therefore may be related to the relationship between low-grade inflammation and IR, hence periodontitis. However, our findings do not show evidence of the influence of systemic inflammation on the association between IR and gingival/periodontal inflammation (Table 4). Adjustments of models for other available biomarkers, such as serum IL-6 or hs-CRP, did not impact the results as well (data not shown).
Data on individual’s weekly vegetable intake was available and used as a surrogate measure of dietary behavior. However, further adjustment of the model for this information did not change the estimates of the association IR- gingival/periodontal inflammation as well.
Unlike other periodontal parameters, such as the Centers for Disease Control and Prevention/American Academy of Periodontology (CDC/AAP) periodontal disease definition44 or attachment loss (AL) alone, which do not distinguish past or present experience of periodontitis and periods of stability/activity, we evaluated the status and progression of gingival/periodontal inflammation by using two case definitions of inflammatory status which indicated clinically the activity or inflammatory status of the gingival tissues or the periodontal pockets at the time of each exam. We were interested in assessing the relationship with IR during the time when gingival and/or periodontal tissue might be inflamed, which could represent the episodic “bursts” of this disease. Ideally, we could have used the tooth sites with both PPD≥4 mm and BOP, but due to high levels of bleeding at each site probing the flow of the blood from one site contaminated the other sites in the pocket, and the exact source of the blood flow was difficult to differentiate. Thus, we only recorded bleeding on two of the six sites of each tooth to increase measurement accuracy. An ideal approach to disease classification would involve the use of current clinical parameters complemented by a radiographic assessment to detect the presence of bone tissue destruction as well as other biologic phenotypes that represent the periodontal pathogenesis at the microbial, cellular, or molecular level.45–48 Importantly, we collected detailed information to measure the HOMA-IR exposure and we conducted a full-mouth probing pocket depth examination.
To summarize, findings from the present study suggest that metabolic abnormalities that long precede diabetes development, such as IR, might be an important mechanism of risk.
CONCLUSION
Our results show that baseline IR is associated with increased risk of gingival/periodontal inflammation at follow-up in this population of overweight/obese Hispanic adults. These findings have public health relevance, as prevention of IR through a healthy lifestyle may help reduce periodontal inflammation as well as diabetes. Alternatively, the presence of gingival/periodontal inflammation in the oral cavity may reflect undetected IR.
One-sentence summary.
Insulin resistance significantly predicts gingival/periodontal inflammation in a Hispanic adult population.
Acknowledgments
This work was fully supported by Award Number R01DE020111 from the National Institute of Dental and Craniofacial Research (NIDCR) and partially supported by award number 2U54MD007587 from the National Institute on Minority Health and Health Disparities (NIMHD), award number 1U54RR026139-01A1 from the National Center for Research Resources (NCRR), and award Number K23 DE025313-01 from the National Institute of Dental and Craniofacial Research (NIDCR). The authors acknowledge the SOALS team (Tania Ginebra, Carla León, Yashira Maldonado, Dr. Sasha Martinez, Xiomara O’Farrill, Samantha Ordaz, Dr. Margarita Ramirez-Vick, Elaine Rodríguez, Rosalyn Román, Rafael Ruiz, Yadiris Santaella, Grace Vélez, José L. Vergara, Lay Wah, Jeanpaul Fernández) and PRCTRC laboratory personnel (Aracelis Arroyo and Nilda González) who contributed to the conduct/oversight/planning of data collection of the study.
Footnotes
BD Vacutainer®, Becton Dickinson and Company, Franklin Lakes, NJ
J & J Vitros 250 Chemistry Analyzer, Ortho-Clinical Diagnostics, Inc, Rochester, NY
AIA-360 Automated Immunoassay Analyzer, Tosoh Bioscience, Inc, South San Francisco, CA.
Meso Scale Discovery (MSD), Meso Scale Diagnostics, LLC, Rockville, MD
Human Adiponectin Ria Kit (HADP-61HK), EMD Millipore Corporation, Billerica, MA
Stata Statistical Software: Release 14, StataCorp LP, College Station, TX.
AUTHOR CONTRIBUTIONS:
O.M. Andriankaja contributed to conception and design of the manuscript, data acquisition and analysis, interpretation, drafted the manuscript, and critically revised the manuscript. F.J. Muñoz-Torres contributed to data acquisition and analysis, and critically revised the manuscript. J.O. Vivaldi contributes to data acquisition, and critically revised the manuscript. B.G. Leroux and M. Campos contributed to data interpretation, and critically revised the manuscript. K. Joshipura and C.M. Pérez contributed to data acquisition, analysis and interpretation, and critically revised the manuscript. All authors gave final approval and agreed to be accountable for all aspects of the work.
DISCLOSURE STATEMENT:
All authors declare that they have no conflict of interests. They have no interests directly or indirectly related to the work under construction. Dr. Brian G. Leroux disclosed that he has a contract as statistician consultant with the University of Puerto Rico. No other interests that go beyond financial interests and compensation, such as non-financial interests were disclosed by the authors.
References
- 1.World Health Organization Global Report on Diabetes. Geneva, Switzerland: WHO; 2016. http://apps.who.int/iris/bitstream/10665/204871/1/9789241565257eng.pdf. [accessed 2016 June 7] [Google Scholar]
- 2.World Health Organization Obesity and Overweight: Fact sheet (Media center) Geneva, Switzerland: WHO; 2016. http://www.who.int/mediacentre/factsheets/fs311/en/ accessed 2016 June 7. [Google Scholar]
- 3.Freemantle N, Holmes J, Hockey A, Kumar S. How strong is the association between abdominal obesity and the incidence of type 2 diabetes? Int J Clin Pract. 2008;62:1391–1396. doi: 10.1111/j.1742-1241.2008.01805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palle AR, Reddy CM, Shankar BS, Gelli V, Sudhakar J, Reddy KK. Association between obesity and chronic periodontitis: a cross-sectional study. J Contemp Dent Pract. 2013;14:168–173. doi: 10.5005/jp-journals-10024-1294. [DOI] [PubMed] [Google Scholar]
- 5.Loe H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care. 1993;16:329–334. [PubMed] [Google Scholar]
- 6.Holt SC, Ebersole JL. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: the ‘red complex’, a prototype polybacterial pathogenic consortium in periodontitis. Periodontol 2000. 2005;38:72–122. doi: 10.1111/j.1600-0757.2005.00113.x. [DOI] [PubMed] [Google Scholar]
- 7.Eke PI, Dye BA, Wei L, et al. Update on Prevalence of Periodontitis in Adults in the United States: NHANES 2009 to 2012. J Periodontol. 2015;86:611–622. doi: 10.1902/jop.2015.140520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 9.Haffajee AD, Socransky SS. Microbial etiological agents of destructive periodontal diseases. Periodontol 2000. 1994;5:78–111. doi: 10.1111/j.1600-0757.1994.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 10.Socransky SS, Haffajee AD. The bacterial etiology of destructive periodontal disease: current concepts. J Periodontol. 1992;63:322–331. doi: 10.1902/jop.1992.63.4s.322. [DOI] [PubMed] [Google Scholar]
- 11.Goodson JM, Tanner AC, Haffajee AD, Sornberger GC, Socransky SS. Patterns of progression and regression of advanced destructive periodontal disease. J Clin Periodontal. 1982;9:472–481. doi: 10.1111/j.1600-051x.1982.tb02108.x. [DOI] [PubMed] [Google Scholar]
- 12.Page RC, Kornman KS. The pathogenesis of human periodontitis: an introduction. Periodontol 2000. 1997;14:9–11. doi: 10.1111/j.1600-0757.1997.tb00189.x. [DOI] [PubMed] [Google Scholar]
- 13.Leite RS, Marlow NM, Fernandes JK. Oral health and type 2 diabetes. Am J Med Sci. 2013;345:271–273. doi: 10.1097/MAJ.0b013e31828bdedf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nascimento GG, Leite FR, Do LG, et al. Is weight gain associated with the incidence of periodontitis? A systematic review and meta-analysis. J Clin Periodontol. 2015;42:495–505. doi: 10.1111/jcpe.12417. [DOI] [PubMed] [Google Scholar]
- 15.Keller A, Rohde JF, Raymond K, Heitmann BL. Association between periodontal disease and overweight and obesity: a systematic review. J Periodontol. 2015;86:766–776. doi: 10.1902/jop.2015.140589. [DOI] [PubMed] [Google Scholar]
- 16.Papageorgiou SN, Reichert C, Jäger A, Deschner J. Effect of overweight/obesity on response to periodontal treatment: systematic review and a meta-analysis. J Clin Periodontol. 2015;42:247–261. doi: 10.1111/jcpe.12365. [DOI] [PubMed] [Google Scholar]
- 17.Chaffee BW, Weston SJ. Association between chronic periodontal disease and obesity: a systematic review and meta-analysis. J Periodontol. 2010;81:1708–1724. doi: 10.1902/jop.2010.100321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim SG, Han K, Kim HA, et al. Association between insulin resistance and periodontitis in Korean adults. J Clin Periodontol. 2014;41:121–130. doi: 10.1111/jcpe.12196. [DOI] [PubMed] [Google Scholar]
- 19.Islam SK, Seo M, Lee YS, Moon SS. Association of periodontitis with insulin resistance, beta-cell function, and impaired fasting glucose before onset of diabetes. Endocr J. 2015;62:981–989. doi: 10.1507/endocrj.EJ15-0350. [DOI] [PubMed] [Google Scholar]
- 20.Benguigui C, Bongard V, Ruidavets JB, et al. Metabolic syndrome, insulin resistance, and periodontitis: a cross-sectional study in a middle-aged French population. J Clin Periodontol. 2010;37:601–608. doi: 10.1111/j.1600-051X.2010.01571.x. [DOI] [PubMed] [Google Scholar]
- 21.Timonen P, Suominen-Taipale L, Jula A, Niskanen M, Knuuttila M, Ylostalo P. Insulin sensitivity and periodontal infection in a non-diabetic, non-smoking adult population. J Clin Periodontol. 2011;38:17–24. doi: 10.1111/j.1600-051X.2010.01642.x. [DOI] [PubMed] [Google Scholar]
- 22.Sweeting LA, Davis K, Cobb CM. Periodontal Treatment Protocol (PTP) for the general dental practice. J Dent Hyg. 2008;82:16–26. [PubMed] [Google Scholar]
- 23.Andriankaja OM, Joshipura K. Potential association between prediabetic conditions and gingival and/or periodontal inflammation. J Diabetes Invest. 2014;5:108–114. doi: 10.1111/jdi.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaves ES, Wood RC, Jones AA, Newbold DA, Manwell MA, Kornman KS. Relationship of “bleeding on probing” and “gingival index bleeding” as clinical parameters of gingival inflammation. J Clin Periodontol. 1993;20:139–143. doi: 10.1111/j.1600-051x.1993.tb00328.x. [DOI] [PubMed] [Google Scholar]
- 25.Andriankaja OM, Jimenez JJ, Munoz-Torres FJ, Perez CM, Vergara JL, Joshipura K. Lipid lowering agents (LLA) use and systemic and oral inflammation in overweight or obese adult Puerto Ricans: the SOALS Study. J Clin Periodontol. 2015;42:1090–1096. doi: 10.1111/jcpe.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rivera R, Andriankaja OM, Perez CM, Joshipura K. Relationship between periodontal disease and asthma among overweight/obese adults. J Clin Periodontol. 2016;43:566–571. doi: 10.1111/jcpe.12553. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perez CM, Munoz F, Andriankaja OM, et al. Cross-sectional associations of impaired glucose metabolism measures with bleeding on probing and periodontitis. J Clin Periodontol. 2017;44:142–149. doi: 10.1111/jcpe.12662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization. Obesity: preventing and managing the global epidemic: report of a WHO consultation. Geneva, Switzerland: WHO; 2000. p. 8. (Technical Report Series 894). http://www.who.int/nutrition/publications/obesity/WHO_TRS_894/en/.WHO_TRS_894.pdf. [PubMed] [Google Scholar]
- 29.Joshipura KJ, Andriankaja MO, Hu FB, Ritchie CS. Relative utility of 1-h Oral Glucose Tolerance Test as a measure of abnormal glucose homeostasis. Diabetes Res Clin Pract. 2011;93:268–275. doi: 10.1016/j.diabres.2011.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 31.Timonen P, Saxlin T, Knuuttila M, et al. Role of insulin sensitivity and beta cell function in the development of periodontal disease in adults without diabetes. J Clin Periodontol. 2013;40:1079–1086. doi: 10.1111/jcpe.12162. [DOI] [PubMed] [Google Scholar]
- 32.CDC. National Health and Nutrition Examination Survey (NHANES): Oral health examiners manual. CDC. 2011-2012 [ Accessed August 15, 2016] www.cdc.gov/nchs/data/nhanes/nhanes_11_12/Oral_Health_Examiners_Manual.pdf.
- 33.Van der Weijden GA, Timmerman MF, Saxton CA, Russell JI, Huntington E, Van der Velden U. Intra-/inter-examiner reproducibility study of gingival bleeding. Journal of periodontal research. 1994;29:236–241. doi: 10.1111/j.1600-0765.1994.tb01217.x. [DOI] [PubMed] [Google Scholar]
- 34.Mojon P, Chung JP, Favre P, Budtz-Jorgensen E. Examiner agreement on periodontal indices during dental surveys of elders. J Clin Periodontol. 1996;23:56–59. doi: 10.1111/j.1600-051x.1996.tb00505.x. [DOI] [PubMed] [Google Scholar]
- 35.Muller HP, Barrieshi-Nusair KM. Gingival bleeding on repeat probing after different time intervals in plaque-induced gingivitis. Clin Oral Investig. 2005;94:278–283. doi: 10.1007/s00784-005-0001-8. [DOI] [PubMed] [Google Scholar]
- 36.World Health Organization. Global Recommendations on Physical Activity for Health. WHO; 20 Avenue Appia, 1211 Geneva 27, Switzerland: 2010. pp. 24–27. [PubMed] [Google Scholar]
- 37.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 38.Silness J, Loe H. Periodontal Disease in Pregnancy. II. Correlation between Oral Hygiene and Periodontal Condition. Acta Odontol Scand. 1964;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 39.Fleiss JL, Park MH, Chilton NW, Alman JE, Feldman RS, Chauncey HH. Representativeness of the “Ramfjord teeth” for epidemiologic studies of gingivitis and periodontitis. Community Dent Oral Epidemiol. 1987;15:221–224. doi: 10.1111/j.1600-0528.1987.tb00525.x. [DOI] [PubMed] [Google Scholar]
- 40.Lawless JF. Negative binomial and mixed Poisson regression. Can J Stat. 1987;15:209–225. [Google Scholar]
- 41.Vickers AJ, Altman DG. Statistics notes: Analysing controlled trials with baseline and follow up measurements. Statistics Notes. Educational and Debates. BMJ. 2001;323:1123–1124. doi: 10.1136/bmj.323.7321.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.King GL. The role of inflammatory cytokines in diabetes and its complications. J periodontol. 2008;79:1527–1534. doi: 10.1902/jop.2008.080246. [DOI] [PubMed] [Google Scholar]
- 43.Yadav A, Kataria MA, Saini V, Yadav A. Role of leptin and adiponectin in insulin resistance. Clin Chim Acta. 2013;417:80–84. doi: 10.1016/j.cca.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 44.Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ. Update of the case definitions for population-based surveillance of periodontitis. J Periodontol. 2012;83:1449–1455. doi: 10.1902/jop.2012.110664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zaki HA, Hoffmann KR, Hausmann E, Scannapieco FA. Is radiologic assessment of alveolar crest height useful to monitor periodontal disease activity? Dent Clin North Am. 2015;59:859–872. doi: 10.1016/j.cden.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muthukumar S, Anand MV, Madhankumar S. Relationship between gingival bleeding and anaerobic periodontal infection assessed by BANA (N-Benzoyl-DL-Arginine-beta-Napthylamide) assay. J Pharm Bioallied Sci. 2014;6:S70–73. doi: 10.4103/0975-7406.137391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Offenbacher S, Barros SP, Beck JD. Rethinking periodontal inflammation. J Periodontol. 2008;79:1577–1584. doi: 10.1902/jop.2008.080220. [DOI] [PubMed] [Google Scholar]
- 48.Dosseva-Panova VT, Popova CL, Panov VE. Subgingival microbial profile and production of proinflammatory cytokines in chronic periodontitis. Folia medica. 2014;56:152–160. doi: 10.2478/folmed-2014-0022. [DOI] [PubMed] [Google Scholar]


