Abstract
Objective
There is no evidence for genetic association of the organic anion transporters (OAT) 1–3 (SLC22A6, SLC22A7, SLC22A8) and multi-drug resistance protein 4 (MRP4; ABCC4) with serum urate or gout. The Māori and Pacific (Polynesian) population of New Zealand (NZ) has the highest prevalence of gout worldwide. Our aim was to determine if there were any Polynesian population-specific genetic variants in the SLC22A6-8 and ABCC4 genes associated with gout.
Participants and Methods
All participants had ≥3 self-reported Māori and/or Pacific grandparents. From the total sample set of 1808 participants, 191 hyperuricaemic and 202 normouricaemic individuals were resequenced over the four genes with the remaining 1415 individuals used for replication. Regression analyses were performed adjusting by age, sex and Polynesian ancestry. To study the functional effect of non-synonymous variants ABCC4 transport assays were done in Xenopus laevis ooctyes.
Results
A total of 39 common variants were detected, with an ABCC4 variant (rs4148500) significantly associated with hyperuricemia and gout. Rs4148500 was monomorphic (for the protective major allele) in Europeans. There was evidence for association for rs4148500 with gout in the resequenced samples (OR=1.62, P=0.012) that was replicated (OR=1.25, P=0.033) and restricted to males (ORMales=1.43, P=0.001; ORFemales=0.98, P=0.89). The gout risk allele was associated with FEUA in males (β=−0.570, P=0.01). A rare population-specific variant (P1036L) with strong functional consequence reduced the uric acid transport activity of ABCC4 by 30%.
Conclusion
Association of ABCC4 with gout and FEUA is consistent with the established role of MRP4 as a unidirectional renal uric acid efflux pump.
Gout is a form of arthritis caused by innate immune system reaction to monosodium urate crystals in individuals with hyperuricemia (HU) (1). It is 3–4 fold more prevalent in males than females (2). Genome-wide association studies (GWAS) have identified genetic variants in loci encoding renal and intestinal uric acid transporters and associated accessory molecules (SLC2A9/GLUT9, ABCG2, SLC22A11/OAT4, SLC22A12/URAT1, SLC17A1/NPT1, PDZK1) that control serum urate levels in Europeans (3–5). Most of these variants associate with serum urate and gout in other ancestral groups (6, 7). However, there remain other known uric acid transporters (SLC22A6-8/OAT1-3, ABCC4/MPR4) (8–12) without evidence at a genome-wide level of significance for genetic control of urate and risk of gout (13). Identifying natural common genetic variants that associate with urate levels and risk of gout in the genes encoding these molecules would both increase the understanding of the molecular basis of HU and gout and highlight the molecules as targets for urate-lowering drugs. For example, small molecules reverse the urate-raising property of the common ABCG2 141K variant (14) and lesinurad is a recently developed drug that promotes uric acid excretion via URAT1 (15, 16).
We hypothesised that the genome of the Māori and Pacific (Polynesian) population of New Zealand (NZ) could contain genetic variants in the SLC22A6-8 and ABCC4 genes, absent in Europeans, that control serum urate levels. This population group has the highest prevalence of gout worldwide (2), driven by inherent HU (17, 18). Furthermore, people of Polynesian ancestry exhibit lower urinary fractional excretion of uric acid (FEUA) (19–21). Therefore these genes were resequenced in 191 Polynesian individuals with the highest, and 202 with the lowest, serum urate levels with the aim of detecting population-specific genetic variants that associate with gout.
Participants and Methods
Participants
All 1808 Polynesian gout cases and controls in this study were drawn from the New Zealand Māori and Pacific Island populations, recruited during the years 2006 to 2013 from within New Zealand. Only people with ≥3 self-reported Polynesian grandparents were included (Table 1). Gout cases fulfilled the 1977 American Rheumatology Association preliminary classification criteria for gout by clinical examination (22). Participants without gout self-reported no history of gout. From this group 191 individuals with the highest serum urate measures (average 0.539 mmol/L in males and 0.511 mmol/L in females) and 202 with the lowest serum urate measures (average 0.330 mmol/L in males and 0.284 mmol/L in females) were selected for resequencing (n=393; ‘Resequence’ sample set). Of the remaining 1415 samples, 1075 comprised the ‘Replication 1’ sample set. The Lower South Ethics Committee (OTA/99/11/098) and New Zealand Multi-region Ethics Committee (MEC/05/10/130) granted ethical approval for analysis of these individuals.
Table 1.
Summary demographic and biochemical characteristics
| Resequencing | Replication 1 | Replication 2 | ||||
|---|---|---|---|---|---|---|
| Gout | Non-gout | Gout | Non-gout | Gout | Non-gout | |
| N | 142 | 251 | 589 | 486 | 181 | 159 |
| Age (average, se, % missing) | 34.2, 0.9, 1.4 | 44.2, 0.9, 0 | 39.1, 0.6, 2.9 | 43.9, 0.7, 0 | 40.4, 1.2, 3.9 | 41.8, 1.2, 0 |
| Sex (% male) | 88.0 | 48.2 | 80.6 | 46.7 | 80.1 | 55.3 |
| Self-reported Polynesian g’parents (average, se) | 0.969, 0.006 | 0.945, 0.006 | 0.949, 0.004 | 0.943, 0.004 | 0.906, 0.008 | 0.905, 0.009 |
| Male serum urate in mmol/L (average, se, % missing) | 0.549, 0.015, 0 | 0.377, 0.008, 0 | 0.403, 0.005, 19.8 | 0.445, 0.005, 18.2 | 0.441, 0.009, 4.2 | 0.404, 0.008, 1.2 |
| Female serum urate in mmol/L (average, se, % missing) | 0.538, 0.015, 0 | 0.309, 0.007, 0 | 0.391, 0.012, 21.2 | 0.389, 0.004, 25.3 | 0.458, 0.022, 5.6 | 0.339, 0.010, 0 |
| Male FEUA (average, se, % missing) | 2.25, 0.27, 33.6 | 5.98, 0.37, 66.1 | 3.64, 0.28, 40.0 | 4.71, 0.25, 60.4 | 4.10, 0.19, 15.9 | 5.33, 0.25, 4.5 |
| Female FEUA (average, se, % missing) | 2.49, 0.83, 29.5 | 6.10, 0.50, 66.9 | 4.09, 0.46, 38.6 | 4.84, 0.24, 59.5 | 4.97, 0.38, 11.2 | 6.21, 0.46, 5.6 |
A separate sample set (‘Replication 2’) comprised of 181 people with gout and 159 people without gout, ascertained with criteria described above. These participants were recruited in collaboration with Ngāti Porou Hauora Charitable Trust (NPHCT), the Māori iwi (tribe) health service provider located in the East Coast (Tairāwhiti) region of the North Island of New Zealand. This separate sample set is comprised nearly exclusively of people of New Zealand Māori (Eastern Polynesian) ancestry (99.5%). The NPHCT study was approved by the Ngāti Porou Hauora Board and ethical approval granted by the Northern Y Region Health Research Ethics Committee (NTY07/07/074).
Urate and creatinine measurements for all sample sets were obtained from serum and a spot urine sample, with participants non-fasting and sampling occurring at convenient times. Serum urate measurements at recruitment are presented for all participants in Table 1. FEUA was calculated using the Simkin index (23). HU was defined as ≥6 mg/dL in women and ≥7 mg/dL in men. Demographic and biochemical characteristics are shown in Table 1.
Resequencing
Illumina indexed libraries were constructed using 250ng of native genomic DNA according to the manufacturer’s protocol (Illumina Inc, San Diego, CA) with the following modifications: 1) DNA was fragmented using a Covaris E220 DNA Sonicator (Covaris, Inc. Woburn, MA) to range in size between 100 and 400bp; 2) Illumina adapter-ligated library fragments were amplified for eight cycles; 3) solid phase reversible immobilisation bead cleanup was used for enzymatic purification throughout the library process, as well as final library size selection targeting 300–500bp fragments. Hybridization was performed with a custom version of the Roche NimbleGen SeqCap kit, according to the manufacturer’s protocol. Ninety dual-indexed samples were combined and captured as a pool and run on a lane of Illumina HiSeq 2000, which produces approximately 36Gb/lane of sequence. The total target capture was 2.59Mb of genomic space that included the SLC22A6-8 and ABCC4 promoters, 5` and 3` untranslated regions and exons (Table S1). The total sequence per sample was 132.56Mb yielding 51.2× average coverage across the targeted space. The resequencing was done at the McDonnell Genome Institute at Washington University, St Louis, USA.
Using the Genome Analysis Tool Kit (GATK) best practice recommendations (24) the FASTQ files were aligned to the human decoy reference genome (build GRCh37) using the Burrows-Wheeler Aligner (BWA v0.7.12 r1039) (25). Resultant BAM files were processed using Picard (v 1.114) to mark reads that originate from PCR duplication. This was followed by INDEL realignment and base recalibration using GATK v3.3.0 (26). GNU Parallel (v20140422) (27) and the GATK HaplotypeCaller (28) were used to generate genomic variant call files (GVCF). These files were merged in batches of 100 using the GATKs MergeGVCFs command, followed by variant calling using the GATK GenotypeGVCF command. The SNPs in the resulting variant call file (VCF) were processed using the GATKs variant quality score recalibration utility taking quality by depth, Fisher score, mapping quality rank sum and read position rank sum into account. This recalibration was applied to the VCF file and SNPs with a truth sensitivity of less than 99.00 were removed.
Each variant was annotated using Plink/SEQ (v0.10) (https://atgu.mgh.harvard.edu/plinkseq/) and for those variants found within the boundary of a gene or genes, the variants were classified into: intronic, exonic-unknown, exonic-missense, exonic-silent, UTR3 and UTR5. Rare variants were additionally annotated (Table S2) with Combined Annotation Dependent Depletion (CADD) (29) scores using Gemini (v 0.12.2) (30).
Genotyping
All samples including those in the resequencing experiment were genotyped using either the Taqman rs4148500 assay or a custom Taqman genotyping assay (for Chr13:95724019) on a Roche Lightcycler 480. The genotypes were autocalled by the Lightcycler 480 software and the reported dye signal plots were visually inspected for correct genotype clustering. There was 100% concordance between Taqman genotypes and genotypes obtained from the resequencing VCF files. No individual or combined sample set departed from Hardy-Weinberg equilibrium (P>0.05).
Association analysis
Logistic regression was performed on the resequence data using PLINK/SEQ with HU as outcome. This model was adjusted for age and sex. Association with gout was tested by logistic regression with adjustment for age, sex, self-reported number of Polynesian grandparents and Polynesian ancestral group (Western, Eastern, mixed) in the resequence, replication and combined sample sets using R v3.2.0 (31). Linear regression, with the same adjustors, was used to test for association with FEUA and serum urate.
ABCC4 functional assay
To study the effect of the rare P1036L coding SNP on the transport function of ABCC4 the relevant point mutant was first generated using site-directed mutagenesis (QuikChange, Stratagene) of Xenopus laevis expression constructs in the pGEMHE vector. Point mutants were also generated for two additional non-synonymous variants, G187W and G487E, which have shown reduced function in a prior study (32). In each case the fidelity of mutagenesis was assessed by sequencing the entire open reading frame. Transport function of wild-type and mutant ABCC4 was then assessed using 14C-urate efflux in individual Xenopus laevis oocytes, as previously described for ABCG2 (12, 33). Collagenase-digested oocytes were thus micro-injected with in vitro-transcribed cRNA (25 ng/oocyte) for each construct, followed by transport assays after two days in culture. For [14C]-urate efflux studies, oocytes were pre-injected with 50 nl of 1500 μM [14C]-urate dissolved in efflux medium (ND96, pH 7.4). Pre-injected oocytes were then incubated in ND96 medium for 30 min at 16°C for recovery. After incubation, the oocytes were washed in ND96 medium four times to remove any external adhering [14C]-urate from the oocytes and were then subjected to efflux for 1h at room temperature (~25°C) in ND96 medium (pH 7.4) in the absence or presence of drug; pharmacological sensitivity was determined for tranilast, benzbromarone, and probenecid. Oocytes were then washed again three times with the ice-cold uptake medium to remove external radioisotope. The radioisotope content of each individual oocyte was measured by scintillation counter after solubilization in 0.3 ml of 10% (v/v) SDS and addition of 2.5 ml of scintillation fluid. The experiments included 12–15 oocytes in each group for Figure 2A and 30 oocytes/group for Figure 2B; given the modest functional effects of coding mutations in ABCC4 we utilized twice the usual number of oocytes for this experiment in Figure 2B. The radioisotope content of each individual oocyte was measured by scintillation counter after solubilization in 0.3 ml of 10% (v/v) SDS and addition of 2.5 ml of scintillation fluid. Statistical significance for individual experiments was defined as two-tailed p < 0.05 in the Mann Whitney rank sum test, and results were reported as means ± S.E.
Figure 2.
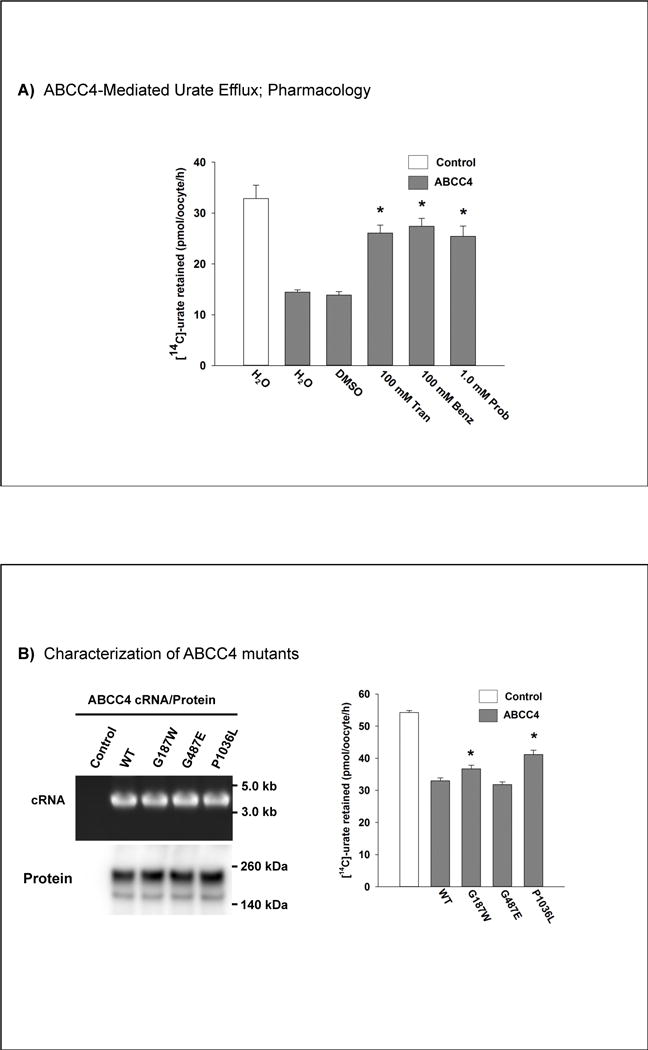
Panel A, ABCC4-mediated urate efflux and pharmacology in Xenopus oocytes. * represents a statistically significant difference (P<0.001 for Tran and Benz, P=0.002 for Prob) to ABCC4 H2O. DMSO, dimethyl sulphoxide; Tran, tranilast; Benz, benzbromarone; Prob, probenecid. Panel B, Characterization of ABCC4 mutants. * represents a statistically significant difference (P=0.011 for G187W, P<0.001 for P1076L) to WT.
ABCC4 Western blotting
Total cellular protein for Western blot analysis was prepared from groups of ~50 X. laevis oocytes injected with relevant cRNAs that were transcribed in vitro from related constructs. After 48 hours of expression of protein from the injected cRNA, oocytes were transferred to 1.7 ml polypropylene microfuge tubes on ice and were lysed using a Teflon homogenizer in lysis buffer (50 mM Tris-HCl, pH 7.5, 50 mM NaCl, 1 mM EDTA, pH 8, 1% Triton X-100) supplemented with protease inhibitors cocktail (Roche, Indianapolis, IN). After clearing the lysate off yolk and cellular debris by centrifugation at 5,000 rpm for 10 min, the supernatant was stored at −80 °C. Western blotting was performed using affinity-purified rabbit polyclonal anti-ABCC4 antibody (Cell Signaling Technology, Danvers, MA) at a titer of 1:1000. Total lysates of proteins were fractionated using 7.5% SDS/PAGE gel electrophoresis (BIO-RAD, Hercules, CA). Proteins were transferred to polyvinylidene difluoride membrane (BIO-RAD, Hercules, CA) at 100 V for 3 h. The membrane was blocked in 5% nonfat dried milk in tris-buffered saline with Tween 20 (TBST). Primary antibodies were diluted in 5% milk in TBST and incubated with the membrane at room temperature for 2h with continuous gentle shaking. Blots were washed in TBST and probed with an HRP-conjugated secondary antibody (BIO-RAD, Hercules, CA) in TBST containing 5% fat-free milk for 1h at RT. The membrane was then washed four times with TBST and chemiluminescence performed using ECL (PIERCE; Rockford, IL), following standard protocols.
Results
Identification of common variants
A total of 39 variants with frequency greater than 0.05 were detected by the resequencing across the four loci, with the majority (n=26) in ABCC4 (Table 2). All variants were tested for association with HU, and six variants in ABCC4 were significantly associated (two being in complete linkage disequilibrium) (Table 2). Accounting for variants in strong linkage disequilibrium (r2>0.90) a total of 33 tests were done (Table 2; Figure 1). Therefore the PCorrected value for significant association with HU was P<1.4×10−3, which only rs4148500 achieved (OR=1.86, P=6×10−4). This variant was monomorphic for the urate-lowering allele in Europeans (Table 2).
Table 2.
Association analysis of 39 variants in the ABCC4, SLC22A6-A8 genes with hyperuricaemia.
| rs id | Position | Ref/Alt | F Reseq | F EUR1 | F EAS1 | OR2 | SE | P | Annotation3 |
|---|---|---|---|---|---|---|---|---|---|
| rs3017670 | chr11:62744899 | A/G | 0.683 | 0.84 | 0.84 | 0.92 | 0.16 | 0.59 | SLC22A6|intronic |
| rs2276300 | chr11:62748699 | G/A | 0.165 | 0 | 0.18 | 0.95 | 0.21 | 0.82 | SLC22A6|intronic |
| rs4149171 | chr11:62752182 | T/C | 0.245 | 0.14 | 0.27 | 1.04 | 0.17 | 0.81 | SLC22A6|UTR5 |
| rs4149170 | chr11:62752289 | C/T | 0.239 | 0.07 | 0.26 | 1.11 | 0.17 | 0.56 | SLC22A6|UTR5 |
| rs2187384 | chr11:62761161 | C/T | 0.099 | 0.19 | 0.28 | 1.04 | 0.25 | 0.88 | SLC22A8|intronic |
| rs953894 | chr11:62762422 | C/T | 0.182 | 0.21 | 0.31 | 1.25 | 0.20 | 0.26 | SLC22A8|intronic |
| rs2276299 | chr11:62766431 | A/T | 0.456 | 0.19 | 0.27 | 0.82 | 0.16 | 0.21 | SLC22A8|exonic-silent |
| rs4149179 | chr11:62782446 | C/T | 0.265 | 0.03 | 0.26 | 0.92 | 0.17 | 0.63 | SLC22A8|UTR5 |
| rs4963227 | chr11:62783150 | C/T | 0.934 | 0.91 | 0.92 | 1.14 | 0.30 | 0.68 | SLC22A8|intronic |
| rs3770 | chr13:95672237 | G/A | 0.662 | 0.38 | 0.50 | 0.94 | 0.16 | 0.72 | ABCC4|UTR3 |
| rs9516519 | chr13:95672457 | T/G | 0.052 | 0.12 | 0 | 0.94 | 0.36 | 0.86 | ABCC4|UTR3 |
| rs9516520 | chr13:95672858 | T/C | 0.052 | 0.12 | 0 | 0.94 | 0.36 | 0.86 | ABCC4|UTR3 |
| rs1059751 | chr13:95672950 | A/G | 0.272 | 0.51 | 0.50 | 1.07 | 0.18 | 0.69 | ABCC4|UTR3 |
| rs9516521 | chr13:95673122 | T/C | 0.052 | 0.12 | 0 | 0.94 | 0.36 | 0.86 | ABCC4|UTR3 |
| rs4148553 | chr13:95673135 | C/T | 0.272 | 0.51 | 0.50 | 1.07 | 0.18 | 0.69 | ABCC4|UTR3 |
| rs4148551 | chr13:95673518 | T/C | 0.662 | 0.37 | 0.50 | 0.94 | 0.16 | 0.72 | ABCC4|UTR3 |
| rs3742106 | chr13:95673791 | A/C | 0.662 | 0.37 | 0.50 | 0.94 | 0.16 | 0.72 | ABCC4|UTR3 |
| . | chr13:95714909 | A/G | 0.091 | . | . | 0.95 | 0.26 | 0.83 | ABCC4|intronic |
| rs1751034 | chr13:95714976 | C/T | 0.784 | 0.81 | 0.75 | 1.10 | 0.18 | 0.60 | ABCC4|exonic-silent |
| rs1189466 | chr13:95726541 | A/G | 0.810 | 0.93 | 0.77 | 0.99 | 0.20 | 0.96 | ABCC4|exonic-silent |
| rs1678339 | chr13:95727780 | T/C | 0.810 | 0.93 | 0.76 | 0.99 | 0.20 | 0.96 | ABCC4|exonic-silent |
| rs45477596 | chr13:95735502 | C/T | 0.145 | 0 | 0 | 0.97 | 0.21 | 0.88 | ABCC4|exonic-missense |
| rs1189437 | chr13:95735604 | G/T | 0.762 | 0.92 | 0.74 | 1.00 | 0.18 | 0.99 | ABCC4|intronic |
| rs6650282 | chr13:95748221 | T/C | 0.504 | 0.41 | 0.28 | 0.88 | 0.15 | 0.41 | ABCC4|UTR3 |
| rs1729770 | chr13:95818193 | C/T | 0.144 | 027 | 0.08 | 0.63 | 0.23 | 0.04 | ABCC4|intronic |
| rs4148500 | chr13:95818288 | G/C | 0.290 | 0 | 0.19 | 1.86 | 0.18 | 6.2×10−4 | ABCC4|intronic |
| rs2274405 | chr13:95818978 | T/C | 0.162 | 0.61 | 0.55 | 0.73 | 0.21 | 0.13 | ABCC4|exonic-silent |
| rs2274406 | chr13:95818996 | T/C | 0.148 | 0.61 | 0.55 | 0.86 | 0.22 | 0.51 | ABCC4|exonic-silent |
| rs2274407 | chr13:95859035 | C/A | 0.749 | 0.07 | 0.20 | 0.65 | 0.18 | 0.017 | ABCC4|csplice-missense |
| rs11568679 | chr13:95861741 | T/C | 0.727 | 0 | 0 | 0.66 | 0.17 | 0.016 | ABCC4|exonic-silent |
| rs899494 | chr13:95861804 | A/G | 0.117 | 0.85 | 0.79 | 1.05 | 0.25 | 0.84 | ABCC4|exonic-silent |
| rs899496 | chr13:95862896 | A/G | 0.233 | 0.82 | 0.66 | 0.83 | 0.19 | 0.32 | ABCC4|intronic |
| rs11568637 | chr13:95886854 | T/C | 0.067 | 0 | 0.08 | 1.47 | 0.33 | 0.24 | ABCC4|intronic |
| rs4148437 | chr13:95899354 | A/G | 0.807 | 0.41 | 0.13 | 0.87 | 0.20 | 0.47 | ABCC4|splice |
| rs11568681 | chr13:95953517 | G/T | 0.810 | 0.39 | 0.45 | 0.89 | 0.20 | 0.54 | ABCC4|exonic-missense |
| rs1574430 | chr6:43269029 | A/C | 0.810 | 0.58 | 0.65 | 0.89 | 0.20 | 0.54 | SLC22A7|intronic |
| rs2841648 | chr6:43269179 | C/A | 0.180 | 0.49 | 0.59 | 1.20 | 0.20 | 0.36 | SLC22A7|intronic |
| rs56401710 | chr6:43269180 | C/A | 0.683 | 0.49 | 0.59 | 0.92 | 0.16 | 0.59 | SLC22A7|intronic |
| rs2270860 | chr6:43270151 | C/T | 0.165 | 0.35 | 0.35 | 0.95 | 0.21 | 0.82 | SLC22A7|splice |
Reference allele frequencies derived from 1000 Genomes data (www.ensembl.org). EUR, European; EAS, East Asian.
Adjusted by age, sex.
Each variant was annotated using PLINK/SEQ (v0.10) (https://atgu.mgh.harvard.edu/plinkseq/) and for those variants found within the boundary of a gene or genes, the variants were further classified into the categories: intronic, exonic-unknown, exonic-missense, exonic-silent, UTR3 and UTR5.
Figure 1.
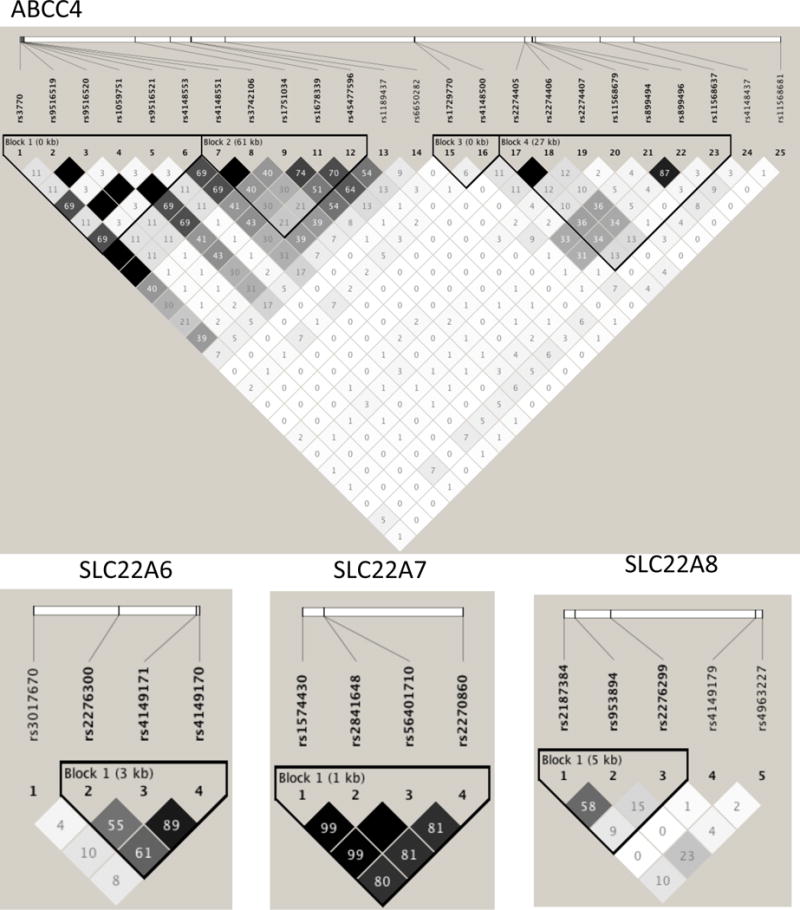
Linkage disequilibrium (r2) relationships between common variants (Table 2) in the resequenced samples in the four genes.
Replication of rs4148500
Rs4148500 was genotyped over the Replication 1 and Replication 2 sample sets and tested for association with gout (Table 3). Gout rather than HU was examined for the pragmatic reason that we had access to a large number of clinically-ascertained gout cases for whom HU could be assumed. Given the differences in allele frequency at ABCG2 rs2231142 between Western (Samoa, Tonga, Niue, Tokelau) and Eastern (NZ and Cook Island Māori) Polynesian (34) the non-gout samples were first subdivided into Eastern and Western Polynesian. The A allele frequency was 0.186 in Eastern Polynesians and 0.314 in Western Polynesians. Therefore, Eastern versus Western versus mixed ancestry was additionally adjusted for in subsequent regression analyses in order to account for any intra-Polynesian stratification. Applying the ancestry adjustment (in addition to number of Polynesian grandparents) to the Resequence data set showed evidence for association of rs4148500 with both gout (Table 3; OR=1.62, P=0.012) and hyperuricaemia (OR=1.80, P=0.003).
Table 3.
Association analysis of ABCC4 rs4148500 with gout
| Sample Set, n (freq) | Gout | Non-gout | OR [95% CI]2 | P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GG | AG | AA | A | GG | AG | AA | A | |||
| Resequence | 54 (0.380) | 69 (0.486) | 19 (0.134) | 107 (0.377) | 141 (0.562) | 98 (0.390) | 12 (0.048) | 122 (0.243) | 1.62 [1.12–2.38] | 0.012 |
| Replication 1+2 | 407 (0.529) | 298 (0.387) | 65 (0.102) | 428 (0.278) | 371 (0.575) | 244 (0.378) | 30 (0.047) | 304 (0.236) | 1.253 [1.02–1.53] | 0.033 |
| Replication 1 | 283 (0.480) | 246 (0.418) | 60 (0.102) | 366 (0.311) | 272 (0.560) | 191 (0.393) | 23 (0.047) | 237 (0.244) | 1.36 [1.08–1.71] | 0.0081 |
| Replication 2 (NPH) | 124 (0.685) | 52 (0.287) | 5 (0.028) | 62 (0.173) | 99 (0.623) | 53 (0.333) | 7 (0.044) | 67 (0.211) | 0.80 [0.49–1.29] | 0.36 |
| All samples | 461 (0.505) | 367 (0.402) | 84 (0.092) | 535 (0.293) | 512 (0.571) | 342 (0.382) | 42 (0.047) | 426 (0.238) | 1.304 [1.09–1.55] | 0.0038 |
|
| ||||||||||
| Males | 365 (0.490) | 303 (0.407) | 77 (0.103) | 457 (0.307) | 253 (0.583) | 163 (0.376) | 18 (0.041) | 199 (0.229) | 1.435 [1.15–1.77] | 0.0011 |
| WP1 males | 119 (0.361) | 158 (0.479) | 53 (0.161) | 264 (0.400) | 64 (0.430) | 71 (0.477) | 14 (0.094) | 99 (0.332) | 1.41 [1.04–1.93] | 0.030 |
| EP1 males | 236 (0.608) | 131 (0.338) | 21 (0.054) | 173 (0.223) | 175 (0.681) | 78 (0.304) | 4 (0.016) | 66 (0.167) | 1.45 [1.06–1.99] | 0.020 |
| Females | 96 (0.575) | 64 (0.383) | 7 (0.042) | 78 (0.234) | 257 (0.561) | 177 (0.386) | 24 (0.052) | 225 (0.246) | 0.986 [0.70–1.36] | 0.89 |
| WP1 females | 16 (0.432) | 19 (0.514) | 2 (0.054) | 23 (0.311) | 57 (0.500) | 48 (0.421) | 9 (0.079) | 66 (0.289) | 1.37 [0.71–2.61] | 0.34 |
| EP1 females | 78 (0.614) | 44 (0.346) | 5 (0.039) | 54 (0.213) | 184 (0.580) | 119 (0.375) | 14 (0.044) | 147 (0.232) | 0.82 [0.54–1.21] | 0.32 |
WP; Western Polynesian, EP; Eastern Polynesian. People of mixed EP/WP ancestry (n=85) were not included in the sex stratification.
Adjusted by age, sex, number of Polynesian grandparents, Polynesian ancestral class (WP, EP, WP/EP).
OR=1.31, P=0.01 by meta-analysis combining Replication 1 and 2 using a fixed effects model and OR=1.15, P=0.59 using a random effects model (PHet=0.048).
OR=1.42, P=1×10−4 by meta-analysis combining Resequence and Replication 1 and 2 using a fixed effects model and OR=1.36, P=0.098 using a random effects model (PHet=0.036).
OR=1.43, P=0.0015 by meta-analysis combining WP and EP males using a fixed effects model and OR=1.43, P=0.0015 using a random effects model (PHet=0.90).
OR=0.94, P=0.73 by meta-analysis combining WP and EP females using a fixed effects model and OR=0.99, P=0.97 using a random effects model (PHet=0.18).
The association with gout was replicated in the combined Replication 1 + 2 sample sets (OR=1.25, P=0.033), with significant association in Replication 1 (OR=1.36, P=0.008) but not Replication 2 (OR=0.85, P=0.49). The minor allele of rs4148500 was also associated with increased risk of gout in the combined Resequence and Replication samples (OR=1.30, P=0.004). Using the combined Resequence and Replication 1 and 2 sample sets, sex-stratified sample sets were generated, revealing that the association was restricted to males only (Table 3: OR=1.43, P=0.001 in males; OR=0.98, P=0.89 in females). Combining the male and female sample sets by meta-analysis demonstrated evidence for a differential strength of effect (PHeterogeneity=0.062 using a fixed effects model). The effect size was similar between Western and Eastern Polynesian males (OR=1.41, P=0.03 versus OR=1.45, P=0.02, respectively).
Association of rs4148500 with FEUA
In the combined sample set rs4148500 was tested for association with FEUA in combined cases and controls and serum urate in controls (Table 4). There was evidence for association of the minor allele with reduced FEUA in the combined sample set (β=−0.378, P=0.03), an effect that was restricted to males (β=−0.570, P=0.01 in males; β=0.071, P=0.82 in females). In the non-gout controls there was a strong trend for association with serum urate in the male combined sample set (Table 4; β=0.013, P=0.07).
Table 4.
The change in FEUA and serum urate per copy of the ABCC4 rs4148500 minor allele in combined sample sets
| Serum urate (non-gout only) | FEUA (gout and non-gout) | |||||
|---|---|---|---|---|---|---|
| β (mmol/L) [95% CI] | SE | P | β [95% CI] | SE | P | |
| Combined | 0.004 [−0.005 – 0.014] | 0.005 | 0.38 | −0.378 [−0.729 - −0.027] | 0.179 | 0.035 |
| Combined males | 0.013 [−0.001 – 0.027] | 0.007 | 0.070 | −0.570 [−1.006 - −0.135] | 0.222 | 0.011 |
| Combined females | −0.003 [−0.016 – 0.010] | 0.007 | 0.67 | 0.071 [−0.529 – 0.671] | 0.306 | 0.82 |
Adjusted by age, sex, number of Polynesian grandparents, Polynesian ancestral class (WP, EP, WP/EP).
Identification of rare variants: ABCC4 P1036L
Eighty rare variants were also detected in the resequenced Polynesian samples. These comprised 37 singleton variants (17 of which were not reported in 1000 Genomes), 19 variants <1% prevalence in controls (5 not reported in 1000 Genomes) and 24 of 1–5% prevalence in controls (3 not reported in 1000 Genomes) (Table S2). Of particular interest was a novel ABCC4 missense variant found in seven Western Polynesian hyperuricaemic individuals and no controls (chr13:95724019, G/A, P1036L). The mutated residue is located within the ABC transporter 2 domain with a scaled CADD score of 24 (top 0.003% of CADD scores in the genome). The chr13:95724019 variant was genotyped over the Replication 1 sample set where the A-allele was restricted to individuals of Western Polynesian ancestry (Table S3). Testing for association with gout revealed over-representation of the A-allele in cases although there was no statistical evidence for association (OR=3.09, P=0.15). The A-allele over-representation in cases was restricted to males (in the unadjusted data ORMales=3.61, P=0.23; ORFemales=1.07, P=0.96).
Functional testing of P1036L
In order to evaluate any effects of P1036L on uric acid transport by ABCC4 the wild-type and mutant alleles were expressed in Xenopus oocytes and efflux of 14C-labelled uric acid measured (Figure 2). ABCC4 exported uric acid at an approximately 2-fold slower rate than ABCG2 (data not shown and (33)). Unlike ABCG2 (33), ABCC4 was sensitive to tranilast and benzbromarone, in addition to 1.0 mM probenecid (Figure 2A). The presence of a leucine residue at position 1036 led to an approximately 35% reduction in uric acid efflux, with no effect on protein expression (Figure 2B).
In contrast to a prior report of coding SNP effects in ABCC4 (32), we found that the G487E coding SNP mutation did not affect ABCC4 function whereas the G187W mutant had reduced function as reported previously (Figure 2B). Additionally, unlike in the prior report (32) which noted reduced protein expression for the 187W mutant, this mutant was fully expressed in oocytes by Western blot analysis; this discrepancy is perhaps a reflection of the different expression systems utilized (transient transfection of HEK293 cells versus direct microinjection of cRNA in Xenopus oocytes). A recent report, also using transient transfection of HEK293T, cells also reported normal expression of the 187W mutant (35).
Discussion
We used a resequencing approach to detect association of the ABCC4 gene, that encodes the multi-drug resistance protein 4 (MRP4), with hyperuricemia (OR=1.80, P=0.003) and gout (OR=1.62, P=0.012) in samples from the NZ Māori and Pacific population. Association with gout was replicated (OR=1.25, P=0.033). These results demonstrate the utility of resequencing approaches in populations with a higher prevalence of phenotype of interest in order to increase understanding of the molecular basis of disease. The reason for non-replication of the association of rs4148500 with gout in Replication 2 sample set could be low power; there was only 43% power to detect an effect at α=0.05 and (based on Replication 1 data) OR=1.36 and minor allele frequency of 0.244. Despite this, the direction of effect in males in the Replication 1 sample set (OR=1.23, P>0.05) was consistent with the combined data whereby the minor A-allele of rs4148500 confers risk to gout in males of Polynesian ancestry.
Consistent with the gout association, the gout-risk A-allele of rs4148500 was associated with reduced FEUA in males (Table 4). This finding is also important as it provides further confirmation of MRP4 as a unidirectional urinary uric acid efflux pump for uric acid (11, 12). In the uricotelic avian proximal tubule, MRP4 is the dominant apical secretory uric acid transporter (36), with ABCG2 not significantly contributing to uric acid secretion in the avian system (36). Interestingly in this system uric acid secretion is inhibited by AMP-activated protein kinase, of which the gene encoding the gamma-2 subunit is associated with urate concentration and gout in European people (5, 7). MRP4 also has been proposed as a hepatic transporter of urate into the circulation owing to its hepatic basolateral expression (11). It has been demonstrated that furosemide and thiazide diuretics and the urate-lowering benzbromarone inhibit, whereas the active metabolite of the urate-lowering allopurinol (oxypurinol) stimulates, MRP4-mediated renal uric acid efflux (37). Consistent with this, we found that ABCC4 was sensitive to benzbromarone and probenecid at a higher concentration (1.0 mM). Thus, MRP4 may transpire to be a clinically important transporter in the management of gout and co-morbid conditions.
The association of ABCC4 with gout and FEUA was restricted to males (Tables 3 and 4). This may be of particular clinical relevance, noting the very high rates of gout in Māori and Pacific men; gout affects more than 25% of Māori and Pacific men over the age of 60 years (2). Differential effects of association of genetic variants with urate and gout between males and females is a phenomenon observed at some other uric acid transporter loci. At ABCG2 association of rs2231142 with gout is weaker in women (OR=1.5) than men (OR=2.0) which at least partly reflects a 50% greater effect of rs2231142 on serum urate levels in men (5). At SLC2A9, while there is a 70% greater effect on serum urate levels in women than men, the effect on gout is similar (5). However at SLC17A1/NPT1, SLC22A11/OAT4 and SLC22A12/URAT1, while effect sizes are up to one-third larger in men, any sex-specific effects remain to be established and are not of the extent observed at SLC2A9, ABCG2 and ABCC4. Understanding the molecular basis of the interaction of sex with genotype at these loci will illuminate the molecular mechanisms of urate control.
The rare (~1%) Polynesian-specific 1036L variant selected for follow-up genotyping was not significantly associated with gout in the combined re-sequence and replication 1 and 2 sample sets (P=0.15), although the direction of effect (OR=3.1) was consistent with the over-representation of the 1036L variant in re-sequenced individuals with hyperuricemia (n=7) compared to none observed in normouricemic individuals. This variant also had an extremely high CADD score, predicting a pathogenic (functional) effect. Consistent with this and with the over-representation of the 1036L allele in hyperuricemic and individuals with gout, the 1036L allele had an ~30% reduced uric acid efflux from Xenopus oocytes. In vivo it can be predicted that individuals with this allele filter less uric acid into the urine. Residue 1036 is intracellular, located in the second nucleotide-binding domain (32). ABCC4 is a very polymorphic gene with, for example, >400 missense variants reported in the Exome Aggregation Consortium database of 60,706 unrelated individuals (38), although 1036L is not present in this database. Some of the variants have been evaluated for effect on functional characteristics of MRP4, for example contributing to intracellular accumulation of antiviral agents (32) and methylated arsenic metabolites (35), and to inappropriate lack of localization to the plasma membrane (35). With urate control and risk of gout as clinical outcomes it will be necessary to systematically test missense variants of ABCC4 for association with urate concentrations and risk of gout, and to evaluate their influence on the ability of MRP4 to transport uric acid.
An extreme sample drawn from the hyperuricaemic Polynesian population was resequenced here under the hypothesis that this population contains a higher prevalence of urate-raising genetic variants in the selected genes (postulated to control urinary uric acid excretion) that had not previously been associated with urate levels or gout by genome-wide association approaches. In the case of ABCC4, the minor risk (A) allele of rs4148500 is not present in Europeans yet has a prevalence of 24% in people of Polynesian descent. Thus rs4148500 is a population-specific variant that highlights the relative importance of genetic variation in ABCC4 as a risk factor for gout in the NZ Māori and Pacific male population. Determining if rs4148500 is the causal variant at the ABCC4 locus will require sequence information from the entire region in the Polynesian genome and genotyping of variants in linkage disequilibrium. Furthermore the risk A-allele is also prevalent in the Asian population (Table 2; 19%) warranting testing for association with gout in populations from this region, with a focus on replicating the male-specific effect observed in the Polynesian population of NZ.
Supplementary Material
Acknowledgments
The New Zealand Health Research Council, Lottery Health New Zealand, Arthritis New Zealand and the National Institute of Health (United States) are thanked for funding this study. Ria Akuhata, Nancy Aupouri, Carol Ford (Ngāti Porou Hauora), Jill Drake (Canterbury District Health Board), Roddi Laurence and Meaghan House (University of Auckland) and Gabrielle Sexton are thanked for assistance in recruitment. Anna Gosling is thanked for helpful discussions on Polynesian migrations. The authors sincerely thank all participants with and without gout who donated time and information for this study. The Genome Institute at the University of Washington St Louis and Bob Fulton are thanked for their help. This research was supported by grant R21 AR065968-01 (Stahl, Merriman, Choi and Mount), NIH R01 AR056291 to Choi, and NZ Health Research Council program grants 08/075, 11/1075 and 14/527 to Merriman, Dalbeth and Stamp.
References
- 1.Dalbeth N, Merriman TR, Stamp LK. Gout. (Review) Lancet. 2016;388:2039–52. doi: 10.1016/S0140-6736(16)00346-9. [DOI] [PubMed] [Google Scholar]
- 2.Winnard D, Wright C, Taylor WJ, Jackson G, Te Karu L, Gow PJ, et al. National prevalence of gout derived from administrative health data in Aotearoa New Zealand. Rheumatology. 2012;51:901–9. doi: 10.1093/rheumatology/ker361. [DOI] [PubMed] [Google Scholar]
- 3.Dehghan A, Köttgen A, Yang Q, Hwang SJ, Kao WL, Rivadeneira F, et al. Association of three genetic loci with uric acid concentration and risk of gout: a genome-wide association study. Lancet. 2008;372:1953–61. doi: 10.1016/S0140-6736(08)61343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merriman TR, Choi HK, Dalbeth N. The genetic basis of gout. Rheum Dis cCin North Am. 2014;40:279–90. doi: 10.1016/j.rdc.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Köttgen A, Albrecht E, Teumer A, Vitart V, Krumsiek J, Hundertmark C, et al. Genome-wide association analyses identify 18 new loci associated with serum urate concentrations. Nature Genet. 2013;45:145–54. doi: 10.1038/ng.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merriman TR. Population heterogeneity in the genetic control of serum urate. (Review) Sem Nephrol. 2011;31(5):420–5. doi: 10.1016/j.semnephrol.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Phipps-Green A, Merriman M, Topless R, Altaf S, Montgomery G, Franklin C, et al. Twenty-eight loci that influence serum urate levels: analysis of association with gout. Ann Rheum Dis. 2016;75:124–30. doi: 10.1136/annrheumdis-2014-205877. [DOI] [PubMed] [Google Scholar]
- 8.Aslamkhan A, Han Y-H, Walden R, Sweet DH, Pritchard JB. Stoichiometry of organic anion/dicarboxylate exchange in membrane vesicles from rat renal cortex and hOAT1-expressing cells. Am J Physiol. 2003;285:F775–F83. doi: 10.1152/ajprenal.00140.2003. [DOI] [PubMed] [Google Scholar]
- 9.Bakhiya N, Bahn A, Burckhardt G, Wolff NA. Human organic anion transporter 3 (hOAT3) can operate as an exchanger and mediate secretory urate flux. Cell Physiol Biochem. 2003;13:249–56. doi: 10.1159/000074539. [DOI] [PubMed] [Google Scholar]
- 10.Sato M, Mamada H, Anzai N, Shirasaka Y, Nakanishi T, Tamai I. Renal secretion of uric acid by organic anion transporter 2 (OAT2/SLC22A7) in human. Biol Pharm Bull. 2010;33:498–503. doi: 10.1248/bpb.33.498. [DOI] [PubMed] [Google Scholar]
- 11.Van Aubel RA, Smeets PH, van den Heuvel JJ, Russel FG. Human organic anion transporter MRP4 (ABCC4) is an efflux pump for the purine end metabolite urate with multiple allosteric substrate binding sites. Am J Physiol. 2005;288:F327–F33. doi: 10.1152/ajprenal.00133.2004. [DOI] [PubMed] [Google Scholar]
- 12.Woodward OM, Köttgen A, Coresh J, Boerwinkle E, Guggino WB, Köttgen M. Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc Natl Acad Sci U S A. 2009;106:10338–42. doi: 10.1073/pnas.0901249106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mandal A, Mount DB. The molecular physiology of uric acid homeostasis. Annu Rev Physiol. 2015;77:323–45. doi: 10.1146/annurev-physiol-021113-170343. [DOI] [PubMed] [Google Scholar]
- 14.Woodward OM, Tukaye DN, Cui J, Greenwell P, Constantoulakis LM, Parker BS, et al. Gout-causing Q141K mutation in ABCG2 leads to instability of the nucleotide-binding domain and can be corrected with small molecules. Proc Natl Acad Sci U S A. 2013;110:5223–8. doi: 10.1073/pnas.1214530110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edwards NL, So A. Emerging therapies for gout. Rheum Dis Clin North Am. 2014;40:375–87. doi: 10.1016/j.rdc.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Tan PK, Liu S, Miner JN. The URAT1 uric acid transporter is important in uric acid homeostasis and its activity may be altered in gout patients and in drug- induced hyperuricemia. Arthritis Rheumatol. 2014;66(S1295):2963. [Google Scholar]
- 17.Robinson P, Taylor W, Merriman T. Systematic review of the prevalence of gout and hyperuricaemia in Australia. Inter Med J. 2012;42:997–1007. doi: 10.1111/j.1445-5994.2012.02794.x. [DOI] [PubMed] [Google Scholar]
- 18.Gosling AL, Matisoo-Smith E, Merriman TR. Hyperuricaemia in the Pacific: why the elevated serum urate levels? Rheumatol Int. 2014;34:743–57. doi: 10.1007/s00296-013-2922-x. [DOI] [PubMed] [Google Scholar]
- 19.Dalbeth N, House ME, Gamble GD, Horne A, Pool B, Purvis L, et al. Population-specific influence of SLC2A9 genotype on the acute hyperuricaemic response to a fructose load. Ann Rheum Dis. 2013;72:1868–73. doi: 10.1136/annrheumdis-2012-202732. [DOI] [PubMed] [Google Scholar]
- 20.Gibson T, Waterworth R, Hatfield P, Robinson G, Bremner K. Hyperuricaemia, gout and kidney function in New Zealand Maori men. Br j Rheumatol. 1984;23:276–82. doi: 10.1093/rheumatology/23.4.276. [DOI] [PubMed] [Google Scholar]
- 21.Simmonds HA, McBride MB, Hatfield PJ, Graham R, McCaskey J, Jackson M. Polynesian women are also at risk for hyperuricaemia and gout because of a genetic defect in renal urate handling. Br J Rheumatol. 1994;33:932–7. doi: 10.1093/rheumatology/33.10.932. [DOI] [PubMed] [Google Scholar]
- 22.Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yu TF. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20:895–900. doi: 10.1002/art.1780200320. [DOI] [PubMed] [Google Scholar]
- 23.Simkin PA. Urate excretion in normal and gouty men. Adv Exp Med Biol. 1977;76B:41–5. doi: 10.1007/978-1-4684-3285-5_5. [DOI] [PubMed] [Google Scholar]
- 24.Auwera GA, Carneiro MO, Hartl C, Poplin R, del Angel G, Levy-Moonshine A, et al. rom FastQ data to high-confidence variant calls: The Genome Analysis Toolkit best practices pipeline. Curr Protoc Bioinformatics. 2013;43 doi: 10.1002/0471250953.bi1110s43. 11.10.1-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H, Durbin R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics. 2009;25:1754–60. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tange O. Gnu parallel-the command-line power tool. The USENIX Magazine. 2011;36:42–7. [Google Scholar]
- 28.DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43:491–8. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kircher M, Witten DM, Jain P, O’Roak BJ, Cooper GM, Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet. 2014;46:310–5. doi: 10.1038/ng.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paila U, Chapman BA, Kirchner R, Quinlan AR. GEMINI: integrative exploration of genetic variation and genome annotations. PLoS Comput Biol. 2013;9:e1003153. doi: 10.1371/journal.pcbi.1003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Team RC. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2012. 2014. [Google Scholar]
- 32.Abla N, Chinn LW, Nakamura T, Liu L, Huang CC, Johns SJ, et al. The human multidrug resistance protein 4 (MRP4, ABCC4): functional analysis of a highly polymorphic gene. J Pharmacol Exp Ther. 2008;325:859–68. doi: 10.1124/jpet.108.136523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mandal AK, Mercado A, Foster A, Zandi-Neiad K, Mount DB. Uricosuric targets of tranilast. Pharmacol Res Perspec. 2017 doi: 10.1002/prp2.291. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phipps-Green AJ, Hollis-Moffatt J, Dalbeth N, Merriman ME, Topless R, Gow PJ, et al. A strong role for the ABCG2 gene in susceptibility to gout in New Zealand Pacific Island and Caucasian, but not Maori, case and control sample sets. Hum Mol Genet. 2010;19:4813–9. doi: 10.1093/hmg/ddq412. [DOI] [PubMed] [Google Scholar]
- 35.Banerjee M, Marensi V, Conseil G, Le XC, Cole SP, Leslie EM. Polymorphic variants of MRP4/ABCC4 differentially modulate the transport of methylated arsenic metabolites and physiological organic anions. Biochemical Pharmacol. 2016;120:72–82. doi: 10.1016/j.bcp.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 36.Bataille AM, Goldmeyer J, Renfro JL. Avian renal proximal tubule epithelium urate secretion is mediated by Mrp4. Am J Physiol. 2008;295:R2024–R33. doi: 10.1152/ajpregu.90471.2008. [DOI] [PubMed] [Google Scholar]
- 37.El-Sheikh AA, Van Den Heuvel JJ, Koenderink JB, Russel FG. Effect of hypouricaemic and hyperuricaemic drugs on the renal urate efflux transporter, multidrug resistance protein 4. Br J Pharmacol. 2008;155:1066–75. doi: 10.1038/bjp.2008.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lek M, Karczewski K, Minikel E, Samocha K, Banks E, Fennell T, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–91. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


