Significance
The selective estrogen receptor β (ERβ) agonist, LY3201, acts on enteric neuronal stem/progenitor cells, enhancing their capacity to generate enteric glial cells and neurons in vitro. Using two murine models of enteric nervous system damage, we showed that in vivo an ERβ agonist elicited accelerated reconstitution of the enteric neuron pool in the myenteric plexus. Our study suggests that selective ERβ agonists might be useful to promote neurogenesis in the damaged enteric nervous system.
Keywords: estrogen receptor β, enteric neurons, enteric glial cells
Abstract
Injury to the enteric nervous system (ENS) can cause several gastrointestinal (GI) disorders including achalasia, irritable bowel syndrome, and gastroparesis. Recently, a subpopulation of enteric glial cells with neuronal stem/progenitor properties (ENSCs) has been identified in the adult ENS. ENSCs have the ability of reconstituting the enteric neuronal pool after damage of the myenteric plexus. Since the estrogen receptor β (ERβ) is expressed in enteric glial cells and neurons, we investigated whether a selective ERβ agonist, LY3201, can influence neuronal and glial cell differentiation. Myenteric ganglia from the murine muscularis externa were isolated and cultured in either glial cell medium or neuronal medium. In glial cell medium, the number of glial progenitor cells (Sox10+) was increased by fourfold in the presence of LY3201. In the neuronal medium supplemented with an antimitotic agent to block glial cell proliferation, LY3201 elicited a 2.7-fold increase in the number of neurons (neurofilament+ or HuC/D+). In addition, the effect of LY3201 was evaluated in vivo in two murine models of enteric neuronal damage and loss, namely, high-fat diet and topical application of the cationic detergent benzalkonium chloride (BAC) on the intestinal serosa, respectively. In both models, treatment with LY3201 significantly increased the recovery of neurons after damage. Thus, LY3201 was able to stimulate glial-to-neuron cell differentiation in vitro and promoted neurogenesis in the damaged myenteric plexus in vivo. Overall, our study suggests that selective ERβ agonists may represent a therapeutic tool to treat patients suffering from GI disorders, caused by excessive neuronal/glial cell damage.
The enteric nervous system (ENS) is composed of a network of neurons and glial cells that regulates essential gastrointestinal (GI) functions like motility, absorption, and secretion independently of the central nervous system (CNS). Defective functioning of the ENS is associated with various GI diseases including ileus, achalasia, irritable bowel syndrome, and gastroparesis (1). Throughout life, various injuries, such as inflammation and aging, can cause damage to the ENS, resulting in some cases in neuronal loss leading to altered GI motility and secretion (2). Recent work has shown that, after physical or inflammatory damage, a subpopulation of enteric glial cells with neuronal stem/progenitor properties (ENSCs) from the undamaged parts of the ENS is able to reconstitute the enteric neuronal network (3). Indeed, neurogenesis from adult enteric glia has been extensively tested in vitro and, even if less consistently, in vivo (4). In this context, Laranjeira et al. (5) showed that, after chemical ablation of myenteric neurons with benzalkonium chloride (BAC), there is regeneration of newly differentiated neurons from a subpopulation of Sox10-expressing glial cells/progenitor cells migrating from the unaffected adjacent myenteric ganglia. In line, Belkind-Gerson et al. (6) demonstrated that, upon injury to the gut, the neurogenic potential of enteric glia is induced through activation of the serotonin signaling pathway via the 5-HT4 receptor. Similarly, Kulkarni et al. (7) showed that, under physiological conditions, the adult ENS is maintained by a dynamic balance between neuronal apoptosis and neurogenesis from neuronal Sox10−Nestin+ precursors that are not mature glial cells. However, the neurogenic potential of Sox10+Nestin− cells is only activated upon injury.
Thus, understanding the molecular mechanisms promoting adult neurogenesis via activation of ENSCs will be essential to develop novel pharmacological agents to treat GI diseases involving alteration to the ENS.
Estrogen receptor α (ERα) and estrogen receptor β (ERβ) are nuclear receptors involved in the modulation of several physiological and biological processes ranging from lipid and glucose homeostasis, cell proliferation and growth, to immunity, reproduction, and CNS homeostasis (8). In particular, both ERα and ERβ have been implicated in neuroprotection (9, 10) and neurogenesis during damage of the CNS such as multiple sclerosis and stroke (11, 12).
So far, little is known about the possible role of estrogens in favoring neurogenesis in the ENS. Both ERα and ERβ are expressed in the gut wall (13). While ERα is only expressed on interstitial cells of Cajal, ERβ is expressed on both neurons and glial cells (14). Based on these findings, we aimed to test whether a synthetic selective ERβ agonist, LY3201, already shown to have neuroprotective (15) and immunomodulating (16) effects in the brain, was promoting neurogenesis in the ENS using two validate models of enteric neuronal damage such as BAC treatment and high-fat diet (HFD) (17, 18).
In the present study, we demonstrate that in vitro activation of ERβ with LY3201 increased glial cell/progenitor cell proliferation, resulting in increased numbers of enteric neurons and glial cells. Moreover, upon intestinal injury, LY3201 treatment resulted in reduced damage of the ENS with decreased neuronal loss in both BAC-treated and HFD mice. Thus, our study provides some insight into neurogenesis in the injured ENS and points toward a possible application of ERβ agonists in several clinical contexts in which damage to the ENS is responsible for the pathogenesis of the disease.
Results
ERβ Is Expressed in Neurons and Glial Cells in Human and Mouse Myenteric Plexus.
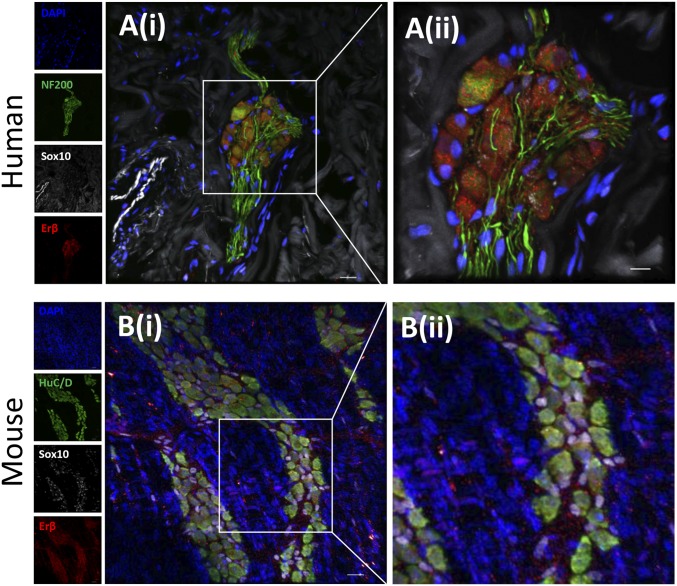
Confocal microscopy of the myenteric plexus in human and mouse colonic samples was used to define the cellular subsets and subcellular localization of ERβ. As shown in Fig. 1, in both humans (A) and mice (B), ERβ is expressed in neurons (HuC/D+ or NF200+) and glial cells (Sox10+) in the myenteric plexus. Of note, ERα expression was not detectable in the human colonic myenteric plexus (SI Appendix, Fig. S1A).
Fig. 1.
Erβ localization in mouse and human myenteric plexus. Two-dimensional projection of a z stack of confocal microscopy images of myenteric plexus from human (A) and mouse colon (B). (A) Human colonic myenteric ganglia stained for Erβ (red), Sox10 (gray), neurofilament (NF 200; green), and nuclei (DAPI; blue). Inset Ai is magnified in Aii. (B) Murine muscularis externa whole mount stained for Erβ (red), Sox10 (gray), HuC/D (green), and nuclei (DAPI; blue). Inset Bi is magnified in Bii.
ERβ Activation Increases the Number of Glial Cells/Progenitor Cells in Vitro.
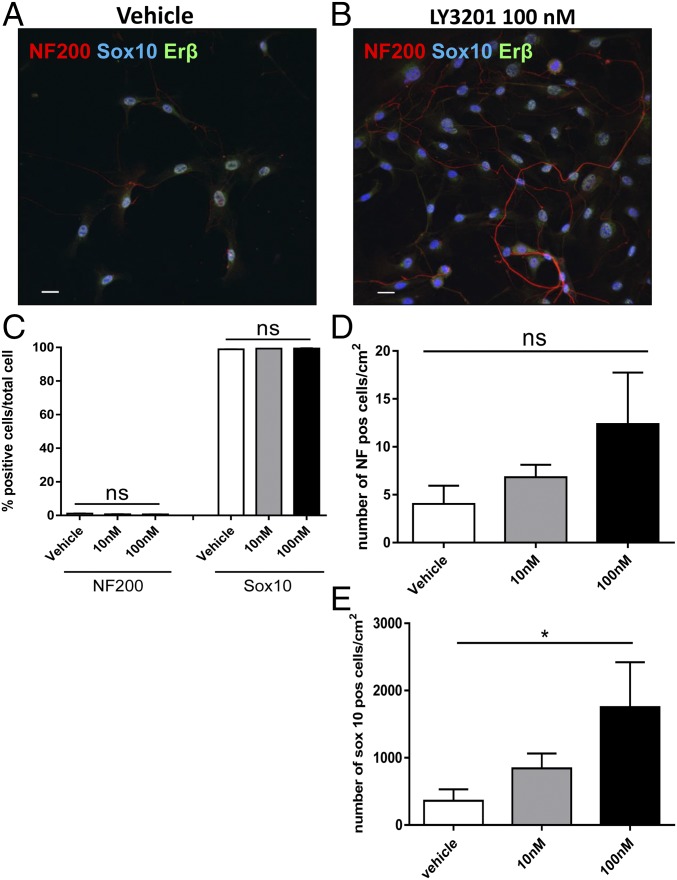
To evaluate whether ERβ activation could enhance neurogenic properties of Sox10+ glial cells in vitro, myenteric ganglia were isolated from adult mice and cultured for 7 d in glial cell medium supplemented with different concentrations of the ERβ agonist LY3201. Glial progenitor cells, identified by immunostaining for Sox10, coexpressed nuclear ERβ (Fig. 2 A and B). Interestingly, stimulation with the ERβ agonist, LY3201, increased the number of Sox10+ cells by fourfold at 100 nM (mean, 1,754 ± 668/cm2) and twofold at 10 nM (mean, 843 ± 221/cm2) compared with vehicle (mean, 361 ± 171/cm2) (Fig. 2E). In contrast to the effects of the ERβ-selective ligand, the specific ERα agonist propylpyrazoletriol (15 nM) had no effect on the proliferation of Sox10+ cells (SI Appendix, Fig. S2) and no ERα was detectable in murine myenteric ganglia cultured in vitro (SI Appendix, Fig. S2).
Fig. 2.
Erβ activation increases proliferation of enteric glial cells in primary culture of murine myenteric ganglia. Myenteric ganglia were isolated via enzymatic digestion of the muscularis externa of adult male C57BL/6 mice and cultured for 7 d with different concentration of LY3201 as indicated. Immunofluorescence images of individual ganglia cultured with vehicle (A) and with 100 nM of LY3201 (B) stained with anti-Sox10 (blue), anti-NF200 (red), and anti-Erβ (green). (C) Percentages of neurons (NF200) and glial cells (Sox10) in myenteric ganglia cultures treated with different concentrations of LY3201 as indicated. (D and E) Number of NF+ cells per square centimeter (D) and Sox10+ cells per square centimeter (E) in myenteric cultured ganglia treated with different concentrations of LY3201 as indicated. Data are representative of three independent experiments. One-way ANOVA followed by Dunnett’s multiple-comparison test (*P < 0.05).
ERβ Activation Promotes Differentiation of Mature Enteric Neurons in Vitro.
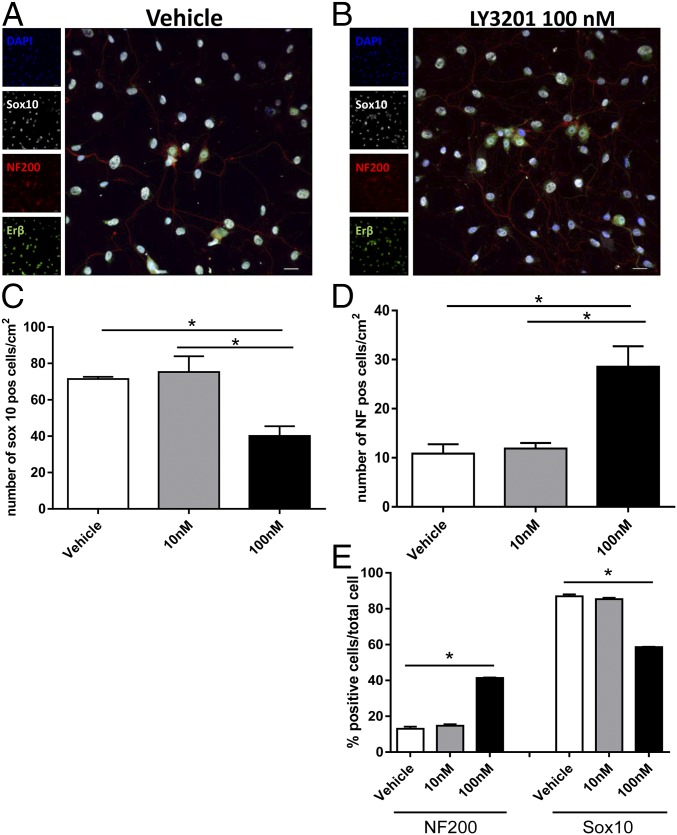
To determine whether ERβ activation was able to promote the generation of neurons from enteric glial progenitor cells, LY3201 was tested in vitro on enteric neuronal cultures. Myenteric ganglia were cultured for 7 d in neuronal medium supplemented with different concentrations of LY3201. LY3201 increased the number of neurons [neurofilament (NF200+)] when glial cell proliferation was inhibited by the addition of the antimitotic agent AraC (Fig. 3 A and B). The number of neurons was increased 2.7-fold when cells were treated with 100 nM LY3201 (vehicle, 11 ± 2/cm2; 100 nM LY3201, 29 ± 4/cm2), while 10 nM LY3201 had no significant effect on neuronal proliferation/differentiation (Fig. 3D). In contrast, the number of glial cells was reduced in the presence of 100 nM LY3201 (vehicle, 71.5 ± 1.2/cm2; 10 nM LY3201, 75.2 ± 8.7/cm2; 100 nM LY3201, 40.2 ± 5.3/cm2) (Fig. 3C). Overall, LY3201 increased the percentage of neurons from 13% in the vehicle-treated samples to 41%, while it decreased the percentage of glial cells from 87 to 59% (Fig. 3E).
Fig. 3.
Erβ activation promotes differentiation of mature neurons in enteric neuronal cultures. Myenteric ganglia were isolated via enzymatic digestion from the muscularis externa of adult male C57BL/6 mice and cultured for 7 d with different concentrations of LY3201 as indicated. Immunofluorescence images of individual ganglia cultured with vehicle (A) and with 100 nM LY3201 (B) stained with anti-Sox10 (gray), anti-NF200 (red), and anti-Erβ (green). To avoid proliferation of glial cells, an antimitotic agent (AraC) was added to the culture. Number of Sox10+ cells per square centimeter (C) and NF+ cells per square centimeter (D) in ganglia treated with vehicle, 10 nM LY3201, and 100 nM LY3201, showing, respectively, a significant decrease of glial cells and a significant increase of neurons upon treatment with 100 nM LY3201, while there was no measurable effect upon 10 nM LY3201 treatment. (E) Percentages of neurons (NF200) and glial cells (Sox10) in cultures treated with vehicle, 10 nM LY3201, and 100 nM LY3201, showing that treatment with 100 nM LY3201 increased the percentage of NF+ cells while decreasing the percentage of Sox10+ cells. Data are representative of three independent experiments. One-way ANOVA followed by Dunnett’s multiple-comparison test (*P < 0.05; **P < 0.001).
LY3201 Increases the Fluorescence Intensity of Sox10.
To investigate whether Sox10 is an ERβ-regulated gene, the effects of 10 and 100 nM LY3201 on glial cells in vitro were examined. The number of Sox10+ cells was evaluated (SI Appendix, Fig. S3 A and B) and the intensity of Sox10 expression was assessed as corrected total cellular fluorescence (CTCF). The fluorescence intensity was significantly increased when cells were treated with LY3201 at concentrations of 10 and 100 nM in a dose-dependent manner (CTCF vehicle, 2,860 ± 1,823; 10 nM, 3,709 ± 2,896; 100 nM, 6,530 ± 2,719) (SI Appendix, Fig. S3C).
ERβ Stimulation Accelerates Repopulation of Neurons in the Myenteric Plexus After Chemical Damage.
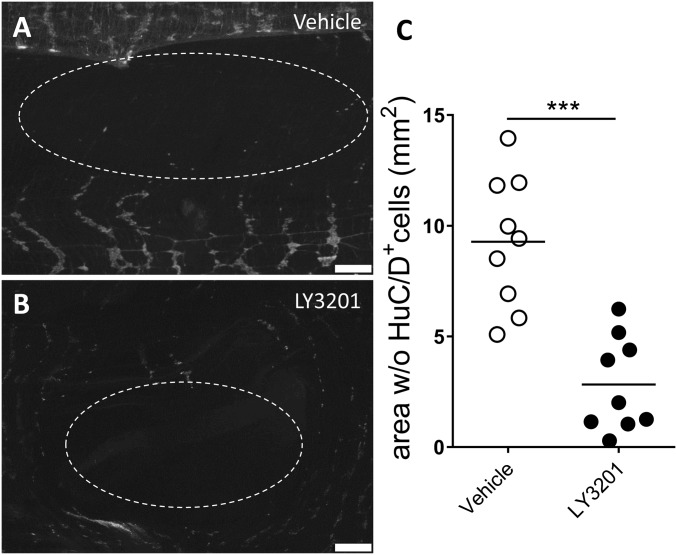
To evaluate whether LY3201 could accelerate repopulation of neurons in the myenteric plexus after local chemical damage, C57BL/6 wild-type mice were treated with local application on the intestinal serosa of the cationic detergent BAC (treated area, 5 mm2) and after 4 d implanted with slow-release pellets containing LY3201 (0.119 mg in 7 d) or vehicle. Both groups were killed at day 11 after BAC treatment and the size of the area (in square millimeters) depleted of neurons was evaluated by loss of staining of the neuronal marker HuC/D (Fig. 4 A and B). As shown in Fig. 4C, treatment with LY3201 significantly reduced the myenteric plexus area depleted of neurons (2.44 ± 2.1/mm2) compared with area in vehicle-treated mice (8.42 ± 2.8/mm2) (Fig. 4C). Thus, ERβ stimulation promoted neurogenesis, leading to a more rapid reconstitution of neurons in the myenteric plexus.
Fig. 4.
Erβ stimulation reduces the size of the area depleted of neurons by benzalkonium chloride (BAC). Adult male C57BL/6 mice were treated with topical administration of BAC on the ileal serosa. After 4 d, mice were s.c. implanted with slow-releasing pellets containing LY3201 or vehicle. Both groups were killed at day 11 after BAC treatment and the size of the area (in square millimeters) depleted of neurons was assessed via immunofluorescence with anti-HuC/D staining. (A and B) Immunofluorescence images of muscularis externa stained for HuC/D from vehicle-treated (A) and LY3201-treated (B) mice. (C) Points represent the area depleted of neurons (in square millimeters) from individual mice. Data are representative of two independent experiments. Nonparametric Mann–Whitney test (***P < 0.0001).
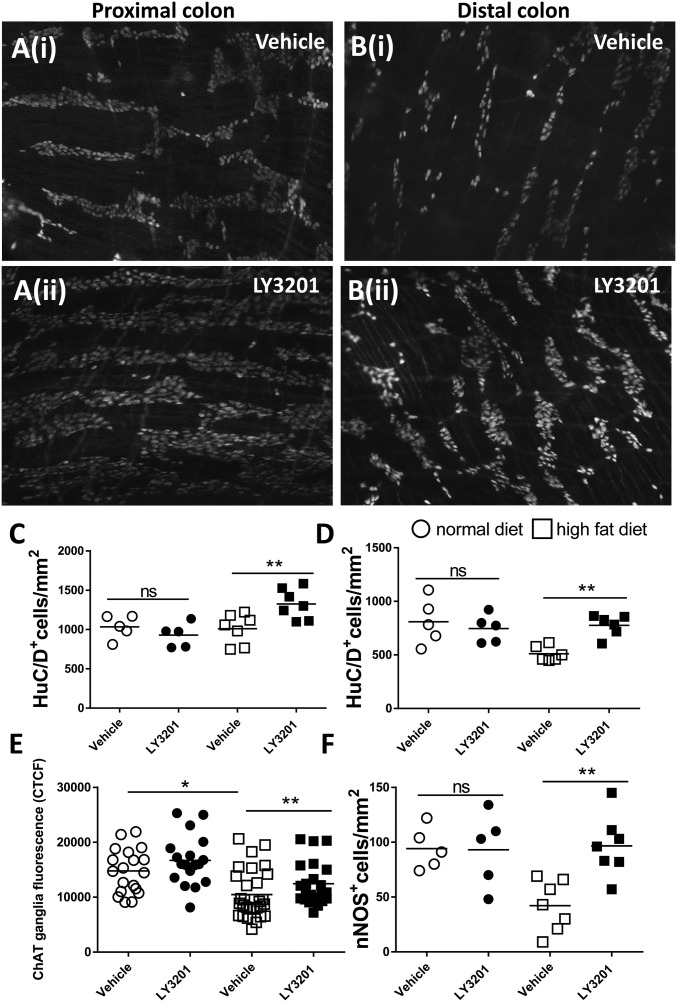
ERβ Reduces Neuronal Loss in Colonic Myenteric Plexus of Mice with HFD-Induced Obesity.
Typically, HFD-induced obese mice suffer from loss of colonic neurons over time. Thus, to evaluate whether LY3201 is able to restore the neuron pool lost upon HFD feeding, obese mice were implanted with LY3201 s.c. pellets, which released the drug for 7 d or with vehicle control pellets. In line with the literature (19), in the distal colon HFD diet reduced the number of neurons by 37% compared with mice on a normal diet (ND) (mean ND+veh, 810 ± 21/mm2, vs. HFD+veh, 510 ± 70/mm2), while HFD diet had no effect on neuronal numbers in the proximal colon (mean ND+veh, 1,034 ± 14/mm2, vs. HFD+veh, 1,011 ± 19/mm2) (Fig. 5 A–D). Interestingly, treatment with LY3201 in the HFD group resulted in an increase in the number of colonic myenteric plexus neurons over that seen in mice treated with vehicle pellets. There was a 30% increase in the proximal colon (mean HFD+veh, 1,011 ± 19/mm2, vs. HFD+LY3201, 1,326 ± 19/mm2) and a 52% increase in the distal colon (mean HFD+veh, 510 ± 70/mm2, vs. HFD+LY3201, 776 ± 99/mm2). To define whether the ERβ agonist also influenced the neurochemical coding profile of the myenteric neurons, the levels of choline acetyltransferase (ChAT) and neuronal nitric oxide synthase (nNOS) were analyzed with immunofluorescence in the distal colon of HFD and ND mice implanted or not with LY3201-releasing pellets (Fig. 5 E and F). In line with the literature, in the distal colon an HFD resulted in a decrease of ChAT fluorescence intensity per ganglion area (CTCF) and of nNOS neurons. Treatment with LY3201 in the HFD group restored the expression of ChAT and resulted in an increase of nNOS+ neurons (Fig. 5 E and F and SI Appendix, Fig. S4). An increase of ChAT and nNOS neurons was not observed in ND mice treated with LY3201, suggesting that ERβ activation has a specific effect only after damage to the myenteric plexus.
Fig. 5.
Erβ activation reduced ChAT and nNOS neurons loss in the colonic myenteric plexus after HFD. Diet-induced-obese mice (HFD) and normal diet control mice (ND) were implanted for 7 d, with s.c. pellets containing LY3201 or vehicle. After 7 d of treatment, colonic muscularis externa whole mounts were stained with anti-HuC/D to detect the number of neurons in the myenteric plexus of proximal (A) and distal colon (B) in mice treated with vehicle (i) or with LY3201 (ii). Points represent number of HuC/D+ cells per square millimeter for individual ND and HFD mice treated with vehicle or LY3201 pellets; proximal (C) and distal (D) colon. (E and F) Myenteric ganglia fluorescence intensity (CTCF) of ChAT (E) and number of nNOS neurons (F) were assessed in the distal colon. Data are representative of two independent experiments. One-way ANOVA followed by Dunnett’s multiple-comparison test (*P < 0.05; **P < 0.001).
Discussion
Considering that the myenteric plexus is exposed to various injuries throughout life, it is not surprising that several GI disorders are accompanied by damage to the ENS. Thus, understanding the mechanism involved in the reconstitution of the neuronal pool from Sox10+ precursors may hold great therapeutic advantages.
Recently, experimental evidence has revealed a role for estrogens in neuronal renewal in the CNS (20). Considering that both components of the ENS, enteric neurons and glial cells, express the estrogen receptor ERβ but not ERα (14), we aimed to define whether an ERβ-selective agonist would modulate injury-induced loss of neurons and/or recovery of neurons after injury.
Using primary murine myenteric glial cell cultures, we found that stimulation of ERβ with the selective ERβ agonist, LY3201, induces glial cell/progenitor cell proliferation. However, since glial cells compete for the same environment with neurons (21), uncontrolled glial cell proliferation in culture may result in inhibition of the neuronal pool expansion, thereby complicating the analysis of the effect of LY3201 on neurogenesis in vitro. For this reason, we tested the effect of LY3201 in an enteric neuronal culture in which an antimitotic agent, namely AraC, blocked glial cell proliferation. The addition of LY3201 to the enteric neuronal culture reduced the percentage of Sox10+ cells, while it increased the percentage of neurons, suggesting that LY3201 is able to enhance the differentiation of neurons from Sox10+ cells. Taken together, these data showed that activation of ERβ leads to proliferation of glial cells/progenitor cells and subsequently promoted their differentiation into neurons.
Sox10 is a member of the high-mobility group (HMG) gene family and is specifically expressed in neural crest cells (NCSCs) (22). Sox10 is required for differentiation of glial cells (23) and constitutive expression of this gene in NCSCs preserves both glial and neuronal differentiation (24). We found that Sox10 expression is increased, in a dose-dependent manner, by LY3201, indicating that it is an ERβ-regulated gene and that its expression can be pharmacologically modulated.
To define whether or not the ERβ-selective ligand could also support neurogenesis in vivo, we used two mouse models of enteric neuronal damage, namely, topical application on the gut serosa of BAC and prolonged feeding of mice with an HFD. These experimental models are associated with alteration of the ENS leading to loss of enteric neurons. Serosal application of BAC resulted in damage of the myenteric plexus with loss of typical neuronal network. Recent studies have shown that, several days after BAC-induced damage, ENSCs migrate from the unaffected edges reconstituting the myenteric ganglia. In the present study, treatment with LY3201 significantly accelerated recovery of the ENS leading to a reduction of the area deprived of neurons. This suggests that ERβ activation may act on ENSCs surrounding the damaged area, leading to neuronal differentiation and reconstitution of myenteric ganglia.
ERβ stimulation was also effective in decreasing neuronal loss in a model of HFD damage to the ENS. Treatment of obese mice with LY3201 restored the number of myenteric neurons. The effect was specific to the distal colon since no neuronal loss was found in the proximal colon. In addition, administration of LY3201 in the HFD group was able to restore the expression of ChAT and resulted in an increase of nNOS+ neurons. Increase of ChAT and nNOS neurons was not observed in ND mice treated with LY3201, suggesting that ERβ activation has a specific effect only after damage to the myenteric plexus.
Overall, our data strongly suggest that selective ERβ agonists might be useful to promote neurogenesis in the damaged ENS. These data may have several possible clinical implications since ERβ agonists might be useful for patients with damaged or reduced enteric neurons due to injury. Considering that selective ERβ stimulation had no significant effect on enteric neurons in normal mice, we have provided evidence that ERβ-mediated neurogenesis only occurs after damage. Future studies will reveal whether ERβ-specific agonists may open new avenues in the treatment of severe cases of gut motor abnormalities.
Materials and Methods
Ethical Approval.
All experimental procedures were approved by the Animal Care and Animal Experiments Committee of the University of Leuven. Human tissue collection was approved by the Ethics Committee of the University Hospital of Leuven (s56330). All patients gave their written informed consent before their participation in the study. After inclusion (signing of informed consent), the data are anonymized and each patient is from then on only identifiable by a unique ID number.
Animals.
Wild-type (WT) (C57BL/6J) male mice were kept at the Katholieke Universiteit (KU) Leuven animal facility under specific pathogen-free conditions on a 12:12-h light–dark cycle. WT mice had ad libitum access to water and were provided with commercially available chow (ssniff R/M-H; ssniff Spezialdiäten). In some experiments, 18-wk-old diet-induced obese male mice (DIO-B6-M) were fed for at least 12 wk with an HFD (60% Kcal; Research Diet) and control mice (DIO-control; Taconic Biosciences) were purchased from Taconic Biosciences.
Myenteric Ganglia Isolation and Culture.
After dissection of the murine small intestine, the luminal content was flushed with cold KREBS buffer (1.2 mmol/L NaH2PO4·2H2O, 117 mmol/L NaCl, 4.7 mmol/L KCl, 25 mmol/L NaHCO3, 11 mmol/L glucose, 2.5 mmol/L CaCl2·2H2O, and 1.2 mmol/L MgCl2·6H2O). The muscularis externa was peeled off from the mucosa using cotton swap and digested for 15 min at 37 °C using DMEM/F12 medium (Lonza) supplemented with 0.144 g/mL NaHCO3, 3.2% H2O, 2.4 mM glutamine, 10% FBS, 50 U/mL penicillin and streptomycin, 2.5 µg/mL gentamicin, 0.01 mg/mL amphotericin B (Thermo Fisher Scientific), 2.5 mg/mL BSA (Sigma-Aldrich), 1 mg/mL protease (Sigma-Aldrich), and 20 mg/mL collagenase type IV (Thermo Fisher Scientific). After digestion, tissue fragments were subjected to mechanical dissociation using the GentleMACS Dissociator (Miltenyi Biotec). Digested tissues were suspended in DMEM/F12 medium without enzymes, and ganglia were collected under a stereotactic microscope. Fifty ganglia per condition were cultured in poly-d-lysine (5 mg/10 mL borate buffer; Sigma-Aldrich)/laminin (20 μg/mL in PBS; Sigma-Aldrich)-coated glass coverslips with the addition of Neuronal Medium (Sigma-Aldrich) supplemented with 10% FBS (Biowest), 0.2% G5 (Thermo Fisher), and 0.05% NGF (Alomone). In some experiments, to reduce glial cell proliferation and favor neuronal cell enrichment, 1% AraC (Sigma-Aldrich) was added to the medium. Cells were cultured for 7 d in the presence or absence of different concentration of (3aS, 4R, 9bR)-2,2-difluoro-4-(4-hydroxyphenyl)-3,3a,4,9b-tetrahydro-1H-cyclopenta[c]chromen-8-ol (LY3201; CAS 787621-78-7); gift from Eli Lilly and 4,4′,4′′-(4-propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol (PTT) (CAS 263717–53-9; Tocris) as indicated in the figures.
In Vivo Treatment with ERβ Agonist.
Pellets of LY3201 were prepared by Innovative Research of America to release 0.119 mg in 7 d. Pellets were made of a matrix containing cholesterol, cellulose, lactose, phosphates, and stearates with or without the active product. Mice were randomly separated into vehicle- or LY3201-treated groups. Pellets were implanted on the lateral side of the neck between the ear and the shoulder. Control vehicle pellets were used as control.
In Vivo Treatment with BAC.
Animals were anesthetized by i.p. injection of ketamine (Ketalar; 100 mg/kg; Pfizer) and xylazine (Rompun; 10 mg/kg; Bayer). A laparotomy with a midline incision was performed, and a portion of the ileum was exposed. Sterile cellulose filter paper disks (5-mm diameter; Whatman) soaked in 0.1% BAC (Sigma-Aldrich) in NaCl solution were placed on the exteriorized intestine, 2 cm from the ileocecal valve. The tissue was incubated for 15 min with BAC, after which the paper disk was removed and the treated area thoroughly rinsed with sterile saline solution. Four days after BAC treatment, animals were implanted with pellets containing vehicle or LY3201. Mice were killed with CO2 overdose 11 d after treatment. The treated segment of bowel and untreated segments oral and aboral to the treated area were harvested from each animal, and the muscularis layer was analyzed via immunofluorescence.
Immunofluorescence.
Tissue culture preparations of the myenteric plexus were fixed with 4% paraformaldehyde (PFA; Sigma-Aldrich) for 10 min. After washing with PBS, coverslips were stored in PBS containing 0.03% sodium azide until usage. Notably, control and treated cells were kept separately on chamber slides before fixation and incubated for 2 h at room temperature in blocking solution with the same concentration of antibody [goat anti-Sox10 (Santa Cruz Biotechnology); chicken anti-neurofilament 200 kDa (NF200; Abcam)]. Immunofluorescence was visualized using a Zeiss LSM780 confocal laser-scanning microscope (Cell Imaging Core, KU Leuven). To quantify Sox10 intensity of fluorescence, a single in-focus plane was acquired. Using ImageJ, an outline was drawn around each cell and circularity, area, and mean fluorescence measured, along with several adjacent background readings. The CTCF [=integrated density − (area of selected cell × mean fluorescence of background readings)] was calculated (25).
For the staining of murine tissues, 3-cm intestinal fragments (jejunum or colon) were stretched on a Sylgard plate and fixed in 4% PFA (Sigma-Aldrich) for 30 min at room temperature. After washing with PBS, tissues were stored in PBS containing 0.03% sodium azide until usage. Next, whole mounts of the muscularis externa were prepared by removal of the mucosa to obtain preparations containing the circular and longitudinal smooth muscle layers. For the staining of human tissues, full-thickness intestinal colonic samples were collected from the healthy mucosa of patients undergoing colon carcinoma resection at Universitaire Ziekenhuizen Leuven (Leuven, Belgium). Intestinal samples were fixed overnight with 4% PFA at 4 °C. After washing with PBS, tissues were incubated in 20% sucrose (Sigma-Aldrich) and embedded in optimum cutting temperature (O.C.T.) compound (Tissue-Tek O.C.T.; Sakura Finetek). Tissue blocks were stored at −80 °C until slides were prepared. Next, both human and murine tissue segments were blocked and permeabilized for 2 h at room temperature in blocking/permeabilizing solution composed of PBS, 1% BSA (Sigma-Aldrich), and 0.3% Triton X-100 (Sigma-Aldrich). Subsequently, the murine muscularis externa whole mounts were incubated overnight at 4 °C with the following primary antibodies in blocking solution supplemented with 0.3% Triton X-100 (Sigma-Aldrich): chicken anti-neurofilament, 200 kDa (NF200; Abcam); human ANNA-1 serum anti-HuC/D (kindly provided by V. A. Lennon, Mayo Clinic, Rochester, MN); goat anti-SOX10 (Santa Cruz Biotechnology); rabbit anti-ERβ antibody [raised in the Laboratory of Jan-Ake Gustafsson (Karolinska Institute, Stockholm, Sweden) and previously characterized] (26); rabbit anti-ERα (Santa Cruz); goat anti-nNOS (Abcam); and rabbit anti-ChAT (Abcam). After washing in PBS, the whole mounts were incubated for 2 h at room temperature in a mixture of fluorescently labeled secondary antibodies in blocking solution supplemented with 0.3% Triton X-100: AF488-conjugated donkey anti-goat (Jackson ImmunoLabs); Cy3-conjugated donkey anti-rabbit (Jackson ImmunoLabs); Cy5-conjugated donkey anti-chicken (Jackson ImmunoLabs); and 4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI) (Sigma-Aldrich). Finally, samples were rinsed in PBS and mounted with SlowFade Diamond Antifade (Invitrogen). Immunofluorescence was visualized using a Zeiss LSM780 confocal laser-scanning microscope (Cell Imaging Core, KU Leuven). For each field of view, a z series spanning the entire depth of the myenteric plexus was imaged and projected for cell counting. In each field, the number of HuC/D-immunoreactive neuronal bodies and the number of nNOS neurons per area were manually counted in a blind fashion. Moreover, in each field, the intensity fluorescence of ChAT was determined drawing around each ganglia an outline and measuring, using ImageJ software (NIH), the area and the mean fluorescence. The mean fluorescence of several adjacent background readings was also measured. The CTCF was determined as follows: integrated density – (area of selected cell × mean fluorescence of background readings) (25).
Statistical Analysis.
Significance between two groups was determined by unpaired nonparametric Mann–Whitney test, while one-way ANOVA followed by Dunnett’s multiple-comparison test was performed to compare multiple mean groups. Graph Pad Prism, version 5.01, software was used to generate graphs and perform statistical analysis (GraphPad Software).
Supplementary Material
Acknowledgments
This work was supported by a grant from the Center for Innovative Medicine and the Swedish Cancer Society (to J.A.G.) and from a Research Foundation—Flanders (FWO) Grant (G.0D83.17N) (to G.M.). J.A.G. is thankful to the Robert A. Welch Foundation (Fellowship E-0004) for their support.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1720267115/-/DCSupplemental.
References
- 1.Goldstein AM, Thapar N, Karunaratne TB, De Giorgio R. Clinical aspects of neurointestinal disease: Pathophysiology, diagnosis, and treatment. Dev Biol. 2016;417:217–228. doi: 10.1016/j.ydbio.2016.03.032. [DOI] [PubMed] [Google Scholar]
- 2.Verheijden S, Boeckxstaens GE. Neuro-immune interaction and the regulation of intestinal immune homeostasis. Am J Physiol Gastrointest Liver Physiol. 2018;314:G75–G80. doi: 10.1152/ajpgi.00425.2016. [DOI] [PubMed] [Google Scholar]
- 3.Coelho-Aguiar JdM, et al. The enteric glia: Identity and functions. Glia. 2015;63:921–935. doi: 10.1002/glia.22795. [DOI] [PubMed] [Google Scholar]
- 4.Belkind-Gerson J, et al. Colitis induces enteric neurogenesis through a 5-HT4-dependent mechanism. Inflamm Bowel Dis. 2015;21:870–878. doi: 10.1097/MIB.0000000000000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laranjeira C, et al. Glial cells in the mouse enteric nervous system can undergo neurogenesis in response to injury. J Clin Invest. 2011;121:3412–3424. doi: 10.1172/JCI58200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belkind-Gerson J, et al. Colitis promotes neuronal differentiation of Sox2+ and PLP1+ enteric cells. Sci Rep. 2017;7:2525. doi: 10.1038/s41598-017-02890-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kulkarni S, et al. Adult enteric nervous system in health is maintained by a dynamic balance between neuronal apoptosis and neurogenesis. Proc Natl Acad Sci USA. 2017;114:E3709–E3718. doi: 10.1073/pnas.1619406114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jia M, Dahlman-Wright K, Gustafsson J-Å. Estrogen receptor alpha and beta in health and disease. Best Pract Res Clin Endocrinol Metab. 2015;29:557–568. doi: 10.1016/j.beem.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Zhao L, Brinton RD. Estrogen receptor α and β differentially regulate intracellular Ca2+ dynamics leading to ERK phosphorylation and estrogen neuroprotection in hippocampal neurons. Brain Res. 2007;1172:48–59. doi: 10.1016/j.brainres.2007.06.092. [DOI] [PubMed] [Google Scholar]
- 10.Zhao L, Wu TW, Brinton RD. Estrogen receptor subtypes alpha and beta contribute to neuroprotection and increased Bcl-2 expression in primary hippocampal neurons. Brain Res. 2004;1010:22–34. doi: 10.1016/j.brainres.2004.02.066. [DOI] [PubMed] [Google Scholar]
- 11.Spence RD, et al. Estrogen mediates neuroprotection and anti-inflammatory effects during EAE through ERα signaling on astrocytes but not through ERβ signaling on astrocytes or neurons. J Neurosci. 2013;33:10924–10933. doi: 10.1523/JNEUROSCI.0886-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki S, et al. Estradiol enhances neurogenesis following ischemic stroke through estrogen receptors α and β. J Comp Neurol. 2007;500:1064–1075. doi: 10.1002/cne.21240. [DOI] [PubMed] [Google Scholar]
- 13.Kawano N, et al. Identification and localization of estrogen receptor alpha- and beta-positive cells in adult male and female mouse intestine at various estrogen levels. Histochem Cell Biol. 2004;121:399–405. doi: 10.1007/s00418-004-0644-6. [DOI] [PubMed] [Google Scholar]
- 14.Bassotti G, et al. An assessment of enteric nervous system and estroprogestinic receptors in obstructed defecation associated with rectal intussusception. Neurogastroenterol Motil. 2012;24:e155–e161. doi: 10.1111/j.1365-2982.2011.01850.x. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki H, et al. Involvement of estrogen receptor β in maintenance of serotonergic neurons of the dorsal raphe. Mol Psychiatry. 2013;18:674–680. doi: 10.1038/mp.2012.62. [DOI] [PubMed] [Google Scholar]
- 16.Wu WF, et al. Targeting estrogen receptor β in microglia and T cells to treat experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2013;110:3543–3548. doi: 10.1073/pnas.1300313110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanani M, et al. Regeneration of myenteric plexus in the mouse colon after experimental denervation with benzalkonium chloride. J Comp Neurol. 2003;462:315–327. doi: 10.1002/cne.10721. [DOI] [PubMed] [Google Scholar]
- 18.Voss U, Sand E, Olde B, Ekblad E. Enteric neuropathy can be induced by high fat diet in vivo and palmitic acid exposure in vitro. PLoS One. 2013;8:e81413. doi: 10.1371/journal.pone.0081413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beraldi EJ, et al. High-fat diet promotes neuronal loss in the myenteric plexus of the large intestine in mice. Dig Dis Sci. 2015;60:841–849. doi: 10.1007/s10620-014-3402-1. [DOI] [PubMed] [Google Scholar]
- 20.Fowler CD, Johnson F, Wang Z. Estrogen regulation of cell proliferation and distribution of estrogen receptor-alpha in the brains of adult female prairie and meadow voles. J Comp Neurol. 2005;489:166–179. doi: 10.1002/cne.20638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delivopoulos E, Murray AF. Controlled adhesion and growth of long term glial and neuronal cultures on Parylene-C. PLoS One. 2011;6:e25411. doi: 10.1371/journal.pone.0025411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Southard-Smith EM, Kos L, Pavan WJ. Sox10 mutation disrupts neural crest development in Dom Hirschsprung mouse model. Nat Genet. 1998;18:60–64. doi: 10.1038/ng0198-60. [DOI] [PubMed] [Google Scholar]
- 23.Paratore C, Goerich DE, Suter U, Wegner M, Sommer L. Survival and glial fate acquisition of neural crest cells are regulated by an interplay between the transcription factor Sox10 and extrinsic combinatorial signaling. Development. 2001;128:3949–3961. doi: 10.1242/dev.128.20.3949. [DOI] [PubMed] [Google Scholar]
- 24.Kim J, Lo L, Dormand E, Anderson DJ. SOX10 maintains multipotency and inhibits neuronal differentiation of neural crest stem cells. Neuron. 2003;38:17–31. doi: 10.1016/s0896-6273(03)00163-6. [DOI] [PubMed] [Google Scholar]
- 25.McCloy RA, et al. Partial inhibition of Cdk1 in G 2 phase overrides the SAC and decouples mitotic events. Cell Cycle. 2014;13:1400–1412. doi: 10.4161/cc.28401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu WF, et al. Estrogen receptor β, a regulator of androgen receptor signaling in the mouse ventral prostate. Proc Natl Acad Sci USA. 2017;114:E3816–E3822. doi: 10.1073/pnas.1702211114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.