Significance
Hippopotami exert a strong influence on the biogeochemistry and ecology of freshwater ecosystems by excreting terrestrially derived organic matter into these systems. These impacts are likely to be strongly controlled by hydrology. In sub-Saharan Africa, anthropogenic water abstraction and climate change are significantly altering water cycles, often reducing dry-season flow. In this study, we report how hippopotami shape water chemistry and biodiversity patterns in a human-altered river. Importantly, we note that during recently prolonged low-flow periods the influence of the hippopotamus was greatly altered such that its nutrient contributions promoted eutrophication and affected biodiversity. These results highlight the extent to which human modification of environmental systems may unexpectedly alter the impacts of ecologically influential species at multiple scales.
Keywords: diversity, eutrophication, fish, hydrology, invertebrates
Abstract
Cross-boundary transfers of nutrients can profoundly shape the ecology of recipient systems. The common hippopotamus, Hippopotamus amphibius, is a significant vector of such subsidies from terrestrial to river ecosystems. We compared river pools with high and low densities of H. amphibius to determine how H. amphibius subsidies shape the chemistry and ecology of aquatic communities. Our study watershed, like many in sub-Saharan Africa, has been severely impacted by anthropogenic water abstraction reducing dry-season flow to zero. We conducted observations for multiple years over wet and dry seasons to identify how hydrological variability influences the impacts of H. amphibius. During the wet season, when the river was flowing, we detected no differences in water chemistry and nutrient parameters between pools with high and low densities of H. amphibius. Likewise, the diversity and abundance of fish and aquatic insect communities were indistinguishable. During the dry season, however, high-density H. amphibius pools differed drastically in almost all measured attributes of water chemistry and exhibited depressed fish and insect diversity and fish abundance compared with low-density H. amphibius pools. Scaled up to the entire watershed, we estimate that H. amphibius in this hydrologically altered watershed reduces dry-season fish abundance and indices of gamma-level diversity by 41% and 16%, respectively, but appears to promote aquatic invertebrate diversity. Widespread human-driven shifts in hydrology appear to redefine the role of H. amphibius, altering their influence on ecosystem diversity and functioning in a fashion that may be more severe than presently appreciated.
Transfers of material and energy across community boundaries shape the ecology of entire landscapes (1–3). Semiaquatic species, which transit between aquatic and terrestrial ecosystems, are good examples of biological vectors of cross-boundary subsidies (4–6). Semiaquatic species that rely on terrestrial sources of energy and nutrients (7, 8) can have large impacts on recipient aquatic habitats, affecting nutrient cycling, food web dynamics, and aquatic community structure, particularly if these recipient habitats are smaller and more contained than the sources of subsidies (9, 10).
The common hippopotamus (Hippopotamus amphibius) is a semiaquatic megaherbivore (>1,000 kg) that consumes large amounts of terrestrial vegetation (40–50 kg wet mass) (11) during nightly foraging bouts. After these foraging bouts, H. amphibius return to their aquatic refuges and spend most of the day resting and defecating. Subalusky, et al. (6) estimated, for example, that the H. amphibius population in the Maasai Mara National Reserve, Kenya, egested 8,563 kg dry matter/d into their diel habitats in the Mara River. Thus, over the course of their daily movements across ecosystem boundaries (terrestrial grazing areas and the aquatic refugia), H. amphibius deliver substantial, and continuous, supplies of terrestrially derived organic matter and nutrients, such as nitrogen and phosphorus, to aquatic ecosystems (5, 6). While the magnitude of these biologically mediated transfers is clearly high, it is important to understand how these inputs shape the chemistry of recipient aquatic systems and how such shifts in turn affect the ecology of aquatic communities.
River hydrology has been identified as a driver that shapes the ecological functioning of rivers (12). River-flow regimes are likely to have a profound impact on the resultant influence that H. amphibius has on the chemistry and ecology of the rivers it inhabits (5). In sub-Saharan Africa, many rivers are ephemeral and experience reduced flow during the dry season (13). Consequently, many species have evolved life-history strategies to deal with this seasonal variation (14). However, natural seasonal variation in river flow has increased drastically in duration and intensity by increased water abstraction and other anthropogenic watershed modifications (15). The resultant unnaturally high variation in seasonal river flow will likely be further exacerbated by global climate change (16, 17).
Increases in the intensity and duration of low-flow events in rivers may greatly amplify the biogeochemical and ecological effects of H. amphibius by inhibiting the downstream transport of organic matter, increasing local nutrient loading, altering microbial respiration, and imposing physiological stress on exposed organisms. Furthermore, a buildup of organic material can increase concentrations of colored dissolved organic matter, reducing primary production and light penetration in the water column (18–21). While it has been hypothesized that H. amphibius-vectored nutrients may promote the abundance and diversity of aquatic life (22), it is possible that this role reverses during no-flow periods when there is a buildup of H. amphibius organic matter. Understanding the chemical and biological impacts of H. amphibius on rivers is especially important during this period because it shapes watershed-level ecology. During extreme drying events, river flow can cease, and river pools become important biological refuges for aquatic life and sources of recolonization after such drying events (23).
Rigorously addressing the interactive relationship between changing river hydrology and the ecological impact of H. amphibius is challenging in many field environments. To empirically address these questions, we used a unique field context where, as a result of human modification, a historically perennial river in central Tanzania now dries down seasonally into a series of isolated physically and hydrologically similar pools that host either high densities of H. amphibius or low or no H. amphibius. We used these high- and low-density H. amphibius pools as experimental replicates where we sampled water chemistry and measured the abundance, diversity, and species composition of fish and aquatic invertebrate communities. These measurements were conducted during both dry and wet seasons to elucidate how hydrology regulates the impacts of H. amphibius. With data collected from this system we asked (i) how do H. amphibius influence river water chemistry and how do these alterations shape core attributes of aquatic biodiversity? (ii) How does seasonal variation in river hydrology regulate the impact of H. amphibius on both river chemistry and biology? (iii) How may localized impacts of H. amphibius scale up to shape entire watersheds? We predicted we would observe the most pronounced differences between pools with high and low densities of H. amphibius during the dry season when there is no flow. To our knowledge, no study has assessed the consequence of season, and subsequent river flow, on the ecological influence of H. amphibius subsidies on aquatic systems. Addressing these questions is vital because hydrological regimes are being altered by anthropogenic water abstraction and climate change across the distribution range of H. amphibius (15–17).
Methods
Site Description.
The study was conducted in the Ruaha National Park in central Tanzania (7°42′ S, 34°54′ E), which forms part of the Great Ruaha ecosystem. The main source of water for wildlife within Ruaha National Park is the Great Ruaha River. Field sampling was conducted along an ∼50-km stretch of the river. Mean annual rainfall in this region is ∼580 mm with most rainfall occurring during the wet season from November/December to May. The extensive dry season spans June to November/December (24). Historical data show that river flow around Msembe camp in Ruaha National Park during the dry season was 1–3 m3⋅s−1 (25). From 1993 to the present day, excessive water abstraction upstream of Ruaha National Park has significantly reduced river flow of the Great Ruaha River during the dry season (25). Consequently, river flow at Msembe camp is zero during the dry season. As a result, large sections of the Great Ruaha River stop flowing and form discrete pools separated by large expanses of dry riverbed. In the peak dry season of 2013 (November–December) and the wet (July) and peak dry (November–December) seasons of 2015, we collected biogeochemical and ecological data from high- and low-density H. amphibius pools, as defined below. For the purpose of our study, we defined July as the wet season. Although there was no precipitation during July 2015, the river pools were hydrologically connected by flowing water from the antecedent rainy season. During both the 2013 and 2015 dry seasons, the Great Ruaha River stopped flowing, and sampled pools were not connected by flowing water (Fig. 1).
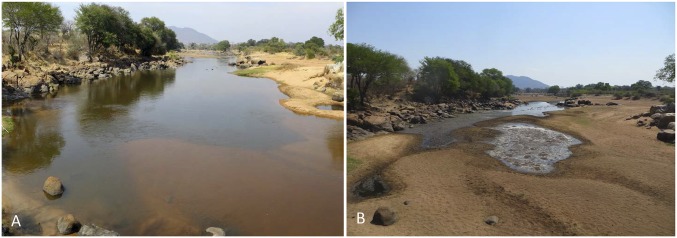
Fig. 1.
The Great Ruaha River in Ruaha National Park, Tanzania observed from the same vantage point during the wet season of 2015 (A) and the dry season of 2015 (B). During the wet season (pool size: length = 185 m, width = 34 m), flow connects pools in the river, whereas in the dry season (pool size: length = 155 m, width = 20 m), the river ceases flowing for several months every year. This results in large stretches of dry riverbed with isolated pools that are completely disconnected.
Hydrological Monitoring.
Monthly rainfall records for Ruaha National Park were obtained from park officials. River hydrology tracked the annual bimodal rainfall distribution (SI Appendix, Fig. S1) and was used to assess seasonal variation in river hydrology.
H. amphibius Surveys and Focal River Pool Selection.
We monitored H. amphibius daily at river pool sites in the Great Ruaha River throughout the 2013 and 2015 dry seasons and the 2015 wet season. At each pool, daily H. amphibius counts were conducted by at least two observers, and the maximum number of H. amphibius observed during a count was recorded. In addition to these direct observations, we also used camera traps (Reconyx HC500) to estimate H. amphibius abundance in pools during daylight hours between 06:00 h and 18:00 h. This resulted in 28,153 photographs during 2013 and 71,312 photographs during 2015. Pool volume was calculated for all focal pools during the wet and dry seasons. Average pool length and width measurements were calculated from three field measurements made using a laser rangefinder. Average pool depth was estimated from three depth measurements made along the midline of the pools using a remotely deployed sounding line. Volume was estimated from average length, width, and depth measurements by assuming pools approximate a hemispheric or semicylindrical shape.
We integrated the observer surveys and camera trap data to determine H. amphibius abundance in focal pools. H. amphibius density for each pool was calculated as average number of H. amphibius in each pool during the sampling season divided by the pool volume for that sampling season. There was a bimodal distribution of H. amphibius data with no clumping around the center of the distribution (SI Appendix, Fig. S2). This indicates a clear segregation between high- and low-density H. amphibius pools. Any density that fell above the 50% percentile for a given season was categorized as a high-density H. amphibius pool, and any value that fell below the 50% percentile was a low-density H. amphibius pool. Within these categories, the majority of pools fell below the 25% percentile or above the 75% percentile (SI Appendix, Fig. S2). In total, we had 11 sampling pools during the dry season of 2013 (n = 5 high-density H. amphibius pools and n = 6 low-density H. amphibius pools). During the 2015 sampling period we had 18 sample pools during the wet season (n = 7 high-density H. amphibius pools and n = 11 low-density H. amphibius pools) and 12 sample pools during the dry season (n = 6 high-density H. amphibius pools and n = 6 low-density H. amphibius pools). The smaller sample size in the dry season versus the wet season of 2015 was a result of excessive drying during this period that dewatered some pools to the point that they were unsuitable habitat. Treatment pools were randomly interspersed along the 50-km stretch of the river (SI Appendix, Fig. S3).
Water-Chemistry Sampling.
To determine how changing hydrology influenced H. amphibius-vectored nutrient concentrations in river pools, we collected water samples during the two peak dry-season sampling periods (2013 and 2015) and during the wet season of 2015. In addition, we continually monitored dissolved oxygen (DO) concentrations on a weekly basis from July to November in 2015 in all pools. All within-season sampling occurred concurrently in high- and low-density H. amphibius pools.
From each pool, we collected two water samples of river surface water (i.e., the top 10 cm): one sample ∼10 m upstream of the H. amphibius congregation (if present) and the other ∼10 m downstream of the congregation. We collected these samples (1 L each) using a 3-m-long pole and collection bottle. From these two water samples, we immediately field-measured DO concentrations (in milligrams per liter) and pH using a handheld, cross-calibrated multiparameter electronic meter (YSI, Inc.). Two additional water samples (collected from the same place and from the top 10 cm of surface water) from each pool were then combined, kept on ice, and returned to the field laboratory where they were filtered using ashed glass-fiber filters (GF/F; Whatman) and thereafter were kept frozen for later nutrient analysis. From these filtered water samples we measured total dissolved nitrogen (TDN) (in milligrams per liter) and dissolved organic carbon (DOC) (in milligrams per liter) concentrations on a Shimadzu Vcpn analyzer (Shimadzu Corporation). We also measured total dissolved phosphorus (TDP) (in micrograms per liter) using the ascorbic acid colorimetric method for soluble reactive phosphorus following persulfate oxidation.
Glass-fiber filters collected during filtrations were then used to analyze particulate phosphorus (PP) (in micrograms per liter), particulate nitrogen (PN) (in milligrams per liter), particulate carbon (PC) (in milligrams per liter), and chlorophyll-a (Chl-a) (in micrograms per liter). Depending on river flow and particulate concentrations in the river water, we filtered 3–80 mL of river water per filter with the volume filtered noted for each. These filters were dried and kept in the dark until analysis (ca. 6 mo later). We analyzed PP colorimetrically using methods similar to those used for TDP analysis. For PC and PN, filters were fumed with acid to remove carbonate, followed by analyses via high-temperature combustion in an elemental analyzer (Costech Analytical Technologies, Inc.). Concentrations of Chl-a were determined using in vitro determination via fluorescence (26).
Biological Diversity Sampling.
We conducted biological diversity surveys for fish and aquatic invertebrates in all sample pools during the peak dry season of 2013 (n = 5 high-density H. amphibius pools and n = 6 low-density H. amphibius pools) and during the wet and peak dry season of 2015 (wet season: n = 7 high-density H. amphibius pools and n = 11 low-density H. amphibius pools; dry season: n = 6 high-density H. amphibius pools and n = 6 low-density H. amphibius pools). Dry-season sampling in both years was conducted approximately 1 mo after cessation of flow to coincide with water-chemistry sampling. As per the water-chemistry data, we collected fish and invertebrate data from both high- and low-density H. amphibius pools concurrently. For fish sampling, we used a throw net (1.8-m diameter, 1-cm mesh) (27) and identified and enumerated all fish that were caught in each sampling event. Fish sampling was conducted near the shore (e.g., ref. 28) because these littoral sites are utilized by both adult and juvenile fishes (29) and because this allowed us to standardize sampling effort in all pools, irrespective of the size of the pool and H. amphibius density. Each pool was sampled using 10 throws in which the net deployed at full diameter. Fish captured during sampling were held temporarily until the conclusion of sampling in a given pool.
To calculate invertebrate diversity, we passed a flat, square-edged net (30-cm width, 500-μm mesh) for 1.5 m through the top 2 cm of sediment layer in each pool, and all invertebrates that were collected were identified to the level of order or class. In each pool, we did five replicate net passes evenly distributed across the pool.
Estimating Watershed-Level Shifts in Water Chemistry and Biological Diversity.
To understand how the local-level impacts of H. amphibius may scale up to influence water chemistry and biodiversity at the whole-ecosystem scale, we coupled data collected on the impacts of H. amphibius in our focal pools with watershed-scale data on H. amphibius and river geomorphology to model H. amphibius impacts on (i) DO concentration, (ii) fish diversity, (iii) aquatic invertebrate diversity, (iv) fish abundance, and (v) abundance of tilapia (an economically and culturally prized fish species) across the Great Ruaha watershed.
Watershed-level data on the abundance and distribution of H. amphibius was obtained using aerial surveys conducted during the dry season of 2015 over a 160-km stretch of the Great Ruaha River extending from the Usangu wetlands to the point where the river is blocked by the Mtera dam, hereafter termed the “Great Ruaha watershed.” We used high-resolution satellite imagery (Planet satellite imagery) to calculate the size of all river pools in this region (n = 60). We applied our measurements of average pool depth drawn from measurements of focal pools to estimate the volumes of all such pools in the watershed beyond our study area. These volumes combined with aerial survey data to estimate the density of H. amphibius of all pools in the Great Ruaha watershed.
Using our field measurements of the aforementioned 12 focal H. amphibius pools during the 2015 dry season in our study region, we estimated the relationship between H. amphibius density and DO concentration, total fish abundance, and specifically tilapia abundance (extended methods for these calculations are described in SI Appendix, SI Materials and Methods). In addition, we used the 2015 dry-season fish and aquatic invertebrate species compositions from these focal pools to calculate both fish and aquatic invertebrate diversity (Hill number and species richness) at the watershed scale (i.e., gamma diversity). Water-quality modeling was based on DO owing to the strong relationship observed between DO and all other water-quality variables that we measured. We did not include aquatic invertebrate abundance in these analyses because we found no significant difference in abundance between our treatment pools in our main analysis (see Aquatic Invertebrate Diversity and Abundance). Watershed-scale diversity was calculated by summing the species-specific abundances across the different treatment pools (SI Appendix, SI Materials and Methods).
To synthesize these observed impacts into predictions about how H. amphibius shape watershed-level biodiversity patterns, we contrasted outputs generated from two scenarios: (i) effects observed using the distribution and density of H. amphibius derived from the 2015 census and (ii) effects that would be observed if H. amphibius were largely removed from the system (i.e., if all pools were considered low-density H. amphibius pools). Observed differences in water chemistry, biological diversity, and fish abundance between these two scenarios were then relied upon to estimate the net impact of H. amphibius across the Great Ruaha watershed.
Statistical Analysis.
To explore how H. amphibius density influenced water chemistry across wet- and dry-season regimes, we performed multidimensional scaling (MDS) using a Euclidean distance matrix on the nine water-chemistry variables (DO, Chl-a, DOC, TDN, TDP, pH, PP, PC, and PN) in PRIMER-E v6 (30). All nine variables were normalized because they are measured using different scales. We used a permutational multivariate analysis of variance (PERMANOVA) to test for significant differences observed between treatments in the MDS plot. If the PERMANOVA found significant separation across treatments, we ran a Mann–Whitney U test for each of the nine water variables to identify which variables contributed to the observed dissimilarity between low- and high-density H. amphibius pools. Finally, we used Pearson’s correlation coefficient matrix to assess the relationship between the nine water-quality variables. To avoid possible type 1 error for the above analyses, we used the Holm (31) correction of α for sequential analysis of the same null model.
We assessed potential differences in pool volume (dependent variable) between treatment pools (independent variable) using a general linear model. Pool volume was log transformed to meet assumptions of normality. We ran a separate model for each of the three sampling periods.
To determine whether biological diversity and abundance differed between high- and low-density H. amphibius pools, we calculated the diversity for fish and aquatic invertebrates using the Hill number metric (32). Hill numbers, also known as the “effective number of species,” have been identified as an appropriate index to measure abundance-based species diversity (32). We compared Hill numbers and abundance (dependent variables in separate models) across treatments (independent variable) using a general linear model. Pool volume was included as a covariate within these models. Hill numbers were log transformed to meet assumptions of normality. These values were then back-transformed for graphical representation. We initially ran full models (including interactions) to test for homogeneity of slopes. Nonsignificant interactions were removed, and the reduced model was rerun (per ref. 33). Separate models were run for fish and invertebrate data.
To detect which taxa contributed to the observed difference in diversity and community composition between treatments, we used one-way similarity percentages (SIMPER) species-contribution analyses based on Bray–Curtis similarity in PRIMER-E. This analysis identifies differences in the relative contribution of each species between low- and high-density H. amphibius treatments. The relative contribution of each species is related to our sampling effort per pool (i.e., 10 net throws per pool for fish and five net sweeps per pool for aquatic invertebrates). Because we included all species in these analyses (i.e., we used no minimum contribution cutoff in the SIMPER), we were also able to identify differences in species composition between treatments. For all analyses, we ran separate models for fish and invertebrates in the wet and dry seasons. Dissimilarity results in the relative contribution of each species in the different treatment pools obtained from the SIMPER analysis were tested for significance using a PERMANOVA.
Finally, we used a Spearman’s ρ nonparametric signed-rank test to examine the correlation between fish diversity, total number of fish, invertebrate diversity, and total number of invertebrates and the nine water-chemistry variables that we collected. This allowed us to determine which of the water variables were related to changes in biological diversity and abundance. Again, we applied the Holm correction of α (31) for sequential analysis of the same null model and present the adjusted P values. Univariate analyses were run using SPSS v. 24 (IBM). Data are available in SI Appendix.
Results
Hydrological Monitoring.
Rainfall ceased in April 2013 and in May 2015 (SI Appendix, Fig. S1). After this period, there was no significant rainfall until the start of the next wet season in November/December. Thus, as the dry season progressed, river flow reduced, and the river stopped flowing in September/October for both years. Overall, 2013 was drier than 2015. In 2013, the dry season lasted 5 mo and received less rainfall in the preceding wet season (mean ± SE: 319 ± 9 mm) compared with the 4-mo dry season in 2015 (preceding wet season rainfall: 451 ± 27 mm). Rainfall in late November/December (after our sampling period) caused certain sections of the Great Ruaha River to resume flow.
H. amphibius Numbers and Density per Pool.
In the dry season of 2013, high-density H. amphibius pools had on average ∼25 times the absolute number of H. amphibius (26 ± 9 H. amphibius per pool) and had ∼112 times higher density (0.03 ± 0.02 H. amphibius/m3 of water) compared with low-density H. amphibius pools (1 ± 0.2 H. amphibius per pool; 0.0003 ± 0.0002 H. amphibius/m3 of water). During the 2015 dry season, high-density H. amphibius pools contained approximately nine times the absolute number of H. amphibius (35 ± 11 H. amphibius per pool) compared with low-density H. amphibius pools (4 ± 3 H. amphibius per pool) and had ∼27 higher density (high-density H. amphibius pools: 0.008 ± 0.003 H. amphibius/m3 of water; low-density H. amphibius pools: 0.0003 ± 0.0001 H. amphibius/m3 of water). During the wet-season sampling period, the increase in water availability decreased the average number of H. amphibius in all sample pools. Despite this decrease, high-density H. amphibius pools still contained approximately eight times the number of H. amphibius (26 ± 5 H. amphibius per pool) and had ∼30 times higher density (0.006 ± 0.002 H. amphibius/m3 of water) compared with low-density H. amphibius pools (3 ± 2 H. amphibius per pool; 0.0002 ± 0.00008 H. amphibius/m3 of water).We found no significant difference in pool volume between treatments for each of the different sampling periods (2013 dry-season ANOVA: F1,9 = 1.160, P = 0.309; 2015 dry-season ANOVA: F1,10 = 0.014, P = 0.907; 2015 wet-season ANOVA: F1,16 = 0.795, P = 0.368).
Effects of H. amphibius on Local-Scale Water Chemistry.
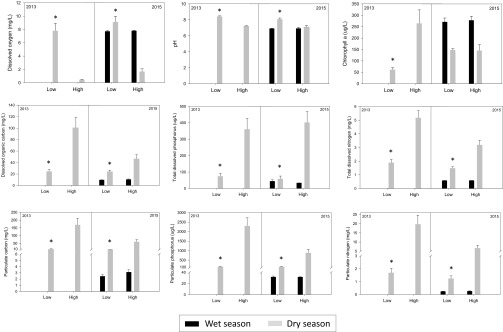
During the peak dry season of 2013 and 2015, multivariate analyses showed significant differences between high- and low-density H. amphibius pools with regard to the water-chemistry attributes that we measured (2013 PERMANOVA pseudo F1,9 = 19.445, P = 0.003; 2015 PERMANOVA pseudo F1,10 = 10.459, adjusted P = 0.002) (Fig. 2). The directionality of difference between treatments was identical in the dry season of both 2013 and 2015: High-density H. amphibius pools had significantly higher levels of particulate and dissolved organic carbon, nitrogen, and phosphorus and significantly lower DO and pH than low-density H. amphibius pools (Fig. 3 and Table 1). In 2013, Chl-a was significantly higher in high-density H. amphibius pools than in low-density H. amphibius pools. However, in 2015, we found no difference in Chl-a between treatment pools (Table 1). There was a significant negative correlation between DO and DOC, TDN, TDP, PP, PC, and PN and a significant positive relationship between DO and pH for both the 2013 and 2015 dry seasons. In 2013 Chl-a was significantly positively correlated with DOC, TDN, PC, PN, and PP and was negatively correlated with DO and pH. There was no correlation between TDP and Chl-a. In 2015, Chl-a was not correlated with any water-quality variable (SI Appendix, Tables S1 and S2).
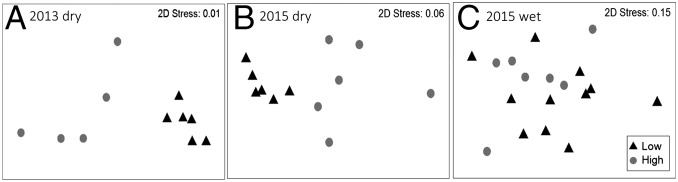
Fig. 2.
MDS plot showing the effect of treatment (high- and low-density H. amphibius pools) on the nine water-chemistry variables (DO, Chl-a, DOC, TDN, TDP, pH, PP, PC, and PN) during the 2013 dry season (n = 5 high-density H. amphibius pools and n = 6 low-density H. amphibius pools) (A), the 2015 dry season (n = 6 high-density H. amphibius pools and n = 6 low-density H. amphibius pools) (B), and the 2015 wet season (n = 7 high-density H. amphibius pools and n = 11 low-density H. amphibius pools) (C). High- and low-density H. amphibius pools were significantly different in both dry seasons but were not different during the wet season when river flow was high.
Fig. 3.
Effect of H. amphibius on nine water-chemistry attributes (mean + SE). Comparisons are made here between high- and low-density H. amphibius treatments in the dry seasons of 2013 (n = 5 high-density H. amphibius pools and n = 6 low-density H. amphibius pools) and 2015 (n = 6 high-density H. amphibius pools and n = 6 low-density H. amphibius pools) and the wet season of 2015 (n = 7 high-density H. amphibius pools and n = 11 low-density H. amphibius pools). The asterisks denote significant differences (P < 0.05) between treatments within the same year and season.
Table 1.
Significance (Mann–Whitney U test) of differences in water-chemistry variables between high- and low-density H. amphibius pools in the dry seasons of 2013 and 2015 and the wet season of 2015
| Variable | 2013 dry season | 2015 dry season | 2015 wet season | |||
| Mann-Whitney U | P value | Mann-Whitney U | P value | Mann-Whitney U | P value | |
| DO | 0 | 0.036 | 0 | 0.009 | 33 | 1.000 |
| DOC | 0 | 0.036 | 2 | 0.024 | 28 | 1.000 |
| TDN | 0 | 0.036 | 2 | 0.024 | 34 | 1.000 |
| TDP | 0 | 0.036 | 0 | 0.009 | 21 | 0.567 |
| Chl-a | 2 | 0.036 | 16 | 0.409 | 22 | 0.600 |
| PC) | 0 | 0.036 | 1 | 0.024 | 23 | 0.179 |
| PN | 1 | 0.036 | 1 | 0.024 | 31 | 1.000 |
| PP | 0 | 0.036 | 0 | 0.024 | 37 | 1.000 |
| pH | 0 | 0.036 | 1 | 0.014 | 32 | 1.000 |
P values are adjusted using Holm’s (31) correction of α for sequential analysis of the same null hypothesis.
By contrast, during the wet season, when all H. amphibius pools were hydrologically connected via surface flow, multivariate analyses showed no difference between low- and high-density H. amphibius pools with respect to overall water chemistry (PERMANOVA pseudo F1,16 = 0.754, P = 0.615) (Fig. 2). Further analysis revealed no difference between treatment pools for each water-quality variable (Fig. 3 and Table 1).
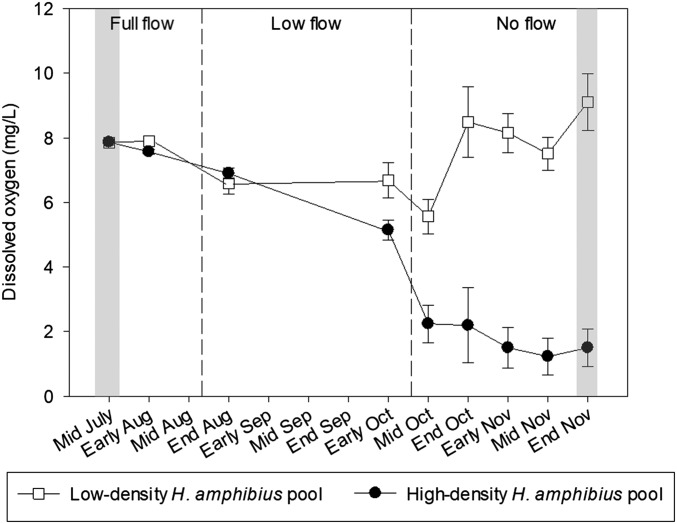
The long-term high-frequency DO sampling conducted in 2015 shows that high- and low-density H. amphibius pools had similar DO concentrations during the wet season when the river was flowing. However, as the dry season progressed, high-density H. amphibius pools experienced a rapid decline in DO concentrations compared with low-density H. amphibius pools, with the greatest differences in DO concentrations between pools being observed when river flow ceased (Fig. 4).
Fig. 4.
DO concentration (mean ± SE) for low- and high-density H. amphibius pools as measured over a period of decreasing river discharge in the Great Ruaha River during 2015. Light gray bars indicate wet-season and peak dry-season sampling periods.
Effects of H. amphibius on Local-Scale Aquatic Biological Diversity.
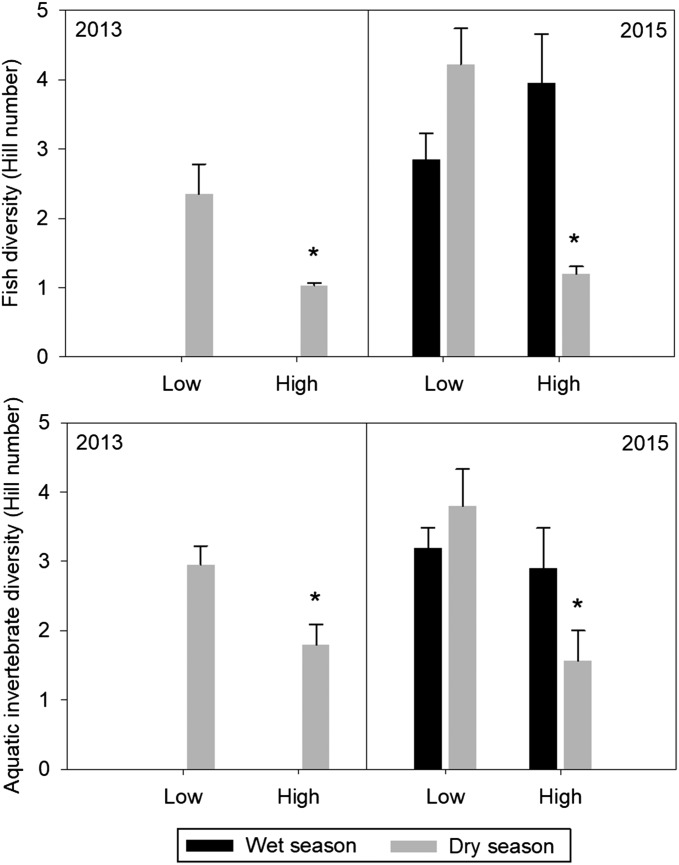
Fish diversity and abundance.
A total of 698 and 1,109 fish were captured and identified in the dry seasons of 2013 and 2015, respectively. In both dry seasons high-density H. amphibius pools had significantly lower fish diversity than the low-density H. amphibius pools [reduced model analysis of covariance (ANCOVA) 2013: F1;8 = 36.798, P < 0.001; reduced model ANCOVA 2015: F1;9 = 197.548, P < 0.001] (Fig. 5). In both years, pool size influenced fish diversity, with diversity decreasing as a function of pool size across both high- and low-density H. amphibius pools. Because the response slopes of diversity to pool volume are homogeneous in both years (i.e., nonsignificant interaction in the full model; ANCOVA 2013: F1;7 = 1.301, P = 0.911; ANCOVA 2015: F1;8 = 0.396, P = 0.547), the observed difference in diversity between high- and low-density H. amphibius pools can be inferred over the range of pool sizes. Although there was a negative relationship between pool volume and diversity, there was no statistical difference in pool volume between the different treatments in each sampling period (see above). Thus, the higher fish diversity in low-density H. amphibius pools is likely due to the density of H. amphibius and not to the relationship between pool volume and diversity. The observed differences in fish diversity for both 2013 and 2015 appear to be related primarily to changes in TDN, TDP, PP, and PC. In addition, 2013 diversity appears to be related in DOC, while 2015 diversity data also appear to be related to changes in DO, PN, and pH (SI Appendix, Table S3).
Fig. 5.
Comparisons of fish (Upper) and aquatic invertebrate (Lower) diversity (Hill numbers; mean + SE adjusted to account for the effect of the covariate, pool size, on diversity) in low- and high-density H. amphibius pools during the peak dry season of 2013 (n = 5 high-density H. amphibius pools and n = 6 low-density H. amphibius pools) and the peak wet and dry seasons of 2015 (wet: n = 7 high-density H. amphibius pools and n = 11 low-density H. amphibius pools; dry: n = 6 high-density H. amphibius pools and n = 6 low-density H. amphibius pools). Significant differences in fish and aquatic invertebrate diversity were observed between low- and high-density H. amphibius pools during both dry seasons. No such differences were evident for either group during the wet season. The asterisks denote significant differences (P < 0.05) between treatment pools for the same year.
High-density H. amphibius pools were not only depauperate in respect to fish species diversity; the average dissimilarity in relative abundance of species to the overall species composition between high- and low-density H. amphibius pools during the dry season was 79.91% and 86.68% for 2013 and 2015, respectively (i.e., treatment pools were different; 2013 PERMANOVA pseudo F1,9 = 2.890, P = 0.004; 2015 PERMANOVA pseudo F1,10 = 2.983, P = 0.017). Despite these differences, high-density H. amphibius pools contained many of the same fish species that were detected in low-density H. amphibius pools. Only one (Clarias gariepinus) and two (C. gariepinus and Synodontis matthesi) fish species were detected only in high-density H. amphibius pools and not in low-density H. amphibius pools during the dry-season sampling in 2013 and 2015, respectively (SI Appendix, Tables S4 and S5). For both sampling years, treatment pools had similar dry-season fish-species compositions. High-density H. amphibius pools were largely dominated by Oreochromis urolepis, Brycinus affinis, S. matthesi, Labeo coubie, and C. gariepinus. Low-density H. amphibius pools were characterized by O. urolepis, B. affinis, Labeo cylindricus, Enteromius radiatus, Enteromius lineomaculatus, Schilbe intermedius, S. matthesi, L. coubie, Labeo congoro, Hydrocynus vittatus, Distichodus petersi, C. gariepinus, and Astatotilapia bloyeti (SI Appendix, Tables S4 and S5).
Fish abundance was also significantly lower in high-density H. amphibius pools than in low-density H. amphibius pools in both the 2013 and 2015 dry seasons (SI Appendix, Fig. S9). In 2013, fish abundance was not influenced by pool size (reduced model ANCOVA: F1;6 = 0.184, P = 0.679). However, in 2015, the full model revealed a significant interaction between the pool size and treatment (full model ANCOVA: F1;8 = 10.151, P = 0.013). This trend suggests that in 2015 the high-density H. amphibius pools showed lower total numbers of fish only at smaller pool sizes, and as pool size increased, the difference between treatments in the total number of fish decreased. At large pool size, high-density H. amphibius pools had higher fish abundance than low-density H. amphibius pools (SI Appendix, Fig. S9).
In the wet season, a total of 886 fish were caught and identified. During the wet season, there was no significant difference in fish diversity between high- and low-density H. amphibius pools (reduced model ANCOVA: F1;15 = 1.801, P = 0.200) (Fig. 5). Furthermore, there was no relationship between pool size and fish diversity during the wet season (reduced model ANCOVA: F1;15 = 1.876, P = 0.191). There was no significant difference in the average dissimilarity in the relative abundance of species to the overall species composition between high- and low-density H. amphibius pools (i.e., treatment pools were similar; PERMANOVA pseudo F1,16 = 1.606, P = 0.189) with high- and low-density pools sharing 10 of 12 fish species (SI Appendix, Table S6). In addition, these pools also contained similar abundances of fish (reduced model ANCOVA F1;16 = 0.033, P = 0.858), irrespective of pool size (reduced model ANCOVA: F1;8 = 1.097, P = 0.312).
Aquatic invertebrate diversity and abundance.
A total of 493 aquatic invertebrates were collected and identified in the dry season of 2013, and 734 were collected and identified in the dry season of 2015. Aquatic invertebrate diversity was significantly lower in high-density H. amphibius pools than in low-density H. amphibius pools in the dry seasons of both 2013 (reduced model ANCOVA: F1;8 = 12.712, P = 0.007) (Fig. 5) and 2015 (reduced model ANCOVA F1;9 = 25.463, P = 0.001) (Fig. 5). In both years, there was no relationship between diversity and pool size (2013 reduced model ANCOVA: F1;8 = 1.431, P = 0.266; 2015 reduced model ANCOVA: F1;9 = 0.092, P = 0.060). In 2013, the average dissimilarity between low- and high-density H. amphibius pools was 63.32%, whereas in 2015, the average dissimilarity was 83.49%. The observed dissimilarity between treatment pools was not significant in 2013 (PERMANOVA pseudo F1,9 = 1.368, P = 0.267) but was significant in 2015 (PERMANOVA pseudo F1,10 = 2.847, P = 0.029). High-density H. amphibius pools were dominated by adult coleopterans in both 2013 and 2015 (SI Appendix, Tables S4 and S5). The observed lack of significance in invertebrate community dissimilarity in 2013 was most likely due to this dominance of coleopterans; if they were removed, the average dissimilarity increased from 63.32 to 81.77%, a significant difference (PERMANOVA pseudo F1,9 = 2.305, P = 0.05). The reduction in diversity between low- and high-density H. amphibius pools appears to be associated with DO, TDP, and pH in 2013 and with DO, TDP, PP, and pH in 2015 (SI Appendix, Table S3). Low-density H. amphibius pools in both 2013 and 2015 were characterized by decapods, coleopterans, gastropods, odonates, dipterans, unionoidans, and hemipterans (SI Appendix, Tables S4 and S5).
Despite differences in diversity, the abundance of aquatic invertebrates during the dry season was similar between high- and low-density H. amphibius pools for both 2013 and 2015 (2013 reduced model ANCOVA: F1;8 = 1.238, P = 0.298; 2015 reduced model ANCOVA: F1;9 = 0.050, P = 0.829). This lack of difference did not appear to be influenced by pool size (2013 reduced model ANCOVA: F1;8 = 1.966, P = 0.198; 2015 reduced model ANCOVA: F1;9 = 3.246, P = 0.105).
In the wet season, a total of 878 aquatic invertebrates were collected and identified. There were no significant differences in aquatic invertebrate diversity (reduced model ANCOVA: F1;15 = 0.689, P = 0.420) (Fig. 3) between high- and low-density H. amphibius pools during the wet season. There was also no relationship between pool size and aquatic invertebrate diversity (reduced model ANCOVA: F1;15 = 0.596, P = 0.452). There was also no significant difference in the community dissimilarity between high- and low-density H. amphibius pools (PERMANOVA pseudo F1,16 = 1.079, P = 0.346) with high- and low-density H. amphibius pools sharing 9 of 10 defined orders/classes of aquatic invertebrates (SI Appendix, Table S6). Furthermore, high-and low-density H. amphibius pools had similar abundances of aquatic invertebrates (reduced model ANCOVA: F1;15 = 2211, P = 0.158) with pool size having no effect on total abundance (reduced model ANCOVA: F1;15 = 0.008, P = 0.930).
Estimating Watershed-Level Effects of H. amphibius on Water Chemistry and Aquatic Biological Diversity.
The 2015 H. amphibius aerial census revealed that H. amphibius are distributed widely across the Great Ruaha watershed. In these surveys, we identified a total of 60 isolated pools across the Great Ruaha watershed that were large enough to be suitable for H. amphibius habitation throughout the dry season, although only 47 such pools were in fact inhabited by H. amphibius at the time of the survey (Fig. 6). These 60 pools also comprise the principal remaining refugia for other aquatic biodiversity because the Great Ruaha River stopped flowing and dried down to isolated pools during the dry season.
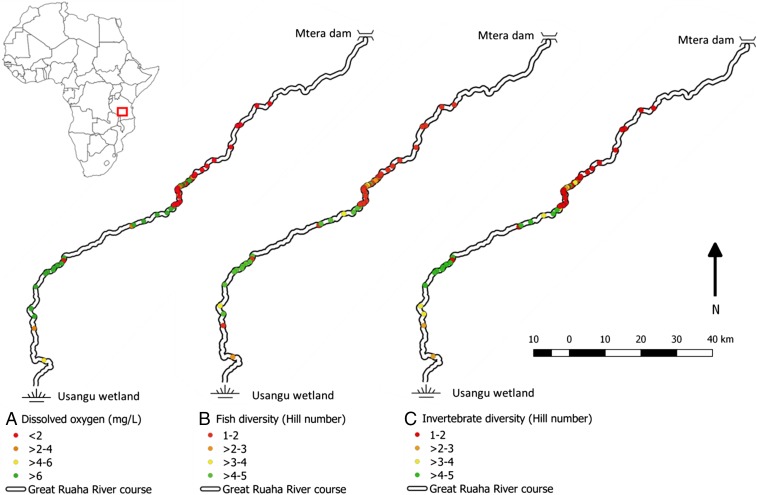
Fig. 6.
Map of the Great Ruaha watershed in Tanzania (from Usangu swamps to the Mtera dam). All colored circles represent river pools that are large enough to provide suitable habitat for H. amphibius during the dry season, as enumerated via aerial surveys. Based on measurements collected in our study region and densities of H. amphibius recorded during aerial surveys during the dry season of 2015, we model the dry-season impacts of H. amphibius on DO concentrations (A), fish diversity (Hill number) (B), and aquatic invertebrate diversity (Hill number) (C) at the watershed scale. The river flows in a northeast direction from the Usangu wetland to the Mtera dam.
Using the observed H. amphibius densities drawn from the 2015 aerial survey and measurements made in our focal study pools, we estimate an average DO concentration of 4.5 mg/L across all 60 pools in the Great Ruaha watershed during the dry season (Fig. 6 and SI Appendix, SI Materials and Methods). However, if H. amphibius were to be largely removed from the Great Ruaha watershed (i.e., all pools in the watershed approximated conditions locally observed in our focal low-density H. amphibius pools), we estimate that average dry-season DO concentrations would increase to 8.7 mg/L.
H. amphibius appear to have variable effects upon fish diversity at the watershed level during the dry season (gamma diversity). When fish diversity is measured using the Hill number diversity index, the presence of H. amphibius appear to drive pronounced decreases in watershed-level fish diversity. Hill number-measured values of fish diversity for the Great Ruaha watershed were estimated to decrease from 6.9, if H. amphibius are largely removed in the watershed, to 5.8 when H. amphibius are present in the watershed. In contrast, species richness across the Great Ruaha watershed is estimated to increase from 10 to 12 species when H. amphibius are present.
We estimate that the relative watershed-level fish abundance during the dry season would decrease by ∼41% in the presence of H. amphibius. In the case of tilapia, specifically, abundance would decrease by ∼41% across the Great Ruaha watershed when H. amphibius are present compared with scenarios in which all watershed pools were set to the approximate values observed in low-density H. amphibius pools.
In contrast, the presence of H. amphibius appeared to increase both Hill number-measured aquatic invertebrate diversity and species richness at the watershed scale during the dry season. Hill number values increased from 2.3, when only low-density H. amphibius pool estimates were applied to the entire Ruaha watershed, to 3.4 when both high- and low-density H. amphibius estimates were used. Similarly, taxonomic richness increased slightly, from 11 different aquatic invertebrate orders or classes in scenarios presuming the absence of H. amphibius in the watershed to 12 different aquatic invertebrate orders or classes when H. amphibius were present in the watershed.
Discussion
The unique experimental context of the Great Ruaha River afforded a special opportunity to examine, in a replicated fashion, the influence that H. amphibius exerts on core attributes of river water chemistry and to determine how these shifts influence aquatic communities at both local and regional scales. Importantly, the dynamic variation in water flow in this system also exhibited how strongly the effects of H. amphibius on river biogeochemistry and biodiversity are regulated by both seasonal and anthropogenic impacts upon river hydrology. Comparisons of water chemistry and fish and aquatic invertebrate community attributes between pools with high and low densities of H. amphibius were vastly different during wet and dry seasons. The large quantities of nutrients and organic matter that H. amphibius vector into aquatic ecosystems are known from other systems to be utilized by aquatic consumers (5, 34). Such subsidies have been assumed, at least implicitly, to have a positive or stimulatory influence on recipient communities (22). However, research on the ecological use of these subsidies has been carried out principally in less water-stressed systems that rarely dry seasonally. Historically, the Great Ruaha River experienced natural seasonal variation in hydrology but was able to maintain river flow even during the peak dry season. However, current anthropogenic water abstraction has greatly exacerbated seasonal river dry-down to the point that the river is unable to maintain dry-season flow. Thus, this anthropogenically driven alteration to river hydrology is influencing the ecological impact of H. amphibius subsidies. H. amphibius may now periodically cause eutrophication that poses challenges to aquatic communities (see also ref. 35).
During both dry seasons in which low- and high-density H. amphibius pools were sampled, concentrations of dissolved and particulate nutrients and organic carbon were significantly elevated in high-density H. amphibius pools (Fig. 3). We posit that the elevated nutrient concentrations result from the increased loading of dung and urine directly to these high-density H. amphibius pools as well as any subsequent remixing and bioturbation of these materials. With high-end estimates of H. amphibius absolute numbers in pools approaching 78 and a single H. amphibius estimated to egest ∼5 kg of organic material per day (6), it is with high confidence that we link H. amphibius to these dry-season nutrient shifts. Differences in all measured attributes of water chemistry between low- and high-density H. amphibius pools were more pronounced during the 2013 dry season, which had smaller pools and therefore a higher density of H. amphibius, as a result of a longer dry season versus the 2015 dry season. This suggests that the intensity of these H. amphibius-caused biogeochemical shifts shows graded responses that track the severity of the reduction in available water.
These dry-season differences in river water chemistry presumably caused by H. amphibius had a profound impact on both fish and aquatic invertebrate communities when measured at the level of the river pool. During the dry season in both observation years the low-density H. amphibius pools hosted more than two times higher fish diversity and approximately two times higher invertebrate diversity than the high-density H. amphibius pools (Fig. 5). The dry-season abundance of fish was found to be up to ∼27 times higher in 2013 in low-density H. amphibius pools than in high-density H. amphibius pools. However, in 2015, the relationship between fish abundance and pool size differed as pool size increased (SI Appendix, Fig. S4). This relationship, however, is primarily driven by one high-density H. amphibius pool that had relatively high fish abundance. Unlike fish, the overall abundance of aquatic invertebrates (all taxa combined) did not differ between pool types during the dry season. This lack of numerical difference was largely driven by the increase in coleopterans, which dominated high-density H. amphibius pools.
Our results suggest that a combination of different H. amphibius-induced chemical shifts contributed to the differences (when observed) in abundance and diversity. The extremely reduced DO concentrations (i.e., <1 mg/L) recorded in the high-density H. amphibius pools during the dry season are likely associated with high rates of microbial respiration in these nutrient-rich high-density pools. The long-term DO trends show that high-density H. amphibius pools experienced low DO concentrations (∼1 mg/L) for approximately 1 mo. While a number of fish and aquatic invertebrate species can tolerate short periods of hypoxic concentrations, fewer species have adaptations that allow them to tolerate such long-term reductions in DO (36, 37). Low DO concentrations can also create negative settlement cues for recruits, impacting the juvenile life stage of many aquatic invertebrates (35).
Increases in nutrient concentrations, especially when they occur in association with depressed DO concentrations, have likewise been associated with reductions in abundance and biological diversity in other freshwater ecosystems (38–40). Even DOC and PC, which can be significant resources for aquatic consumers (41), are known in high concentrations to suppress the production of attached algae (42), zooplankton (43), and fish (44).
The observed river pool-level reductions in dry-season diversity (fish and aquatic invertebrates) and abundance (fish only) could be mediated through two pathways: (i) via the emigration of species that are sensitive to reduced DO or other shifts in pool chemistry that occurred before complete dry down and isolation of these pools or (ii) via direct mortality of sensitive species. We believe both pathways may be at play in the Great Ruaha River. Fish kills were regularly observed in high-density H. amphibius pools during the onset of the dry season. Species that remained in the high-density H. amphibius pools often were those that have physiological and behavioral strategies that permit tolerance to these shifts in water chemistry. For example, C. gariepinus and O. urolepis, common in the dry-season high-density H. amphibius pools, are capable of aerial respiration and aquatic surface respiration, respectively (45, 46). Similarly, some coleopterans are able to trap air bubbles from the water surface and therefore do not rely solely on DO (47).
The extremely divergent chemical and biological differences observed between low- and high- density H. amphibius pools during the dry season were almost completely erased during the wet season when the river resumed flow. No significant differences in any of the nine water-chemistry variables that we monitored were observed during the wet season. Similarly, all wet-season–measured attributes of fish or aquatic insect communities were statistically indistinguishable between pool types when flow resumed. While H. amphibius continued to vector nutrients into these study pools during the wet season, these inputs were presumably diluted and redistributed by the increased flow. The tendency for H. amphibius to spread out slightly more across the watershed during the wet season likely also contributed to these patterns. These observed patterns illustrate how temporally variable the influence of H. amphibius is upon aquatic ecosystems and the important role that hydrology plays in regulating the intensity of these impacts.
The aforementioned dry-season impacts of H. amphibius on river biodiversity measured at the level of individual river pools in our study area very likely scale up to become biologically, ecologically, and economically consequential at the level of whole watersheds. Such impacts become evident when we compare how dry-season fish and invertebrate diversity and abundance differ between watershed scenarios in which H. amphibius are present (i.e., the scenario in which the distribution and density of H. amphibius in watershed pools is modeled to match the values observed during the 2015 H. amphibius aerial census) versus when they are absent (i.e., the scenario in which all watershed pools are considered to be low-density H. amphibius pools). Comparisons of outputs from these two different scenarios of H. amphibius density suggest that the presence of H. amphibius approximately halves DO concentrations across the entire watershed. Likely related, the presence of H. amphibius appears to drive down watershed-level dry-season fish abundance by ∼41% and Hill number fish diversity by ∼16%. These reductions occur despite a slight increase in fish species richness (from 10 to 12 fish species) in watershed scenarios with H. amphibius present (SI Appendix, Table S5). Watershed-level species richness increases as Hill number-measured diversity decreases because the two fish species (C. gariepinus and S. matthesi) that were detectable only in high-density H. amphibius pools occur only at very low abundances. In contrast to the watershed-level patterns observed for fish, H. amphibius appears to increase dry-season aquatic invertebrate diversity (Hill number) by ∼47% as well as slightly increase richness from 11 to 12 orders or classes (SI Appendix, Table S5). This increase in Hill number-measured aquatic invertebrate diversity appears to be primarily due to particularly elevated abundances of coleopterans in H. amphibius pools. The observation, however, that the abundances of all other aquatic invertebrate taxa in the watershed were negatively affected by H. amphibius suggests that some caution be used when interpreting this conclusion that H. amphibius promote aquatic invertebrate diversity (SI Appendix, Table S5).
Shifts in riverine fish and invertebrate diversity and abundance observed at both the local and regional level may proceed to affect important ecological processes such as alterations in nutrient cycling, detrital breakdown, predator–prey dynamics, and the patterns by which emergent aquatic biomass influences terrestrial ecosystem dynamics (2, 48–52). The watershed-level impacts predicted by these modeling exercises influence not only ecological dynamics but likely also human communities that are reliant on fish resources from rivers for food and income. In Tanzania, and across East Africa, for example, tilapia are the preferred and most widely consumed fish species (53). Across the Great Ruaha watershed we estimated that the dry-season effects of H. amphibius are likely to reduce tilapia abundance by ∼41%. These diverse and far-reaching impacts of H. amphibius on changing rivers are by no means specific to southern Tanzania. Across sub-Saharan Africa, the majority of rivers that are inhabited by H. amphibius are, like the Great Ruaha River, experiencing anthropogenically driven reductions in river flow or are predicted to soon experience such hydrological impacts (as reviewed in SI Appendix, Table S7). Because this amounts to impacts shaping a minimum of 63 watersheds that collectively host ∼94% of the global H. amphibius population (SI Appendix, Table S7 and ref. 54), the impacts observed in the Great Ruaha watershed very likely are indicative of emerging issues relevant to the whole of Africa.
Modified source-sink models have been applied to river systems that experience periods of low/no flow and periodic disconnection between river pools to try to understand aquatic population dynamics (38, 55). In our study, low-density H. amphibius pools likely serve as source pools during the dry season because of their ability to retain aquatic biodiversity (Fig. 5). Our observations that during the wet season the once depauperate high-density H. amphibius pools were rapidly recolonized to levels of species diversity and abundance comparable to those in low-density H. amphibius pools suggest some resilience in this system. However, exactly how local-scale losses of biodiversity and abundance in high-density H. amphibius pools impact the long-term persistence of biodiversity across the Great Ruaha watershed remains unclear. Recent records of local species extinction in the Great Ruaha watershed have already been recorded for such groups as freshwater invertebrates (e.g., Ostreidae), freshwater mammals (Aonyx capensis), and a variety of fish species that were common in the Great Ruaha watershed before the onset of severe anthropogenically driven drying which commenced in the early 1990s (25).
Discerning the impacts of H. amphibius upon this ecosystem is made more complex, and yet more important, because of its recent history of human modification. As has been documented elsewhere, H. amphibius positively influence aquatic communities via nutrient provisioning during the wet season (5, 6). However, as river discharge wanes, H. amphibius switch, especially at the local scale, from being providers of resources for aquatic communities to largely serving as an agent of eutrophication and biodiversity loss. The amplified negative effects observed during the dry season are likely new and may be shifting the balance of the net impacts that H. amphibius have on watershed-level biodiversity. Climate change and accelerating water abstraction associated with rapidly expanding human populations across the range of H. amphibius (SI Appendix, Table S7 and refs. 15–17) appear poised to further exacerbate deleterious eutrophication caused by H. amphibius. This case study calls attention to the profound ways by which human alteration of environmental variables can drastically reshape the magnitude and directionality of species’ effects on the chemistry and ecology of the ecosystems they inhabit.
Supplementary Material
Acknowledgments
We thank the Director General of Tanzania National Parks, the Tanzanian Wildlife Research Institute, Tanzania Commission for Science and Technology, the Chief Park Warden of Ruaha National Park Dr. C. D. Timbuka, and Ruaha National Park Ecologist Paul Banga for allowing us to conduct our research in Ruaha National Park. We also thank Robert Philemon Kivuyo and Melissa H. Schmitt for invaluable assistance in the field and Sandra Brovold and Michelle Rorer for their assistance with laboratory analyses. This research was funded by National Science Foundation (NSF) International Research Fellowship Program Office of International Science and Engineering Grant 1064649, NSF Division of Environmental Biology Grant 1146247, the Safari Club International Foundation, and a University of California Faculty Research Grant.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1800407115/-/DCSupplemental.
References
- 1.Nakano S, Miyasaka H, Kuhara N. Terrestrial-aquatic linkages: Riparian anthropod inputs alter trophic cascades in a stream food web. Ecology. 1999;80:2435–2441. [Google Scholar]
- 2.Sabo JL, Power ME. River-watershed exchange: Effects of riverine subsidies on riparian lizards and their terrestrial prey. Ecology. 2002;83:1860–1869. [Google Scholar]
- 3.Baxter CV, Fausch KD, Murakami M, Chapman PL. Fish invasion restructures stream and forest food webs by interrupting reciprocal prey subsidies. Ecology. 2004;85:2656–2663. [Google Scholar]
- 4.Roe AM, Meyer CB, Nibbelink NP, Ben-David M. Differential tree and shrub production in response to fertilization and disturbance by coastal river otters in Alaska. Ecology. 2010;91:3177–3188. doi: 10.1890/09-1216.1. [DOI] [PubMed] [Google Scholar]
- 5.McCauley DJ, et al. Carbon stable isotopes suggest that hippopotamus-vectored nutrients subsidize aquatic consumers in an East African river. Ecosphere. 2015;6:52. [Google Scholar]
- 6.Subalusky AL, Dutton CL, Rosi-Marshall EJ, Post DM. The hippopotamus conveyor belt: Vectors of carbon and nutrients from terrestrial grasslands to aquatic systems in sub-Saharan Africa. Freshw Biol. 2015;50:512–525. [Google Scholar]
- 7.Vannote RL, Minshall GW, Cummins KW, Sedell JR, Cushing CE. The river continuum concept. Can J Fish Aquat Sci. 1980;37:130–137. [Google Scholar]
- 8.Doucett RR, Marks JC, Blinn DW, Caron M, Hungate BA. Measuring terrestrial subsidies to aquatic food webs using stable isotopes of hydrogen. Ecology. 2007;88:1587–1592. doi: 10.1890/06-1184. [DOI] [PubMed] [Google Scholar]
- 9.Wallace JB, Eggert SL, Meyer JL, Webster JR. Multiple trophic levels of a forest stream linked to terrestrial litter inputs. Science. 1997;277:102–104. [Google Scholar]
- 10.Arthington AH, Balcombe SR, Wilson GA, Thoms MC, Marshall J. Spatial and temporal variation in fish assemblage structure in isolated waterwholes during the 2001 dry season of an arid-zone river, Cooper Creek, Australia. Mar Freshw Res. 2005;56:25–35. [Google Scholar]
- 11.Lewison RL, Carter J. Exploring behaviour of an unusual megaherbivore: A spatially explicit foraging model of the hippopotamus. Ecol Modell. 2004;171:127–138. [Google Scholar]
- 12.Power ME, Sun A, Parker G, Dietrich WE, Wootton JT. Hydraulic food-chain models. Bioscience. 1995;45:159–167. [Google Scholar]
- 13.Thieme ML, et al. Freshwater Ecoregions of Africa and Madagascar: A Conservation Assessment. Island; Washington, DC: 2005. [Google Scholar]
- 14.Bunn SE, Arthington AH. Basic principles and ecological consequences of altered flow regimes for aquatic biodiversity. Environ Manage. 2002;30:492–507. doi: 10.1007/s00267-002-2737-0. [DOI] [PubMed] [Google Scholar]
- 15.Snoussi M, et al. Downstream and coastal impacts of damming and water abstraction in Africa. Environ Manage. 2007;39:587–600. doi: 10.1007/s00267-004-0369-2. [DOI] [PubMed] [Google Scholar]
- 16.Arnell NW, Gosling SN. The impacts of climate change on river flow regimes at the global scale. J Hydrol (Amst) 2013;486:351–364. [Google Scholar]
- 17.McClain ME. Balancing water resources development and environmental sustainability in Africa: A review of recent research findings and applications. Ambio. 2013;42:549–565. doi: 10.1007/s13280-012-0359-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schindler DW, Curtis PJ. The role of DOC in protecting freshwaters subjected to climatic warming and acidification from UV exposure. Biogeochemistry. 1997;36:1–8. [Google Scholar]
- 19.Williamson CE, Morris DP, Pace ML, Olson AG. Dissolved organic carbon and nutrients as regulators of lake ecosystems: Resurrection of a more integrated paradigm. Limnol Oceanogr. 1999;44:795–803. [Google Scholar]
- 20.Williamson CE, Neale PJ, Grad G, De Lange HJ, Hargreaves BR. Beneficial and detrimental effects of UV on aquatic organisms: Implications of spectral variation. Ecol Appl. 2001;11:1843–1857. [Google Scholar]
- 21.Woodward G, et al. Continental-scale effects of nutrient pollution on stream ecosystem functioning. Science. 2012;336:1438–1440. doi: 10.1126/science.1219534. [DOI] [PubMed] [Google Scholar]
- 22.Mosepele K, Moyle PB, Merron GS, Purkey DR, Mosepele B. Fish, floods, and ecosystem engineers: Aquatic conservation in the Okavango Delta, Botswana. Bioscience. 2009;59:53–64. [Google Scholar]
- 23.Covich AP, Crowl TA, Scatena FN. Effects of extreme low flows on freshwater shrimps in a perennial tropical stream. Freshw Biol. 2003;48:1199–1206. [Google Scholar]
- 24.Coppolillo PB, Kashaija L, Moyer DC, Knapp E. 2004 Technical report on water availability in the Ruaha River and the state of the Usangu Game Reserve, November 2003. Available at https://nanopdf.com/download/water-resources-in-the-ruaha-river_pdf. Accessed March 7, 2017.
- 25.Mtahiko MGG, et al. Towards an ecohydrology-based restoration of the Usangu wetlands and the Great Ruaha River, Tanzania. Wetlands Ecol Manage. 2006;14:489–503. [Google Scholar]
- 26.Arar EJ, Collins GB. Method 445.0 in Vitro Determination of Chlorophyll a and Pheophytin a in Marine and Freshwater Algae by Fluorescence. US Environmental Protection Agency; Washington, DC: 1997. [Google Scholar]
- 27.Medeiros ESF, Silva MJ, Figueiredo BRS, Ramos TPA, Ramos RTC. Effects of fishing technique on assessing species composition in aquatic systems in semi-arid Brazil. Braz J Biol. 2010;70:255–262. doi: 10.1590/s1519-69842010000200004. [DOI] [PubMed] [Google Scholar]
- 28.Cao Y, Larsen DP, Hughes RM. Evaluating sampling efficiency in fish assemblage surveys: A similarity-based approach. Can J Fish Aquat Sci. 2001;58:1782–1793. [Google Scholar]
- 29.Dauble DD, Gray RH. Comparison of a small seine and a backpack electroshocker to evaluate nearshore fish populations in rivers. Prog Fish-Cult. 1980;42:93–95. [Google Scholar]
- 30.Clarke KR, Gorley RN. Primer-E User Manual and Tutorial. Primer-E Limited; Plymouth, UK: 2006. [Google Scholar]
- 31.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 32.Chao A, Chiu C-H, Jost L. Unifying species diversity, phylogenetic diversity, functional diversity, and related similarity and differential measures through Hill numbers. Annu Rev Ecol Evol Syst. 2014;45:297–324. [Google Scholar]
- 33.Engqvist L. The mistreatment of covariate interaction terms in linear model analyses of behavioural and evolutionary ecology studies. Anim Behav. 2005;70:967–971. [Google Scholar]
- 34.Grey J, Harper DM. Using stable isotope analyses to identify allochthonous inputs to Lake Naivasha mediated via the hippopotamus gut. Isotopes Environ Health Stud. 2002;38:245–250. doi: 10.1080/10256010208033269. [DOI] [PubMed] [Google Scholar]
- 35.Dawson J, Pillay D, Roberts PJ, Perissinotto R. Declines in benthic macroinvertebrate community metrics and microphytobenthic biomass in an estuarine lake following enrichment by hippo dung. Sci Rep. 2016;6:37359. doi: 10.1038/srep37359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis JC. Minimal dissolved oxygen requirements of aquatic life with emphasis on Canadian species: A review. J Fish Res Board Can. 1975;32:2295–2332. [Google Scholar]
- 37.Matthews WJ. Patterns in Freshwater Fish Ecology. Chapman & Hall; New York: 1998. [Google Scholar]
- 38.Magoulick DD, Kobza RM. The role of refugia for fishes during drought: A review and synthesis. Freshw Biol. 2003;48:1186–1198. [Google Scholar]
- 39.Grimm NB, et al. Merging aquatic and terrestrial perspectives of nutrient biogeochemistry. Oecologia. 2003;137:485–501. doi: 10.1007/s00442-003-1382-5. [DOI] [PubMed] [Google Scholar]
- 40.Keeler BL, et al. Linking water quality and well-being for improved assessment and valuation of ecosystem services. Proc Natl Acad Sci USA. 2012;109:18619–18624. doi: 10.1073/pnas.1215991109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karlsson J, Jansson M, Jonsson A. Similar relationships between pelagic primary and bacterial production in clearwater and humic lakes. Ecology. 2002;83:2902–2910. [Google Scholar]
- 42.Vadeboncoeur Y, et al. From Greenland to green lakes: Cultural eutrophication and its loss of benthic patchways in lakes. Limnol Oceanogr. 2003;48:1408–1418. [Google Scholar]
- 43.Kelly PT, Solomon CT, Weidel BC, Jones SE. Terrestrial carbon is a resource, but not a subsidy, for lake zooplankton. Ecology. 2014;95:1236–1242. doi: 10.1890/13-1586.1. [DOI] [PubMed] [Google Scholar]
- 44.Karlsson J, et al. Terrestrial organic matter input suppresses biomass production in lake ecosystems. Ecology. 2015;96:2870–2876. doi: 10.1890/15-0515.1. [DOI] [PubMed] [Google Scholar]
- 45.Hengsawat K, Ward FJ, Jaruratjamorn P. The effect of stocking density on yield, growth and mortality of African catfish (Clarias gariepinus Burchell 1822) cultured in cages. Aquaculture. 1997;152:67–76. [Google Scholar]
- 46.Chapman LJ, Kaufman LS, Chapman CA, McKenzie FE. Hypoxia tolerance in twelve species of East African cichlids: Potential for low oxygen refugia in Lake Victoria. Conserv Biol. 1995;9:1274–1287. doi: 10.1046/j.1523-1739.1995.9051262.x-i1. [DOI] [PubMed] [Google Scholar]
- 47.Cooper MJ, Uzarski DG, Burton TM. Benthic invertebrate fauna, wetland ecosystems. In: Likens GE, editor. Encyclopedia of Inland Waters. Elsevier; Boston: 2009. pp. 232–241. [Google Scholar]
- 48.Covich AP, Palmer MA, Crowl TA. The role of benthic invertebrate species in freshwater ecosystems. Bioscience. 1999;49:119–127. [Google Scholar]
- 49.Nakano S, Murakami M. Reciprocal subsidies: Dynamic interdependence between terrestrial and aquatic food webs. Proc Natl Acad Sci USA. 2001;98:166–170. doi: 10.1073/pnas.98.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chase JM, Knight TM. Drought-induced mosquito outbreaks in wetlands. Ecol Lett. 2003;6:1017–1024. [Google Scholar]
- 51.Knight TM, McCoy MW, Chase JM, McCoy KA, Holt RD. Trophic cascades across ecosystems. Nature. 2005;437:880–883. doi: 10.1038/nature03962. [DOI] [PubMed] [Google Scholar]
- 52.Finlay JC, Vredenburg VT. Introduced trout sever trophic connections in watersheds: Consequences for a declining amphibian. Ecology. 2007;88:2187–2198. doi: 10.1890/06-0344.1. [DOI] [PubMed] [Google Scholar]
- 53.MALF 2016. The Tanzanian fisheries sector: Challenges and opportunities (United Republic of Tanzania: Ministry of Agriculture, Livestock and Fisheries, Dar es Salaam, Tanzania)
- 54.Lewison RL, Pluháček J. 2017. Hippopotamus amphibius. The IUCN Red List of Threatened Species 2017 (International Union for Conservation of Nature and Natural Resources, Gland, Switzerland), e.T10103A18567364.
- 55.Perry GLW, Bond NR. Spatially explicit modeling of habitat dynamics and fish population persistence in an intermittent lowland stream. Ecol Appl. 2009;19:731–746. doi: 10.1890/08-0651.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.