Significance
Protein AMPylation in eukaryotes is a comparatively understudied posttranslational modification. With the exception of yeast, all eukaryotes have the enzymatic machinery required to execute this modification. Members of the heat shock protein family in different cellular compartments appear to be preferred targets for AMPylation, but it has proven challenging to adduce its biological function. We show that genetic modifications that affect AMPylation status, through generation of null alleles and a constitutively active version of the AMPylase FIC-1, can have a major impact on the susceptibility of Caenorhabditis elegans to neurodegenerative conditions linked to protein aggregation.
Keywords: AMPylation, chaperones, Hsp70, protein aggregation, proteostasis
Abstract
Proteostasis is critical to maintain organismal viability, a process counteracted by aging-dependent protein aggregation. Chaperones of the heat shock protein (HSP) family help control proteostasis by reducing the burden of unfolded proteins. They also oversee the formation of protein aggregates. Here, we explore how AMPylation, a posttranslational protein modification that has emerged as a powerful modulator of HSP70 activity, influences the dynamics of protein aggregation. We find that adjustments of cellular AMPylation levels in Caenorhabditis elegans directly affect aggregation properties and associated toxicity of amyloid-β (Aβ), of a polyglutamine (polyQ)-extended polypeptide, and of α-synuclein (α-syn). Expression of a constitutively active C. elegans AMPylase FIC-1(E274G) under its own promoter expedites aggregation of Aβ and α-syn, and drastically reduces their toxicity. A deficiency in AMPylation decreases the cellular tolerance for aggregation-prone polyQ proteins and alters their aggregation behavior. Overexpression of FIC-1(E274G) interferes with cell survival and larval development, underscoring the need for tight control of AMPylase activity in vivo. We thus define a link between HSP70 AMPylation and the dynamics of protein aggregation in neurodegenerative disease models. Our results are consistent with a cytoprotective, rather than a cytotoxic, role for such protein aggregates.
Neurodegenerative diseases (NDs), such as Alzheimer’s disease (AD), Huntington’s disease (HD), and Parkinson’s disease (PD), are afflictions of aging that share a common hallmark: protein aggregates. In healthy cells, chaperones and other heat shock proteins (HSPs) work to maintain protein homeostasis (proteostasis) by reducing the burden of unfolded proteins and overseeing the formation, triage, and degradation of protein aggregates. In particular, the HSP70 family of chaperones populates some of the most critical nodes in the proteostasis network, and, as such, their expression level and activity are highly regulated (1–4). Because of their ability to modulate protein aggregation, several HSPs were suggested to play key roles in the development and progression of NDs. These often involve clogging of the target cells and tissues with accumulations of peptides or proteins, such as α-synuclein (α-syn) in PD; mutant huntingtin (mHtt), which contains extended polyglutamine (polyQ) repeats, in HD; and amyloid-β (Aβ) peptide in AD (5–9). The unifying theme that connects these NDs is their strong association with a failure of cellular maintenance mechanisms to control pathological protein aggregation. Inside cells, such disease-associated, aggregation-prone proteins start out as monomers, only then to participate in a continuous conversion into soluble toxic oligomers. The sequestration of these toxic oligomers into large, insoluble aggregates and inclusion bodies is part of a cellular coping mechanism that gradually depletes these toxic intermediate species of α-syn, Aβ, and mHtt (10–16). Molecular chaperones (e.g., HSP40, HSP70) and chaperonins (e.g., TCP-1 ring complex) orchestrate the continuum of mHtt, Aβ, and α-syn oligomerization and alleviate associated cytotoxicity, by preventing monomers from oligomerizing, by facilitating degradation of oligomers via the ubiquitin-proteasome and autophagy-lysosomal pathways or through enhancement of oligomer deposition into large, insoluble aggregates (17–19). Because of their prominent role in the modulation of protein aggregation, the master transcriptional regulator of the heat shock response (HSR), HSF-1, and its downstream targets, HSP70 and HSP90, are considered prime targets for intervention in NDs (20–22). However, while up-regulation of HSPs may be beneficial in the context of NDs, excessive activity of HSPs favors fast, uncontrolled cell division cycles and is suspected to be a contributing factor to cancer (23–26).
Protein AMPylation regulates HSP70 activity in the endoplasmic reticulum (ER) and the cytoplasm (27–32). The addition of AMP to the side chain of a threonine or serine residue is catalyzed by conserved enzymes (AMPylases) that are present in a single copy in most metazoans, including Caenorhabditis elegans (FIC-1), Mus musculus (mFICD), and humans (HYPE), but is absent from yeast. HYPE preferentially AMPylates the ER-resident HSP70 family chaperone Grp78/BiP in its substrate-free, ATP-bound conformation (30). BiP AMPylation disfavors cochaperone-dependent ATP hydrolysis, believed to be a prerequisite for client binding. AMPylation “locks” BiP in a primed state (33, 34), ready to engage in client refolding immediately after BiP’s deAMPylation (35). The C. elegans HYPE ortholog FIC-1 modifies a number of ER and cytoplasmic targets, including the HSP70 family members HSP-1 (cytosolic) as well as HSP-3 and HSP-4 (the two C. elegans Grp78/BiP orthologs) (29). AMPylase-deficient fic-1(n5823) animals show enhanced susceptibility to Pseudomonas aeruginosa infections, while worms that express a mutant AMPylase with enhanced activity under the control of the endogenous fic-1 promotor {nIs733[Pfic-1::FIC-1(E274G)]} display increased pathogen tolerance, suggesting a link between HSP AMPylation, innate immunity, and stress tolerance in vivo (29). Expression of active FIC-1(E274G) in yeast, a eukaryote that lacks endogenous AMPylation enzymes, induces massive protein aggregation, which was alleviated by the overexpression of Ssa2, a cytosolic HSP70 protein (27). These data indicate a novel mode of HSP70 inactivation by AMPylation and point toward a broad role for protein AMPylation in the regulation of proteostasis.
Here, we explored AMPylation of HSP70 and implicate it in protein aggregation and toxicity in C. elegans models of NDs. We find that changes in AMPylation levels cause altered aggregation dynamics in vitro and in vivo, with beneficial or detrimental outcomes, depending on the ND model examined. Expression of active FIC-1(E274G) significantly increases survival of Aβ-expressing worms despite enhanced aggregate formation. RNAi-mediated ablation of HSP-1, HSP-3, and HSP-4 phenocopies these results. Increased AMPylation also alleviates α-syn toxicity. Conversely, we find that expression of aggregation-prone polyQ proteins in an AMPylase-deficient fic-1(n5823) background worsens associated symptoms. Our work indicates a role for HSP70 AMPylation in the control of protein aggregation in vivo and highlights the potential of HSP70 as a target for modulation of pathological protein aggregation.
Results
Changes in Cellular AMPylation Levels Alter Aβ Aggregation and Toxicity.
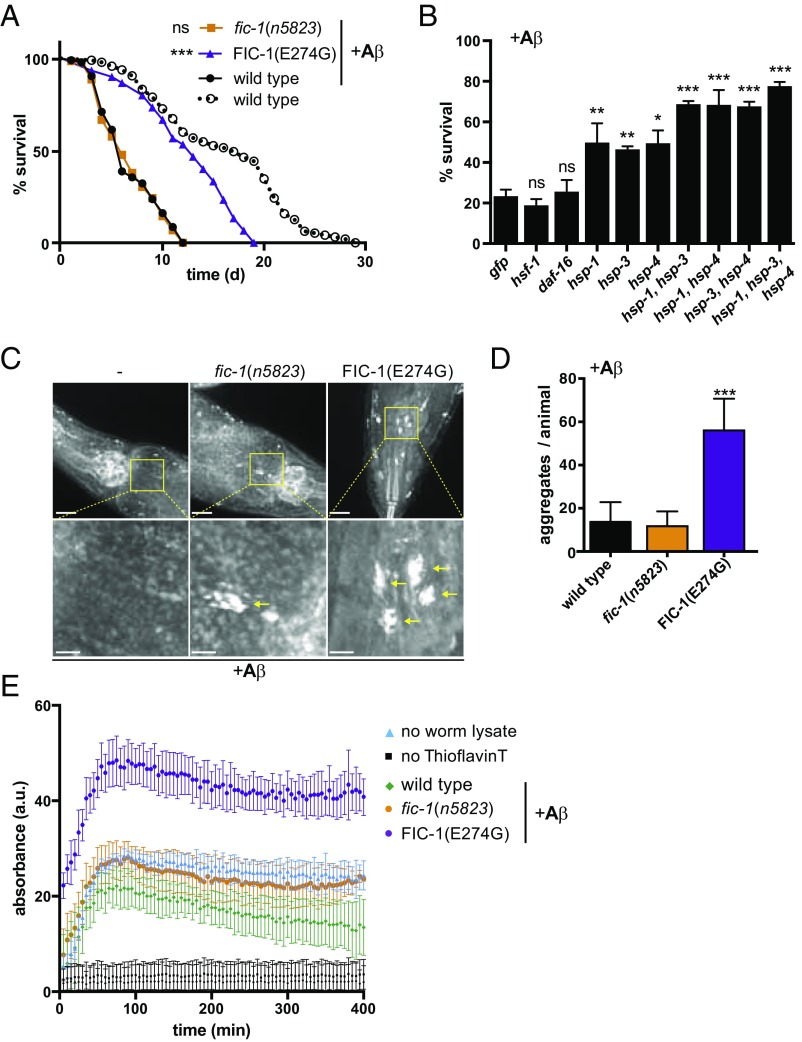
AMPylation of HSP70 family proteins prevents them from participating in protein quality control (30). As such, we hypothesized that intracellular AMPylation levels could affect protein aggregation and associated toxicity of the AD-associated (Aβ) peptide, by reducing or enhancing the available pool of active HSP70s. To test this, we mated C. elegans strains with constitutive muscular expression of Aβ (CL2006) with previously established AMPylase-deficient fic-1(n5823) worms or integrated transgenic nematode strains that express constitutively active FIC-1(E274G) and analyzed homozygous F2 offspring. Constitutively active FIC-1(E274G) was controlled by the endogenous fic-1 promotor, which is very weak yet active in all cells (29, 36). When transferred from permissive (15 °C) to inducing (20 °C) conditions, Aβ aggregation in C. elegans body wall muscle cells causes paralysis and significantly shortens lifespan (36). The presence of FIC-1(E274G) was sufficient to alleviate Aβ toxicity under inducing conditions, while AMPylation deficiency did not alter survival or impaired mobility of Aβ-expressing animals (Fig. 1A and SI Appendix, Fig. S1A). Analysis of total Aβ content confirmed that Aβ levels remained unaltered in response to changes in AMPylation (SI Appendix, Fig. S1B). Because we previously identified HSP70 family members HSP-1, HSP-3, and HSP-4 as prime targets for FIC-1 in C. elegans, we asked whether RNAi-mediated reduction of HSP-1, HSP-3, HSP-4, or combinations thereof would suppress Aβ toxicity. While individual knockdown of HSP-1, HSP-3, and HSP-4 enhanced survival of Aβ-expressing worms only slightly, combined knockdown of HSP-1, HSP-3, and HSP-4 in pairs significantly improved survival, with a triple knockdown of HSP-1, HSP-3, and HSP-4 resulting in ∼80% survival after 4 d at 22 °C, compared with approximately ∼20% survival in controls (Fig. 1B). Similarly, the introduction of an hsp-3 null allele into Aβ-expressing strains ameliorated its fitness, consistent with a beneficial effect of reduced HSP-3 function (SI Appendix, Fig. S1C). Ablation of hsf-1 or daf-16, two transcription factors implicated in the control of HSP transcription (37, 38), as well as skn-1, a major transcriptional regulator implicated in the control of aging, did not reduce the beneficial effect of FIC-1(E274G) expression in Aβ worms. This result is consistent with the notion that FIC-1(E274G) acts on preexisting chaperone pools rather than by changing the overall abundance of HSF-1–, SKN-1–, or Daf-16–dependent HSPs (SI Appendix, Fig. S1D).
Fig. 1.
Changes in cellular AMPylation levels alter Aβ aggregation and toxicity. (A) Lifespan analysis of wild-type, fic-1(n5823), and FIC-1(E274G) animals expressing human Aβ peptide from a minigene. (B) Survival assays of wild-type animals expressing human Aβ peptide upon RNAi-mediated ablation of the indicated genes (x axis). Thioflavin S staining (C, Upper) and quantification of protein aggregates (D) of wild-type (n = 31), fic-1(n5823) (n = 34), and FIC-1(E274G) (n = 23) animals expressing human Aβ peptide are shown. (C, Lower) Higher magnification images of the boxed areas. (Scale bars: Upper, 10 μm; Lower, 2 μm.) (E) In vitro Abeta aggregation assay. Lysates of wild-type, fic-1(n5823), and FIC-1(E274G) animals were supplemented with recombinant Abeta1-42. Protein aggregation was monitored by Thioflavin absorbance. In A–D, error bars represent SD. Statistical significance (P values) was calculated using the Gehan–Breslow–Wilcoxon test (A) or Mann–Whitney U test (B and D) compared with wild-type control (A and D) or anti-GFP RNAi control (B). *P < 0.05; **P < 0.01; ***P < 0.001; not significant (ns), P > 0.05.
To test whether enhanced survival of FIC-1(E274G) worms was accompanied by changes in Aβ aggregation, we stained worms grown under inducing conditions with Thioflavin S to visualize amyloid plaque formation. FIC-1(E274G) strains contained significantly more aggregates than wild-type and fic-1(n5823) worms (Fig. 1 C and D). Similarly, knockdown of hsp-1, hsp-3, or hsp-4 enhanced aggregation of Aβ (SI Appendix, Fig. S1E). In vitro aggregation of Aβ in the presence of post-debris worm supernatants of FIC-1(E274G) strains was enhanced in comparison to fic-1(n5823) or wild-type controls (Fig. 1E). AMPylation of HSP-1, HSP-3, and HSP-4 may thus limit their ability to prevent Aβ aggregation, pushing the balance away from toxic intermediate oligomers and toward the formation of large, cytoprotective over intermediate, toxic Aβ aggregates.
AMPylation Deficiency Enhances Survival Under Heat Stress Conditions.
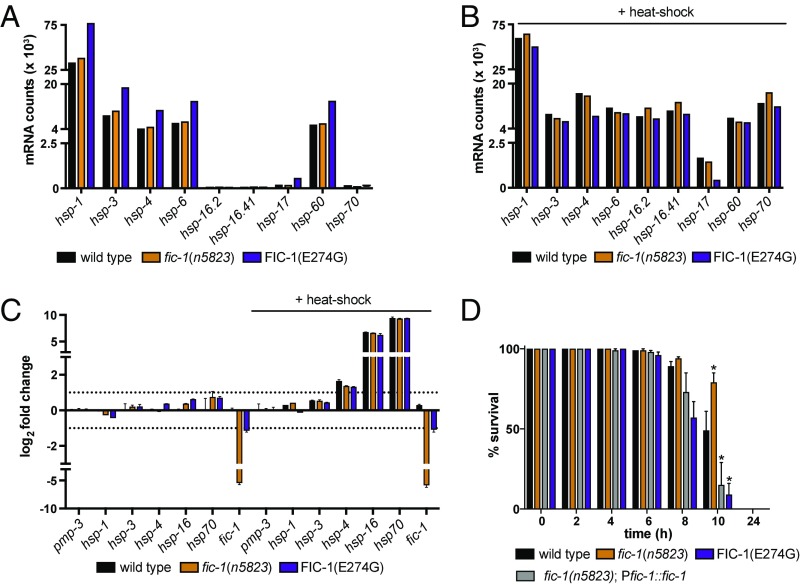
When overexpressed in Saccharomyces cerevisiae, an organism that lacks endogenous AMPylase activity, the constitutively active C. elegans AMPylase FIC-1(E274G) transfers AMP to the yeast cytosolic HSP70 family protein Ssa2 (27). AMPylation-mediated inhibition of Ssa2 induces the HSR, enhances protein aggregation, and eventually causes cell death (27). To determine whether fic-1 contributes to the HSR in its endogenous environment, we first introduced a transgenic heat-shock reporter (Phsp-16.2::gfp) into fic-1(n5823) and FIC-1(E274G) worms. Neither in the absence nor following a heat shock (30 min at 35 °C) did we observe genotype-dependent changes in reporter activation (SI Appendix, Fig. S2 A and B). We next performed RNA-sequencing (RNAseq) experiments to compare wild-type, fic-1(n5823), and FIC-1(E274G) strains exposed to heat stress or left untreated. In the absence of extrinsic stress, transcription profiles of the tested strains showed no major transcriptional alterations in response to hypo-AMPylation (fic-1 n5823) or hyper-AMPylation [FIC-1(E274G)] (Fig. 2A and SI Appendix, Fig. S2 C and D). Worms exposed to acute heat stress, as expected, showed a substantial up-regulation of hsf-1–regulated hsp genes (e.g., hsp-16.2, hsp16.41, hsp-70), but this was not affected by the individual genotypes of the tested strains (Fig. 2B and SI Appendix, Fig. S2 E and F). However, a small set of genes, particularly those coding for collagens, showed altered transcript levels that correlated with enhanced or decreased protein AMPylation (SI Appendix, Fig. S2G). The qPCR experiments confirmed the absence of AMPylation-related changes in HSR-associated gene expression (Fig. 2C) and corroborated a link between protein AMPylation and collagen gene transcription in FIC-1(E274G) and fic-1(n5823) strains.
Fig. 2.
AMPylation deficiency enhances survival under heat stress conditions. (A) Heat stress tolerance of wild-type, fic-1(n5823), and FIC-1(E274G) animals cultured at 33 °C at the indicated time points. Normalized mRNA counts of indicated genes in wild-type, fic-1(n5823), and FIC-1(E274G) animals in the absence of (B) or following (C) a 30-min heat shock at 35 °C are shown. (D) qPCR validation of transcriptional changes in untreated or heat-stressed wild-type, fic-1(n5823), and FIC-1(E274G) animals. In A–C, error bars represent SD. Statistical significance (P values) was calculated using the Mann–Whitney U test compared with wild-type control. *P < 0.01.
To check whether protein AMPylation might play a physiological role in heat stress tolerance beyond induction of the HSR, we tested survival of fic-1(n5823) and FIC-1(E274G) worms (29) incubated at 33 °C. Under chronic heat stress (33 °C for 24 h), fic-1(n5823) strains showed enhanced thermoresistance compared with wild-type controls, while FIC-1(E274G) worms, as well as worms expressing fic-1 from an extrachromosomal array (nIs734) in a fic-1(n5823) background, were significantly more sensitive (Fig. 2D). Consistent with this finding, fic-1(n5823) embryos survived extended exposure to 33 °C substantially better than wild-type or FIC-1(E274G) controls (SI Appendix, Fig. S2H). In contrast, short-term exposure to heat stress (30 min at 35 °C) affected neither worm development nor adult survival after heat shock in any of the genetic backgrounds tested (SI Appendix, Fig. S2 I and J).
Together, these results suggest that under prolonged (heat) stress exposure, when the function of HSPs is under constant high demand, HSP AMPylation is critical for survival by imposing an additional layer of HSP regulation that is independent of the transcriptional HSR. The observed changes in collagen expression are likely explained by minor differences in the developmental stage of the samples analyzed.
Protein AMPylation Affects Aggregation Behavior of PolyQ-Repeat–Containing Proteins in C. elegans.
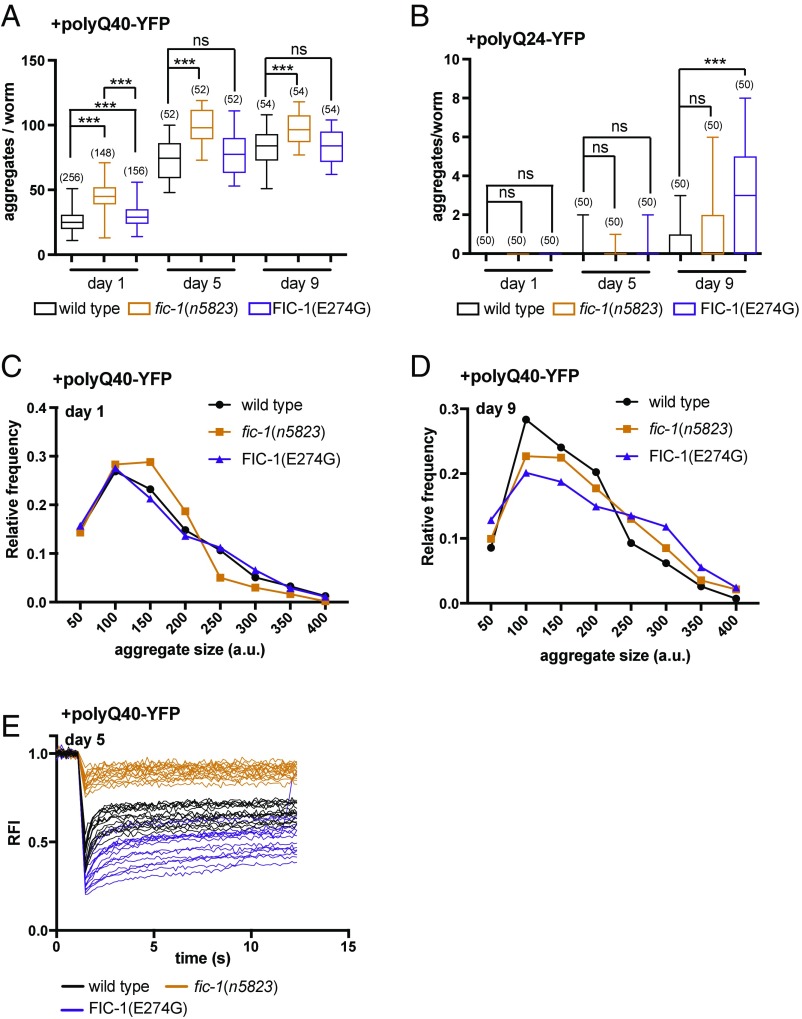
Transgenic expression of FIC-1(E274G) extended survival of Aβ-positive worms despite enhancing Aβ formation. This suggested that changes in intracellular AMPylation levels directly influence the dynamics of protein aggregation in vivo. To test this, we asked how aggregation of polyQ-YFP fusion proteins was affected when expressed in fic-1(n5823) or FIC-1(E274G) worms. Aggregation of polyQ-repeat variants of YFP in C. elegans is dependent on age and polyQ length: Glutamine repeats of 40 or more residues will promote the formation of discrete, fluorescent foci from early larval stages onward, while the formation of visible polyQ24-YFP aggregates within 10 d of adulthood is insignificant (39). Transgenic day 1 adult worms with muscular expression of polyQ40-YFP in a fic-1(n5823) background showed a marked increase in the total number of discrete aggregates (45 ± 0.9) compared with controls (26 ± 0.5) (Fig. 3A and SI Appendix, Fig. S3A) Total levels of polyQ40-YFP, as well as transcription of hsp-1, hsp-3, hsp-4, or the hsf-1–regulated HSPs F44H5.4/5, were unchanged (SI Appendix, Fig. S3 B and C). This distinction, albeit less pronounced with increasing age, persisted on day 5 and day 9 (Fig. 3A and SI Appendix, Fig. S3D). The number of aggregates in 9-d-old FIC-1(E274G) worms that express polyQ24-YFP increased significantly (2.9 ± 0.4) compared with controls (0.5 ± 0.1) (Fig. 3B). We also noted a tendency of altered polyQ24-YFP aggregation in AMPylase-deficient fic-1(n5823) worms (1 ± 0.2) (Fig. 3B). Consistent with these findings, cellulose acetate filter trap assays of worm lysates corroborated enhanced aggregation of polyQ40-YFP in fic-1(n5823) strains, as well as altered polyQ24-YFP aggregation in FIC-1(E274G) and fic-1(n5823) strains (SI Appendix, Fig. S3E). These findings emphasize the regulatory impact of HSP AMPylation on orchestrating protein aggregation.
Fig. 3.
AMPylation controls aggregation of polyQ40-repeat–containing proteins in C. elegans. Time-dependent aggregation of polyQ40-YFP (A) and polyQ24-YFP (B) in wild-type, fic-1(n5823), and FIC-1(E274G) animals is shown. The number of tested animals is given in parentheses. Error bars represent SD. Statistical significance (P value) was calculated using the Mann–Whitney U test. ***P < 0.05; not significant (ns), P > 0.05. The distribution profiles of polyQ40-YFP aggregate sizes on day 1 (C) and day 9 (D) of adulthood are shown. The bin size is 50 a.u. (E) PolyQ40-YFP aggregate mobility on day 5 of adulthood as measured by FRAP. The y axis depicts relative fluorescence intensity (RFI).
Since the presence of FIC-1(E274G) enhanced protein aggregation in Aβ-expressing worms, whereas fic-1(n5823) animals showed enhanced polyQ40-YFP aggregate counts, we hypothesized that AMPylation-mediated inhibition of HSPs might change the size and mobility of individual polyQ40-YFP aggregates, rather than promote the formation of more foci. We evaluated microscopic images that showed polyQ40-YFP aggregates formed in wild-type, fic-1(n5823), or FIC-1(E274G) animals and found that on day 1, where the discrepancy between the number of aggregates is maximal, fic-1(n5823) worms contained an increased proportion of small aggregates and a decreased proportion of larger aggregates compared with wild-type worms (Fig. 3C). The presence of FIC-1(E274G) did not alter the overall size distribution of polyQ40-YFP aggregates (Fig. 3C). As worms aged, FIC-1(E274G) expression supported the assembly of larger polyQ40-YFP foci, while the size profile of polyQ40-YFP aggregates in fic-1(n5823) strains became more wild type-like (Fig. 3D and SI Appendix, Fig. S3F). AMPylase deficiency enhanced the mobility of polyQ40-YFP foci, while the presence of FIC-1(E274G) significantly decreased it, as measured by fluorescence recovery after photobleaching (FRAP) microscopy (Fig. 3E and SI Appendix, Fig. S3 G and H). Changes in intracellular AMPylation levels therefore suffice to modify the abundance/size/mobility relationship of polyQ-repeat protein aggregates.
AMPylation-Mediated Changes in PolyQ-YFP Aggregation Alter Associated Toxicity.
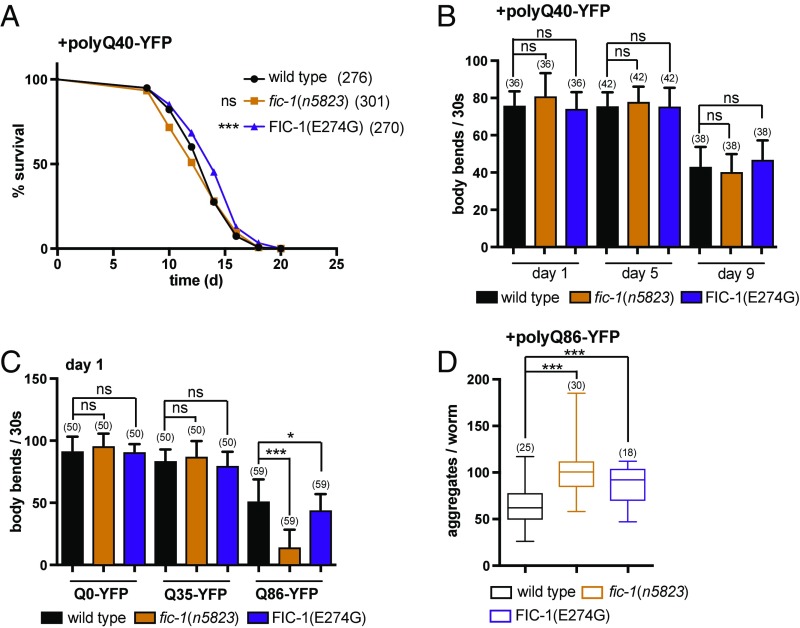
Since progressive polyQ-YFP aggregation reduces worm motility and shortens their lifespan (39), we next tested if the severity of the polyQ-YFP–dependent impairments would diverge in a fic-1(n5823) or FIC-1(E274G) background. Based on our previous results, we predicted that FIC-1(E274G) strains would exhibit changes in fitness relative to wild-type or fic-1(n5823) strains. Indeed, enhanced AMPylation levels showed a slight trend toward lifespan extension of FIC-1(E274G); polyQ40-YFP animals (Fig. 4A). We did not observe changes in worm motility over the course of 9 d in relation to AMPylation status or expression of polyQ protein (Fig. 4B and SI Appendix, Fig. S4A) However, polyQ24-YFP–expressing worms seemed particularly sensitive to changes in protein AMPylation, as both FIC-1(E274G) as well as fic-1(n5823) significantly shortened the lifespan of the animals tested (SI Appendix, Fig. S4B).
Fig. 4.
AMPylation affects polyQ-YFP toxicity in C. elegans. (A) Lifespan analysis of polyQ40-YFP–expressing wild-type, fic-1(n5823), and FIC-1(E274G) animals. (B) Motility assay of polyQ40-YFP–expressing wild-type, fic-1(n5823), and FIC-1(E274G) worms at the indicated time points. (C) Motility of wild-type, fic-1(n5823), and FIC-1(E274G) worms panneurally expressing the indicated polyQ-YFP proteins at day 1 of adulthood. (D) Number of polyQ86-YFP aggregates in the indicated genomic backgrounds. In A–D, the number of tested animals per group is shown in parentheses. Error bars represent SD. Statistical significance (P values) was calculated using the Mann–Whitney U test (motility) or Gehan–Brislow–Wilcoxon test (lifespan) compared with wild-type control. *P < 0.05; ***P < 0.001; not significant (ns), P > 0.05.
To evaluate the impact of protein AMPylation on polyQ-YFP aggregation in neurons, we monitored aggregate formation, as well as the mobility of transgenic nematode lines, with pan-neural polyQ0-YFP, polyQ35-YFP, or polyQ86-YFP expression. Loss of fic-1 potentiated the motility defects (Fig. 4C) and enhanced aggregate formation in polyQ86-YFP–expressing day 1 adults (Fig. 4D and SI Appendix, Fig. S4C), but it did not affect polyQ0-YFP or polyQ40-YFP strains. FIC-1(E274G) also significantly altered the number of aggregates and motility in polyQ86-YFP–expressing strains (Fig. 4 C and D).
Together, our data suggest that AMPylation-mediated regulation of HSPs impacts worm fitness in the presence of polyQ-repeat proteins, yet to a lesser extent than observed for Aβ-expressing animals. Our results support the hypothesis that not only the total number of protein aggregates but also their relative size directly impacts animal fitness.
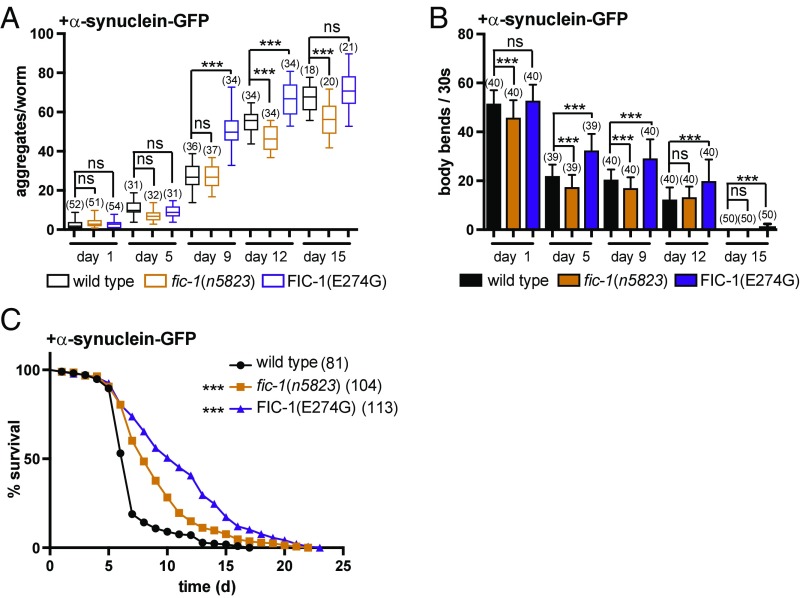
AMPylation Alters Toxicity of α-Syn Aggregates in C. elegans.
Intrigued by the impact of AMPylation on Aβ and polyQ-YFP toxicity, we asked whether α-syn aggregation might be similarly sensitive to changes in FIC-1 activity. We assessed age-dependent α-syn–GFP aggregation in fic-1(n5823) or FIC-1(E274G) background, and found that FIC-1(E274G) expression significantly expedited aggregate formation, such that the number of α-syn–GFP foci in 8-d-old α-syn–GFP; FIC-1(E274G) worms matched total numbers of aggregates observed in α-syn–GFP at day 15 of adulthood (Fig. 5A and SI Appendix, Fig. S5A). FIC-1 deficiency attenuated the assembly of α-syn–GFP foci as worms aged. This yielded significantly fewer aggregates on day 12 or day 15. Consistent with a beneficial role for larger α-syn–GFP aggregates as a means of reducing the toxicity elicited by intermediate species, FIC-1(E274G) worms remained significantly more motile (Fig. 5B) and outlived fic-1(n5823) or wild-type animals expressing α-syn–GFP in standard lifespan assays (Fig. 5C). While fic-1 deficiency slightly reduced motility (Fig. 5B), it enhanced survival of worms expressing α-syn–GFP, but not to the extent seen for FIC-1(E274G) animals (Fig. 5C).
Fig. 5.
AMPylation alters α-syn toxicity in C. elegans. (A) Time-dependent aggregation of α-syn in wild-type, fic-1(n5823), and FIC-1(E274G) animals. (B) Motility tests of α-syn–expressing wild-type, fic-1(n5823), and FIC-1(E274G) worms at the indicated time points. (C) Survival assay of α-syn–expressing wild-type, fic-1(n5823), and FIC-1(E274G) animals. In A–C, the number of tested animals per group is shown in parentheses. Error bars represent SD. Statistical significance (P values) was calculated using the Mann–Whitney U test (motility) or Gehan–Brislow–Wilcoxon test (lifespan) compared with wild-type control. ***P < 0.001; not significant (ns), P > 0.05.
These data not only support the capacity of fic-1 in the regulation of α-syn aggregation but also indicate additional control of the aggregation/motility/survival relationship beyond protein AMPylation.
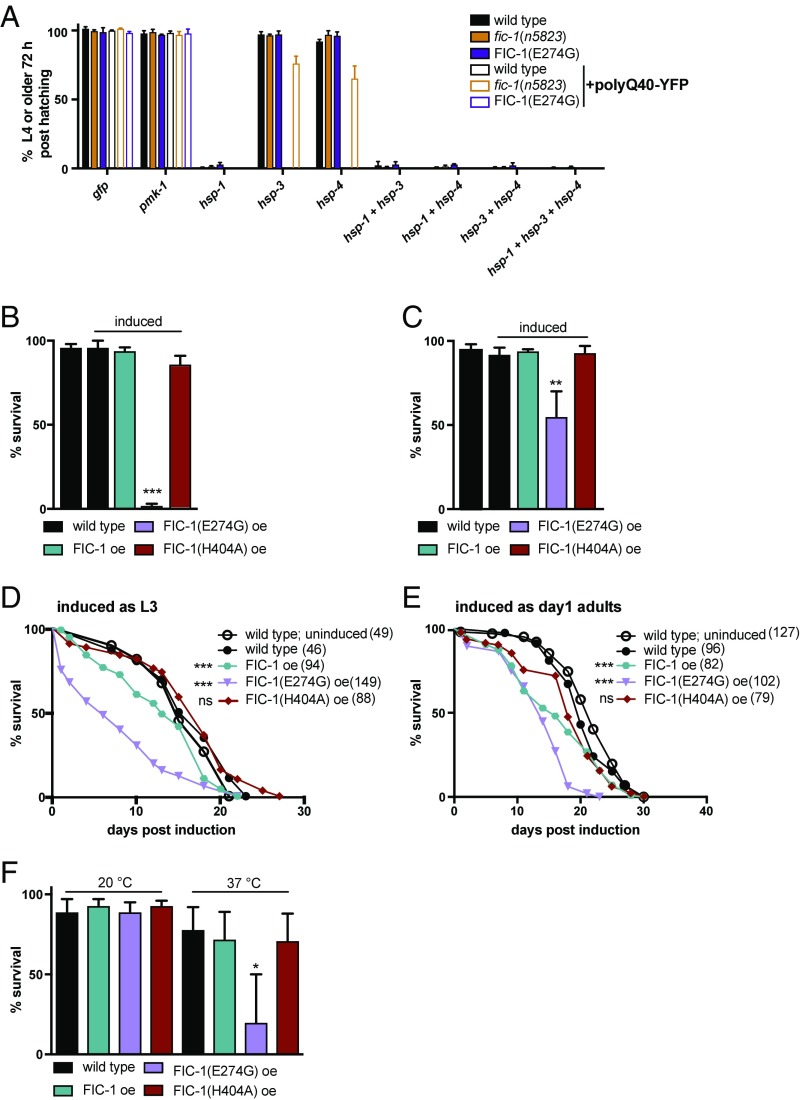
Fic-1 Is Essential to Balance HSP Activity During Larval Development.
While endogenous-level FIC-1(E274G) expression does not affect survival of wild-type animals (29), it improves worm fitness in the presence of aggregation-prone proteins. Because evolution likely favored tight regulation of FIC-1, we suspected that enhanced AMPylation levels might affect larval development, where rapid cell division depends on maximally active proteostasis machinery. We asked if AMPylation-mediated inhibition of HSP-1, HSP-3, and HSP-4 could be detrimental during early animal development in worms when proteostasis is challenged by aggregation-prone proteins. Using RNAi, we depleted HSP-1, HSP-3, and HSP-4 in polyQ40-YFP and control strains and tested whether larval development was impaired. Whereas development was inhibited upon ablation of hsp-3 or hsp-4 in polyQ40-YFP–expressing wild-type and FIC-1(E274G) larval 1 (L1)-stage larvae, it was unaffected in fic-1(n5823) worms (Fig. 6A). Neither hsp-3 nor hsp-4 knockdown affected larval development in the absence of polyQ40-YFP in any of the genetic backgrounds tested, consistent with the previously identified compensatory regulation of the two C. elegans BiP orthologs (40). Hsp-1 or combinatorial hsp-3/hsp-4 ablation was lethal at the L1 stage, independent of the presence polyQ40-YFP (Fig. 6A). When transferred to RNAi conditions at the L3 stage, knockdown of hsp-1, hsp-3, and hsp-4 did not interfere with aging (SI Appendix, Fig. S6A). These results are consistent with an increased demand for chaperone activity to buffer proteostasis in rapidly dividing cells, particularly in the presence of stress caused by protein aggregation, and suggest a regulatory role for FIC-1–mediated HSP AMPylation during early development.
Fig. 6.
Extensive protein AMPylation interferes with larval development and heat stress tolerance in C. elegans. (A) Development assay depicting the proportion of wild-type, fic-1(n5823), and FIC-1(E274G) embryos that do or do not express polyQ40-YFP and are able to reach adulthood within 72 h at 20 °C when grown on RNAi plates posthatching. Forty-eight-hour survival of L1 (B) and L3 (C) larvae following induction of overexpression (oe) of indicated proteins is shown. Lifespan assays of worms that overexpress the indicated proteins as L3 larvae (D) or day 1 adults (E) are shown. (F) Forty-eight-hour survival of day 1 adults induced to express the indicated proteins. In D and E, the number of tested animals per group is shown in parentheses. Error bars represent SD. Statistical significance (P values) was calculated using the Mann–Whitney U test (motility) or Gehan–Brislow–Wilcoxon test (lifespan) compared with wild-type control. *P < 0.05; **P < 0.01; ***P < 0.001, not significant (ns), P > 0.05.
In light of the above, we predicted that HSP hyper-AMPylation might be detrimental in the early larval stages in C. elegans. As our attempts to obtain constitutively expressing Peft-3::FIC-1(E274G) strains were unsuccessful, Peft-3::fic-1 lines were readily obtained. We therefore expressed FIC-1, FIC-1(E274G), and the catalytically incapacitated mutant enzyme FIC-1(H404A) equipped with a C-terminal HA-tag under the inducible hsp-16.2p promotor. If overexpression of FIC-1(E274G) were to lead to systemic HSP70 AMPylation and associated inhibition, then the consequences of overexpression should be most pronounced when induced in early larval stages (L1–L2), where rapid cell division is essential. Indeed, we observed an onset-dependent susceptibility to hyper-AMPylation that declined with age: Overexpression of FIC-1(E274G) in synchronized L1 larvae was acutely lethal, while overexpression of FIC-1 or FIC-1(H404A) did not affect larval fitness or development (Fig. 6B), recapitulating acute toxicity of FIC-1(E274G) as previously described in S. cerevisiae (27). When induced at the L3 stage, FIC-1(E274G) significantly impacted larval development (Fig. 6C) and only about 50% of FIC-1(E274G) worms reached adulthood within 48 h. The lifespan of this fraction of FIC-1(E274G) animals was significantly reduced compared with FIC-1 or wild-type controls (Fig. 6D). Overexpression of FIC-1(E274G) in day 1 adult worms attenuated survival yet did not alter motility (Fig. 6E and SI Appendix, Fig. S6 B and C). Notably, overexpression of FIC-1 significantly reduced survival without impairing larval development, possibly because of a higher abundance of endogenous FIC-1 activators in (late) adulthood.
Because AMPylation deficiency conferred enhanced heat tolerance (Fig. 2D), we tested if FIC-1(E274G) overexpression might limit the worm’s ability to sustain prolonged heat stress through inhibition of HSP activity by AMPylation. We induced overexpression of FIC-1, FIC-1(E274G), and FIC-1(H404A); rested the worms for 2 h at 20 °C to allow protein expression; and then exposed the worms to either 20 °C or 33 °C. Overexpression of FIC-1(E274G) significantly reduced heat tolerance, although it did not affect short-term survival at 20 °C (Fig. 6F).
Our results indicate a particular sensitivity to HSP70 dysregulation in early life and highlight a critical regulatory role for FIC-1 in balancing cellular HSP activity to accommodate aggregation or heat stress at a stage of rapid cell division.
Discussion
As cells age, their ability to control the many facets of protein folding declines, producing an environment prone to protein aggregation. Likewise, the cytoprotective HSR and unfolded protein response in the ER are attenuated in older animals (41–43). HSP70 chaperones are essential in protein (re)folding (44, 45), but whether posttranslational HSP70 modifications contribute to the coordination of the cellular chaperoning machinery remained unknown. Our results provide evidence that FIC-1–mediated AMPylation of HSP70 affects the dynamics of protein aggregation by negatively regulating its activity.
Using C. elegans models for Aβ, polyQ, and α-syn aggregation, we found that both reduction and enhancement of intracellular AMPylation alter protein aggregation behavior. Aggregate size and mobility are affected, causing changes in lifespan and motility. While we anticipate that AMPylation of HSPs is essentially eliminated in fic-1(n5823) animals, expression of constitutively active FIC-1(E274G) from its (weak) endogenous fic-1 promotor is unlikely to result in continuous and complete AMPylation of cellular HSP70 pools. Rather, the cellular chaperoning machinery will be impaired but not completely shut down in these animals. However, overexpression of FIC-1(E274G) will result in near-complete HSP70 AMPylation, essentially switching off the HSP70-dependent chaperoning machinery. As a result, any process dependent on the chaperoning system, such as cell division, is prone to failure, such as early embryonic development in C. elegans (as shown in Fig. 6B) or propagation of yeast (27).
We had previously identified HSP-1, HSP-3, and HSP-4 as substrates for the C. elegans AMPylase FIC-1 (29). AMPylation inhibits the activity of HSP-1, HSP-3, and HSP-4, an outcome we recapitulated by their ablation by means of RNAi. We therefore suggest that changes in AMPylation of additional FIC-1 substrates, including the translation elongation factor EEF-1A.2 or core histones (29), may be less important in this context.
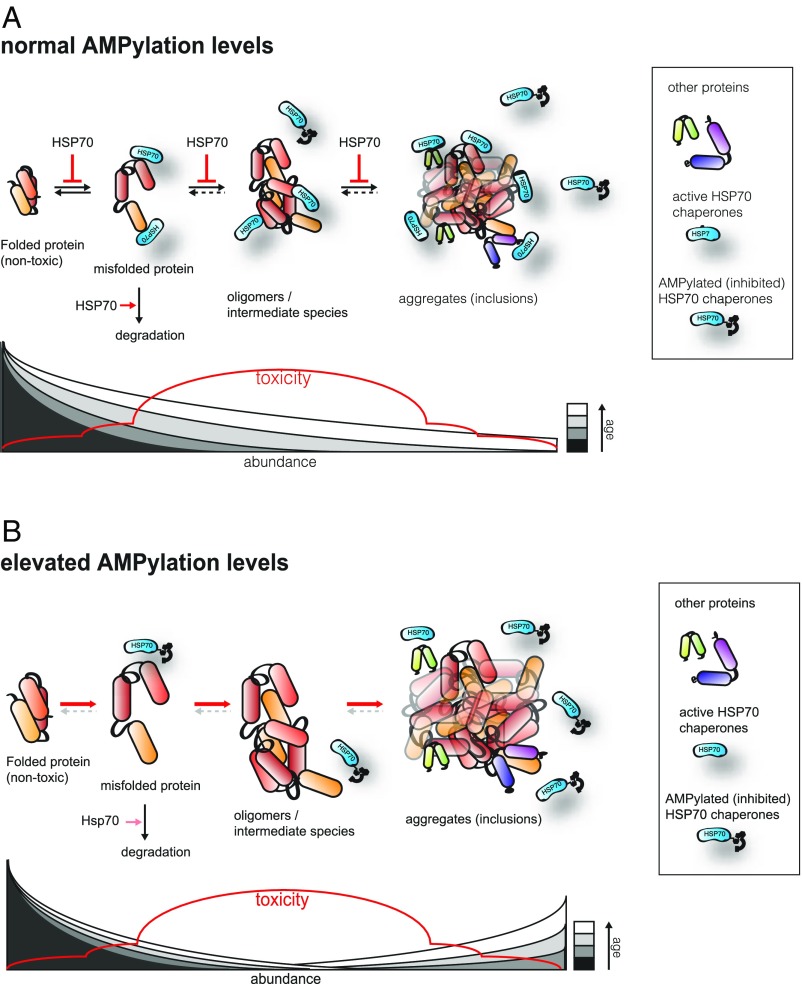
AMPylation impairs the activity of HSP-1, HSP-3, and HSP-4, all of which interact with Aβ plaques in C. elegans (46), and promotes the formation of larger aggregates. This extends lifespan. Intermediate-sized, soluble protein aggregates are thought to be the major toxic aggregate species, whereas larger, insoluble foci inflict less harm (13–15, 18, 47, 48). Dampening HSP70 activity through AMPylation may thus favor assembly of larger foci, as HSP70s are no longer in sufficient supply to counteract protein aggregation (Fig. 7 A and B). AMPylation of HSP-3/HSP-4 might impair the turnover of misfolded secretory proteins and enhance the cell’s vulnerability to aggregate formation. Likewise, we propose that AMPylation of HSP-1, an HSP70 family member that shuttles between the cytoplasm and the nuclear lumen, renders the cytoplasmic and nuclear compartments more vulnerable to protein aggregation.
Fig. 7.
AMPylation levels regulate aggregation behavior. Under normal conditions (A), HSP70 proteins prevent the unfolding and support the refolding of misfolded proteins, while suppressing the formation of protein oligomers/intermediates as well as aggregates. Elevated AMPylation levels (B) limit the available pool of active HSP70 proteins, hence increasing the rate of protein aggregation and favoring the formation of large foci over smaller oligomers.
Connections between HSP70 and Grp78/BiP activity and protein aggregation in mammals are well established. Several HSP70 activators are currently being tested for their potential to slow the progression of AD, HD, and PD (21, 22, 49–52). Our results suggest that HSP70 inhibitors, rather than activators, might represent an interesting alternative route to explore. What, then, is the possible explanation for why both HSP70 activation and inhibition can be advantageous in the context of aggregation-associated toxicity? Under the assumption that soluble, intermediate aggregates represent the major toxic species, both the avoidance of aggregate formation, achieved by HSP overexpression or HSP activators, as well as the expedited assembly of large, cytoprotective aggregates, as stimulated by transient HSP inhibition ( i.e., through enhanced HSP70 AMPylation), would efficiently detoxify affected cells (52, 53) (Fig. 7). Thus, we propose that pharmacological interventions that target the human AMPylase HYPE may have therapeutic potential.
Enhanced AMPylation levels are beneficial in the context of Aβ and α-syn aggregation. For polyQ-repeat proteins, the absence of fic-1, more so than FIC-1(E274G) expression, affects the formation of aggregation foci. The minimal fitness gain seen for polyQ-YFP; FIC-1(E274G) strains suggests that AMPylation levels achieved by endogenous FIC-1 activity might suffice for proteostasis in the presence of this polyQ-YFP species. A likely explanation for this observation would be that polyQ-YFP proteins directly or indirectly promote the activation of endogenous FIC-1. At this time, however, we do not understand the fundamental mechanisms by which an activating signal for FIC-1 is generated or how the enzyme perceives it. Differences in protein aggregation propensities between Aβ, α-syn, and polyQ-YFP species (54) may constrain how aggregate formation is regulated by HSP70 activity, and therefore determine the susceptibility of aggregate formation to changes in AMPylation of HSP70.
The impact of HSP AMPylation on protein aggregation and larval development implies a well-balanced regulatory regimen. We further note that FIC-1 expression is elevated in the C. elegans germline and in embryos, consistent with an important role of protein AMPylation during larval development (29). We find that ablation of hsp-1 at early larval stages is lethal, confirming previous reports (55). However, individual knockdown of hsp-3 or hsp-4 is well tolerated even in a fic-1 background. The ability of hsp-3 and hsp-4 to cross-complement and substitute for each other in the ER provides a likely explanation (40, 56).
In conclusion, our findings challenge the long-standing paradigm that proteostasis is regulated solely by the abundance of HSP proteins. Rather, a dynamic interplay between expression level and posttranslational modifications, such as AMPylation, coordinates stress tolerance and contributes to age-associated pathologies. Pharmacological modulation of AMPylation may thus provide a new toehold to modulate the proteostasis network for therapeutic benefit in a wide range of age-related diseases.
Materials and Methods
A detailed description of the materials and methods used in this study is provided in SI Appendix, including the following: C. elegans strains and growth conditions, germline transformation, worm synchronization, in vivo Aβ aggregation assays, in vivo polyQ-YFP and α-syn–GFP aggregation assays, RNAi, motility assays, development assays, stress tolerance assays, fitness and longevity assays, RNAseq, RT-qPCR, Thioflavin S staining of amyloid plaques, microscopy, in vitro aggregation assays, plasmid construction, immunoblotting, FRAP, and experimental statistics.
Supplementary Material
Acknowledgments
We thank the members of the H.L.P., Horvitz, and D.P. laboratories for helpful comments and discussion. The Whitehead genome core facility is acknowledged for processing RNAseq and genomic DNA samples. We thank Justin Reemer for technical assistance and the Whitehead fluorescence-activated cell sorting facility for support with the reporter assays. George Bell and the Whitehead bioinformatics group are acknowledged for their help with the analysis of RNAseq data. M.C.T. is a recipient of a Young Investigator Award from the Emerald Foundation, Inc. This work was supported by an NIH Early Independence Award (DP5 OD017941-01 to D.P.) and a Cancer Research Fellowship from the Alexander and Margaret Stewart Trust (to D.P.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1801989115/-/DCSupplemental.
References
- 1.Haynes CM, Ron D. The mitochondrial UPR - protecting organelle protein homeostasis. J Cell Sci. 2010;123:3849–3855. doi: 10.1242/jcs.075119. [DOI] [PubMed] [Google Scholar]
- 2.Oakes SA, Papa FR. The role of endoplasmic reticulum stress in human pathology. Annu Rev Pathol. 2015;10:173–194. doi: 10.1146/annurev-pathol-012513-104649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Senft D, Ronai ZA. UPR, autophagy, and mitochondria crosstalk underlies the ER stress response. Trends Biochem Sci. 2015;40:141–148. doi: 10.1016/j.tibs.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Westerheide SD, Raynes R, Powell C, Xue B, Uversky VN. HSF transcription factor family, heat shock response, and protein intrinsic disorder. Curr Protein Pept Sci. 2012;13:86–103. doi: 10.2174/138920312799277956. [DOI] [PubMed] [Google Scholar]
- 5.Chen S, Brown IR. Neuronal expression of constitutive heat shock proteins: Implications for neurodegenerative diseases. Cell Stress Chaperones. 2007;12:51–58. doi: 10.1379/CSC-236R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kopito RR, Ron D. Conformational disease. Nat Cell Biol. 2000;2:E207–E209. doi: 10.1038/35041139. [DOI] [PubMed] [Google Scholar]
- 7.Shah TM, Gupta SM, Chatterjee P, Campbell M, Martins RN. Beta-amyloid sequelae in the eye: A critical review on its diagnostic significance and clinical relevance in Alzheimer’s disease. Mol Psychiatry. 2017;22:353–363. doi: 10.1038/mp.2016.251. [DOI] [PubMed] [Google Scholar]
- 8.Del Tredici K, Braak H. Review: Sporadic Parkinson’s disease: Development and distribution of α-synuclein pathology. Neuropathol Appl Neurobiol. 2016;42:33–50. doi: 10.1111/nan.12298. [DOI] [PubMed] [Google Scholar]
- 9.Adegbuyiro A, Sedighi F, Pilkington AW, 4th, Groover S, Legleiter J. Proteins containing expanded polyglutamine tracts and neurodegenerative disease. Biochemistry. 2017;56:1199–1217. doi: 10.1021/acs.biochem.6b00936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winner B, et al. In vivo demonstration that alpha-synuclein oligomers are toxic. Proc Natl Acad Sci USA. 2011;108:4194–4199. doi: 10.1073/pnas.1100976108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colla E, et al. Accumulation of toxic α-synuclein oligomer within endoplasmic reticulum occurs in α-synucleinopathy in vivo. J Neurosci. 2012;32:3301–3305. doi: 10.1523/JNEUROSCI.5368-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi BK, et al. Large α-synuclein oligomers inhibit neuronal SNARE-mediated vesicle docking. Proc Natl Acad Sci USA. 2013;110:4087–4092. doi: 10.1073/pnas.1218424110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leitman J, Ulrich Hartl F, Lederkremer GZ. Soluble forms of polyQ-expanded huntingtin rather than large aggregates cause endoplasmic reticulum stress. Nat Commun. 2013;4:2753. doi: 10.1038/ncomms3753. [DOI] [PubMed] [Google Scholar]
- 14.Kayed R, et al. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- 15.Cleary JP, et al. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- 16.Arrasate M, Mitra S, Schweitzer ES, Segal MR, Finkbeiner S. Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature. 2004;431:805–810. doi: 10.1038/nature02998. [DOI] [PubMed] [Google Scholar]
- 17.Behrends C, et al. Chaperonin TRiC promotes the assembly of polyQ expansion proteins into nontoxic oligomers. Mol Cell. 2006;23:887–897. doi: 10.1016/j.molcel.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 18.Schaffar G, et al. Cellular toxicity of polyglutamine expansion proteins: Mechanism of transcription factor deactivation. Mol Cell. 2004;15:95–105. doi: 10.1016/j.molcel.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 19.Wacker JL, Zareie MH, Fong H, Sarikaya M, Muchowski PJ. Hsp70 and Hsp40 attenuate formation of spherical and annular polyglutamine oligomers by partitioning monomer. Nat Struct Mol Biol. 2004;11:1215–1222. doi: 10.1038/nsmb860. [DOI] [PubMed] [Google Scholar]
- 20.Fujikake N, et al. Heat shock transcription factor 1-activating compounds suppress polyglutamine-induced neurodegeneration through induction of multiple molecular chaperones. J Biol Chem. 2008;283:26188–26197. doi: 10.1074/jbc.M710521200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evans CG, Chang L, Gestwicki JE. Heat shock protein 70 (hsp70) as an emerging drug target. J Med Chem. 2010;53:4585–4602. doi: 10.1021/jm100054f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evans CG, Wisén S, Gestwicki JE. Heat shock proteins 70 and 90 inhibit early stages of amyloid beta-(1-42) aggregation in vitro. J Biol Chem. 2006;281:33182–33191. doi: 10.1074/jbc.M606192200. [DOI] [PubMed] [Google Scholar]
- 23.Murphy ME. The HSP70 family and cancer. Carcinogenesis. 2013;34:1181–1188. doi: 10.1093/carcin/bgt111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jego G, Hazoumé A, Seigneuric R, Garrido C. Targeting heat shock proteins in cancer. Cancer Lett. 2013;332:275–285. doi: 10.1016/j.canlet.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 25.Goloudina AR, Demidov ON, Garrido C. Inhibition of HSP70: A challenging anti-cancer strategy. Cancer Lett. 2012;325:117–124. doi: 10.1016/j.canlet.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Truman AW, et al. CDK-dependent Hsp70 Phosphorylation controls G1 cyclin abundance and cell-cycle progression. Cell. 2012;151:1308–1318. doi: 10.1016/j.cell.2012.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Truttmann MC, et al. Unrestrained AMPylation targets cytosolic chaperones and activates the heat shock response. Proc Natl Acad Sci USA. 2017;114:E152–E160. doi: 10.1073/pnas.1619234114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Truttmann MCPHL, Ploegh HL. rAMPing up stress signaling: Protein AMPylation in metazoans. Trends Cell Biol. 2017;27:608–620. doi: 10.1016/j.tcb.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Truttmann MC, et al. The Caenorhabditis elegans protein FIC-1 is an AMPylase that covalently modifies heat-shock 70 family proteins, translation elongation factors and histones. PLoS Genet. 2016;12:e1006023. doi: 10.1371/journal.pgen.1006023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Preissler S, et al. AMPylation matches BiP activity to client protein load in the endoplasmic reticulum. eLife. 2015;4:e12621. doi: 10.7554/eLife.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ham H, et al. Unfolded protein response-regulated dFic reversibly AMPylates BiP during endoplasmic reticulum homeostasis. J Biol Chem. 2014;289:36059–36069. doi: 10.1074/jbc.M114.612515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanyal A, et al. A novel link between Fic (filamentation induced by cAMP)-mediated adenylylation/AMPylation and the unfolded protein response. J Biol Chem. 2015;290:8482–8499. doi: 10.1074/jbc.M114.618348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Misselwitz B, Staeck O, Rapoport TA. J proteins catalytically activate Hsp70 molecules to trap a wide range of peptide sequences. Mol Cell. 1998;2:593–603. doi: 10.1016/s1097-2765(00)80158-6. [DOI] [PubMed] [Google Scholar]
- 34.De Los Rios P, Barducci A. Hsp70 chaperones are non-equilibrium machines that achieve ultra-affinity by energy consumption. eLife. 2014;3:e02218. doi: 10.7554/eLife.02218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Preissler S, Rato C, Perera L, Saudek V, Ron D. FICD acts bifunctionally to AMPylate and de-AMPylate the endoplasmic reticulum chaperone BiP. Nat Struct Mol Biol. 2017;24:23–29. doi: 10.1038/nsmb.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Link CD. Expression of human beta-amyloid peptide in transgenic Caenorhabditis elegans. Proc Natl Acad Sci USA. 1995;92:9368–9372. doi: 10.1073/pnas.92.20.9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manière X, et al. High transcript levels of heat-shock genes are associated with shorter lifespan of Caenorhabditis elegans. Exp Gerontol. 2014;60:12–17. doi: 10.1016/j.exger.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 38.Singh V, Aballay A. Regulation of DAF-16-mediated innate immunity in Caenorhabditis elegans. J Biol Chem. 2009;284:35580–35587. doi: 10.1074/jbc.M109.060905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morley JF, Brignull HR, Weyers JJ, Morimoto RI. The threshold for polyglutamine-expansion protein aggregation and cellular toxicity is dynamic and influenced by aging in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2002;99:10417–10422. doi: 10.1073/pnas.152161099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kapulkin WJ, Hiester BG, Link CD. Compensatory regulation among ER chaperones in C. elegans. FEBS Lett. 2005;579:3063–3068. doi: 10.1016/j.febslet.2005.04.062. [DOI] [PubMed] [Google Scholar]
- 41.Labbadia J, Morimoto RI. Repression of the heat shock response is a programmed event at the onset of reproduction. Mol Cell. 2015;59:639–650. doi: 10.1016/j.molcel.2015.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor RC. Aging and the UPR(ER) Brain Res. 2016;1648:588–593. doi: 10.1016/j.brainres.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 43.Martínez G, Duran-Aniotz C, Cabral-Miranda F, Vivar JP, Hetz C. Endoplasmic reticulum proteostasis impairment in aging. Aging Cell. 2017;16:615–623. doi: 10.1111/acel.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mayer MP, Bukau B. Hsp70 chaperones: Cellular functions and molecular mechanism. Cell Mol Life Sci. 2005;62:670–684. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kampinga HH, Craig EA. The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat Rev Mol Cell Biol. 2010;11:579–592. doi: 10.1038/nrm2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fonte V, et al. Interaction of intracellular beta amyloid peptide with chaperone proteins. Proc Natl Acad Sci USA. 2002;99:9439–9444, and erratum (2013) 110:4853. doi: 10.1073/pnas.152313999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saarikangas J, Barral Y. Protein aggregation as a mechanism of adaptive cellular responses. Curr Genet. 2016;62:711–724. doi: 10.1007/s00294-016-0596-0. [DOI] [PubMed] [Google Scholar]
- 48.Saarikangas J, Barral Y. Protein aggregates are associated with replicative aging without compromising protein quality control. eLife. 2015;4:e06197. doi: 10.7554/eLife.06197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang Y, Turner RS, Gaut JR. The chaperone BiP/GRP78 binds to amyloid precursor protein and decreases Abeta40 and Abeta42 secretion. J Biol Chem. 1998;273:25552–25555. doi: 10.1074/jbc.273.40.25552. [DOI] [PubMed] [Google Scholar]
- 50.Hoshino T, et al. Suppression of Alzheimer’s disease-related phenotypes by expression of heat shock protein 70 in mice. J Neurosci. 2011;31:5225–5234. doi: 10.1523/JNEUROSCI.5478-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Magrané J, Smith RC, Walsh K, Querfurth HW. Heat shock protein 70 participates in the neuroprotective response to intracellularly expressed beta-amyloid in neurons. J Neurosci. 2004;24:1700–1706. doi: 10.1523/JNEUROSCI.4330-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang AM, et al. Activation of Hsp70 reduces neurotoxicity by promoting polyglutamine protein degradation. Nat Chem Biol. 2013;9:112–118. doi: 10.1038/nchembio.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jinwal UK, et al. Hsp70 ATPase modulators as therapeutics for Alzheimer’s and other neurodegenerative diseases. Mol Cell Pharmacol. 2010;2:43–46. [PMC free article] [PubMed] [Google Scholar]
- 54.Villar-Piqué A, Ventura S. Protein aggregation propensity is a crucial determinant of intracellular inclusion formation and quality control degradation. Biochim Biophys Acta. 2013;1833:2714–2724. doi: 10.1016/j.bbamcr.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 55.Morley JF, Morimoto RI. Regulation of longevity in Caenorhabditis elegans by heat shock factor and molecular chaperones. Mol Biol Cell. 2004;15:657–664. doi: 10.1091/mbc.E03-07-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shen X, et al. Complementary signaling pathways regulate the unfolded protein response and are required for C. elegans development. Cell. 2001;107:893–903. doi: 10.1016/s0092-8674(01)00612-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.