Abstract
In the absence of the RNA-templated reverse transcriptase, telomerase, the predominant means of terminal addition, arises from break-induced replication (BIR) at multiple homologous subtelomeric Y′ loci and among internal homeologous (imperfect) (polyG1-3T) tracts. These last tracts are interspersed between subtelomeric Y′ direct repeats. One major survivor class contains very short (~50 bp) terminal telomere repeats. This size is sufficient for slow growth and partial telomere functionality and cell viability. However, in cells carrying the mre11A470T allele, adjacent to the predicted Rad50/Mre11 junction, cells thrive at wild-type rates, with small, but reproducible, increases in telomere length. We have proposed that the increase in telomere size and growth rate are causally linked. To understand the BIR process at the telomere, we initiated studies of large-tract (RAD51-sensitive) homologous BIR in MRE11 and mre11A470T cells in a model color assay coupled with CHEF gel analysis and marker retention. Wild-type and mutant homologous BIR rates are maintained at the same level as the rates between wild-type and mutant homeologous BIR. However, the fidelity of BIR products was severely altered in mre11A470T cells. We find that 95% of homologous BIR in MRE11 cells gives rise to the expected product size, while 25% of BIR products in mre11A470T cells were of unpredicted size (error-prone), most of which initiated at an aberrant site. However, ~25% of homeologous MRE11 cells and 1/7 of homeologous mre11A470T cells underwent error-prone BIR. This class is initiated erroneously, followed by secondary events that elongate or truncate the telomere. We conclude that error-prone BIRs are increased in homeologous recombination in wild-type and in mre11A470T cells. This finding may explain the bypass of senescence in telomerase-negative cells.
Keywords: recombination, telomere, homeologous, BIR, Mre11, error-prone BIE
1. Introduction
Telomeres are the ribonucleoprotein particles bound to the termini of most eukaryotic chromosomes. Telomeres are required for end protection against non-specific degradation, rearrangement, or uncontrolled sizes of G+T-rich repeats and act as a “dynamic cap” (Soudet et al. 2014). In yeast and most eukaryotes, telomerase is responsible for the addition of G+T repeats through its reverse transcriptase activity (Est2) (Counter et al. 1997, Lingner et al. 1997), using a template in the telomerase RNA component (TLC1) (Singer and Gottschling 1994).
In the absence of telomerase, two major survivor classes arise. The first, Type-I, consists of rapid variation in the amplification of subtelomeric long-repeat Y′ elements and maintenance of the short 50 bp telomere. The 50 bp telomere tract is near the size required for minimal function, accounting for the slow growth and low viability of Type-I suppressors (Lundblad and Blackburn 1993). Indeed, complete suppression of senescence is associated with only an additional 50 bp increase in telomere length (Joseph et al. 2010). The mechanism of this process, break induced replication (BIR), is the invasion of single stranded DNA between either homologous Y elements or the irregular polyG1-3T telomeric repeats, followed by DNA replication to the end of the chromosome. The Type-I dependence on both the strand invasion enzyme, Rad51, and the DNA polymerase subunit, Pol32, support a BIR mechanism. (Lundblad and Blackburn 1993, Teng and Zakian 1999, McEachern and Haber 2006). Type-I survivors are also dependent on Rad52 and two other components of the Rad52 epistasis group, Rad54 and Rad57 (Chen et al. 2001). Rad59, in contrast, may be involved in an alternative BIR pathway (Churikov et al. 2014).
The second major form of recombination (Type-II) is independent of Rad51 but requires Rad52 and the Mre11/Rad50/Xrs2-NBS1 (Mre11 complex) (Lundblad and Blackburn 1993, Teng and Zakian1999). Type II recombinants arise at later points in senescence through either telomere/telomere recombination and/or a rolling-circle replication (Natarajan and McEachern 2002), followed by BIR recombination between chromosomal telomeres. The Mre11 complex is involved in multiple processes that involve MR or MRX complexes in telomere homeostasis (Gao et al. 2014). These include the formation of the telomeric anti-checkpoint that maintains the presence of the double strand break at the telomere. The anti-checkpoint also results in an equilibrium between the Mre11/Tel1 activators of telomerase on short telomeres (Hector et al. 2007) and repression of elongation by the telomere-length dependent proteins Rap1, Rif1 and Rif2 (Yang et al. 2017). This equilibrium can also involve recombinational elongation of the telomere. This anti-checkpoint acts as a “dynamic cap” by its stringent maintenance of telomere size homeostasis (Dubrana et al. 2001).
The frequencies of homeologous recombination and BIR are influenced by both the degree of homeology and mismatch repair (Mirzoeva et al. 2006, Nicholson et al. 2006). The absence of some mismatch components such as Pms1, elevates the homeologous recombination rate. Pms1, therefore, helps to block homeologous recombination (Bailis and Rothstein 1990). This trait is shared by three additional mismatch repair enzymes, Msh2, Msh3 and Msh6 (Nicholson et al. 2006). The MRE11 allele, mre11A470T, is a missense allele that is a member of the thirteen-amino-acid A470T motif. Based on crystallization studies of Mre11/Rad50/DNA complexes, this motif is located at the Rad50/Mre11 interface (Seifert et al. 2016). These correlations suggest that the Mre11/Rad50 interface has an important regulatory function. In telomerase-negative cells, the Mre11A470T complex confers slightly longer telomeres, termed Type-IA, based on Type-I. This increase in size, thought to be mediated through BIR, is sufficient to confer robust cellular growth and a bypass of senescence. We, therefore, sought first to investigate the basic properties of homologous and homeologous BIR in telomerase-positive cells.
To begin to understand the mre11A470T suppression of senescence, we exploited a colony color assay for BIR coupled with CHEF (Contour Clamped Homogeneous Field Electrophoresis; Bio-Rad) to determine the influence of mre11A470T in BIR in telomerase-positive cells. In most cases, BIR gives rise to the predicted products. However, we have also found and characterized “error-prone” BIR products present in both homeologous and mre11A470T-dependent BIR.
2.0 Materials and methods
2.1 Yeast Strains
W303 and its isogenic derivative, KMM4, contain the MRE11 and mre11A470T allele, respectively (Joseph et al. 2010). MRE11 sam1Δ and mre11A470T sam1Δ null alleles were created by replacement of the SAM1 gene with the Kan resistance cassette (Open Resources). The chromosome III left sequences (D8B and MRC1) have been described previously (Davis and Symington 2004).
2.2 Plasmids
Briefly, the D8B plasmid contains a sequence on the left arm of chromosome III (D8B), URA3, SUP11, and a centromeric plasmid backbone. Plasmids were linearized and oligonucleotides containing telomere seed sequence and the SnaB1 site were ligated and re-circularized. This D8B-tg chromosome fragmentation vector (CFV) contains a unique sequence from the left arm of chromosome III that can act as the substrate for the invasion of plasmid homologous sequences to undergo BIR (Figure 2). Similarly, MRC-tg CFV can initiate BIR at homologous sites, telomere-proximal to D8B sequences. Both cloned plasmid centromeres were used in the formation of BIR products.
Figure 2.
Plasmids Used in The Study of cIII and SAM2 Recombination. The plasmids D8B1-tg and MRC1-tg were digested with SnaB1 and transformed into MRE11 or mre11A470T cells, producing the 110-kb and 30-kb cIII fragments, respectively. In the presence of sam1Δ, recombination of SAM1-tg is restricted to SAM2 and gives rise to an 80-kb BIR product. CEN (blue). ARS (origin of replication, SUP (SUP11). SAM1 is transcribed counterclockwise, while the cIII sequences are clockwise.
A 1.35-kb portion of SAM1 (cXII bp 515322–516303) was cloned from 5′ to 3′ end into Bam HI- and BglII-sites of pYCF2, respectively (Morrow et al. 1997), forming SAM1 CFV. The resultant plasmid was digested with BglII and HindIII and ligated to the annealed tg-oligo1/ca-oligo2 carrying the SnaB1 enzyme as described (Davis and Symington 2004) and religated to form the plasmid SAM1-tg CFV (Figure 2). DNA sequencing (Beckman Coulter) confirmed the ligation junction of the plasmid products. After digestion with SnaBI, Sam1-tg CFV was transformed into a sam1Δ kanr strain carrying either the MRE11 or mre11A470T gene. The presence of the null allele allows only interaction between the plasmid SAM1-tg and the chromosomal SAM2 locus on cIV.
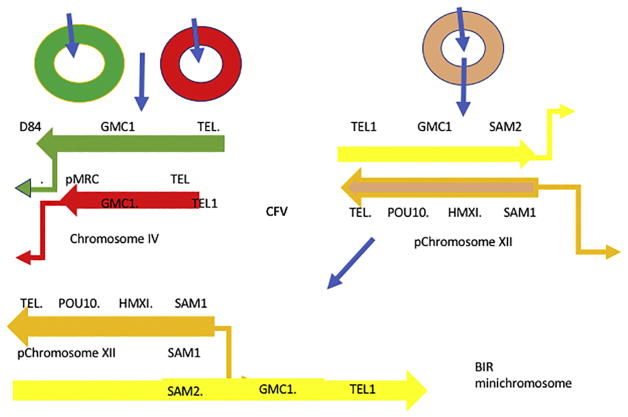
Normally, the chromosome XII and chromosome IV SAM genes are located in opposite transcriptional orientations relative to the centromere, and therefore can only form translocations. To overcome this problem, we placed the SAM1-tg CFV and SAM2 in the same transcriptional orientation, so that BIR products were possible (Figure 1).
Figure 1.
Homologous and Homeologous Recombination Assays. (Left) Digestion of D8B-tg CFV (green) or MRC-tg CFV (red) with SnaBI (top blue arrow), both containing URA3 and CEN, sequences lead to one end having telomere seed sequences (Tel). The other end consists of the D8B and MRC1 sequences for strand invasion and replication into homologous chromosome III left (cIII; blue) sequences. These BIR products are 110-kb and 30-kb product, respectively. SAM1-tg CFV (orange) can only pair with SAM2 on cIV (yellow) in sam1Δ strains, giving rise to the 80-kb BIR containing the GMC1 locus. The SAM1/SAM2 hybrid protein is indicated in red.
2.3 BIR plasmid assay system
We found that normalization with circular DNA transformation and gap-repair recombination of MRC-tg could act as controls. Gap repair controls displayed a greater statistical confidence and less inter-experimental variation.
Following SnaB1 cleavage and gel extraction, transformation of D8B-tg and MRC-tg linearized plasmids were transformed into MRE11 or mre11A470T strains, allowing both loci to pair with chromosome (c) IIIL sequences. The same amount of DNA was transformed into MRE11 and mre11A470T strains for each experiment and plated on uracil omission media as a measure of homologous BIR. In contrast, SAM1-tg CFV invades SAM2 after transformation into sam1Δ SAM2 cells, allowing an analysis of homeologous BIR.
Transformants were plated onto uracil omission media and grown at 30°C for 72 hours. The transformation efficiency is defined by the #Ura+ colonies/μg DNA. The control gapped plasmid was formed by digesting MRC-tg CFV. Control MRC-tg plasmids were identical in structure to the original circular plasmids before SnaB1 cleavage.
For both control gap repair and circular plasmids, progenitor linear molecules were transformed with identical amounts of DNA. For BIR analysis, transformations were plated onto uracil omission plates for 24 hours. We then transferred replicas of the plates onto rich media (YPD), for color development, and adenine omission media, as an internal monitor of the color assay. We assayed the YPD patch color after 48 hours at 30°C. In wild-type cells, both white and pink colonies represented BIR events, while only white colonies were indicative of BIR positive events in mre11A470T cells, based on CHEF gel analysis. A final BIR rate, normalized against the rate of circular or gap repair, was calculated as (#BIR positive transformants/μg)/(#transformants/μg control DNA). The color assay is also dependent on the batch of yeast extract. Each batch must be tested for color and CHEF analysis on a small scale.
The products of the plasmids, D8B-tg and MRC-tg CFVs, are 110-kb and 30-kb BIR products, respectively. SAM1-tg CFV, transformed into sam1Δ SAM2, resulted in strand invasion from the beginning of the SAM2 gene towards the telomere. This generated an ~ 80-kb BIR product (Figures 1& 2) on the right arm of cIV.
2.4 CHEF (Contour Clamped Homogeneous Field Electrophoresis) analysis
CHEF gel conditions (Schwartz and Koval 1989, Sambrook and Russell 2006) were dependent on the different size ranges. For the lower size range (5–75-kb) samples were embedded into agarose and loaded on a 1% pulse-field-certified agarose by the manufacturer’s procedure in 0.5xTBE at 14°C for 11 hours at a switch time from 1–6 seconds, an angle of 120°, and a voltage gradient of 6V/cm. For the larger size range (240–2,200-kb), the same procedure was followed, except that electrophoresis was carried out for 24 hours at a switch time of 60–120 seconds. Gels were stained with 1.0 μg/ml EtBr for 30 minutes under rotation. Following electrophoresis, the Genie Blotter (Bio-Rad), was used to transfer a gel to a positively-charged nylon membrane for one hour at 12V. The presence of loci adjacent to SAM2 would help in the identification of proper initiation. For the SAM1-tg plasmid, after introduction into a sam1Δ SAM2 strain, the invaded site has a linked telomere-proximal GMC1 sequence, as identified by Southern blots.
3.0 RESULTS
3.1 The BIR Color/CHEF/Linkage assay
BIR was measured through a modification of previous techniques (Davis and Symington 2004). Homologous BIR products were formed by the transformation of linear CFVs (D8B-tg or MRC-tg) containing a telomere seed sequence at one end and homologous sequences to invade loci at their cIII genomic position. Similarly, homeologous BIR products were formed by the invasion of SAM1-tg into the SAM2 locus on cIV (Figure 1, 2).
We transformed SnaBI-digested D8B-tg and MRC1-tg into MRE11 or mre11A470T cells for homologous BIR. For analysis of homeologous transformation, SAM1-tg was transformed into MRE11 sam1Δ SAM2 or mre11A470T sam1Δ SAM2 with the SAM2 locus on cIV. The BIR products were assayed by the SUP11 suppression of the red colony color of the ade2-1 locus. Since large BIR products are stable, cells containing the BIR product were white and pink in MRE11 cells, and white colonies in mre11A470T cells. In contrast, undigested or gapped circular plasmids appear as Ade-− sectored colonies due to the high rate of SUP11 loss.
The expected size of the BIR product was confirmed by CHEF analysis. The products of the D8B-tg and MRC-tg CPVs are 80-kb and 30-kb BIR products, respectively. SAM1-tg CFV transformed into sam1Δ SAM2 resulted in strand invasion from the beginning of the SAM2 gene towards the telomere of cIV, generating an ~ 80-kb BIR product (Figures 1& 2) on the right arm of cIV. Transformants are presented as median value of multiple trials and normally analyzed by the F-test. All of the expected size of the SAM1-tg transformants maintained the expected GMC1 markers, indicating the fidelity of invasion to form normal products.
3.2 mre11A470T does not influence DSB recombination
We first studied the simple hypothesis that mre11A470T simply influences multiple classes of homologous recombination (Table 1). The frequency of gapped repair in mre11A470T is eight-fold lower than in MRE11 cells. However, circular plasmids gave rise to transformants that were also at frequencies 10-fold less in mre11A470T than in wild type. While the specific reason for this decrease is unknown, we can conclude that mre11A470T confers no direct effect on gap repair. Approximately 8–10% of the frequency of circular transformants were capable of undergoing gap repair in both MRE11 and mre11A470T cells. Hence, mre11A470T does not stimulate multiple classes of recombination.
Table 1.
Wild type and mre11A470T strains no not differ in the relative fractions after transformation with gapped or circular D8B-tg and MRC1-tg plasmids. N represents the sample size
| Strain | Gapped Plasmid | Circular Plasmid |
|---|---|---|
| MRE11 | 56.98 × 102 (n=6) 7.8× | 197.00 × 102 10× |
| mre11A470T | 7.21 × 102 (n=6) | 19.27 × 102(n=6) |
3.3 The Efficiency of BIR formation from transformants is increased in mre11A470T Cells
The overall frequencies of BIR recombinants from D8B-tg and MRC-tg plasmids are identical when normalized to the number of cells competent for transformation, i.e. (#BIR positive transformants/μg) / (#transformants/μg control DNA). However, the fraction of BIR present in transformants of linear plasmids (#BIR products/μg linear plasmid) / (#transformants/μg linear plasmid) were 25% and 75% in MRE11 and mre11A470T cells, respectively (Table 2).
Table 2.
Two areas of homology are present within chromosome III:DB8 telomere proximal to MRC1. The plasmids DB8tg CFV or MRC1tg CFV were transformed into wild type and mutant cells. The number of BIR events per number of regained linear plasmids is shown.
| Site | Allele | [#BIR/#linear products] × 100 |
|---|---|---|
| D8B | MRE11 | 29 +/− 4.8% |
| D8B | mre11A470T | 75 +/− 8.2% |
| MRC1 | MRE11 | 34 +/− 22.8% |
| MRC1 | mre11A470T | 84 +/− 3.8% |
One possible explanation for this result is a higher rate of plasmid circularization (Orr-Weaver et al. 1983) or other uncharacterized product of transformation in wild-type cells. In this event, mre11A470T cells must be restricted in or kinetically favored over the competing product present in wild-type cells. Another possibility is a decreased fidelity of BIR recombinants in mre11A470T cells (see Discussion).
3.4 Homologous and homeologous break-induced replication frequencies in mre11 and mre11A470T cells
We found the distribution of data points did not give a close fit between trials. However, the values are well separated (Figure 4). These data suggest that other factors, apart from those leading to a binary distribution, are entering into the final value. To accommodate this stipulation, we used the F test (Huang et al. 1996) as the best statistical test for significance of the results (Table 3). We note that in this system, we are limited in statistical comparison of the homologous and homeologous systems, since the sites of invasion differ. However, since we observed no major difference between MRC and D8B, this is unlikely to be significant (Figure 3).
Figure 4.
Graph of the percentage homology relative to mre11A470T homologous BIR median data from Table 3. Wild-type and mre11A470T homologous rates were similar to other homologous rates, but quite distinct from homeologous rates. Homeologous rates were quite low, but similar in abundances. The F-test was used in this case because of non-binomial and population deviations influencing the rate determination. The visualization is presented to provide the raw data.
Table 3.
Data from independent experiments of homologous and homeologous recombination in MRE11 and mre11A470T(A470T). For each experiment (red), six to seven samples were used). The median of pooled data (green and the sample size is shown in the parentheses. Values are in number of transformants/μg of circular or gapped DNA plasmid. *Comparisons of pooled median values are shown on right by F-test P values between the four cumulative medians as shown in the text. Ho=homologous, He=homeologous.
| Strain | Homologous Median | Homeologous Median | Median1 | Median 2 | F-test P values |
|---|---|---|---|---|---|
| MRE11 | 0.026 (7) | 0.0034 (7) | |||
| MRE11 | 0.08 (7) | 0.001 (7) | |||
| MRE11 | 0.03 (12) | 0.0023 (8) | |||
| MRE11 | 0.046 (26) | 0.0029 (22) | Ho Mre11 | HeMre11 | P<0.0001 |
| A470T | 0.056 (7) | 0.0061 (8) | Ho A470T | He A470T | P<0.001 |
| A470T | 0.05 (7) | 0.000048 (7) | Ho Mre11 | He Mre11 | No sig |
| A470T | 0.026 (12) | 0.00087 (7) | He Mre11 | He A470T | No sig |
| A470T | 0.038 (26) | 0.0032 (22) |
Figure 3.
(Left) Plates Derived from Transformation of D8B-tg into MRE11 (top) and mre11A470T (bottom). White colors indicated the presence of a BIR product in MRE11 cells, while both pink and white mre11A470T colonies represent cells containing a BIR product. (Right) A subset of cells were analyzed by CHEF gels. D8B-tg was digested and transformed into wild-type and mre11A470T cells to produce the 110-kb BIR products and anomalous fragments of larger size, a subset of which is shown on the right, after probing a Southern blot with URA3 from cV, an internal control in the Southern blot. Both homologous and homologous error-prone BIR are shown by the blue arrow. All of MRE11 and 5/6 mre11A470T homologous BIR were at the expected size. The error prone BIR was 100-kb larger (see Table 4). The size marker control is the leftmost lane in the gel (data not shown). The BIR product size was, as expected, 80-kb. Fragment sizes were determined from an adjacent left lane of both lanes that contained yeast chromosome size markers [Bio-Rad] in the original gel. Abbreviations: w=white colony, p=pink colony and r=red colony. Arrows refer to the expected BIR product and an atypical error-borne BIR product.
3.41 Insignificant differences
In MRE11 strains, no significant difference was observed in the BIR frequencies from D8B CFV-tg and MRC1 CFV-tg transformants. Similarly, in mre11A470T cells, D8B-tg and MRC1-tg transformants did not significantly differ (median D8B =77.5; median MRC1=83 n=4). However, we used only the D8B-tg plasmid for the large-scale transformation frequencies. The BIR frequency in wild-type and in mre11A470T homologous BIR (median=0.046 n=26 and median 0.032 n =26, respectively), revealed no significant differences (Table 3, Figure 3, Figure 4). Similarly, a comparison of MRE11 and mre11A470T revealed no significant effect on the frequency of homeologous BIR: median=0.0029 (n=22) and median=0.0012 (n=22), respectively (P=0.459, respectively).
3.42 Significant differences
We then tested the alternative hypothesis that mre11A470T might increase homeologous BIR events, thereby helping to explain the bypass of senescence (Table 3, Figures 4, Figure 5B). When the SAM1-tg CFV is transformed into sam1Δ SAM2 cells, the alignment of SAM1-tg with the SAM2 gene on cIV gives rise to an 80-kb product (Figure 1). A comparison between MRE11 homologous and homeologous BIR leads to a median=0.046/n=26 and a median=0.0032/n=22, respectively. These distributions are different with high confidence (F-test P<0.0001). A similar change (F-test, P <0.0001) was observed between mre11A470T homologous and homeologous BIR (median= 0.032 n=26 and median=0.0032 n=22, respectively, with an F-test P value <0.001). Both wild-type and mutant strains have 13.8 to 16-fold higher rates of homologous versus homeologous BIR rates, respectively. These data suggest a similar degree of difference between homologous and homeologous BIR of wild type or mutant. Thus, mre11A470T is likely to require the absence of telomerase, or act in a pathway different than the Rad51-dependent BIR pathway (Table 3, Figure 3, Figure 5B).
Figure 5.
The Absence of Adjacent Markers in Error-Prone products. Maps of BIR product with SAM1 invading SAM2 and replicating chromosome IV with the GMC1 marker after transformation into mre11Δ SAM1 are shown at the bottom. When SAM1-tg was transformed into MRE11 sam1Δ and mre11A470Tsam1Δ cells, 3/5 and 3/3 species were the expected BIR products of 80-kb when probed with URA3 (right gel). A second gel (left) was probed with upstream GMC1 marker. The larger forms hybridized to URA3, but not to GMC1, indicating the presence of an error-prone product. Contrast and brightness was adjusted so that the gel appeared as the original. Fragment sizes were determined in an adjacent left lane of both lanes that contained yeast chromosome size markers [Bio-Rad] in the original gel. Two photographic images of the autoradiograms differed, although the original data were of similar size. Thus, we re-adjusted the gel size to reflect that fact. C=control yeast DNA Abbreviations: w=white colony, p=pink colony, r=red colony (bottom). A schematic of the chromosome IV BIR product with markers noted.
3.5 “Error-prone” plasmid/chromosomal BIR is significantly elevated in mre11A470T cells and by the presence of homeology
BIR events are intrinsically subject to mutations and rearrangements under the best of circumstances (Deem et al. 2011). Phenotypically, we identified “error-prone” class of BIRs as those that do not maintain the expected site of origin or are a larger size than expected.
To follow the site of invasion of SAM1-tg into SAM2, we use the adjacent GMC1 locus to determine the expected initiation and extension by DNA replication (Table 4, Figure 5). Only one example of an elongated product, the homologous wild-type BIR, was initiated from D8B-tg invasion into the chromosome III chromosomal site (1/29 tested, Table 4). In contrast, 50% of the wild-type homeologous products were larger than expected (5/21) (Fisher Exact Test; P<0/05) and not initiated from the expected site, although plasmid markers were retained.
Table 4.
Normal and error-prone frequencies determined by the Fisher exact test for homologous and homeologous MRE11 and mre11A470T. Horizontally, the homologous DNA is present on the top line MRE11 and expected/error prone BIRs is compared with the values obtained in mre11A470T. Similarly, lines 3 vs line 5 are the values of a vertical comparison of BIRs wild-type/homologous vs homeologous/wild type BIRs (left) and a comparison between homologous/mre11A470T vs homeologous/mre11A470T (right, with the P, value on the bottom. Significant values are in red, while ns=no significant difference.
| Homologous MRE11 | Homologous mre11A470T | Fisher Exact | ||
|---|---|---|---|---|
| Expected structure | Error prone | Expected structure | Error prone | |
| 28 | 1 | 16 | 5 | P<0.01 |
| Homeologous MRE11 sam1Δ | Error Prone | Homeologous mre11A470T sam1Δ | Error Prone | |
| 6 | 6 | 8 | 1 | ns |
| Fisher Exact | P<0.05 | P=0.087 |
There are likely to be additional weak homology sites of invasion, or multiple rounds of invasion. That notion comes from a low homology region to SAM1 on cXV, using low stringency parameters in BLAST analysis (Tatusova and Madden 1999). This sequence would be predicted to produce a ~560-kb chromosome. Many products were indeed observed among the elongated BIR species (Figure 3, Figure 5B). The presence of these sites suggests that alternative invasion can occur with highly diverged sequences, particularly in mre11A470T cells and in cells with greater homeology.
Two conclusions can be reached (Table 4). First, the frequencies of error-prone BIR products are increased in the presence of homeology (6/12) compared to homologous BIR in wild-type cells (28/29). Similarly, a comparison of homeologous (8/9) versus homologous BIR in mre11A470T cells (16/21) may be P=.087. The latter result may be significant with higher sample sizes. Most error-prone events did not maintain the primary site of invasion on chromosome IV and were elongated by secondary processes. Hence, at least in wild-type cells, homeology most likely increases the error rate due to misalignment during invasion, a process that may be relevant to the bypass of senescence.
Second, an analysis of wild-type (28/29) and mre11A470T (6/12) homologous BIR events at MRE11D8B revealed a major increase in homologous error prone BIR events. (Fisher exact analysis (P<0.01)). In an analysis of wild-type (6/12) and mre11A470T (8/9), homeologous BIR could not be shown to be significant, although the presence of small samples in both groups may be masking significant results (Fisher Exact Test, P=0.637). Hence, the differences between the mutant and wild-type strains were clear for homologous BIR but could not be shown to be significant in homeologous cells. Nonetheless, this result does show that mutant cells have far lower fidelity of homologous BIR than in wild-type homologous BIR and may therefore contribute to the bypass of senescence.
4. Discussion
We introduced (Davis and Symington 2004) plasmids D8B-tg and MRC1 into MRE11 or mre11A470T cells or SAM1-tg into MRE11sam1Δ or mre11A470T sam1Δ cells and determined BIR frequencies, using a color assay to measure the frequency of different classes of products. A subset of transformants were analyzed by CHEF gel electrophoresis and by Southern blots for GMC1 marker retention.
One of our hypotheses for the bypass of senescence was that mre11A470T is hyper-recombinational in a homeologous and/or homologous recombination. We present evidence that this hypothesis is incorrect at least in terms of the range of gap repair to BIR. Similarly, the mre11A470T mutation had no effect on gap-repair. Hence, a general alteration in recombination was not involved in mre11A470T function. Homeologous recombination as assayed by SAM1-tg interacting with cIV SAM2 was decreased similarly in wild-type and mutant BIR products (Bailis and Rothstein 1990). The same phenotype of identical decreases in frequency was observed between wild-type and mutant homologous recombination (Table 3).
However, a more detailed study of the products of homologous or homeologous BIR led to some additional considerations. First, we found that high molecular weight BIR products, 200–600-kb greater in size, are present in only 1/27 BIR product in wild-type cells. We refer to these alternative products as “error-prone” BIR. Specifically, we found that these large products were increased in abundance in the mre11A470T strains and by the introduction of homeology.
Multiple studies have indicated that the BIR process itself is intrinsically error-prone, as defined by the accumulation of mutations near the invasion site. This would explain data that mutations in mismatch repair enzymes increase the efficiency of homeologous BIR, an observation also made in other types of homeologous recombination. (Bailis and Rothstein 1990). There is some indication that excision repair is also required for homeologous recombination (Nicholson et al. 2000). More severe errors in micro template switching can cause rearrangement in homologous BIR (Vissers et al. 2009). Any of these processes would give rise to the large products that we have observed. These latter processes may explain some of the elongated products.
The BIR products initiated by D8B-tg on chromosome III were 110-kb in length and, in the case of MRC, 30-kb. The BIR product initiated by SAM1-tg of the expected length was 80-kb flanked by the expected GMC1 marker. All other transformants of different size failed to contain the cIV GMC1 sequences but did maintain at least a part of the plasmid. These events increased the frequency of error-prone BIR significantly in the presence of the mre11A470T mutation or homeologous DNA, independently.
However, in some cases, we found that products were consistent with the expected size of a very low homology site. We used BLAST analysis placing under weak homology conditions and identified only one region that maintained some homology with SAM1 or SAM2. No other fragments were identified under any BLAST conditions. The expected product for invasion at the cXV site predicts the expected 560 product of a single BIR invasion that we observe in many contexts (Figure 5B). Since this is only deduced from BLAST results, we cannot determine whether such a site is actually utilized. We can only conclude that the results are consistent with a low fidelity of SAM1 CFV-tg. In what state the plasmid end is present is not known, except that it still contains URA3, SUP11, and centromere sequences. In many cases, the site of invasion may be imprecise, with products only showing the flanking plasmid markers maintained. The lack of observable homology may be due to an unknown region of homeology or products that have undergone major rearrangements such as template switching, or mutagenesis (Schmidt et al. 2006). These forms may be coupled with the presence of unstable BIR products (see below).
Another finding is consistent with the behavior of mre11A470T homeologous error-prone BIR. We found that linear BIR products were formed more efficiently from linear transformants in mre11A470T cells than in wild-type cells. Although kinetic models may explain the differences, the invasion of DNA at promiscuous sites is also consistent with the “error prone model”. The lack of fidelity in the invasion of a homeologous recombination some cases may explain larger fragments. In addition, other unknown sites of invasion may also be associated with the same forms of mitigating mutations and massive re-invasion, polymerase slippage or template switching.
BIR has also been associated with microsatellite instability. In context of telomere simple sequence repeats, such a class of BIR could give rise to an expansion of telomere tract sizes. BIR-related expansion of triplets has been observed previously (Leffak 2017) and is a possible means of senescence bypass if the regions are unstable, giving rise to short intrachromatid or interchromatid extensions. Regardless, the mre11A470T mutation is associated with compromising rearrangements and maintenance that may play a role in expansion in a telomerase negative background.
In this paper, we tested the hypothesis that mre11A470T mutation alone increased homeologous recombination. This increase could explain the bypass of senescence exhibited in mre11A470T telomerase-negative cells. However, these studies have demonstrated that mre11A470T is not sufficient to explain this phenotype. A reanalysis of the bypass is still required using gene mutations that encompass all of the known short-tract BIR and single-strand annealing enzymes, such as Rad59. This study nonetheless provided important information on the properties of homologous and homeologous recombination in the wild-type and mre11A470T cells. We can make three conclusions.
Bailis and Rothstein (Bailis and Rothstein 1990) assayed the mitotic frequency of homeologous recombination between the SAM1 and SAM2 genes encoding the two S-adenosyl-methionine synthetase genes. The SAM1 and SAM2 genes are 83% identical at the DNA sequence level, but recombine ectopically, at 8.4 × 10-9/cell division. The mismatch repair gene PMS1 reduces homeologous recombination between the SAM1 and SAM2. However, telomere rapid deletion (TRD) during intrachromatid excision of long telomeres in yeast is not influenced by loss of the pms1, msh2, and msh3, and mre11-rad50 mutations, while mutations in structural components of the telomere, including yKu70 and Sir3, abolish and alter the fidelity of TRD, respectively. (Li and Lustig 1996, Polotnianka et al. 1998). However, telomeric senescence is also blocked by a presumptive increase in recombination that results in bypass of senescence in mre11A470T cells (Joseph et al. 2010). Now that we have the control data of long-tract repeats, we can investigate the telomere more closely in TRD and during senescence with the suspected involvement of Rad59 (Churikov et al. 2014).
Interestingly, rad50 (Porter et al. 1996, Ellahi et al.) (most likely present as mre11-rad50) has no effect on homeologous recombination nor in the TRD process. Yet mre11A470T, at the interface of the two proteins, seems to have a major effect on homeologous BIR compared to the wild-type protein. These results raise the possibility that short tract homeologous recombination follows a different pathway, possibly defined by the RAD59 pathways.
Acknowledgments
We would like to thank Drs. Sue Jinks-Robertson and Astrid Engel for advice and Ms. Bonnie Hoffman for critical comments. This study was funded by 5F32GM103034 (to CP), R01GM069943-6A1, and institutional funds from both the Department of Biochemistry and Molecular Biology and from the Tulane Cancer Center.
Abbreviation List
- aa
amino acid
- BIR
break-induced replication
- CHEF electrophoresis
contour-clamped homogeneous electric field electrophoresis
- D
deoxyribo-
- Ds
double-strand(ed)
- Kb
kilobase(s) or 1000 bp
- Kan
kanamycin
- Kd
kilodalton(s)
- Nt
nucleotides
- Oligo
olignonucleotides
- ORF
open reading frame(s)
- P
plasmid
- Pr
promoter
- PA
polyacrylamide
- PAGE
polyacrylamide gel electrophoresis
- R
recombinant
- SDS
sodium dodecyl sulfate
- Ss
single-stranded
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bailis AM, Rothstein R. A defect in mismatch repair in Saccharomyces cerevisiae stimulates ectopic recombination between homeologous genes by an excision repair dependent process. Genetics. 1990;126(3):535–547. doi: 10.1093/genetics/126.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Imam A, Greider CW. Two survivor pathways that allow growth in the absences of telomerase are generated by distinct telomere recombination events. Mol Cell Biol. 2001;21(5):1819–1827. doi: 10.1128/MCB.21.5.1819-1827.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churikov D, Charifi F, Simon MN, Geli V. Rad59-facilitated acquisition of Y′ elements by short telomeres delays the onset of senescence. PLoS Genet. 2014;10(11):e1004736. doi: 10.1371/journal.pgen.1004736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counter CM, Meyerson M, Eaton EN, Weinberg RA. The catalytic subunit of yeast telomerase. Proc Natl Acad Sci U S A. 1997;94(17):9202–9207. doi: 10.1073/pnas.94.17.9202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AP, Symington LS. RAD51-dependent break-induced replication in yeast. Mol Cell Biol. 2004;24(6):2344–2351. doi: 10.1128/MCB.24.6.2344-2351.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deem A, Keszthelyi A, Blackgrove T, Vayl A, Coffey B, Mathur R, Chabes A, Malkov A. Break-induced replication is highly inaccurate. PLoS biology. 2011;9(2):e1000594. doi: 10.1371/journal.pbio.1000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrana K, Perrod S, Gasser SM. Turning telomeres off and on. Curr Opin Cell Biol. 2001;13(3):281–289. doi: 10.1016/s0955-0674(00)00210-6. [DOI] [PubMed] [Google Scholar]
- Ellahi A, Thurtle DM, Rine J. The Chromatin and Transcriptional Landscape of Native Saccharomyces cerevisiae Telomeres and Subtelomeric Domains. Genetics. 2015;200(2):505–521. doi: 10.1534/genetics.115.175711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hector RE, Shtofman RL, Ray A, Chen BR, Nyun T, Berkner KL, Runge KW. Tel1p preferentially associates with short telomeres to stimulate their elongation. Mol Cell. 2007;27(5):851–858. doi: 10.1016/j.molcel.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Huang QQ, Lee KY, Wang JH. A novel yeast protein showing specific association with the cyclin-dependent kinase 5. FEBS Lett. 1996;378(1):48–50. doi: 10.1016/0014-5793(95)01394-6. [DOI] [PubMed] [Google Scholar]
- Joseph IS, Kumari A, Bhattacharyya MK, Gao H, Li B, Lustig AJ. An mre11 mutation that promotes telomere recombination and an efficient bypass of senescence. Genetics. 2010;185(3):761–770. doi: 10.1534/genetics.110.117598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffak M. Break-induced replication links microsatellite expansion to complex genome rearrangements. Bioessays. 2017;39(8) doi: 10.1002/bies.201700025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Lustig AJ. A novel mechanism for telomere size control in Saccharomyces cerevisiae. Genes Dev. 1996;10(11):1310–1326. doi: 10.1101/gad.10.11.1310. [DOI] [PubMed] [Google Scholar]
- Lingner J, Cech TR, Hughes TR, Lundblad V. Three Ever Shorter Telomere (EST) genes are dispensable for in vitro yeast telomerase activity. Proc Natl Acad Sci U S A. 1997;94(21):11190–11195. doi: 10.1073/pnas.94.21.11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundblad V, Blackburn EH. An alternative pathway for yeast telomere maintenance rescues est1-senescence. Cell. 1993;73(2):347–360. doi: 10.1016/0092-8674(93)90234-h. [DOI] [PubMed] [Google Scholar]
- McEachern MJ, Haber JE. Break-induced replication and recombinational telomere elongation in yeast. Annu Rev Biochem. 2006;75:111–135. doi: 10.1146/annurev.biochem.74.082803.133234. [DOI] [PubMed] [Google Scholar]
- Mirzoeva OK, Kawaguchi T, Pieper RO. The Mre11/Rad50/Nbs1 complex interacts with the mismatch repair system and contributes to temozolomide-induced G2 arrestand cytotoxicity. Mol Cancer Ther. 2006;5(11):2757–2766. doi: 10.1158/1535-7163.MCT-06-0183. [DOI] [PubMed] [Google Scholar]
- Morrow DM, Connelly C, Hieter P. ”Break copy” duplication: a model for chromosome fragment formation in Saccharomyces cerevisiae. Genetics. 1997;147(2):371–382. doi: 10.1093/genetics/147.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan S, McEachern MJ. Recombinational telomere elongation promoted by DNA circles. Mol Cell Biol. 2002;22(13):4512–4521. doi: 10.1128/MCB.22.13.4512-4521.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson A, Fabbri RM, Reeves JW, Crouse GF. The effects of mismatch repair and RAD1 genes on interchromosomal crossover recombination in Saccharomyces cerevisiae. Genetics. 2006;173(2):647–659. doi: 10.1534/genetics.105.055244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson A, Hendrix M, Jinks-Robertson S, Crouse GF. Regulation of mitotic homeologous recombination in yeast. Functions of mismatch repair and nucleotide excision repair genes. Genetics. 2000;154(1):133–146. doi: 10.1093/genetics/154.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr-Weaver TL, Szostak JW, Rothstein RJ. Genetic applications of yeast transformation with linear and gapped plasmids. Methods Enzymol. 1983;101:228–245. doi: 10.1016/0076-6879(83)01017-4. [DOI] [PubMed] [Google Scholar]
- Polotnianka RM, Li J, Lustig AJ. The yeast Ku heterodimer is essential forprotection of the telomere against nucleolytic and recombinational activities. Curr Biol. 1998;8(14):831–834. doi: 10.1016/s0960-9822(98)70325-2. [DOI] [PubMed] [Google Scholar]
- Porter G, Westmoreland J, Priebe S, Resnick MA. Homologous and homeologous intermolecular gene conversion are not differentially affected by mutations in the DNA damage or the mismatch repair genes RAD1, RAD50, RAD51, RAD52, RAD54, PMS1 and MSH2. Genetics. 1996;143(2):755–767. doi: 10.1093/genetics/143.2.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Russell DW. Pulsed-field Gel Electrophoresis via Contour-clamped Homogeneous Electric Field Gels. CSH Protoc. 2006;2006(1) doi: 10.1101/pdb.prot4033. [DOI] [PubMed] [Google Scholar]
- Schmidt KH, Wu J, Kolodner RD. Control of translocations between highly diverged genes by Sgs1, the Saccharomyces cerevisiae homolog of the Bloom’s syndrome protein. Mol Cell Biol. 2006;26(14):5406–5420. doi: 10.1128/MCB.00161-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz DC, Koval M. Conformational dynamics of individual DNA molecules during gel electrophoresis. Nature. 1989;338(6215):520–522. doi: 10.1038/338520a0. [DOI] [PubMed] [Google Scholar]
- Seifert FU, Lammens K, Stoehr G, Kessler B, Hopfner KP. Structural mechanism of ATP-dependent DNA binding and DNA end bridging by eukaryotic Rad50. Embo Journal. 2016;35(7):759–772. doi: 10.15252/embj.201592934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer MS, Gottschling DE. TLC1: template RNA component of Saccharomyces cerevisiae telomerase. Science. 1994;266(5184):404–409. doi: 10.1126/science.7545955. [DOI] [PubMed] [Google Scholar]
- Soudet J, Jolivet P, Teixeira MT. Elucidation of the DNA end-replication problem in Saccharomyces cerevisiae. Mol Cell. 2014;53(6):954–964. doi: 10.1016/j.molcel.2014.02.030. [DOI] [PubMed] [Google Scholar]
- Tatusova TA, Madden TL. BLAST 2 Sequences, a new tool for comparing protein and nucleotide sequences. FEMS Microbiol Lett. 1999;174(2):247–250. doi: 10.1111/j.1574-6968.1999.tb13575.x. [DOI] [PubMed] [Google Scholar]
- Teng SC, V, Zakian A. Telomere-telomere recombination is an efficient bypass pathway for telomere maintenance in Saccharomyces cerevisiae. Mol Cell Biol. 1999;19(12):8083–8093. doi: 10.1128/mcb.19.12.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissers LE, Bhatt SS, Janssen IM, Xia Z, Lalani SR, Pfundt R, Derwinska K, de Vries BB, Gilissen C, Hoischen A, Nesteruk M, Wisniowiecka-Kowalnik B, Smyk M, Brunner HG, Cheung SW, van Kessel AG, Veltman JA, Stankiewicz P. Rare pathogenic microdeletions and tandem duplications are microhomology-mediated and stimulated by local genomic architecture. Hum Mol Genet. 2009;18(19):3579–3593. doi: 10.1093/hmg/ddp306. [DOI] [PubMed] [Google Scholar]
- Yang CW, Tseng SF, Yu CJ, Chung CY, Chang CY, Pobiega S, Teng SC. Telomere shortening triggers a feedback loop to enhance end protection. Nucleic Acids Res. 2017 doi: 10.1093/nar/gkx503. [DOI] [PMC free article] [PubMed] [Google Scholar]