Abstract
Background
Current prevention guidelines recommend using the Pooled Cohort Equation (PCE) for 10-year atherosclerotic cardiovascular disease (CVD) risk assessment. However, the PCE has serious limitations in older adults: it excludes heart failure (HF) hospitalization, estimates 10-year risk which may not be the most relevant time frame, and is not indicated for individuals aged >79 years.
Objectives
To determine whether adding biomarkers to PCE variables improves global CVD (coronary heart disease [CHD], stroke, and HF) risk prediction in older adults over a shorter time period.
Methods
Atherosclerosis Risk in Communities Study participants without prevalent CVD including HF (n=4760; mean±SD age=75.4±5.1 years) were followed for incident global CVD events. Adding N-terminal pro–B-type natriuretic peptide, high-sensitivity cardiac troponin T, and high-sensitivity C-reactive protein to the PCE and a “lab model” with the biomarkers, age, race, and gender were assessed for prediction improvement. Area under the receiver operating characteristic curve (AUC) and net reclassification index (NRI) were calculated.
Results
Over median follow-up of ~4 years, incident HF was the leading CVD event (n=193 vs 118 CHD and 81 stroke events). Compared to the PCE, each biomarker improved risk prediction. The largest improvement in risk prediction metrics was with the addition of all 3 biomarkers (ΔAUC 0.103; continuous NRI 0.484). The lab model also performed better than the PCE model (ΔAUC 0.091, continuous NRI 0.355).
Conclusions
Adding biomarkers to the PCE or a simpler “lab model” improves short-term global CVD risk prediction and may be useful to inform short-term preventive strategies in older adults.
Keywords: biomarkers, cardiovascular disease, elderly, heart failure, prevention, risk assessment
Introduction
The American College of Cardiology (ACC)/American Heart Association (AHA) cholesterol (1) and hypertension (2) guidelines recommend using the Pooled Cohort Equation (PCE) to estimate a 10-year risk for atherosclerotic cardiovascular disease (ASCVD) events to guide the decision-making process for both the initiation and intensity of pharmacotherapy in the management of cholesterol and blood pressure. However, application of the PCE is limited in older adults. The PCE does not include heart failure (HF) hospitalization, a leading cardiovascular disease event in older populations (4). Moreover, a 10-year time frame may be less relevant for older adults given competing causes of mortality besides ASCVD events. Further, the PCE is not recommended in individuals >79 years of age because of insufficient data in this population from the pooled cohorts, including the Atherosclerosis Risk in Communities (ARIC) study.
The guidelines recommend clinician–patient discussions in lieu of definitive primary prevention recommendations for individuals aged >75 years. However, it is difficult to discuss potential benefits and risks of therapy without comprehensive risk assessment. The association of traditional risk factors with ASCVD events may be less robust in older adults (5), which may be secondary to the high prevalence of risk factors in older adults; hence, comprehensive risk assessment in these individuals may warrant use of additional measures beyond traditional risk factors. A high-sensitivity cardiac troponin T (hs-cTnT) assay, approved in 2017 in the United States for clinical use in detection of acute coronary syndromes, can measure plasma concentrations that are 10-fold lower than widely used fourth-generation assays (6). In prior studies, the addition of both hs-cTnT and N-terminal pro–B-type natriuretic peptide (NT-proBNP) improved risk prediction for incident coronary heart disease (CHD) and HF beyond traditional risk factors used in the ARIC study risk equations for CHD and HF (6,7).
Evidence is limited on the use of these biomarkers for assessing risk for incident CHD, stroke, and HF in older adults being treated with contemporary medical therapy. We hypothesized that addition of three candidate biomarkers—hs-cTnT, NT-proBNP, and high-sensitivity C-reactive protein (hs-CRP)—to the PCE clinical variables would be a better model for risk prediction of global CVD events (including CHD, stroke, and HF) over a shorter time frame than 10 years.
Methods
See also Online Supplement.
Study Population
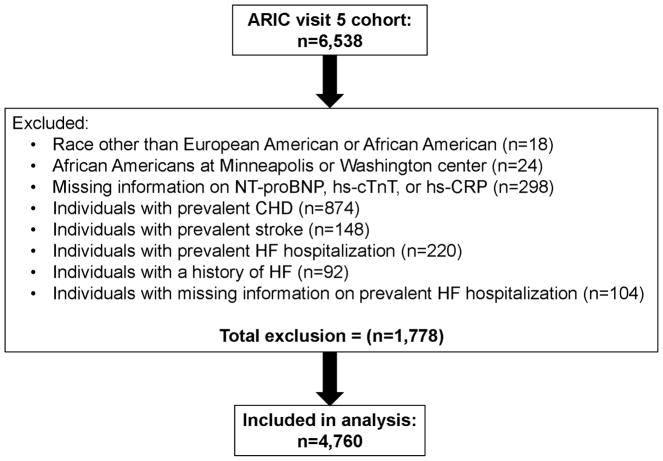
The ARIC study is a prospective community-based study of CVD incidence in 15,792 middle-aged (45–64 years at visit 1) adults recruited from four U.S. communities in 1987–1989. A detailed description of the ARIC study design and methods has been published elsewhere (8). This study was conducted among individuals who participated in ARIC visit 5 (aged 69–88 years), conducted between June 1, 2011, and August 30, 2013. Of 6,538 individuals who completed visit 5, we excluded those with self-reported race neither African American nor Caucasian (n=18), any African American participants at the Minnesota and Washington County field centers (n=24) because of small enrollment numbers, individuals with missing data for NT-proBNP, hs-cTnT, or hs-CRP (n=298), and those with prevalent CHD (n=874), prevalent ischemic stroke (n=148), prevalent HF (n=220), history of HF (n=92), or missing information on HF (n=104). Thus, a total of 4,760 individuals were included in all analyses (Figure 1).
Figure 1. Flow diagram for eligible cohort selection from Atherosclerosis Risk in Communities (ARIC) visit 5.
CHD, coronary heart disease; HF, heart failure; CHD, coronary heart disease; HF, heart failure; NT-proBNP: N-terminal pro–B-type natriuretic peptide; hs-CRP, high-sensitivity C-reactive protein; hs-cTnT, high-sensitivity cardiac troponin T.
Prevalent CHD, stroke, and HF were defined as self-reported myocardial infarction, ischemic stroke, or HF before ARIC visit 1 or adjudicated myocardial infarction (including silent myocardial infarction), coronary revascularization, stroke, or HF between ARIC visits 1 and 5 based on detailed review of abstracted medical records (9–12). Medical history, demographic data, anthropometric data, blood pressure measurements, and fasting lipid assessments were obtained during ARIC visit 5 at the same time as the blood draw for the biomarker measurements. Lipid measurements were performed on fasting plasma samples that were stored at −70°C with ethylenediaminetetraacetic acid as the anticoagulant. Plasma total cholesterol, high-density lipoprotein cholesterol, and triglycerides were measured using enzymatic measures (13). Low-density lipoprotein cholesterol was calculated using the Friedewald equation (14). Diabetes mellitus was defined as a fasting glucose level ≥126 mg/dL, a self-reported physician diagnosis of diabetes, or use of diabetes medications. Hypertension in the ARIC study is defined as systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg or use of blood pressure–lowering therapies.
Research protocols were approved by institutional review boards at each center; all participants provided written informed consent.
Biomarker Measurements
hs-cTnT was measured in 2013 using a highly sensitive assay (Elecsys Troponin T Gen 5 STAT; Roche Diagnostics, Indianapolis, IN, USA) in stored samples that were collected at visit 5. As per the manufacturer package insert, the limit of detection of this assay is 5 ng/L and limit of quantification is 6 ng/L. We measured NT-proBNP using electrochemiluminescent immunoassay (Roche Diagnostics) with a measurement range of 5–35,000 pg/mL and a limit of quantification of 35 pg/mL (7). Participants with NT-proBNP and hs-cTnT levels below the lower limits of detection were assigned values equal to half the lower limits of detection. The interassay coefficient of variance for hs-cTnT was 6.4% for a mean control level of 29 ng/L. The interassay coefficient of variance for NT-proBNP was 7.4% for a mean control level of 134 pg/mL. The variability in NT-proBNP and hs-cTnT concentrations related to frozen storage and freeze–thaw cycles as well as the reliability coefficient and interassay coefficient of variance for other biomarkers have been described previously (15). hs-CRP levels were assessed by the immunoturbidimetric CRP-Latex (II) high-sensitivity assay (Denka Seiken, Tokyo, Japan) using a Hitachi 911 analyzer (Roche Diagnostics).
Outcomes Assessed
The methods of assessing incident CVD in the ARIC study have been described previously (9–12). Briefly, medical records were abstracted for all CVD outcomes (CHD, stroke, and HF) and adjudicated by an endpoints committee; incident CHD events included fatal CHD, definite or probable myocardial infarction, silent myocardial infarction between examinations as determined by electrocardiography, and coronary revascularization. Incident stroke events included ischemic and thrombotic strokes, defined as validated definite or probable hospitalized embolic or thrombotic strokes. HF hospitalization consisted of definite and probable acute decompensated HF based on medical chart review of signs and symptoms of decompensation, elevation of natriuretic peptides, cardiac systolic and/or diastolic dysfunction, and HF-specific therapy by certified physicians (12). Deaths were also ascertained via active surveillance.
Incident global CVD was defined as a composite of incident CHD, incident stroke, and incident HF hospitalization; incident ASCVD was defined as a composite endpoint of incident CHD and incident stroke. All outcomes were assessed after ARIC visit 5 with follow-up through December 31, 2015. The median (25th, 75th percentile) follow-up period for each outcome was global CVD 3.55 (3.11, 4.03) years, ASCVD 3.56 (3.15, 4.03), incident CHD 3.57 (3.16, 4.04) years, stroke 3.57 (3.17, 4.04) years, and HF 3.56 (3.15, 4.04) years.
Statistical Analysis
Descriptive data were presented as the mean±SD for normally distributed variables and median (25th, 75th) for non-normally distributed variables. Log-transformed variables are also described using geometric mean (95% confidence intervals [CIs]). All three biomarkers (hs-cTnT, NT-proBNP, and hs-CRP) were modeled as both categorical and continuous variables. Continuous associations were tested for linearity. For the categorical analysis, we used splines to verify cut-points. Each biomarker concentration was divided in three categories: hs-cTnT <6 ng/L, 6–13 ng/L, and ≥14 ng/L; NT-proBNP <100 pg/mL, 100–299 pg/mL, and ≥300 pg/mL; and hs-CRP <1 mg/L, 1–<3 mg/L, and ≥3 mg/L.
Incidence rates were calculated as events per 1000 person-years. Using Cox proportional hazards models, we estimated the hazard ratios (HRs) and 95% CIs for the associations of hs-cTnT, NT-proBNP, and hs-CRP with the primary global CVD outcome, as well as the secondary outcome of ASCVD and tertiary outcomes of incident CHD, incident stroke, and incident HF. Model 1 was adjusted for all variables included in the PCE (age, gender, race, systolic blood pressure, total cholesterol, high-density lipoprotein cholesterol, antihypertensive medication use, current smoking, and diabetes mellitus). Model 2 included all the variables in model 1 plus estimated glomerular filtration rate. P-values for trends were calculated.
To assess the utility of hs-cTnT, NT-proBNP, and hs-CRP for risk stratification and prediction, we used statistical measures of discrimination including the area under the receiver operating characteristic curve (AUC) (16), net reclassification index (NRI), and integrated discrimination index (IDI) (17) to calculate the incremental value of adding the individual biomarkers separately to the “PCE model” (including all variables of the PCE risk equation) and then all three biomarkers to the PCE model (“complete model”). Further, we tested the performance of the PCE model against a basic model (including age, gender, and race) and the “complete model.” A simple “lab model” that included age, gender, race and all three candidate biomarkers was also tested against the PCE model. Kaplan–Meier estimate was applied to calculate absolute risk over the 4-year follow-up period. We then described the 4-year risk of global CVD events for the various models by deciles of estimated risk and the estimated percentage of global CVD events occurring within each decile.
Results
Baseline characteristics of the included population are shown in Table 1. The mean age of the population was 75.4±5.1 years, and 62.3% were women (Table 1). Approximately 50% of the cohort was on lipid-lowering agents, 45% on statins, 66% on aspirin, and 70% on antihypertensive treatment. Incident global CVD, ASCVD, and HF events occurred in 7.1 % (n=336), 4.1% (n=195), and 4.1 % (n=193) of the population, Baseline characteristic across each biomarker category are shown in Online Tables 1A–C.
Table 1.
Baseline Characteristics of Study Population (Atherosclerosis Risk in Communities [ARIC], Visit 5 [2011–2013])
| Baseline Characteristic | (n=4760) |
|---|---|
| Age (years) | 75.4±5.1 |
| Female (%) | 62.3 |
| Black (%) | 22.4 |
| Hypertension (%) | 71.2 |
| Diabetes (%) | 29.2 |
| Antihypertensive drug use (%) | 70.4 |
| Dyslipidemic drug use (%) | 50.2 |
| Statin use (%) | 45.5 |
| Aspirin use (%) | 65.5 |
| hs-cTnT (ng/L) | 10 [7, 15] |
| NT-proBNP (pg/mL) | 118.9 [62.2, 225.9] |
| hs-CRP (mg/L) | 1.9 [0.9, 4.2] |
Data presented as mean±SD, median [25th, 75th percentiles], or percentage.
Abbreviations: hs-CRP, high-sensitivity C-reactive protein; hs-cTnT, high-sensitivity cardiac troponin T; NT-proBNP, N-terminal pro–B-type natriuretic peptide.
When examined as continuous variables, both hs-cTnT and NT-proBNP were significantly associated with each outcome, whereas hs-CRP showed less robust associations (Online Tables 2–3). In model 1 (PCE variables), hs-cTnT was significantly associated with incident global CVD (HR=2.10 [95% CI 1.73–2.54]), ASCVD (HR=1.61 [95%CI 1.25–2.09]), CHD (HR=1.71 [95% CI 1.23–2.37]), stroke [HR=1.49 [95% CI 1.00–2.22]), and HF (HR=2.86 [95% CI 2.24–3.65]). NT-proBNP also was significantly associated with incident global CVD (HR=2.05 [95% CI 1.83–2.29]), ASCVD (HR=1.48 [95% CI 1.27–1.72]), CHD (HR=1.52 [95% CI 1.26–1.84]), stroke (HR=1.43 [95% CI 1.12–1.81]), and HF (HR=2.87 [95% CI 2.49–3.31]). hs-CRP was significantly associated with incident global CVD (HR=1.29 [95% CI 1.16–1.43]), ASCVD (HR=1.15 [95% CI 1.00–1.32)], CHD (HR=1.21 [95% CI 1.01–1.44]), and HF (HR=1.39 [95% CI 1.21–1.60]), but not incident stroke (HR=1.07 [95% CI 0.86–1.34], p=0.52).
In the categorical analysis (Online Table 3), the HRs for all outcomes significantly increased across increasing concentrations of hs-cTnT. The highest category of hs-cTnT (≥14 ng/L) showed the highest risk for each outcome: HRgCVD=5.40 (95% CI 2.97–9.80), HRASCVD=3.94 (95% CI 1.83–8.48), HRCHD=3.79 (95% CI 1.43–10.00), HRstroke=4.52 (1.30–15.69), and HRHF=8.17 (3.46–19.30). The 4-year absolute risk for global CVD, ASCVD, CHD, stroke, and HF events also significantly increased in a graded manner across hs-cTnT categories, with the highest absolute risk for global CVD (15% [95% CI 12–17]) for individuals with hs-cTnT ≥14 ng/L.
NT-proBNP was also robustly associated with global CVD and ASCVD as well as incident CHD, stroke, and HF in a graded manner (Online Table 3). As with hs-cTnT, the highest category of NT-proBNP (≥300 pg/mL) had the strongest association: HRgCVD=5.18 (95% CI 3.78–7.09), HRASCVD=2.74 (95% CI 1.82–4.15), HRCHD=2.98 (95% CI 1.75–5.06), HRstroke=2.59 (95% CI 1.36–4.94), and HRHF=11.33 (95% CI 7.17–17.92). The 4-year absolute risk for each outcome significantly increased across increasing NT-proBNP levels, with the highest 4-year absolute risk for global CVD (20% [95% CI 17–24]) in the highest category.
The association of hs-CRP concentrations in the highest category (≥3 mg/L) with events was significant for incident global CVD events (HRgCVD=1.77 [95% CI 1.29–2.44]) and HF (HRHF=2.61 [95% CI 1.61–4.22]). The 4-year absolute risk increased across hs-CRP categories for incident HF and global CVD (Online Table 3).
Because hs-cTnT and NT-proBNP had the strongest associations, we examined the risk of incident global CVD with combined hs-cTnT and NT-proBNP categories defined as low (hs-cTnT <6 ng/L and NT-proBNP <100 pg/mL), medium (hs-cTnT 6–13 ng/L and NT-proBNP ≥100–<300 pg/mL), and high (hs-cTnT ≥14 ng/L and NT-proBNP ≥300 pg/mL) risk (Online Figure 1). More than a quarter (n=97) of all global CVD events occurred in individuals with hs-cTnT of ≥14 ng/L and NT-proBNP ≥300 pg/mL: HR=11.5 (95% CI 5.5–24.5) compared with individuals with low levels of both.
Risk Prediction
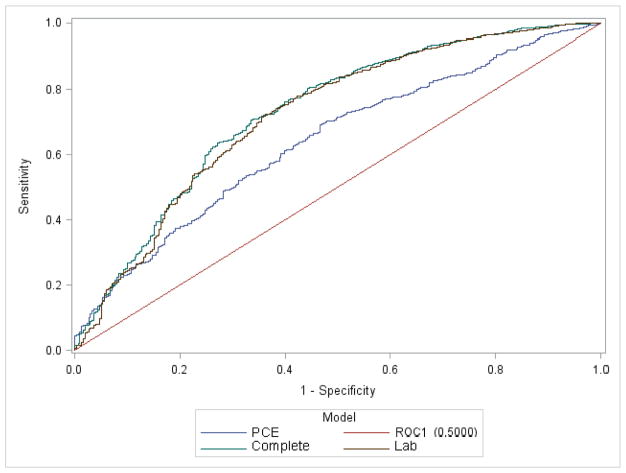
For risk prediction of global CVD, the PCE model showed fair discrimination: C-statistic of 0.647 vs 0.597 for the basic model (Table 2). Evaluation for the incremental benefit of adding each biomarker to the PCE model, however, showed significantly improved discrimination with analysis of the C-statistics, category-free NRI, and IDI. Each biomarker significantly increased the C-statistic when added to the PCE model; however, addition of NT-proBNP improved the C-statistic the most from baseline (ΔAUC 0.092, Table 2). The largest improvement in risk prediction metrics was seen with the “complete model” (PCE +all three biomarkers) (ΔAUC 0.103, category-free NRI 0.484, and IDI 0.075). The simpler “lab”model (age, gender, and race+all three biomarkers) also performed well (ΔAUC 0.091, category-free NRI 0.355, and IDI 0.068) (Figure 2). As categories are not established for 4-year global CVD risk in the elderly, multiple cutpoints were examined; NRI values were all significant but varied slightly depending on the cutpoint chosen (Online Appendix).
Table 2.
Comparisons of biomarkers with clinical variables of the PCE and other models with differences in AUC, NRI, and IDI for risk prediction of global CVD
| C-statistics Primary Model (95% CI) | C-statistics Extended Comparison Model (95% CI) | ΔAUC (95% CI) | NRI (95% CI) | Continuous NRI (95% CI) | IDI (95% CI) | |
|---|---|---|---|---|---|---|
| Basic model vs PCE | 0.597 (0.57, 0.63) | 0.647 (0.62, 0.68) | 0.050 (0.028, 0.084) | 0.057 (0.005, 0.184) | 0.274 (0.174, 0.434) | 0.012 (0.006, 0.023) |
| PCE vs hs-CRP model | 0.647 (0.623, 0.679) | 0.665 (0.642, 0.6991) | 0.018 (0.006, 0.034) | 0.035 (−0.022, 0.104) | 0.167 (0.026, 0.299) | 0.007 (0.002, 0.015) |
| PCE vs hs-cTnT model | 0.647 (0.623, 0.679) | 0.686 (0.663, 0.720) | 0.039 (0.022, 0.057) | 0.137 (0.051, 0.248) | 0.361 (0.226, 0.496) | 0.019 (0.011, 0.030) |
| PCE vs NT-proBNP model | 0.647 (0.623, 0.679) | 0.739 (0.709, 0.771) | 0.092 (0.063, 0.117) | 0.304 (0.190, 0.401) | 0.407 (0.270, 0.538) | 0.066 (0.040, 0.099) |
| PCE vs Complete model | 0.647 (0.623, 0.679) | 0.750 (0.724, 0.781) | 0.103 (0.074, 0.129) | 0.370 (0.228, 0.455) | 0.484 (0.359, 0.634) | 0.075 (0.051, 0.112) |
| PCE model vs Lab model | 0.647 (0.623, 0.679) | 0.738 (0.710, 0.7683) | 0.091 (0.055, 0.121) | 0.302 (0.145, 0.412) | 0.355 (0.177, 0.550) | 0.068 (0.041, 0.104) |
NRI was calculated using cutpoints of 2.4% and 8% over 4 years to categorize participants as low, intermediate, or high risk.
Basic model: age, gender, and race
PCE model: age, gender, race, total cholesterol, HDL-C, SBP, antihypertensive medication use, current smoking, and diabetes status
hs-CRP model: PCE variables + hs-CRP
hs-cTnT model: PCE variables + hs-cTnT
NT-proBNP model: PCE variables + NT-proBNP
Complete model: = PCE variables + hs-cTnT, NT-pro-BNP, and hs-CRP
Lab model: age, gender, race + hs-cTnT, NT-pro-BNP, and hs-CRP
Abbreviations: AUC, area under the receiver operative characteristic curve (C-statistics); IDI, integrated discrimination index; NRI, net reclassification index.
Figure 2.
Receiver operating characteristic curves for models analyzed based on logistic regression results of incident global CVD events.
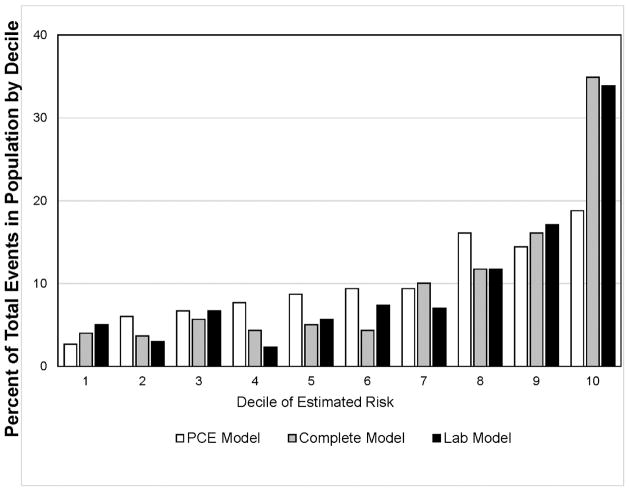
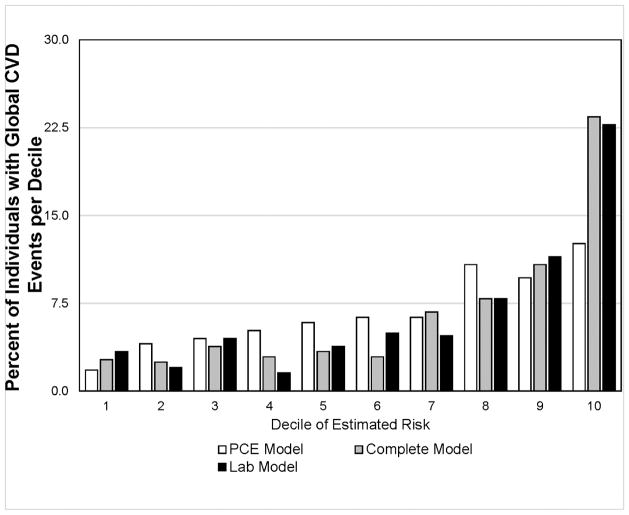
As shown in the Central Illustration, Panel A, individuals in the highest decile of estimated risk assessed by the complete model or the lab model accounted for ~35% of all CVD events, while 52% of all CVD events occurred in individuals in the upper two deciles of risk. The PCE risk model overestimated individuals with low risk and underestimated high-risk individuals over a 4-year period compared with the models using biomarkers. The addition of biomarkers to the PCE resulted in much better identification of higher risk individuals. As shown in the Central Illustration, Panel B, among individuals in the highest decile of risk, >22% had an incident global CVD event during a 4-year period. In marked contrast, <4% of individuals in the lower 5 deciles had an incident global CVD event.
Central Illustration. Global CVD events within ~4 years by deciles of risk estimates.
Pooled Cohort Equation (PCE) model: age, gender, race, hypertension, antihypertensive medication use, diabetes status, total cholesterol, and high-density lipoprotein cholesterol; complete model: PCE model + hs-cTnT, hs-CRP, and NT-proBNP; lab model: age, gender, race, hs-cTnT, hs-CRP, and NT-proBNP. A: Distribution of global CVD events by deciles of estimated risk; B: Percent of individuals in each estimated risk decile with incident global CVD event.
Discussion
In this study, we assessed the addition of three biomarkers—hs-cTnT, NT-proBNP, and hs-CRP, which are now routinely available for clinical use in the United States—to traditional risk factors used in the PCE to examine short-term risk stratification for global CVD events in a biracial cohort of older adults without known CVD at baseline. Over a follow-up period of ~4 years, all three biomarkers showed significant associations with global CVD events as well as with HF events in models adjusted for traditional CVD risk factors. The addition of all three biomarkers to the PCE model significantly improved parameters of discrimination and risk classification for global CVD event risk in older adults. Findings of the current study show that addition of hs-cTnT, NT-proBNP, and hs-CRP to PCE variables or to a simple “lab model” with age, gender, and race robustly improves short-term global CVD risk prediction in older adults.
Although older individuals have the highest absolute risk for CVD events, application of the 2013 ACC/AHA cholesterol guidelines (1) and the most recent 2017 ACC/AHA hypertension management guidelines (2) remains challenging in clinical practice for this group. Current recommendations base risk stratification on 10-year ASCVD risk calculated by the PCE (18). However, the PCE does not account for HF hospitalization, the most common incident event in our study in individuals without established CVD and a leading healthcare expenditure in the United States (4). In 2010, there were ~1 million hospitalizations for HF, with 71% in individuals aged ≥65 years and 53% in those aged ≥75 years (19). Given the expected rise in HF incidence in Americans (20,21), the need for HF prevention has recently been highlighted (22). A comprehensive risk assessment and identification of older adults at risk for global CVD events including HF is the first step in this direction. Indeed, the use of NT-proBNP was recently recommended for HF prevention in high-risk individuals (23). As shown in our study, risk stratification may be greatly improved by a combination of biomarkers.
The PCE does not apply to ages >79 years. The ACC/AHA cholesterol guidelines recommend using PCE-based risk for persons aged 76–79 years and take a conservative approach to initiation of statin therapy for primary prevention in persons >75 years of age. Per the guidelines, the decision to start statin therapy “requires consideration of additional factors, including increasing comorbidities, safety considerations, and priorities of care” because limited data are available from randomized controlled trials (1). Thus, statin therapy initiation in adults >75 years for primary prevention has been a subject of debate among physicians for several years (24). The concepts of the Precision Medicine Initiative (25) are especially relevant to CVD prevention. Quantitative data on risk for global CVD events are particularly important in older individuals, who are vulnerable to polypharmacy and at greater risk of side effects than younger individuals.
The current hypertension guidelines state, “it can be assumed that the vast majority of older adults have a 10-year ASCVD risk ≥10%, placing them in the high risk category that requires initiation of antihypertensive drug therapy at [blood pressure] ≥130/80 mm Hg.” In the Systolic Blood Pressure Intervention Trial (SPRINT), patients assigned to an intensive systolic blood pressure treatment target of <120 mm Hg versus the “standard” systolic blood pressure treatment goal of <140 mm Hg had a 25% lower relative risk of major CVD events and associated mortality (26); however, there were concerns about the side effects of pharmacotherapy in older adults (≥75 years) (27), particularly hypotension.
Recent trials (28,29) have shown that older participants have greater risk reduction in CVD events with more aggressive risk factor modification, including blood pressure treatment and the use of high-intensity statins for dyslipidemia management. A study from the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) showed that intensive statin therapy in a higher risk population, as determined by multiple biomarkers, provided a greater absolute risk reduction for primary cardiovascular events (30).
The association of traditional risk factors for CVD has been shown to attenuate in older adults (31). When the PCE variables were used at ARIC visit 4 (median age ~62 years), the AUC for global CVD was 0.724 (95% CI 0.711–0.737) vs 0.647 (95% CI 0.627–0.682) at visit 5 (median age ~74 years). Furthermore, at visit 5, AUC tended to be higher for age <80 years (0.659 [95% CI 0.634–0.705] vs 0.596 [95% CI 0.582–0.6891] for age ≥80 years). Both the complete and lab models outperformed the PCE model in adults >80 years with AUCs of 0.751 (95% CI 0.707–0.816) and 0.743 (95% CI 0.688–0.802), respectively, versus 0.596 (95% CI 0.582–0.689) for the PCE model (data not shown). We postulate that traditional risk factors work better in younger individuals because subclinical CVD tracks very closely with the presence of clinical risk factors, whereas in older individuals, risk factors such as hypertension are present in the vast majority of the population (70% in this study), and thus markers of subclinical “injury” such as hs-cTnT and NT-proBNP provide greater benefit in predicting short-term risk.
Further, the 10-year risk estimate provided by the PCE may not be the most relevant time frame for older individuals because of other causes of mortality besides cardiovascular events. Assessing short-term risk can enable physician patient discussions to focus on preventive strategies for shorter term goals that are modifiable on a practical annual basis.
Providing older adults with more precise estimation of short-time risk for global CVD events, and for the individual components such as HF, may provide useful information for the physician–patient discussion on the risks and benefits of initiation of statin therapy, dose of statin, or use of multiple drugs to treat hypertension and aid in joint decision making. For example, an individual with a global CVD risk of >20% over a 4-year period and elevated HF risk may be more motivated for both lifestyle modification and adherence to triple therapy to achieve a systolic blood pressure goal of <120 mm Hg. In addition, noninvasive testing such as echocardiogram may be useful to evaluate left ventricular function. Newer and more expensive therapies for dyslipidemia (32) and diabetes mellitus (33), which have shown CVD event reduction, may also be targeted to high-risk individuals. Alternatively, patients with low CVD risk of <4% over 4 years, approximately half of our study population, may choose not to take a high-dose statin and multiple drugs for hypertension.
The ARIC study and others have previously shown the incremental value of biomarkers and other diagnostic modalities in prediction of CHD and stroke events (6,7,34). A global risk model including both ASCVD and HF was defined in the Framingham cohort, but with mean age of 49 years, and only traditional CVD risk factors as covariates (35). Recently, global risk scores combining HF with ASCVD have been described (36) that incorporate cardiac biomarkers and coronary calcium computed tomography scores in US population studies in younger cohorts (mean 62 years in the Multi-Ethnic Study of Atherosclerosis and 44 years in the Dallas Heart Study).
Strengths and Limitations
There are several strengths of our study. The ARIC study is a well-characterized biracial population with a relatively large sample size, rigorously measured cardiovascular risk factors, and a large number of adjudicated global CVD events occurring within a 4-year period while on contemporary medical therapy. The three candidate biomarkers—hs-cTnT, hs-CRP, and NT-proBNP— are all available for clinical use in the United States.
Our study also has several limitations. All three biomarkers were measured as a one-time measurement from samples obtained during 2011–2013 for ARIC visit 5 and were not available in 4.6% of the population. Intraindividual variability (biological variability) is high for NT-proBNP. This was an observational study and our findings would need to be validated in another cohort; a prospective trial would be necessary to examine the clinical effectiveness of an approach in which biomarkers were used for risk stratification to determine initiation and intensity of pharmacotherapy along with the cost-effectiveness of such an approach; particularly given the paucity of data in older adults, our results could be used to design clinical trials with more reasonable sample sizes, endpoints, and duration to test preventive strategies in this understudied population. Also, our risk model was derived from 4-year follow-up in individuals that included statin users and therefore cannot be directly compared to the PCE or the current hypertension guidelines for 10-year risk based on non-statin-treated cohorts. Finally, no cutpoints for short-term global CVD risk are established in the elderly; therefore we examined continuous NRI and multiple cutpoints.
Conclusions
The addition of biomarkers to the PCE or use of biomarkers in a simpler “lab model” markedly improves short-term global CVD risk prediction in older adults. Such information may be useful in joint decision making on both the initiation and intensity of preventive therapies in older Americans. Confirmation of these findings in other cohorts and further studies to examine the cost-effectiveness of such an approach in older adults are warranted.
Supplementary Material
Online Table 1a. Baseline characteristics according to hs-cTnT categories
Online Table 1b: Baseline characteristics according to NT-proBNP categories
Online Table 1c: Baseline characteristics across hs-CRP categories
Online Table 2: The association of hs-cTnT, NT-proBNP, and hs-CRP as continuous variables with incident CHD, stroke, HF, and global CVD
Online Table 3: The association (presented as hazards ratio) and 4-year absolute risk for categories of hs-cTnT, NT-proBNP, and hs-CRP with incident CHD, stroke, HF, and global CVD
Online Table 4: Association of incident global CVD and biomarkers in PCE model + eGFR, hazard ratio and 95% CI for all covariates
Online Table 5: Baseline characteristics of individuals excluded for missing biomarker data
Online Table 6: Baseline characteristics of individuals excluded for missing data on prevalent HF hospitalization
Online Figure 1: Four-year risk of global CVD by hs-cTnT and NT-proBNP concentrations
The hazard ratios were calculated for Model 1; data adjusted for the clinical variables of the pooled cohort equation; age, gender, race, total cholesterol, smoking, high density lipoprotein cholesterol, hypertension, use of antihypertensive therapy and diabetes mellitus.
CLINICAL PERSPECTIVES.
Heart failure is a leading CVD event in the older adults and should be included as an outcome in CVD risk models. The Pooled Cohort Equation is a comprehensive risk assessment tool; however, it has several limitations applicable to older adults. Addition of readily measurable biomarkers—hs-cTnT, NT-proBNP and hs-CRP—improved the prediction of primary global CVD events in an older cohort of individuals on contemporary medical therapy. A more comprehensive risk stratification of older adults may be relevant to identify higher risk individuals who are candidates for more aggressive preventive strategies and to provide more cost-effective medical care.
TRANSLATIONAL OUTLOOK.
Future studies should test the reproducibility and confirm risk prediction improvement by a biomarker-based model in other elderly cohorts for both short-term and longer time periods. Further studies should also examine the cost-effectiveness of such an approach in older adults.
Acknowledgments
Funding: The Atherosclerosis Risk in Communities study has been funded in whole or in part with federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under contract nos. HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I). E.S. was supported by R01-DK089174. C.M.B. was supported by R01-HL134320.
The authors thank the ARIC participants and staff for their important contributions.
Abbreviations
- AUC
area under the receiver operating characteristic curve
- CHD
coronary heart disease
- CVD
cardiovascular disease
- HF
heart failure
- hs-CRP
high-sensitivity C-reactive protein
- hs-cTnT
high-sensitivity cardiac troponin T
- IDI
integrated discrimination index
- NRI
net reclassification index, NT-proBNP, N-terminal pro–B-type natriuretic peptide
- PCE
Pooled Cohort Equation
Footnotes
Disclosures: A. Saeed: None. V. Nambi: Research Grant; Significant; Roche Patent. Expert Witness; Modest; Siemens (compensation for event adjudication). W. Sun: None. S.S. Virani: Research Grant; Significant; AHA, ADA, VA. Honoraria; Significant; American College of Cardiology, National Lipid Association. Other; Significant; Steering comittee: PALM Registry at Duke University (no financial remuneration). G.E. Taffet: None. A. Deswal: None. E. Selvin: Research Grant; Significant; NIH, FNIH. K. Matsushita: None. L.E. Wagenknecht: None. R. Hoogeveen: Research Grant; Modest; Denka Seiken. J. Coresh: None. J. De Lemos: Consultant/Advisory Board; Modest; Roche Diagnostics, Abbott Diagnostics, Ortho Clinical Diagnostics, Amgen, Novo Nordisc, Regeneron. C.M. Ballantyne: Other Research Support; Significant; Abbott Diagnostic, Amarin, Amgen, Eli Lilly, Esperion, Novartis, Pfizer, Regneron, Roche, Sanofi, Takeda. Provisional patent (patent no. 61721475) entitled “Biomarkers to Improve Prediction of Heart Failure Risk” filed by Baylor College of Medicine and Roche (V. Nambi, R. Hoogeveen, CM Ballantyne).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 2.Whelton PK, Carey RM, Aronow WS, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017 doi: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Werner CA. [Accessed 30 January 2018];The older population. 2010 https://www.census.gov/prod/cen2010/briefs/c2010br-09.pdf.
- 4.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whelton SP, Roy P, Astor BC, et al. Elevated high-sensitivity C-reactive protein as a risk marker of the attenuated relationship between serum cholesterol and cardiovascular events at older age: the ARIC Study. Am J Epidemiol. 2013;178:1076–84. doi: 10.1093/aje/kwt086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saunders JT, Nambi V, de Lemos JA, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation. 2011;123:1367–76. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nambi V, Liu X, Chambless LE, et al. Troponin T and N-terminal pro-B-type natriuretic peptide: a biomarker approach to predict heart failure risk--the Atherosclerosis Risk in Communities study. Clin Chem. 2013;59:1802–10. doi: 10.1373/clinchem.2013.203638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 9.Rosamond WD, Folsom AR, Chambless LE, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30:736–43. doi: 10.1161/01.str.30.4.736. [DOI] [PubMed] [Google Scholar]
- 10.Rosamond WD, Chambless LE, Folsom AR, et al. Trends in the incidence of myocardial infarction and in mortality due to coronary heart disease, 1987 to 1994. N Engl J Med. 1998;339:861–7. doi: 10.1056/NEJM199809243391301. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal SK, Chambless LE, Ballantyne CM, et al. Prediction of incident heart failure in general practice: the Atherosclerosis Risk in Communities (ARIC) Study. Circ Heart Fail. 2012;5:422–9. doi: 10.1161/CIRCHEARTFAILURE.111.964841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosamond WD, Chang PP, Baggett C, et al. Classification of heart failure in the Atherosclerosis Risk in Communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012;5:152–9. doi: 10.1161/CIRCHEARTFAILURE.111.963199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharrett AR, Patsch W, Sorlie PD, Heiss G, Bond MG, Davis CE. Associations of lipoprotein cholesterols, apolipoproteins A-I and B, and triglycerides with carotid atherosclerosis and coronary heart disease. The Atherosclerosis Risk in Communities (ARIC) Study. Arterioscler Thromb. 1994;14:1098–104. doi: 10.1161/01.atv.14.7.1098. [DOI] [PubMed] [Google Scholar]
- 14.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 15.Oluleye OW, Folsom AR, Nambi V, Lutsey PL, Ballantyne CM for the ARIC Study Investigators. Troponin T, B-type natriuretic peptide, C-reactive protein, and cause-specific mortality. Ann Epidemiol. 2013;23:66–73. doi: 10.1016/j.annepidem.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–72. doi: 10.1002/sim.2929. discussion 207–12. [DOI] [PubMed] [Google Scholar]
- 17.Pencina MJ, D’Agostino RB, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Statistics in Medicine. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American College of Cardiology. [Accessed 13 January 2018];ASCVD risk estimator plus calculator. http://tools.acc.org/ASCVD-Risk-Estimator-Plus/#!/calculate/estimate/
- 19.Hall MJ, Levant S, DeFrances CJ. [Accessed 13 January 2018];Hospitalization for congestive heart failure: United States, 2000–2010. http://www.cdc.gov/nchs/data/databriefs/db108.pdf. [PubMed]
- 20.Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nature reviews Cardiology. 2011;8:30–41. doi: 10.1038/nrcardio.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. Jama. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 22.Nambi V, Deswal A, Ballantyne CM. Prevention of “Failure”: Is It a Failure of Prevention? Circulation. 2018;137:106–108. doi: 10.1161/CIRCULATIONAHA.117.030645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70:776–803. doi: 10.1016/j.jacc.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 24.Strandberg TE, Kolehmainen L, Vuorio A. Evaluation and treatment of older patients with hypercholesterolemia: A clinical review. JAMA. 2014;312:1136–1144. doi: 10.1001/jama.2014.10924. [DOI] [PubMed] [Google Scholar]
- 25.Precision Medicine Initiative Working Group. [Accessed 13 January 2018];The Precision Medicine Initiative Cohort Program – building a research foundation for 21st century medicine. http://www.nih.gov/sites/default/files/research-training/initiatives/pmi/pmi-working-group-report-20150917-2.pdf.
- 26.SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–16. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: A randomized clinical trial. JAMA. 2016;315:2673–2682. doi: 10.1001/jama.2016.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381–386. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 29.Everett BM, Zeller T, Glynn RJ, Ridker PM, Blankenberg S. High-sensitivity cardiac troponin I and B-type natriuretic peptide as predictors of vascular events in primary prevention: impact of statin therapy. Circulation. 2015;131:1851–60. doi: 10.1161/CIRCULATIONAHA.114.014522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Everett BM, Kurth T, Buring JE, Ridker PM. The relative strength of C-reactive protein and lipid levels as determinants of ischemic stroke compared with coronary heart disease in women. J Am Coll Cardiol. 2006;48:2235–42. doi: 10.1016/j.jacc.2006.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whelton SP, Roy P, Astor BC, et al. Elevated high-sensitivity C-reactive protein as a risk marker of the attenuated relationship between serum cholesterol and cardiovascular events at older age. The ARIC Study. Am J Epidemiol. 2013;178:1076–84. doi: 10.1093/aje/kwt086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med. 2017;376:1713–22. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 33.Zinman B, Lachin JM, Inzucchi SE. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2016;374:1094. doi: 10.1056/NEJMc1600827. [DOI] [PubMed] [Google Scholar]
- 34.Yeboah J, Young R, McClelland RL, et al. Utility of Nontraditional Risk Markers in Atherosclerotic Cardiovascular Disease Risk Assessment. J Am Coll Cardiol. 2016;67:139–147. doi: 10.1016/j.jacc.2015.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D’Agostino RB, Vasan RS, Pencina MJ, et al. General Cardiovascular Risk Profile for Use in Primary Care: The Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 36.de Lemos JA, Ayers CR, Levine B, et al. Multimodality Strategy for Cardiovascular Risk Assessment: Performance in 2 Population-Based Cohorts. Circulation. 2017;135:2119–2132. doi: 10.1161/CIRCULATIONAHA.117.027272. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Table 1a. Baseline characteristics according to hs-cTnT categories
Online Table 1b: Baseline characteristics according to NT-proBNP categories
Online Table 1c: Baseline characteristics across hs-CRP categories
Online Table 2: The association of hs-cTnT, NT-proBNP, and hs-CRP as continuous variables with incident CHD, stroke, HF, and global CVD
Online Table 3: The association (presented as hazards ratio) and 4-year absolute risk for categories of hs-cTnT, NT-proBNP, and hs-CRP with incident CHD, stroke, HF, and global CVD
Online Table 4: Association of incident global CVD and biomarkers in PCE model + eGFR, hazard ratio and 95% CI for all covariates
Online Table 5: Baseline characteristics of individuals excluded for missing biomarker data
Online Table 6: Baseline characteristics of individuals excluded for missing data on prevalent HF hospitalization
Online Figure 1: Four-year risk of global CVD by hs-cTnT and NT-proBNP concentrations
The hazard ratios were calculated for Model 1; data adjusted for the clinical variables of the pooled cohort equation; age, gender, race, total cholesterol, smoking, high density lipoprotein cholesterol, hypertension, use of antihypertensive therapy and diabetes mellitus.