Significance
Photosynthetic water oxidation provides oxygen and maintains aerobic life on earth. The Mn4CaO5 cluster is the catalytic site for this reaction in Photosystem II. During water oxidation, a redox-active tyrosine, YZ, is oxidized and reduced in a proton-coupled electron transfer reaction (PCET). We provide spectroscopic evidence that the essential calcium ion interacts with YZ in its radical and singlet states. In addition, multiple conformational states of the YZ radical/singlet are identified. Calcium is shown to select for a PCET reaction in a subset of these states. Conformational targeting could be a key to control of the PCET pathway and may occur in other enzymes, such as ribonucleotide reductase, which use tyrosine as a PCET cofactor.
Keywords: water oxidation, photosynthesis, manganese–calcium cofactor, vibrational spectroscopy, radical
Abstract
In Photosystem II (PSII), YZ (Tyr161D1) participates in radical transfer between the chlorophyll donor and the Mn4CaO5 cluster. Under flashing illumination, the metal cluster cycles among five Sn states, and oxygen is evolved from water. The essential YZ is transiently oxidized and reduced on each flash in a proton-coupled electron transfer (PCET) reaction. Calcium is required for function. Of reconstituted divalent ions, only strontium restores oxygen evolution. YZ is predicted to hydrogen bond to calcium-bound water and to His190D1 in PSII structures. Here, we report a vibrational spectroscopic study of YZ radical and singlet in the presence of the metal cluster. The S2 state is trapped by illumination at 190 K; flash illumination then generates the S2YZ radical. Using reaction-induced FTIR spectroscopy and divalent ion depletion/substitution, we identify calcium-sensitive tyrosyl radical and tyrosine singlet bands in the S2 state. In calcium-containing PSII, two CO stretching bands are detected at 1,503 and 1,478 cm−1. These bands are assigned to two different radical conformers in calcium-containing PSII. At pH 6.0, the 1,503-cm−1 band shifts to 1,507 cm−1 in strontium-containing PSII, and the band is reduced in intensity in calcium-depleted PSII. These effects are consistent with a hydrogen-bonding interaction between the calcium site and one conformer of radical YZ. Analysis of the amide I region indicates that calcium selects for a PCET reaction in a subset of the YZ conformers, which are trapped in the S2 state. These results support the interpretation that YZ undergoes a redox-coupled conformational change, which is calcium dependent.
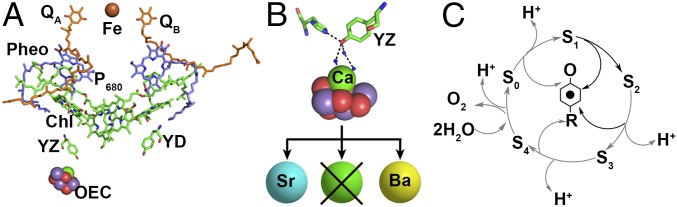
In plants, cyanobacteria, and algae, Photosystem II (PSII) carries out light-driven photosynthetic oxygen evolution (Fig. 1A). This reaction is essential for the maintenance of aerobic life on earth and is a model in the development of sustainable, alternative energy sources. Absorption of light by PSII induces a charge separation between the dimeric chlorophyll (chl) donor, P680, and a bound plastoquinone, QA or QB (Fig. 1A) (1, 2). P680+ subsequently oxidizes Tyr161D1 (YZ), which then removes an electron from the nearby Mn4CaO5 cluster. The Mn4CaO5 cluster is the site of water binding and oxidation. The YZ radical/YZ singlet redox couple acts as the interface between the one-electron chemistry of the reaction center and the four-electron–four proton reaction, which is required to release oxygen from water. On each light-driven step, YZ, which is tyrosine in the D1 polypeptide (Tyr161-D1, Fig. 1B) is transiently oxidized and reduced by a proton-coupled electron transfer (PCET) mechanism (Fig. 1C). YZ is essential for oxygen evolution. In X-ray structures from cyanobacteria and cryo-EM structures from higher plants, YZ is hydrogen-bonded to His190 in the D1 polypeptide. YZ is also hydrogen-bonded to a calcium-bound water molecule (Fig. 1B) (1–5). The extensive, calcium-dependent hydrogen-bonding network has been proposed to be important in PCET reactions (6–9).
Fig. 1.
(A) Redox cofactors in PSII. In the OEC, manganese atoms are shown in purple, calcium is shown in green, and oxygens are shown in red [Protein Data Bank (PDB) ID code 4UB6]. (B) YZ and its hydrogen-bonding partners. Water is represented by blue spheres (PDB ID code 4UB6). Illustration of divalent ion substitution and calcium depletion, starting with Ca-PSII (Sr2+ is depicted in cyan sphere, and Ba2+ in yellow). (C) S-state cycle of OEC; the black arrow indicates the only S-state transition allowed at 190 K. A proton-release pattern is shown (58). (Inset) YZ is oxidized and reduced by a PCET reaction.
With flash illumination, the oxygen-evolving complex (OEC) cycles through five different oxidation states, named the Sn states, where n = 0–4 (10). The S1 state is the dark-stable state, and oxygen is produced during the S3-to-S0 transition (Fig. 1C). Cryogenic temperatures inhibit oxygen evolution by blocking some of these transitions. At 190 K, the S1-to-S2 transition can occur, but the other S-state transitions cannot. When a flash is given to the S2 state at this temperature, YZ is oxidized by P680+, but, subsequently, YZ radical fails to oxidize the OEC (11). The QA− to QB transition is also inhibited at 190 K (12). Therefore, in the 190 K trapped S2 state, a laser flash generates the YZ radical and QA− state, which recombines via a PCET reaction (9, 13, 14).
The role of calcium in the YZ PCET reactions has not been fully elucidated. It is known that the decay time and midpoint potential of YZ radical are altered by manganese removal (for examples, see refs. 15 and 16). From X-ray and cryo-EM structures, calcium depletion is also expected to change hydrogen-bonding interactions between bound water molecules and YZ (1–5) (Fig. 1B). Calcium depletion can be readily reversed by the readdition of calcium alone (reviewed in ref. 17). Although calcium depletion is expected to change hydrogen bonding to water (18, 19), substantial changes in manganese ligation and manganese–-manganese distances have not been detected in calcium-depleted samples (3, 20, 21). Among the divalent ions, only reconstituted strontium is able to replace calcium and supports oxygen evolution activity (reviewed in refs. 22, 23). From an X-ray structure, strontium is known to bind at the calcium site (24). While there are no large changes compared with calcium-containing PSII, this structure does exhibit a slightly elongated Sr–water distance (24). Barium, with its larger ionic radius, is not effective in restoring oxygen evolution (9, 25), and has been proposed to be a noncompetitive inhibitor of water oxidation (26) and to disrupt hydrogen-bonding interactions in the proton transfer network (9).
Here, we study the structure and interactions of YZ radical and YZ singlet using reaction-induced Fourier transform infrared (RIFT-IR) spectroscopy (27). The bands of radical and singlet are distinguishable, due to the expected dramatic changes in force constants caused by oxidation of the aromatic ring (28). Notably, this is a report of the vibrational spectrum, YZ radical minus YZ singlet, in an oxygen-evolving PSII preparation. To slow the decay of YZ radical, cryogenic conditions were used to trap the OEC in the S2 state.
Results
Samples.
PSII preparations were purified and characterized by methods described in SI Appendix, Fig. S1). PSII was depleted of calcium, and this preparation was used for CD-PSII experiments at pH 6.0 and 7.5. This preparation was also used as the “parent” preparation for all reconstitution experiments. This preparation was reconstituted either with strontium or calcium, generating Sr-PSII and Ca-PSII, respectively. The preparation was also treated with barium to give Ba-PSII.
Spectroscopic Methods.
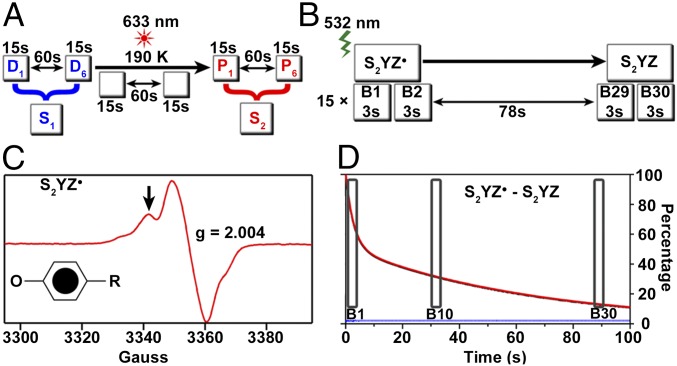
In the presence of an intact metal cluster, YZ radical is generated by a flash on the nanosecond time scale (via oxidation by P680+) and reduced on the microsecond-to-millisecond time scale by the Mn4CaO5 cluster (29). To prolong the lifetime of YZ radical and to obtain the YZ radical-minus-singlet spectrum in the presence of the Mn4CaO5 cluster, we first trapped the S2 state by continuous illumination at 190 K (Fig. 2A). This approach has been used in our published EPR studies to generate the S2 state and to monitor the decay kinetics of YZ radical after a laser flash (SI Appendix, Fig. S2) (9, 13, 14).
Fig. 2.
Monitoring the YZ·QA− recombination reaction in the S2 state. (A) The S2 state is generated by continuous, red (633-nm) illumination at 190 K. RIFT-IR data collection for the S1-to-S2 state transition is illustrated. Each data block is 15 s, and the S2QA−-minus-S1QA spectrum is generated as a control, P6-minus-D6. (B) A laser flash given to the S2 state at 190 K generating the YZ radical. RIFT-IR data collection monitoring the YZ·QA− recombination reaction is illustrated. Each data block is 3 s, and the YZ·QA−-minus-YZQA spectrum is generated, B1-minus-B30. To probe the reaction at later times, B10-B30 is used. There were 15 flashes and 15 rounds of rapid-scan FTIR data acquisition per sample. (C) Representative EPR spectrum of S2YZ· in Ca-PSII. Black arrow indicates the field position used to monitor YZ·QA− recombination in D. (Inset) Structure of tyrosine radical. (D) Representative EPR transient data monitoring YZ· in Ca-PSII at pH 6.0. The boxes show the data blocks and time scales used to construct YZ·QA− RIFT-IR spectra (B1-minus-B30; B10-minus-B30).
In the experiment described here, which monitors the RIFT-IR signal of YZ radical, the experiment was performed similarly to the published EPR studies. A 532-nm flash was given to the S2 trapped sample (Fig. 2B) after a dark adaptation. This flash generates YZ·QA− (Fig. 2C), as detected by the EPR control experiments (Fig. 2 C and D and SI Appendix, Fig. S2); this species decays on the seconds time scale. For RIFT-IR studies, the decay of YZ·QA− was monitored for a total of 90 s (Fig. 2D). To emphasize vibrational contributions from YZ radical, IR data were recorded in 3-s time blocks. These blocks were used to generate the difference RIFT-IR spectrum; for example, 3 s at time point B1 (0 s) and 3 s at time point B30 (87 s) (B1-minus-B30, Fig. 2B). These data are expected to reflect a YZ radical contribution because the control EPR transients, recorded under the same conditions, provide evidence for YZ radical decay on this time scale (Fig. 2D and SI Appendix, Fig. S2) (9). The decay of the S2 state is minimal under these conditions. Further, QA− to QB transfer is blocked at this temperature (12). For more information, see methods described previously (9, 13, 14, 30). As an additional time point, data obtained in B10 (30 s) and B30 (87 s) were also used to construct a difference RIFT-IR spectrum from Ca-PSII. This second difference spectrum, B10-B30, is expected to reflect additional YZ radical decay after the flash, compared with B1-minus-B30.
RIFT-IR Spectra Associated with the Production of the S2 State, with YZ·QA− Recombination in the S2 State, and Comparison with Controls.
SI Appendix, Fig. S3A presents the S2QA−-minus-S1QA spectrum obtained from Ca-PSII at pH 6.0, according to the method in Fig. 2A. The S2QA−-minus-S1QA spectra are characteristic of this transition and have been reported previously under these conditions (30). Any YZ radical contribution to these S2QA−-minus-S1QA spectra will be minimal because there is an ∼2-min dark adaptation time after the red illumination is discontinued. This dark time allows time for YZ radical to decay. The YD radical contribution is minimized due to a preflash, which is given at room temperature before the sample is cooled to 190 K. The preflash is given to minimize the YD radical contribution and assist in synchronization in the S1 state.
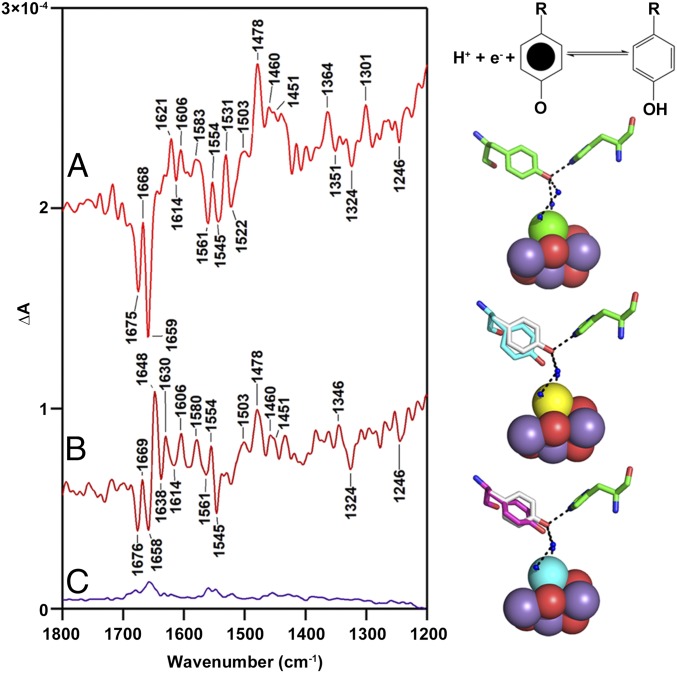
SI Appendix, Fig. S3B presents spectra obtained of YZ·QA−-minus-YZQA (Fig. 2B, B1-minus-B30), as recorded from the S2 state at 190 K. The spectrum in SI Appendix, Fig. S3B has been expanded by a factor of 5 relative to SI Appendix, Fig. S3A for comparison purposes. As shown, in some regions of the mid-IR spectrum, the S2- and YZ-associated difference spectra are easily distinguishable by comparing the relative intensities of vibrational bands. For example, bands at 1,606, 1,324, and 1,301 cm−1 are characteristic of YZ·QA−-minus-YZQA and are not observed in the S2QA−-minus-S1QA spectrum. The relative intensity differences at 1,718, 1,579, 1,554, and 1,534 cm−1 also distinguish the YZ·QA−-minus-YZQA from the S2QA−-minus-S1QA spectrum.
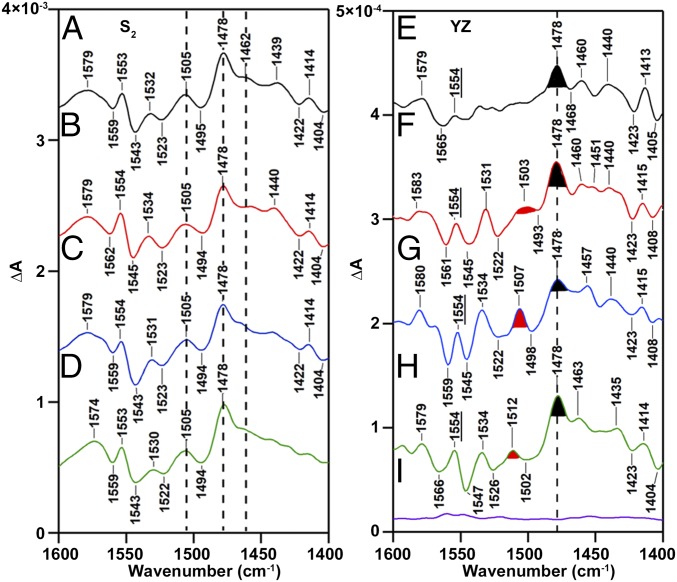
The spectrum in SI Appendix, Fig. S3B (Ca-PSII) is repeated in Fig. 3F and compared with a spectrum acquired from CD-PSII (Fig. 3E) in the 1,600–1,400-cm−1 region. Importantly, YZ radical yield and decay are similar in these two preparations, as assessed by EPR control experiments (SI Appendix, Fig. S2). Therefore, Fig. 3 E and F are both expected to contain ring and CO stretching modes of YZ radical. In model tyrosyl radicals generated by 77K UV photolysis and detected by FT-IR spectroscopy, unique radical bands are observed at 1,577 (ring stretch), 1,516 (CO stretch), and 1,448 (ring stretch) cm−1 (28). The CO stretch is at 1,514 cm−1, as detected by Raman spectroscopy in solution (31, 32). These bands are shifted in position from their frequencies in the singlet state and thus will appear in the difference spectra as unique positive signals.
Fig. 3.
Spectra associated with the S1 to S2 transition (Left) and YZ·QA− recombination (Right). RIFT-IR spectra (1,600–1,400 cm−1) were acquired at 190 K and pH 6.0. In each panel, CD-PSII is black (A and E); Ca-PSII is red (B and F); Sr-PSII is blue (C and G), and Ba-PSII is green (D and H). (Left) Representing the S1-to-S2 transition, spectra are averages of 12 (A), 13 (B), 11 (C), and 14 (D). (Right) Representing YZ·QA− recombination, a baseline, purple (I), is generated from S2-minus-S2, and spectra are averages of 17 (E), 15 (F), 15 (G), 18 (H), and 12 (I) samples. Spectra were constructed as B1-minus-B30 (Fig. 2D). The filled and marked bands are discussed in the text.
In CD-PSII at pH 6.0 (Fig. 3E), spectra resemble data recorded using red illumination from manganese-depleted PSII, which cannot oxidize water, at higher temperature. See ref. 27 for a discussion. In previous work, selective isotopic labeling of QA− or YZ radical showed that both species contribute in the 1,480-cm−1 region (33, 34). To assess their contributions under the conditions used here, SI Appendix, Fig. S4B presents a control experiment using hydroxylamine to trap QA−. In this experiment, hydroxylamine was added to Ca-PSII, and a laser flash was given to a dark-adapted sample. This experiment is expected to accumulate QA−, and YZ radical formation is blocked under this condition (35). In SI Appendix, Fig. S4B, a band at 1,484 cm−1 is observed. Previously, isotopic labeling of plastoquinone, density-functional theory (DFT) calculations, and RIFT-IR spectroscopy at 80 K led to the assignment of a 1,482-cm−1 band to the ring CC antisymmetric stretch of QA− (33). Observation of a 1,484-cm−1 band in SI Appendix, Fig. S4B is, therefore, in agreement with that previous isotopic labeling work.
A control spectrum, associated with YZ radical decay, was also obtained from manganese-depleted (Tris-washed) PSII (SI Appendix, Fig. S4 C–E) using 532-nm laser flashes. Comparison of SI Appendix, Fig. S4 C–E follows the decay of the signal as a function of time. As expected, the manganese-depleted spectrum is distinct in some regions from the intact S2YZ radical spectrum (compare SI Appendix, Fig. S4 A and C). However, both spectra exhibit a 1,478-cm−1 band. A band with this frequency was previously assigned to the CO vibrational band of YZ radical by selective isotopic labeling of tyrosine and kinetic analysis (36–39). Taken together, these control experiments support the use of previous isotopic labeling assignments in the 1,480-cm−1 region under these conditions.
In Ca-PSII, the pattern of bands, detected in the RIFT-IR spectrum, supports assignment to tyrosyl radical reduction after comparison with model compounds (SI Appendix, Fig. S3B). Bands are observed in regions of the spectrum corresponding to the CO stretching vibrations of the radical (∼1,520–1,470 cm−1) and of the singlet (1,270–1,240 cm−1). This large frequency shift is due to the delocalization of spin density in the radical (28). The appearance of amide I bands is also diagnostic of radical formation in a peptide or protein (40). These regions of the spectrum are discussed below.
CO Stretching Bands of the YZ Radical at pH 6.0 and 7.5.
As shown in Fig. 3 A–D, removal of calcium and replacement with other cations does not have a significant effect on the spectrum associated with S2QA−-minus-S1QA (see additional spectral regions, SI Appendix, Fig. S5). However, in the YZ·QA−-minus-YZQA spectrum derived from Ca-PSII (Fig. 3F), a positive band is observed at 1,503 cm−1, which is calcium-sensitive. For example, the intensity of this band decreases when calcium is depleted (Fig. 3E). This is attributed to broadening, due to conformational flexibility of the radical in the absence of calcium (see discussion of amide I region below). Further, strontium replacement (Fig. 3G) shifts this positive band to 1,507 cm−1. In barium-treated PSII (Fig. 3H), the positive band is observed at 1,512 cm−1. The frequency of this band in barium-treated PSII is similar to the frequency in a model compound, i.e., tyrosyl radicals produced by UV photolysis of the amino acid in solution, 1,514 ± 2 cm−1 (32).
Experiments were also conducted at pH 7.5 (SI Appendix, Fig. S6). At this pH, CD-PSII and Ba-PSII exhibit bands with frequencies, which are similar, at 1,507 and 1,508 cm−1. This similarity between calcium-depleted and barium-treated preparations is consistent with the proposed role of barium as a noncompetitive inhibitor (26) and with disruption of hydrogen-bonding interactions when barium interacts with PSII (9, 25). At pH 7.5, calcium reconstitution gives a broad band at 1,503 cm1, which is the same frequency observed in Ca-PSII at pH 6.0. Strontium reconstitution and pH 7.5 give a band at 1,504 cm−1, down-shifted from the pH 6.0 Sr-PSII result. This pH dependence is attributed to protonation of amino acid residues, such as His190D1, near YZ at pH 6.0 (1–5). Based on frequency and the EPR control experiments, we assign the Ca-PSII 1,503 cm−1 to a υ7a stretching mode of YZ radical, in the presence of the Mn4CaO5 cluster and in one trapped conformational state. The observation of divalent ion-dependent shifts provides strong support for the assignment of the 1,503-cm−1 CO band to a calcium-interacting conformer of YZ.
Based on previous isotopic labeling studies, the 1,478-cm−1 band (Fig. 3 E–H) is also assigned to the CO stretching mode of the YZ radical. This form of YZ radical is in a distinct environment due to an alteration in the trapped conformational state. The 1,478-cm−1 band is not calcium sensitive and appears in the calcium-depleted PSII spectrum. This band is observed both at pH 6.0 and 7.5 (SI Appendix, Fig. S6). This interpretation suggests that there are at least two conformational states of YZ radical, which differ in the degree of their interaction with the calcium ion. Note that in the S2QA−-minus-S1QA spectra (Fig. 3 A–D), overlapping contributions from other redox active species, amino acids, and peptide bonds contribute. While a complete assignment of the S2 spectrum at 190 K is not yet available (41), Fig. 3 A–D shows that calcium sensitivity is not observed in this region.
CO Stretching Bands of YZ Singlet at pH 6.0.
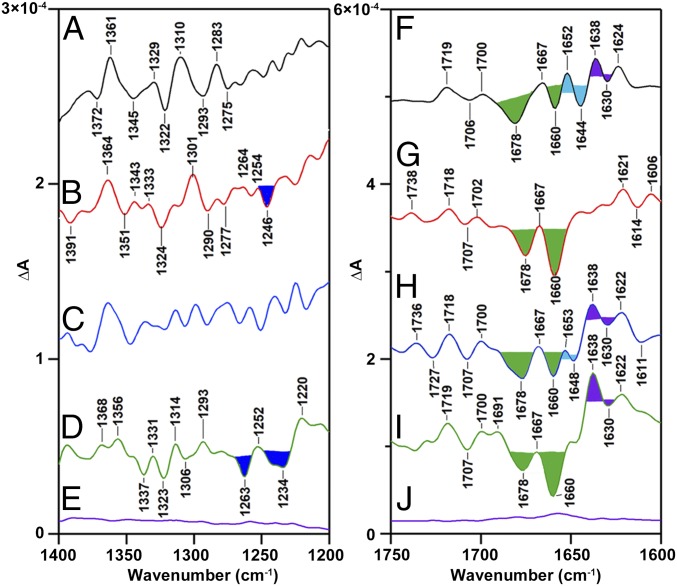
Fig. 4 (Left) presents the expanded region from 1,400 to 1,200 cm−1 of the YZ·QA−-minus-YZQA spectrum. This region is predicted by model compound studies to contain a negative band, corresponding to the CO stretching mode of the YZ singlet state. The CO stretching mode of tyrosine absorbs between 1,260 and 1,240 cm−1, depending on the strength and type of hydrogen bonding to the phenolic oxygen (42). A negative band is observed at 1,245 cm−1 in Ca-PSII (Fig. 4B), but is absent in CD-PSII (Fig. 4A). In Sr-PSII (Fig. 4C), the spectra are complex in this region, but in Ba-PSII (Fig. 4D) two bands are observed. These data support assignment of a calcium-dependent 1,245-cm−1 band to the YZ singlet state. The frequency is suggestive of a protonated YZ singlet state, which is hydrogen bonding as a proton acceptor (42). While bands are observed in this region in the S2QA−-minus-S1QA spectra (SI Appendix, Fig. S5, Left), the S2/S1-derived bands are not identical in frequency and not calcium dependent, and so can be distinguished from the YZ singlet band. RIFT-IR spectra were also acquired at pH 7.5 and compared with the pH 6.0 spectra (SI Appendix, Fig. S7). The 1,245-cm−1 band is observed in Ca-PSII either at pH 6 or pH 7.5 (SI Appendix, Fig. S7 B and G).
Fig. 4.
Divalent cation effects on spectra associated with YZ·QA− recombination. RIFT-IR spectra (1,400–1,200 cm−1, Left and 1,750–1,600 cm−1, Right) were acquired at 190 K and pH 6.0. Samples: CD-PSII is black (A and F); Ca-PSII is red (B and G); Sr-PSII is blue (C and H); Ba-PSII is green (D and I), and baselines, purple (E and J), are generated from S2-minus-S2. Spectra are averages of 17 (A and F), 15 (B and G), 15 (C and H), 18 (D and I), and 12 (E and J) samples. Spectra (A–D and F–I) were constructed as B1-minus-B30 (Fig. 2D). The filled bands are discussed in the text.
Amide I Region.
Fig. 4 (Right) shows the 1,750–1,600-cm−1 region of the YZ·QA− recombination spectrum; in this region, amide I bands are expected at ∼1,650 cm−1. In model peptides and ribonucleotide reductase (RNR), oxidation/reduction of tyrosine is associated with amide I and II contributions to the spectrum (40, 43). These contributions are characteristic of sequence and will also reflect the detailed backbone and dihedral angles associated with the trapped conformer. The observation of two different CO vibrational bands, as discussed above, is consistent with the presence of at least two YZ radical conformers. All of the spectra in Fig. 4 F–I exhibit negative, positive, negative bands at 1,678, 1,667, and 1,660 cm−1, respectively, which can be attributed to a redox reaction in a conformer, with one defined set of backbone and dihedral angles (green fill). In Ba-PSII (Fig. 4I), the amide region exhibits these 1,678-, 1,667-, and 1,660-cm−1 bands and additional bands at positive 1,638 and negative 1,630 cm−1 (dark-blue fill). These additional bands are considered here as markers for a conformer, preferentially populated in Ba-PSII. Comparison of spectra acquired in Ba-PSII with data acquired in Sr-PSII (Fig. 4H) shows that while some of the amide bands are similar, Sr-PSII exhibits additional amide bands at positive 1,653 and negative 1,648 cm−1 (light-blue fill). This reflects a third conformational state, populated by strontium binding at the calcium site. In CD-PSII, the amide region of the spectrum contains amide bands corresponding to all of the conformers discussed above (Fig. 4F), attributable to increased conformational flexibility, acquired in the absence of calcium.
As described here, the amide I region of Ca-PSII is distinct from that of Sr-PSII and Ba-PSII, when YZ·QA− data are compared immediately following the laser flash (B1-minus-B30, Fig. 2). Notably, in Ca-PSII, additional amide I conformational marker bands appear at later times (B10-minus-B30 spectrum, Fig. 5B). These data show that calcium occupancy selects for a PCET reaction in a subset of YZ conformational states. When the pH 6.0 experiments are compared with those recorded at pH 7.5 (SI Appendix, Fig. S8), the amide band frequencies are shifted, but the same overall patterns apply. For example, the amide I region of the CD-PSII spectrum appears to be the most complex at pH 7.5, as also noted at pH 6.0.
Fig. 5.
Time dependence of the YZ·QA− RIFT-IR signal (Left) and a conformational model (Right) showing divalent cation effects on the amide I region. (Left) RIFT-IR spectra acquired at 190 K, showing YZ·QA−-minus-YZQA spectra from Ca-PSII at two different times after the laser flash. The spectra are (A) red, B1-minus-B30, corresponding to 0 s minus 87 s, and (B) brown, B10-minus-B30, corresponding to 30 s minus 87 s), at pH 6.0. See method, Fig. 2. Spectra in A and B are averages derived from 15 samples. C is a baseline, generated from S2-minus-S2 (12 samples). (Right) Calcium-dependent, conformational selection and the YZ·QA− recombination reaction. Waters are shown as blue spheres (colors as Fig. 1). The conformer at the top is taken from PDB ID code 4UB6. The other conformers are speculative and were generated using the mutagenesis function.
1,550- and 1,750-cm−1 Regions.
In the YZ difference spectra, bands are observed at 1,740–1,710 cm−1 (Fig. 4 and SI Appendix, Fig. S8). We attribute these bands to the transfer of Bohr protons to aspartate and glutamate residues in response to the YZ PCET reactions. In model compounds, the ν8a ring stretching vibrational band of the radical is expected as a positive band at 1,556 cm−1 (28). A positive 1,554-cm−1 band in the YZ recombination spectra (Fig. 3 E–H) has a frequency consistent with a radical ring stretching assignment. This band is observed at pH 6.0 and 7.5 (SI Appendix, Fig. S6).
Discussion
Biological radical transfer reactions are important in many enzymatic processes and are often facilitated by redox-active tyrosines. In proteins such as PSII and RNR, conserved tyrosine side chains act as electron transfer intermediates and reaction initiators, respectively. In PSII, YZ radical accepts an electron from the Mn4CaO5 cluster and drives transitions in the S-state cycle. The midpoint potential of tyrosine depends on pH (44). The driving force for each S-state transition is small (45). Therefore, the tyrosyl radical must be reprotonated on each electron transfer to preserve the high-midpoint potential of YZ.
To control the high reactivity of the radical state, a conformational control mechanism could be adopted. For example, a translation of the aromatic ring, when the radical and singlet states are compared, can make/break hydrogen bonds or change the accessibility of the site to water. These changes can then be crucial to the mechanism, since the making and breaking of a hydrogen bond (∼3 kcal/mol) is significant in the energetics of PSII reactions. Redox-coupled structural changes have been proposed to occur at two points in the RNR PCET pathway, Y122 and Y731 (43, 46, 47). In addition, redox-coupled changes in secondary structure have been observed and simulated in beta hairpin peptides (48). Thus, conformational gating could be a general method of targeting and controlling PCET pathways in enzymes.
This report provides a vibrational spectroscopic study of the YZ radical and YZ singlet states in the presence of the Mn4CaO5 cluster and a report of conformational selection in PSII. To obtain this information, PSII sample was trapped in the S2 state, and the recombination of YZ radical and QA− was monitored at 190 K. These are conditions in which YZ radical cannot oxidize the S2 state, and the cycle is blocked. However, the metal cluster is intact, and relevant hydrogen-bonding interactions to the YZ radical and singlet state are preserved. Using this approach, we present evidence for calcium-induced changes in the vibrational frequencies of a subset of YZ radical and YZ singlet bands. In the presence of calcium, a positive band at 1,503 cm−1 is observed, assigned here to a conformer of YZ radical that interacts with the calcium ion. The band at 1,503 cm−1 shifts to 1,507 cm−1 with strontium replacement and to 1,512 cm−1 after barium treatment. The 1,512-cm−1 frequency observed with barium treatment is similar to the frequency observed in model tyrosyl radical in solution with no divalent ion interaction. This result is consistent with disruption of the hydrogen-bond network after barium treatment, as previously proposed (9). The expected pKas of metal-bound water for calcium and strontium are 12.7 and 13.2 (see refs. 22, 25, 26, and references therein). The observed shift when strontium-YZ (1,507 cm−1) is compared with calcium-YZ (1,503 cm−1) is consistent with a change in hydrogen bonding between the YZ phenoxyl oxygen and calcium/strontium-bound water. The intensity of the 1,503-cm−1 band is decreased in CD-PSII at pH 6.0, representing broadening caused by conformational flexibility at the YZ site induced in the absence of calcium. At pH 7.5 in CD-PSII, the band is observed at 1,507 cm−1, which is similar to the CO frequency observed in Ba-PSII at this pH value.
For YZ singlet, divalent ion replacement or removal alters the frequency of a negative band at 1,245 cm−1. In Sr-PSII at pH 6, this band is shifted and reduced in intensity, attributable to the presence of multiple interactions. In Ba-PSII, two bands are observed at 1,263 and 1,234 cm−1. These results are consistent with a hydrogen-bonding interaction between the calcium site and the YZ singlet state.
The YZ radical bands are down-shifted from those of tyrosyl radical in solution, where the band is observed at 1,514 ± 2 cm−1 (28, 31, 32). However, in a matrix experiment, phenoxyl radical has been reported to have a CO vibrational band at 1,481 cm−1 (49). This change was attributed to a decrease in the double-bond character of the CO bond. Interactions between phenoxyl radicals and metal complexes have been shown to down-shift the radical CO frequency in model compounds (50). In Escherichia coli RNR, the Y122 radical has a CO stretching mode at 1,499 cm−1 (32, 51). The Y122 radical is not hydrogen bonded, but is located in a hydrophobic environment (46). Therefore, the unique CO frequencies of YZ at 1,503 and 1,478 cm−1 (pH 6.0) radical reflect hydrogen bonding in a high dielectric environment and the unique interactions of YZ with H190D1 and calcium-bound water. The YZ singlet band at 1,245 cm−1 is characteristic of a strongly hydrogen-bonded, protonated tyrosine, which accepts a hydrogen bond. Failure to observe the singlet CO band in CD-PSII and Sr-PSII is attributed to broadening and distribution of hydrogen-bonding interactions.
One focus of our study is on the coupling of the YZ PCET reactions with backbone and dihedral angle changes. This can be assessed from the amide I region. These data are consistent with a calcium-dependent selection mechanism. It has been shown previously that oxidation of tyrosine in dipeptides (40), pentapeptides (52), and 18-mer beta hairpins (31) leads to appearance of amide I vibrational bands in the RIFT-IR spectrum, associated with UV photolysis and radical generation. For RNR, 13C1 isotopic labeling of the tyrosine backbone and DFT simulations led to the conclusion that the spectral pattern in the amide I and II regions is diagnostic of the backbone and dihedral angle change. The B-radical to A-singlet conformational change corresponded to an ∼100° change of backbone dihedral angle and was modeled to translate the phenolic oxygen and make/break a hydrogen bond to D84 (43).
To explain our PSII results, cryogenic illumination is proposed to trap multiple conformers of the YZ-Mn4CaO5 cofactor. In Ca-PSII, the pattern of bands in the amide I region is characterized by two negative bands at 1,678 and 1,660 cm−1. These marker bands are observed in Sr-PSII, but Sr-PSII also exhibits additional amide bands. We hypothesize that these bands are characteristic of another YZ radical conformation. Distinctive amide I marker bands appear in the Ca-PSII spectra on a longer time scale. Calcium depletion alters the amide region, suggesting that interactions with calcium are important in conformer selection in the PCET reaction.
In structures of PSII, YZ is hydrogen bonded to His190 in the D1 polypeptide and to calcium-bound water (1–5). Our previous EPR results led us to propose a two-pathway model for proton transfer to YZ radical (9). One, most likely dominant at low pH, involves His190 in the D1 polypeptide. A second pathway was proposed to involve water bound to calcium. These current results are consistent with this picture and with hydrogen bonding between calcium-bound water and YZ conformers both in the radical and singlet states. Such an outcome is consistent with a role for this water in proton transfer. Our results also provide evidence for a distinct local conformational change in the YZ side chain, which is controlled in Ca-PSII. This strategy of a controlled, conformational rearrangement may be important in targeting PCET pathways in PSII and in other redox enzymes, which conduct radical transfer with aromatic amino acid cofactors.
Experimental Methods
Highly active PSII was purified from market spinach using Triton X-100, followed by octylthioglucoside (OTG) treatment to give OTG-PSII samples (53, 54). The light-induced oxygen evolution rate (55) was greater than 1,000 µmol O2 (mg chl·h)−1 at pH 6.0. Ethylene glycol-bis(β-aminoethyl ether)-N,N′,N′,N′-tetraacetic acid treatment at pH 7.5 was then used to generate a CD-PSII sample (see SI Appendix, Fig. S1 for additional details). Calcium and strontium reconstitution (Ca-PSII and Sr-PSII) (9, 30), barium treatment (Ba-PSII) (9, 25, 26, 30), and Tris-treatment, generating manganese-depleted or “Tris-washed” PSII, were performed (56). EPR and RIFT-IR spectroscopy were performed at pH 6.0 or pH 7.5 and 190 K (9, 13, 14, 30, 41, 57). See SI Appendix.
Supplementary Material
Acknowledgments
The authors thank Ms. Udita Brahmachari for helpful discussions. The authors acknowledge National Science Foundation Grant MCB-14-11734 (to B.A.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1800758115/-/DCSupplemental.
References
- 1.Umena Y, Kawakami K, Shen JR, Kamiya N. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature. 2011;473:55–60. doi: 10.1038/nature09913. [DOI] [PubMed] [Google Scholar]
- 2.Suga M, et al. Native structure of photosystem II at 1.95 Å resolution viewed by femtosecond X-ray pulses. Nature. 2015;517:99–103. doi: 10.1038/nature13991. [DOI] [PubMed] [Google Scholar]
- 3.Young ID, et al. Structure of photosystem II and substrate binding at room temperature. Nature. 2016;540:453–457. doi: 10.1038/nature20161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei X, et al. Structure of spinach photosystem II-LHCII supercomplex at 3.2 Å resolution. Nature. 2016;534:69–74. doi: 10.1038/nature18020. [DOI] [PubMed] [Google Scholar]
- 5.Su X, et al. Structure and assembly mechanism of plant C2S2M2-type PSII-LHCII supercomplex. Science. 2017;357:815–820. doi: 10.1126/science.aan0327. [DOI] [PubMed] [Google Scholar]
- 6.Saito K, Shen JR, Ishida T, Ishikita H. Short hydrogen bond between redox-active tyrosine Y(Z) and D1-His190 in the photosystem II crystal structure. Biochemistry. 2011;50:9836–9844. doi: 10.1021/bi201366j. [DOI] [PubMed] [Google Scholar]
- 7.Polander BC, Barry BA. A hydrogen-bonding network plays a catalytic role in photosynthetic oxygen evolution. Proc Natl Acad Sci USA. 2012;109:6112–6117. doi: 10.1073/pnas.1200093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ugur I, Rutherford AW, Kaila VR. Redox-coupled substrate water reorganization in the active site of photosystem II-The role of calcium in substrate water delivery. Biochim Biophys Acta. 2016;1857:740–748. doi: 10.1016/j.bbabio.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 9.Guo Z, Barry BA. Calcium, ammonia, redox-active tyrosine YZ, and proton-coupled electron transfer in the photosynthetic oxygen-evolving complex. J Phys Chem B. 2017;121:3987–3996. doi: 10.1021/acs.jpcb.7b01802. [DOI] [PubMed] [Google Scholar]
- 10.Kok B, Forbush B, McGloin M. Cooperation of charges in photosynthetic O2 evolution-I. A linear four step mechanism. Photochem Photobiol. 1970;11:457–475. doi: 10.1111/j.1751-1097.1970.tb06017.x. [DOI] [PubMed] [Google Scholar]
- 11.Ioannidis N, Zahariou G, Petrouleas V. The EPR spectrum of tyrosine Z* and its decay kinetics in O2-evolving photosystem II preparations. Biochemistry. 2008;47:6292–6300. doi: 10.1021/bi800390r. [DOI] [PubMed] [Google Scholar]
- 12.de Paula JC, Innes JB, Brudvig GW. Electron transfer in photosystem II at cryogenic temperatures. Biochemistry. 1985;24:8114–8120. doi: 10.1021/bi00348a042. [DOI] [PubMed] [Google Scholar]
- 13.Keough JM, Jenson DL, Zuniga AN, Barry BA. Proton coupled electron transfer and redox-active tyrosine Z in the photosynthetic oxygen-evolving complex. J Am Chem Soc. 2011;133:11084–11087. doi: 10.1021/ja2041139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keough JM, Zuniga AN, Jenson DL, Barry BA. Redox control and hydrogen bonding networks: Proton-coupled electron transfer reactions and tyrosine Z in the photosynthetic oxygen-evolving complex. J Phys Chem B. 2013;117:1296–1307. doi: 10.1021/jp3118314. [DOI] [PubMed] [Google Scholar]
- 15.Zhang M, et al. Structural insights into the light-driven auto-assembly process of the water oxidizing Mn4CaO5-cluster in photosystem II. eLife. 2017;6:e26933. doi: 10.7554/eLife.26933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dekker JP, van Gorkom HJ, Brok M, Ouwehand L. Optical characterization of photosystem II electron donors. Biochim Biophys Acta. 1984;764:301–309. [Google Scholar]
- 17.Miqyass M, van Gorkom H. Calcium requirement for S-state transitions. Photosynth Res. 2007;91:175. [Google Scholar]
- 18.Siegbahn PEM. Water oxidation energy diagrams for photosystem II for different protonation states, and the effect of removing calcium. Phys Chem Chem Phys. 2014;16:11893–11900. doi: 10.1039/c3cp55329a. [DOI] [PubMed] [Google Scholar]
- 19.Saito K, Ishikita H. Influence of the Ca(2+) ion on the Mn4Ca conformation and the H-bond network arrangement in Photosystem II. Biochim Biophys Acta. 2014;1837:159–166. doi: 10.1016/j.bbabio.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Latimer MJ, DeRose VJ, Yachandra VK, Sauer K, Klein MP. Structural effects of calcium depletion on the manganese cluster of photosystem II: Determination by x-ray absorption spectroscopy. J Phys Chem B. 1998;102:8257–8265. doi: 10.1021/jp981668r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lohmiller T, Cox N, Su JH, Messinger J, Lubitz W. The basic properties of the electronic structure of the oxygen-evolving complex of photosystem II are not perturbed by Ca2+ removal. J Biol Chem. 2012;287:24721–24733. doi: 10.1074/jbc.M112.365288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yocum CF. The calcium and chloride requirements of the O2 evolving complex. Coord Chem Rev. 2008;252:296–305. [Google Scholar]
- 23.Miqyass M, Marosvölgyi MA, Nagel Z, Yocum CF, van Gorkom HJ. S-state dependence of the calcium requirement and binding characteristics in the oxygen-evolving complex of photosystem II. Biochemistry. 2008;47:7915–7924. doi: 10.1021/bi8006059. [DOI] [PubMed] [Google Scholar]
- 24.Koua FHM, Umena Y, Kawakami K, Shen JR. Structure of Sr-substituted photosystem II at 2.1 A resolution and its implications in the mechanism of water oxidation. Proc Natl Acad Sci USA. 2013;110:3889–3894. doi: 10.1073/pnas.1219922110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polander BC, Barry BA. Calcium and the hydrogen-bonded water network in the photosynthetic oxygen-evolving complex. J Phys Chem Lett. 2013;4:786–791. doi: 10.1021/jz400071k. [DOI] [PubMed] [Google Scholar]
- 26.Vrettos JS, Stone DA, Brudvig GW. Quantifying the ion selectivity of the Ca2+ site in photosystem II: Evidence for direct involvement of Ca2+ in O2 formation. Biochemistry. 2001;40:7937–7945. doi: 10.1021/bi010679z. [DOI] [PubMed] [Google Scholar]
- 27.Pujols-Ayala I, Barry BA. Tyrosyl radicals in photosystem II. Biochim Biophys Acta. 2004;1655:205–216. doi: 10.1016/j.bbabio.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 28.Range K, Ayala I, York D, Barry BA. Normal modes of redox-active tyrosine: Conformation dependence and comparison to experiment. J Phys Chem B. 2006;110:10970–10981. doi: 10.1021/jp061503f. [DOI] [PubMed] [Google Scholar]
- 29.Renger G, Kühn P. Reaction pattern and mechanism of light induced oxidative water splitting in photosynthesis. Biochim Biophys Acta. 2007;1767:458–471. doi: 10.1016/j.bbabio.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Guo Z, Barry BA. Cryogenic trapping and Isotope editing identify a protonated water cluster as an intermediate in the photosynthetic oxygen-evolving reaction. J Phys Chem B. 2016;120:8794–8808. doi: 10.1021/acs.jpcb.6b05283. [DOI] [PubMed] [Google Scholar]
- 31.Pagba CV, Barry BA. Redox-induced conformational switching in photosystem-II-inspired biomimetic peptides: A UV resonance Raman study. J Phys Chem B. 2012;116:10590–10599. doi: 10.1021/jp303607b. [DOI] [PubMed] [Google Scholar]
- 32.Pagba CV, et al. A tyrosine-tryptophan dyad and radical-based charge transfer in a ribonucleotide reductase-inspired maquette. Nat Commun. 2015;6:10010. doi: 10.1038/ncomms10010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Razeghifard MR, et al. In vivo, in vitro, and calculated vibrational spectra of plastoquinone and the Plastosemiquinone anion radical. J Phys Chem B. 1999;103:9790–9800. [Google Scholar]
- 34.Kim S, et al. Isotope-based discrimination between the infrared modes of plastosemiquinone anion radicals and neutral tyrosyl radicals in photosystem II. J Phys Chem B. 2000;104:9720–9727. [Google Scholar]
- 35.Ghanotakis DF, Babcock GT. Hydroxylamine as an inhibitor between Z and P680 in photosystem-II. FEBS Lett. 1983;153:231–234. [Google Scholar]
- 36.MacDonald GM, Bixby KA, Barry BA. A difference Fourier-transform infrared study of two redox-active tyrosine residues in photosystem II. Proc Natl Acad Sci USA. 1993;90:11024–11028. doi: 10.1073/pnas.90.23.11024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim SY, Ayala I, Steenhuis JJ, Gonzalez ET, Barry BA. Infrared spectroscopic identification of the C-O stretching vibration associated with the tyrosyl Z center dot and D center dot radicals in photosystem II. Biochim Biophys Acta. 1998;1364:337–360. doi: 10.1016/s0005-2728(98)00016-4. [DOI] [PubMed] [Google Scholar]
- 38.Ayala I, Kim S, Steenhuis JJ, Razeghifard MR, Barry BA. A kinetic study of the vibrational spectrum associated with the reduction of tyrosyl radical Z center dot in photosystem II. Biophys J. 1999;76:A248. [Google Scholar]
- 39.Pujols-Ayala I, Sacksteder CA, Barry BA. Redox-active tyrosine residues: Role for the peptide bond in electron transfer. J Am Chem Soc. 2003;125:7536–7538. doi: 10.1021/ja035005l. [DOI] [PubMed] [Google Scholar]
- 40.Ayala I, Range K, York D, Barry BA. Spectroscopic properties of tyrosyl radicals in dipeptides. J Am Chem Soc. 2002;124:5496–5505. doi: 10.1021/ja0164327. [DOI] [PubMed] [Google Scholar]
- 41.Steenhuis JJ, Barry BA. Protein and ligand environments of the S2 state in photosynthetic oxygen evolution: A difference FT-IR study. J Phys Chem B. 1997;101:6652–6660. [Google Scholar]
- 42.Takeuchi H, Watanabe N, Satoh Y, Harada I. Effects of hydrogen-bonding on the tyrosine Raman bands in the 1300-1150 cm-1 region. J Raman Spectrosc. 1989;20:233–237. [Google Scholar]
- 43.Offenbacher AR, Burns LA, Sherrill CD, Barry BA. Redox-linked conformational control of proton-coupled electron transfer: Y122 in the ribonucleotide reductase β2 subunit. J Phys Chem B. 2013;117:8457–8468. doi: 10.1021/jp404757r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harriman A. Further comments on the redox potentials of tryptophan and tyrosine. J Phys Chem B. 1987;91:6102–6104. [Google Scholar]
- 45.Krishtalik LI. Energetics of multielectron reactions–photosynthetic oxygen evolution. Biochim Biophys Acta. 1986;849:162–171. [Google Scholar]
- 46.Högbom M, et al. Displacement of the tyrosyl radical cofactor in ribonucleotide reductase obtained by single-crystal high-field EPR and 1.4-A x-ray data. Proc Natl Acad Sci USA. 2003;100:3209–3214. doi: 10.1073/pnas.0536684100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kasanmascheff M, Lee W, Nick TU, Stubbe J, Bennati M. Radical transfer in E. coli ribonucleotide reductase: A NH2Y731/R(411)A-alpha mutant unmasks a new conformation of the pathway residue 731. Chem Sci (Camb) 2016;7:2170–2178. doi: 10.1039/c5sc03460d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hwang H, et al. Redox-driven conformational dynamics in a photosystem-II-inspired β-hairpin maquette determined through spectroscopy and simulation. J Phys Chem B. 2017;121:3536–3545. doi: 10.1021/acs.jpcb.6b09481. [DOI] [PubMed] [Google Scholar]
- 49.Spanget-Larsen J, et al. Vibrations of the phenoxyl radical. J Am Chem Soc. 2001;123:11253–11261. doi: 10.1021/ja0113596. [DOI] [PubMed] [Google Scholar]
- 50.Schnepf R, et al. Resonance Raman spectroscopic study of phenoxyl radical complexes. J Am Chem Soc. 1998;120:2352–2364. [Google Scholar]
- 51.Barry BA, et al. Proton coupled electron transfer and redox active tyrosines: Structure and function of the tyrosyl radicals in ribonucleotide reductase and photosystem II. J Phys Chem Lett. 2012;3:543–554. doi: 10.1021/jz2014117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vassiliev IR, Offenbacher AR, Barry BA. Redox-active tyrosine residues in pentapeptides. J Phys Chem B. 2005;109:23077–23085. doi: 10.1021/jp054159f. [DOI] [PubMed] [Google Scholar]
- 53.Berthold DA, Babcock GT, Yocum CF. A highly resolved, oxygen-evolving photosystem-II preparation from spinach thylakoid membranes: EPR and electron-transport properties. FEBS Lett. 1981;134:231–234. [Google Scholar]
- 54.Mishra RK, Ghanotakis DF. Selective extraction of CP 26 and CP 29 proteins without affecting the binding of the extrinsic proteins (33, 23 and 17 kDa) and the DCMU sensitivity of a Photosystem II core complex. Photosynth Res. 1994;42:37–42. doi: 10.1007/BF00019056. [DOI] [PubMed] [Google Scholar]
- 55.Barry BA. Tyrosyl radicals in photosystem II. Methods Enzymol. 1995;258:303–319. doi: 10.1016/0076-6879(95)58053-0. [DOI] [PubMed] [Google Scholar]
- 56.Yamamoto Y, Tamura N, Nishimura M. Release of polypeptides from highly active O2‐evolving photosystem II preparation by this treatment. FEBS Lett. 1981;133:265–268. [Google Scholar]
- 57.Steenhuis JJ, Barry BA. A difference infrared study of protein structural changes in the photosynthetic water-oxidizing complex. J Am Chem Soc. 1996;118:11927–11932. [Google Scholar]
- 58.Junge W, Haumann M, Ahlbrink R, Mulkidjanian A, Clausen J. Electrostatics and proton transfer in photosynthetic water oxidation. Philos Trans R Soc Lond B Biol Sci. 2002;357:1407–1417, discussion 1417–1420. doi: 10.1098/rstb.2002.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.