Significance
An individual’s socioeconomic status (SES) is a central feature of their environmental surroundings and has been shown to relate to the development and maturation of their brain in childhood. Here, we demonstrate that an individual’s present (adult) SES relates to their brain function and anatomy across a broad range of middle-age adulthood. In middle-aged adults (35–64 years), lower SES individuals exhibit less organized functional brain networks and reduced cortical thickness compared with higher SES individuals. These relationships cannot be fully explained by differences in health, demographics, or cognition. Additionally, childhood SES does not explain the relation between SES and brain network organization. These observations provide support for a powerful relationship between the environment and the brain that is evident in adult middle age.
Keywords: socioeconomic status, aging, lifespan, resting-state networks, cortical thickness
Abstract
An individual’s environmental surroundings interact with the development and maturation of their brain. An important aspect of an individual’s environment is his or her socioeconomic status (SES), which estimates access to material resources and social prestige. Previous characterizations of the relation between SES and the brain have primarily focused on earlier or later epochs of the lifespan (i.e., childhood, older age). We broaden this work to examine the relationship between SES and the brain across a wide range of human adulthood (20–89 years), including individuals from the less studied middle-age range. SES, defined by education attainment and occupational socioeconomic characteristics, moderates previously reported age-related differences in the brain’s functional network organization and whole-brain cortical structure. Across middle age (35–64 years), lower SES is associated with reduced resting-state system segregation (a measure of effective functional network organization). A similar but less robust relationship exists between SES and age with respect to brain anatomy: Lower SES is associated with reduced cortical gray matter thickness in middle age. Conversely, younger and older adulthood do not exhibit consistent SES-related difference in the brain measures. The SES–brain relationships persist after controlling for measures of physical and mental health, cognitive ability, and participant demographics. Critically, an individual’s childhood SES cannot account for the relationship between their current SES and functional network organization. These findings provide evidence that SES relates to the brain’s functional network organization and anatomy across adult middle age, and that higher SES may be a protective factor against age-related brain decline.
Socioeconomic status (SES) is a multifaceted construct that not only represents social standing but also plays a prominent role in shaping our environment by defining our access to healthcare, nutrition, and enrichment (1, 2). To this end, an individual’s SES is strongly associated with his or her well-being, including physical health (3, 4), mental health (5, 6), neurodevelopment (7, 8), cognitive ability (4), and the structure and function of his or her brain (for review, see ref. 9).
Examination of the relationship between SES and the brain has primarily focused on the earlier or later stages of the lifespan, which are considered sensitive periods that are typified by significant changes in brain anatomy and function (10). Throughout childhood and adolescence, lower SES has been shown to negatively impact neurodevelopment (for review, see ref. 9), wherein children from lower SES backgrounds exhibit smaller brain volume (e.g., ref. 11) and altered brain function (e.g., refs. 8 and 12). While SES-related brain differences are pronounced in cases of poverty (e.g., refs. 13–15), they also exist across the SES continuum, providing evidence for a SES gradient relationship to measures of the brain and associated cognition (e.g., refs. 8 and 11). On the other end of the age spectrum, studies of older adults have demonstrated a relationship between SES, brain structure, and health. SES differences are associated with differences in regional and global brain structure (16–20), neuropathology (21), and incidence of dementia (22). While the direction of some of these relationships in older adults varies across studies, it has been hypothesized that higher SES may serve as a “reserve” or protective factor, delaying or minimizing age-related brain deterioration (16, 23). Notably, this work in older adults aligns with studies in animals (rodents and nonhuman primates; refs. 24 and 25) as well as humans (26–28) that have demonstrated how numerous lifestyle and environment factors, such as exercise and environmental stimulation, can maintain or even enhance different aspects of brain structure and function over the course of aging.
It is possible that SES–brain relationships are most pronounced when the brain is most vulnerable: during the earlier and later stages of life. Alternatively, an individual’s SES may represent one of a number of life course factors that continually influences brain and cognition over time (29–31), either serving to amplify or limit progressive changes in brain structure and function over age (32–36). Preliminary evidence for this latter hypothesis exists but is incomplete; while a relationship between SES and the brain in both younger adulthood (37, 38) and middle-age adulthood (39–41) have been reported, these studies have typically focused on different brain measures representing separate features of the brain (e.g., functional activation during specific tasks, regional brain anatomy), and few studies have simultaneously examined SES–brain relationships across different segments of age. Obtaining a deep understanding of whether and how SES is differentially associated with the brain across adulthood thus requires piecing together results from independent studies that have used distinct measures of SES (e.g., education, income, community disadvantage, constructs of multiple variables) and different measures of brain function and anatomy. This approach has fallen short in producing a clear conclusion (for a recent review, see ref. 9) and limits our ability to understand both the nature and extent of SES’s association with brain function and anatomy over distinct portions of the adult lifespan.
In the present study, we evaluate whether SES relates to previously established differences in brain function (33, 42, 43) and anatomy (44, 45) across a wide range of the adult lifespan (age, 20–89 y). Given that SES represents a surrogate measure for diverse domains of life (e.g., education, income, occupational social prestige; ref. 1), it is likely that it relates to the function and structure of multiple brain regions that are distributed across distinct brain systems (e.g., see ref. 9). However, it is possible that different parts of the brain will be differently affected at distinct phases of adulthood, making it difficult to isolate and compare these effects across age. Accordingly, we focused our efforts on examining relationships between SES and “global” measures of an individuals’ brain function and structure, as opposed to properties of specific brain regions. Measures of brain function and anatomy were operationalized using neuroimaging-based quantification of functional network organization and cortical gray matter thickness, respectively.
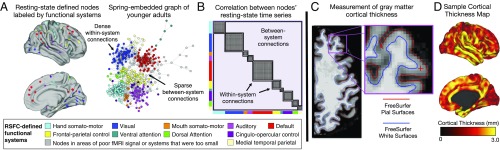
To measure brain function, we leveraged recent methodological developments, which have permitted quantification and evaluation of the brain’s large-scale functional network organization (46, 47). A measure of brain network organization, system segregation, was defined using formal network models applied to functional interactions between areas of the brain as measured at rest [resting-state functional correlations (RSFCs) (48, 49); Fig. 1 A and B]. RSFC patterns are malleable over both shorter and longer timescales (i.e., development and aging) in relation to the changing statistics of the environment (47), thus making these functional brain signals a useful target for understanding differences in an individual’s SES and life experiences. Importantly, examining the brain at rest minimizes the influence of differences in task performance that might relate to differences in SES (e.g., refs. 50 and 51). The RSFCs of brain areas are organized into a large-scale brain network that consists of multiple segregated subnetworks or modules, many of which correspond to known functionally dissociable brain systems (e.g., the visual system, the default system, the frontal-parietal control system; ref. 52). The segregation of these brain systems reflects an important organizational feature of an individual’s brain network and how it functions (53). We and others have demonstrated that RSFC system segregation decreases with increasing age (for review, see ref. 53) and relates to the task-related functional activity of brain areas (54). Reduced brain system segregation is also associated with poorer cognitive ability across age (33), and training studies have shown that increases in system segregation are associated with improvement in cognitive ability (55).
Fig. 1.
Functional network and structural measures of the brain. (A) Functional network organization was measured using resting-state network analysis. The set of nodes is depicted on a “midthickness” brain surface (Left) and with a spring-embedded graph (Right). The spring-embedded graph depicts the network organization of the mean younger adult (20–34 y) brain network, where nodes in the same functional system are more connected with each other (i.e., closer in distance) than with nodes from other systems. The nodes shown in the figure are colored by younger adults’ functional system assignments. (B) The mean resting-state fMRI time series of each node was extracted to form a node-to-node cross-correlation data matrix. Edges between nodes of the same brain system are within-system connections (shaded), whereas edges between nodes of different systems (e.g., connection from a default system node to a frontal-parietal control system node) are between-system connections (not shaded). For each participant’s resting-state data matrix, system segregation is calculated using the mean within-system and mean between-system connection strength (see Materials and Methods for additional details). (C) Cortical gray matter thickness was measured as the distance between the pial (CSF–gray matter boundary; red) and white (gray matter–white matter boundary; blue) surfaces of the brain. (Magnification: 3.87×.) (D) An example of a single participant’s cortical thickness map is shown on a midthickness surface rendering of the right hemisphere.
Following the prior work that has demonstrated relationships between SES and brain anatomy in both childhood (56–59) and adulthood (17, 37, 40, 60), we also evaluated whether SES moderates age-associated differences in brain anatomy. Measurements of brain anatomy were estimated from T1-weighted images of each individual’s brain (Fig. 1 C and D). Automated image segmentation of structural MRI scans is capable of deriving high-precision measurements of an individual’s neuroanatomy (e.g., thickness, surface area, volume). This method has been externally validated with both manual tracing and histological analyses of postmortem brains (61–63) to reveal strong associations with cellular neuroanatomy (e.g., neuronal count, cell density, cortical thickness; refs. 64–66). In keeping with this, image-based estimation of gray matter thickness values has been shown to correlate strongly with adult aging (45) and early diagnoses of dementia (67).
Accumulating evidence has demonstrated how both RSFC system segregation and cortical gray matter thickness each exhibit systematic differences across adult age. It is unclear whether and how an individual’s environment may be related to differences in these important global measures of brain function and anatomy during adulthood. Here, we tested the hypothesis that SES moderates age-related differences of functional network organization and brain anatomy across a wide age sampling of community-dwelling adult individuals. We provide evidence that an individual’s SES relates to their functional network organization and brain structure during their adulthood.
Results
SES Moderates Age-Related Differences in Functional Network Organization.
Participants’ ages ranged from 20 to 89 y (n = 304), with relatively dense sampling across each of the decades (Table 1). All participants were cognitively normal [Mini-Mental State Examination (MMSE) ≥ 26]. SES was defined from a combination of participants’ education years and their occupational socioeconomic index defined for their present occupation (68) [see Materials and Methods and additional details in SI Appendix, Supplemental Results 1.1, for comparison of the SES measure to other relevant measures (e.g., weighted household income, subjective social standing)]. For retired participants, occupational socioeconomic index was defined from their preretirement occupation. Although education level differed across age, with older age being associated with fewer years of formal education, the derived measure of SES was comparable across age [SES by age correlation: r(303) = −0.06, P = 0.271; see Table 1 for descriptive statistics within specific age ranges].
Table 1.
Demographic information
| Age groups | |||||
| Variable | Younger (20–34 y) | Middle early (35–49 y) | Middle late (50–64 y) | Older (65–89 y) | P value |
| N | 44 | 43 | 85 | 132 | NA |
| % Female | 59% | 63% | 66% | 55% | NS |
| % Minority | 34% | 21% | 12% | 4% | <0.001*** |
| SES (SD) | 0.18 (1.30) | 0.21 (1.21) | −0.11 (1.19) | −0.05 (1.22) | NS |
| Education years (SD) | 16.52 (2.46) | 16.29 (2.26) | 15.65 (2.13) | 15.50 (2.29) | 0.028* |
| Occupational socioeconomic index (SD) | 45.73 (12.96) | 47.44 (13.02) | 45.30 (12.99) | 47.20 (11.79) | NS |
| MMSE (SD) | 28.41 (1.23) | 28.67 (1.13) | 28.39 (1.13) | 28.11 (1.21) | 0.035* |
| BMI | 25.20 (4.82) | 24.99 (3.91) | 26.54 (4.00) | 25.80 (3.64) | NS |
| SF-36 PCS | 56.74 (4.88) | 56.99 (4.10) | 55.38 (6.93) | 53.05 (5.77) | <0.001*** |
| % with chronic physical health issues | 23% | 14% | 20% | 31% | NS |
| % Hypertension | 0% | 2% | 24% | 32% | <0.001*** |
| % Never smoker | 64% | 67% | 73% | 56% | NS |
| Alcohol per week (SD) | 2.88 (3.73) | 2.42 (2.74) | 2.31 (3.84) | 2.77 (3.75) | NS |
| Head motion (mean FD; SD) | 0.12 (0.03) | 0.14 (0.04) | 0.16 (0.06) | 0.18 (0.06) | <0.001*** |
Statistical tests between age groups were conducted with χ2 test for distributions of gender, minority (self-reporting as nonwhite/Caucasian), participants with chronic physical health issues, participants with hypertension, and smoker status; ANOVAs were used for SES, education years, occupational socioeconomic index, Mini-Mental State Examination (MMSE), SF-36 Physical Component Score (PCS), alcohol consumption (drinks per week), and in-scanner head motion during resting state (BOLD fMRI) scan [mean frame displacement (FD)]. Asterisks denote statistical significance (*P < 0.05, **P < 0.01, ***P < 0.001); NS, not significant; NA, statistical test was not performed.
We evaluated whether SES relates to RSFC brain system segregation as a function of age. A general linear model was conducted to test for the main effects of SES, age, and their interaction on RSFC brain system segregation (controlling for measures of head motion in the scanner). Both SES and age were treated as continuous variables. The main effect of SES was marginally significant in predicting brain system segregation [β = 0.32, t(299) = 1.83, P = 0.069], and the main effect of age was significant [β = −0.43, t(299) = −7.61, P < 0.001]. Notably, a significant interaction between SES and age was observed in brain system segregation [β = −0.0003, t(299) = −1.98, P = 0.049].
To clearly interpret the SES by age interaction on brain system segregation, participants were divided into four independent age groups defined across adulthood [young adults (YA), 20–34 y; middle-early adults (ME), 35–49 y; middle-late adults (ML), 50–64 y; older adults (OA), 65–89 y]. The age groups coincided with the age-based divisions that were used to derive the functional systems of the brain networks, an approach that was also important for minimizing any possible age-related biases when estimating brain system segregation (33, 54) (see Materials and Methods for details). This analysis confirmed that SES (included as a continuous variable) and age group exhibited a significant interaction on system segregation [F(3,295) = 3.16, P = 0.025, = 0.03].
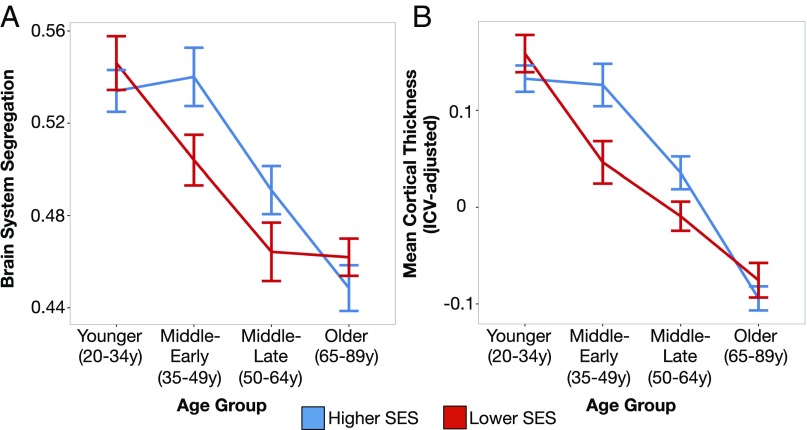
Additional post hoc analyses were conducted to unpack the SES relationships further. Overall, the SES by age group interaction was characterized by higher SES being associated with greater brain system segregation in the middle-age segments (35–64 y) but not the younger or older age segments. The SES by age interaction is summarized in Fig. 2A, where SES is also stratified into higher and lower groups defined by the median SES of the entire sample. As depicted in the figure, higher SES was associated with greater brain system segregation in the earlier portion of middle age (ME, t(41) = 2.15, P = 0.038, 95% CI = [0.002, 0.070]), with a similar trend in the later portion of middle age (ML, t(83) = 1.62, P = 0.109, 95% CI = [−0.006, 0.060]). Significant SES-related differences were not present for system segregation in the youngest and oldest age groups (values of t < 1.05, values of P > 0.296, 95% CIs cross zero). We note that while these post hoc tests do not survive correction for multiple comparisons, they served to better understand the nature of the differing relationships that drove the SES by age interaction [see SI Appendix, Supplemental Results 1.2–1.3, for additional analyses including a broader range of middle age that matches previous work (40, 41)].
Fig. 2.
Lower SES adults exhibit reduced segregation of their resting-state functional brain networks and lower mean cortical thickness in middle-age adulthood. For each age group, brain system segregation (A) and mean cortical thickness (B) are plotted for higher and lower SES (stratified using a median split across the entire participant sample; error bars depict standard error of the mean). Higher SES is associated with greater brain system segregation and mean cortical thickness in middle-age groups (ME, 35–49 y; ML, 50–64 y). Primary statistical models were completed using general linear modeling, where SES was modeled continuously (see SES Moderates Age-Related Differences in Functional Network Organization and SES Moderates Differences in Brain Anatomy Across Age Groups for details).
In addition, examination of Fig. 2A also suggests that brain system segregation was comparable between lower SES adults at earlier middle age and higher SES adults at later middle age. Direct comparisons of these adults confirmed the observation: brain system segregation did not differ between lower SES ME (35–49 y) and higher SES ML (50–64 y: M (SD) = 0.50 (0.05) vs. 0.49 (0.07), respectively; t(60) = 0.79, P = 0.434, 95% CI = [−0.020, 0.046]). This comparison suggests that middle-aged adults of lower SES may exhibit earlier signs of brain aging; however, the observations should be interpreted with caution given the cross-sectional study design.
SES Moderates Differences in Brain Anatomy Across Age Groups.
A parallel set of analyses examined the relationship between SES and age on mean cortical thickness [adjusting for head size and controlling for measures of head motion in the scanner (69)]. Although the main effect of age was significant for mean cortical thickness [β = −0.58, t(299) = −12.15, P < 0.001], neither the main effect of SES [β = −0.08, t(299) = 0.52, P = 0.604] nor the SES by age interaction was significant for mean cortical thickness [β = −0.00007, t(299) = −0.61, P = 0.545]. However, when age was examined using the categorical divisions described above, a significant interaction emerged between SES and age group on mean cortical thickness [F(3,295) = 2.67, P = 0.048, = 0.03].
Consistent with the functional network observations, the SES and age group interaction was characterized by higher SES being associated with greater mean cortical thickness in the middle-age segments (35–64 y; Fig. 2B). Post hoc comparisons demonstrated that higher SES was associated with greater gray matter thickness in middle age (ME, t(41) = 2.58, P = 0.014, 95% CI = [0.017, 0.142]; ML, t(83) = 1.98, P = 0.050, 95% CI = [0.000, 0.090]), but not in younger or older age groups (values of t < 1.12, values of P > 0.271, 95% CIs cross zero). Also similar to the observations in brain system segregation, cortical thickness was comparable between lower SES adults at early middle age and higher SES adults at late middle age [M (SD) = 0.05 (0.10) vs. 0.04 (0.11), respectively; t(60) = 0.38, P = 0.703, 95% CI = [−0.046, 0.067]; also see SI Appendix, Supplemental Results 1.2 and 1.3, for additional related analyses].
SES by Age Interactions on Global Brain Measures Are Largely Maintained After Controlling for Participant Demographics, Health, or Cognitive Ability.
A broad collection of life factors varies in relation to an individual’s SES (1, 4, 9), increasing age (70, 71), or both (e.g., refs. 72 and 73). It is critical to examine whether individual differences in these variables might explain any of the described brain observations. Independent statistical models were constructed to include participants’ demographics, measures of physical health, measures of mental health, and cognitive ability, while simultaneously controlling for head motion in evaluating the SES by age group interactions on both system segregation and cortical thickness (see Table 2 for a summary).
Table 2.
Covariate analysis summary
| SES × age group (n = 304) | SES × age group controlling for childhood SES (n = 168) | |||||||
| Brain system segregation | Cortical thickness | Brain system segregation | Cortical thickness | |||||
| Covariates | F | P | F | P | F | P | F | P |
Head motion only |
3.16 | 0.025* | 2.67 | 0.048* | 2.85 | 0.039* | 0.65 | NS |
| Demographic + head motion | 2.47 | 0.062+ | 2.81 | 0.040* | 2.79 | 0.042* | 0.97 | NS |
| Physical health + head motion | 3.11 | 0.027* | 2.26 | 0.082+ | 2.58 | 0.056+ | 0.78 | NS |
| Mental health + head motion | 3.15 | 0.026* | 2.81 | 0.040* | 2.59 | 0.055+ | 1.22 | NS |
Fluid intelligence + head motion |
2.97 | 0.032* | 2.23 | 0.085+ | 2.73 | 0.046* | 0.56 | NS |
Long-term episodic memory + head motion |
3.41 | 0.018* | 2.67 | 0.048* | 2.98 | 0.033* | 0.68 | NS |
Summary of SES by age group interactions on brain system segregation and mean cortical thickness: controlling for multiple sets of covariates (left), controlling for covariates and childhood SES in a subsample of participants who had childhood-SES information available (right). Head motion refers to estimated measures of participants’ in-scanner head motion (mean FD); demographic variables include sex and race; physical health variables include BMI, SF-36 PCS, hypertension, smoker status, alcohol consumption, and chronic health issues; mental health variables include depressive symptoms and life satisfaction; fluid intelligence and episodic memory are factor scores generated from multiple tasks (see SI Appendix, Supplemental Methods). Asterisks denote statistical significance, *P < 0.05; crosses denote marginal effects, +P < 0.10; NS, not significant.
Controlling for gender and whether or not the participant was classified as a racial minority (i.e., self-reporting as nonwhite/Caucasian) revealed a marginally significant interaction between SES and age group (SES treated as a continuous variable) on brain system segregation [F(3,284) = 2.47, P = 0.062, = 0.03] and a significant interaction on cortical thickness [F(3,284) = 2.81, P = 0.040, = 0.03]. A statistical model incorporating differences in physical health included a broad range of available variables [i.e., SF-36 Physical Component Score (PCS), body mass index (BMI), hypertension, smoking status, alcohol consumption, and chronic physical health issues; see Materials and Methods and SI Appendix, Supplemental Methods, for description of each covariate]. The SES by age group interaction remained significant for brain system segregation [F(3,288) = 3.11, P = 0.027, = 0.03] and attenuated to marginal significance for cortical thickness [F(3,288) = 2.26, P = 0.082, = 0.02]. Finally, controlling for two mental health variables, depressive symptoms and subjective well-being, also did not qualitatively alter the SES by age group interaction on brain system segregation [F(3,292) = 3.15, P = 0.026, = 0.03] or cortical thickness [F(3,292) = 2.81, P = 0.040, = 0.03].
Broad domains of cognitive ability (e.g., episodic memory, fluid intelligence) vary across age (70, 74), SES (even in middle-age adulthood, the time window where we observed the strongest SES–brain effect; ref. 75), and in relation to brain anatomy (36, 76) and brain network organization (53). In light of these observations, it is important to determine whether the present SES–brain findings apply to individuals across levels of cognitive ability. Aggregate measures representing both long-term episodic memory and fluid processing (intelligence) were incorporated as covariates in statistical models testing for an interaction between SES and age group on brain anatomy and functional network organization. Controlling for episodic memory, the interaction remained significant for brain system segregation [F(3,294) = 3.41, P = 0.018, = 0.03] and cortical thickness [F(3,294) = 2.67, P = 0.048, = 0.03]; controlling for fluid intelligence, the SES by age group interaction remained significant for brain system segregation [F(3,294) = 2.97, P = 0.032, = 0.03] and was marginally significant for cortical thickness [F(3,294) = 2.23, P = 0.085, = 0.02].
Individual Differences in Childhood SES Do Not Explain SES-Related Brain Network Organization Differences in Adulthood.
Given the strong influence of childhood SES on brain maturation and function (i.e., shown in children and adults; refs. 5, 20, 57, 59, and 77), it is possible that the presently observed SES-related brain differences are a consequence of preestablished brain differences carrying forward from childhood. Information related to childhood SES (parental education) was available for a subsample of our study participants (n = 168). Childhood SES was defined as the highest educational degree completed by either parent (Materials and Methods). The childhood-SES measure was significantly associated with an individual’s present (“adult”) SES [F(6,161) = 2.94, P = 0.010, = 0.10], and also age group (χ2 = 43.78, P < 0.001), where lower present SES and older age were associated with having parents with less education (SI Appendix, Table S1).
Controlling for childhood SES, the interaction between the participants’ present SES and age group remained significant for brain system segregation [F(3,153) = 2.85, P = 0.039, = 0.05]. Furthermore, this SES by age group interaction on segregation largely remained significant, or showed very minor attenuation in effects, when controlling for childhood SES in conjunction with the numerous sets of covariates (Table 2 and SI Appendix, Supplemental Results 1.4). The interaction between SES and age group was not significant for cortical thickness when controlling for childhood SES [F(3,153) = 0.65, P = 0.583, = 0.01], and the interaction remained nonsignificant when controlling for additional covariates (Table 2).
It is possible that there exists a cumulative effect of an individual’s childhood SES and their present SES on their functional network organization and brain anatomy, as a function of age. When modeled together with age group, the main effect of childhood SES [values of F(6,145) ≤ 1.76, values of P > 0.111] and its interaction with age group did not significantly predict either brain measure [values of F(12,145) ≤ 1.13, values of P > 0.337]. Additionally, no significant interactions were observed between childhood SES, present SES, and age group for either brain measure [values of F(9,126) < 0.51, values of P > 0.863]. We note that a high collinearity was observed between childhood SES and age group, possibly influencing the observations involving childhood SES with respect to age (SI Appendix, Supplemental Results 1.5).
Discussion
SES moderated previously reported age-related differences in large-scale resting-state functional network organization. A similar but less robust interaction was evident between SES and age on brain anatomy. SES was operationalized as a combination of educational attainment and occupational socioeconomic characteristics, factors that have been previously shown to relate to health and well-being outcomes (1). Middle-aged participants with lower SES exhibited reduced segregation of the systems in their large-scale functional brain networks and thinner mean cortical gray matter, compared with higher SES individuals in an equivalent age range. Critically, the relationships between SES and both brain measures were largely present while controlling for differences in participant demographics, physical and mental health, and cognitive ability. A measure of childhood SES was available for a subset of participants and was included in statistical models: the interactions between an individual’s present (adult) SES and age persisted while controlling for childhood SES on brain system segregation. By measuring features of brain function and anatomy within individuals who have been densely sampled across the adult lifespan, these collective observations provide support for a compelling relationship between the socioeconomic aspects of the environment and the brain that is evident in middle-age adulthood.
SES Relates to Global Measures of Brain Function and Anatomy.
In addition to its adult lifespan focus, a unique aspect of the present work is the inclusion of a measure of large-scale functional network organization. Studies of SES and brain function have primarily examined task-related functional activity in specific regions of the brain and under specific task demands (8, 78, 79). Due to the specificity of these observations, it has been difficult to gain a broader understanding of SES–brain function results and their potential impact on cognitive ability. We hypothesized that SES should be associated with brain function across distributed and widespread regions, as SES represents broad environmental and lifestyle variation in a person’s life. Accordingly, we examined the relationship between SES and a brain measure that captures the brain’s large-scale functional network interactions (for additional follow-up analysis focusing on more specific system distinctions, see SI Appendix, Supplemental Results 1.6). The segregated organization of brain systems (i.e., subnetworks) observed in healthy young adults facilitates functional specialization and efficient communication across systems (80). Multiple studies have shown that brain systems exhibit less segregation with increasing age in healthy adults (33, 42, 43). Reduction in segregation is related to poorer cognitive performance (33) and differences in brain function (54) in older adults. In the present study, we found that SES moderates the relationship between age and brain system segregation. Specifically, lower SES individuals exhibited reduced system segregation in middle-age adulthood (35–64 y), compared with higher SES individuals. Critically, this relationship remained significant after controlling for cognitive abilities related to aging and system segregation.
A less segregated functional brain network reflects a “blurring” of distinct and modular systems of the brain. One of us (G.S.W.) has previously speculated that reduced brain system segregation may characterize or result in diminished resilience against focal brain damage (53). When brain systems are segregated, the impact of localized damage is more likely to be confined within specific brain systems (81–83), whereas comparable damage to a less segregated network should result in a failure of function that propagates across multiple systems. This idea echoes previously described “brain reserve” concepts (23) and frames brain reserve as a network property. In this view, individuals who exhibit lower system segregation earlier in adulthood (in the present case, lower SES individuals, on average) may be more prone to cognitive decline when faced with age-related neurodegeneration or insult. While the present interpretations are constrained by the correlational nature of the observations, it is possible that an individual’s SES either serves to protect, enhance, or deteriorate their brain network organization. We expand on these ideas in a subsequent section below. However, coupled with evidence demonstrating that higher measures of cognitive ability are associated with greater system segregation (33), the present results support the proposal that greater RSFC brain system segregation is associated with more positive aspects of an individual’s cognition, health, and environment (53).
SES moderated age-related differences in mean cortical thickness, although the relationship did not persist in the subsample analysis that included childhood SES as a covariate. Keeping in mind this limitation, there existed a positive association between SES and mean cortical thickness in middle age (35–64 y), paralleling the observations with brain system segregation. This adds to the evidence that an individual’s social–cultural environment interacts with their brain anatomy in middle-aged adults (age range, 30–54 y; ref. 40). Prior work has revealed SES-related anatomical differences in specific regions of the brain including those implicated in executive control (e.g., refs. 37 and 84), long-term episodic memory (e.g., refs. 38 and 85), and verbal ability (e.g., ref. 11) (for review, see ref. 9). While our focus here was on global measures of brain anatomy, we conducted additional analyses to examine regional SES–brain relationships, which revealed some parallels with previous work and also our present functional network observations (SI Appendix, Fig. S1 and Supplemental Results 1.6).
SES Modifies Age-Related Differences in the Brain During Middle-Age Adulthood.
The present observations suggest that SES is more directly related to resting-state brain system segregation compared with cortical thickness, especially in middle-age adulthood. Resting-state signals represent a statistical marker of experience-dependent coactivation patterns, which are sculpted across the lifespan (for review, see ref. 47). Our data support the view that an individual’s SES constitutes a valid experiential marker that relates to their brain, despite the potential coarseness of SES as an individual-difference measure. Conversely, given that SES-related anatomical differences did not survive inclusion of childhood SES (whereas functional network organization differences did), it appears that adult SES shares a considerable amount of variance with childhood SES, as measured by parental education, in relation to cortical thickness (but see ref. 86). An alternate but related possibility is that cortical anatomy is particularly prone to environmental modulation during specific phases of life (e.g., childhood and adolescence; ref. 59), whereas functional networks exhibit greater and continual sensitivity to SES-related modulation across an individual’s adulthood (87).
Neither younger nor older adults exhibited positive relationships between SES and either brain measure. It is important to point out that for both younger and older adults, the consensus of previous literature has yet to converge on a clear and consistent pattern of SES–brain anatomy relationships (e.g., for younger adults, see refs. 38 vs. 37 vs. 77; for older adults, see refs. 18 and 88 vs. 16 and 17); the relation between SES and large-scale functional network organization in any age segment has not been investigated, to the best of our knowledge. Keeping in mind the variability in observations, we consider additional possible reasons for the SES-related observations in younger and older age groups. First, it is possible that SES impacts volunteer status such that the lower SES participants are more highly selected in older adults (89, 90). This type of participant “survivorship” could result in a highly selected sample of lower SES elderly who are extremely healthy, therefore exhibiting similar or even better (e.g., ref. 17) measures of the brain compared with higher SES individuals, for whom poor health is better managed and often manifests later in life (73). In fact, a trend for a negative relationship between SES and system segregation was present in our older adult sample (SI Appendix, Supplemental Results 1.2). While a negative relationship was not present when comparing SES to mean cortical thickness in our older adult sample, this direction of relationship has been reported in previous studies of brain anatomy (16, 17). Second, in the present sample, it is possible that both older and younger adult groups exhibited unique differences in basic feature of their data (e.g., sample size, distribution of SES, distribution of the two brain measures) that limited detection of SES-related differences. However, close inspection of the data suggests that this is not the case (SI Appendix, Supplemental Results 1.7). Despite the similarities of SES across groups and the fact that we actively attempted to recruit community-dwelling individuals across a wide range of SES, it is entirely possible that greater SES-related brain differences would be identified with a greater range of SES sampling (i.e., particularly focusing on individuals living in poverty; refs. 91 and 92). Third, education and occupation may be more poorly suited for capturing SES effects in younger and older cohorts. Younger adulthood is a time during which occupation and financial stability are typically highly dynamic, thus prohibiting reliable assessment of SES. Conversely, assessment of preretirement occupation for older adults may not adequately capture an individual’s environment postretirement (financially and socially; see ref. 93 for discussion). Finally, it is important to acknowledge and consider the possible cohort effects that can exist in cross-sectional studies. Group-related differences in the present study may reflect generational differences relevant to SES [e.g., cross-generational differences in SES mobility (94), differences in various aspects of the social–cultural environments, and other objective/subjective measures of SES that relate to individuals from different generations (SI Appendix, Supplemental Results 1.1)]. Of course, these alternative explanations are not mutually exclusive and may all contribute to the present set of observations.
Possible Mediators Underlying the SES–Brain Relationship in Adulthood.
It has been proposed that an individual’s sustained experience over the course of their life continuously sculpts his or her brain structure and function (29). These ideas are consistent with studies in rodents (for review, see ref. 25) and primates (e.g., ref. 24), which have demonstrated how living in enriched and complex environments can induce changes in brain structure (e.g., neurogenesis, increased dendritic tree complexity and dendritic spine density) and function (e.g., ref. 95). In humans, SES is one measure that represents aspects of “sustained experience,” combining objective (e.g., education and income) and subjective factors (e.g., social standing), which influence an individual’s environment across their lifespan (4). There are multiple paths by which SES may interact with the brain, including but not limited to (i) mediating difference in physical health promoted by an individual’s access to resources including adequate healthcare, nutrition, and leisurely exercise (4, 96, 97); (ii) representing engagement in activities related to continuous and sustained learning (25, 29, 98, 99); and (iii) signaling exposure to environmental and social stressors (30, 100, 101).
To unpack this idea further, sustained experience over the life course can exert both positive and negative impacts on the brain (29, 30). While our results parallel previous findings that conceptualize higher SES as a form of reserve (i.e., conferring protection to the typical course of brain changes that accompany aging; refs. 23 and 102), it is conceivable that instead of higher SES enhancing or protecting brain function and anatomy, environmental aspects of lower SES are a detrimental factor that accelerate the course of brain aging. Specifically, the factors correlated with lower SES, including inadequate (healthy) nutrition, limited access to decent healthcare, and a less stimulating environment, are detrimental to maintaining a healthy brain (for reviews, see refs. 12 and 31). Relatedly, individuals with lower SES may systematically experience greater amount of continual stress related to their environment in comparison with higher SES individuals resulting in allostatic load (for review, see ref. 103), a factor known to result from chronic dysregulation of physiological systems (e.g., neuroendocrine, immune, and autonomic nervous function; refs. 104 and 105) and impact brain anatomy (e.g., refs. 106 and 107). While the brain observations reported here would look identical under either of these scenarios, this latter possibility suggests an entirely different mechanism to the presently reported SES–brain relationships.
Relevant evidence has been provided from studies that have examined the relationship between neighborhood-level factors and brain anatomy. For example, a recent study of middle-aged adults (30–54 y) revealed a relationship between community-level socioeconomic factors and cortical anatomy (40). Reduced cortical volume and greater community disadvantage was mediated by cardiovascular risk and neuroendocrine function. This finding aligned with an earlier study of middle-age adulthood (35–64 y) demonstrating that the relationship between neighborhood level deprivation and regional anatomical differences (cortical thickness in supramarginal gyrus, superior temporal gyrus, and lateral sulcus; described as “Wernicke’s area,” and its right homolog) was mediated by an inflammation factor (a cardiometabolic risk marker) (41). These studies reveal how the relationship between socioeconomic factors and brain anatomy may be mediated by both neuroendocrine and cardiometabolic pathways. While the present study controlled for measures of physical health in each individual (i.e., BMI, hypertension), other physiological risk factors that were not surveyed may be found to mediate the relationship between an individual’s SES and their brain.
Although the design of the current study could not distinguish the possible difference between “protective” higher SES vs. “harmful” lower SES, the results demonstrate that SES is an important factor to account for when investigating age-related differences in the brain. Furthermore, as the stratification of SES in our study is not defined by a specific threshold (e.g., the poverty line), our results suggest that the relationship between SES and the brain in middle-aged adults may exhibit a gradient pattern where individuals with relatively higher SES reap environmental benefits compared with those with lower SES, similar to SES gradients in health and cognition (1, 108). Altogether, SES may relate to the aging brain in multiple ways. Whereas engaging and resourceful environments associated with higher SES may provide a buffer (or delay) against aging, inadequate health conditions associated with lower SES environments (e.g., exposure to toxins, poorer nutrition) together with continual stress may accelerate the aging process (e.g., refs. 30, 100, and 106) and/or make the brain more vulnerable to damage.
Limitations and Future Directions.
It is important to acknowledge that while considerable effort was placed to recruit participants with lower SES, the necessary exclusion criteria of the study can still result in a relatively selective sample that prohibits examination of a broader range of SES due to the relation between SES and certain exclusion criteria (e.g., BMI, psychiatric disorders; refs. 109 and 110).
In addition, it is possible that some of the brain differences observed across participants in middle age relate to their environment during childhood and adolescence. We attempted to control for this possible source of influence using a subsample of participants with childhood-SES data. Childhood SES (when modeled together with age) did not exhibit a significant relationship with either brain measure, and there was an absence of any three-way interactions between an individual’s childhood SES, their present SES, and age on either brain measure. However, given the limitations in both the childhood-SES measure and number of participants with available childhood-SES data, additional work is needed to further understand the possible cumulative and interactive relationships between childhood SES and present SES on the brain.
Importantly, the cross-sectional design of this study prohibits conclusions to be made regarding age-related changes in brain function and anatomy within an individual as a function of their SES. In keeping with this, and as noted earlier, we cannot rule out the possibility of cohort-specific effects. Cross-cohort comparisons enable the examination of age-related differences across a broad segment of ages, but these differences in age are also inherently tied to differences in the sociocultural environments under which the individuals were born and raised (e.g., propensity for SES mobility, wartime, times of broad economic hardship). Relatedly, the correlational aspects of analysis also prohibit conclusions about SES–brain directionality. For example, it is entirely possible that differences in brain functional organization or anatomy earlier in the lifespan modify the SES of middle-aged or older participants. The SES observations reported here motivate future studies examining brain aging using longitudinal designs to investigate whether SES moderates the rate and direction of brain change across the adult lifespan and, critically, how these changes relate to changes in cognition.
Given the relation between SES and both brain measures during middle age, a promising goal for future studies is to dissect and even manipulate the features that are represented by SES and its relation to the brain in adults. As SES represents a complex and multifaceted construct, it is extremely difficult to manipulate as a whole (e.g., ref. 111). However, there are numerous opportunities to influence certain aspects of an individual’s environment that may relate to the features differentiated by SES. These interventions include programs designed to enhance social and cognitive engagement (98, 112), increase active learning (100), reduce stress (113), and improve physical fitness (28, 97). While many of these interventional studies have targeted older adults, our results suggest that expanding the focus to middle age is an important goal.
Conclusion
An individual’s SES, defined by educational attainment and occupational socioeconomic characteristics, relates to their functional network organization and brain anatomy across broad segments of life. Compared with middle-aged adults from higher SES, lower SES adults exhibited signs of both functional and structural brain aging earlier in adulthood. Prevailing work has highlighted the impact that SES has on brain and cognition in both childhood but also advanced age. We provide evidence that there exists a powerful relationship between an individual’s present environment and their brain well beyond these specific segments of life.
Materials and Methods
Participants.
Participants were recruited from the Dallas–Fort Worth community and provided written consent before participating. Study procedures were reviewed and approved by the Institutional Review Boards at the University of Texas at Dallas and the University of Texas Southwestern Medical Center. Participants were part of the Dallas Lifespan Brain Study (DLBS), a study designed to examine the effects of healthy aging on brain and cognition. The current study includes data from participants that completed both an anatomical and a resting-state fMRI scan, and reported their occupation/occupation-before-retirement in their demographic survey (n = 359; age range, 20–89 y; mean age, 59.79; SD, 16.89).
The entire participant sample shares the exclusion criteria typically employed in studies involving MRI: BMI of >35, loss of consciousness of >10 min, radiation/chemotherapy in the last 5 years, various diseases (e.g., epilepsy, lupus, stroke, multiple sclerosis, Parkinson’s, Alzheimer’s) or major psychiatric disorders [e.g., manic–depressive (bipolar) disorder, schizophrenia, depression (including if individuals were in remission without treatment for >6 mo), attention deficit or learning disorder (including if the disorder was resolved by high school graduation)], electroshock therapy for depression, brain surgery, excessive alcohol or caffeine consumption (self-report), blood pressure of >160/90, unprescribed/illegal drug use, use of sedatives, use of benzodiazepines, use of antipsychotics, coronary bypass, and an MMSE (114) score of ≤25. Despite these restrictions, an important feature of the DLBS is that it includes recruitment of participants who more broadly represent the education level and health condition of middle-aged and older adults in the general population. The recruitment of this subset of participants targeted individuals that were 50 y and older with lower educational attainment (i.e., no college degree; ≤14 y of formal education); exceptions were made for individuals with education of 15 or more years, if they also have a chronic health condition (e.g., hypertension, diabetes, cancer, or heart surgery).
Brain imaging data were subjected to substantial quality control to remove participants with poor data quality or excessive head movement (see below); 304 participants remained in the final study sample. Participants were separated into four age groups for the identification of age group-specific functional brain systems (see below), and the statistical analyses used to further understand the continuous SES by age interaction. Each age group contained 15 y until the typical cutoff age for older adults that corresponds to the average age of retirement in the United States (65 y): YA, 20–34 y; ME, 35–49 y; ML, 50–64 y; OA, 65–89 y. See Table 1 for detailed demographic information per age group.
SES was assessed, on average, within 3 wk of the MRI scan (mean time, 22 d; range, 1–205 d; 236 out of 304 participants were tested within 30 d). Accordingly, all participants’ anatomical and functional neuroimaging data were collected in close proximity to the measurement of SES-related variables (education and occupation or occupation before retirement).
Experimental Design and Data Acquisition.
The DLBS consists of multiple data acquisition sessions of cognitive and neuropsychological testing, MRI scanning, as well as take-home surveys on demographic and psychosocial data. All functional and anatomical MRI scans were acquired on a Phillips Achieva 3T scanner. Brief descriptions of acquisition and processing of structural and functional images are provided below; additional details are available in SI Appendix, Supplemental Methods.
Structural Imaging Acquisition and Preprocessing.
For each participant, a T1-weighted magnetization-prepared rapid-acquisition gradient echo structural image was obtained (repetition time, 8.1 ms; echo time, 3.7 ms; flip angle, 12°; field of view, 204 × 256 mm; 160 slices with 1 × 1 × 1-mm voxels). The volumetric image was used to construct the participant’s cortical surface, which was then deformed to the fsaverage surface using FreeSurfer, version 5.3 (115, 116). The left and right fsaverage surfaces were then registered to a hybrid left-right fsaverage atlas (fs_LR; ref. 117).
Resting-State fMRI Acquisition and Preprocessing.
Participants completed an eye-open fixation resting-state blood oxygen level-dependent (BOLD) scan. The functional images went through standard fMRI preprocessing to reduce artifacts (e.g., slice-timing correction, realignment). Then, RSFC preprocessing was used to reduce spurious variance unlikely to reflect neuronal activity: (i) demean and detrending; (ii) multiple regression of the BOLD data to remove variance related to the whole-brain signal, ventricular signal, white matter signal, their derivatives, and the “Friston24” motion regressors (118); and (iii) bandpass filtering (0.009–0.08 Hz). Last, motion-contaminated resting-state fMRI volumes [i.e., if frame displacement (FD) > 0.3 mm] were flagged (“scrubbing”), discarded, and interpolated for subsequent nuisance regression and bandpass filtering (119). Preprocessed resting-state data were registered to the fs_LR (32 k) left and right hemisphere surfaces for analysis (116).
Brain Graph Construction and Brain System Assignments.
Nodes and edges definition.
RSFC brain graphs were constructed using a modified set of published nodes applied to the surface-mapped resting-state data (Fig. 1A) (33). The cross-correlation of each node’s mean time course was incorporated into a node-to-node correlation matrix with Fisher’s z-transformed correlation values for each participant (Fig. 1B; for details, see SI Appendix, Supplemental Methods). Due to possible artifactual negative correlations (120, 121) introduced by a necessary processing step used to ensure removal of motion-related artifacts (119, 122), negative z values were excluded from the data matrix in accordance with past studies (33, 123).
Age group-specific functional brain system assignments.
To eliminate biases introduced by using younger adult-defined brain systems (123), which may provide a better fit for the younger compared with the older adults, large-scale functional brain systems were identified in each age group by applying Infomap community detection (124) to bootstrapped, thresholded mean matrices of each age group (2–10% edge density, 1,000 iterations). The final system assignment was based on the most common (modal) assignment across 2–10% edge density (see assignments for younger adults in Fig. 1A). The community assignments were labeled based on their overlap with a set of published RSFC functional systems (123). While age was treated as a continuous variable in the primary analyses, follow-up analyses treated age as a categorical variable to maintain the same level of granularity used in calculating system segregation from age group-specific system assignments.
Measures.
Brain measures.
Measure of functional network organization: System segregation score.
System segregation (33) takes the differences in mean within-system and mean between-system correlations (Fig. 1B) as a proportion of mean within-system correlation, as noted in the following formula:
where is the mean Fisher’s z-transformed Pearson’s correlation (z) across nodes within the same system, and is the mean z between nodes of one system to all nodes in other systems. Values along the diagonal of the matrix were not included. Importantly, the measure of system segregation retains the weight of all positive edges in a graph, allowing weak connections to contribute to the characterization of system interactions.
Measure of anatomical brain structure: Cortical thickness.
Cortical thickness was measured for each participant by estimating the distance between the pial and white matter surfaces at every brain vertex generated by FreeSurfer following quality-control procedures, which included manual editing to correct for brain segmentation errors (Fig. 1 C and D and ref.69). The mean whole-brain cortical thickness was calculated by averaging across the left and right hemispheres’ average cortical thickness values. All statistical analyses were conducted on mean cortical thickness that was adjusted for intracranial volume (ICV) (obtained from automated FreeSurfer output): ICV-adjusted cortical thickness.
SES.
Using a combination of estimated years of education and occupational socioeconomic index (68), an SES score was derived for each participant (125). Years of education and occupational socioeconomic index were significantly correlated [r(302) = 0.49, P < 0.001]. Principal component analysis was used to extract a common factor between the two variables, with the first factor score representing SES (75% variance explained by the first factor).
Education attainment.
Years of education were estimated by degree completion (e.g., high school/GED, college, graduate school) plus the extra years of education beyond the completed degree that were self-reported. Extra education years were capped to be one less than the next possible degree. For example, a participant with 5 additional years after a high school degree (estimated to take 12 y) but never completing college would have an estimated 15 y of education (1 y less than completion of college, which is estimated to take 16 y). The maximum years of education was capped at 22 (e.g., PhD, MD).
Occupational socioeconomic characteristics.
Participants’ self-reported occupation (current or preretirement) was matched to a corresponding US census occupation code, and then assigned a gender-specific socioeconomic index score derived from predicted prestige score (i.e., composite score based on estimated occupational wages, occupational education, and a wage–occupation–prestige index; ref. 68). While this index is somewhat subjective, among our participants with additional SES measures (n = 168), it is correlated with weighted household income [r(166) = 0.27, P < 0.001] and the MacArthur’s scale of subjective socioeconomic status (126) [r(166) = −0.22, P = 0.004; see SI Appendix, Supplemental Results 1.1, for more details]. If an occupation was not listed, the code from the most related job was used. Consensus of coding for nonlisted occupations was established between two coders (M.Y.C. and J.N.). Since full-time student and homemaker status do not provide a clear approximation of occupation-related income and prestige, participants listing their occupation as student (n = 19) or homemaker (n = 5) were not included in the current study.
Childhood SES.
For a subset of participants with available information (n = 168), parental education was used to determine their SES during childhood. Information regarding parental occupation was not available. For analyses controlling for childhood SES as a covariate, participants’ childhood SES was defined by the highest degree either parent completed, coded as a seven-level categorical variable (SI Appendix, Table S1).
Measures of demographics, health, and cognitive ability.
An extensive set of measures was used as covariates to control for participants’ demographics, health (physical and mental), and cognition. For each set of variables, statistical models were created to examine the relationship between SES and age with respect to each of the brain measures while controlling for the corresponding covariates of interest. A summary of each set of covariates is provided in SI Appendix, Supplemental Methods.
Supplementary Material
Acknowledgments
This work was supported by the James S. McDonnell Foundation (G.S.W.) and NIH Grant 5R37AG-006265-30 (to D.C.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. P.J.G. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1714021115/-/DCSupplemental.
References
- 1.Adler NE, et al. Socioeconomic status and health. The challenge of the gradient. Am Psychol. 1994;49:15–24. doi: 10.1037//0003-066x.49.1.15. [DOI] [PubMed] [Google Scholar]
- 2.Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: Concepts, methodologies, and guidelines. Annu Rev Public Health. 1997;18:341–378. doi: 10.1146/annurev.publhealth.18.1.341. [DOI] [PubMed] [Google Scholar]
- 3.Adler NE, Rehkopf DH. U.S. disparities in health: Descriptions, causes, and mechanisms. Annu Rev Public Health. 2008;29:235–252. doi: 10.1146/annurev.publhealth.29.020907.090852. [DOI] [PubMed] [Google Scholar]
- 4.Hurst L, et al. Lifetime socioeconomic inequalities in physical and cognitive aging. Am J Public Health. 2013;103:1641–1648. doi: 10.2105/AJPH.2013.301240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo Y, Waite LJ. The impact of childhood and adult SES on physical, mental, and cognitive well-being in later life. J Gerontol B Psychol Sci Soc Sci. 2005;60:S93–S101. doi: 10.1093/geronb/60.2.s93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinquart M, Sörensen S. Influences of socioeconomic status, social network, and competence on subjective well-being in later life: A meta-analysis. Psychol Aging. 2000;15:187–224. doi: 10.1037//0882-7974.15.2.187. [DOI] [PubMed] [Google Scholar]
- 7.Hackman DA, Farah MJ. Socioeconomic status and the developing brain. Trends Cogn Sci. 2009;13:65–73. doi: 10.1016/j.tics.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raizada RD, Richards TL, Meltzoff A, Kuhl PK. Socioeconomic status predicts hemispheric specialisation of the left inferior frontal gyrus in young children. Neuroimage. 2008;40:1392–1401. doi: 10.1016/j.neuroimage.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farah MJ. The neuroscience of socioeconomic status: Correlates, causes, and consequences. Neuron. 2017;96:56–71. doi: 10.1016/j.neuron.2017.08.034. [DOI] [PubMed] [Google Scholar]
- 10.Hedman AM, van Haren NE, Schnack HG, Kahn RS, Hulshoff Pol HE. Human brain changes across the life span: A review of 56 longitudinal magnetic resonance imaging studies. Hum Brain Mapp. 2012;33:1987–2002. doi: 10.1002/hbm.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noble KG, Houston SM, Kan E, Sowell ER. Neural correlates of socioeconomic status in the developing human brain. Dev Sci. 2012;15:516–527. doi: 10.1111/j.1467-7687.2012.01147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hackman DA, Farah MJ, Meaney MJ. Socioeconomic status and the brain: Mechanistic insights from human and animal research. Nat Rev Neurosci. 2010;11:651–659. doi: 10.1038/nrn2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mackey AP, et al. Neuroanatomical correlates of the income-achievement gap. Psychol Sci. 2015;26:925–933. doi: 10.1177/0956797615572233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hair NL, Hanson JL, Wolfe BL, Pollak SD. Association of child poverty, brain development, and academic achievement. JAMA Pediatr. 2015;169:822–829. doi: 10.1001/jamapediatrics.2015.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim P, et al. Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. Proc Natl Acad Sci USA. 2013;110:18442–18447. doi: 10.1073/pnas.1308240110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coffey CE, Saxton JA, Ratcliff G, Bryan RN, Lucke JF. Relation of education to brain size in normal aging: Implications for the reserve hypothesis. Neurology. 1999;53:189–196. doi: 10.1212/wnl.53.1.189. [DOI] [PubMed] [Google Scholar]
- 17.Fotenos AF, Mintun MA, Snyder AZ, Morris JC, Buckner RL. Brain volume decline in aging: Evidence for a relation between socioeconomic status, preclinical Alzheimer disease, and reserve. Arch Neurol. 2008;65:113–120. doi: 10.1001/archneurol.2007.27. [DOI] [PubMed] [Google Scholar]
- 18.Foubert-Samier A, et al. Education, occupation, leisure activities, and brain reserve: A population-based study. Neurobiol Aging. 2012;33:423.e15-25. doi: 10.1016/j.neurobiolaging.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 19.Kim JP, et al. Effects of education on aging-related cortical thinning among cognitively normal individuals. Neurology. 2015;85:806–812. doi: 10.1212/WNL.0000000000001884. [DOI] [PubMed] [Google Scholar]
- 20.Staff RT, et al. Childhood socioeconomic status and adult brain size: Childhood socioeconomic status influences adult hippocampal size. Ann Neurol. 2012;71:653–660. doi: 10.1002/ana.22631. [DOI] [PubMed] [Google Scholar]
- 21.Bennett DA, et al. Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology. 2003;60:1909–1915. doi: 10.1212/01.wnl.0000069923.64550.9f. [DOI] [PubMed] [Google Scholar]
- 22.Yaffe K, et al. Health ABC Study Effect of socioeconomic disparities on incidence of dementia among biracial older adults: Prospective study. BMJ. 2013;347:f7051. doi: 10.1136/bmj.f7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012;11:1006–1012. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kozorovitskiy Y, et al. Experience induces structural and biochemical changes in the adult primate brain. Proc Natl Acad Sci USA. 2005;102:17478–17482. doi: 10.1073/pnas.0508817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- 26.Landau SM, et al. Association of lifetime cognitive engagement and low β-amyloid deposition. Arch Neurol. 2012;69:623–629. doi: 10.1001/archneurol.2011.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lövdén M, et al. Experience-dependent plasticity of white-matter microstructure extends into old age. Neuropsychologia. 2010;48:3878–3883. doi: 10.1016/j.neuropsychologia.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 28.Voss MW, et al. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front Aging Neurosci. 2010;2:32. doi: 10.3389/fnagi.2010.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reuter-Lorenz PA, Park DC. How does it STAC up? Revisiting the scaffolding theory of aging and cognition. Neuropsychol Rev. 2014;24:355–370. doi: 10.1007/s11065-014-9270-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sapolsky RM. Social status and health in humans and other animals. Annu Rev Anthropol. 2004;33:393–418. [Google Scholar]
- 31.Tost H, Champagne FA, Meyer-Lindenberg A. Environmental influence in the brain, human welfare and mental health. Nat Neurosci. 2015;18:1421–1431. doi: 10.1038/nn.4108. [DOI] [PubMed] [Google Scholar]
- 32.Cabeza R. Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychol Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- 33.Chan MY, Park DC, Savalia NK, Petersen SE, Wig GS. Decreased segregation of brain systems across the healthy adult lifespan. Proc Natl Acad Sci USA. 2014;111:E4997–E5006. doi: 10.1073/pnas.1415122111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Damoiseaux JS, et al. Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex. 2008;18:1856–1864. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- 35.Park DC, et al. Aging reduces neural specialization in ventral visual cortex. Proc Natl Acad Sci USA. 2004;101:13091–13095. doi: 10.1073/pnas.0405148101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raz N, Rodrigue KM. Differential aging of the brain: Patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev. 2006;30:730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang J, et al. Regional gray matter volume mediates the relationship between family socioeconomic status and depression-related trait in a young healthy sample. Cogn Affect Behav Neurosci. 2016;16:51–62. doi: 10.3758/s13415-015-0371-6. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, et al. Pathway to neural resilience: Self-esteem buffers against deleterious effects of poverty on the hippocampus. Hum Brain Mapp. 2016;37:3757–3766. doi: 10.1002/hbm.23273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Butterworth P, Cherbuin N, Sachdev P, Anstey KJ. The association between financial hardship and amygdala and hippocampal volumes: Results from the PATH through life project. Soc Cogn Affect Neurosci. 2012;7:548–556. doi: 10.1093/scan/nsr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gianaros PJ, et al. Community socioeconomic disadvantage in midlife relates to cortical morphology via neuroendocrine and cardiometabolic pathways. Cereb Cortex. 2017;27:460–473. doi: 10.1093/cercor/bhv233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krishnadas R, et al. Socioeconomic deprivation and cortical morphology: Psychological, social, and biological determinants of ill health study. Psychosom Med. 2013;75:616–623. doi: 10.1097/PSY.0b013e3182a151a7. [DOI] [PubMed] [Google Scholar]
- 42.Betzel RF, et al. Changes in structural and functional connectivity among resting-state networks across the human lifespan. Neuroimage. 2014;102:345–357. doi: 10.1016/j.neuroimage.2014.07.067. [DOI] [PubMed] [Google Scholar]
- 43.Geerligs L, Renken RJ, Saliasi E, Maurits NM, Lorist MM. A brain-wide study of age-related changes in functional connectivity. Cereb Cortex. 2015;25:1987–1999. doi: 10.1093/cercor/bhu012. [DOI] [PubMed] [Google Scholar]
- 44.Raz N, et al. Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- 45.Salat DH, et al. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- 46.Bullmore E, Sporns O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- 47.Wig GS, Schlaggar BL, Petersen SE. Concepts and principles in the analysis of brain networks. Ann N Y Acad Sci. 2011;1224:126–146. doi: 10.1111/j.1749-6632.2010.05947.x. [DOI] [PubMed] [Google Scholar]
- 48.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 49.Raichle ME. The restless brain: How intrinsic activity organizes brain function. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140172. doi: 10.1098/rstb.2014.0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Finn AS, et al. Functional brain organization of working memory in adolescents varies in relation to family income and academic achievement. Dev Sci. 2017;20:e12450. doi: 10.1111/desc.12450. [DOI] [PubMed] [Google Scholar]
- 51.Sheridan MA, How J, Araujo M, Schamberg MA, Nelson CA. What are the links between maternal social status, hippocampal function, and HPA axis function in children? Dev Sci. 2013;16:665–675. doi: 10.1111/desc.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petersen SE, Sporns O. Brain networks and cognitive architectures. Neuron. 2015;88:207–219. doi: 10.1016/j.neuron.2015.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wig GS. Segregated systems of human brain networks. Trends Cogn Sci. 2017;21:981–996. doi: 10.1016/j.tics.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 54.Chan MY, Alhazmi FH, Park DC, Savalia NK, Wig GS. Resting-state network topology differentiates task signals across the adult life span. J Neurosci. 2017;37:2734–2745. doi: 10.1523/JNEUROSCI.2406-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gallen CL, et al. Modular brain network organization predicts response to cognitive training in older adults. PLoS One. 2016;11:e0169015. doi: 10.1371/journal.pone.0169015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lawson GM, Duda JT, Avants BB, Wu J, Farah MJ. Associations between children’s socioeconomic status and prefrontal cortical thickness. Dev Sci. 2013;16:641–652. doi: 10.1111/desc.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Romeo RR, et al. Socioeconomic status and reading disability: Neuroanatomy and plasticity in response to intervention. Cereb Cortex. June 7, 2017 doi: 10.1093/cercor/bhx131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Noble KG, et al. Hippocampal volume varies with educational attainment across the life-span. Front Hum Neurosci. 2012;6:307. doi: 10.3389/fnhum.2012.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Noble KG, et al. Family income, parental education and brain structure in children and adolescents. Nat Neurosci. 2015;18:773–778. doi: 10.1038/nn.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Velsen EF, et al. Brain cortical thickness in the general elderly population: The Rotterdam scan study. Neurosci Lett. 2013;550:189–194. doi: 10.1016/j.neulet.2013.06.063. [DOI] [PubMed] [Google Scholar]
- 61.Kennedy KM, et al. Age-related differences in regional brain volumes: A comparison of optimized voxel-based morphometry to manual volumetry. Neurobiol Aging. 2009;30:1657–1676. doi: 10.1016/j.neurobiolaging.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rosas HD, et al. Regional and progressive thinning of the cortical ribbon in Huntington’s disease. Neurology. 2002;58:695–701. doi: 10.1212/wnl.58.5.695. [DOI] [PubMed] [Google Scholar]
- 63.Cardinale F, et al. Validation of FreeSurfer-estimated brain cortical thickness: Comparison with histologic measurements. Neuroinformatics. 2014;12:535–542. doi: 10.1007/s12021-014-9229-2. [DOI] [PubMed] [Google Scholar]
- 64.Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science. 1997;278:412–419. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- 65.Pakkenberg B, Gundersen HJ. Neocortical neuron number in humans: Effect of sex and age. J Comp Neurol. 1997;384:312–320. [PubMed] [Google Scholar]
- 66.Terry RD, DeTeresa R, Hansen LA. Neocortical cell counts in normal human adult aging. Ann Neurol. 1987;21:530–539. doi: 10.1002/ana.410210603. [DOI] [PubMed] [Google Scholar]
- 67.Querbes O, et al. Alzheimer’s Disease Neuroimaging Initiative Early diagnosis of Alzheimer’s disease using cortical thickness: Impact of cognitive reserve. Brain. 2009;132:2036–2047. doi: 10.1093/brain/awp105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hauser RM, Warren JR. Socioeconomic indexes for occupations: A review, update, and critique. Sociol Methodol. 1997;27:177–298. [Google Scholar]
- 69.Savalia NK, et al. Motion-related artifacts in structural brain images revealed with independent estimates of in-scanner head motion. Hum Brain Mapp. 2017;38:472–492. doi: 10.1002/hbm.23397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baltes PB, Lindenberger U. Emergence of a powerful connection between sensory and cognitive functions across the adult life span: A new window to the study of cognitive aging? Psychol Aging. 1997;12:12–21. doi: 10.1037//0882-7974.12.1.12. [DOI] [PubMed] [Google Scholar]
- 71.Steptoe A, Deaton A, Stone AA. Subjective wellbeing, health, and ageing. Lancet. 2015;385:640–648. doi: 10.1016/S0140-6736(13)61489-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.House JS, Lantz PM, Herd P. Continuity and change in the social stratification of aging and health over the life course: Evidence from a nationally representative longitudinal study from 1986 to 2001/2002 (Americans’ changing lives study) J Gerontol B Psychol Sci Soc Sci. 2005;60:15–26. doi: 10.1093/geronb/60.special_issue_2.s15. [DOI] [PubMed] [Google Scholar]
- 73.House JS, et al. The social stratification of aging and health. J Health Soc Behav. 1994;35:213–234. [PubMed] [Google Scholar]
- 74.Park DC, et al. Models of visuospatial and verbal memory across the adult life span. Psychol Aging. 2002;17:299–320. [PubMed] [Google Scholar]
- 75.Turrell G, et al. Socioeconomic position across the lifecourse and cognitive function in late middle age. J Gerontol B Psychol Sci Soc Sci. 2002;57:S43–S51. doi: 10.1093/geronb/57.1.s43. [DOI] [PubMed] [Google Scholar]
- 76.Fjell AM, Walhovd KB. Structural brain changes in aging: Courses, causes and cognitive consequences. Rev Neurosci. 2010;21:187–221. doi: 10.1515/revneuro.2010.21.3.187. [DOI] [PubMed] [Google Scholar]
- 77.Yu Q, et al. Socioeconomic status and hippocampal volume in children and young adults. Dev Sci. 2018;21:e12561. doi: 10.1111/desc.12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Muscatell KA, et al. Social status modulates neural activity in the mentalizing network. Neuroimage. 2012;60:1771–1777. doi: 10.1016/j.neuroimage.2012.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shaywitz SE, et al. Neural systems for compensation and persistence: Young adult outcome of childhood reading disability. Biol Psychiatry. 2003;54:25–33. doi: 10.1016/s0006-3223(02)01836-x. [DOI] [PubMed] [Google Scholar]
- 80.Sporns O, Betzel RF. Modular brain networks. Annu Rev Psychol. 2016;67:613–640. doi: 10.1146/annurev-psych-122414-033634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.He BJ, et al. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron. 2007;53:905–918. doi: 10.1016/j.neuron.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 82.Nomura EM, et al. Double dissociation of two cognitive control networks in patients with focal brain lesions. Proc Natl Acad Sci USA. 2010;107:12017–12022. doi: 10.1073/pnas.1002431107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Siegel JS, et al. Disruptions of network connectivity predict impairment in multiple behavioral domains after stroke. Proc Natl Acad Sci USA. 2016;113:E4367–E4376. doi: 10.1073/pnas.1521083113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gianaros PJ, et al. Perigenual anterior cingulate morphology covaries with perceived social standing. Soc Cogn Affect Neurosci. 2007;2:161–173. doi: 10.1093/scan/nsm013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Janowitz D, et al. Genetic, psychosocial and clinical factors associated with hippocampal volume in the general population. Transl Psychiatry. 2014;4:e465. doi: 10.1038/tp.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cavanagh J, et al. Socioeconomic status and the cerebellar grey matter volume. Data from a well-characterised population sample. Cerebellum. 2013;12:882–891. doi: 10.1007/s12311-013-0497-4. [DOI] [PubMed] [Google Scholar]
- 87.Biswal BB, et al. Toward discovery science of human brain function. Proc Natl Acad Sci USA. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu Y, et al. AddNeuroMed Consortium Education increases reserve against Alzheimer’s disease–evidence from structural MRI analysis. Neuroradiology. 2012;54:929–938. doi: 10.1007/s00234-012-1005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bassuk SS, Berkman LF, Amick BC., 3rd Socioeconomic status and mortality among the elderly: Findings from four US communities. Am J Epidemiol. 2002;155:520–533. doi: 10.1093/aje/155.6.520. [DOI] [PubMed] [Google Scholar]
- 90.Huisman M, et al. Socioeconomic inequalities in mortality among elderly people in 11 European populations. J Epidemiol Community Health. 2004;58:468–475. doi: 10.1136/jech.2003.010496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rank MR, Hirschl TA. The likelihood of poverty across the American adult life span. Soc Work. 1999;44:201–216. [Google Scholar]
- 92.Sandoval DA, Rank MR, Hirschl TA. The increasing risk of poverty across the American life course. Demography. 2009;46:717–737. doi: 10.1353/dem.0.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Grundy E, Holt G. The socioeconomic status of older adults: How should we measure it in studies of health inequalities? J Epidemiol Community Health. 2001;55:895–904. doi: 10.1136/jech.55.12.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Biblarz TJ, Bengtson VL, Bucur A. Social mobility across three generations. J Marriage Fam. 1996;58:188–200. [Google Scholar]
- 95.Wainwright PE, Lévesque S, Krempulec L, Bulman-Fleming B, McCutcheon D. Effects of environmental enrichment on cortical depth and Morris-maze performance in B6D2F2 mice exposed prenatally to ethanol. Neurotoxicol Teratol. 1993;15:11–20. doi: 10.1016/0892-0362(93)90040-u. [DOI] [PubMed] [Google Scholar]
- 96.Chaddock L, Pontifex MB, Hillman CH, Kramer AF. A review of the relation of aerobic fitness and physical activity to brain structure and function in children. J Int Neuropsychol Soc. 2011;17:975–985. doi: 10.1017/S1355617711000567. [DOI] [PubMed] [Google Scholar]
- 97.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 98.Carlson MC, et al. Evidence for neurocognitive plasticity in at-risk older adults: The experience corps program. J Gerontol A Biol Sci Med Sci. 2009;64:1275–1282. doi: 10.1093/gerona/glp117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Park DC, et al. The impact of sustained engagement on cognitive function in older adults: The synapse project. Psychol Sci. 2014;25:103–112. doi: 10.1177/0956797613499592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 101.McEwen BS. Brain on stress: How the social environment gets under the skin. Proc Natl Acad Sci USA. 2012;109:17180–17185. doi: 10.1073/pnas.1121254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jones RN, et al. Conceptual and measurement challenges in research on cognitive reserve. J Int Neuropsychol Soc. 2011;17:593–601. doi: 10.1017/S1355617710001748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Ann N Y Acad Sci. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 105.McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Arch Intern Med. 1993;153:2093–2101. [PubMed] [Google Scholar]
- 106.Liston C, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McEwen BS. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 108.Noble KG, McCandliss BD, Farah MJ. Socioeconomic gradients predict individual differences in neurocognitive abilities. Dev Sci. 2007;10:464–480. doi: 10.1111/j.1467-7687.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- 109.Hudson CG. Socioeconomic status and mental illness: Tests of the social causation and selection hypotheses. Am J Orthopsychiatry. 2005;75:3–18. doi: 10.1037/0002-9432.75.1.3. [DOI] [PubMed] [Google Scholar]
- 110.Tyrrell J, et al. Height, body mass index, and socioeconomic status: Mendelian randomisation study in UK Biobank. BMJ. 2016;352:i582. doi: 10.1136/bmj.i582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ludwig J, et al. Neighborhoods, obesity, and diabetes—a randomized social experiment. N Engl J Med. 2011;365:1509–1519. doi: 10.1056/NEJMsa1103216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Stine-Morrow EA, Parisi JM, Morrow DG, Park DC. The effects of an engaged lifestyle on cognitive vitality: A field experiment. Psychol Aging. 2008;23:778–786. doi: 10.1037/a0014341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Carlson LE, Speca M, Faris P, Patel KD. One year pre-post intervention follow-up of psychological, immune, endocrine and blood pressure outcomes of mindfulness-based stress reduction (MBSR) in breast and prostate cancer outpatients. Brain Behav Immun. 2007;21:1038–1049. doi: 10.1016/j.bbi.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 114.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 115.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 116.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 117.Van Essen DC, Glasser MF, Dierker DL, Harwell J, Coalson T. Parcellations and hemispheric asymmetries of human cerebral cortex analyzed on surface-based atlases. Cereb Cortex. 2012;22:2241–2262. doi: 10.1093/cercor/bhr291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. Magn Reson Med. 1996;35:346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- 119.Power JD, et al. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–341. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gotts SJ, et al. The perils of global signal regression for group comparisons: A case study of autism spectrum disorders. Front Hum Neurosci. 2013;7:356. doi: 10.3389/fnhum.2013.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: Are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Satterthwaite TD, et al. Heterogeneous impact of motion on fundamental patterns of developmental changes in functional connectivity during youth. Neuroimage. 2013;83:45–57. doi: 10.1016/j.neuroimage.2013.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Power JD, et al. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rosvall M, Bergstrom CT. Maps of random walks on complex networks reveal community structure. Proc Natl Acad Sci USA. 2008;105:1118–1123. doi: 10.1073/pnas.0706851105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Na J, Chan MY, Lodi-Smith J, Park DC. Social-class differences in self-concept clarity and their implications for well-being. J Health Psychol. 2016;2016:1359105316643597. doi: 10.1177/1359105316643597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Adler NE, Epel ES, Castellazzo G, Ickovics JR. Relationship of subjective and objective social status with psychological and physiological functioning: Preliminary data in healthy white women. Health Psychol. 2000;19:586–592. doi: 10.1037//0278-6133.19.6.586. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.