Significance
The lateral habenula, a brain region that has been implicated in depression, receives inputs from brain nuclei associated with basic emotions and drives. In this report, using fiber photometry and optogenetics on behaving rats, we show that one major lateral habenula output pathway controls the motivation to exert effort in both aversive and appetitive contexts. Overactivity of this pathway could contribute to the reduced motivation seen in human depression.
Keywords: lateral habenula, rostromedial tegmental nucleus, motivation, optogenetics, fiber photometry
Abstract
The neural mechanisms conferring reduced motivation, as observed in depressed individuals, is poorly understood. Here, we examine in rodents if reduced motivation to exert effort is controlled by transmission from the lateral habenula (LHb), a nucleus overactive in depressed-like states, to the rostromedial tegmental nucleus (RMTg), a nucleus that inhibits dopaminergic neurons. In an aversive test wherein immobility indicates loss of effort, LHb→RMTg transmission increased during transitions into immobility, driving LHb→RMTg increased immobility, and inhibiting LHb→RMTg produced the opposite effects. In an appetitive test, driving LHb→RMTg reduced the effort exerted to receive a reward, without affecting the reward’s hedonic property. Notably, LHb→RMTg stimulation only affected specific aspects of these motor tasks, did not affect all motor tasks, and promoted avoidance, indicating that LHb→RMTg activity does not generally reduce movement but appears to carry a negative valence that reduces effort. These results indicate that LHb→RMTg activity controls the motivation to exert effort and may contribute to the reduced motivation in depression.
Depressive disorders cause significant morbidity and mortality in the human population (1). A number of potentially aberrant neural mechanisms have been characterized, which may reflect the multiplicity of depressive symptoms (2–9). While recent studies in humans (10–14) and rodents (15–23) suggest that excessive lateral habenula (LHb) activity may contribute to depression, the impact of LHb hyperactivity on an individual’s level of motivation has not been examined.
Motivation can be defined as the propensity of an organism to exert effort to move toward a rewarding, or away from an aversive, stimulus (24). The amount of effort an individual exerts to achieve a goal is believed to depend on a complex calculation of the cost required to perform a defined action and the perceived benefit gained from that action (24). Maladaptive dysfunction in neural pathways encoding such information (e.g., if the cost of performing an action is overvalued or if the perceived benefit is undervalued) can lead to behavioral deficits such as the reduced motivation seen in depression (25, 26). The specific neural pathways underlying such motivational deficits in depression are unknown.
The LHb, a predominantly glutamatergic nucleus, receives inputs from several limbic nuclei associated with motivational states (27–31). It provides a major disynaptic inhibitory output, through the midbrain GABAergic rostromedial tegmental nucleus (RMTg) to monoaminergic centers (32). In particular, the RMTg transmits reward-related signals from the LHb to dopamine neurons, which are suggested to play a central role in reinforcing or discouraging ongoing action (33, 34). LHb→RMTg signals have been shown to promote active, passive, and conditioned behavioral avoidance (35). However, the relation between these signals and motivation has not been examined.
It is notable that monoaminergic output has been associated with increased motivated behavior and positive affective states (36, 37). Increasing dopaminergic cell activity in the ventral tegmental area increases motivated behavioral responses in a challenging task, and their negative modulation decreases such responses (38). The impact of reduced mesolimbic system activity has been characterized as an inflation in the perceived cost of exerting effort, leading to immobility (24). We thus reasoned that the LHb→RMTg pathway, by inhibiting monoaminergic centers, could control motivated behavior; in particular, we hypothesized that this pathway controls the motivation to exert effort.
Results
LHb→RMTg Activity Increases with Transitions to Immobility in the Forced Swim Test.
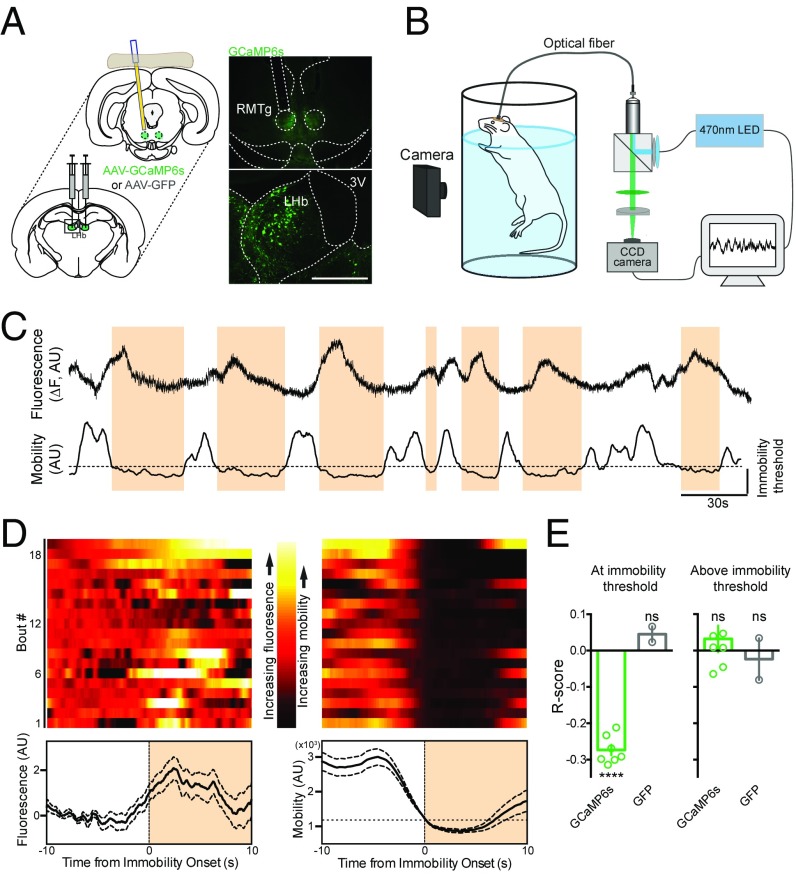
To examine LHb→RMTg activity in a behaving animal, the LHb of rats was injected with an adeno-associated virus (AAV) encoding the calcium indicator GCaMP6s (AAV-hSyn-GCaMP6s). An optical fiber was implanted over the RMTg to measure the activity of axon terminals from LHb→RMTg (Fig. 1A, Fig. S1, and SI Experimental Procedures). Control rats received the same surgery but were injected with an AAV encoding GFP (AAV-hSyn-GFP). Four weeks later, GCaMP6s expression was detected in cell bodies of the LHb and at the axon terminals of those fibers in the RMTg (Fig. 1A). Changes in fluorescence, indicating changes in neural activity (39), were recorded with a custom-built fiber photometry system (SI Experimental Procedures and ref. 40). To assess motivation, rats were subjected to the forced swim test (FST) (41), an aversive inescapable environment in which a rat’s effort is indicated by the persistence of its movement (Fig. 1B). Over the course of the test, rats spend a larger fraction of time immobile. These periods of immobility can be used as a measure of reduced motivation in rodents (29, 38, 42). The rats’ behavior was captured using a digital camera, and immobility bouts were determined using an unbiased MATLAB script that was validated against human scorers (Fig. S2 and SI Experimental Procedures).
Fig. 1.
Activity of LHb→RMTg is coincident with immobility bouts in the FST. (A, Left) AAV encoding GCaMP6s were injected into LHb, and a 400-µm optical fiber was implanted over the RMTg. (A, Right) Diagram and fluorescence images. (Scale bar, 500 µm.) 3V, third ventricle. (B) Diagram of recording setup. Video and CCD cameras captured rat mobility and changes in fluorescence in RMTg, respectively. (C) Representative example of change in fluorescence (Top) and mobility (Bottom) during FST; immobility threshold is indicated. (D) Representative examples (Top) and mean ± SEM (Bottom) of change in fluorescence (Left) and mobility (Right), aligned to onset of immobility bout. (E) Graph of correlation (Pearson’s) between fluorescence and mobility for rats (circles indicate individuals; bar indicates mean) expressing indicated constructs, aligned about the onset of immobility bouts (Left) or during periods of mobility (Right). ****P < 0.0001. AU, arbitrary unit; ns, not significant.
The onset of immobility bouts coincided with increased fluorescence signal (Fig. 1 C and D), as indicated by a significant negative correlation between the rat’s movement about the onset of an immobility bout and the neural activity of the LHb→RMTg (Fig. 1E, Left; r = −0.27 ± 0.01, P < 0.0001; rat n = 7, test n = 12, bout n = 298; see SI Experimental Procedures for measurements and statistical tests). Such correlation was not observed in rats injected with AAV-GFP (r = 0.05 ± 0.02; rat n = 2, test n = 5, bout n = 167). When the same analysis was made at periods of mobility (above threshold), no correlation was measured in rats expressing GCaMP6s (Fig. 1E, Right; r = 0.03 ± 0.04, P > 0.05; rat n = 7, test n = 12, bout n = 424) or GFP (r = −0.02 ± 0.06, P > 0.05; rat n = 2, test n = 5, bout n = 101). These findings support the view that increased neural activity in the LHb→RMTg correlates with reduced motivation to exert effort in an aversive context; furthermore, this correlation is not generally related to movement, as it occurs only during specific periods (i.e., at immobility threshold, when an animal would be expected to have low motivation).
Driving LHb→RMTg Increases Immobility in the FST.
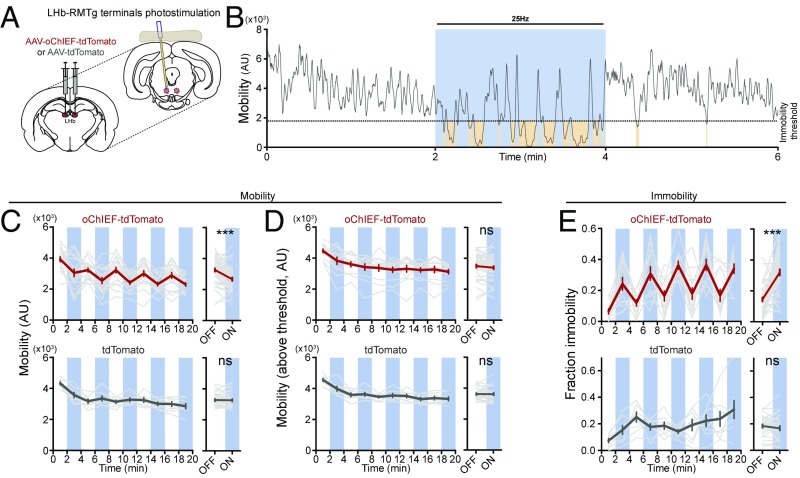
To test the hypothesis that the activity of the LHb→RMTg is sufficient to reduce the motivation to exert effort, the LHb was injected with an AAV encoding a light-dependent excitatory opsin, oChIEF (43), fused to the red fluorescent protein tdTomato (AAV-hSyn-oChIEF-tdTomato) or a control fluorophore (AAV-hSyn-tdTomato), and an optical fiber was implanted over the RMTg (Fig. 2A and Fig. S3). Single-unit recordings in anesthetized rats confirmed that brief, 5-ms pulses of blue light (∼15 mW at optical fiber tip) delivered at 25 Hz was sufficient to drive postsynaptic activity in RMTg neurons (Fig. S4).
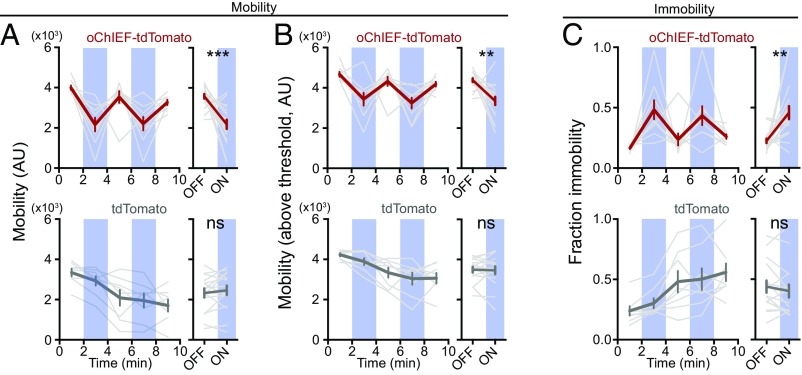
Fig. 2.
Stimulation of LHb→RMTg increases immobility in FST. (A) AAV encoding the light-sensitive cation channel oChIEF-tdTomato (n = 13) (or tdTomato alone, n = 7) was injected into the LHb, and a 200-µm optical fiber was implanted over the RMTg. (B) Representative example of change in mobility during light delivery (blue); brown shading indicates periods of immobility. (C–E, Top): (C, Left) Plot of mean mobility (gray indicates individual rats; red indicates mean ± SEM) during periods of light (blue) or no light (white). (C, Right) Mean ± SEM for indicated periods. (D) Same as C, for mobility values above immobility threshold. (E) Same as C, for time spent immobile. (C–E, Bottom) Same as C–E, Top, for rats expressing tdTomato. ***P < 0.001; paired Student’s t test. AU, arbitrary unit; ns, not significant.
Rats were subjected to 20-min FST sessions, as with the fiber photometry recordings, with each session consisting of alternating 2-min epochs with and without unilateral 25-Hz optical stimulation (Fig. 2B). Stimulation of the LHb→RMTg was sufficient to decrease a rat’s mobility during 2-min epochs, compared with the average mobility of the preceding and proceeding nonstimulation epochs [Fig. 2C; 3,300 ± 100 significant motion pixels (smp) (44)] without light vs. 2,700 ± 100 smp with light, P < 0.001). Notably, during stimulation periods, an animal’s average mobility (when the animal was above the immobility threshold) was unaffected (Fig. 2D; 3,500 ± 100 smp without light vs. 3,400 ± 100 smp with light, P > 0.05). This suggests that stimulation did not affect a rat’s general ability to move. Rather, the overall effect of stimulation on mobility was mostly due to an increase in the fraction of time a rat spent below the mobility threshold (Fig. 2E; 0.15 ± 0.01 without light vs. 0.32 ± 0.02 with light, P < 0.001). We observed that these immobility events occurred more often (18 ± 3 events without light vs. 33 ± 1 events with light, P < 0.001) and were longer in duration (3.7 ± 0.6 s without light vs. 5.6 ± 0.3 s with light, P < 0.05) during activation of the LHb→RMTg. Stimulation had no significant effect on the mobility of control rats expressing tdTomato (Fig. 2 C–E; mobility: 3,300 ± 70 smp without light vs. 3,300 ± 80 smp with light, P > 0.05; mobility above threshold: 3,600 ± 60 smp without light vs. 3,600 ± 80 smp with light, P > 0.05; fraction immobility: 0.19 ± 0.01 without light vs. 0.17 ± 0.02 with light, P > 0.05), with no change in the number (30 ± 4 events without light vs. 26 ± 3 events with light, P > 0.05) or the duration (3.4 ± 0.3 s without light vs. 7 ± 3 s with light, P > 0.05) of immobility events. These results support the view that stimulation of the LHb→RMTg neural pathway is sufficient to reduce motivation to exert effort in an aversive context.
Inhibiting LHb→RMTg Reduces Immobility in the FST.
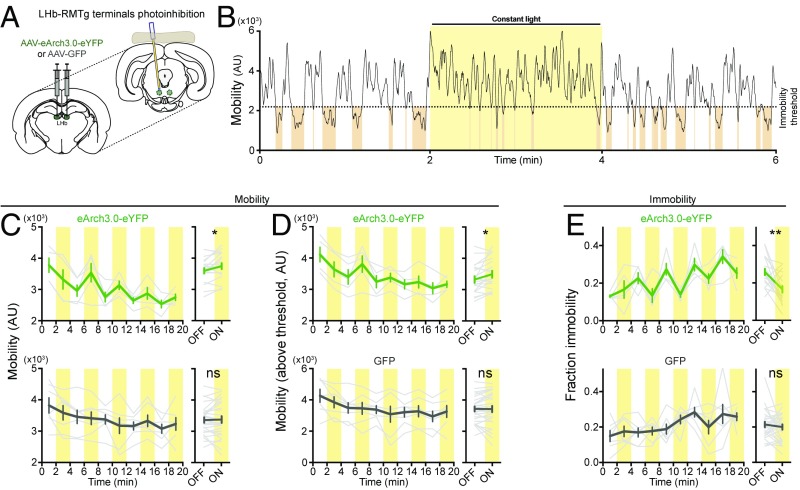
To test the hypothesis that activity in the LHb→RMTg is necessary to drive immobility in the same aversive swim test, rats were injected in the LHb with an AAV encoding the light-dependent proton pump eArch3.0 fused to a yellow fluorescent protein (AAV-hSyn-eArch3.0-eYFP) or with GFP (AAV-EF1α-GFP) as a control. Optical fibers (200-µm diameter) were implanted over the RMTg (Fig. 3A and Fig. S5). As above, rats were examined during a 20 min swim session with alternating 2-min epochs of no light and constant green light. When light was applied, a rat’s mobility in an epoch with light was significantly higher than the average mobility of the flanking epochs without light (Fig. 3 B and C; 3,620 ± 90 smp without light vs. 3,770 ± 90 smp with light, P < 0.05). Furthermore, light delivery decreased the fraction of time the rat spent immobile (Fig. 3E; 0.25 ± 0.02 without light vs. 0.17 ± 0.02 with light, P < 0.01). We observed that these immobility events occurred less often (33 ± 1 events without light vs. 22 ± 2 events with light, P < 0.05) and were shorter in duration (4.7 ± 0.2 s without light vs. 3.8 ± 0.1 s with light, P < 0.05) when light was delivered to the RMTg. No significant change was observed in rats expressing GFP (Fig. 3 C–E; mobility: 3,360 ± 90 smp without light vs. 3,400 ± 100 smp with light, P > 0.05; mobility above threshold: 3,710 ± 80 smp without light vs. 3,700 ± 100 smp with light, P > 0.05; fraction immobility: 0.21 ± 0.02 without light vs. 0.20 ± 0.01 with light, P > 0.05), with no change in the number of events (33 ± 2 events without light vs. 31 ± 4 events with light, P > 0.05) or their duration (3.8 ± 0.3 s without light vs. 3.4 ± 0.2 s with light, P > 0.05).
Fig. 3.
Reducing LHb→RMTg activity decreases immobility in FST. (A) AAV encoding the hyperpolarizing proton pump eArch3.0 (n = 5) or GFP (n = 7) was injected into the LHb, and a 200-µm optical fiber was implanted over the LHb. (B) Representative example of change in mobility during light delivery (yellow); brown shading indicates periods of immobility. (C–E, Top): (C, Left) Plot of mean mobility (gray indicates individual rats; green indicates mean ± SEM) during periods of light (yellow) or no light (white). (C, Right) Mean ± SEM for indicated periods. (D) Same as C, for mobility values above immobility threshold. (E) Same as C, for time spent immobile. (C–E, Bottom) Same as C–E, Top, for rats expressing GFP. *P < 0.05, **P < 0.01; paired Student’s t test. AU, arbitrary unit; ns, not significant.
These results are generally opposite compared with those observed with blue light activation of oChIEF, supporting the views that green light delivery to eArch3.0-expressing terminals inhibits their activity (45, 46) and that LHb→RMTg activity is necessary to produce the normal level of immobility in the aversive context of the FST.
Activating LHb→RMTg Reduces Effort Exerted to Gain Rewards in an Appetitive Test.
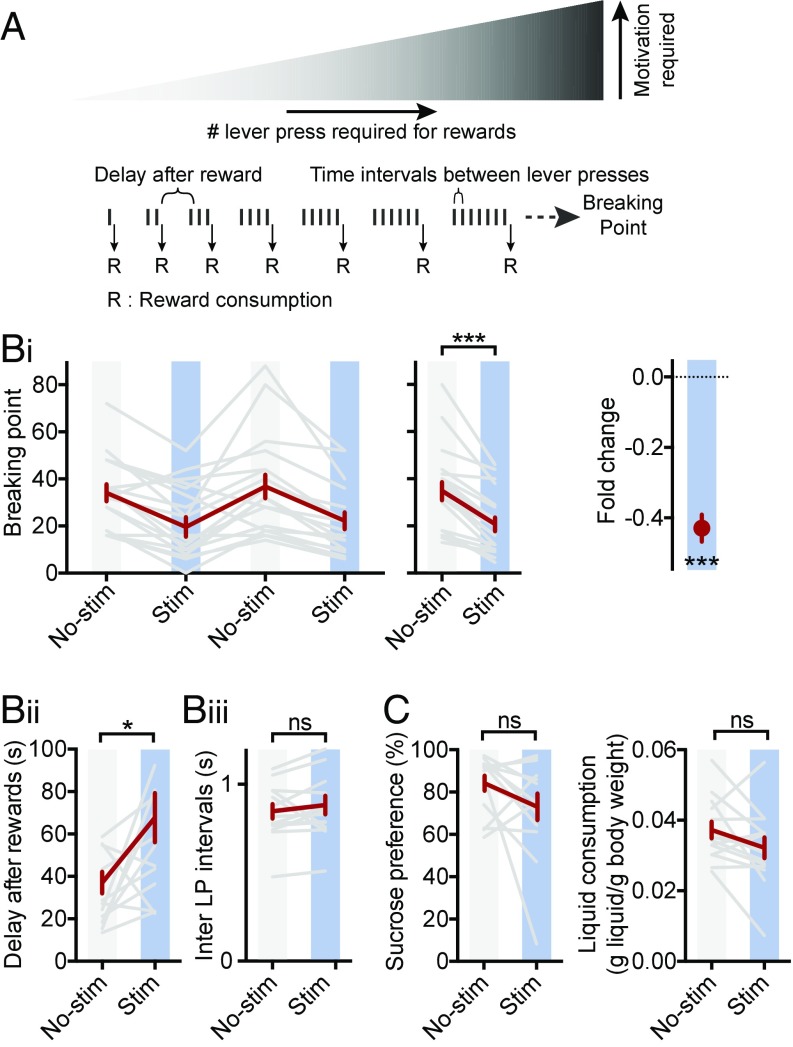
To examine whether increased activity at the LHb→RMTg was sufficient to decrease motivation in an appetitive context, rats were tested in a progressive ratio (PR) operant task, which is commonly used to evaluate motivation in rodents (47). In this test, increasing work (more lever presses) is required to receive a reward as trials proceed. The maximal work a rat exerts to receive a reward, the breaking point (BP), is used as a measure of its motivation (Fig. 4A).
Fig. 4.
Stimulation of LHb→RMTg reduces motivation to receive reward, but not reward value. (A) Diagram of PR reinforcement schedule; see SI Experimental Procedures. (B i–iii) Plots of BP (i), delay after rewards (ii), and inter–lever-press (LP) intervals (iii) for mean of daily trials (i, Left), indicated conditions (i, Middle; ii; and iii), and fold change by stimulation (i, Right, one sample Student’s t test); gray indicates individual rats; red indicates mean ± SEM. (C) Plots of sucrose preference (Left) and total liquid consumed (Right) for indicated conditions during SPT; see SI Experimental Procedures. Same rats as used in Fig. 2. *P < 0.05, ***P < 0.001; paired Student’s t test, unless otherwise indicated. ns, not significant; stim; stimulation.
We trained rats injected and implanted as in Fig. 2 to press a lever to obtain a sucrose reward. After training, we tested rats with a PR schedule of reinforcement (SI Experimental Procedures). In alternating sessions (one session per day), rats were or were not exposed to blue light through an optical fiber (trains of 25 Hz for 1 s every 2 s) during the entire session. With stimulation, rats’ BPs were significantly reduced by more than 40% compared with nonstimulation sessions (Fig. 4 B, i; BP without light 35 ± 4 vs. BP with light 21 ± 3, P < 0.001). This indicates that driving LHb→RMTg activity is sufficient to reduce the work performed by a rat to receive a reward. LHb→RMTg stimulation significantly increased the time between receiving a reward and the subsequent lever press (Fig. 4 B, ii; 36 ± 5 s without light vs. 67 ± 12 s with light, P < 0.05). Interestingly, during a bout of lever presses before a reward, the time interval between lever presses was unaffected (Fig. 4 B, iii; 0.85 ± 0.04 s without light vs. 0.88 ± 0.05 s with light, P > 0.05), suggesting that once a threshold motivation to work is achieved, the vigor of a rat’s performance is not modified. Stimulation did not affect a rat’s preference for sucrose water over plain water [sucrose preference test (SPT)], indicating that the hedonic value of the reward is unaffected by stimulation (Fig. 4C; 84 ± 4% of water consumed contained sucrose without light vs. 73 ± 6% with light, P > 0.05). Additionally, LHb→RMTg activation did not reduce thirst as revealed by the total liquid consumed (Fig. 4C; 0.037 ± 0.002 g of liquid per gram of body weight without light vs. 0.032 ± 0.003 g of liquid per gram of body weight with light, P > 0.05). These results indicate that the motivation to exert effort to receive a reward, rather than the value of the reward or the ability to perform the task, is affected by LHb→RMTg activation.
Activating LHb→RMTg During an Open Field Test.
We next tested animals in the open field (OF) (38), normally used to determine if a manipulation has nonspecific effects on movement. Rats expressing oChIEF-tdTomato or the control fluorophore tdTomato were placed in an OF, and movement was monitored during a 10-min period during which alternating 2-min epochs with and without unilateral 25-Hz optical stimulation were delivered (Fig. 5). When light was applied, a rat’s mobility in an epoch with light was significantly lower than that of the flanking epochs without light (Fig. 5). Both low and high levels of movement were affected by light (Fig. 5; mobility: 3,600 ± 100 smp without light vs. 2,200 ± 200 smp with light, P < 0.001; mobility above threshold: 4,400 ± 100 smp without light vs. 3,300 ± 200 smp with light, P < 0.01; fraction immobility: 0.23 ± 0.02 without light vs. 0.46 ± 0.05 with light, P < 0.001). Stimulation had no significant effect on the mobility of control rats expressing tdTomato (Fig. 5; mobility: 2,300 ± 200 smp without light vs. 2,400 ± 200 smp with light, P > 0.05; mobility above threshold: 3,500 ± 100 smp without light vs. 3,500 ± 200 smp with light, P > 0.05; fraction immobility: 0.44 ± 0.05 without light vs. 0.40 ± 0.05 with light, P > 0.05).
Fig. 5.
Stimulation of LHb→RMTg increases immobility in the OF. (A–C, Top): (A, Left) Plot of mean mobility for rats expressing oChIEF (gray indicates individual rats; red indicates mean ± SEM) during periods of light (blue) or no light (white). (A, Right) Mean ± SEM for indicated periods. (B) Same as A, for mobility values above immobility threshold. (C) Same as A, for time spent immobile. (A–C, Bottom) Same as A–C, Top, for rats expressing tdTomato. Same rats as used in Figs. 2 and 4. **P < 0.01, ***P < 0.001; paired Student’s t test. AU, arbitrary unit; ns, not significant.
While the OF test has been used to measure basic motor function, this test is also a measure of a rat’s motivation to explore an environment (48, 49). Since there was no effect of LHb→RMTg stimulation on suprathreshold mobility in the FST nor on the inter–lever-press interval in the PR test, the more parsimonious interpretation is that the effect of such stimulation on the OF test is due to reduced motivation to explore (50, 51) and is not an effect on general motor function.
Activating LHb→RMTg Has No Effect on Motor Coordination and Is Aversive.
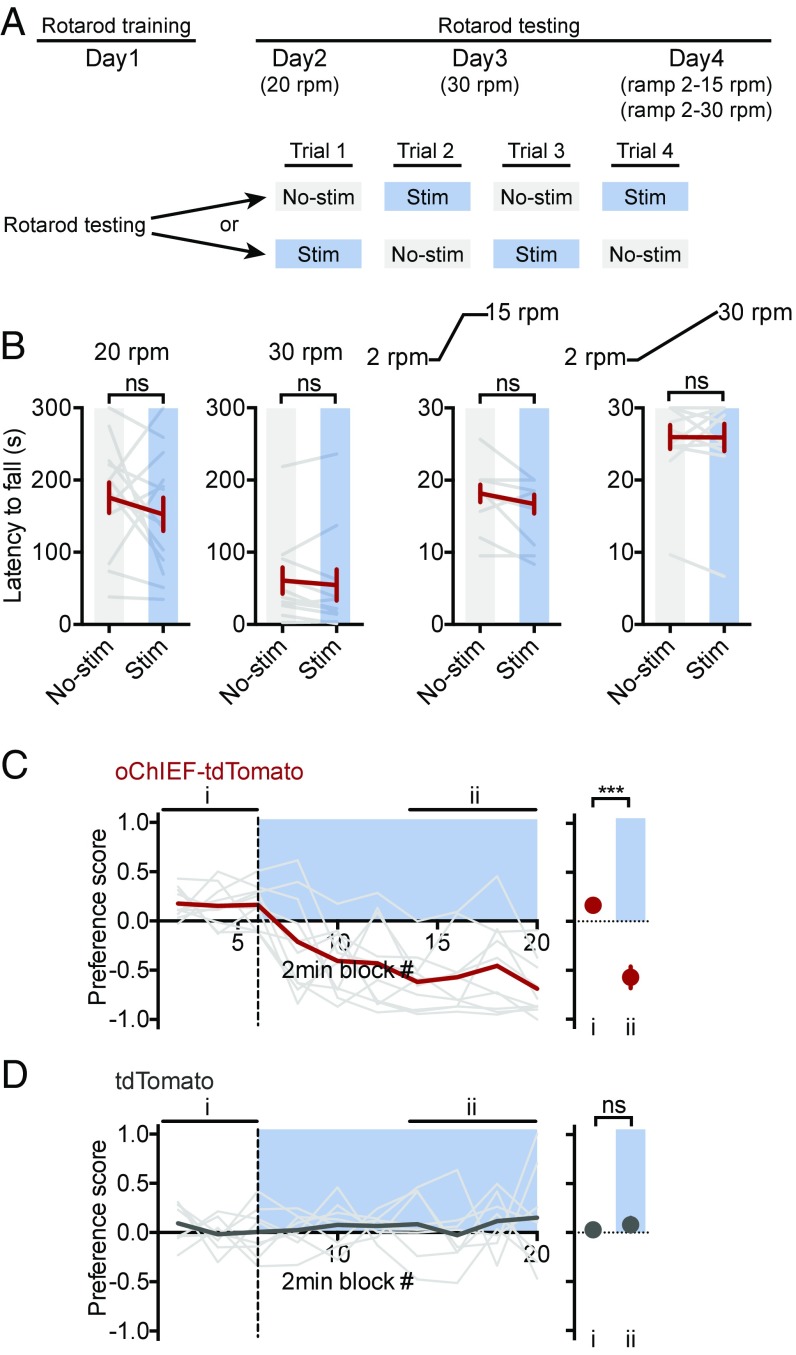
As a further test to distinguish between an effect of LHb→RMTg activity on the motivation to move rather than an effect on general motor function, rats injected with AAV-oChIEF-tdTomato, which had already been tested on the FST (Fig. 2), were tested on the rotarod, a task requiring quick and coordinated movements. After an initial training period, rats’ latencies to fall were measured under a variety of conditions (slow or fast constant speed and slow or fast ramping speed) (Fig. 6A). Under no condition did LHb→RMTg stimulation have a significant effect on rats’ latencies to fall [Fig. 6B; 20 rpm (rotations per minute): 180 ± 20 s without light vs. 150 ± 20 s with light, P > 0.05; 30 rpm: 60 ± 20 s without light vs. 50 ± 20 s with light, P > 0.05; ramp from 2 to 15 rpm: 18 ± 1 s without light vs. 17 ± 1 s with light, P > 0.05; ramp from 2 to 30 rpm: 26 ± 2 s without light vs. 26 ± 2 s with light, P > 0.05]. Notably, these animals had shown specific deficits in the FST. This indicates that LHb→RMTg stimulation does not have a generalized effect on motor activity, but rather has an effect only under specific conditions.
Fig. 6.
Stimulation of the LHb→RMTg had no effect on motor coordination and is aversive. (A) Diagram of rotarod test (SI Experimental Procedures) schedule. (B) Plot of latency to fall for indicated conditions. Real-time place preference test for rats expressing oChIEF (C) and tdTomato (D). (C and D, Left) Plots of preference score [light gray indicates individual rats; red (C) or dark gray (D) indicates mean] for the indicated conditions. (C and D, Right) Mean for values during indicated periods (i and ii); blue indicates period of light stimulation while rat is in the side of box unpaired (above x axis) or paired (below x axis) with stimulation. Same rats as used in Figs. 2 and 4. ***P < 0.001; paired Student’s t test. ns, not significant; stim; stimulation.
Lastly, we asked whether stimulation of the LHb→RMTg was aversive, as would be expected for the experience of reduced motivational states (52). In this test (the real-time place preference test), rats are placed in a box with two compartments. After an initial habituation period, rats receive optical stimulation (25 Hz) only when they were present in one compartment. Rats expressing oChIEF actively avoided the compartment paired with stimulation (Fig. 6C; preference score: 0.16 ± 0.03 without light vs. −0.6 ± 0.1 with light, P < 0.001). Stimulation had no significant effect on control rats expressing tdTomato (Fig. 6D; preference score: 0.03 ± 0.04 without light vs. 0.08 ± 0.07 with light, P > 0.05). These results indicate that overactivity of the LHb→RMTg is aversive (Fig. 6C) (35).
Discussion
Major depression is a leading cause of disability worldwide (1). It encompasses a heterogeneous set of disorders with distinct features (e.g., depressed mood, anhedonia, altered weight and/or sleep, fatigue, guilt, etc.). Abnormalities in several brain regions, such as the ventral tegmental area, the nucleus accumbens, and, more recently, the LHb, have been linked to depression (2–4, 53).
A deficit in motivation is a central symptom of depression that can impact behavior in aversive and rewarding environments (54, 55). This deficit could be the result of inflating the perceived cost, or undervaluing the potential benefit, of avoiding punishment or receiving a reward (24–26). In any of these cases, a deficit in motivation suggests an abnormality in the functional link between brain regions that evaluate reward-related events and those that control motivated behavior. Anatomically, the LHb is well suited to fill this role, as it receives afferents from brain nuclei signaling reward-related events and provides signals to monoaminergic centers known to control motivated behaviors (27–31, 56–58). Functionally, the activity of LHb neurons is affected by stimuli that contain motivational value; for example, they are excited by stimuli that predict punishment or the absence of rewards (31, 56, 57). The LHb is thus well positioned, both anatomically and functionally, to have an impact on motivated behaviors based on expected outcomes. In light of these findings and the proposed role of the LHb in depression, we tested whether the projection from the LHb to the RMTg, an important LHb output that inhibits dopaminergic centers, can control motivation in aversive and appetitive contexts.
In an initial set of experiments, we examined whether the motivation to exert effort in an aversive environment is controlled by the LHb→RMTg. We found that this pathway displays increased activity coinciding with the animal’s entry into states of immobility in the FST—states that are classically described as “behavioral despair” and are a measure of reduced motivation in an aversive and energetically demanding environment (29, 38, 42). Notably, activity of this pathway did not correlate with fluctuations in mobility that do not contain an immobility bout, suggesting that this pathway does not simply control movement. Driving or inhibiting this pathway increased or decreased, respectively, a rat’s mobility, specifically by changing the frequency and duration of inactive states, with no effect on its ability to move vigorously. Combined, these data establish a causal relationship between activity of the LHb→RMTg pathway and immobility, supporting the view that this pathway controls the motivation to sustain effort in an aversive context. Interestingly, stimulation of medial prefrontal cortex (mPFC) axons terminating in the LHb also reduced mobility in the FST (29). Our results suggest that mPFC→LHb inputs may target LHb neurons that project to the RMTg.
We also tested whether the motivation to exert effort in a rewarding environment is controlled by the LHb→RMTg. Given the complexity of the brain circuitry known to encode reward and aversion, where specific circuit components have been identified for each (58), the motivation to exert effort in these different contexts could be mediated by separate or overlapping mechanisms. Here, we show that activation of the LHb→RMTg pathway reduces the motivation to work for a reward in an appetitive operant task, suggesting that at least some overlapping neural pathways control motivation in both aversive and appetitive contexts.
The effects observed in our behavioral tasks measuring the impact of LHb→RMTg stimulation on motivation are unlikely to be caused by a general reduction in mobility, as suggested by its effect in the OF test. This conclusion is supported by several observations: LHb→RMTg stimulation (i) produced no change in suprathreshold mobility in the FST; (ii) did not affect the inter–lever-press interval in the PR task; (iii) did not affect the total liquid consumed in the SPT; (iv) had no effect in the rotarod test; and (v) drove, rather than inhibited, movement in the real-time place preference test. Furthermore, LHb→RMTg activity correlated with transitions into immobility, but not generally with movement. Together, these findings support the view that increasing LHb→RMTg activity does not have a general effect on motor behavior, but rather controls motivation to exert effort. The effects in the OF test are more likely due to a reduced motivation to explore (50, 51). In this regard, it will be interesting to compare the effects of LHb→RMTg stimulation on an animal’s OF behavior in a familiar or novel environment.
Our results are consistent with pharmacological and dopamine-depletion manipulations, which have been shown to reduce the amount of effort made in challenging or energetically demanding conditions, and to decrease spontaneous (non–goal-oriented) locomotor function (e.g., OF) while leaving intact some motor behaviors (24, 25, 50). Other studies examining inputs to, or outputs from, the LHb (27–30), are also consistent with LHb→RMTg activity reducing motivation.
In summary, our data indicate that activity of neurons that project from the LHb to the RMTg control the motivation to perform behaviors requiring effort, irrespective of whether those behaviors are directed toward escaping an aversive condition or toward acquiring a reward. Aberrant overactivity of this pathway, previously examined in other contexts (35, 59), may be responsible for some of the motivational deficits seen in depression.
Experimental Procedures
All procedures involving animals were approved by the Institutional Animal Care and Use Committees of the University of California, San Diego. Detailed methods describing stereotaxic injections of AAVs and optic fiber cannula implantation, fiber photometry in vivo calcium imaging, behavioral assays, and statistical methods are described in SI Experimental Procedures.
Supplementary Material
Acknowledgments
We thank all members of the R.M. laboratory for their discussions regarding this project. C.D.P. was supported by a Canadian Institutes of Health Research postdoctoral scholarship and the Brain & Behavior Research Foundation (National Association for Research on Schizophrenia and Depression Young Investigator Grant). This work was supported by NIH Grant R01-MH091119 (R.M.) and the Shiley-Marcos Endowment (R.M.).
Footnotes
Conflict of interest statement: C.D.P., R.M., and Okihide Hikosaka are coauthors on a review article published in 2014. S.A. founded the company Neurophotometrics Ltd., which manufactures fiber photometry systems.
Data deposition: All relevant data and code supporting the findings of this study have been deposited on GitHub and are available at https://github.com/NotAnHerb/LHbRMTg.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1801837115/-/DCSupplemental.
References
- 1.Ferrari AJ, et al. Burden of depressive disorders by country, sex, age, and year: Findings from the global burden of disease study 2010. PLoS Med. 2013;10:e1001547. doi: 10.1371/journal.pmed.1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: Implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Airan RD, et al. High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science. 2007;317:819–823. doi: 10.1126/science.1144400. [DOI] [PubMed] [Google Scholar]
- 4.Berton O, Nestler EJ. New approaches to antidepressant drug discovery: Beyond monoamines. Nat Rev Neurosci. 2006;7:137–151. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- 5.Pittenger C, Duman RS. Stress, depression, and neuroplasticity: A convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- 6.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- 8.Parker KJ, Schatzberg AF, Lyons DM. Neuroendocrine aspects of hypercortisolism in major depression. Horm Behav. 2003;43:60–66. doi: 10.1016/s0018-506x(02)00016-8. [DOI] [PubMed] [Google Scholar]
- 9.Mill J, Petronis A. Molecular studies of major depressive disorder: The epigenetic perspective. Mol Psychiatry. 2007;12:799–814. doi: 10.1038/sj.mp.4001992. [DOI] [PubMed] [Google Scholar]
- 10.Morris JS, Smith KA, Cowen PJ, Friston KJ, Dolan RJ. Covariation of activity in habenula and dorsal raphé nuclei following tryptophan depletion. Neuroimage. 1999;10:163–172. doi: 10.1006/nimg.1999.0455. [DOI] [PubMed] [Google Scholar]
- 11.Sartorius A, et al. Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biol Psychiatry. 2010;67:e9–e11. doi: 10.1016/j.biopsych.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 12.Ranft K, et al. Evidence for structural abnormalities of the human habenular complex in affective disorders but not in schizophrenia. Psychol Med. 2010;40:557–567. doi: 10.1017/S0033291709990821. [DOI] [PubMed] [Google Scholar]
- 13.Lawson RP, et al. Disrupted habenula function in major depression. Mol Psychiatry. 2017;22:202–208. doi: 10.1038/mp.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carlson PJ, et al. Neural correlates of rapid antidepressant response to ketamine in treatment-resistant unipolar depression: A preliminary positron emission tomography study. Biol Psychiatry. 2013;73:1213–1221. doi: 10.1016/j.biopsych.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amat J, et al. The role of the habenular complex in the elevation of dorsal raphe nucleus serotonin and the changes in the behavioral responses produced by uncontrollable stress. Brain Res. 2001;917:118–126. doi: 10.1016/s0006-8993(01)02934-1. [DOI] [PubMed] [Google Scholar]
- 16.Yang L-M, Hu B, Xia Y-H, Zhang B-L, Zhao H. Lateral habenula lesions improve the behavioral response in depressed rats via increasing the serotonin level in dorsal raphe nucleus. Behav Brain Res. 2008;188:84–90. doi: 10.1016/j.bbr.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 17.Caldecott-Hazard S, Mazziotta J, Phelps M. Cerebral correlates of depressed behavior in rats, visualized using 14C-2-deoxyglucose autoradiography. J Neurosci. 1988;8:1951–1961. doi: 10.1523/JNEUROSCI.08-06-01951.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shumake J, Gonzalez-Lima F. Brain systems underlying susceptibility to helplessness and depression. Behav Cogn Neurosci Rev. 2003;2:198–221. doi: 10.1177/1534582303259057. [DOI] [PubMed] [Google Scholar]
- 19.Li B, et al. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature. 2011;470:535–539. doi: 10.1038/nature09742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shabel SJ, Proulx CD, Piriz J, Malinow R. Mood regulation. GABA/glutamate co-release controls habenula output and is modified by antidepressant treatment. Science. 2014;345:1494–1498. doi: 10.1126/science.1250469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li K, et al. βCaMKII in lateral habenula mediates core symptoms of depression. Science. 2013;341:1016–1020. doi: 10.1126/science.1240729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lecca S, Meye FJ, Mameli M. The lateral habenula in addiction and depression: An anatomical, synaptic and behavioral overview. Eur J Neurosci. 2014;39:1170–1178. doi: 10.1111/ejn.12480. [DOI] [PubMed] [Google Scholar]
- 23.Lecca S, et al. Rescue of GABAB and GIRK function in the lateral habenula by protein phosphatase 2A inhibition ameliorates depression-like phenotypes in mice. Nat Med. 2016;22:254–261. doi: 10.1038/nm.4037. [DOI] [PubMed] [Google Scholar]
- 24.Salamone JD, Correa M. The mysterious motivational functions of mesolimbic dopamine. Neuron. 2012;76:470–485. doi: 10.1016/j.neuron.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Treadway MT, et al. Dopaminergic mechanisms of individual differences in human effort-based decision-making. J Neurosci. 2012;32:6170–6176. doi: 10.1523/JNEUROSCI.6459-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hollon NG, Burgeno LM, Phillips PEM. Stress effects on the neural substrates of motivated behavior. Nat Neurosci. 2015;18:1405–1412. doi: 10.1038/nn.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim U, Lee T. Topography of descending projections from anterior insular and medial prefrontal regions to the lateral habenula of the epithalamus in the rat. Eur J Neurosci. 2012;35:1253–1269. doi: 10.1111/j.1460-9568.2012.08030.x. [DOI] [PubMed] [Google Scholar]
- 28.Stamatakis AM, et al. Lateral hypothalamic area glutamatergic neurons and their projections to the lateral habenula regulate feeding and reward. J Neurosci. 2016;36:302–311. doi: 10.1523/JNEUROSCI.1202-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Warden MR, et al. A prefrontal cortex-brainstem neuronal projection that controls response to behavioural challenge. Nature. 2012;492:428–432. doi: 10.1038/nature11617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baker PM, et al. The lateral habenula circuitry: Reward processing and cognitive control. J Neurosci. 2016;36:11482–11488. doi: 10.1523/JNEUROSCI.2350-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stephenson-Jones M, et al. A basal ganglia circuit for evaluating action outcomes. Nature. 2016;539:289–293. doi: 10.1038/nature19845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barrot M, et al. Braking dopamine systems: A new GABA master structure for mesolimbic and nigrostriatal functions. J Neurosci. 2012;32:14094–14101. doi: 10.1523/JNEUROSCI.3370-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hong S, Jhou TC, Smith M, Saleem KS, Hikosaka O. Negative reward signals from the lateral habenula to dopamine neurons are mediated by rostromedial tegmental nucleus in primates. J Neurosci. 2011;31:11457–11471. doi: 10.1523/JNEUROSCI.1384-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009;61:786–800. doi: 10.1016/j.neuron.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stamatakis AM, Stuber GD. Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nat Neurosci. 2012;15:1105–1107. doi: 10.1038/nn.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamid AA, et al. Mesolimbic dopamine signals the value of work. Nat Neurosci. 2016;19:117–126. doi: 10.1038/nn.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 38.Tye KM, et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2013;493:537–541. doi: 10.1038/nature11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen T-W, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim CK, et al. Simultaneous fast measurement of circuit dynamics at multiple sites across the mammalian brain. Nat Methods. 2016;13:325–328. doi: 10.1038/nmeth.3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Porsolt RD, Le Pichon M, Jalfre M. Depression: A new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 42.Rygula R, et al. Anhedonia and motivational deficits in rats: Impact of chronic social stress. Behav Brain Res. 2005;162:127–134. doi: 10.1016/j.bbr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 43.Lin JY, Lin MZ, Steinbach P, Tsien RY. Characterization of engineered channelrhodopsin variants with improved properties and kinetics. Biophys J. 2009;96:1803–1814. doi: 10.1016/j.bpj.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kopec CD, et al. A robust automated method to analyze rodent motion during fear conditioning. Neuropharmacology. 2007;52:228–233. doi: 10.1016/j.neuropharm.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 45.Mattis J, et al. Principles for applying optogenetic tools derived from direct comparative analysis of microbial opsins. Nat Methods. 2011;9:159–172. doi: 10.1038/nmeth.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vento PJ, Burnham NW, Rowley CS, Jhou TC. Learning from one’s mistakes: A dual role for the rostromedial tegmental nucleus in the encoding and expression of punished reward seeking. Biol Psychiatry. 2017;81:1041–1049. doi: 10.1016/j.biopsych.2016.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aberman JE, Ward SJ, Salamone JD. Effects of dopamine antagonists and accumbens dopamine depletions on time-constrained progressive-ratio performance. Pharmacol Biochem Behav. 1998;61:341–348. doi: 10.1016/s0091-3057(98)00112-9. [DOI] [PubMed] [Google Scholar]
- 48.Archer J. Tests for emotionality in rats and mice: A review. Anim Behav. 1973;21:205–235. doi: 10.1016/s0003-3472(73)80065-x. [DOI] [PubMed] [Google Scholar]
- 49.Roth KA, Katz RJ. Stress, behavioral arousal, and open field activity–A reexamination of emotionality in the rat. Neurosci Biobehav Rev. 1979;3:247–263. doi: 10.1016/0149-7634(79)90012-5. [DOI] [PubMed] [Google Scholar]
- 50.Baldo BA, Sadeghian K, Basso AM, Kelley AE. Effects of selective dopamine D1 or D2 receptor blockade within nucleus accumbens subregions on ingestive behavior and associated motor activity. Behav Brain Res. 2002;137:165–177. doi: 10.1016/s0166-4328(02)00293-0. [DOI] [PubMed] [Google Scholar]
- 51.Winn P, Robbins TW. Comparative effects of infusions of 6-hydroxydopamine into nucleus accumbens and anterolateral hypothalamus induced by 6-hydroxydopamine on the response to dopamine agonists, body weight, locomotor activity and measures of exploration in the rat. Neuropharmacology. 1984;24:25–31. doi: 10.1016/0028-3908(85)90091-7. [DOI] [PubMed] [Google Scholar]
- 52.Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: Rewarding, aversive, and alerting. Neuron. 2010;68:815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Proulx CD, Hikosaka O, Malinow R. Reward processing by the lateral habenula in normal and depressive behaviors. Nat Neurosci. 2014;17:1146–1152. doi: 10.1038/nn.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Treadway MT, Zald DH. Reconsidering anhedonia in depression: Lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35:537–555. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salamone JD, Koychev I, Correa M, McGuire P. Neurobiological basis of motivational deficits in psychopathology. Eur Neuropsychopharmacol. 2014;25:1225–1238. doi: 10.1016/j.euroneuro.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 56.Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- 57.Matsumoto M, Hikosaka O. Representation of negative motivational value in the primate lateral habenula. Nat Neurosci. 2009;12:77–84. doi: 10.1038/nn.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hu H. Reward and aversion. Annu Rev Neurosci. 2016;39:297–324. doi: 10.1146/annurev-neuro-070815-014106. [DOI] [PubMed] [Google Scholar]
- 59.Knowland D, et al. Distinct ventral pallidal neural populations mediate separate symptoms of depression. Cell. 2017;170:284–297.e18. doi: 10.1016/j.cell.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.