Abstract
Intimate Partner Violence (IPV) perpetration may induce cardiovascular reactivity and risk markers thereby precipitating early onset cardiovascular disease (CVD). However, this relationship has been largely under-researched in comparison to the health impacts of IPV victimisation. We therefore aimed to systematically review the current evidence investigating the relationship between IPV perpetration and CV risk. Six databases (CINAHL, Ovid MEDLINE, Pubmed, Scopus, ProQuest, Google Scholar) were searched between August 2016 and August 2017 using a predefined search strategy. Inclusion criteria were studies of cross sectional and longitudinal design published since 2010, presenting IPV status by perpetrators (as distinct from victims) and an outcome of CVD (e.g. cardiac disease, stroke), CV risk markers (e.g. blood pressure) and/or a composite CV risk score. Twenty two potentially eligible studies were identified and full texts recovered. After ineligible studies were excluded, four remained (total n = 10,665). Positive relationships were observed between IPV perpetration and (i) short term CV reactivity markers (higher heart rate, lower vagal ratios, shorter pre-ejection periods) and (ii) longer term CV risk factors and outcomes including greater systolic blood pressure, incident hypertension, elevated 30 year CV risk score and self-report cardiac disease. Despite being a neglected area of research characterised by a high degree of heterogeneity, the early evidence suggests that IPV perpetration may be associated with elevated risk of CVD. We discuss these findings in the context of CVD prevention from the individual, family and inter-generational perspectives and directions for future studies.
Keywords: Intimate Partner Violence, Perpetrators, Cardiovascular risk
Highlights
-
•
Intimate Partner Violence (IPV) perpetration may affect cardiovascular (CV) risk.
-
•
4 studies have investigated the relationship between IPV perpetration and CV risk.
-
•
IPV perpetration was positively associated with short and long term CV markers.
-
•
CVD prevention may require individual, family and inter-generational perspectives.
1. Introduction
Family violence (FV) is a pervasive source of environmental stress that affects one in three women, globally (World Health Organization, 2013). It is most commonly perpetrated by men against current or former intimate partners (Intimate Partner Violence; IPV) (Taft et al., 2001). IPV victimisation has been associated with a range of deleterious mental and physical health effects including cardiovascular (CV) risk behaviours and outcomes (Stene et al., 2013). The CV effects of IPV victimisation have been extensively researched and include higher rates of carotid atherosclerosis, Takotsubo cardiomyopathy (“broken heart syndrome”), obesity, high triglycerides, low HDL-C, and higher cigarette, drug and alcohol consumption compared with women who are not exposed (Stene et al., 2013).
In 2015, Suglia et al. conducted a systematic review investigating the long term association between exposure to violence in either childhood or adulthood and subsequent CV outcomes including hypertension, blood pressure, stroke, coronary heart disease or myocardial infarction (Suglia et al., 2015). The authors found a consistent and significant relationship between childhood exposure to physical or sexual violence and cardiovascular endpoints, documenting heterogeneity in exposure and endpoint measures. The association was less clear for violent exposure in adulthood and CV health.
By comparison, the cardiotoxic effects of IPV perpetration remain largely unknown, largely due to a lack of research. Research from the fields of personality and social psychology and criminology support the hypothesis that violent behaviours have negative effects on the CV system, particularly over the short term. For example, aspects of control (i.e., dominance) have been shown to predict higher systolic and diastolic blood pressure (SBP, DBP) in men (Baron et al., 2016). Others have demonstrated that high-hostile men show elevated and more prolonged increases in BP, forearm blood flow and vascular resistance, and norepinephrine compared with low-hostile men (Suarez et al., 1998). When compared with low-hostile men, high hostility independently predicts vascular sympathetic drive (Virtanen et al., 2003) and higher skin conductance responses (Carmona et al., 2008).
In 2010, Pinto et al., conducted a review of the literature that identified potential biological correlates of IPV perpetration more specifically, including physiological reactivity (e.g. changes in biomarkers in response to a stressor). The objective of the review of the evidence (generated between 1979 and 2010) was to investigate physiological reactivity, specifically arousal levels, in order to identify subtypes of IPV perpetration. Research assessing the relationship between IPV perpetration and CV risk and markers has rarely been considered in the context of CV disease progression. Indeed, it is plausible that chronic, maladaptive CV responses owing to the use of IPV perpetration could induce subclinical CV disease such as carotid atherosclerosis and peripheral vascular disease, CV risk factors such as hypertension or hyperlipidemia, or CV reactivity. Yet traditionally, this has been a neglected area of public health research.
The aim of this paper was therefore to systematically review the current evidence based investigating the relationship between IPV perpetration (distinct from victimisation) and CVD, discuss candidate pathophysiologic mechanisms and consider how better understanding the CV system of perpetrators may have utility in both IPV and CVD prevention.
2. Methods
2.1. Selection of studies
This review built on that of Pinto et al., published in 2010. Studies were considered for inclusion if they were: (i) published between 2010 and August 2017 (ii) full-text articles; (iii) cross sectional and longitudinal cohort study designs; (iv) examined associations between IPV perpetration (self-reported, identified through administrative records, partner reported) as distinct from victimisation; (v) included a CV outcome (e.g. cardiac disease, stroke), markers of CV risk or reactivity (e.g. blood pressure, heart rate variability) and/or a composite CV risk score identified by self-report, medical records, data linkage; and (vi) studies that defined IPV as form(s) of physical, verbal, emotional, sexual, religious, technological, and/or financial abuse where a current or previous intimate partner was victimised. We excluded studies which did not present the aforementioned CV outcomes, and randomised controlled trials.
2.2. Search strategy and data extraction
Between August 2016 and August 2017, six databases for medical, health, psychiatric and social sciences (CINAHL, Ovid MEDLINE, Pubmed, SCOPUS, Pro Quest and Google Scholar) were searched using a computer-generated search strategy. The full search strategy is provided in Appendix A. In order to identify grey literature, reference lists of relevant reviews and studies were searched. AO conducted the electronic search strategy, which was replicated by AJS. Once abstracts of potentially relevant papers were identified and full-text copies obtained, each author finalised the list of included articles based on predetermined inclusion and exclusion criteria. The review was conducted in accordance with the guidelines outlined in the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement 2009 (Moher et al., 2009).
3. Results
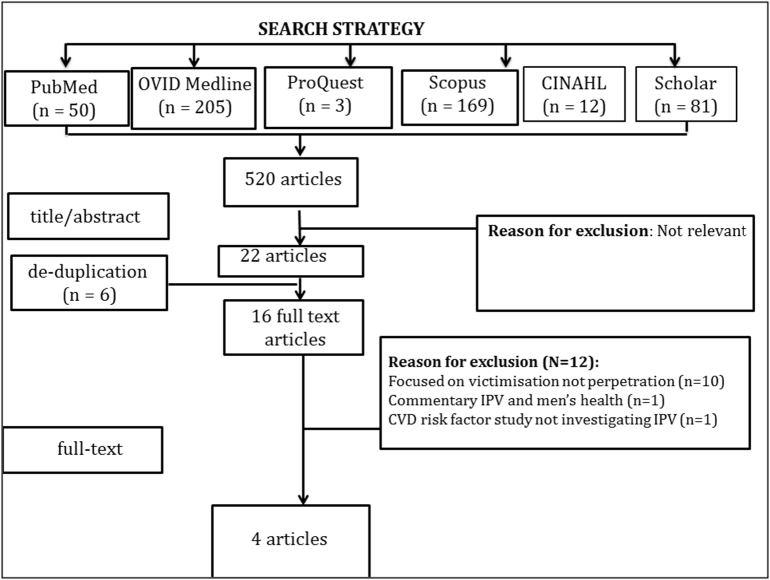
The initial search strategy yielded 520 citations. Of these, 22 were identified as potentially relevant. After removing five duplicates, 16 full text articles were retrieved. Of these, 12 were excluded, leaving four eligible studies for inclusion in this review (total n = 10,665). The consensus between authors was 100%. Fig. 1 displays a summary of the results of the systematic search.
Fig. 1.
Summary of search results (QUOROM Flowchart).
3.1. Population and design
Key characteristics of the included studies are displayed in Table 1. One study investigating short term CV reactivity used a laboratory based, repeated measures study design of healthy males; half of whom had a criminal history of IPV (n = 17) and half did not (n = 17) (mean age: 36.88 ± 2.59 and 34.82 ± 1.47, respectively). Two of the included studies used data from the National Longitudinal Study of Adolescent to Adult Health; (n = 9976; n = 9699, respectively) a nationally representative sample of US adolescents in grades 7–12. Participants were interviewed across four waves of assessment between 1994–95 and 2008–09. The fourth study used a cross-sectional design investigating the association between IPV and physical health conditions in offenders with alcohol disorders (mean age 33.4 ± 10.9). This study treated cardiac and other conditions as a predictor of IPV perpetration.
Table 1.
Key characteristics and findings of included studies.
| Author and year | Study design/setting | Population | IPV measure | CV measure | Covariates | Key findings |
|---|---|---|---|---|---|---|
| Clark et al. (2014) | Two waves of population based data (National Longitudinal Study of Adolescent Health) | 9699 healthy young adults/late adolescents | Revised Conflict Tactics Scales (Wave 3). | Systolic and diastolic blood pressure/self-reported use of anti-hypertensives (Wave 4) | Sex, race/ethnicity, and wave 3 age, educational attainment, financial distress | Men experiencing severe perpetration and victimisation had a 2.66 mm Hg (95% CI: 0.05, 5.28) higher SBP and a 59% increased odds of incident hypertension. |
| Clark et al. (2016) | Two waves of population based data (National Longitudinal Study of Adolescent Health) | 9976 healthy young adults/late adolescents | Revised Conflict Tactics Scales (Wave 3). | Framingham Risk Prediction model to assess 30-year risk of developing CVD (Wave 4). | Sex, race/ethnicity, and wave 3 age, respondent educational attainment, financial stress, and child maltreatment and wave 1 indicators of parental education and neighborhood poverty. | Perpetration positively associated with CVD risk (beta: 0.33, 95% CI: 0.03, 0.62). |
| Romero-Martínez et al. (2014) | Healthy male volunteers. Repeated measures design in laboratory setting | 34 (17 IPV perpetrators; 17 controls) | Criminal record | Pre-ejection period Heart rate reactivity (beats per minute) and variability (vagal ratio) |
Time × group interaction | IPV perpetrators had higher HRs, lower vagal ratios and shorter PEPs than controls. |
| Crane and Easton (2017) | Cross-sectional study comprising offenders with alcohol use disorder | 655 | Revised Conflict Tactics Scale (CTS-2) | Self-reported cardiac issues (cardiovascular or heart disease, angina, hypercholesterolemia, hypertension) during clinical interview, confirmed with review of medical records | Ethnicity, gender, and employment status | Unadjusted OR 1.99 [1.07, 3.72] Adjusted OR 1.93 [1.02, 3.66] |
3.2. Measures of IPV
Three of the four studies used a subset of questions from the revised Conflict Tactics Scales (Straus et al., 1996) to assess IPV perpetration, e.g. Have you threatened your partner with violence, pushed or shoved, or thrown something that could hurt; slapped, hit or kicked your partner; made your partner have sexual relations when they did not want to; caused an injury, such as a sprain, bruise, or cut because of a fight with your partner. Both Clark studies categorised IPV as follows: no IPV experience, victimisation only, perpetration only and bi-directional (both victimisation and perpetration) (Clark et al., 2014, Clark et al., 2016). Crane and Easton (2017) categorised participants as violent (any physical IPV) or non-violent (no physical IPV). The Romero-Martinez study identified IPV perpetration history through criminal records (Romero-Martínez et al., 2014).
3.3. Measures of CVD
Romero-Martínez et al.'s (2014) study used short term CV reactivity in response to a stressful task as the outcome of interest (heart rate (HR), vagal ratio and pre-ejection period (PEP) before, during and after exposure). Clark et al. (2014) used the primary endpoint of systolic blood pressure (SBP) as a continuous variables as well incident hypertension (as diagnosed by participant self-report). Clark et al. (2016) used the Framingham Risk Prediction model (Pencina et al., 2009) (calculated at wave 4 (2008/09) when participants were 29 years) to assess 30-year risk of developing incident CVD. Crane and Easton (2017) identified existing cardiac issues (CV or heart disease, angina, hypercholesterolemia, hypertension) by self-report during clinical interview, confirmed with review of medical records.
3.4. Relationship between IPV perpetration and CVD outcome
All four studies identified a positive association between IPV perpetration and the CV measure of interest. Romero-Martínez et al. (2014) found that men with a history of IPV had poorer CV reactivity compared with men with no history. A significant “time × group” interaction effect was found where IPV perpetrators had higher HR [0.68; F (2.05, 65.58). 3.17, p. 0.047] and lower vagal ratio [0.64, F (1.92, 61.49). 3.08, p. 0.050] during the recovery time, compared to controls. A significant “group” effect was found for PEP [F (1, 32). 3.93, p. 0.05], where IPV perpetrators had shorter PEP than controls. Those with a history of IPV perpetration and those without did not differ on any socio-demographic factors. Clark et al. (2014) found that men who identified as severe perpetrators (combined with victims) had a 2.66 mm Hg (95% CI: 0.05, 5.28) higher SBP and a 59% increased odds of incident hypertension. When looking at CVD risk scores, Clark et al. (2016) found that IPV perpetration, specifically in late adolescence and young adulthood, was associated with an increased risk of incident CVD in the ensuing 7–14 years (b 0.33 (95% CIs: 0.03, 0.62)). Both Clark et al., 2014, Clark et al., 2016 papers adjusted for socio-demographic factors, including age, ethnicity, educational attainment and financial stress.
3.5. Relationship between CVD and IPV perpetration outcome
Crane and Easton (2017) was the only study to investigate the contribution of cardiac disease on IPV perpetration. Having self-reported cardiac issues was significantly associated with greater odds of IPV perpetration in men with alcohol use disorders (OR 1.99 [1.07, 3.72]); an association that held true following adjustment for ethnicity, gender and employment (adj OR 1.93 [1.02, 3.66]).
4. Discussion
This review revealed positive relationships between IPV perpetration and (i) short term CV reactivity markers (higher HR, lower vagal ratios, shorter PEP) and (ii) longer term CV risk factors and outcomes (greater SBP, incident hypertension, elevated 30 year CV risk score, self-report cardiac disease). Despite being a neglected area of research characterised by a high degree of heterogeneity, the early evidence suggests that IPV perpetration may be associated with elevated risk of CVD. While there is vast research demonstrating the long term association between incident CVD and exposure to IPV (Suglia et al., 2015) – both from the perspective of partners and children – further explicating this relationship for perpetrators is likely to be of significance.
Assuming the association between IPV use and CV risk observed here is replicated by other studies in the future, there are numerous pathways by which IPV use could be a determinant of CV health. Persistent behavioural patterns that are associated with IPV likely trigger a cascade of stress-induced alterations in immuno-inflammatory, autonomic and endocrine responses that elevate CV later life risk. Indeed, IPV could be a risk marker for maladaptive stress responses that lead to cardiovascular events. One recently identified candidate mechanism linking stress and CVD is amygdala activation (Tawakol et al., 2017). The Lancet study found the acute effects of stress to occur via bone-marrow activity and arterial inflammation. In the context of violent behaviours, perceived challenges to social status have been shown to elicit a testosterone response that precipitates increased physiological activation and aggressive behaviours (Porges et al., 2015). As a form of intervention, yoga, meditation and mindfulness therapy have been shown to be effective in improving stress resilience and self-awareness in vulnerable youth (Ramadoss and Bose, 2010) and as an adjunctive treatment in forensic settings (Sistig et al., 2015). Walton et al. (2002) demonstrated that the specific meditation technique of Transcendental Meditation (TM) can reverse or delay the progression of pathophysiological changes that underlie CVD; blood pressure, carotid artery intima-media thickness, myocardial ischemia and left ventricular hypertrophy (Walton et al., 2002).
Better understanding the CV system of IPV perpetrators could have a number of clinical implications for IPV prevention. Indeed, the cardiovascular physiology of IPV perpetrators could serve both as a target and outcome for intervention. Past research has revealed IPV perpetration is characterised by alterations in CV markers including blood flow and galvanic skin response, during build up, enactment and aftermath (Ali et al., 2016). The autonomic nervous system, specifically, cardiac vagal control has been implicated. Compared with healthy controls, Umhau et al. (2002) found an absence of posture-elicited changes in a measure of decreased cardiac vagal activity (respiratory sinus arrhythmia; RSA) in perpetrators, suggesting differences in the neural regulation of heart rate and may be related to difficulties in controlling autonomic state. Yet others have shown that these relationships are not always linear; IPV perpetration can be accompanied by hyper-arousal for most, but hypo-arousal for others (Jacobson and Gottman, 1998). Monitoring return to baseline autonomic function during the resolution phase could guide biofeedback based interventions that draw on individual patterns of CV markers in order to predict and prevent a subsequent IPV episode. With the advent of everyday digital devices for heart rate monitoring such as Fitbits, this approach could be utilised as part of an early IPV intervention strategy adjunctive to clinical treatment programs like Men's Behaviour Change.
Eliminating or mitigating a critical and common source of environmental stress like IPV may have cardio-protective effects that reverberate through families. Adverse childhood events (ACE) are robust predictors of CV problems in later life, including onset (Rich-Edwards et al., 2012) and recurrent CVD (Le Carolyn et al., 2013). Developmentally, trauma that occurs in childhood and teenage years - particularly during critical growth periods - predicts a range of deleterious behavioural and health outcomes including pronounced chronic disease risk in later life. This association is such that early life trauma has a population attributable risk of 26.1% for adult CVD onset (Bear, 2014); a magnitude greater than that of diabetes, history of hypertension or abdominal obesity. The generational effects of trauma caused by IPV are far reaching. There is preliminary epigenetic evidence that ancestral trauma can influence later generations' health and development on variables such as birth weight in the absence of direct exposure (Vågerö and Rajaleid, 2017). The relatively new concept of ‘transgenerational response’ considers how traumatic exposures that occur in early life impact subsequent generations via two principal, non-mutually exclusive types of pathways; social pathways wherein individuals learns from the environment created by their caregivers and epigenetic pathways whereby exposures in previous generations can modify gene expression in future generations (Yehuda and Bierer, 2007). For example, maternal Post Traumatic Stress Disorder (PTSD) is predictive of increased risk for PTSD in offspring (Yehuda et al., 2008). Animal models demonstrate the potency of pre-natal maternal chronic stress on a range of behavioural, physiological and psychological outcomes of offspring. It is thus plausible that IPV use and the associated trauma for victims has intergenerational effects on CV risk of offspring that occurs via epigenetic pathways. To this end, taking an inter-generational lifecourse approach to CVD prevention that targets IPV perpetration as a source of early life stress could yield CV benefits at the individual, familial and generational levels.
The major limitation of this review is the dearth of available evidence in this area. Research and resource provision for IPV perpetrators has traditionally been neglected with the imperative to support victims; as evident by the number of studies yielded from this review compared with that of Suglia et al. (2015). As discussed above, the benefits of greater investment in perpetrators will likely extend to victims and others exposed to IPV, including children and even grandchildren with potential to mitigate the source of an environmental stressor like IPV and its deleterious health consequences. Another limitation of this review was the assessment tool used to measure IPV in three of the included studies; the revised Conflicts Tactics Scale (CTS2). Despite being the most commonly used scale to assess IPV, it has been criticised extensively. Hegarty et al. (1999) argues: (i) the psychological aggression scale does not include any items measuring the emotional abuse which is a prominent feature of partner abuse against women; (ii) the sexual coercion scale omitted some sexual abuse acts against women (e.g., put foreign objects in my vagina) and (iii) the definition of partner abuse is often operationalized as one episode of minor violence. In addition, the decision for the Clark studies to exclude forms of abuse such as controlling behaviours, may have concealed a more pronounced relationship between IPV use and CV risk than that observed. Indeed, prior research indicates that domineering traits predict rises in CV risk like blood pressure parameters. It is also plausible that the young age of the sample in the Clark studies diluted any effects that may have been observed in an older population in which clinical manifestations of CVD are more likely to have occurred. Given the small number of included studies in the review, a further limitation was the inability to explore different combinations of IPV exposure and CVD outcomes. For example, whether being both a perpetrator and a victim has cumulative effects on health, when compared to individuals who are victims or perpetrators, exclusively. It is important to note that while these studies all addressed the confounds of socio-demographics, further research into the independent effects of socio-demographic factors on the relationship between CVD and IPV perpetration would be beneficial. Lastly, none of the studies reviewed included non-heterosexual relationships. This highlights a common problem in IPV research, despite evidence that IPV occurs at a similar rate in homosexual and heterosexual relationships (Langhinrichsen-Rohling et al., 2012). Future research should aim to include non-heterosexual relationships in their studies to ensure their sample is representative.
5. Conclusion
Preliminary evidence from this review suggests positive relationships may exist between IPV perpetration and short term CV reactivity markers and longer term CV risk factors and outcomes. Indeed, the CV health of IPV perpetrators is an almost completely neglected area of preventive medicine. The likely benefits of further explicating this relationship are of relevance to public health, family violence and epigenetic fields.
Acknowledgments
AO is supported by a Future Leader Fellowship (#101160) from the Heart Foundation, Australia. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors declare no conflicts of interest.
Contributor Information
Adrienne O'Neil, Email: adrienne.oneil@unimelb.edu.au.
Anna J. Scovelle, Email: anna.scovelle@unimelb.edu.au.
Appendix A. Full search strategy
-
1)
OVID
“family violen*” or “domestic violen*” or “intimate partner violen*” or wife batter* or “domestic abuse” or “domestic assault” AND cardiovascular or cardiac or stroke or myocardial infarct* or coronary or blood pressure or lipid or cholesterol or hypertensi* or heart rate*
-
2)
PUB MED (Title abstract)
“family violen*” or “domestic violen*” or “intimate partner violen*” or wife batter* or “domestic abuse” or “domestic assault” AND cardiovascular
-
3)
CINAHL
Limit Full text, Human, English, Abstract.
cardiovascular AND “family violen*” or “domestic violen*” or “intimate partner violen*” or wife batter* or “domestic abuse” or “domestic assault”
-
4)
SCOPUS =
(TITLE-ABS-KEY (family AND violen*) OR TITLE-ABS-KEY (domestic AND violen*) OR TITLE-ABS-KEY (domestic AND assault) OR TITLE-ABS-KEY (wife AND batter*) OR TITLE-ABS-KEY (intimate AND partner AND violen*) OR TITLE-ABS-KEY (domestic AND abuse) AND TITLE-ABS-KEY (cardiovascular))
(Title, Abstract Keywords)
-
5)
PROQUEST Central
(refined to Keywords in Title only; Scientific Journals, Reports, Theses)
ti(domestic violen*) AND ti(cardiovascular)
ti(intimate partner violen*) AND ti(cardiovascular) domestic abuse and CV
ti(wife batter*) AND ti(cardiovascular)
ti(domestic assault*) AND ti(cardiovascular)
-
6)
Google Scholar
Limit Since 2017
“family violence” “domestic violence” “intimate partner violence” “cardiovascular”
References
- Ali P.A., Dhingra K., McGarry J. A literature review of intimate partner violence and its classifications. Aggress. Violent Behav. 2016;31:16–25. [Google Scholar]
- Baron C.E., Smith T.W., Uchino B.N., Baucom B.R., Birmingham W.C. Getting along and getting ahead: affiliation and dominance predict ambulatory blood pressure. Health Psychol. 2016;35:253. doi: 10.1037/hea0000290. [DOI] [PubMed] [Google Scholar]
- Bear T.M. 142nd APHA Annual Meeting and Exposition (November 15–November 19, 2014) APHA; 2014. Population attributable fractions of cardiovascular disease and serious mental illness associated with childhood adversity. [Google Scholar]
- Carmona J.E., Holland A.K., Stratton H.J., Harrison D.W. Sympathetic arousal to a vestibular stressor in high and low hostile men. Brain Cogn. 2008;66:150–155. doi: 10.1016/j.bandc.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Clark C.J., Everson-Rose S.A., Alonso A. Effect of partner violence in adolescence and young adulthood on blood pressure and incident hypertension. PLoS One. 2014;9 doi: 10.1371/journal.pone.0092204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C.J., Alonso A., Everson-Rose S.A. Intimate partner violence in late adolescence and young adulthood and subsequent cardiovascular risk in adulthood. Prev. Med. 2016;87:132–137. doi: 10.1016/j.ypmed.2016.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane C.A., Easton C.J. Physical health conditions and intimate partner violence perpetration among offenders with alcohol use diagnoses. J. Interpers. Violence. 2017;32:1678–1691. doi: 10.1177/0886260515590124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegarty K., Sheehan M., Schonfeld C. A multidimensional definition of partner abuse: development and preliminary validation of the Composite Abuse Scale. J. Fam. Violence. 1999;14:399–415. [Google Scholar]
- Jacobson N.S., Gottman J.M. Simon and Schuster; 1998. When Men Batter Women: New Insights Into Ending Abusive Relationships. [Google Scholar]
- Langhinrichsen-Rohling J., Selwyn C., Rohling M.L. Rates of bidirectional versus unidirectional intimate partner violence across samples, sexual orientations, and race/ethnicities: a comprehensive review. Partn. Abus. 2012;3:199–230. [Google Scholar]
- Le Carolyn M.H., Neylan T.C., Na B., Regan M., Zhang Q., Cohen B.E. Lifetime trauma exposure and prospective cardiovascular events and all-cause mortality: findings from the Heart and Soul Study. Psychosom. Med. 2013;75:849. doi: 10.1097/PSY.0b013e3182a88846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- Pencina M.J., D'Agostino R.B., Larson M.G., Massaro J.M., Vasan R.S. Predicting the 30-year risk of cardiovascular disease: the Framingham heart study. Circulation. 2009;119:3078–3084. doi: 10.1161/CIRCULATIONAHA.108.816694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto L.A., Sullivan E.L., Rosenbaum A. Biological correlates of intimate partner violence perpetration. Aggress. Violent Behav. 2010;15:387–398. doi: 10.1016/j.avb.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges E.C., Smith K.E., Decety J. Individual differences in vagal regulation are related to testosterone responses to observed violence. Front. Psychol. 2015;6 doi: 10.3389/fpsyg.2015.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadoss R., Bose B. Transformative life skills: pilot study of a yoga model for reduced stress and improving self-control in vulnerable youth. Int. J. Yoga Ther. 2010;20:73–78. [Google Scholar]
- Rich-Edwards J.W., Mason S., Rexrode K., Spiegelman D., Hilbert E., Kawachi I., Jun H.J., Wright R.J. Physical and sexual abuse in childhood as predictors of early-onset cardiovascular events in women. Circulation. 2012;126:920–927. doi: 10.1161/CIRCULATIONAHA.111.076877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Martínez A., Nunes-Costa R., Lila M., González-Bono E., Moya-Albiol L. Cardiovascular reactivity to a marital conflict version of the Trier social stress test in intimate partner violence perpetrators. Stress. 2014;17:321–327. doi: 10.3109/10253890.2014.919448. [DOI] [PubMed] [Google Scholar]
- Sistig B., Friedman S.H., McKenna B., Consedine N.S. Mindful yoga as an adjunct treatment for forensic inpatients: a preliminary evaluation. J. Forensic Psychiatry Psychol. 2015;26:824–846. [Google Scholar]
- Stene L.E., Jacobsen G.W., Dyb G., Tverdal A., Schei B. Intimate partner violence and cardiovascular risk in women: a population-based cohort study. J. Women's Health. 2013;22:250–258. doi: 10.1089/jwh.2012.3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus M.A., Hamby S.L., Boney-McCoy S., Sugarman D.B. The revised Conflict Tactics Scales (CTS2) J. Fam. Issues. 1996;17:283–316. [Google Scholar]
- Suarez E.C., Kuhn C.M., Schanberg S.M., Williams R.B., Zimmermann E.A. Neuroendocrine, cardiovascular, and emotional responses of hostile men: the role of interpersonal challenge. Psychosom. Med. 1998;60:78–88. doi: 10.1097/00006842-199801000-00017. [DOI] [PubMed] [Google Scholar]
- Suglia S.F., Sapra K.J., Koenen K.C. Violence and cardiovascular health: a systematic review. Am. J. Prev. Med. 2015;48:205–212. doi: 10.1016/j.amepre.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taft A., Hegarty K., Flood M. Are men and women equally violent to intimate partners? Aust. N. Z. J. Public Health. 2001;35:498–500. doi: 10.1111/j.1467-842x.2001.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Tawakol A., Ishai A., Takx R.A. Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study. Lancet. 2017;389:834–845. doi: 10.1016/S0140-6736(16)31714-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umhau J.C., George D.T., Reed S., Petrulis S.G., Rawlings R., Porges S.W. Atypical autonomic regulation in perpetrators of violent domestic abuse. Psychophysiology. 2002;39:117–123. doi: 10.1017/S0048577202990669. [DOI] [PubMed] [Google Scholar]
- Vågerö D., Rajaleid K. Does childhood trauma influence offspring's birth characteristics? Int. J. Epidemiol. 2017;46:219–229. doi: 10.1093/ije/dyw048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen R., Jula A., Salminen J.K. Anxiety and hostility are associated with reduced baroreflex sensitivity and increased beat-to-beat blood pressure variability. Psychosom. Med. 2003;65:751–756. doi: 10.1097/01.psy.0000088760.65046.cf. [DOI] [PubMed] [Google Scholar]
- Walton K.G., Schneider R.H., Nidich S.I., Salemo J.W., Nordstrom C.K., Merz C.N.B. Psychosocial stress and cardiovascular disease part 2: effectiveness of the transcendental meditation program in treatment and prevention. Behav. Med. 2002;28:106–123. doi: 10.1080/08964280209596049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2013. London School of Hygiene and Tropical Medicine and South African Research Council, Global and Regional Estimates of Violence Against Women: Prevalence and Health Effects of Intimate Partner Violence and Non-Partner Sexual Violence. [Google Scholar]
- Yehuda R., Bierer L.M. Transgenerational transmission of cortisol and PTSD risk. Prog. Brain Res. 2007;167:121–135. doi: 10.1016/S0079-6123(07)67009-5. [DOI] [PubMed] [Google Scholar]
- Yehuda R., Bell A., Bierer L.M., Schmeidler J. Maternal, not paternal, PTSD is related to increased risk for PTSD in offspring of Holocaust survivors. J. Psychiatr. Res. 2008;42:1104–1111. doi: 10.1016/j.jpsychires.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]