Abstract
Screening for melanoma may save lives, but may also cause patient distress. One key reason that preventative visual skin examinations for skin cancer are not currently recommended is the inadequate available evidence to assess potential harm to psychosocial wellbeing. We investigated potential psychological harms and benefits of skin examinations by conducting telephone surveys in 2015 of 187 screened participants; all were ≥35 years old. Participants had their skin examined by practitioners who had completed INFORMED, a validated web-based training for detection of skin cancers, particularly melanoma. Participants underwent the Spielberger State-Trait Anxiety Inventory (STAI), Psychological Consequences of Screening (PCQ), Hospital Anxiety and Depression (HAD) scale, and the 12-Item Short Form Health Survey (SF-12). Analyses were conducted in 2017. Of the entire study sample, 40% were thoroughly screened as determined by patient-reported level of undress and skin areas examined. Participants who were thoroughly screened: did not differ on negative psychosocial measures; scored higher on measures of positive psychosocial wellbeing (PCQ); and were more motivated to conduct monthly self-examinations and seek annual clinician skin examinations, compared to other participants (p < 0.05). Importantly, thoroughly screened patients were more likely to report skin prevention practices (skin self-examinations to identify a concerning lesion, practitioner provided skin exam), recommend skin examinations to peers, and feel satisfied with their skin cancer education than less thoroughly screened individuals (p < 0.01). Our results suggest that visual screening for skin cancer does not worsen patient psychosocial wellbeing and may be associated with improved skin cancer-related practices and attitudes.
Keywords: Cancer, Melanoma, Cancer prevention, Screening
1. Introduction
Melanoma incidence continues to increase and accounts for over 79% of skin cancer-related deaths (Trask et al., 2001; de Vries et al., 2007). The 5-year survival rate is very high among early stages; >96% for in situ melanomas and 92% at stage I (Balch et al., 2009; Balch et al., 2011). However, survival decreases markedly to 67% at stage II, and 49% at stage III (Balch et al., 2009; Balch et al., 2011). Thus the importance of early diagnosis of melanoma is paramount. Full-body visual skin examination is the primary tool for secondary prevention of skin cancer, particularly melanoma. Regular whole-body skin examinations are associated with reduced melanoma thickness at diagnosis and improved survival rate (Aneja et al., 2012), which has been found for both provider (Berwick et al., 1996) and self-administered skin exams (Aitken et al., 2010).
Though many providers use whole body skin exams as a standard method of skin cancer detection (Tsao & Weinstock, 2016), surprisingly little research has examined additional benefits, or potential harms of screening (Bibbins-Domingo et al., 2016; Wernli et al., 2016). This relative gap in the literature contributed to consecutive “insufficient” (“I”) ratings by the US Preventative Services Task Force (USPSTF) (Bibbins-Domingo et al., 2016). In 2016, the USPSTF issued another “insufficient” statement regarding the utility of visual skin examinations for skin cancer screening of asymptomatic healthy adults (Bibbins-Domingo et al., 2016) in primary care settings. Some of the concerns mentioned by USPSTF included the over-diagnosis and an increase in unnecessary skin biopsies, which we addressed in previous work (Weinstock et al., 2016). They also cited an inability to adequately compare the benefits and harms of skin examinations, including potential harm on psychosocial wellbeing (Bibbins-Domingo et al., 2016).
Potential psychosocial effects of cancer screening, such as anxiety and distress have contributed to revised screening recommendations for some cancer types, including breast and colon cancer (Chad-Friedman et al., 2017; Brennan & Houssami, 2016). Screenings for several types of cancer have been found to be associated with largely beneficial or non-harmful results (Niv et al., 2012; Taupin et al., 2006; McCaffery et al., 2010; Wardle et al., 2015). However, the effects on these outcomes have not been reported for skin cancer screening.
The purpose of this paper is to document the results of a survey assessment of the positive and negative psychosocial consequences, as well as post-screening skin cancer prevention attitudes and behaviors of patients who were screened by primary care providers at the University of Pittsburgh Medical Center (UPMC) initiative to screen for melanoma. Prior assessments of this initiative have shown that skin surgery and dermatology visits are not increased among patients seen by participating providers (Weinstock et al., 2016) and detected melanomas were more numerous and thinner among screened patients (Ferris et al., 2017).
As has been described elsewhere (Weinstock et al., 2016), the screening was conducted by primary care providers (PCPs; ie, physicians and other clinicians) during routine visits. These clinicians were offered online training using a modified version of the INFORMED (INternet course FOR Melanoma Early Detection) program (Eide et al., 2013). INFORMED (available at www.visualdx.com/educational-resources) has been previously shown to improve PCP skills related to melanoma detection, including the ability to appropriately reassure patients who have benign lesions that may resemble melanoma, such as seborrheic keratoses (Mykletun et al., 2001; Singer et al., 2009). It was anticipated that the ability to appropriately reassure patients would reduce the risk of screening-induced harms.
2. Methods
In the UPMC melanoma screening program (described elsewhere (Weinstock et al., 2016)), UPMC PCPs completed a modified version of the INFORMED training beginning in January 2014 to improve the early detection of melanoma and keratinocyte carcinomas (basal and squamous cell carcinomas of the skin) (Eide et al., 2013; Shaikh et al., 2012). The electronic medical record (EMR) included a health maintenance function (a new check box) to indicate screening for melanoma. For this study, UPMC staff drew a sample of all patients ≥35 years of age who were indicated in the EMR having a visit where a screen was done in the 2 PCP practices.
Letters were sent to patients offering an opportunity to opt out of the survey. Consent for the survey was conducted by telephone at the time of the survey. The protocol for obtaining verbal consent from all participants was approved by the appropriate IRB committees. Baseline surveys, representing the first contact with patients, were conducted in 2015 in batches to minimize a response bias of only completing surveys of easy-to-reach patients. Initial surveys were conducted an average of 5 months after the index PCP appointment, with a second survey following three months after the baseline contact or after a subsequent dermatology appointment.
Despite the consistent EMR presence of a checked box to indicate screening, not all patients reported in their baseline survey that they had had their skin thoroughly examined for early detection of cancer. Screening was then defined based on patient responses to several questions including: whether screening was performed, the level of undress during examination and whether certain body parts were examined. One hundred and twenty-two patients (65%) reported having their skin examined; 60 patients reported that their entire skin was examined specifically for skin cancer, and 76 patients reported being screened, and reported being completely undressed with or without undergarments and had at least two out of three body parts examined (the back, abdomen, and calves). For the purposes of these analyses, “thoroughly screened,” patients were those 76 (41%), and, “not thoroughly screened patients,” were those 111 patients (59%) who did not indicate that they had their whole body screened for skin cancer, did not disrobe or have two of the three body parts examined.
2.1. Measures
Demographics: Characteristics queried in the baseline survey included: gender; household composition (lived with both adults and children, just children, just adults, you live alone); education (8th grade or less, some high school, high school graduate or General Education Degree (GED), technical school or junior college graduate, some college, college graduate, post graduate or professional degree, other); ethnicity (Hispanic, yes or no); Race (American Indian/Alaska Native, Asian, Native Hawaiian or Pacific Islander, Black or African American, White/Caucasian or other); Household income (<$20,000, $20,000–$40,000, $40,001–$80,000, >$80,000).
The Hospital Anxiety and Depression rating scale (HADS) is a 14-item questionnaire, with 7 questions measuring anxiety (HADS-A) and 7 questions measuring depression (HADS-D) on a self-reporting scale running from 0 to 3 (Snaith & Zigmond, 1986; Mykletun et al., 2001; Singer et al., 2009). Total scores ranged from 0 to 21, with higher scores indicating more severe anxiety or depression. Based on previous data, a cut-off score of 8 or more is considered to be optimal for allocating patients into groups with high and low depressive and anxiety symptoms (Bjelland et al., 2002).
The Spielberger State-Trait Anxiety Index – form 6 (STAI-6), is a validated 6-item version of the State Trait Anxiety Inventory, a self-administered measure to assess general anxiety. Scores range from 20 to 80 with higher scores indicating more severe anxiety. An individual is considered highly anxious with a score of over 44 (Millar et al., 1995; Marteau & Bekker, 1992).
Psychological Consequences Questionnaire (PCQ) is a self-administered questionnaire designed to measure positive and negative psychological impact of a mammogram (Cockburn et al., 1992; Rijnsburger et al., 2006). The PCQ measures the consequence of screening on three major life domains: emotional, defined as the psychological aspects of a person's behavior; physical, defined as the impact on a person's physical functioning, including activities of daily living; and social functioning, defined as the effect on a person's social functioning and how she relates to others. Scoring of the negative consequences within each dimension vary from 0 to 3 with higher score indicating more distress associated with screening (PCQ – negative). Cumulative scores could range in the emotional dimension from 0 to 15, physical from 0 to 12, and social from 0 to 9.
The positive emotional, physical, and social functioning consequences of the screening experience (Cockburn et al., 1992) are also queried with scores in each dimension varying from 0 to 3 with higher score indicating less distress and more positive consequence associated with screening. Cumulative scores could range in the emotional dimension from 0 to 15, physical from 0 to 9, and social from 0 to 6.
The 12-Item Short Form Health Survey (SF-12) is a self-administered questionnaire developed to measure health-related quality of life across age, disease, and treatment group (Gandek et al., 1998). The SF-12 consists of 12 items in the physical and mental domains referring to thoughts and feelings in the past. The original form refers to one week as the reference time period; this study modified to ask about thoughts and feelings in the past four weeks. The Physical Component Summary (PCS) is an index of overall physical functioning and the Mental Component Summary (MCS) is an index of emotional and mental health. Standardized scores range from 0 to 100, with higher scores indicating better self-perceived health.
Other questions included the experience of their skin exam (embarrassment, skin biopsies done), degree of education provided at their visit (ability to identify a concerning lesion, written materials), potential concerns regarding skin cancer (seriousness of diagnosis and financial burden), and skin cancer prevention practices (UV protection, monthly and annual self-skin exams, quality of self-skin exam, desire for future skin exams).
2.2. Statistical analysis
Patients were not obligated to answer all questions so some missing values are represented. Differences between the thoroughly screened and not thoroughly screened cohorts were evaluated using Fisher's exact test. For continuous variables, normality was assessed using the Shapiro-Wilk test. The SF-12 PCS follow-up screen met assumptions of normality (p > 0.05 for Shapiro-Wilk test of normality for both groups). Assumptions of normality were not met for scores on the HADS, STAI-6, PCQ, and SF-12 MCS screens (p < 0.001 for Shapiro-Wilk test of normality for both groups). Accordingly, non-parametric statistics were used for analysis. Welch's two sample t-test was used to test for differences between the thoroughly screened and not thoroughly screened groups for normally distributed variables and Wilcoxon rank sum test was used to test for differences between groups for variables that were non-normally distributed. The alpha criterion was set at <0.05. Statistical analysis was performed during 2017 using R 3.2.3 (R Core Development Team, 2015).
3. Results
3.1. Sample population
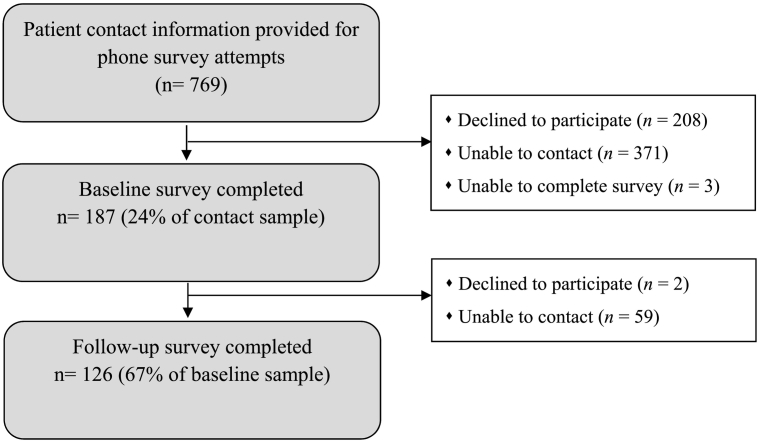
The 187 participants surveyed at baseline represented 24% of the patient lists; 126 (67%) of those reached at baseline completed the follow up survey (Fig. 1). At baseline, 27% of patients called declined to complete the survey, whereas 49% were not able to be reached or complete the survey; at follow-up 1% refused the survey, and 32% were not able to be reached.
Fig. 1.
Consort diagram for patients contacted for baseline and follow-up surveys.
Demographic characteristics are presented in Table 1. The patient population included 57% men, which differed between the thoroughly screened (70%) and not thoroughly screened (49%) groups (p < 0.01). The sample was also predominantly Caucasian (90%) and college educated (71%) with an annual household income of >$80,000 (56%); 32% had incomes $40,000–$80,000. While the majority of patients (79%) did not have a personal history of skin cancer 59% reported having a family history of melanoma, and 26% reported knowing someone who died from melanoma. There were no significant differences in history of skin examination or personal or family history of melanoma between those who were thoroughly screened and not thoroughly screened.
Table 1.
Characteristics of participants grouped by screening status.
| All % (n) | Thoroughly screened % (n) | Not thoroughly screened % (n) | p-Value (2 sided) | ||
|---|---|---|---|---|---|
| Sex * | Female | 42.8 (80) | 30.3 (23) | 51.4 (57) | 0.0042 |
| Male | 57.2 (107) | 69.7 (53) | 48.6 (54) | ||
| Race | Black or African American | 5.3 (10) | 4.1 (5) | 7.7 (5) | 0.4378 |
| White | 89.8 (168) | 90.2 (110) | 89.2 (58) | ||
| Other | 4.8 (9) | 5.7 (7) | 3.1 (2) | ||
| Education | Some high school, High school grad or GED | 9.7 (18) | 10.7 (13) | 7.7 (5) | 0.5275 |
| Technical school or junior college grad, some college | 18.8 (35) | 19.8 (24) | 16.9 (11) | ||
| College graduate | 59.1 (110) | 55.4 (67) | 66.2 (43) | ||
| Post grad or professional degree | 12.4 (23) | 14 (17) | 9.2 (6) | ||
| Annual household income | <$20,000 | 4.8 (8) | 2.9 (2) | 6.1 (6) | 0.4390 |
| $20,001–40,000 | 7.2 (12) | 7.4 (5) | 7.1 (7) | ||
| $40,001–80,000 | 31.9 (53) | 38.2 (26) | 27.6 (27) | ||
| >$80,000 | 56 (93) | 51.5 (35) | 59.2 (58) | ||
| Personal history of skin cancer | Yes | 20.9 (39) | 21.1 (16) | 20.7 (23) | 0.9562 |
| No | 79.1 (148) | 78.9 (60) | 79.3 (88) | ||
| Family history of melanoma | Yes | 58.6 (109) | 61.8 (47) | 56.4 (62) | 0.4559 |
| No | 41.4 (77) | 38.2 (29) | 43.6 (48) | ||
| Knows someone who died from melanoma | Yes | 25.7 (48) | 30.3 (23) | 22.5 (25) | 0.2339 |
| No | 74.3 (139) | 69.7 (53) | 77.5 (86) | ||
| Personal history of skin exam | Yes | 40.1 (75) | 39.5 (30) | 40.5 (45) | 0.8838 |
| No | 59.9 (112) | 60.5 (46) | 59.5 (66) | ||
Note: * indicates statistical significance (p < 0.01).
Psychosocial outcome measures by screening status are summarized in Table 2. On the baseline survey participants reporting that they were thoroughly screened scored significantly higher on positive emotional consequences compared with those who were not thoroughly screened (p < 0.001), but this difference did not persist to the follow-up measure approximately three months later. There were no significant differences between thoroughly screened and not thoroughly screened patients at baseline or follow-up for the HADS anxiety and depression, STAI-6, negative PCQ, and SF-12 PCS. Though similar at baseline, at follow-up thoroughly screened patients scored slightly, but significantly lower on the SF-12 compared with not thoroughly screened patients (p = 0.026).
Table 2.
Psychosocial measures in participants thoroughly screened and not thoroughly screened at baseline and follow-up.
| Scales | Baseline |
Follow-up |
||||
|---|---|---|---|---|---|---|
| Thoroughly screened n = 76 |
Not thoroughly screened n = 111 |
2 sided p-value | Thoroughly screened n = 53 |
Not thoroughly screened n = 73 |
2 sided p-value | |
| Median (range) | Median (range) (n) | Median (range) (n) | Median (range) (n) | |||
| HADS-A | 3.5 (0–15) | 4.0 (0–18) | 0.9 | 3 (0–14) | 4 (0–16) | 0.6 |
| HADS-D | 1 (0−20) | 2 (0–15) | 0.1 | 1 (0–19) | 2 (0−12) | 0.3 |
| STAI-6 | 23.3 (20–70) | 26.7 (20–80) | 0.6 | 23.3 (20–80) | 23.3 (20–63.7) | 0.6 |
| Negative emotional consequences PCQ | 0 (0–9) | 0 (0–8) | 0.5 | 0 (0−10) | 0 (0−11) | 0.8 |
| Negative physical consequences PCQ | 0 (0–5) | 0 (0–4) | 0.3 | 0 (0–8) | 0 (0–10) | 0.8 |
| Negative social consequences PCQ | 0 (0–8) | 0 (0–6) | 0.7 | 0 (0–7) | 0 (0–9) | 0.8 |
| Positive emotional consequences PCQ | 6 (0–15) | 4 (0–15) | 0.0002 | 7 (0–15) | 6 (0–15) | 0.6 |
| Positive physical consequences PCQ | 0 (0–9) | 0 (0–9) | 0.4 | 0 (0–9) | 0 (0–9) | 0.9 |
| Positive social consequences PCQ | 0 (0–6) | 0 (0–6) | 0.5 | 0 (0–6) | 0 (0–6) | 0.8 |
| SF-12 PCSa | 40.6 (30.9–47.3)⁎ | 40.5 (31.4–48.7) | 0.7 | 41.0 (4.0) | 40.4 (3.8) | 0.4 |
| SF-12 MCS | 49.3 (36.9–56.6)⁎ | 49.1 (28.5–60.5) | 0.4 | 49.2 (28.6–56.9) | 49.5 (35.5–59.2) | 0.02 |
n = 74 for SF-12 PCS and SF-12 MCS initial screening data of those thoroughly screened.
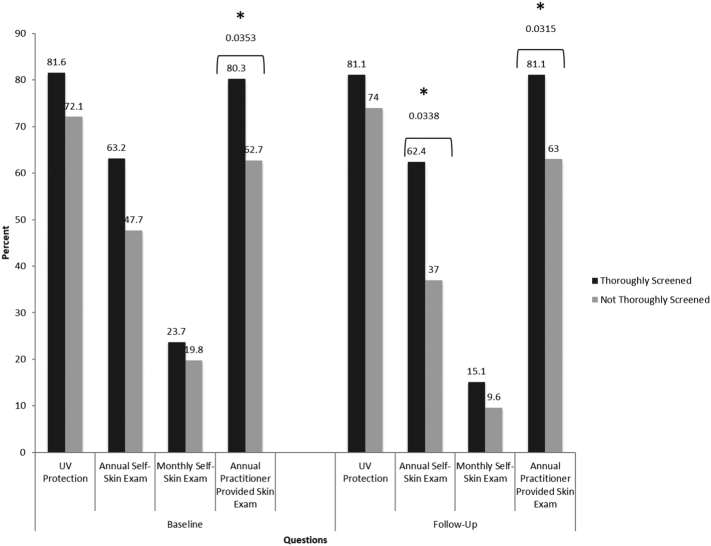
Skin cancer prevention practices by screening status at baseline and follow-up are summarized in Fig. 2. At both baseline and follow-up, thoroughly screened participants were more likely to have their skin annually examined by a provider than patients who were not thoroughly screened (p = 0.035, and 0.031 respectively). Thoroughly screened patients more likely than patients who were not thoroughly screened to report that they will perform previous skin self-examinations in the next year (p = 0.034), though the difference at baseline was not significant. Also, the likelihood of that they will perform skin self-examinations monthly was not different between groups at either baseline or follow-up survey.
Fig. 2.
Participants “very likely” to perform prevention practices by baseline and follow-up screening status.
Skin cancer education by screening status at baseline is summarized in Table 3. Overall thoroughly screened participants received the education they desired by their providers and were more likely than those not thoroughly screened to be recommended by their provider to perform regular total body skin self-exams, and to receive written information about skin cancer screening (all p < 0.0001). No significant differences were found between groups regarding satisfaction and of written materials as well as perception of adequacy of written materials regarding skin cancer. Thoroughly screened individuals reported increased ability to identify a concerning skin lesion after their skin exam compared to those not thoroughly screened (p = 0.0041), and were more likely to recommend others to have their skin examined (p = 0.011).
Table 3.
Skin cancer education provided in patients thoroughly screened and not thoroughly screened at baseline.
| Question | Response options | Baseline |
p-Value (2 sided) | |
|---|---|---|---|---|
| Thoroughly screened % (n) |
Not thoroughly screened % (n) |
|||
| In your appointment, did you get the information you needed about skin cancer? | Yes | 72.9 (51) | 28.8 (32) | <0.0001 |
| No | 27.1 (19) | 71.2 (79) | ||
| At your visit, did your provider recommend that you perform regular total body skin self-exams? | Yes | 56.9 (41) | 25.9 (28) | <0.0001 |
| No | 43.1 (31) | 74.1 (80) | ||
| After your visit, how likely would you be able to identify a concerning spot on your skin? | Very likely | 40 (30) | 26.1 (29) | 0.0026 |
| Somewhat likely | 46.7 (35) | 38.7 (43) | ||
| Not likely | 13.33 (10) (8) | 35.1 (39) | ||
| After receiving your skin cancer screening, did you suggest that anyone else get screened? | Yes | 21.1 (16) | 8.1 (9) | 0.01 |
| No | 86.6 (162) | 78.9 (60) | ||
| Was written information on skin cancer screening provided to you by your provider's office? | Yes | 31.3 (21) | 5.6 (6) | <0.0001 |
| No | 68.7 (46) | 94.4 (101) | ||
| How well did you understand the written information that was provided to you about skin cancer screening? | Very well | 76.2 (16) | 60 (3) | 0.07 |
| Somewhat well or a little bit | 23.8 (5) | 40 (3) | ||
| How satisfied were you with the written materials that you received? | Very satisfied | 71.4 (15) | 66.7 (4) | 0.8 |
| Somewhat satisfied | 28.6 (6) | 33.3 (2) | ||
| How adequate did you find the written materials that you received? | Very adequate | 61.9 (13) | 66.7 (4) | 0.8 |
| Somewhat or not very adequate | 38.1 (8) | 33.3 (2) | ||
4. Discussion
We examined psychosocial and behavioral consequences of skin cancer screening in primary care and found no evidence of harms in terms of distress and anxiety, but did find indications of benefit in terms of skin cancer prevention intentions or activities. Numerous worldwide studies have examined the benefits of physician-led skin screening on early melanoma detection (Aitken et al., 2010; Swetter et al., 2012; Terushkin & Halpern, 2009; Aitken et al., 2002; Janda et al., 2006; Aitken et al., 2006; Breitbart et al., 2012; Katalinic et al., 2012). However, there are no known previous studies that investigate the potential psychological benefits or harms from skin cancer screening (Wernli et al., 2016). The USPSTF cited these unknown potential psychological harms in support for its “insufficient” rating (Bibbins-Domingo et al., 2016). Our results suggest that screening was not associated with negative emotions and may positively influence emotional wellbeing. While we note differences between patients thoroughly screened and not, the reported HADS, STAI-6, PCQ, and SF-12 scores for all patients were within normal range, well below any threshold suggestive of anxiety, depression, emotional and social distress, and physical impediment.
The absence of psychological harm and even potential future prevention benefits from visual skin cancer screening is consistent with evidence of psychological impact found for other cancer screens. Mammographic breast cancer screening has been associated with low levels of distress immediately before, during or after the screening (Chad-Friedman et al., 2017). The mammogram itself does not inflict any psychosocial harm; and a negative result can even reduce anxiety (Brett et al., 2005).
Screenings for other cancers are associated with largely beneficial or non-harmful results (Niv et al., 2012; Taupin et al., 2006; McCaffery et al., 2010; Wardle et al., 2015). Few studies have found any psychosocial harm associated with colonoscopies (Chad-Friedman et al., 2017; Niv et al., 2012; Robb et al., 2013). Improved health-related quality of life (Taupin et al., 2006; Pizzo et al., 2011) and reduced anxiety (Wardle et al., 2003; Thiis-Evensen et al., 1999) have even been found after colonoscopies or fecal occult blood tests. Similar to mammograms, patients who receive a negative colorectal cancer screening result report significant decreases in anxiety and improvement in health-related quality of life (Kirkoen et al., 2016). For cervical cancer, most investigators have found no negative impact of HPV testing on psychosocial wellbeing (McCaffery et al., 2010; Kitchener et al., 2008).
The greatest psychological cost of cancer screening is likely to derive from false positive results. In the case of mammography, most of the evidence suggests that an abnormal result, not the mammogram itself, is a significant cause of anxiety, at least in the short term (Brennan & Houssami, 2016; Wardle et al., 2015; Brett et al., 2005; Schonberg et al., 2014). Similarly, for other invasive cancer screenings like for cervical and colorectal cancer, psychosocial harm has mostly been associated with a false positive result which decreases over time and with patient education (Maissi et al., 2004; Sharp et al., 2013; Kitchener et al., 2004; Bobridge et al., 2014; Laing et al., 2014; Parker et al., 2002; Brasso et al., 2010). It has been noted that in the long term these effects lessen, especially after patient education, follow-up examination, and reassurance (Wardle et al., 2015; Brett et al., 2005; Lee et al., 2016). Our own research has shown that no detrimental differences were found between survey respondents in this study who were biopsied compared to those who were not (Matthews et al., 2018), though the sample size was not large enough to examine those with false-positive results compared with others.
Our results, particularly regarding intention to practice skin cancer prevention after being screened contrast to some studies that examine the psychological impact of a melanoma diagnosis. The diagnosis of melanoma, even at early stages is known to negatively affect psychosocial wellbeing (Tesio et al., 2017; Bourdon et al., 2016; Bowen et al., 2012), and may even affect recovery and disease morbidity (Chida et al., 2008; Kendall et al., 2011), as well as prevent skin cancer prevention practices like regular skin examinations and UV protection (Bowen et al., 2012; Cornish et al., 2009; Kasparian, 2013), though other research has found positive skin self-examination practices after diagnosis (McLoone et al., 2013; Bird et al., 2015). We found that intention to practice skin cancer prevention in the future was significantly greater for participants who were thoroughly screened compared to those who were not (Fig. 2), though this practice was not the primary objective of the screening practices. These results suggest that beyond providing potential benefits in psychosocial wellbeing, practitioner provided skin cancer screening may promote better skin cancer prevention practices outside of the clinic.
The quality of skin cancer education varied greatly between participants who were thoroughly screened and not thoroughly screened at their primary care visit. The greater post-screening prevention behaviors between groups may stem from provider counseling, the screening itself, or both. Compared with patients who were not thoroughly screened, patients who were thoroughly screened reported that they received the information they wanted about skin cancer, written information on skin cancer, and recommendations to perform regular skin self-exams that resulted in higher confidence to identify a concerning lesion on their body and to recommend skin cancer screening to others. Patients who receive practitioner-provided skin cancer screening may be more capable of performing skin self-exams, and may be better equipped at identifying high-risk lesions.
5. Limitations
The present study has several limitations that must be considered along with these results. This study only evaluates patients of two practices within the UPMC setting. We do not have demographic information and other characteristics of the providers and patients of those practices to determine the potential generalizability to the entire UPMC system. Additionally, providers selected which patients were screened and documented their screening status in their chart based on their own clinical judgement, which may have been biased. However, we are not able to compare the patients who were and were not screened by providers to evaluate those potential biases. Also, within the set of patients screened, our survey was completed with a relatively small proportion of all patients screened in that time period. Without demographic or other information on patients who did not complete the survey, we are not able to describe the potential selection biases introduced by participation. Also, the time between the patient's visit and each of the surveys varied, making it difficult to clearly assess effects of time on performance of outcome measures. However, all scores for psychosocial wellbeing were well below threshold, and a difference of a few weeks would likely have had minimal impact on the outcome. Our survey study was also non-representative of at-risk Caucasian patients as a very high proportion were highly educated and of higher income. More than half of the respondents were male, which is curious given the contrast with the higher proportion of female respondents in our previous work with primary care providers (Weinstock et al., 2007; Markova et al., 2013). Highly educated women, compared to women with low education levels, are less likely to report anxiety related to breast cancer (Mainiero et al., 2001), but it is not clear if the same relationship exists between education and anxiety related to skin examination. Our statistical power to detect differences between patients thoroughly screened and others was limited. Finally, none of the psychosocial measures were designed specifically for skin cancer screening, although there are no validated measures for psychosocial wellbeing after skin cancer screening. Furthermore, these scales have been more successful at highlighting psychosocial wellbeing after a false-positive result from a screening exam, which is an important outcome of skin screening by primary care providers, but was not within the scope of this study. A much larger study would be required to assess anxiety and other psychosocial measures in association with biopsy, and more narrowly with false positive results.
6. Conclusions
In the current climate of healthcare reform and cost-cutting, recommendations from the USPSTF and other organizations are crucial to ensure that patients receive appropriate screening. Without recommendations for screening, primary care providers are less likely to provide skin cancer screening, potentially resulting in missed skin cancer diagnoses, with missed opportunities to encourage skin cancer prevention practices. Our results suggest that provider screening results in important benefits to future skin cancer self and provider screening.
Acknowledgments
Acknowledgments
Work on this manuscript was generously supported by funds from the Melanoma Research Alliance #305493 and the New England Melanoma Foundation #2014.
No financial disclosures were reported by the authors of this paper.
Conflict of interest
The authors declare there is no conflict of interest.
Funding and conflict of interest information
Research was supported by a grant from the Melanoma Research Alliance. The authors state no conflict of interest. The contents do not represent the views of the U.S. Department of Veteran Affairs or the United States Government.
Conflict of interest and financial disclosure
Patricia Markham Risica, Natalie H. Matthews, Laura Dionne, Jennifer Mello, Laura K. Ferris, Melissa Saul, Alan Geller, Francis Solano, John M. Kirkwood, and Martin A. Weinstock have no conflicts of interest or financial disclosures.
References
- Aitken J.F., Elwood M., Baade P.D., Youl P., English D. Clinical whole-body skin examination reduces the incidence of thick melanomas. Int. J. Cancer. 2010;126(2):450–458. doi: 10.1002/ijc.24747. [DOI] [PubMed] [Google Scholar]
- Aitken J.F., Elwood J.M., Lowe J.B., Firman D.W., Balanda K.P., Ring I.T. A randomised trial of population screening for melanoma. J. Med. Screen. 2002;9(1):33–37. doi: 10.1136/jms.9.1.33. [DOI] [PubMed] [Google Scholar]
- Aitken J.F., Janda M., Elwood M., Youl P.H., Ring I.T., Lowe J.B. Clinical outcomes from skin screening clinics within a community-based melanoma screening program. J. Am. Acad. Dermatol. 2006;54(1):105–114. doi: 10.1016/j.jaad.2005.08.072. [DOI] [PubMed] [Google Scholar]
- Aneja S., Aneja S., Bordeaux J.S. Association of increased dermatologist density with lower melanoma mortality. Arch. Dermatol. 2012;148(2):174–178. doi: 10.1001/archdermatol.2011.345. [DOI] [PubMed] [Google Scholar]
- Balch C.M., Gershenwald J.E., Soong S.J., Thompson J.F. Update on the melanoma staging system: the importance of sentinel node staging and primary tumor mitotic rate. J. Surg. Oncol. 2011;104(4):379–385. doi: 10.1002/jso.21876. [DOI] [PubMed] [Google Scholar]
- Balch C.M., Gershenwald J.E., Soong S.J. Final version of 2009 AJCC melanoma staging and classification. J. Clin. Oncol. 2009;27(36):6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berwick M., Begg C.B., Fine J.A., Roush G.C., Barnhill R.L. Screening for cutaneous melanoma by skin self-examination. J. Natl. Cancer Inst. 1996;88(1):17–23. doi: 10.1093/jnci/88.1.17. [DOI] [PubMed] [Google Scholar]
- Bibbins-Domingo K., Grossman D.C., Curry S.J. Screening for skin cancer: US preventive services task force recommendation statement. JAMA. 2016;316(4):429–435. doi: 10.1001/jama.2016.8465. [DOI] [PubMed] [Google Scholar]
- Bird J., Coleman P., Danson S. Coping with melanoma-related worry: a qualitative study of the experiences and support needs of patients with malignant melanoma. J. Clin. Nurs. 2015;24(7–8):937–947. doi: 10.1111/jocn.12758. [DOI] [PubMed] [Google Scholar]
- Bjelland I., Dahl A.A., Haug T.T., Neckelmann D. The validity of the hospital anxiety and depression scale. An updated literature review. J. Psychosom. Res. 2002;52(2):69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- Bobridge A., Bampton P., Cole S., Lewis H., Young G. The psychological impact of participating in colorectal cancer screening by faecal immuno-chemical testing—the Australian experience. Br. J. Cancer. 2014;111(5):970–975. doi: 10.1038/bjc.2014.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdon M., Blanchin M., Tessier P. Changes in quality of life after a diagnosis of cancer: a 2-year study comparing breast cancer and melanoma patients. Qual. Life Res. 2016;25(8):1969–1979. doi: 10.1007/s11136-016-1244-3. [DOI] [PubMed] [Google Scholar]
- Bowen D., Jabson J., Haddock N., Hay J., Edwards K. Skin care behaviors among melanoma survivors. Psychooncology. 2012;21(12):1285–1291. doi: 10.1002/pon.2017. [DOI] [PubMed] [Google Scholar]
- Brasso K., Ladelund S., Frederiksen B.L., Jorgensen T. Psychological distress following fecal occult blood test in colorectal cancer screening—a population-based study. Scand. J. Gastroenterol. 2010;45(10):1211–1216. doi: 10.3109/00365521.2010.485355. [DOI] [PubMed] [Google Scholar]
- Breitbart E.W., Waldmann A., Nolte S. Systematic skin cancer screening in northern Germany. J. Am. Acad. Dermatol. 2012;66(2):201–211. doi: 10.1016/j.jaad.2010.11.016. [DOI] [PubMed] [Google Scholar]
- Brennan M., Houssami N. Discussing the benefits and harms of screening mammography. Maturitas. 2016;92:150–153. doi: 10.1016/j.maturitas.2016.08.003. [DOI] [PubMed] [Google Scholar]
- Brett J., Bankhead C., Henderson B., Watson E., Austoker J. The psychological impact of mammographic screening. A systematic review. Psychooncology. 2005;14(11):917–938. doi: 10.1002/pon.904. [DOI] [PubMed] [Google Scholar]
- Chad-Friedman E., Coleman S., Traeger L.N. Psychological distress associated with cancer screening: a systematic review. Cancer. 2017;123(20) doi: 10.1002/cncr.30904. (Oct 15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida Y., Hamer M., Wardle J., Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat. Clin. Pract. Oncol. 2008;5(8):466–475. doi: 10.1038/ncponc1134. [DOI] [PubMed] [Google Scholar]
- Cockburn J., De Luise T., Hurley S., Clover K. Development and validation of the PCQ: a questionnaire to measure the psychological consequences of screening mammography. Soc. Sci. Med. 1992;34(10):1129–1134. doi: 10.1016/0277-9536(92)90286-y. [DOI] [PubMed] [Google Scholar]
- Cornish D., Holterhues C., van de Poll-Franse L.V., Coebergh J.W., Nijsten T. A systematic review of health-related quality of life in cutaneous melanoma. Ann. Oncol. 2009;20(Suppl. 6):vi51–8. doi: 10.1093/annonc/mdp255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide M.J., Asgari M.M., Fletcher S.W. Effects on skills and practice from a web-based skin cancer course for primary care providers. J. Am. Board Fam. Med. 2013;26(6):648–657. doi: 10.3122/jabfm.2013.06.130108. [DOI] [PubMed] [Google Scholar]
- Eide N., Hoifodt H.K., Nesland J.M. Disseminated tumour cells in bone marrow of patients with uveal melanoma. Acta Ophthalmol. 2013;91(4):343–348. doi: 10.1111/j.1755-3768.2012.02449.x. [DOI] [PubMed] [Google Scholar]
- Ferris L.K., Saul M.I., Lin Y. A large skin cancer screening quality initiative: description and first-year outcomes. JAMA Oncol. 2017;3(8):1112–1115. doi: 10.1001/jamaoncol.2016.6779. (Aug 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandek B., Ware J.E., Aaronson N.K. Cross-validation of item selection and scoring for the SF-12 health survey in nine countries: results from the IQOLA project. International quality of life assessment. J. Clin. Epidemiol. 1998;51(11):1171–1178. doi: 10.1016/s0895-4356(98)00109-7. [DOI] [PubMed] [Google Scholar]
- Janda M., Lowe J.B., Elwood M., Ring I.T., Youl P.H., Aitken J.F. Do centralised skin screening clinics increase participation in melanoma screening (Australia)? Cancer Causes Control. 2006;17(2):161–168. doi: 10.1007/s10552-005-0419-y. [DOI] [PubMed] [Google Scholar]
- Kasparian N.A. Psychological stress and melanoma: are we meeting our patients' psychological needs? Clin. Dermatol. 2013;31(1):41–46. doi: 10.1016/j.clindermatol.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Katalinic A., Waldmann A., Weinstock M.A. Does skin cancer screening save lives?: an observational study comparing trends in melanoma mortality in regions with and without screening. Cancer. 2012;118(21):5395–5402. doi: 10.1002/cncr.27566. [DOI] [PubMed] [Google Scholar]
- Kendall J., Glaze K., Oakland S., Hansen J., Parry C. What do 1281 distress screeners tell us about cancer patients in a community cancer center? Psychooncology. 2011;20(6):594–600. doi: 10.1002/pon.1907. [DOI] [PubMed] [Google Scholar]
- Kirkoen B., Berstad P., Botteri E. Do no harm: no psychological harm from colorectal cancer screening. Br. J. Cancer. 2016;114(5):497–504. doi: 10.1038/bjc.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchener H.C., Burns S., Nelson L. A randomised controlled trial of cytological surveillance versus patient choice between surveillance and colposcopy in managing mildly abnormal cervical smears. BJOG. 2004;111(1):63–70. doi: 10.1046/j.1471-0528.2003.00007.x. [DOI] [PubMed] [Google Scholar]
- Kitchener H.C., Fletcher I., Roberts C., Wheeler P., Almonte M., Maguire P. The psychosocial impact of human papillomavirus testing in primary cervical screening—a study within a randomized trial. Int. J. Gynecol. Cancer. 2008;18(4):743–748. doi: 10.1111/j.1525-1438.2007.01113.x. [DOI] [PubMed] [Google Scholar]
- Laing S.S., Bogart A., Chubak J., Fuller S., Green B.B. Psychological distress after a positive fecal occult blood test result among members of an integrated healthcare delivery system. Cancer Epidemiol. Biomark. Prev. 2014;23(1):154–159. doi: 10.1158/1055-9965.EPI-13-0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Hardesty L.A., Kunzler N.M., Rosenkrantz A.B. Direct interactive public education by breast radiologists about screening mammography: impact on anxiety and empowerment. J. Am. Coll. Radiol. 2016;13(11s):R89–r97. doi: 10.1016/j.jacr.2016.09.033. [DOI] [PubMed] [Google Scholar]
- Mainiero M.B., Schepps B., Clements N.C., Bird C.E. Mammography-related anxiety: effect of preprocedural patient education. Womens Health Issues. 2001;11(2):110–115. doi: 10.1016/s1049-3867(00)00071-2. [DOI] [PubMed] [Google Scholar]
- Maissi E., Marteau T.M., Hankins M., Moss S., Legood R., Gray A. Psychological impact of human papillomavirus testing in women with borderline or mildly dyskaryotic cervical smear test results: cross sectional questionnaire study. BMJ. 2004;328(7451):1293. doi: 10.1136/bmj.328.7451.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marteau T.M., Bekker H. The development of a six-item short-form of the state scale of the Spielberger state-trait anxiety inventory (STAI) Br. J. Clin. Psychol. 1992;31(Pt 3):301–306. doi: 10.1111/j.2044-8260.1992.tb00997.x. [DOI] [PubMed] [Google Scholar]
- Markova A., Weinstock M.A., Risica P. Effect of a web-based curriculum on primary care practice: basic skin cancer triage trial. Fam. Med. 2013;45(8):558–568. [PubMed] [Google Scholar]
- Matthews N.H.R.P., Ferris L.K., Saul M. Psychosocial impact of skin biopsies in the setting of melanoma screening — a retrospective nested case-control study. Br. J. Dermatol. 2018 doi: 10.1111/bjd.17134. (Under Review) [DOI] [PubMed] [Google Scholar]
- McCaffery K.J., Irwig L., Turner R. Psychosocial outcomes of three triage methods for the management of borderline abnormal cervical smears: an open randomised trial. BMJ. 2010;340:b4491. doi: 10.1136/bmj.b4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoone J.K., Watts K.J., Menzies S.W., Barlow-Stewart K., Mann G.J., Kasparian N.A. Melanoma survivors at high risk of developing new primary disease: a qualitative examination of the factors that contribute to patient satisfaction with clinical care. Psychooncology. 2013;22(9):1994–2000. doi: 10.1002/pon.3243. [DOI] [PubMed] [Google Scholar]
- Millar K., Jelicic M., Bonke B., Asbury A.J. Assessment of preoperative anxiety: comparison of measures in patients awaiting surgery for breast cancer. Br. J. Anaesth. 1995;74(2):180–183. doi: 10.1093/bja/74.2.180. [DOI] [PubMed] [Google Scholar]
- Mykletun A., Stordal E., Dahl A.A. Hospital anxiety and depression (HAD) scale: factor structure, item analyses and internal consistency in a large population. Br. J. Psychiatry. 2001;179:540–544. doi: 10.1192/bjp.179.6.540. [DOI] [PubMed] [Google Scholar]
- Niv Y., Bogolavski I., Ilani S. Impact of colonoscopy on quality of life. Eur. J. Gastroenterol. Hepatol. 2012;24(7):781–786. doi: 10.1097/MEG.0b013e328352deff. [DOI] [PubMed] [Google Scholar]
- Parker M.A., Robinson M.H., Scholefield J.H., Hardcastle J.D. Psychiatric morbidity and screening for colorectal cancer. J. Med. Screen. 2002;9(1):7–10. doi: 10.1136/jms.9.1.7. [DOI] [PubMed] [Google Scholar]
- Pizzo E., Pezzoli A., Stockbrugger R., Bracci E., Vagnoni E., Gullini S. Screenee perception and health-related quality of life in colorectal cancer screening: a review. Value Health. 2011;14(1):152–159. doi: 10.1016/j.jval.2010.10.018. [DOI] [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing. Austria; Vienna: 2014. R: A language and environment for statistical computing.http://www.R-project.org/ [Google Scholar]
- Rijnsburger A.J., Essink-Bot M.L., van As E., Cockburn J., de Koning H.J. Measuring psychological consequences of screening: adaptation of the psychological consequences questionnaire into Dutch. Qual. Life Res. 2006;15(5):933–940. doi: 10.1007/s11136-005-5093-8. [DOI] [PubMed] [Google Scholar]
- Robb K.A., Lo S.H., Power E. Patient-reported outcomes following flexible sigmoidoscopy screening for colorectal cancer in a demonstration screening programme in the UK. J. Med. Screen. 2013;19(4):171–176. doi: 10.1258/jms.2012.012129. (Dec) [DOI] [PubMed] [Google Scholar]
- Schonberg M.A., Silliman R.A., Ngo L.H. Older women's experience with a benign breast biopsy-a mixed methods study. J. Gen. Intern. Med. 2014;29(12):1631–1640. doi: 10.1007/s11606-014-2981-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S., Kuhnt S., Gotze H. Hospital anxiety and depression scale cutoff scores for cancer patients in acute care. Br. J. Cancer. 2009;100(6):908–912. doi: 10.1038/sj.bjc.6604952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh W.R., Geller A., Alexander G. Developing an interactive web-based learning program on skin cancer: the learning experiences of clinical educators. J. Cancer Educ. 2012;27(4):709–716. doi: 10.1007/s13187-012-0378-4. [DOI] [PubMed] [Google Scholar]
- Sharp L., Cotton S., Carsin A.E. Factors associated with psychological distress following colposcopy among women with low-grade abnormal cervical cytology: a prospective study within the trial of management of borderline and other low-grade abnormal smears (TOMBOLA) Psychooncology. 2013;22(2):368–380. doi: 10.1002/pon.2097. [DOI] [PubMed] [Google Scholar]
- Snaith R.P., Zigmond A.S. The hospital anxiety and depression scale. Br. Med. J. (Clin. Res. Ed.) 1986;292(6516):344. doi: 10.1136/bmj.292.6516.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swetter S.M., Pollitt R.A., Johnson T.M., Brooks D.R., Geller A.C. Behavioral determinants of successful early melanoma detection: role of self and physician skin examination. Cancer. 2012;118(15):3725–3734. doi: 10.1002/cncr.26707. [DOI] [PubMed] [Google Scholar]
- Taupin D., Chambers S.L., Corbett M., Shadbolt B. Colonoscopic screening for colorectal cancer improves quality of life measures: a population-based screening study. Health Qual. Life Outcomes. 2006;4:82. doi: 10.1186/1477-7525-4-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terushkin V., Halpern A.C. Melanoma early detection. Hematol. Oncol. Clin. North Am. 2009;23(3):481–500. doi: 10.1016/j.hoc.2009.03.001. (viii) [DOI] [PubMed] [Google Scholar]
- Tesio V., Ribero S., Castelli L., Bassino S., Leombruni P., Caliendo V., Grassi M., Lauro D., Macripò G., Torta R.G.V. Psychological characteristics of early-stage melanoma patients: a cross-sectional study on 204 patients. Melanoma Res. 2017;27(3):277–280. doi: 10.1097/CMR.0000000000000348. (Jun) [DOI] [PubMed] [Google Scholar]
- Thiis-Evensen E., Wilhelmsen I., Hoff G.S., Blomhoff S., Sauar J. The psychologic effect of attending a screening program for colorectal polyps. Scand. J. Gastroenterol. 1999;34(1):103–109. doi: 10.1080/00365529950172916. [DOI] [PubMed] [Google Scholar]
- Trask P.C., Paterson A.G., Hayasaka S., Dunn R.L., Riba M., Johnson T. Psychosocial characteristics of individuals with non-stage IV melanoma. J. Clin. Oncol. 2001;19(11):2844–2850. doi: 10.1200/JCO.2001.19.11.2844. [DOI] [PubMed] [Google Scholar]
- Tsao H., Weinstock M.A. Visual inspection and the US preventive services task force recommendation on skin cancer screening. JAMA. 2016;316(4):398–400. doi: 10.1001/jama.2016.9850. [DOI] [PubMed] [Google Scholar]
- de Vries E., Houterman S., Janssen-Heijnen M.L. Up-to-date survival estimates and historical trends of cutaneous malignant melanoma in the south-east of the Netherlands. Ann. Oncol. 2007;18(6):1110–1116. doi: 10.1093/annonc/mdm087. [DOI] [PubMed] [Google Scholar]
- Wardle J., Robb K., Vernon S., Waller J. Screening for prevention and early diagnosis of cancer. Am. Psychol. 2015;70(2):119–133. doi: 10.1037/a0037357. [DOI] [PubMed] [Google Scholar]
- Wardle J., Williamson S., Sutton S. Psychological impact of colorectal cancer screening. Health Psychol. 2003;22(1):54–59. doi: 10.1037//0278-6133.22.1.54. [DOI] [PubMed] [Google Scholar]
- Wernli K.J., Henrikson N.B., Morrison C.C., Nguyen M., Pocobelli G., Blasi P.R. Screening for skin cancer in adults: updated evidence report and systematic review for the US preventive services task force. JAMA. 2016;316(4):436–447. doi: 10.1001/jama.2016.5415. [DOI] [PubMed] [Google Scholar]
- Weinstock M.A., Ferris L.K., Saul M.I. Downstream consequences of melanoma screening in a community practice setting: first results. Cancer. 2016;122(20):3152–3156. doi: 10.1002/cncr.30177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock M.A., Risica P.M., Martin R.A. Melanoma early detection with thorough skin self-examination: the “check it out” randomized trial. Am. J. Prev. Med. 2007;32(6):517–524. doi: 10.1016/j.amepre.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]