Abstract
This study investigated the effect of radiofrequency hyperthermia combined with chemotherapy in the treatment of advanced ovarian cancer.
Methods
Seventy-five patients with advanced ovarian cancer were grouped into observation (n = 36) and control (n = 37) groups according to different treatment methods. The age of the patients in the control and the experimental groups were (55 + 11) and (53 + 12) years old, respectively. The control group was received chemotherapy alone (paclitaxel + cisplatin chemotherapy), and on the basis of systemic chemotherapy, the observation group was administered therapy in conjunction with abdominal pelvic radiofrequency hyperthermia.
Results
The tumor remission rate, ascites, serum CA125 levels, pain control, quality of life enhancement, III+IV bone marrow suppression and improvement of gastrointestinal reaction in the observation group were better than those of the control group (all P< 0.05).
Conclusion
Radiofrequency hyperthermia combined with chemotherapy in the treatment of advanced ovarian cancer has significantly improved the tumor remission rate, ascite control and CA125 levels, and substantially reduced the gastrointestinal reaction and bone marrow suppression rate, which is worthy of intensive clinical application.
Keywords: Ovarian cancer, Radiofrequency hyperthermia, Drug therapy, Paclitaxel
1. Introduction
Ovarian cancer is one of the most common malignant tumors of the female reproductive system, and it is the most common skin cancer [1]. Because of the special location, complicated anatomy and function of the ovary, the onset is occult, and the early symptoms of the patient are atypical or not obvious, and the early diagnosis is very difficult [2,3]. Although carbohydrate antigen-125 (CA125) and human epididymis secretory protein 4 (HE4) were already used for early diagnosis of ovarian cancer [4], which is highest in mortality rate among all gynecological tumors [5] . About two thirds of ovarian cancer patients come to the hospital for advanced symptoms every year. After treatment, the condition has been improved, but the recurrence or metastasis of the tumor leads to the failure of most of the patients, resulting in death. Radiofrequency hyperthermia, as a new therapy for cancer, cannot only kill the tumor but also damage the normal tissue by radiofrequency wave heating, and its treatment effect is obvious [6]. This study therefore set out to assess the curative effect of advanced ovarian cancer patients by radiofrequency hyperthermia combined with chemotherapy regimens in 73 cases of ovarian cancer patients.
2. Materials and methods
2.1. General information
A total of 73 patients with advanced ovarian cancer admitted in our hospital from October 2013 to August 2015 were divided into 37 groups, 36 cases in the control group and two cases in the experimental group. The age of the patients was (55 + 11) and (53 + 12) in the control and the experimental groups, respectively.
Pathological type (control/ experimental cases per each group, respectively):
20/21 cases of serous adenocarcinoma,4/5 cases of mucinous adenocarcinoma, 3/2 cases of endometrial adenocarcinoma, 3/4 cases of clear cell carcinoma, 5/3 cases of squamous cellcarcinoma and 2/1 cases of undifferentiated carcinoma.
Clinical staging of ovarian carcinoma (control / experimental groups, respectively): 9/8 cases of stage IIIA, 12/13 cases of stage IIIB, 16/15 cases of stage IV; KPS scores were >67.
Routine examination was normal in the two groups prior to beginning of the treatment. Furthermore, the general data of the experimental group and the control group were not significantly different (P>0.05).
Ethical approval: The research related to human use has been complied with all the relevant national regulations, institutional policies and in accordance the tenets of the Helsinki Declaration, and has been approved by the authors’ institutional review board or equivalent committee.
Informed consent: Informed consent has been obtained from all individuals included in this study.
2.2. Methods
Control group: Thirty seven patients served as the control group was treated with routine chemotherapy only. The patients were treated with anti-allergic drugs to prevent allergic reactions in the course of chemotherapy before the application of chemotherapy drugs. Cisplatin (75 mg/ m2) and paclitaxel (135 mg/m2) were received over for 24 h for 21 cycles of chemotherapy, with 6 courses of treatment. Meanwhile, the patients in the control group were monitored closely for any changes in the rate of treatment and blood pressure to prevent the patients who are at a high risk of shock and even death due to abnormal blood pressure.
Experimental group: Patients with a variety of pathologically confirmed cancer were treated with radiofrequency hyperthermia and chemotherapy for 7 days, and then underwent hyperthermia chemotherapy for 3 days. Rotational program of hyperthermia and chemotherapy treatment were performed for 2-3 weeks.
In this study, the SR1000 tumor radiofrequency hyperthermia apparatus (Xianke, Shenzhen, China) was adopted, and the non-contact treatment method was conducted. The tissue was heated by radiofrequency wave, the temperature was set at (42.5-43.5), and the duration was 60-120 min.
In the course of the 8 cycles of treatment, each group received 1 curative evaluation after 2 cycles of treatment, 6D / cycle [3]. The evaluation indexes of the experimental group and the control group were compared.
2.3. Assessment criteria
The composite of chemotherapy in patients with solid tumors was evaluated according to the changes of the tumor (30%), clinical symptoms (15%), physical condition (15%), and survival rate (40%), assigned a score of 0-100 as follows: invalid (<25), stable (25~49) and effective (50~75) and effective (>75) 4 grades, tumor remission rate, which equals to effective remission rate and effective remission rate.
Bone marrow suppression: it was divided into 4 levels: I, IV, IV, and graded by evaluation of thrombocytopenia and decrease of wbc [8].
Gastrointestinal reaction: the reaction includes the following symptoms: constipation, diarrhea, vomiting, nausea and severe degree I ~ IV [8].
Results of CA125 biomarker for early detection [9] and pain control are divided into 3 categories. Reference WHO cancer leachate treatment standard was employed to divide the assessment for the improvement of the quality of life in ascites in combination with Karnofsky integral [10,11] standard (Table 1).
Table 1.
Evaluation form of CA125 test results.
| Experimental / control groups | Effective / Very effective | Improvement / Stability | Invalid |
|---|---|---|---|
| CA125 | |||
| Detection level of CA125 | Decrease >50% or normal | Rise <25% or <50% | Elevation >50% |
| Duration (days) | ≤ 4 | Less than or equal to 4 | - |
| Ascites | |||
| Ascite ratio before and after | >1/2 | - | ≤1/2 or elevation |
| Duration (weeks) | >4 | - | - |
| Life improvement | |||
| Score before treatment (score) | >20 | ≥10 and s19 | <10 |
| Pain symptom control | |||
| Taking a painkiller before treatment | Greater than 1/3 | >1/3 and <2/3 | Greater than 2/3 |
| Duration (days) | ≥3 | ≥3 | - |
Table 2.
The comparison of efficacy between traditional method (control group) and combined therapy (experimental group).
| Groups | Cases | Tumor alleviation | Ascite regulation | CA125 control |
|---|---|---|---|---|
| Experimental group | 36 | 30 (83.3) | 27 (75.0) | 30 (83.3) |
| Control group | 37 | 16 (43.2) | 11 (29.7) | 18 (48.6) |
| X2 | - | 12.58 | 14.98 | 9.75 |
| P -value | - | <0.05 | <0.05 | < 0.05 |
2.4. Statistical method
All patients were followed up from the first treatment, and the survival rate was calculated by kaplan-meier method. Single-factor analysis was performed with the log-rank. All the data were analyzed by SPSS 16 software., Qualitative data were verified by Pearson’s chi-squared test (x2) of statistics; A P- value of less than 0.05 was considered statistically significant.
3. Results
3.1. Comparison of the short-term curative effect between the experimental and control groups
Compared to the control group, the tumor control rate of ascites in the experimental group (combined chemotherapy), has been effectively alleviated, and CA125 level inspection indications have been significantly improved (P<0.05).
3.2. Comparison of the toxicity and side effects of the experimental and control groups
No significant differences in the nausea and vomiting, white blood cells (WBCs), serum creatinine and fatty induration were found between the two groups (Table 3).
Table 3.
Toxicity and side effects of the experimental group in comparison with the control group.
| Groups | Cases | Nausea and vomiting | WBCs | Serum creatinine | Fatty induration |
|---|---|---|---|---|---|
| Experimental group | 36 | 30 | 14 | 5 | 4 |
| Control group | 37 | 28 | 11 | 7 | 0 |
3.3. Comparison of pain control between the two groups
In this study, the Fisher exact probability method was used to verify the statistical significance (P<0.05) as shown in Table 4. There were 10 patients with pain control in the experimental group, accounting for 77.8%. The control group received 4 cases of pain control, accounting for 54.1%.
Table 4.
Pain control in the two groups.
| Groups | Cases | Significant effect | Effective | No effect |
|---|---|---|---|---|
| Experimental group | 36 | 10 | 18 | 8 |
| Control group | 37 | 4 | 16 | 17 |
3.4. Comparison of the quality of life in the two groups
As presented in Table 5, the quality of life in the experimental group was substantially improved in 24 cases, accounting for 66.7%, while the quality of life in the control group was significantly enhanced in 6 cases, constituting 27.3% (P<0.05).
Table 5.
Quality of life in the two groups.
| Groups | Cases | Significant effect | Effective | No effect |
|---|---|---|---|---|
| Experimental group | 36 | 4 | 20 | 12 |
| Control group | 37 | 1 | 9 | 27 |
Table 6.
Comparison of myelosuppression and gastrointestinal response between the two groups [case (%)].
| Groups | Cases | Marrow suppression of grade III + IV | Third or fourth degree gastrointestinal response |
|---|---|---|---|
| Experimental group | 36 | 4 (11.1) | 8 (22.2) |
| Control group | 37 | 17 (45.9) | 18 (48.6) |
| X2 | - | 10.81 | 5.56 |
| P-value | - | <0.05 | < 0.05 |
| Survival rate: | |||
| Sample | Survival rate | Death rate | |
| Control group | 37 | 15 | 22 |
| Case group | 36 | 23* | 13 |
Compared with the control group, P<0.05
3.5. Comparison of myelosuppression stages III and IV disease and gastrointestinal response levels between the two groups
The experimental group with substantial difference in the myelosuppression stages of disease (III + IV) and gastrointestinal reaction levels compared to the control one (P<0.05) (Table 5).
Mild scalds was occurred in local skin of 4 patients within the control group before heat treatment, but by wiping the area of the scalds with ointment and reducing the temperature properly, it was cured within a week.
4. Discussion
The early diagnosis of ovarian cancer is difficult and the clinical symptoms of advanced stage are confirmed. The mortality rate is relatively high [12], meanwhile; the special anatomic location of the ovary leads to an increase in the probability of diffuse metastasis of the abdomen [13].
There are many ways to treat ovarian cancer; however, only a few methods are effective. The overall efficiency is still no more than 30% [14], and the patients have a high recurrence rate within a period of time after operation. Intraperitoneal direct transfer is the main route [15], and the invasion of deep tissue is rare. The scheme of intraperitoneal thermal chemotherapy is very effective in killing cancer cells [16].
In recent years, it has become a new topic to investigate a regimen that can effectively control ovarian cancer without adverse effects to a certain extent during the treatment [17].
In this study, the experimental group was received radiofrequency hyperthermia combined with chemotherapy, while the control group was treated by chemotherapy alone.
The patients in the experimental group have a higher tumor remission rate of ascites and examination index than those of the control group, and the toxicities of the pointer patients are lower than that of the control group.
Our results match those observed in earlier studies [18].
It has been reported that RF hyperthermia heating plays an important role in tumor therapy scheme, promotion of chemotherapy drugs and, inhibition of DNA repair [19] and multidrug resistance P-glycoprotein expression, meanwhile, it can improve the efficacy of chemotherapy.
It has been reported that radiofrequency hyperthermia and other hyperthermia treatments play a significant role in tumor treatment, which is more obvious in promoting addition reaction between chemotherapy drugs and cancer cell DNA.
DNA repair is inhibited by addition reaction of cancer cell DNA, and increases the addition reaction to inhibit the expression of multidrug resistant p-glycoprotein. Thereby, chemotherapeutic efficacy of ovarian cancer patients has been improved [19].
Figure 1.
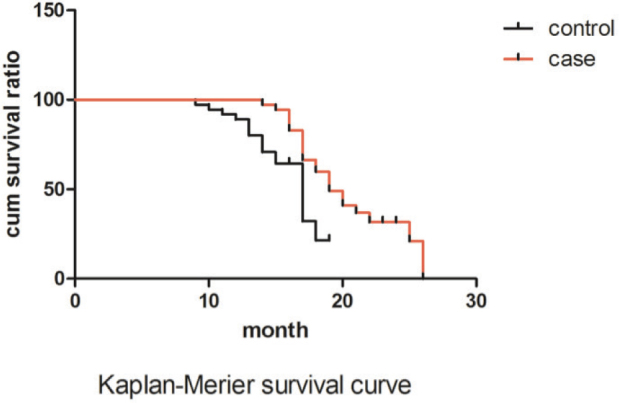
Kaplan-Merier survival curve
In addition, a disorder of immune Th1/Th2 cytokines in the patients with ovarian cancer in peripheral blood leads to the immune escape of tumor cells [21] of the expression of heat shock proteins in patients during radiofrequency hyperthermia enhances the antitumor activity of chemotherapeutic drugs and elicits the immune surveillance function.
5. Conclusion
Radiofrequency hyperthermia [22] as a new cancer treatment, promotes the greater omentum to absorb drugs, improves the utilization rate of chemotherapy drugs. Moreover, it can effectively kill cancer cells in abdominal cavity [23,24]. Previous studies have found that cisplatin has synergistic effects with hyperthermia with no threshold effect drug [6]. Its main target is DNA, which acts on DNA strand and intra-strand junction, forming DDP~DNA complex, interfering with DNA replication, or combining with nuclear protein and cytoplasmic protein.
Hyperthermia hinders the repair of DNA damage induced by cisplatin and improves the utilization rate of cisplatin.
The machine SR1000 tumor radio-frequency hyperthermia machine uses non-contact therapy to heat the tissue to the temperature that can kill the tumor cells. It can kill the tumor cells without damaging the normal tissues.
There were 4 cases of fatty induration in the experimental group, accounting for 11.1%, which was lower than that of RF-8.
Radiofrequency hyperthermia combined with chemotherapy is more effective treatment and results in vascular changes in the tumor tissue and temperature differences.
Normal tissue can tolerate high temperature for a long period of time 42.5-43.5 (safety limit is 45 - 1 DEG C), thereby killing tumor cells without affecting normal cells.
Hyperthermia does not cause adverse reactions, such as myelosuppression or alopecia. Since the number of samples collected in our hospital is smaller after grouping, the results of this clinical observation need to be further verified. It needs to be confirmed by large sample data in order to obtain more reliable indicators.
In summary, radiofrequency hyperthermia combined with chemotherapy is more effective chemotherapy in the treatment of patients with advanced ovarian cancer, considers an alternative for anticancer drug resistance cases, increases the susceptibility of patients for the treatment, and reduces the toxicity of patients and promotes the rehabilitation of patients, which is worthy of comprehensive clinical application.
Acknowledgements
The present study was funded by the Jilin provincial health science research plan (2014Z081)
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
References
- [1].Liu R., Cao X.Y., Xiao Y.. et al. Nedaplatin Combined with gemcitabine in the treatment of elderly patients with recurrent ovarian cancer the curative effect of China. J. gerontol. 2012;32:670–672. [Google Scholar]
- [2].Oberaigner W., Minicozzi P., Bielskalasota M.. et al. Survival for Ovarian Cancer in Europe: The across-country variation did not shrink in the past decade. J.Acta. Oncol.. 2012;51:441–53. doi: 10.3109/0284186X.2011.653437. [DOI] [PubMed] [Google Scholar]
- [3].Wang L., Jiang C., Chen CY.. The value of soluble B7-H4 in the diagnosis of early ovarian cancer. J. Chin. J. Mod. Med. 2014;24:107–109. [Google Scholar]
- [4].Whitehouse C., Solomon E.. Current status of the molecular characterization of the ovarian cancer antigen CA125 and implications for its use in clinical screening. J. Gynecol. Oncol. 2003;88:152–157. doi: 10.1006/gyno.2002.6708. [DOI] [PubMed] [Google Scholar]
- [5].Wei LW., Li L.. Research progress of multidrug resistance related signal transduction pathway and its targeted drugs in epithelial ovarian cancer. J. Clin.Oncol. 2016;43:396–399. [Google Scholar]
- [6].Wang X., Yu Y., Lv F.. et al. Effects of hyperthermia combined with hyperthermia on proliferation of human breast cancer cells. J. Chinese Journal of physical medicine and rehabilitation. 2014;36:577–582. [Google Scholar]
- [7].Zhou HM., Huang HF., Pan LY.. et al. Clinical study of the change of serum CA125 value in judging the curative effect and prognosis of epithelial ovarian cancer. J. Chinese Journal of Practical Gynecology and obstetrics. 2008;24:204–206. [Google Scholar]
- [8].Zhu LN., Fan QX., Zong H.. et al. Clinical application of hyperthermia combined with chemotherapy in advanced gastric cancer. J. Chinese Journal of physical medicine and rehabilitation. 2013;35:326–329. [Google Scholar]
- [9].heng X., Gou HF., Liu JY.. et al. Clinical significance of serum CA125 in diffuse malignant mesothelioma. J. SpringerPlus. 2016;5:1–8. doi: 10.1186/s40064-016-1998-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Shi J., Wei T.. CNKI literature of TCM treatment of ascites cancer drug law research. J. Journal of traditional Chinese medicine information based on Chinese. 2014;21:22–25. [Google Scholar]
- [11].Timmermann C.. the Karnofsky scaleand the history of quality of life measurements in cancer trials. J. Chronic Illness. 2013;9:179–190. doi: 10.1177/1742395312466903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jacobs IJ., Menon U., Ryan A.. et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. J. Lancet. 2016;387:945–956. doi: 10.1016/S0140-6736(15)01224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wu WH., Wang H., Cui MH.. Progress in the study of CD4+CD25+ regulatory T cells and ovarian cancer. J. Maternal and Child Health Care of China. 2016;31:3192–3195. [Google Scholar]
- [14].Li XH., Chen XJ., Ou WB.. et al. Knockdown of creatine kinase B inhibits ovarian cancer progression by decreasing glycolysis. J. The International Journal of Biochemistry and Cell Biology. 2013;45:979–986. doi: 10.1016/j.biocel.2013.02.003. [DOI] [PubMed] [Google Scholar]
- [15].Lili LN., Matyunina LV., Walker DE.. et al. Molecular profiling supports the role of epithelial-to-mesenchymal transition (EMT) in ovarian cancer metastasis. J. Journal of Ovarian Research. 2013;6:49–5249. doi: 10.1186/1757-2215-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhang Y.. Effect of intraperitoneal hyperthermic perfusion chemotherapy on ovarian malignancies. J. Henan Medical Research. 2015;24:103–104. [Google Scholar]
- [17].Ren X., Wang F., Shi Q.. cisplatin intraperitoneal perfusion combined with gemcitabine intravenous chemotherapy in the treatment of malignant ascites of ovarian cancer. J. Journal of Shanxi Medical Journal. 2010;39:531–532. [Google Scholar]
- [18].Rao M., Wu J., Lin S.. et al. Clinical observation of the effect of deep hyperthermia combined with chemotherapy on advanced ovarian cancer. J. Chinese Journal of clinicians (Electronic Edition). 2014;8:1997–2001. [Google Scholar]
- [19].Ma S., Wu Z.. Application of hyperthermia in tumor therapy. J. Chinese Medical Journal. 2004;39:8–11. [Google Scholar]
- [20].Muenyi CS., Trivedi AP., Helm CW.. et al. Cisplatin plus sodium arsenite and hyperthermia induces pseudo-G1 associated apoptotic cell death in ovarian cancer cells. J. Toxicological Sciences An Official Journal of the Society of Toxicology. 2014;139:74. doi: 10.1093/toxsci/kfu029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Xin X., Liu Z., Zhao R.. et al. Clinical study of Th1/Th2 cytokines in peripheral blood of patients with ovarian cancer. J. Journal of Practical Medicine. 2008;24:1154–1156. [Google Scholar]
- [22].Xu W., Lv X., Zhang Y.. et al. Short-term therapeutic effect of radiofrequency hyperthermia combined with intraperitoneal hyperthermic perfusion chemotherapy on malignant peritoneal effusion. J. Chinese Journal of physical medicine and rehabilitation. 2006;28:786–787. [Google Scholar]
- [23].Nicoletto MO., Padrini R., Galeotti F.. et al. Pharmacokinetics of intraperitoneal hyperthermic perfusion with mitoxantrone in ovarian cancer. J. Cancer Chemotherapy and Pharmacology. 2000;45:457–462. doi: 10.1007/s002800051019. [DOI] [PubMed] [Google Scholar]
- [24].Inoue Y., Yamashiro H., Sawada T.. et al. Therapeutic results and pharmacokinetics of combined used anticancer drug in intraperitoneal hyperthermo-chemotherapy (CHPP) J. Gan to Kagaku Ryoho Cancer & Chemotherapy. 1990;17:1551. [PubMed] [Google Scholar]


