Abstract
Purpose of review
Waterborne enteric pathogens remain a global health threat. Increasingly, quantitative microbial risk assessment (QMRA) and infectious disease transmission modeling (IDTM) are used to assess waterborne pathogen risks and evaluate mitigation. These modeling efforts, however, have largely been conducted independently for different purposes and in different settings. In this review, we examine the settings where each modeling strategy is employed.
Recent findings
QMRA research has focused on food contamination and recreational water in high-income countries (HICs) and drinking water and wastewater in low- and middle-income countries (LMICs). IDTM research has focused on large outbreaks (predominately LMICs) and vaccine-preventable diseases (LMICs and HICs).
Summary
Human ecology determines the niches that pathogens exploit, leading researchers to focus on different risk assessment research strategies in different settings. To enhance risk modeling, QMRA and IDTM approaches should be integrated to include dynamics of pathogens in the environment and pathogen transmission through populations.
Keywords: quantitative microbial risk assessment, infectious disease transmission modeling, waterborne pathogen, enteric disease, human ecology
Introduction
Waterborne pathogens that cause enteric and diarrheal disease remain a global public health threat. The Global Burden of Disease study estimates that 1.3 million people, including 0.5 million children under five, died from diarrheal disease in 2015 [1]. Much of this burden is in low- and middle-income countries (LMICs) [2], largely due to inadequate water and sanitation infrastructure. Although the waterborne disease burden in high-income countries (HIC) is lower, outbreaks caused by emerging pathogens and aging infrastructure have resulted in an increase in research and public health activity. In both LMICs and HICs, global climate change (and resulting changes in temperature, rainfall, and extreme weather) has increased the burden of waterborne disease [3, 4]. In the coming decades, climate change is expected to continue to increase this burden and complicate control efforts [5, 6]. To address these public health issues, models have been used for assessing risks and evaluating mitigation efforts. The specific choice of modeling strategy depends on policy question of interest, which will vary by setting. Here, we conduct a literature review to examine the extent to which modeling strategy, as well as the pathogen and transmission pathways of interest, is influenced by the income setting of the study site.
Waterborne enteric pathogens include bacteria, viruses, and protozoa and can exploit a variety of transmission pathways. The major pathogens of concern are pathovars of Escherichia coli (EIEC, ETEC, EPEC, EHEC, EAEC, etc.), Salmonella spp., Shigella spp., Campylobacter spp., Vibrio cholerae, Cryptosporidium spp., Giardia spp., rotavirus, norovirus, poliovirus, and hepatitis A. These pathogens are transmitted through multiple pathways, including water (drinking or recreational water), food, fomites, soil, and contact with animals, which complicates intervention strategies and assessments of disease risk. Moreover, these multiple pathways can be interdependent [7]; e.g., water can be the driver of contamination even when it is not the proximate route of exposure to humans, as in the case of produce contaminated by irrigation. This complex system of transmission pathways can hinder pinpointing the “cause” of the disease, but it can also create opportunities for interventions at different points along the transmission chain. Increasingly, models are being used to examine modes of transmission and optimal intervention strategies. The choice of model framework is often dependent on the specific transmission pathways and patterns of disease observed in a given context.
In their seminal paper, Levine and Levine [8] argued that the distinct human ecologies in developing and developed country contexts have led to distinct patterns of enteric disease epidemiology. In LMICs, for example, increasing urbanization has driven increased population densities, often with insufficient water and sanitation infrastructure; decreasing breast-feeding has increased children’s susceptibility to infection and disease; changing agriculture practices have increased fecal contamination and exposure to zoonotic pathogens; and increasingly disruptive and destructive periods of violence and political strife can devastate infrastructure and create high pathogen transmission conditions. In HICs, on the other hand, access to developed and advanced water and sanitation infrastructure has largely eliminated waterborne enteric bacterial disease. Waterborne viruses and certain protozoa (e.g., rotavirus, Cryptosporidium), however, are still of concern. Ecological niches for pathogen transmission in HIC settings include concentrated animal feeding operations (CAFOs) and large-scale food-service operations; agricultural and food-based operations remain a vehicle for outbreaks of (largely bacterial) enteric illness. Moreover, the increasing dependence on care institutions (e.g., child or geriatric care centers) has left certain populations vulnerable to both acute and chronic enteric infections. These different ecological niches across different national income strata have resulted in different research priorities and strategies. Specifically, different risk models are implemented depending on many factors, including disease burden and environmental contamination levels. This review seeks to clarify how these niches have resulted in divergent use of two modeling strategies. Quantitative microbial risk assessment (QMRA) and infectious disease transmission modeling (IDTM) have both been used to better understand the magnitude and source of disease risk, the contributions of different transmission pathways, and the opportunities for interventions.
QMRA is a modeling framework for characterizing pathogen exposure from the environment through an exposure assessment and for characterizing human risk from that exposure using a dose–response model [9]. QMRA models are comprised of a series of probabilistic steps informed by the literature or primary data collection; the whole model is run many times to generate a distribution of outcomes (Figure 1a). QMRA was initially applied to estimate risk of disease from waterborne pathogens in the 1980s [10–12], using dose–response functions fit to data from the literature [13, 14], and it has since been used in a variety of risk settings. Technological and methodological advances have continued to improve QMRA, and it has been adopted as an assessment framework in many regulatory settings. QMRA models can be limited by lack of information for the specific context; for example, 1) the dose-response function may be estimated based on an animal model or on a different exposure medium; 2) the treatment efficacy may be only known under low turbidity conditions; or 3) the human-environment contact network may not be well-characterized. Furthermore, the uncertainty at each step is compounded. Even with these limitations, QMRA models excel at representing how heterogeneities interact to produce rare, stochastic harm events.
Figure 1.
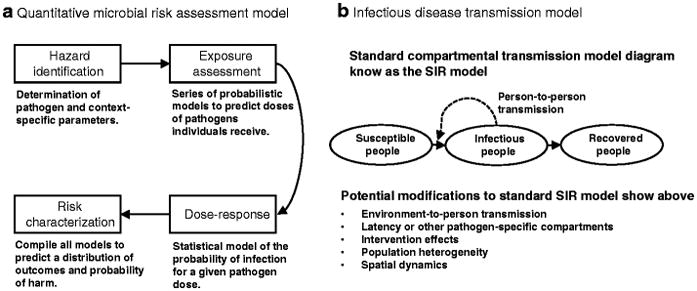
Schematics of typical a) quantitative microbial risk assessment (QMRA) and b) infectious disease transmission model (IDTM) research studies.
IDTM has long been used as a tool in mathematical epidemiology to connect underlying transmission mechanisms to observed outbreaks, to make predictions of incidence, and to investigate the likely impact of control strategies. The fundamental framework is the susceptible–infectious–recovered, or SIR, model developed in the early 1900s [15] that models person-to-person contact and infection transmission in a population of individuals (Figure 1b). Although preliminary work modeling waterborne pathogens in humans began to gain traction in the early 2000s, including retrospective analysis of the 1994 Cryptosporidium outbreak in Milwaukee, WI [16, 17], it was the 2010 cholera outbreak in Haiti that marked a turning point for the field. The 2010 Haiti outbreak prompted an increased interest in IDTM methods for waterborne risk characterization and methods development. For waterborne enteric pathogens, direct person-to-person transmission is typically only one of multiple exposure pathways; environmental exposures—through water, food, or fomites—are a significant, or even the primary, source of infection in many contexts. Modelers have only recently begun to develop and adopt SIR-type models that include an explicit description of an environmental transmission pathway to address this concern [18–21]. In particular, the field of cholera modeling (responding to the 2010 outbreak in Haiti), has been an important adopter of this environmental framework and has a significant presence in the literature (citations are included in supplementary information). While IDT model are typically limited to describing average behaviors, they have been powerful tools for analyzing population level-dynamics.
Despite similarities in conceptual framework and goals, there has been relatively little methodological crossover between QMRA and IDTM. For instance, QMRA rarely considers possible secondary transmission in estimates of risk, while IDTM is only beginning to incorporate pathogen measurement and environmental surveillance. Through a literature review, we explore how research in different settings has leveraged varied modeling strategies and how these strategies differ specifically according to income context (HIC or LMIC), pathogens of study, and environmental transmission pathway.
Literature search methods
We identified references for this review through a search of PubMed for articles published between January 1, 2012 and September 16, 2017 with search terms “E. coli” or “Escherichia coli” or “Salmonella” or “typhoid” or “Shigella” or “Campylobacter” or “Cholera” or “Vibrio cholerae” or “Norovirus” or “Cryptosporidium” or “Giardia” or “polio” or “hepatitis A” or “waterborne pathogens” or “enteric pathogens” or “enteropathogens” AND “risk assessment” or “quantitative microbial risk assessment” or “QMRA” or “transmission dynamics” or “mathematical model” or “infectious disease model” or “transmission model”. We chose this set of waterborne pathogens for their important role in enteric disease globally; although other pathogens may share some common transmission pathways (e.g., foodborne pathogens), we only included pathogens for which water was a significant transmission pathway. After reviewing the results of this initial search, we identified 220 publications that form the basis of this review. Although not every reviewed paper is discussed, a full bibliography is provided in the supplementary material. Because there were relatively few IDTM compared with QMRA studies, citations for IDTM papers prior to 2012 are also included in the supplementary material. This review does not include studies using IDTM models with environmental pathways if they were published in journals not indexed by PubMed or if they were not used in a waterborne disease context.
Since environmental pathways exploited by pathogens are multiple and interdependent, the pathway involved in contamination is not necessarily the pathway involved in proximate exposure. For example, contaminated agricultural runoff may be the source of river contamination, though exposure occurs through recreational activities in or on the river, such as swimming or boating. In this review, we classify the transmission pathway of an article by its main emphasis, which, in this example, might be wastewater if it focuses on agricultural contamination or might be recreational water if the focus is on recreational exposure.
Results
QMRA and IDTM Research Objectives
There were 149 QMRA articles that met our study criteria, 93 of which were conducted in HICs (62%). The number of QMRA papers in HICs has been slowly increasing over the past 5 years but the number of published articles in LMICs has remained largely constant (supplement Figure S1a). There were 72 IDTM articles that met our study criteria; most were grounded in LMIC contexts (47%), with the remainder split between HIC (24%) and generic/unspecified (29%) contexts (supplement Figure S1b). QMRA studies explicitly focus on environmental risk and are designed to assess risk and probability of rare events under heterogeneous conditions. They are, therefore, largely focused on evaluation of an existing, large-scale process or treatment or a novel, smaller-scale treatment. The research objectives for IDTM studies, on the other hand, are focused on population-level dynamics. IDTM studies, therefore, are concerned with understanding epidemiological patterns of outbreaks or evaluating the impacts of possible policies or interventions, primarily vaccination. The advancement of modeling methods was explored for both QMRA and IDTM. These papers are not discussed in this review, but are included in the supplementary information.
QMRA studies that evaluated treatment or intervention strategies disproportionately came from HICs. In the HIC context, most studies considered wastewater treatment or other water management strategies [22–32] or the treatment of food products before consumption [33–38]. A handful of papers considered hand or fomite disinfection [39–41], household-level water treatment [42], or vaccination of animals against waterborne pathogens [43]. In LMICs, there was more emphasis on household water treatment [44–47] and less on wastewater treatment or water management [48–52], the latter primarily coming from upper-middle-income countries. Studies also considered treatment of livestock waste [53] and disinfection of kitchen clothing [54]. Papers evaluating current practices or infrastructure, on the other hand, were dominated by assessment of wastewater irrigation in both HICs [55–64] and LMICs [30, 65–73]. Studies evaluating other wastewater management practices [74, 75], rainwater collection [76–78], and food preparation and supply management [79–81] were done in both HICs and LMICs.
The research objectives for IDTM studies were primarily outbreak assessment and vaccination or other intervention assessment. Outbreak assessment papers were dominated by cholera [82–93] and polio [94–96]. Cholera [97–102] and polio [103–108] were also well represented among vaccination and other policy assessments; vaccination (and vaccine cost-effectiveness in particular) was the primary focus of rotavirus IDTM analyses [109–113]. Other objectives included dynamics analysis or risk-of-outbreak assessment.
Pathways and pathogens: research priorities in LMIC and HIC settings
QMRA
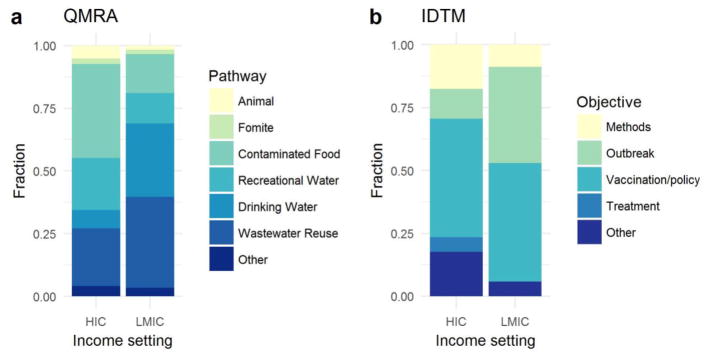
When comparing research using QMRA in LMIC and HIC research settings, we find a greater focus on food (38% HIC vs. 16% LMIC) and recreational water (21% HIC vs. 12% LMIC) in HICs (N=93) relative to LMICs (Figure 2a). These exposure pathways are exploited in developed countries because of the high-intensity farming practices and remaining vulnerabilities with non-point and point source contamination. In LMICs (N=50), on the other hand, the focus shifts toward wastewater contamination (36% LMIC vs. 23% HIC) and drinking water (29% LMIC vs. 7% HIC), likely because of the prevalent use of wastewater in agriculture and the limited availability of effective water treatment. These priorities are consistent with the human–pathogen ecology described by Levine and Levine [8].
Figure 2.
Distribution of a) quantitative microbial risk assessment (QMRA) research studies by transmission pathway and b) infectious disease transmission model (IDTM) research studies by study objective. The distributions are stratified by income context (low- and middle-income (LMIC) and high-income (HIC) countries). IDTM articles without a specific country context are not included in this figure.
HICs have robust drinking water and sanitation infrastructures but also have industrialized food systems that are vulnerable to large-scale contamination and citizens with generally more leisure time to enjoy recreational water through swimming, paddling, or other activities on rivers, lakes, and beaches. The most commonly studied waterborne pathogens in the HIC context, therefore, were either associated with food (Campylobacter (N=28), norovirus (N=24), and non-typhi Salmonella (N=22)), recreational water (Cryptosporidium (N=28)), or both (E. coli (N=30)). Even if food is the means of transmission of the pathogen to the population, it is not necessarily the source of contamination. Contamination sources may be wastewater irrigation, food handlers, etc. Foodstuffs of concern were primarily related to animal products—pork [35, 81, 114–117], beef [118–121], chicken [79, 122–125], shellfish [37, 126], and eggs and dairy [127–133]—but contaminated vegetable crops, such as nuts [33, 34, 134] and greens [29, 58, 61, 63, 135–137] were also studied. The pathogens most often investigated in food-related transmission context were Salmonella (N=8), Campylobacter (N=7), and E. coli (N=6). There are many other pathogens (e.g., Listeria) and toxins that are of concern in food-based risk assessment, but they were not included in this review as they are not considered waterborne pathogens.
Risk from exposure to water, particularly recreational water but also drinking water, was another major focus of QMRA in HICs and includes exposure from rivers, streams, or beaches [62, 138–154], urban infrastructure [155–157], and swimming pools or water parks [158, 159]. Sources of contamination ranged from agricultural to urban, and the primary pathogens of concern were Cryptosporidium (N=14), Giardia (N=10), E. coli (N=11), norovirus (N=8), and Campylobacter (N=9). Drinking water risk is still a concern in HICs because agencies like the U.S. EPA regulate drinking water treatment plants at low risk thresholds, on the order of 1 case in 10,000. Most work in this area has focused on wastewater reuse [23–25, 64, 74, 160], drinking water sourced from surface water [27, 32, 146, 161], and agricultural [57] or urban run-off or discharge [28, 142]. The pathogens most often considered in this context are Cryptosporidium (N=9) and E. coli (N=5), with lesser representation of norovirus (N=4) and Giardia (N=3), among others.
In LMICs, on the other hand, water and sanitation infrastructure can vary quite widely within a country, ranging from unimproved to safely managed. Increased pathogen load and transmission caused by poor infrastructure, as well as an international focus on epidemics flourishing in the wake of natural disasters or war—such as cholera in Haiti and Yemen and polio in Syria [162]—has resulted in research efforts directed at drinking water (29%) and wastewater (36%) (Figure 1). The major pathogens studied in the LMIC context are E. coli (N=20), Giardia (N=15), Cryptosporidium (N=13), Salmonella (including S. typhi; N=13), and rotavirus (N=12).
Drinking water was the major exposure pathway of concern in LMICs. Recent risk assessments have also emphasized the source of the drinking water contamination pathway. For example, studies in LMICs have considered contaminated surface water [163–166], groundwater [167, 168], rainwater [78], treated water [169], and multisource periurban water [48, 170, 171]. Researchers have also considered the impacts of intermittent water supplies [172] and household water treatment [45–47, 173]. Major pathogens considered in the drinking water context were E. coli (N=10), Cryptosporidium (N=7), Giardia (N=6), rotavirus (N=4), and Salmonella (N=4).
Wastewater was another common focus of risk assessment in LMIC, covering wastewater irrigation [30, 51, 52, 66, 67, 71–73, 174], livestock waste [53, 68], non-potable reuse or greywater [44, 175], treatment failure [49], and effluent discharge [50]. The pathogens of interest were varied and included E. coli (N=5), Giardia (N=4), Salmonella (N=3), rotavirus (N=3), and Cryptosporidium (N=3). The majority of wastewater risk assessments considered crop irrigation as the final exposure route, but we classify these as focusing on wastewater rather than foodborne exposures because of the focus on the source of contamination and the consideration of other relevant pathways, such as accidental ingestion by farm workers and their children.
IDTM
In this review, we found IDTM analyses contributing a wide variety of insights, from the role of drug resistance in recent Typhoid fever outbreaks [111], to optimization of oral cholera vaccination campaigns [98], to the risk of spread of polio from Hajj pilgrims and other international travelers [106]. The IDTM papers in our literature search differed from the QMRA papers in their conceptualization of transmission pathways. Compared to QMRA, exposure pathways are typically abstracted in IDTM into direct, person-to-person transmission and, sometimes, indirect, environmentally mediated transmission. Even when the transmission pathways are contextualized for the pathogen and population, the modeling mechanics typically remain the same. Unlike QMRA, inclusion of any kind of environmental data was rare, even when an environmental compartment was explicitly included in the model.
Recent IDTM work differs from QMRA not only in terms of transmission pathways but also in the set of pathogens than that it focuses on: cholera [82–93, 97–102, 176–179], polio [94–96, 103–108, 180–184], rotavirus [109–113, 185], and hepatitis A [186–190] were the most common, though Salmonella typhi [191–193] and norovirus [194–196] were also represented. The pathogens represented in IDTM papers were primarily either associated with recent, highly politicized outbreaks (e.g., cholera) or vaccine-preventable diseases (e.g., polio, rotavirus), in stark contrast to the pathogen distribution among QMRA papers. IDTM studies were overwhelmingly set in LMICs, perhaps due to the fact that there are more epidemiological studies in LMICs collecting health data that are exploited by IDTM and less of the environmental surveillance data that is generally exploited by QMRA studies. Exceptions where we see IDTM studies in HIC settings are for those pathogens where a vaccine has been or is currently being developed, such as rotavirus and norovirus, and for those pathogens where this is risk of an outbreak after importation into a HIC (e.g., [181]). Indeed, while outbreak assessment was primarily seen in the LMIC context, policy-driven analyses made up a large fraction of papers in both HIC and LMIC context (Figure 2b). IDTM methods papers often did not specify an income context.
Conclusions
Human ecology shapes the ecological niches exploited by human pathogens [8], which has led to different risk assessment research objectives in LMICs and HICs. In HICs, food and recreational water are the major sources of exposure, and risk assessment is often closely tied to regulation. In LMICs, research has focused on drinking water and wastewater and is particularly concerned with sources of contamination and developing intervention strategies. While these specific research objectives are largely natural extensions of the human and pathogen ecology, it is important to recognize that risks of waterborne disease are not homogeneous, particularly in LMICs.
We found very little crossover between QMRA and IDTM approaches to waterborne risk characterization and assessment. Only one recent QMRA study explicitly assessed an outbreak (salmonellosis in beef burgers in France [120]). Secondary transmission is typically neglected in QMRA, especially in HICs, and, when it is considered, it is typically in the form of a secondary attack rate parameter (e.g., [63]) and not dynamic modeling. Although the benefits of including IDTM thinking and techniques into QMRA have been previously examined [19], the practice has not yet caught on. The lack of consideration of secondary transmission may stem from the perception that secondary transmission is negligible, particularly in HICs where conditions force outbreaks to die out. However, even if an outbreak is not transiently amplified, there maybe be a significant burden of secondary infections as the outbreak dies out (e.g., as much as 20% of E. coli cases in outbreaks [197]). Moreover, vulnerable or high-risk populations can sustain outbreaks in settings where an outbreak would not take-off among general population. For example, 2017–18 Hepatitis A outbreaks in California, Michigan, and Utah were predominantly concentrated among homeless people and drug users [198]. Increasing rates of vaccine refusal have also created pockets of higher transmission potential.
IDTM can also benefit by adopting some of the exposure assessment approaches used in of QMRA, such as the modeling of environmental pathways. Although a number of waterborne disease IDTMs now include environmental compartments and transmission pathways, these choices have largely been driven by the desire to improve fitting and parameter estimation (e.g., [91]) or to better model spatial spread (e.g., [199, 200]). Leveraging environmental sampling to inform and verify modeled exposures has thus far been rare (e.g., [201]) but has potential to significantly increase the impact of and confidence in IDTM results.
Real advances in risk assessment will soon be possible by incorporating faster and more precise environmental pathogen measurement into both QMRA and IDTM. Although many recent QMRAs do measure pathogens in the environment as a basis for risk assessment, many do not, and the use of proxy indicator pathogens is still prevalent despite being an unreliable surrogate. Assays, such as those using the TaqMan Array Card [202], are being developed to quickly quantify multiple pathogens of interest. Methodological work, such as hydrodynamic modeling or the refinement of the assumptions of exposure quantification, has also advanced and is an important part of improving modeling practices. Ultimately, by reducing the uncertainty in exposure, risk assessors will be able to more precisely characterize and quantify risks from waterborne pathogens.
Historically, QMRA and IDTM studies have been developed and conducted independently with differing research objectives. We argue that risk modeling activities will be enhanced if these modeling approaches are integrated to include dynamics of both the fate and transport of pathogens in the environment as well as the transmission of pathogen through populations.
Supplementary Material
Footnotes
Compliance with Ethical Standards
Conflict of Interest
Nina B. Masters declares that she has no conflict of interest. Andrew F. Brouwer and Joseph N. S. Eisenberg reports grants from NIH/NIGMS, during the course of this study.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
* Of importance
* Of major importance
- 1.GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet. 2016;388(10053):1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Julian TR. Environmental transmission of diarrheal pathogens in low and middle income countries. Environmental Science: Processes & Impacts. 2016;18(8):944–955. doi: 10.1039/c6em00222f. [DOI] [PubMed] [Google Scholar]
- 3.Curriero FC, Patz JA, Rose JB, Lele S. The Association Between Extreme Precipitation and Waterborne Disease Outbreaks in the United States, 1948–1994. American Journal of Public Health. 2001;91(8):1194–1199. doi: 10.2105/ajph.91.8.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wade TJ, Sandhu SK, Levy D, Lee S, LeChevallier MW, Katz L, et al. Did a Severe Flood in the Midwest Cause an Increase in the Incidence of Gastrointestinal Symptoms? American Journal of Epidemiology. 2004;159(4):398–405. doi: 10.1093/aje/kwh050. [DOI] [PubMed] [Google Scholar]
- 5.Levy K, Woster AP, Goldstein RS, Carlton EJ. Untangling the Impacts of Climate Change on Waterborne Diseases: A Systematic Review of Relationships between Diarrheal Diseases and Temperature, Rainfall, Flooding, and Drought. Environmental Science and Technology. 2016;50(10):4905–4922. doi: 10.1021/acs.est.5b06186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lo Iacono G, Armstrong B, Fleming LE, Elson R, Kovats S, Vardoulakis S, et al. Challenges in developing methods for quantifying the effects of weather and climate on water-associated diseases: A systematic review. PLOS Neglected Tropical Diseases. 2017;11(6):e0005659. doi: 10.1371/journal.pntd.0005659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eisenberg JNS, Trostle J, Sorensen RJD, Shields KF. Toward a Systems Approach to Enteric Pathogen Transmission: From Individual Independence to Community Interdependence. Annual Review of Public Health. 2012;33(1):239–257. doi: 10.1146/annurev-publhealth-031811-124530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levine MM, Levine OS. Changes in human ecology and behavior in relation to the emergence of diarrheal diseases, including cholera. Proceedings of the National Academy of Sciences. 1994;91(7):2390–4. doi: 10.1073/pnas.91.7.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haas CN, Rose JB, Gerba CP. Quantitative Microbial Risk Assessment. Hoboken, NJ: John Wiley & Sons, Inc; 2014. [Google Scholar]
- 10.Haas CN. Estimation of risk due to low doses of microorganisms: a comparison of alternative methodologies. American Journal of Epidemiology. 1983;118(4):573–582. doi: 10.1093/oxfordjournals.aje.a113662. [DOI] [PubMed] [Google Scholar]
- 11.Rose JB, Haas CN, Regli S. Risk assessment and control of water borne giardiasis. American Journal of Public Health. 1991;81(6):709–713. doi: 10.2105/ajph.81.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haas CN, Rose JB, Gerba C, Regli S. Risk assessment of virus in drinking water. Risk analysis: an official publication of the Society for Risk Analysis. 1993;13(5):545–52. doi: 10.1111/j.1539-6924.1993.tb00013.x. [DOI] [PubMed] [Google Scholar]
- 13.Teunis PFM, van der Heijden OG, van der Giessen JWB, Havelaar AH. The dose-response relation in human volunteers for gastrointestinal pathogens. Bilthoven, The Netherlands: National Institute of Public Health and the Environment; 1996. [Google Scholar]
- 14.Teunis PFM, Nagelkerke NJD, Haas CN. Dose response models for infectious gastroenteritis. Risk Analysis. 1999;19(6):1251–1260. doi: 10.1023/a:1007055316559. [DOI] [PubMed] [Google Scholar]
- 15.Kermack WO, McKendrick AG. A Contribution to the Mathematical Theory of Epidemics. Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences. 1927;115(772):700–721. [Google Scholar]
- 16.Eisenberg JNS, Brookhart MA, Rice G, Brown M, Colford JM. Disease transmission models for public health decision making: Analysis of epidemic and endemic conditions caused by waterborne pathogens. Environmental Health Perspectives. 2002;110(8):783–790. doi: 10.1289/ehp.02110783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisenberg JNS, Lei X, Hubbard AH, Brookhart MA, Colford JM. The role of disease transmission and conferred immunity in outbreaks: Analysis of the 1993 Cryptosporidium outbreak in Milwaukee, Wisconsin. American Journal of Epidemiology. 2005;161(1):62–72. doi: 10.1093/aje/kwi005. [DOI] [PubMed] [Google Scholar]
- 18.Codeço CT. Endemic and epidemic dynamics of cholera: the role of the aquatic reservoir. BMC infectious diseases. 2001;1:1. doi: 10.1186/1471-2334-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chick SE, Koopman JS, Soorapanth S, Brown ME. Infection transmission system models for microbial risk assessment. Science of the Total Environment. 2001;274(1–3):197–207. doi: 10.1016/s0048-9697(01)00749-5. [DOI] [PubMed] [Google Scholar]
- 20.Li S, Spicknall IH, Koopman JS, Eisenberg JNS. Dynamics and control of infections transmitted from person to person through the environment. American Journal of Epidemiology. 2009;170(2):257–265. doi: 10.1093/aje/kwp116. [DOI] [PubMed] [Google Scholar]
- 21.Tien JH, Earn DJD. Multiple transmission pathways and disease dynamics in a waterborne pathogen model. Bulletin of Mathematical Biology. 2010;72(6):1506–33. doi: 10.1007/s11538-010-9507-6. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi N, Oshiki M, Ito T, Segawa T, Hatamoto M, Kato T, et al. Removal of human pathogenic viruses in a down-flow hanging sponge (DHS) reactor treating municipal wastewater and health risks associated with utilization of the effluent for agricultural irrigation. Water Research. 2017;110:389–398. doi: 10.1016/j.watres.2016.10.054. [DOI] [PubMed] [Google Scholar]
- 23.Pecson BM, Triolo SC, Olivieri S, Chen EC, Pisarenko AN, Yang CC, et al. Reliability of pathogen control in direct potable reuse: Performance evaluation and QMRA of a full-scale 1 MGD advanced treatment train. Water Research. 2017;122:258–268. doi: 10.1016/j.watres.2017.06.014. [DOI] [PubMed] [Google Scholar]
- 24.Chaudhry RM, Hamilton KA, Haas CN, Nelson KL. Drivers of microbial risk for direct potable reuse and de facto reuse treatment schemes: The impacts of source water quality and blending. International Journal of Environmental Research and Public Health. 2017;14(6):1–20. doi: 10.3390/ijerph14060635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amoueyan E, Ahmad S, Eisenberg JNS, Pecson B, Gerrity D. Quantifying pathogen risks associated with potable reuse: A risk assessment case study for Cryptosporidium. Water Research. 2017;119:252–266. doi: 10.1016/j.watres.2017.04.048. [DOI] [PubMed] [Google Scholar]
- 26.Chhipi-Shrestha G, Hewage K, Sadiq R. Microbial quality of reclaimed water for urban reuses: Probabilistic risk-based investigation and recommendations. Science of the Total Environment. 2017;576:738–751. doi: 10.1016/j.scitotenv.2016.10.105. [DOI] [PubMed] [Google Scholar]
- 27.Krkosek W, Reed V, Gagnon GA. Assessing protozoan risks for surface drinking water supplies in Nova Scotia, Canada. Journal of Water and Health. 2016;14(1):155–166. doi: 10.2166/wh.2015.034. [DOI] [PubMed] [Google Scholar]
- 28*.Sokolova E, Petterson SR, Dienus O, Nystrom F, Lindgren PE, Pettersson TJR. Microbial risk assessment of drinking water based on hydrodynamic modelling of pathogen concentrations in source water. Science of the Total Environment. 2015;526:177–186. doi: 10.1016/j.scitotenv.2015.04.040. This analysis incorporated hydrological dynamics into a QMRA of norovirus in drinking water. [DOI] [PubMed] [Google Scholar]
- 29.Amha YM, Kumaraswamy R, Ahmad F. A probabilistic QMRA of Salmonella in direct agricultural reuse of treated municipal wastewater. Water Science and Technology. 2015;71(8):1203–1211. doi: 10.2166/wst.2015.093. [DOI] [PubMed] [Google Scholar]
- 30.Mok HF, Barker SF, Hamilton AJ. A probabilistic quantitative microbial risk assessment model of norovirus disease burden from wastewater irrigation of vegetables in Shepparton, Australia. Water Research. 2014;54:347–362. doi: 10.1016/j.watres.2014.01.060. [DOI] [PubMed] [Google Scholar]
- 31.Agulló-Barceló M, Casas-Mangas R, Lucena F. Direct and indirect QMRA of infectious cryptosporidium oocysts in reclaimed water. Journal of Water and Health. 2012;10(4):539–548. doi: 10.2166/wh.2012.082. [DOI] [PubMed] [Google Scholar]
- 32.Pintar KDM, Fazil A, Pollari F, Waltner-Toews D, Charron DF, Mcewen SA, et al. Considering the risk of infection by cryptosporidium via consumption of municipally treated drinking water from a surface water source in a southwestern Ontario community. Risk Analysis. 2012;32(7):1122–1138. doi: 10.1111/j.1539-6924.2011.01742.x. [DOI] [PubMed] [Google Scholar]
- 33.Farakos SMS, Pouillot R, Johnson R, Spungen J, Son I, Anderson N, et al. A Quantitative assessment of the risk of human salmonellosis arising from the consumption of almonds in the United States: The impact of preventive treatment levels. Journal of Food Protection. 2017;80(5):863–878. doi: 10.4315/0362-028X.JFP-16-403. [DOI] [PubMed] [Google Scholar]
- 34.Farakos SMS, Pouillot R, Johnson R, Spungen J, Son I, Anderson N, et al. A Quantitative assessment of the risk of human salmonellosis arising from the consumption of pecans in the United States. Journal of Food Protection. 2017;80(9):1574–1591. doi: 10.4315/0362-028X.JFP-16-511. [DOI] [PubMed] [Google Scholar]
- 35.Møller COdA, Nauta MJ, Schaffner DW, Dalgaard P, Christensen BB, Hansen TB. Risk assessment of Salmonella in Danish meatballs produced in the catering sector. International Journal of Food Microbiology. 2015;196:109–125. doi: 10.1016/j.ijfoodmicro.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 36.Gayán E, Torres JA, Ávarez I, Condón S. Selection of process conditions by risk assessment for apple juice pasteurization by UV-heat treatments at moderate temperatures. Journal of Food Protection. 2014;77(2):207–215. doi: 10.4315/0362-028X.JFP-13-255. [DOI] [PubMed] [Google Scholar]
- 37.Praveen C, Dancho BA, Kingsley DH, Calci KR, Meade GK, Mena KD, et al. Susceptibility of murine norovirus and hepatitis a virus to electron beam irradiation in oysters and quantifying the reduction in potential infection risks. Applied and Environmental Microbiology. 2013;79(12):3796–3801. doi: 10.1128/AEM.00347-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Espinosa AC, Jesudhasan P, Arredondo R, Cepeda M, Mazari-Hiriart M, Mena KD, et al. Quantifying the reduction in potential health risks by determining the sensitivity of poliovirus type 1 chat strain and rotavirus SA-11 to electron beam irradiation of iceberg lettuce and spinach. Applied and Environmental Microbiology. 2012;78(4):988–993. doi: 10.1128/AEM.06927-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tamimi AH, Maxwell S, Edmonds SL, Gerba CP. Impact of the use of an alcohol-based hand sanitizer in the home on reduction in probability of infection by respiratory and enteric viruses. Epidemiology and Infection. 2015;143(15):3335–3341. doi: 10.1017/S0950268815000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ryan MO, Haas CN, Gurian PL, Gerba CP, Panzl BM, Rose JB. Application of quantitative microbial risk assessment for selection of microbial reduction targets for hard surface disinfectants. American Journal of Infection Control. 2014;42(11) doi: 10.1016/j.ajic.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 41.Schaffner DW, Bowman JP, English DJ, Fischler GE, Fuls JL, Krowka JF, et al. Quantitative Microbial Risk Assessment of Antibacterial Hand Hygiene Products on Risk of Shigellosis. Journal of Food Protection. 2014;77(4):574–582. doi: 10.4315/0362-028X.JFP-13-366. [DOI] [PubMed] [Google Scholar]
- 42.Benami M, Busgang A, Gillor O, Gross A. Quantification and risks associated with bacterial aerosols near domestic greywatertreatment systems. Science of the Total Environment. 2016;562:344–352. doi: 10.1016/j.scitotenv.2016.03.200. [DOI] [PubMed] [Google Scholar]
- 43.Matthews L, Reeve R, Gally DL, Low JC, Woolhouse MEJ, McAteer SP, et al. Predicting the public health benefit of vaccinating cattle against Escherichia coli O157. Proceedings of the National Academy of Sciences. 2013;110(40):16265–16270. doi: 10.1073/pnas.1304978110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dalahmeh SS, Lalander C, Pell M, Vinnerås B, Jönsson H. Quality of greywater treated in biochar filter and risk assessment of gastroenteritis due to household exposure during maintenance and irrigation. Journal of Applied Microbiology. 2016;121(5):1427–1443. doi: 10.1111/jam.13273. [DOI] [PubMed] [Google Scholar]
- 45.Petterson SR. Application of a QMRA framework to inform selection of drinking water interventions in the developing context. Risk Analysis. 2016;36(2):203–214. doi: 10.1111/risa.12452. [DOI] [PubMed] [Google Scholar]
- 46.Reygadas F, Gruber JS, Ray I, Nelson KL. Field efficacy evaluation and post-treatment contamination risk assessment of an ultraviolet disinfection and safe storage system. Water Research. 2015;85:74–84. doi: 10.1016/j.watres.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 47.Coulliette AD, Enger KS, Weir MH, Rose JB. Risk reduction assessment of waterborne Salmonella and Vibrio by a chlorine contact disinfectant point-of-use device. International Journal of Hygiene and Environmental Health. 2013;216(3):355–61. doi: 10.1016/j.ijheh.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 48.Rodriguez-Alvarez MS, Weir MH, Pope JM, Seghezzo L, Rajal VB, Salusso MM, et al. Development of a relative risk model for drinking water regulation and design recommendations for a peri urban region of Argentina. International Journal of Hygiene and Environmental Health. 2015;218(7):627–638. doi: 10.1016/j.ijheh.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y, Chen Z, An W, Xiao S, Yuan H, Zhang D, et al. Risk assessment of Giardia from a full scale MBR sewage treatment plant caused by membrane integrity failure. Journal of Environmental Sciences (China) 2015;30:252–258. doi: 10.1016/j.jes.2014.09.033. [DOI] [PubMed] [Google Scholar]
- 50.Teklehaimanot GZ, Genthe B, Kamika I, Momba MNB. Prevalence of enteropathogenic bacteria in treated effluents and receiving water bodies and their potential health risks. Science of the Total Environment. 2015;518–519:441–449. doi: 10.1016/j.scitotenv.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 51.Symonds EM, Verbyla ME, Lukasik JO, Kafle RC, Breitbart M, Mihelcic JR. A case study of enteric virus removal and insights into the associated risk of water reuse for two wastewater treatment pond systems in Bolivia. Water Research. 2014;65:257–270. doi: 10.1016/j.watres.2014.07.032. [DOI] [PubMed] [Google Scholar]
- 52.Cutolo SA, Piveli RP, Santos JG, Montes CR, Sundefeld G, Campos F, et al. Parasitological risk assessment from wastewater reuse for disposal in soil in developing countries. Water Science and Technology. 2012;65(8):1357–1367. doi: 10.2166/wst.2012.012. [DOI] [PubMed] [Google Scholar]
- 53.Kinyua MN, Wald I, Camacho-Céspedes F, Izurieta R, Haas CN, Ergas SJ. Does the use of tubular digesters to treat livestock waste lower the risk of infection from Cryptosporidium parvum and Giardia lamblia? Journal of Water and Health. 2016;14(5):738–753. doi: 10.2166/wh.2016.032. [DOI] [PubMed] [Google Scholar]
- 54.Chaidez C, Soto-Beltran M, Gerba CP, Tamimi AH. Reduction of risk of Salmonella infection from kitchen cleaning clothes by use of sodium hypochlorite disinfectant cleaner. Letters in Applied Microbiology. 2014;59(5):487–492. doi: 10.1111/lam.12321. [DOI] [PubMed] [Google Scholar]
- 55.Burch TR, Spencer SK, Stokdyk JP, Kieke BA, Larson RA, Firnstahl AD, et al. Quantitative microbial risk assessment for spray irrigation of dairy manure based on an empirical fate and transport model. Environmental Health Perspectives. 2017;125(8):1–11. doi: 10.1289/EHP283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Courault D, Albert I, Perelle S, Fraisse A, Renault P, Salemkour A, et al. Assessment and risk modeling of airborne enteric viruses emitted from wastewater reused for irrigation. Science of the Total Environment. 2017;592:512–526. doi: 10.1016/j.scitotenv.2017.03.105. [DOI] [PubMed] [Google Scholar]
- 57.Clarke R, Peyton D, Healy MG, Fenton O, Cummins E. A quantitative microbial risk assessment model for total coliforms and E. coli in surface runoff following application of biosolids to grassland. Environmental Pollution. 2017;224:739–750. doi: 10.1016/j.envpol.2016.12.025. [DOI] [PubMed] [Google Scholar]
- 58.Makkaew P, Miller M, Fallowfield HJ, Cromar NJ. Microbial risk in wastewater irrigated lettuce: Comparing Escherichia coli contamination from an experimental site with a laboratory approach. Water Science and Technology. 2016;74(3):749–755. doi: 10.2166/wst.2016.237. [DOI] [PubMed] [Google Scholar]
- 59.Beaudequin D, Harden F, Roiko A, Mengersen K. Utility of Bayesian networks in QMRA-based evaluation of risk reduction options for recycled water. Science of the Total Environment. 2016;541:1393–1409. doi: 10.1016/j.scitotenv.2015.10.030. [DOI] [PubMed] [Google Scholar]
- 60.Dungan RS. Estimation of infectious risks in residential populations exposed to airborne pathogens during center pivot irrigation of dairy wastewaters. Environmental Science and Technology. 2014;48(9):5033–5042. doi: 10.1021/es405693v. [DOI] [PubMed] [Google Scholar]
- 61.Barker SF, Amoah P, Drechsel P. A probabilistic model of gastroenteritis risks associated with consumption of street food salads in Kumasi, Ghana: Evaluation of methods to estimate pathogen dose from water, produce or food quality. Science of the Total Environment. 2014;487(1):130–142. doi: 10.1016/j.scitotenv.2014.03.108. [DOI] [PubMed] [Google Scholar]
- 62.Schmidt PJ, Emelko MB, Thompson ME. Analytical recovery of protozoan enumeration methods: Have drinking water QMRA models corrected or created bias? Water Research. 2013;47(7):2399–2408. doi: 10.1016/j.watres.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 63.Barker SF, O’Toole J, Sinclair MI, Leder K, Malawaraarachchi M, Hamilton AJ. A probabilistic model of norovirus disease burden associated with greywater irrigation of home-produced lettuce in Melbourne, Australia. Water Research. 2013;47(3):1421–1432. doi: 10.1016/j.watres.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 64.Aiello R, Cirelli GL, Consoli S, Licciardello F, Toscano A. Risk assessment of treated municipal wastewater reuse in Sicily. Water Science and Technology. 2013;67(1):89–98. doi: 10.2166/wst.2012.535. [DOI] [PubMed] [Google Scholar]
- 65.Shrestha S, Haramoto E, Shindo J. Assessing the infection risk of enteropathogens from consumption of raw vegetables washed with contaminated water in Kathmandu Valley, Nepal. Journal of Applied Microbiology. 2017 doi: 10.1111/jam.13573. [DOI] [PubMed] [Google Scholar]
- 66.Henao-Herreño LX, López-Tamayo AM, Ramos-Bonilla JP, Haas CN, Husserl J. Risk of illness with Salmonella due to consumption of raw unwashed vegetables irrigated with water from the Bogota River. Risk Analysis. 2017;37(4):733–743. doi: 10.1111/risa.12656. [DOI] [PubMed] [Google Scholar]
- 67.Owusu-Ansah EdGJ, Sampson A, Amponsah SK, Abaidoo RC, Dalsgaard A, Hald T. Probabilistic quantitative microbial risk assessment model of norovirus from wastewater irrigated vegetables in Ghana using genome copies and fecal indicator ratio conversion for estimating exposure dose. Science of the Total Environment. 2017;601–602:1712–1719. doi: 10.1016/j.scitotenv.2017.05.168. [DOI] [PubMed] [Google Scholar]
- 68.Le-Thi T, Pham-Duc P, Zurbrügg C, Luu-Quoc T, Nguyen-Mai H, Vu-Van T, et al. Diarrhea risks by exposure to livestock waste in Vietnam using quantitative microbial risk assessment. International Journal of Public Health. 2017;62:83–91. doi: 10.1007/s00038-016-0917-6. [DOI] [PubMed] [Google Scholar]
- 69.Verbyla ME, Symonds EM, Kafle RC, Cairns MR, Iriarte M, Mercado Guzmán A, et al. Managing Microbial Risks from Indirect Wastewater Reuse for Irrigation in Urbanizing Watersheds. Environmental Science and Technology. 2016;50(13):6803–6813. doi: 10.1021/acs.est.5b05398. [DOI] [PubMed] [Google Scholar]
- 70.Krzyzanowski F, de Souza Lauretto M, Nardocci AC, Sato MIZ, Razzolini MTP. Assessing the probability of infection by Salmonella due to sewage sludge use in agriculture under several exposure scenarios for crops and soil ingestion. Science of the Total Environment. 2016;568:66–74. doi: 10.1016/j.scitotenv.2016.05.129. [DOI] [PubMed] [Google Scholar]
- 71.Antwi-Agyei P, Cairncross S, Peasey A, Price V, Bruce J, Baker K, et al. A farm to fork risk assessment for the use of wastewater in agriculture in Accra, Ghana. PLOS One. 2015;10(11):1–19. doi: 10.1371/journal.pone.0142346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Seidu R, Abubakari A, Dennis IA, Heistad A, Stenstrom TA, Larbi JA, et al. A probabilistic assessment of the contribution of wastewater-irrigated lettuce to Escherichia coli O157:H7 infection risk and disease burden in Kumasi, Ghana. Journal of Water and Health. 2015;13(1):217–229. doi: 10.2166/wh.2014.108. [DOI] [PubMed] [Google Scholar]
- 73.Pavione DMS, Bastos RKX, Bevilacqua PD. Quantitative microbial risk assessment applied to irrigation of salad crops with waste stabilization pond effluents. Water Science and Technology. 2013;67(6):1208–1215. doi: 10.2166/wst.2013.674. [DOI] [PubMed] [Google Scholar]
- 74.Kobayashi Y, Peters GM, Ashbolt NJ, Heimersson S, Svanström M, Khan SJ. Global and local health burden trade-off through the hybridisation of quantitative microbial risk assessment and life cycle assessment to aid water management. Water Research. 2015;79:26–38. doi: 10.1016/j.watres.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 75.Yapo RI, Koné B, Bonfoh B, Cissé G, Zinsstag J, Nguyen-Viet H. Quantitative microbial risk assessment related to urban wastewater and lagoon water reuse in Abidjan, Cote d’Ivoire. Journal of Water and Health. 2014;12(2):301–309. doi: 10.2166/wh.2013.051. [DOI] [PubMed] [Google Scholar]
- 76.Hamilton KA, Ahmed W, Palmer A, Sidhu JPS, Hodgers L, Toze S, et al. Public health implications of Acanthamoeba and multiple potential opportunistic pathogens in roof-harvested rainwater tanks. Environmental Research. 2016;150:320–327. doi: 10.1016/j.envres.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 77.Lim KY, Hamilton AJ, Jiang SC. Assessment of public health risk associated with viral contamination in harvested urban stormwater for domestic applications. Science of the Total Environment. 2015;523:95–108. doi: 10.1016/j.scitotenv.2015.03.077. [DOI] [PubMed] [Google Scholar]
- 78.Jesmi Y, Rahiman KMM, Hatha AAM, Deepu L, Jyothi S. Risk assessment of rooftop-collected rainwater for individual household and community use in central Kerala, India. Journal of Environmental Health. 2014;76(6):114–21. [PubMed] [Google Scholar]
- 79.Oscar TP. Risk of Salmonellosis from chicken parts prepared from whole chickens sold in flow pack wrappers and subjected to temperature abuse. Journal of Food Protection. 2017;80(9):1496–1505. doi: 10.4315/0362-028X.JFP-17-097. [DOI] [PubMed] [Google Scholar]
- 80.Maffei DF, Sant’Ana AS, Franco BDGM, Schaffner DW. Quantitative assessment of the impact of cross-contamination during the washing step of ready-to-eat leafy greens on the risk of illness caused by Salmonella. Food Research International. 2017;92:106–112. doi: 10.1016/j.foodres.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 81.Swart AN, van Leusden F, Nauta MJ. A QMRA model for Salmonella in pork products during preparation and consumption. Risk Analysis. 2016;36(3):516–530. doi: 10.1111/risa.12522. [DOI] [PubMed] [Google Scholar]
- 82.Finger F, Genolet T, Mari L, de Magny GC, Manga NM, Rinaldo A, et al. Mobile phone data highlights the role of mass gatherings in the spreading of cholera outbreaks. Proceedings of the National Academy of Sciences. 2016;113(23):6421–6426. doi: 10.1073/pnas.1522305113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mari L, Bertuzzo E, Finger F, Casagrandi R, Gatto M, Rinaldo A. On the predictive ability of mechanistic models for the Haitian cholera epidemic. Journal of the Royal Society, Interface. 2015;12(104):20140840. doi: 10.1098/rsif.2014.0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kirpich A, Weppelmann TA, Yang Y, Ali A, Morris JG, Longini IM. Cholera Transmission in Ouest Department of Haiti: Dynamic Modeling and the Future of the Epidemic. PLOS Neglected Tropical Diseases. 2015;9(10):1–12. doi: 10.1371/journal.pntd.0004153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mukandavire Z, Morris JG. Modeling the epidemiology of cholera to prevent disease transmission in developing Countries. Microbiology Spectrum. 2015;3(3):898264. doi: 10.1128/microbiolspec.VE-0011-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Njagarah JBH, Nyabadza F. Modelling Optimal Control of Cholera in Communities Linked by Migration. Computational and Mathematical Methods in Medicine. 2015;2015 doi: 10.1155/2015/898264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Collins OC, Govinder KS. Incorporating heterogeneity into the transmission dynamics of a waterborne disease model. Journal of Theoretical Biology. 2014;356:133–43. doi: 10.1016/j.jtbi.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 88.Yang J, Qiu Z, Li XZ. Global stability of an age-structured cholera model. Mathematical Biosciences and Engineering. 2014;11(3):641–65. doi: 10.3934/mbe.2014.11.641. [DOI] [PubMed] [Google Scholar]
- 89.Kong JD, Davis W, Wang H. Dynamics of a cholera transmission model with immunological threshold and natural phage control in reservoir. Bulletin of Mathematical Biology. 2014;76(8):2025–2051. doi: 10.1007/s11538-014-9996-9. [DOI] [PubMed] [Google Scholar]
- 90.Sardar T, Mukhopadhyay S, Bhowmick AR, Chattopadhyay J. An optimal cost effectiveness study on Zimbabwe cholera seasonal data from 2008–2011. PLOS One. 2013;8(12):e81231. doi: 10.1371/journal.pone.0081231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Eisenberg MC, Shuai Z, Tien JH, van den Driessche P. A cholera model in a patchy environment with water and human movement. Mathematical Biosciences. 2013;246(1):105–12. doi: 10.1016/j.mbs.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 92.Rinaldo A, Bertuzzo E, Mari L, Righetto L, Blokesch M, Gatto M, et al. Reassessment of the 2010–2011 Haiti cholera outbreak and rainfall-driven multiseason projections. Proceedings of the National Academy of Sciences. 2012;109(17):6602–6607. doi: 10.1073/pnas.1203333109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Righetto L, Casagrandi R, Bertuzzo E, Mari L, Gatto M, Rodriguez-Iturbe I, et al. The role of aquatic reservoir fluctuations in long-term cholera patterns. Epidemics. 2012;4(1):33–42. doi: 10.1016/j.epidem.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 94.Yaari R, Kaliner E, Grotto I, Katriel G, Moran-Gilad J, Sofer D, et al. Modeling the spread of polio in an IPV-vaccinated population: lessons learned from the 2013 silent outbreak in southern Israel. BMC medicine. 2016;14(1):95. doi: 10.1186/s12916-016-0637-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Famulare M. Has Wild poliovirus been eliminated from Nigeria? PLOS One. 2015;10(8):1–13. doi: 10.1371/journal.pone.0135765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Blake IM, Martin R, Goel A, Khetsuriani N, Everts J, Wolff C, et al. The role of older children and adults in wild poliovirus transmission. Proceedings of the National Academy of Sciences. 2014;111(29):10604–10609. doi: 10.1073/pnas.1323688111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim JH, Mogasale V, Burgess C, Wierzba TF. Impact of oral cholera vaccines in cholera-endemic countries: A mathematical modeling study. Vaccine. 2016;34(18):2113–20. doi: 10.1016/j.vaccine.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 98.Azman AS, Luquero FJ, Ciglenecki I, Grais RF, Sack DA, Lessler J. The impact of a one-dose versus two-dose oral cholera vaccine regimen in outbreak settings: A modeling study. PLOS Medicine. 2015;12(8):1–18. doi: 10.1371/journal.pmed.1001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Posny D, Wang J, Mukandavire Z, Modnak C. Analyzing transmission dynamics of cholera with public health interventions. Mathematical Biosciences. 2015;264(1):38–53. doi: 10.1016/j.mbs.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 100.Dimitrov DT, Troeger C, Halloran ME, Longini IM, Chao DL. Comparative effectiveness of different strategies of oral cholera vaccination in bangladesh: a modeling study. PLOS neglected tropical diseases. 2014;8(12):e3343. doi: 10.1371/journal.pntd.0003343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Safi MA, Melesse DY, Gumel AB. Dynamics analysis of a multi-strain cholera model with an imperfect vaccine. Bulletin of Mathematical Biology. 2013;75(7):1104–37. doi: 10.1007/s11538-013-9845-2. [DOI] [PubMed] [Google Scholar]
- 102.Mukandavire Z, Smith DL, Morris JG. Cholera in Haiti: Reproductive numbers and vaccination coverage estimates. Scientific Reports. 2013;3:997. doi: 10.1038/srep00997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dénes A, Székely L. Global dynamics of a mathematical model for the possible re-emergence of polio. Mathematical Biosciences. 2017;293:64–74. doi: 10.1016/j.mbs.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 104.Houy N. The case for periodic OPV routine vaccination campaigns. Journal of Theoretical Biology. 2016;389:20–27. doi: 10.1016/j.jtbi.2015.09.032. [DOI] [PubMed] [Google Scholar]
- 105.Thompson KM, Duintjer Tebbens RJ. The differential impact of oral poliovirus vaccine formulation choices on serotype-specific population immunity to poliovirus transmission. BMC Infectious Diseases. 2015;15(1):376. doi: 10.1186/s12879-015-1116-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wilder-Smith A, Leong WY, Lopez LF, Amaku M, Quam M, Khan K, et al. Potential for international spread of wild poliovirus via travelers. BMC Medicine. 2015;13(1):133. doi: 10.1186/s12916-015-0363-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wagner BG, Behrend MR, Klein DJ, Upfill-Brown AM, Eckhoff PA, Hu H. Quantifying the impact of expanded age group campaigns for polio eradication. PLOS One. 2014;9(12):1–14. doi: 10.1371/journal.pone.0113538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Duintjer Tebbens RJ, Kalkowska DA, Wassilak SG, Pallansch MA, Cochi SL, Thompson KM. The potential impact of expanding target age groups for polio immunization campaigns. BMC Infectious Diseases. 2014;14(1):45. doi: 10.1186/1471-2334-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hansen Edwards C, de Blasio BF, Salamanca BV, Flem E. Re-evaluation of the cost-effectiveness and effects of childhood rotavirus vaccination in Norway. PLOS One. 2017;12(8):e0183306. doi: 10.1371/journal.pone.0183306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bilcke J, Chapman R, Atchison C, Cromer D, Johnson H, Willem L, et al. Quantifying parameter and structural uncertainty of dynamic disease transmission models using MCMC: An application to rotavirus vaccination in England and Wales. Medical Decision Making. 2015;35(5):633–47. doi: 10.1177/0272989X14566013. [DOI] [PubMed] [Google Scholar]
- 111.Pitzer VE, Bilcke J, Heylen E, Crawford FW, Callens M, De Smet F, et al. Did large-scale vaccination drive changes in the circulating rotavirus population in Belgium? Scientific Reports. 2015;5:1–14. doi: 10.1038/srep18585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pitzer VE, Atkins KE, de Blasio BF, van Effelterre T, Atchison CJ, Harris JP, et al. Direct and indirect effects of rotavirus vaccination: Comparing predictions from transmission dynamic models. PLOS One. 2012;7(8) doi: 10.1371/journal.pone.0042320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Atkins KE, Shim E, Carroll S, Quilici S, Galvani AP. The cost-effectiveness of pentavalent rotavirus vaccination in England and Wales. Vaccine. 2012;30(48):6766–76. doi: 10.1016/j.vaccine.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 114.Bollerslev AM, Nauta M, Hansen TB, Aabo S. A risk modelling approach for setting microbiological limits using enterococci as indicator for growth potential of Salmonella in pork. International Journal of Food Microbiology. 2017;240:102–107. doi: 10.1016/j.ijfoodmicro.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 115.Snary EL, Swart AN, Simons RRL, Domingues ARC, Vigre H, Evers EG, et al. A Quantitative Microbiological Risk Assessment for Salmonella in Pigs for the European Union. Risk Analysis. 2016;36(3):437–449. doi: 10.1111/risa.12586. [DOI] [PubMed] [Google Scholar]
- 116.Vigre H, Barfoed K, Swart AN, Simons RRL, Hill AA, Snary EL, et al. Characterization of the Human Risk of Salmonellosis Related to Consumption of Pork Products in Different E.U. Countries Based on a QMRA. Risk Analysis. 2016;36(3):531–545. doi: 10.1111/risa.12499. [DOI] [PubMed] [Google Scholar]
- 117.Lee H, Kim K, Choi KH, Yoon Y. Quantitative microbial risk assessment for Staphylococcus aureus in natural and processed cheese in Korea. Journal of Dairy Science. 2015;98(9):5931–45. doi: 10.3168/jds.2015-9611. [DOI] [PubMed] [Google Scholar]
- 118.Jeong J, Lee J, Lee H, Lee S, Kim S, Ha J, et al. Quantitative microbial risk assessment for campylobacter foodborne illness in raw beef offal consumption in South Korea. Journal of Food Protection. 2017;80(4):609–618. doi: 10.4315/0362-028X.JFP-16-159. [DOI] [PubMed] [Google Scholar]
- 119.Kiermeier A, Jenson I, Sumner J. Risk assessment of Escherichia coli O157 illness from consumption of hamburgers in the United States made from Australian manufacturing beef. Risk Analysis. 2015;35(1):77–89. doi: 10.1111/risa.12248. [DOI] [PubMed] [Google Scholar]
- 120.Guillier L, Danan C, Bergis H, Delignette-Muller ML, Granier S, Rudelle S, et al. Use of quantitative microbial risk assessment when investigating foodborne illness outbreaks: The example of a monophasic Salmonella Typhimurium 4,5,12: I: - Outbreak implicating beef burgers. International Journal of Food Microbiology. 2013;166(3):471–478. doi: 10.1016/j.ijfoodmicro.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 121.Hurd HS, Malladi S. An outcomes model to evaluate risks and benefits of Escherichia coli vaccination in beef cattle. Foodborne Pathogens and Disease. 2012;9(10):952–961. doi: 10.1089/fpd.2012.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Smadi H, Sargeant JM. Quantitative risk assessment of human salmonellosis in Canadian broiler chicken breast from retail to consumption. Risk Analysis. 2013;33(2):232–248. doi: 10.1111/j.1539-6924.2012.01841.x. [DOI] [PubMed] [Google Scholar]
- 123.Boysen L, Nauta M, Duarte ASR, Rosenquist H. Human risk from thermotolerant Campylobacter on broiler meat in Denmark. International Journal of Food Microbiology. 2013;162(2):129–134. doi: 10.1016/j.ijfoodmicro.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 124.Signorini ML, Zbrun MV, Romero-Scharpen A, Olivero C, Bongiovanni F, Soto LP, et al. Quantitative risk assessment of human campylobacteriosis by consumption of salad cross-contaminated with thermophilic Campylobacter spp. from broiler meat in Argentina. Preventive Veterinary Medicine. 2013;109(1–2):37–6. doi: 10.1016/j.prevetmed.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 125.Smadi H, Sargeant JM. Review of Canadian Literature to Estimate Risks Associated with Salmonella in Broilers from Retail to Consumption in Canadian Homes. Critical Reviews in Food Science and Nutrition. 2013;53(7):694–705. doi: 10.1080/10408398.2011.555017. [DOI] [PubMed] [Google Scholar]
- 126.Thebault A, Le Saux JC, Pommepuy M, Le Guyader S, Lailler R, Denis JB. Quantitative approach of risk management strategies for hepatitis a virus-contaminated oyster production areas. Journal of Food Protection. 2012;75(7):1249–57. doi: 10.4315/0362-028X.JFP-11-411. [DOI] [PubMed] [Google Scholar]
- 127.Singer RS, Ruegg PL, Bauman DE. Quantitative risk assessment of antimicrobial-resistant foodborne infections in humans due to recombinant bovine somatotropin usage in dairy cows. Journal of Food Protection. 2017;80(7):1099–1116. doi: 10.4315/0362-028X.JFP-16-404. [DOI] [PubMed] [Google Scholar]
- 128.Giacometti F, Bonilauri P, Piva S, Scavia G, Amatiste S, Bianchi DM, et al. Paediatric HUS Cases Related to the Consumption of Raw Milk Sold by Vending Machines in Italy: Quantitative Risk Assessment Based on Escherichia coli O157 Official Controls over 7 years. Zoonoses and Public Health. 2016:505–516. doi: 10.1111/zph.12331. [DOI] [PubMed] [Google Scholar]
- 129.Giacometti F, Bonilauri P, Amatiste S, Arrigoni N, Bianchi M, Losio MN, et al. Human campylobacteriosis related to the consumption of raw milk sold by vending machines in Italy: Quantitative risk assessment based on official controls over four years. Preventive Veterinary Medicine. 2015;121(1–2):151–158. doi: 10.1016/j.prevetmed.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 130.Giacometti F, Bonilauri P, Albonetti S, Amatiste S, Arrigoni N, Bianchi M, et al. Quantitative risk assessment of human salmonellosis and listeriosis related to the consumption of raw milk in Italy. Journal of food protection. 2015;78(1):13–21. doi: 10.4315/0362-028X.JFP-14-171. [DOI] [PubMed] [Google Scholar]
- 131.Perrin F, Tenenhaus-Aziza F, Michel V, Miszczycha S, Bel N, Sanaa M. Quantitative risk assessment of haemolytic and uremic syndrome linked to O157: H7 and Non-O157: H7 shiga-toxin producing Escherichia coli strains in raw milk soft cheeses. Risk Analysis. 2015;35(1):109–128. doi: 10.1111/risa.12267. [DOI] [PubMed] [Google Scholar]
- 132.Pouillot R, Hoelzer K, Ramirez GA, DeGraft-Hanson J, Dennis SB. Assessment of the risk of salmonellosis from internally contaminated shell eggs following initial storage at 18°C (65°F), compared with 7°C (45°F) Food Microbiology. 2014;43:16–19. doi: 10.1016/j.fm.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 133.Giacometti F, Serraino A, Bonilauri P, Ostanello F, Daminelli P, Finazzi G, et al. Quantitative risk assessment of verocytotoxinproducing Escherichia coli O157 and Campylobacter jejuni related to consumption of raw milk in a province in northern Italy. Journal of Food Protection. 2012;75(11):2031–2038. doi: 10.4315/0362-028X.JFP-12-163. [DOI] [PubMed] [Google Scholar]
- 134.Lambertini E, Barouei J, Schaffner DW, Danyluk MD, Harris LJ. Modeling the risk of salmonellosis from consumption of pistachios produced and consumed in the United States. Food Microbiology. 2017;67:85–96. doi: 10.1016/j.fm.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 135.Pang H, Lambertini E, Buchanan RL, Schaffner DW, Pradhan AK. Quantitative microbial risk assessment for Escherichia coli O157:H7 in fresh-cut lettuce. Journal of Food Protection. 2017;80(2):302–311. doi: 10.4315/0362-028X.JFP-16-246. [DOI] [PubMed] [Google Scholar]
- 136.Bouwknegt M, Verhaelen K, Rzezutka A, Kozyra I, Maunula L, von Bonsdorff CH, et al. Quantitative farm-to-fork risk assessment model for norovirus and hepatitis A virus in European leafy green vegetable and berry fruit supply chains. International Journal of Food Microbiology. 2015;198:50–58. doi: 10.1016/j.ijfoodmicro.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 137.Pielaat A, van Leusden FM, Wijnands LM. Microbiological risk from minimally processed packaged salads in the Dutch food chain. Journal of Food Protection. 2014;77(3):395–403. doi: 10.4315/0362-028X.JFP-13-136. [DOI] [PubMed] [Google Scholar]
- 138.Soller JA, Schoen M, Steele JA, Griffith JF, Schiff KC. Incidence of gastrointestinal illness following wet weather recreational exposures: Harmonization of quantitative microbial risk assessment with an epidemiologic investigation of surfers. Water Research. 2017;121:280–289. doi: 10.1016/j.watres.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 139.Adell AD, McBride G, Wuertz S, Conrad PA, Smith WA. Comparison of human and southern sea otter (Enhydra lutris nereis) health risks for infection with protozoa in nearshore waters. Water Research. 2016;104:220–230. doi: 10.1016/j.watres.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 140.Eregno FE, Tryland I, Tjomsland T, Myrmel M, Robertson L, Heistad A. Quantitative microbial risk assessment combined with hydrodynamic modelling to estimate the public health risk associated with bathing after rainfall events. Science of the Total Environment. 2016;548–549:270–279. doi: 10.1016/j.scitotenv.2016.01.034. [DOI] [PubMed] [Google Scholar]
- 141.Corsi SR, Borchardt MA, Carvin RB, Burch TR, Spencer SK, Lutz MA, et al. Human and bovine viruses and bacteria at three Great Lakes beaches: Environmental variable associations and health risk. Environmental Science and Technology. 2016;50(2):987–995. doi: 10.1021/acs.est.5b04372. [DOI] [PubMed] [Google Scholar]
- 142.Liao H, Krometis LAH, Kline K. Coupling a continuous watershed-scale microbial fate and transport model with a stochastic dose-response model to estimate risk of illness in an urban watershed. Science of the Total Environment. 2016;551–552:668–675. doi: 10.1016/j.scitotenv.2016.02.044. [DOI] [PubMed] [Google Scholar]
- 143.Vergara GGRV, Rose JB, Gin KYH. Risk assessment of noroviruses and human adenoviruses in recreational surface waters. Water Research. 2016;103:276–282. doi: 10.1016/j.watres.2016.07.048. [DOI] [PubMed] [Google Scholar]
- 144.Sterk A, de Man H, Schijven JF, de Nijs T, de Roda Husman AM. Climate change impact on infection risks during bathing downstream of sewage emissions from CSOs or WWTPs. Water Research. 2016;105:11–21. doi: 10.1016/j.watres.2016.08.053. [DOI] [PubMed] [Google Scholar]
- 145.Timm C, Luther S, Jurzik L, Hamza IA, Kistemann T. Applying QMRA and DALY to assess health risks from river bathing. International Journal of Hygiene and Environmental Health. 2016;219(7):681–692. doi: 10.1016/j.ijheh.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 146.Jacob P, Henry A, Meheut G, Charni-Ben-Tabassi N, Ingr V, Helmi K. Health risk assessment related to waterborne pathogens from the river to the tap. International Journal of Environmental Research and Public Health. 2015;12(3):2967–2983. doi: 10.3390/ijerph120302967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Prez VE, Gil PI, Temprana CF, Cuadrado PR, Martínez LC, Giordano MO, et al. Quantification of human infection risk caused by rotavirus in surface waters from Cordoba, Argentina. Science of the Total Environment. 2015;538:220–229. doi: 10.1016/j.scitotenv.2015.08.041. [DOI] [PubMed] [Google Scholar]
- 148.Lodder WJ, Schijven JF, Rutjes SA, de Roda Husman AM, Teunis PFM. Entero- and parechovirus distributions in surface water and probabilities of exposure to these viruses during water recreation. Water Research. 2015;75:25–32. doi: 10.1016/j.watres.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 149.Soller JA, Schoen ME, Varghese A, Ichida AM, Boehm AB, Eftim S, et al. Human health risk implications of multiple sources of faecal indicator bacteria in a recreational waterbody. Water Research. 2014;66:254–264. doi: 10.1016/j.watres.2014.08.026. [DOI] [PubMed] [Google Scholar]
- 150.Wilkes G, Brassard J, Edge TA, Gannon V, Jokinen CC, Jones TH, et al. Bacteria, viruses, and parasites in an intermittent stream protected from and exposed to pasturing cattle: Prevalence, densities, and quantitative microbial risk assessment. Water Research. 2013;47(16):6244–6257. doi: 10.1016/j.watres.2013.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.McBride GB, Stott R, Miller W, Bambic D, Wuertz S. Discharge-based QMRA for estimation of public health risks from exposure to stormwater-borne pathogens in recreational waters in the United States. Water Research. 2013;47(14):5282–5297. doi: 10.1016/j.watres.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 152.Kundu A, McBride G, Wuertz S. Adenovirus-associated health risks for recreational activities in a multi-use coastal watershed based on site-specific quantitative microbial risk assessment. Water Research. 2013;47(16):6309–6325. doi: 10.1016/j.watres.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 153.Schippmann B, Schernewski G, Grawe U. Escherichia coli pollution in a Baltic Sea lagoon: A model-based source and spatial risk assessment. International Journal of Hygiene and Environmental Health. 2013;216(4):408–420. doi: 10.1016/j.ijheh.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 154.Dickinson G, ying Lim K, Jiang SC. Quantitative microbial risk assessment of pathogenic vibrios in marine recreational waters of Southern California. Applied and Environmental Microbiology. 2013;79(1):294–302. doi: 10.1128/AEM.02674-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sales-Ortells H, Medema G. Microbial health risks associated with exposure to stormwater in a water plaza. Water Research. 2015;74:34–46. doi: 10.1016/j.watres.2015.01.044. [DOI] [PubMed] [Google Scholar]
- 156.Sales-Ortells H, Medema G. Screening-level microbial risk assessment of urban water locations: A tool for prioritization. Environmental Science and Technology. 2014;48(16):9780–9789. doi: 10.1021/es5020407. [DOI] [PubMed] [Google Scholar]
- 157.De Man H, Van Den Berg HHJL, Leenen EJTM, Schijven JF, Schets FM, Van Der Vliet JC, et al. Quantitative assessment of infection risk from exposure to waterborne pathogens in urban floodwater. Water Research. 2014;48(1):90–99. doi: 10.1016/j.watres.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 158.Suppes LM, Canales RA, Gerba CP, Reynolds KA. Cryptosporidium risk from swimming pool exposures. International Journal of Hygiene and Environmental Health. 2016;219(8):915–919. doi: 10.1016/j.ijheh.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 159.De Man H, Bouwknegt M, van Heijnsbergen E, Leenen EJTM, van Knapen F, de Roda Husman AM. Health risk assessment for splash parks that use rainwater as source water. Water Research. 2014;54:254–261. doi: 10.1016/j.watres.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 160.Barker SF, Packer M, Scales PJ, Gray S, Snape I, Hamilton AJ. Pathogen reduction requirements for direct potable reuse in Antarctica: Evaluating human health risks in small communities. Science of the Total Environment. 2013;461–462:723–733. doi: 10.1016/j.scitotenv.2013.05.059. [DOI] [PubMed] [Google Scholar]
- 161.Hoyer AB, Schladow SG, Rueda FJ. A hydrodynamics-based approach to evaluating the risk of waterborne pathogens entering drinking water intakes in a large, stratified lake. Water Research. 2015;83:227–236. doi: 10.1016/j.watres.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 162.Raslan R, El Sayegh S, Chams S, Chams N, Leone A, Hajj Hussein I. Re-emerging vaccine-preventable diseases in war-affected peoples of the eastern Mediterranean region—An update. Frontiers in Public Health. 2017;5:1–8. doi: 10.3389/fpubh.2017.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bartak R, Page D, Sandhu C, Grischek T, Saini B, Mehrotra I, et al. Application of risk-based assessment and management to riverbank filtration sites in India. Journal of Water and Health. 2015;13(1):174–189. doi: 10.2166/wh.2014.075. [DOI] [PubMed] [Google Scholar]
- 164.Chigor VN, Sibanda T, Okoh AI. Assessment of the risks for human health of adenoviruses, hepatitis A virus, rotaviruses and enteroviruses in the Buffalo River and three source water dams in the Eastern Cape. Food and Environmental Virology. 2014;6(2):87–98. doi: 10.1007/s12560-014-9138-4. [DOI] [PubMed] [Google Scholar]
- 165.Sato MIZ, Galvani AT, Padula JA, Nardocci AC, Lauretto MdS, Razzolini MTP, et al. Assessing the infection risk of Giardia and Cryptosporidium in public drinking water delivered by surface water systems in Sao Paulo State, Brazil. Science of the Total Environment. 2013;442:389–396. doi: 10.1016/j.scitotenv.2012.09.077. [DOI] [PubMed] [Google Scholar]
- 166.An W, Zhang D, Xiao S, Yu J, Yang M. Risk assessment of Giardia in rivers of southern China based on continuous monitoring. Journal of Environmental Sciences. 2012;24(2):309–313. doi: 10.1016/s1001-0742(11)60768-7. [DOI] [PubMed] [Google Scholar]
- 167.Balderrama-Carmona AP, Gortáres-Moroyoqui P, Álvarez-Valencia LH, Castro-Espinoza L, Balderas-Cortés JDJ, MondacaFernández I, et al. Quantitative microbial risk assessment of Cryptosporidium and Giardia in well water from a native community of Mexico. International Journal of Environmental Health Research. 2015;25(5):570–582. doi: 10.1080/09603123.2014.989492. [DOI] [PubMed] [Google Scholar]
- 168.Shrestha S, Haramoto E, Malla R, Nishida K. Risk of diarrhoea from shallow groundwater contaminated with enteropathogens in the Kathmandu Valley, Nepal. Journal of Water and Health. 2015;13(1):259–269. doi: 10.2166/wh.2014.036. [DOI] [PubMed] [Google Scholar]
- 169.Xiao S, An W, Chen Z, Zhang D, Yu J, Yang M. The burden of drinking water-associated cryptosporidiosis in China: The large contribution of the immunodeficient population identified by quantitative microbial risk assessment. Water Research. 2012;46(13):4272–4280. doi: 10.1016/j.watres.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 170.Thomas K, McBean E, Shantz A, Murphy HM. Comparing the microbial risks associated with household drinking water supplies used in peri-urban communities of Phnom Penh, Cambodia. Journal of Water and Health. 2015;13(1):243–258. doi: 10.2166/wh.2014.214. [DOI] [PubMed] [Google Scholar]
- 171.Machdar E, van der Steen NP, Raschid-Sally L, Lens PNL. Application of Quantitative Microbial Risk Assessment to analyze the public health risk from poor drinking water quality in a low income area in Accra, Ghana. Science of the Total Environment. 2013;449:134–142. doi: 10.1016/j.scitotenv.2013.01.048. [DOI] [PubMed] [Google Scholar]
- 172.Bivins AW, Sumner T, Kumpel E, Howard G, Cumming O, Ross I, et al. Estimating Infection Risks and the Global Burden of Diarrheal Disease Attributable to Intermittent Water Supply Using QMRA. Environmental Science and Technology. 2017;51(13):7542–7551. doi: 10.1021/acs.est.7b01014. [DOI] [PubMed] [Google Scholar]
- 173.Enger KS, Nelson KL, Clasen T, Rose JB, Eisenberg JNS. Linking quantitative microbial risk assessment and epidemiological data: Informing safe drinking water trials in developing countries. Environmental Science and Technology. 2012;46(9):5160–5167. doi: 10.1021/es204381e. [DOI] [PubMed] [Google Scholar]
- 174.Ferrer A, Nguyen-Viet H, Zinsstag J. Quantification of diarrhea risk related to wastewater contact in Thailand. EcoHealth. 2012;9(1):49–59. doi: 10.1007/s10393-012-0746-x. [DOI] [PubMed] [Google Scholar]
- 175.Gao T, Chen R, Wang X, Ngo HH, Li YY, Zhou J, et al. Application of disease burden to quantitative assessment of health hazards for a decentralized water reuse system. Science of the Total Environment. 2016;551–552(13):83–91. doi: 10.1016/j.scitotenv.2016.01.210. [DOI] [PubMed] [Google Scholar]
- 176.Koepke AA, Longini IM, Halloran ME, Wakefield J, Minin VN. Predictive modeling of cholera outbreaks in Bangladesh. Annals of Applied Statistics. 2016;10(2):575–595. doi: 10.1214/16-AOAS908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Fung ICH. Cholera transmission dynamic models for public health practitioners. Emerging Themes in Epidemiology. 2014;11(1):1. doi: 10.1186/1742-7622-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Okosun KO, Makinde OD. A co-infection model of malaria and cholera diseases with optimal control. Mathematical Biosciences. 2014;258:19–32. doi: 10.1016/j.mbs.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 179.Misra AK, Singh V. A delay mathematical model for the spread and control of water borne diseases. Journal of theoretical biology. 2012;301:49–56. doi: 10.1016/j.jtbi.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 180.Kim JH, Rho SH. Transmission dynamics of oral polio vaccine viruses and vaccine-derived polioviruses on networks. Journal of Theoretical Biology. 2015;364:266–274. doi: 10.1016/j.jtbi.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 181.Kisjes KH, Tebbens RJD, Wallace GS, Pallansch MA, Cochi SL, Wassilak SGF, et al. Individual-based modeling of potential poliovirus transmission in connected religious communities in North America with low uptake of vaccination. Journal of Infectious Diseases. 2014;210(Suppl 1):S424–S433. doi: 10.1093/infdis/jit843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mayer BT, Eisenberg JNS, Henry CJ, Gomes MGM, Ionides EL, Koopman JS. Successes and shortcomings of polio eradication: A transmission modeling analysis. American Journal of Epidemiology. 2013;177(11):1236–1245. doi: 10.1093/aje/kws378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Duintjer Tebbens RJ, Pallansch MA, Kalkowska DA, Wassilak SGF, Cochi SL, Thompson KM. Characterizing poliovirus transmission and evolution: Insights from modeling experiences with wild and vaccine-related polioviruses. Risk Analysis. 2013;33(4):703–749. doi: 10.1111/risa.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Thompson KM, Wallace GS, Tebbens RJD, Smith PJ, Barskey AE, Pallansch MA, et al. Trends in the risk of U.S. polio outbreaks and poliovirus vaccine availability for response. Public Health Reports. 2012;127(1):23–37. doi: 10.1177/003335491212700104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Martinez PP, King AA, Yunus M, Faruque ASG, Pascual M. Differential and enhanced response to climate forcing in diarrheal disease due to rotavirus across a megacity of the developing world. Proceedings of the National Academy of Sciences. 2016;113(15):4092–7. doi: 10.1073/pnas.1518977113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Van Effelterre T, Guignard A, Marano C, Rojas R, Jacobsen KH. Modeling the hepatitis A epidemiological transition in Brazil and Mexico. Human Vaccines and Immunotherapeutics. 2017;13(8):1942–1951. doi: 10.1080/21645515.2017.1323158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Van Effelterre T, Marano C, Jacobsen KH. Modeling the hepatitis A epidemiological transition in Thailand. Vaccine. 2016;34(4):555–562. doi: 10.1016/j.vaccine.2015.11.052. [DOI] [PubMed] [Google Scholar]
- 188.Curran D, de Ridder M, Van Effelterre T. The impact of assumptions regarding vaccine-induced immunity on the public health and cost-effectiveness of hepatitis A vaccination: Is one dose sufficient? Human Vaccines and Immunotherapeutics. 2016;12(11):2765–2771. doi: 10.1080/21645515.2016.1203495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Dhankhar P, Nwankwo C, Pillsbury M, Lauschke A, Goveia MG, Acosta CJ, et al. Public health impact and cost-effectiveness of hepatitis A vaccination in the United States: A disease transmission dynamic modeling approach. Value in Health. 2015;18(4):358–367. doi: 10.1016/j.jval.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 190.Van Effelterre T, De Antonio-Suarez R, Cassidy A, Romano-Mazzotti L, Marano C. Model-based projections of the population-level impact of hepatitis A vaccination in Mexico. Human Vaccines and Immunotherapeutics. 2012;8(8):1099–1108. doi: 10.4161/hv.20549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Saad NJ, Bowles CC, Grenfell BT, Basnyat B, Arjyal A, Dongol S, et al. The impact of migration and antimicrobial resistance on the transmission dynamics of typhoid fever in Kathmandu, Nepal: A mathematical modelling study. PLOS Neglected Tropical Diseases. 2017;11(5):1–16. doi: 10.1371/journal.pntd.0005547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Tilahun GT, Makinde OD, Malonza D. Modelling and optimal control of pneumonia disease with cost-effective strategies. Journal of Biological Dynamics. 2017;11:400–426. doi: 10.1080/17513758.2017.1337245. [DOI] [PubMed] [Google Scholar]
- 193*.Pitzer VE, Feasey NA, Msefula C, Mallewa J, Kennedy N, Dube Q, et al. Mathematical modeling to assess the drivers of the recent emergence of typhoid fever in Blantyre, Malawi. Clinical Infectious Diseases. 2015;61(Suppl 4):S251–S258. doi: 10.1093/cid/civ710. This IDTM analysis uses a compartmental model with an environmental reservoir to make clinically relevant conclusions about the role of drug resistance in recent Typhoid fever outbreaks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Miura F, Watanabe T, Watanabe K, Takemoto K, Fukushi K. Comparative assessment of primary and secondary infection risks in a norovirus outbreak using a household model simulation. Journal of Environmental Sciences (China) 2016;50:13–20. doi: 10.1016/j.jes.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 195.Lopman B, Simmons K, Gambhir M, Vinjé J, Parashar U. Epidemiologic implications of asymptomatic reinfection: A mathematical modeling study of norovirus. American Journal of Epidemiology. 2014;179(4):507–512. doi: 10.1093/aje/kwt287. [DOI] [PubMed] [Google Scholar]
- 196.MIilbrath MO, Spicknall IH, Zelner JL, Moe CL, Eisenberg JNS. Heterogeneity in norovirus shedding duration affects community risk. Epidemiology and Infection. 2013;141(08):1572–1584. doi: 10.1017/S0950268813000496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Snedeker KG, Shaw DJ, Locking ME, Prescott RJ. Primary and secondary cases in Escherichia coli O157 outbreaks: A statistical analysis. BMC Infectious Diseases. 2009;9:144. doi: 10.1186/1471-2334-9-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.for Disease Control USC, Prevention. 2017 – Outbreaks of hepatitis A in multiple states among people who are homeless and people who use drugs. 2017 Available from: www.cdc.gov/hepatitis/outbreaks/2017March-HepatitisA.htm.
- 199.Mari L, Bertuzzo E, Righetto L, Casagrandi R, Gatto M, Rodriguez-Iturbe I, et al. Modelling cholera epidemics: The role of waterways, human mobility and sanitation. Journal of the Royal Society, Interface. 2012;(67):376–388. doi: 10.1098/rsif.2011.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Gatto M, Mari L, Bertuzzo E, Casagrandi R, Righetto L, Rodriguez-Iturbe I, et al. Spatially explicit conditions for waterborne pathogen invasion. The American Naturalist. 2013;182(3):328–46. doi: 10.1086/671258. [DOI] [PubMed] [Google Scholar]
- 201*.Brouwer AF, Weir MH, Eisenberg MC, Meza R, Eisenberg JNS. Dose-response relationships for environmentally mediated infectious disease transmission models. PLOS Computational Biology. 2017;13(4):1–28. doi: 10.1371/journal.pcbi.1005481. This methodological analysis incorporates QMRA dose-response functions into IDT models with environmental compartments. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Liu J, Gratz J, Amour C, Kibiki G, Becker S, Janaki L, et al. A laboratory-developed Taqman Array Card for simultaneous detection of 19 enteropathogens. Journal of Clinical Microbiology. 2013;51(2):472–480. doi: 10.1128/JCM.02658-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.