Significance
Ecological processes at regional geographic scales can be connected to those in far distant locations by teleconnections, or interactions between species and systems far removed from one another. Macrosystem ecology views such interactions as elements of much larger ecosystems than either component. We have identified a remarkable example of a transhemispheric macrosystem spanning 15,000 kilometers of the Pacific Ocean maintained by a migratory species of seabird that nests in the South Pacific and winters in the North Pacific. It highlights another example in a growing list of ecosystem disservices of an abundant species of North Pacific salmon, and the need to include ecosystem processes at such geographic scales in conservation and management considerations for this northern open ocean.
Keywords: teleconnection, short-tailed shearwater, carryover effect, interaction chain, ecosystem management
Abstract
Pink salmon (Oncorhynchus gorbuscha) in the North Pacific Ocean have flourished since the 1970s, with growth in wild populations augmented by rising hatchery production. As their abundance has grown, so too has evidence that they are having important effects on other species and on ocean ecosystems. In alternating years of high abundance, they can initiate pelagic trophic cascades in the northern North Pacific Ocean and Bering Sea and depress the availability of common prey resources of other species of salmon, resident seabirds, and other pelagic species. We now propose that the geographic scale of ecosystem disservices of pink salmon is far greater due to a 15,000-kilometer transhemispheric teleconnection in a Pacific Ocean macrosystem maintained by short-tailed shearwaters (Ardenna tenuirostris), seabirds that migrate annually between their nesting grounds in the South Pacific Ocean and wintering grounds in the North Pacific Ocean. Over this century, the frequency and magnitude of mass mortalities of shearwaters as they arrive in Australia, and their abundance and productivity, have been related to the abundance of pink salmon. This has influenced human social, economic, and cultural traditions there, and has the potential to alter the role shearwaters play in insular terrestrial ecology. We can view the unique biennial pulses of pink salmon as a large, replicated, natural experiment that offers basin-scale opportunities to better learn how these ecosystems function. By exploring trophic interaction chains driven by pink salmon, we may achieve a deeper conservation conscientiousness for these northern open oceans.
Linkages between ecosystems and the importance to animal and plant populations, production processes, and community characteristics within and between them are known from a variety of examples at regional geographic scales (e.g., refs. 1–4). On larger scales, linkages, or teleconnections, across broadly separated regions of Earth have been described in numerous fields, including atmospheric sciences, marine and terrestrial ecology, social-ecological systems, and economic markets (e.g., refs. 5–15), and are a foundational element of the emerging subdiscipline of macrosystem ecology (e.g., refs. 16−17, and references therein). Macrosystem ecology draws attention to interactions spanning spatially distant regions that, taken together, have ecosystem characteristics, and indirectly addresses the difficulty in defining ecosystem space. Here we describe a remarkable example of a transhemispheric macrosystem that integrates processes at five geographic scales and six trophic levels spanning some 15,000 km of the Pacific Ocean, with links between meteorology and marine climate in the Northern Hemisphere; the abundance of pink salmon (Oncorhynchus gorbuscha) and marine ecology in the northern North Pacific Ocean and Bering Sea (NP/BS); the ecology of a transhemispheric migrant seabird, the short-tailed shearwater (Ardenna tenuirostris); and terrestrial ecology and social systems in the Tasman Sea in the South Pacific Ocean (SP/TS). Interesting in its own right, this macrosystem is important because it further exposes concern over the growing abundance of wild and hatchery produced salmon, competition for finite common prey resource pools in the NP/BS, and international management and conservation responsibilities for these little-known pelagic ecosystems.
Wild pink salmon stocks began to increase in the 1970s across much of their range in the North Pacific Ocean in association with a shift in the mean state of the Aleutian Low pressure system, the dominant meteorological feature affecting ocean climate over this broad region (e.g., ref. 18). The ensuing regime was favorable for pink salmon (19, 20), and despite subsequent meteorological state shifts (21), their abundance continued to grow. Two other species of Pacific salmon also increased during this time, sockeye (Oncorhynchus nerka) and chum (Oncorhynchus keta), but to far lesser degrees (22). These increasing trends contrast with widespread declines in the abundance of coho salmon (Oncorhynchus kisutch) and the iconic Chinook, or king, salmon (Oncorhynchus tshawytscha) that are of great concern in many ways (e.g., refs. 22–25).
Returns of wild stocks of pink salmon have been augmented 10–20% by hatchery production since the 1980s, primarily in Russia and the United States (22). Pink salmon now constitute ∼70% of the total of all species of Pacific salmon combined and have annual returns in recent years of up to 650 × 106 fish (22). The mechanisms linking pink salmon population dynamics to meteorology and ocean climate are not fully known, but the principal hypotheses focus on early marine survival that is determined in large measure by seawater temperature, the abundance of zooplanktonic prey, and the abundance of piscine predators (e.g., refs. 26 and 27).
Among the Pacific salmon, pink salmon have a unique 2-y life cycle between egg and spawning adult, and most stocks alternate in abundance between years; the majority are much more abundant in odd years than in even years (22, 28). Overall, odd-year stocks are now over twice as abundant as they were in the 1970s (22, 29). It has been argued since the mid-1990s that pink salmon have been having important negative effects on other resident species in the NP/BS through exploitative competition for common prey resources, on the structure of pelagic food webs, and on ecosystem function (30–39).
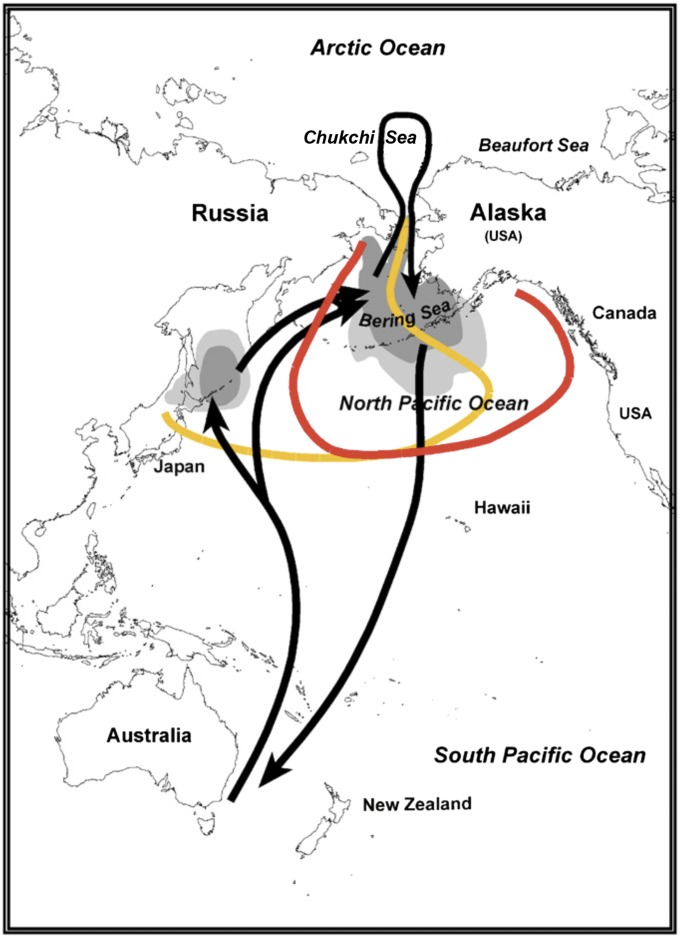
Pink salmon also have been reported to be an important factor in the ecology of short-tailed shearwaters, seabirds that breed in the SP/TS, specifically southeastern Australia, Bass Strait, and Tasmania (SI Appendix, Fig. S1), and spend the austral winter primarily in the NP/BS, although some continue north into the Chukchi Sea (Fig. 1). Roughly 23 × 106 short-tailed shearwaters (45), one of the most abundant species of seabirds in the world, undertake annual migrations of some 30,000-km round trip between the Southern and Northern Hemispheres. Short-tailed shearwaters were shown to be in poorer physical condition and to succumb in greater numbers in the northwestern North Pacific Ocean and central Bering Sea in odd years than in even years (46, 47). Numbers of dead shearwaters encountered on St. Paul Island (Pribilof Islands, eastern Bering Sea) were more than an order-of-magnitude greater in odd years than in even years between 2006 and 2010: odd-year average of 0.57 versus even-year average of 0.022 birds per standardized beach survey (from data reported in ref. 48). Diets of pink salmon and short-tailed shearwaters overlap (49–54), and the biennial pattern in shearwater body condition has been linked to competition with pink salmon for common prey (47). These patterns are distinct from occasional mass mortalities (wrecks), which are comprised primarily of immature birds that occur off Japan in spring as they arrive from the Southern Hemisphere (55), and in the Bering Sea, where wrecks have occurred at least twice in late summer, in 1983 and 1997 (56, 57). Both years were odd years but were further beset by strong El Niño conditions. Birds found in wrecks off Japan and in the Bering Sea appeared to have starved to death. The return migration from the NP/BS to the SP/TS takes about 18 d in September to October (42), and is fueled by fat stores accumulated on the wintering grounds. Because both fall and spring migrations are nonstop, short-tailed shearwaters would not be a factor in, or be affected by, food web processes in the intervening ocean.
Fig. 1.
Generalized open ocean ranges of pink salmon from Japan and Russia (yellow) and Alaska (red) as depicted by refs. 40 and 41, and generalized migration routes of short-tailed shearwaters (arrows) and distribution in the northern North Pacific Ocean, Bering Sea, and Chukchi Sea (after refs. 42 and 43, and information presented in ref. 44). Shearwater distribution depicted in gray, with higher concentrations in darker shades.
Results
Trends in Pink Salmon Abundance.
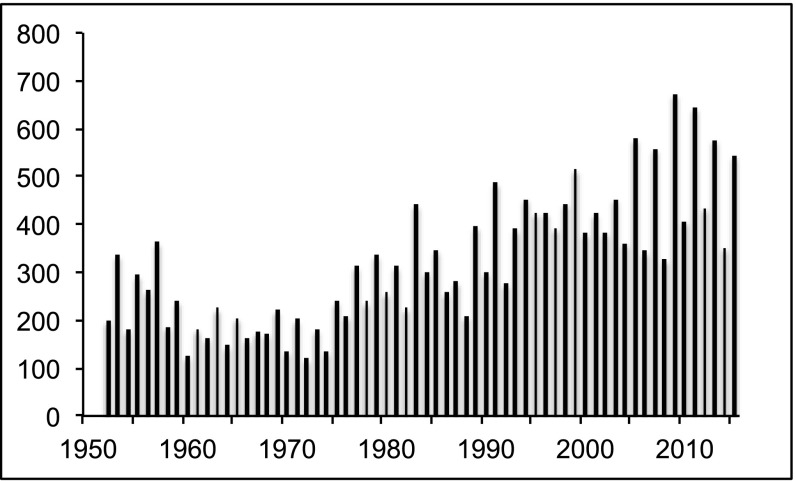
The overall abundance of pink salmon varied over a comparatively small, low range from the early 1950s to the mid-1970s, then increased markedly through about 1990 (Fig. 2). That increase was followed by a second period of relative stability to about 2004. Beginning in 2005, odd-year stocks increased substantially, whereas even-year stocks remained about the same as in the previous interval: the odd-year mean in 2005–2015 increased by 35% over the odd-year mean for 1990–2004 (from 440 × 106 to 595 × 106 fish, P = 0.0001), with odd-year stocks averaging 60% greater than even year stocks (595 × 106 fish vs. 372 × 106 fish, P = 0.0001).
Fig. 2.
Returns (catch plus escapement, millions) of pink salmon in the North Pacific Ocean, 1952–2015. Data from ref. 22.
Long-term trends in abundance of the four groups of salmon we used in our analyses (Methods) were generally similar (SI Appendix, Fig. S2). The greatest difference from the overall pattern was for salmon from the Western Kamchatka Peninsula.
Trends in Shearwater Abundance.
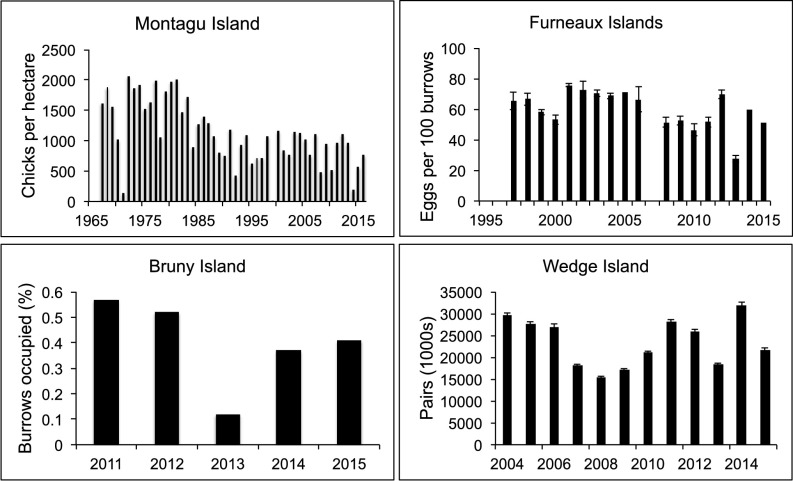
The abundance of short-tailed shearwaters at Montagu Island fell by about 50% during the 1980s (Fig. 3 and SI Appendix); Montagu Island is one of the longest systematic annual monitoring sites for the species. There was no trend in the interval 1990–2016 (R2 = 0.043, P = 0.86), but abundance declined at about −4% y−1 during 2000–2015 (R2 = 0.21, P = 0.063). The slope of the regression for the most recent interval 2005–2016 indicated a decline in abundance of −4.7% y−1, but had little predictive power (R2 = 0.11, P = 0.29), perhaps because of the short time series. Abundance at colonies monitored in the Furneaux Islands declined by −2.1% y−1 across the full sampling interval of 1997–2015 (R2 = 0.26, P = 0.032). Although the slope of the regression was greater after 2005 (−3.4% y−1), it too had little predictive power (R2 = 0.17, P = 0.23). Numbers at Wedge Island fell from ∼30,000 pairs in 2004, when systematic censusing began, to 15,000 pairs in 2008. Since then population estimates have been variable and there was no trend across the full interval (R2 = 0.002, P = 0.88). A short time series of population estimates in 2011–2015 at the main colony on Bruny Island showed no overall trend in abundance (R2 = 0.064, P = 0.68), even though the slope of the regression was – 10% y−1, but did reveal a sharp drop in 2013 that was apparent at all of the other locations where data were available.
Fig. 3.
Trends in abundance of short-tailed shearwaters at four breeding locations in southeastern Australia: Montagu Island, the Furneaux Islands, Wedge Island, and Bruny Island. See Methods for data sources and metrics for abundance estimates.
Interannual Variability in Shearwater Abundance.
The mean and median abundances of short-tailed shearwaters in even years and odd years at Montagu Island were not different in the full interval 1967–2016 (Table 1). However, as the intervals became shorter and more recent, the difference between even- and odd-year abundances grew such that by 2005–2016, when odd year pink salmon stocks were largest, ∼40–50% more shearwaters nested in even years than in odd years.
Table 1.
Mean and median abundances of short-tailed shearwaters at Montagu Island in even years and odd years
| Interval | Mean (±SE) even | Mean (±SE) odd | P | Median even | Median odd | Mean ratio | Median ratio |
| 1967–2016 | 1,237 (92) | 1,092 (101) | 0.30 | 1,144 | 1,066 | 1.13 | 1.07 |
| 1980–2016 | 1,064 (89) | 876 (80) | 0.12 | 966 | 831 | 1.22 | 1.16 |
| 1990–2016 | 916 (57) | 783 (87) | 0.22 | 952 | 769 | 1.17 | 1.24 |
| 2000–2016 | 942 (64) | 716 (111) | 0.10 | 962 | 764 | 1.31 | 1.26 |
| 2005–2016 | 907 (91) | 639 (127) | 0.13 | 957 | 641 | 1.42 | 1.49 |
Chicks per hectare. First series without 1967 and 1999. Second two series without 1999, as explained in Methods.
There was no evidence of differences in mean values of even years and odd years in 1997–2015 or 2005–2015 at the Furneaux Islands based on P values, although the mean and median values in even years in 2005–2015 were 16% greater than in odd years (Table 2). Similarly, means and medians in the single interval at Wedge Island, 2004–2015, were 15% and 32%, respectively, higher in even years than in odd years, but the significance level of the difference between mean values also was low (Table 3). The time series at Bruny Island was too short for meaningful analysis.
Table 2.
Mean and median values of short-tailed shearwater abundance at the Furneaux Islands in even years and odd years (eggs per 100 burrows)
| Interval | Mean (±SE) even | Mean (±SE) odd | P | Median even | Median odd | Mean ratio | Median ratio |
| 1997–2015 | 61.7 (3.1) | 58.1 (4.9) | 0.55 | 66.4 | 58.0 | 1.06 | 1.14 |
| 2005–2015 | 58.9 (4.4) | 50.1 (8.9) | 0.41 | 60.1 | 51.6 | 1.16 | 1.16 |
Table 3.
Mean and median abundances of short-tailed shearwaters at Wedge Island in even years and odd years (adults)
| Interval | Mean (±SE) even | Mean (±SE) odd | P | Median even | Median odd | Mean ratio | Median ratio |
| 2004–2015 | 25,154 (2,462) | 21,903 (2,008) | 0.33 | 26,498 | 20,074 | 1.15 | 1.32 |
Shearwater–Pink Salmon Correlations.
The small number of years in our time series, in the two most recent intervals in particular, limited the power to make inferences about the importance of local summer rainfall (Methods) and salmon to shearwater abundance. With that in mind, rainfall and pink salmon abundance explained from 50% to over 70% of the variability in the abundance of short-tailed shearwaters on Montagu Island in four of the five intervals we analyzed (Table 4). The relationship was weaker in the interval 2000–2016. Rainfall and Alaska salmon were both important predictors of shearwater abundance in the first three intervals, as was Eastern Kamchatka Peninsula salmon in the last three intervals. The importance of December to February rainfall and Alaska salmon declined in 2000–2016 and 2005–2016. In the most recent interval (2005–2016), when pink salmon abundance was highest, 73% of the variability in shearwater abundance at Montagu Island was explained, primarily by rainfall, Eastern Kamchatka Peninsula salmon, and Alaska salmon. Japan + Sea of Okhotsk salmon were marginally significant only in 1990–2016, and Western Kamchatka Peninsula salmon only in 2005–2016.
Table 4.
Results of multiple regression analysis of short-tailed shearwater abundance at Montagu Island versus summer (December to February) rainfall at Montagu Island and pink salmon abundance in four regions of the North Pacific Ocean
| Interval | Adjusted R2 (P) | December to February rain, t (P) | A*, t (P) | B†, t (P) | C‡, t (P) | D§, t (P) |
| 1967–2016 | 0.61 (<0.0001) | −5.5 (<0.0001) | −1.2 (0.24) | −1.3 (0.21) | 0.63 (0.53) | −5.1 (<0.0001) |
| 1980–2016 | 0.50 (0.0001) | −3.7 (0.001) | −1.4 (0.17) | −0.77 (0.44) | 1.2 (0.24) | −3.7 (0.0009) |
| 1990–2016 | 0.65 (0.0001) | −5.6 (<0.0001) | −1.8 (0.08) | 0.028 (0.98) | 2.8 (0.013) | −4.2 (0.0006) |
| 2000–2016 | 0.33 (0.11) | −1.6 (0.14) | −1.5 (0.15) | 0.28 (0.79) | 2.1 (0.064) | −2.2 (0.053) |
| 2005–2016 | 0.73 (0.031) | −1.7 (0.14) | −1.4 (0.21) | 2.0 (0.10) | 3.5 (0.017) | −2.9 (0.033) |
Japan + Sea of Okhotsk, excluding Western Kamchatka Peninsula.
Western Kamchatka Peninsula.
Eastern Kamchatka Peninsula.
Alaska.
The positive relationship between Eastern Kamchatka Peninsula salmon and shearwaters in all intervals, particularly the apparently highly significant ones in the most recent three intervals, was not expected. Because of this, and because Japan + Sea of Okhotsk and Western Kamchatka Peninsula stocks did not contribute importantly in any intervals, we reran the model using only December to February rainfall and Eastern Kamchatka Peninsula and Alaska salmon (Table 5). The adjusted R2 value for 2005–2016 fell somewhat but remained strongly significant, while all other interval values were similar to those in the original model. The importance of December to February rainfall remained about the same, and Alaska salmon tended to be more important. In contrast, the importance of Eastern Kamchatka Peninsula salmon declined markedly in the two most recent intervals, although it remained important in the single interval 1990–2016. When shearwater abundance in the five time intervals was regressed against Eastern Kamchatka Peninsula pink salmon alone, the only marginally significant relationship was for the full interval (1967–2016, P = 0.08), but it explained very little of the variability in shearwater abundance (R2 = 0.06). There were no relationships for the other four intervals: all R2 ≤ 0.03 and all P ≥ 0.33.
Table 5.
Results of multiple regression analysis of short-tailed shearwater abundance at Montagu Island versus summer (December to February) rainfall at Montagu Island and pink salmon abundance in two regions of the North Pacific Ocean
| Interval | Adjusted R2 (P) | December to February rain, t (P) | C*, t (P) | D†, t (P) |
| 1967–2016 | 0.60 (<0.0001) | −5.6 (<0.0001) | 0.76 (0.45) | −6.3 (<0.0001) |
| 1980–2016 | 0.50 (<0.0001) | −4.2 (0.0002) | 1.2 (0.23) | −4.4 (0.0001) |
| 1990–2016 | 0.62 (<0.0001) | −5.4 (<0.0001) | 2.5 (0.019) | −4.1 (0.0005) |
| 2000–2016 | 0.29 (0.069) | −1.7 (0.10) | 1.7 (0.11) | −2.9 (0.012) |
| 2005–2016 | 0.53 (0.040) | −1.5 (0.18) | 1.9 (0.10) | −3.8 (0.007) |
Eastern Kamchatka Peninsula.
Alaska.
There was no relationship between shearwater abundance at the Furneaux Islands and rainfall and salmon in 1997–2015 (adjusted R2 = −0.02, P = 0.49) or 2005–2015 (adjusted R2 = −0.40, P = 0.77). Similarly, there was no relationship between shearwaters and rainfall and salmon at Wedge Island in 2004–2015 (adjusted R2 = −0.17, P = 0.64).
Shearwater Wrecks in Australia.
Wrecks of short-tailed shearwaters shortly after they returned to nesting colonies in Australia and Tasmania have been reported occasionally since the mid-1800s, and dead birds were typically emaciated (e.g., refs. 58 and 59). Environmental conditions in the broader marine region of the Tasman Sea as the birds return from the Northern Hemisphere may at times be responsible: for example, anomalously warm sea surface temperatures in October 2000 may have reduced the availability of important prey at a critical time that caused a major wreck that year (59).
However, beginning in 2007 and coinciding with the increasing abundance of odd-year pink salmon, wrecks occurred in every odd year to at least 2013. The wreck in 2013 was extreme, when very high numbers of birds were stranded in Australia and Tasmania, and even in New Zealand, where they do not nest (60), and corresponded with extremely high abundances of pink salmon returning to United States waters (ref. 22 and www.npafc.org/new/science_statistics.html). Birds in those wrecks also were emaciated, suggesting that they failed to accumulate sufficient fat before departure on their southward migration to sustain them until they could refuel upon their return. Because the wrecks have been occurring in odd years, pink salmon may be implicated as the cause. Negative carryover effects of poor nutrition, apparently due to competition with pink salmon in odd years, also have been seen in resident seabirds that nest in the Aleutian Islands and Bering Sea (37).
Discussion
Short-tailed shearwaters depart their wintering areas beginning in mid to late September (42, 43), about 1 to 2 mo after the peak spawning runs of pink salmon (61, 62). However, their distributions broadly overlap during the time in summer when salmon are feeding voraciously and growing rapidly as they return to their spawning rivers. We believe correlations between the abundance of short-tailed shearwaters and pink salmon in this study provide strong support for the hypothesis that exploitative competition by the fish is having negative effects on the birds, with carryover effects on them at their nesting colonies in the SP/TS. The evidence includes observations of poor physical condition and elevated mortality in the western and eastern Bering Sea in odd years that have been noted previously and that we report here, and in recent years: (i) the wrecks in odd years in southeastern Australia as birds return to their nesting grounds; (ii) the tendency for greater numbers of shearwaters to nest in even years than in odd years, with the biennial difference growing as pink salmon abundance has increased in odd years; (iii) the negative trends in shearwater abundance at Montagu Island and the Furneaux Islands; and (iv) after accounting for the important effect of rainfall, the strong inverse relationship between the abundance of nesting shearwaters at Montagu Island and the abundance of Alaska pink salmon in all five time intervals we examined. That Alaska pink salmon, and not Asian pink salmon, explained most of the variability in shearwater abundance at Montagu Island is perhaps not surprising, since the range of Alaska pink salmon at sea appears to overlap most with the wintering range of the birds. We do not know why Eastern Kamchatka Peninsula salmon were positively related to shearwater abundance at Montagu Island in the full model, but the strength of the relationship was weak or absent after removing Japan + Sea of Okhotsk and Western Kamchatka Peninsula from our analysis. Moreover, there was no evidence of a relationship of the birds to Eastern Kamchatka Peninsula pink salmon in the latter four time intervals when only those fish were considered.
Nor do we know why correlations between pink salmon and shearwaters nesting at the Furneaux Islands and Wedge Island were not identified in our analysis, and refrain here from speculating about possible reasons. However, we believe that this does not materially controvert our hypothesis, based on the totality of evidence, that competition by pink salmon leads to negative effects on overwintering and nesting short-tailed shearwaters.
Our hypothesis is bolstered by compelling correlations between pink salmon abundance and the phenologies, productivity, and diets of several species of resident seabirds in the Bering Sea (37). The weaker strength of correlations between shearwaters and salmon compared with resident seabirds and salmon may be explained by the broad distribution of shearwaters during their nonbreeding season and the smaller spatial, and briefer temporal, overlap with foraging areas occupied by pink salmon. Interannual variability in shearwater distribution in the Bering Sea due to changing sea ice conditions (63) may further mask the appearance of relationships to pink salmon.
The proposed role of pink salmon in the ecology of short-tailed shearwaters does not preclude other negative or positive impacts on the abundance or productivity of the birds that may derive from seasonal or annual vagaries in ocean climate or longer-term changes in the marine environment of the Tasman Sea or the Southern Ocean due to ocean warming or other causes (e.g., refs. 64–66).
Shearwaters and Terrestrial Ecology.
The putative negative effects of pink salmon on short-tailed shearwaters carry beyond just the birds. Many species of seabirds can be important to terrestrial ecology on islands where they nest, chiefly by the transport of marine-derived nitrogen and phosphorus that fertilize soils and streams, and by bioturbation by burrowing species, such as shearwaters, which mixes and aerates soils, all of which alter the composition and productivity of floral communities (e.g., refs. 3 and 67–69). The effects of guano deposition and bioturbation by short-tailed shearwaters on physical and chemical soil properties and on terrestrial vegetation are well documented, as they are for two closely related species, wedge-tailed shearwaters (Ardenna pacifica) in Western Australia and sooty shearwaters (Ardenna grisea) in New Zealand, and are important influences on island ecology (69–73). At Bruny Island, for example, changes in abundances of short-tailed shearwaters between 1977 and 1992 led to marked changes in the composition of the floral community (71).
Shearwaters and Society.
Short-tailed shearwaters also are important to cultural, social, and economic systems in Tasmania, as are other species of shearwaters and petrels there and in New Zealand (74, 75). A commercial harvest of short-tailed shearwater chicks by Aboriginal residents averaged about 72,000 ± 8,800 birds each season in 2007–2017 (76). Chicks also are harvested under recreational licenses available to the public: for example, an average of 884 ± 44 licenses were sold annually in 2011–2016 that resulted in an average annual harvest of about 34,500 ± 5,300 chicks (77).
As a consequence of the massive wreck in 2013 and the dramatic drop in abundance of nesting adults, only 3,300 chicks were taken in the commercial harvest and only 10,913 in the recreational harvest during the ensuing season in 2014 (76, 77).
Teleconnections.
The conceptual model of the macrosystem we propose integrates processes at five geographic scales: local, regional, basin, hemispheric, and transhemispheric. At local scales, individual stocks of pink salmon prosper or not depending on factors that influence early marine survival: that is, prey and predator fields that are in turn determined by local and regional ocean climate (26, 27). At basin scales, atmospheric forcing sets up regional and local ocean climate conditions (78) that influence trends in pink salmon abundance (79) and the effect salmon have on basin-scale prey fields as they mature and migrate in a consumer front back to their spawning rivers. At hemispheric scales, teleconnections between atmospheric systems over the Pacific Ocean, North America, and the Arctic drive basin-scale physical forcing in the NP/BS that condition local-, regional-, and basin-scale ocean climate (refs. 18 and 80, and references therein). At transhemispheric scales, shearwaters migrate 15,000 km from wintering grounds in the NP/BS to nesting colonies in the SP/TS, where in odd years they arrive in poorer physical condition, may experience wrecks, and tend to nest in fewer numbers than in even years. And back to local and regional scales, where wrecks and an apparent decline in shearwater abundance is of concern to Aboriginal residents in regard to their subsistence economy, cultural identity, and recreational and commercial harvests, and stand to impact ecosystem services in the form of soil fertilization and aeration that are important to vegetation community structure on islands where they nest.
The model also integrates interactions across at least six trophic levels in the NP/BS and three in the SP/TS. In the NP/BS pink salmon apparently can initiate pelagic trophic cascades by depleting the abundance of herbivorous zooplankton that leads to elevated standing stocks of phytoplankton. In the other trophic direction, reductions of zooplankton stocks impact predatory micronekton and mesonekton, including other species of salmon that prey upon them. Many species of zooplankton and micronekton are important prey of resident NP/BS seabirds and migratory short-tailed shearwaters. In the SP/TS, shearwaters are important in several ways to indigenous residents and to terrestrial vegetation patterns and ecology.
Conservation of Ecosystems.
Competition among wild pink salmon and numerous other species for finite, common prey resources in the NP/BS appears to have been increasing as salmon abundance has grown. The addition of hatchery-produced salmon that are further filling the ocean is becoming a particular cause for concern; for example, since 1990 in the order of 1.2–1.5 × 109 juvenile pink salmon have been released annually into the northern North Pacific Ocean (22). In 2016 ∼0.64 × 109 smolts were released into Prince William Sound, Alaska alone, where they appear to be having negative impacts on wild pink salmon, sockeye salmon (O. nerka), and Pacific herring (Clupea pallasi) (81–83). In 2013 an estimated 103 × 106 adult pink salmon returned to Prince William Sound, of which ∼30% were wild and 70% were hatchery fish (84).
Salmon and seabirds are conspicuous and have high ecological, economic, cultural, and societal values, thus a great amount of research is devoted to them. But there are other crucial species in the oceanic ecosystems of the NP/BS, including mesopelagic squids, myctophids (Myctophidae), and deep-sea smelts (Bathylagidae) that also compete for the same prey (85–89). They are of particular ecological value in a variety of ways, from sustaining numerous species of salmon, seabirds, and marine mammals (90–96), to being important engines in the oceanic biological pump as vertically migrating predators (97). The biomass of myctophids and bathylagids in the eastern Bering Sea basin alone has been placed in the order of 1–8 × 106 tons each (97, 98), but nothing is known about trends in abundance and other fundamental elements of their ecology because they have no direct economic value.
The desire to continue to raise production levels of wild and hatchery salmon is understandable; the overall annual multinational economic value of Pacific salmon is in the order of 109 US$ and the industry employs tens of thousands of people (99). But it is now time to take stock of the consequences—the ecosystem disservices of salmon—of doing so on other economic, social, cultural, and ecological values in the NP/BS (100, 101) and, because of the teleconnection described here, in the SP/TS as well.
The short-tailed shearwater is not a species in peril, but the apparent response of these birds, as well as responses of humans, resident NP/BS seabirds, other salmon, herring, and likely species yet to be identified, to ecological forcing by pink salmon suggests that pink salmon are altering the distribution of wealth stored in this macrosystem (in the sense of ref. 102). Together, these responses emphasize that we must develop a deeper conservation conscientiousness for this entire oceanic system and more informed approaches for the management of the whole.
This large, replicated, natural experiment is not strictly a replicated natural experiment, since conditions in the NP/BS vary on annual and multiyear timescales for reasons other than pink salmon, for example weather and climate have large influences over patterns of annual production, including those of pink salmon. However, it is perhaps as near as we can come to experimental replication at ocean basin scales, and we should therefore use this unique opportunity to delve into trophic interaction chains driven by pink salmon and help remedy the conspicuous and unfortunate lack of knowledge about marine ecology in these important realms.
Methods
Shearwater Abundance.
The abundance of adult shearwaters arriving at the nesting colonies and the numbers that lay eggs are most meaningful to address correlations with pink salmon abundance. However, this information is very difficult to acquire and is lacking for most sites. Thus, we used proxies of abundance for Montagu Island, the Furneaux Islands, and Bruny Island (SI Appendix, Fig. S1). The proxy at Montagu Island (1967–2016) was the density of chicks on three representative study plots in late March, just before fledging of chicks, as reported by refs. 103–107 for 1967–2003, and by ref. 108 for 2004–2015; P.F. provided data for 2016. Single annual values were derived by summing the numbers of chicks on the plots, calculating the density by dividing that number by the total area of the three plots (1,014 m2), and converting that value to chicks per hectare. Burrow searches were thorough and counts were made only once each year to reduce disturbance and the possibility of desertion by adults.
The proxy of abundance at the Furneaux Islands (1997–2015) was the mean number of eggs per 100 burrows on standardized survey transects on four islands in the island group (East Kangaroo, Little Green, Little Dog, and Big Green) provided by R.M. Counts were made in all but 1 y at East Kangaroo and Big Green, and in 14 of 19 y at Little Green and Little Dog. The proxy at Bruny Island was the number of burrows occupied by nesting birds per total number of burrows examined. Data from 2011 were provided by B. Edwards, Bruny Island, Parks and Wildlife Service, Hobart, Tasmania, Australia; data from 2012 and 2013 were provided by N.B.; and data from 2015 were provided by P. Vertigan, BirdLife Tasmania, Hobart, Tasmania, Australia. We used the actual number of breeding pairs on Wedge Island (2004–2015) as reported by (109) for 2004–2010 and provided by N.B. for 2011–2015. All census data used here for the Furneaux Islands, Wedge Island, and Bruny Island were collected in December. As at Montagu Island, burrow searches at those locations were thorough and were made only once each year.
We compared shearwater abundance between even years and odd years at Montagu Island during five intervals: (i) 1967–2016, (ii) 1980–2016, (iii) 1990–2016, (iv) 2000–2016, and (v) 2005–2016. The intervals correspond to: (i) the full sampling interval, (ii) the approximate beginning of the decline in abundance in 1980, (iii) the approximate end of the decline in 1990 and ensuing period of markedly higher salmon abundance, (iv) a period of relative stability in shearwater abundance following a partial recovery during the 1990s, and (v) an interval of very high salmon abundance (Fig. 3 and SI Appendix, Fig. S2). Near or total nesting failures occurred in 1971 and 1999 due to heavy rainfall (107), so we did not use those years in intervals one to three. We used the intervals 1997–2015 and 2005–2015 at the Furneaux Islands and the single interval 2004–2015 at Wedge Island. The time series at Bruny Island was too short for these analyses.
Shearwater Abundance Versus Pink Salmon Abundance.
We used annual catch plus escapement data reported by ref. 22 for the estimate of pink salmon abundance. These data do not have confidence intervals associated with them, as the methods used to derive the values do not lend themselves to variance statistics.
We compared the abundance of shearwaters at Montagu Island, the Furneaux Islands, and Wedge Island in the same intervals to summer rainfall and the abundance of four groups of pink salmon that were aggregated based on the winter ranges and return spawning migration corridors of stocks, as depicted by refs. 40 and 41. We included rainfall data because it has been shown to be important to chick survival at Montagu Island (107). Rainfall data for Montagu Island came from the Montagu Island Lighthouse, for the Furneaux Islands from the Flinders Island airport, and for Wedge Island from Tarana, Tasmania. All rainfall data are available at www.bom.gov.au/climate/data/index.shtml?bookmark=136. The pink salmon groups were: (i) Japan + Sea of Okhotsk, excluding Western Kamchatka Peninsula; (ii) Western Kamchatka Peninsula; (iii) Eastern Kamchatka Peninsula; and (iv) Alaska. British Columbia and Washington pink salmon were not included in the models because their at-sea range overlaps little with the winter distribution of the majority of short-tailed shearwaters. Although the winter distribution of pink salmon from Western Kamchatka Peninsula generally overlaps those of fish from Japan and elsewhere in the Sea of Okhotsk, they are predominantly even-year dominant stocks so were considered separately from the other stocks in the northwestern Pacific Ocean that are predominantly odd-year dominant. Also, their trend in abundance generally differs from trends of the other groups (SI Appendix, Fig. S2). Again, there were too few years for this analysis at Bruny Island.
Data Analysis.
Mean values of shearwater abundances in even and odd years were compared using Student’s t test. Shearwater annual abundance data were natural log-transformed to calculate trends in abundance using linear regression.
We compared the abundance of shearwaters to rainfall and the abundance of pink salmon using multiple linear regression. We first assessed the extent of collinearity among the salmon groups, but found little evidence for it, as variance inflation factors for all groups were ≤2.4. We used summer (December–February) rainfall as a fifth independent variable in the model for Montagu Island because chicks were counted there in March, and used only December rainfall in models for the Furneaux Islands and Wedge Island because census counts at those locations were made in December.
We compared shearwater chick counts at Montagu Island in March to pink salmon abundance values from the previous calendar year. Shearwater counts at the Furneaux Islands and Wedge Island were made in the same calendar year as salmon abundance estimates.
We did not select an a priori strict threshold for statistical significance. Instead, all correlation coefficients and significance values are presented and considered, along with temporal patterns in change and values of group means and medians, to make biological inferences.
Research Permits.
BirdLife Tasmania holds all animal research and scientific permits required by law to undertake the surveys and research described in the study. All surveys were approved by the Animal Ethics Committee of the Department of Primary Industries, Parks, Water, and Environment. The University of Tasmania Animal Ethics Committee also approved research at Wedge Island and Fisher Island.
Supplementary Material
Acknowledgments
We thank C. Davey and P. Heyligers for tireless efforts in the field at Montagu Island; B. Edwards and P. Vertigan for allowing us to report their unpublished data; A. Blanchard and J. Sterling for advice on statistical analysis; M. Hindell, who has been instrumental in organizing shearwater research in Tasmania and who supervises N.B. and C.P.; C. McMahon, who cosupervises N.B. and C.P.; and G. Ruggerone, two anonymous reviewers, and the editor for helpful and collegial comments on an earlier draft of this manuscript.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
2Retired.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1720577115/-/DCSupplemental.
References
- 1.Estes JA, Tinker MT, Williams TM, Doak DF. Killer whale predation on sea otters linking oceanic and nearshore ecosystems. Science. 1998;282:473–476. doi: 10.1126/science.282.5388.473. [DOI] [PubMed] [Google Scholar]
- 2.Rose DR, Polis GA. The distribution and abundance of coyotes: The effects of allochthonous food. Ecology. 1998;79:998–1007. [Google Scholar]
- 3.Croll DA, Maron JL, Estes JA, Danner EM, Byrd GV. Introduced predators transform subarctic islands from grassland to tundra. Science. 2005;307:1959–1961. doi: 10.1126/science.1108485. [DOI] [PubMed] [Google Scholar]
- 4.McCauley DJ, et al. From wing to wing: The persistence of long ecological interaction chains in less-disturbed ecosystems. Sci Rep. 2012;2:409. doi: 10.1038/srep00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deser C, Phillips AS, Hurrell JW. Pacific interdecadal climate variability: Linkages between the tropics and the North Pacific during boreal winter since 1900. J Clim. 2004;17:3109–3124. [Google Scholar]
- 6.Shaffer SA, et al. Migratory shearwaters integrate oceanic resources across the Pacific Ocean in an endless summer. Proc Natl Acad Sci USA. 2006;103:12799–12802. doi: 10.1073/pnas.0603715103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Divine DV, et al. Tropical Pacific–high latitude south Atlantic teleconnections as seen in ∂18O variability in Antarctic coastal ice cores. J Geophys Res. 2009;114:1–14. [Google Scholar]
- 8.Schwing FB, Mendelsson R, Bograd SJ, Overland JE. Climate change, teleconnection patterns, and regional processes forcing marine populations in the Pacific. J Mar Syst. 2010;79:245–257. [Google Scholar]
- 9.Algeo TJ, Chen ZQ, Fraiser ML, Twitchett RJ. Terrestrial–marine teleconnections in the collapse and rebuilding of Early Triassic marine ecosystems. Palaeogeogr Palaeoclimatol Palaeoecol. 2011;308:1–11. [Google Scholar]
- 10.Ruggerone GT, Agler BA, Nielsen JL. Evidence for competition at sea between Norton Sound chum salmon and Asian hatchery chum salmon. Environ Biol Fishes. 2012;94:149–163. [Google Scholar]
- 11.Chailles E, Newig J, Lenschow A. What role for social-ecological systems research in governing global teleconnections? Glob Environ Change. 2014;27:32–40. [Google Scholar]
- 12.Hoell A, Funk C, Barlow M. The regional forcing of Northern hemisphere drought during recent warm tropical west Pacific Ocean La Niña events. Clim Dyn. 2014;42:3289–3311. [Google Scholar]
- 13.Peterson SL, Rockwell RF, Witte CR, Koons DN. Legacy effects of habitat degradation by lesser snow geese on nesting Savannah sparrows. Condor. 2014;116:527–537. [Google Scholar]
- 14.Moser C, Hart JF. The long arm of climate change: Societal teleconnections and the future of climate change impacts studies. Clim Change. 2015;129:13–26. doi: 10.1007/s10584-015-1328-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knox RG, et al. Hydrometeorological effects of historical land-conversion in an ecosystem-atmosphere model of Northern South America. Hydrol Earth Syst Sci. 2015;19:241–273. [Google Scholar]
- 16.Heffernan JB, et al. Macrosystems ecology: Understanding ecological patterns and processes at continental scales. Front Ecol Environ. 2014;12:5–14. [Google Scholar]
- 17.Fei S, Guo Q, Potter K. Macrosystems ecology: Novel methods and new understanding of multi-scale patterns and processes. Landsc Ecol. 2016;31:1–6. [Google Scholar]
- 18.Overland JE, Adams JM, Bond NA. Decadal variability in the Aleutian Low and its relation to high-latitude circulation. J Clim. 1999;12:1542–1548. [Google Scholar]
- 19.Beamish RJ, Bouillon DR. Salmon production trends in relation to climate. Can J Fish Aquat Sci. 1993;50:1002–1016. [Google Scholar]
- 20.Mantua NJ, Hare SR, Zhang Y, Wallace JM, Francis RC. A Pacific interdecadal climate oscillation with impacts on salmon production. Bull Am Meteorol Soc. 1997;78:1069–1079. [Google Scholar]
- 21.Overland J, Rodionov S, Minobe S, Bond N. North Pacific regime shifts: Definitions, issues and recent transitions. Prog Oceanogr. 2008;77:92–102. [Google Scholar]
- 22.Ruggerone GT, Irvine JR. Numbers and biomass of natural- and hatchery-origin pink, chum, and sockeye salmon in the North Pacific Ocean, 1925–2015. Mar Coast Fish. 2018;10:152–168. [Google Scholar]
- 23.Riddell BR, et al. 2013 Assessment of status and factors for decline of Southern BC Chinook Salmon: Independent panel’s report. Available at www.psc.org/publications/workshop-reports/southern-bc-chinook-expert-panel-workshop. Accessed April 26, 2018.
- 24.Schindler D, et al. 2013 Arctic-Yukon-Kuskokwim Chinook salmon research action plan: Evidence of decline of Chinook salmon populations and recommendations for future research. Available at www.aykssi.org/wp-content/uploads/AYK-SSI-Chinook-Salmon-Action-Plan-83013.pdf. Accessed April 26, 2018.
- 25.Irvine JR, Fukuwaka M. Pacific salmon abundance trends and climate change. ICES J Mar Sci. 2011;68:1122–1130. [Google Scholar]
- 26.Cooney RT. Pink salmon. In: Spies RB, editor. Long-term Ecological Change in the Northern Gulf of Alaska. Elsevier; Oxford: 2007. pp. 76–81. [Google Scholar]
- 27.Davis ND, Chan C, editors. 2011. International workshop on explanations for the high abundance of pink and chum salmon and future trends (North Pacific Anadromous Fish Commission, Vancouver), Technical Report 8.
- 28.Neave F. “Even-year” and “odd-year” pink salmon populations. Proc Trans Royal Soc Can Ser. 1952;3:55–70. [Google Scholar]
- 29.Gritsenko AV, Kharenko EN. Relation between biological parameters of Pacific salmons of the genus Oncorhynchus and their population dynamics off the northeastern Kamchatka Peninsula. J Ichthyol. 2015;55:430–441. [Google Scholar]
- 30.Ruggerone GT, Connors BM. Productivity and life history of sockeye salmon in relation to competition with pink and sockeye salmon in the North Pacific Ocean. Can J Fish Aquat Sci. 2015;72:1–16. [Google Scholar]
- 31.Shiomoto A, Tadokoro K, Nagasawa K, Ishida Y. Trophic relations in the subarctic North Pacific ecosystem: Possible feeding effect from pink salmon. Mar Ecol Prog Ser. 1997;150:75–85. [Google Scholar]
- 32.Sugimoto T, Tadokoro K. Interannual-interdecadal variations in zooplankton biomass, chlorophyll concentration and physical environment in the subarctic Pacific and Bering Sea. Fish Oceanogr. 1997;6:74–93. [Google Scholar]
- 33.Azumaya T, Ishida Y. Density interactions between pink salmon (Oncorhynchus gorbuscha) and chum salmon (O. keta) and their possible effects on distribution and growth in North Pacific Ocean and Bering Sea. N Pac Anad Fish Com Bull. 2000;2:165–174. [Google Scholar]
- 34.Ishida Y, Azumaya T, Fukura M, Davis N. Interannual variability in stock abundance and body size of Pacific salmon in the central Bering Sea. Prog Oceanogr. 2002;55:223–234. [Google Scholar]
- 35.Kaga T, Sato S, Azumaya T, Davis ND, Fukuwaka M-a. Lipid content of chum salmon Oncorhynchus keta affected by pink salmon O. gorbuscha abundance in the central Bering Sea. Mar Ecol Prog Ser. 2013;478:211–221. [Google Scholar]
- 36.Ruggerone GT, Nielsen JL. Evidence for competitive dominance of pink salmon (Oncorhynchus gorbuscha) over other salmonids in the North Pacific Ocean. Rev Fish Biol Fish. 2004;14:371–390. [Google Scholar]
- 37.Springer AM, van Vliet GB. Climate change, pink salmon, and the nexus between bottom-up and top-down forcing in the subarctic Pacific Ocean and Bering Sea. Proc Natl Acad Sci USA. 2014;111:E1880–E1888. doi: 10.1073/pnas.1319089111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruggerone GT, et al. Competition between pink and sockeye salmon at sea and its influence on Bristol Bay sockeye salmon forecast error. N Pac Anad Fish Comm Bull. 2016;6:349–361. [Google Scholar]
- 39.Batten S, Ruggerone GT, Ortiz I. Pink salmon induce a trophic cascade in plankton populations in the southern Bering Sea and around the Aleutian Islands. Fish Oceanogr. 2018 doi: 10.1111/fog.12276. [DOI] [Google Scholar]
- 40.Takagi K, Aro KV, Hartt AC, Dell MB. 1981. Distribution and origin of pink salmon (Oncorhynchus gorbuscha) in offshore waters of the north Pacific Ocean. Int North Pac Fish Comm Bull, Vol 40.
- 41.Myers KW, Klovach NV, Gritsenko OF, Urawa S, Royer TC. Stock-specific distributions of Asian and North American salmon in the open ocean, interannual changes, and oceanographic conditions. N Pac Anad Fish Comm Bull. 2007;4:159–177. [Google Scholar]
- 42.Carey MJ, Phillips RA, Silk JRD, Shaffer SA. Trans-equatorial migration of short-tailed shearwaters revealed by geolocators. Emu. 2014;114:352–359. [Google Scholar]
- 43.Yamamoto T, et al. Annual and seasonal movements of migrating short-tailed shearwaters reflect environmental variation in sub-Arctic and Arctic waters. Mar Biol. 2014;162:413–424. [Google Scholar]
- 44.DeGange AR, Sanger GA. Marine birds. In: Hood DW, Zimmerman ST, editors. The Gulf of Alaska: Physical Environment and Biological Resources. US Department of Commerce, US Department of the Interior; Washington, DC: 1987. pp. 479–524. [Google Scholar]
- 45.Skira IJ, Brothers NP, Pemberton D. Distribution, abundance and conservation status of short-tailed shearwaters Puffinus tenuirostris in Tasmania, Australia. Mar Ornithol. 1996;24:1–14. [Google Scholar]
- 46.Lobkov EG. 1991. Phenomenon of the cyclic increase of mortality of seabirds in coastal Kamchatka. Proceedings of Tenth All-Union Ornithol Conference, Minsk, Belarus: Navuka i Tekhnika (Navuka i Tekhnika, Minsk, Belarus), pp 99–101.
- 47.Toge K, et al. The relationship between pink salmon biomass and the body condition of short-tailed shearwaters in the Bering Sea: Can fish compete with seabirds? Proc R Soc B. 2011;278:2584–2590. doi: 10.1098/rspb.2010.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thompson G, Drummond BA, Romano MD. 2014. Biological monitoring at St. Paul Island, Alaska in 2014, US Fish and Wildlife Service Report, AMNWR 2014/12.
- 49.Ito J. Food and feeding habit of Pacific salmon (genus Oncorhynchus) in their oceanic life. Hokkaido Reg Fish Res Lab Bull. 1964;29:85–97. [Google Scholar]
- 50.Ogi H, Kubodera T, Nakamura K. The pelagic feeding ecology of the short-tailed shearwater Puffinus tenuirostris in the subarctic Pacific region. Rep Yamashina Inst Ornith. 1980;12:157–182. [Google Scholar]
- 51.Tadokoro K, Ishida Y, Davis ND, Ueyanagi S, Sugimoto T. Change in chum salmon (Oncorhynchus keta) stomach contents associated with fluctuation of pink salmon (O. gorbuscha) abundance in the central subarctic Pacific and Bering Sea. Fish Oceanogr. 1996;5:89–99. [Google Scholar]
- 52.Kaeriyama M, et al. Feeding ecology of sockeye and pink salmon in the Gulf of Alaska. N Pac Anad Fish Comm Bull. 2000;2:55–63. [Google Scholar]
- 53.Hunt GL, Jr, Baduini C, Jahncke J. Diets of short-tailed shearwaters in the southeastern Bering Sea. Deep Sea Res Part II Top Stud Oceanogr. 2002;49:6147–6156. [Google Scholar]
- 54.Davis ND, Fukuwaka M, Armstrong JL, Myers KW. Salmon food habits studies in the Bering Sea, 1960 to present. N Pac Anad Fish Comm Tech Rep. 2005;6:24–28. [Google Scholar]
- 55.Oka N, Maruyama N. Mass mortality of short-tailed shearwaters along the Japanese coast. Tori. 1986;34:97–104. [Google Scholar]
- 56.Hatch SA. Did the 1982–1983 El Nino-Southern oscillation affect seabirds in Alaska? Wilson Bull. 1987;99:468–474. [Google Scholar]
- 57.Baduini CL, et al. Mass mortality of short-tailed shearwaters in the south-eastern Bering Sea during summer 1997. Fish Oceanogr. 2001;10:117–130. [Google Scholar]
- 58.Gibson JD, Sefton AR. Mortality of shearwaters. Emu. 1955;55:259–262. [Google Scholar]
- 59.Skira IJ. Large mortality of short-tailed shearwaters Puffinus tenuirostris in Australian and New Zealand seas in October 2000. Corella. 2003;27:81–84. [Google Scholar]
- 60.Gough D. 2013 Dead birds ‘not just a freak event.’ The Sydney Morning Herald. Available at www.smh.com.au/environment/animals/dead-birds-not-just-a-freak-event-20131030-2wgzd.html. Accessed April 26, 2018.
- 61.Heard WR. Life history of pink salmon (Oncorhynchus gorbuscha) In: Groot C, Margolis L, editors. Pacific Salmon Life Histories. UBC Press; Vancouver: 1991. pp. 119–230. [Google Scholar]
- 62.Myers KW, Aydin KY, Walker RV. 1996. Known Ocean Ranges of Stocks of Pacific Salmon and Steelhead as Shown by Tagging Experiments, 1956-1995. (NPAFC Doc. 192) (Univ of Washington. Seattle, WA)
- 63.Renner M, et al. Timing of ice retreat alters seabird abundances and distributions in the southeast Bering Sea. Biol Lett. 2016;12:20160276. doi: 10.1098/rsbl.2016.0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Woehler EJ, Raymond B, Watts DJ. Convergence or divergence: Where do short-tailed shearwaters forage in the Southern Ocean? Mar Ecol Prog Ser. 2006;324:261–270. [Google Scholar]
- 65.Raymond B, et al. Shearwater foraging in the Southern Ocean: The roles of prey availability and winds. PLoS One. 2010;5:e10960. doi: 10.1371/journal.pone.0010960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chambers LE, et al. Determining trends and environmental drivers from long-term marine mammal and seabird data: Examples from Southern Australia. Reg Environ Change. 2015;15:197–209. [Google Scholar]
- 67.Furness RW. The occurrence of burrow-nesting among birds and its influence on soil fertility and stability. In: Meadows PS, Meadows A, editors. Symposia of the Zoological Society of London—The Environmental Impact of Burrowing Animals and Animal Burrows. Clarendon Press; London: 1991. pp. 53–67. [Google Scholar]
- 68.Sanchez-Pinero F, Polis GA. Bottom-up dynamics of allochthonous input: Direct and indirect effects of seabirds on islands. Ecology. 2000;81:3117–3132. [Google Scholar]
- 69.Bancroft WJ, Garkaklis MJ, Roberts JD. Burrow building in seabird colonies: A soil-forming process in island ecosystems. Pedobiologia (Jena) 2005;49:149–165. [Google Scholar]
- 70.Brown MJ, Maruyama N, Williams KJ. Ecological studies of vegetation in short-tailed shearwater colonies in Tasmania. Pap Proc R Soc Tasman. 1993;127:11–16. [Google Scholar]
- 71.Walsh D, Kirkpatrick JB, Skira IJ. Vegetation patterns, environmental correlates and vegetation change in a Puffinus tenuirostris breeding colony at Cape Queen Elizabeth, Tasmania. Aust J Bot. 1997;45:71–79. [Google Scholar]
- 72.Hawke DJ, Newman J. Inventories and elemental accumulation in peat soils of forested seabird breeding islands, southern New Zealand. Aust J Soil Res. 2004;42:45–48. [Google Scholar]
- 73.Stuart I. To kill a mutton bird: The archaeology of birding in the outer Furneaux islands. Hist Environ. 1998;14:19–28. [Google Scholar]
- 74.Lyver PO’B. Use of traditional knowledge by Rakiura Maori to guide sooty shearwater harvests. Wildl Soc Bull. 2002;30:29–40. [Google Scholar]
- 75.Jones CJ, et al. Reinstatement of customary seabird harvests after a 50-year moratorium. J Wildl Manage. 2015;79:31–38. [Google Scholar]
- 76.Department of Primary Industries, Parks, Water, Environment 2017. Commercial Muttonbirds Harvest (DPIPWE, Wildlife Management Branch, Hobart, TAS, Australia), 1 p.
- 77.Department of Primary Industries, Parks, Water, Environment . Game Tracks. DPIPWE, Wildlife Management Branch; Hobart, TAS, Australia: 2017. pp. 24–25. [Google Scholar]
- 78.Johnstone JA, Mantua NJ. Atmospheric controls on northeast Pacific temperature variability and change, 1900-2012. Proc Natl Acad Sci USA. 2014;111:14360–14365. doi: 10.1073/pnas.1318371111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mueter FJ, Ware DM, Peterman RM. Spatial correlation patterns in coastal environmental variables and survival rates of salmon in the north-east Pacific Ocean. Fish Oceanogr. 2002;11:205–218. [Google Scholar]
- 80.Mantua NJ, Hare SR. The Pacific decadal oscillation. J Oceanogr. 2002;58:35–44. [Google Scholar]
- 81.Pearson WH, et al. Hypotheses concerning the decline and poor recovery of Pacific herring in Prince William Sound, Alaska. Rev Fish Biol. 2012;22:95–135. [Google Scholar]
- 82.Amoroso RO, Tillotson MD, Hilborn R. Measuring the net biological impact of fisheries enhancement: Pink salmon hatcheries can increase yield, but with apparent costs to wild populations. Can J Fish Aquat Sci. 2017;74:1233–1242. [Google Scholar]
- 83.Ward EJ, et al. Evaluating signals of oil spill impacts, climate, and species interactions in Pacific herring and Pacific salmon populations in Prince William Sound and Copper River, Alaska. PLoS One. 2017;12:e0172898. doi: 10.1371/journal.pone.0172898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Knudsen E, et al. 2015. Interactions of wild and hatchery pink salmon and chum salmon in Prince William Sound and Southeast Alaska, Final Progress Report for 2013 (Prince William Sound Science Center, Cordova, AK; Sitka Sound Science Center, Sitka, AK)
- 85.Furuhashi M, Shimazaki K. 1989. Vertical distribution and diet of Stenobrachius nannochir (Myctophidae) in the Southern Bering Sea, Summer, 1987. Proceedings of the NIPR Symposium on Polar Biolology, 2:94–104.
- 86.Balanov AA. Diet of common mesopelagic fishes of the Bering Sea. J Ichthyol. 1994;34:73–82. [Google Scholar]
- 87.Nishimura A, Nagasawa K, Asanuma T, Aoki H, Kubota T. Age, growth, and feeding habits of lanternfish, Stenobrachius leucopsarus (Myctophidae), collected from the near-surface layer in the Bering Sea. Fish Sci. 1999;65:11–15. [Google Scholar]
- 88.Tanimata N, Yamamura O, Sakurai Y, Azumaya T. Feeding habits and distribution of Stenobrachius leucopsarus (Myctophidae) in the central Bering Sea during late summer. N Pac Anad Fish Comm Tech Rep. 2005;6:29–30. [Google Scholar]
- 89.Hunsicker ME, Essington TE, Aydin KY, Ishida B. Predatory role of the commander squid Berryteuthis magister in the eastern Bering Sea: Insights from stable isotopes and food habits. Mar Ecol Prog Ser. 2010;415:91–108. [Google Scholar]
- 90.Nagasawa K, Nishimura A, Asanuma T, Marubayashi T. Myctophids in the Bering Sea: Distribution, abundance, and significance as food for salmonids. Proceedings of the International Symposium on the Role of Forage Fishes in Marine Ecosystems, Lowell Wakefield Fish Symposium Series. 1996;14:337–350. [Google Scholar]
- 91.Springer AM, Piatt JF, van Vliet G. Seabirds as proxies of marine habitats and food webs in the western Aleutian Arc. Fish Oceanogr. 1996;5:45–55. [Google Scholar]
- 92.Ohizumi H, Kuramochi T, Kubodera T, Yoshioka M, Miyazaki N. Feeding habits of Dall’s porpoises (Phocoenoides dalli) in the subarctic North Pacific and the Bering Sea basin and the impact of predation on mesopelagic micronekton. Deep Sea Res Part I Oceanogr Res Pap. 2003;50:593–610. [Google Scholar]
- 93.Zeppelin TK, Ream RR. Foraging habitats based on the diet of female northern fur seals (Callorhinus ursinus) on the Pribilof Islands, Alaska. J Zool (Lond) 2006;270:565–576. [Google Scholar]
- 94.Davis ND, Myers K, Fournier WJ. Winter food habits of chinook salmon in the eastern Bering Sea. N Pac Anad Fish Comm Bull. 2009;5:243–253. [Google Scholar]
- 95.Sakai O, Yamamura O, Sakurai Y, Azumaya T. Temporal variation in chum salmon, Oncorhynchus keta, diets in the central Bering Sea in summer and early autumn. Environ Biol Fishes. 2012;93:319–331. [Google Scholar]
- 96.Waite JN, Burkanov VN, Andrews RD. Prey competition between sympatric Steller sea lions (Eumetopias jubatus) and northern fur seals (Callorhinus ursinus) on Lovushki Island, Russia. Can J Zool. 2012;90:110–127. [Google Scholar]
- 97.Radchenko V. Mesopelagic fish community supplies “biological pump.”. Raffles Bull Zool. 2007;14:265–271. [Google Scholar]
- 98.Balanov AA, Il’inski EN. Species composition and biomass of mesopelagic fishes in the Sea of Okhotsk and the Bering Sea. J Ichthyol. 1992;32:85–93. [Google Scholar]
- 99.The Research Group 2009 North Pacific Salmon Fisheries Economic Measurement Estimates (The Research Group, Corvallis, OR), Version 1.2. Available at https://mbstp.org/wp-content/uploads/2013/10/Salmon_Economic_Valuation.pdf. Accessed April 26, 2018.
- 100.Holt CA, Rutherford MB, Peterman RM. International cooperation among nation-states of the North Pacific Ocean on the problem of competition among salmon for a common pool of prey resources. Mar Policy. 2008;32:607–617. [Google Scholar]
- 101.Peterman RL, Holt CA, Rutherford MR. The need for international cooperation to reduce competition among salmon for a common pool of prey resources in the North Pacific Ocean. N Pac Anad Fish Comm Tech Rep. 2012;8:99–101. [Google Scholar]
- 102.Yun SD, Hutniczak B, Abbott JK, Fenichel EP. Ecosystem-based management and the wealth of ecosystems. Proc Natl Acad Sci USA. 2017;114:6539–6544. doi: 10.1073/pnas.1617666114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fullagar PJ, Davey CC, van Tets GF, Heyligers PC. Is the short-tailed shearwater colonizing New South Wales? Nature in Eurobodalla. 1991;5:51–56. [Google Scholar]
- 104.Fullagar PJ, Heyligers PC, Crowley MA, van Tets GF, Davey CC. The breeding birds of Montagu Island, NSW. Nature in Eurobodalla. 1993;7:57–64. [Google Scholar]
- 105.Fullagar PJ, Perkins HD, Heyligers PC. 45th Annual assessment of shearwater breeding success on Montagu Island, 26–29 March 2004. Nature in Eurobodalla. 2004;18:57–63. [Google Scholar]
- 106.Heyligers PC, Perkins HD, Tiller CJ. 42nd Annual assessment of shearwater breeding success on Montagu Island, 26–30 March 2001. Nature in Eurobodalla. 2002;15:66–72. [Google Scholar]
- 107.Tiller CJ, Klomp NI, Fullagar PJ, Heyligers PC. Catastrophic breeding failure caused by heavy rainfall in a shearwater colony. Mar Ornithol. 2013;41:75–77. [Google Scholar]
- 108.Crowley MA, Davey CC, Fullagar PJ, Priddel D. 57th Annual assessment of shearwater breeding success on Montagu Island, 17–23 March 2016. Nature in Eurobodalla. 2016;30:56–63. [Google Scholar]
- 109.Vertigan C. 2010. The life history of short-tailed shearwaters (Puffinus tenuirostris) in response to spatio-temporal environmental variation. PhD dissertation (University of Tasmania, Hobart, TAS, Australia)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.