Abstract
Objective
The purpose of this study was to evaluate the incidence rate of post-stroke upper limb spasticity and its correlation with cerebral infarction site.
Methods
A total of 498 inpatient and outpatient cases are included in the present study. The post-stroke upper limb spasticity rate of different cerebral infarction site was calculated.
Results
A total of 498 patients with cerebral infarction are enrolled in this study. Of these patients, 91 have dropped out and 407 have completed the study. Of the completed cases, 172 are in the spasm group and 235 are in the non-spasm group. The total incidence of upper limb spasticity is 34.5%. The incidences of upper extremity spasms are 12.5%, 20%, 22.5%, 35%, 40%, and 42.5% in 2 weeks, 1 month, 2 months, 3 months, 6 months, and 12 months, respectively. The incidence of upper extremity spasms increases with time. The incidences of upper limb spasticity are 12.1%, 63.3%, 58.5%, 9.4% and 8.3% when cerebral infarction occurs in the cortical and subcortical mixed areas, basal ganglia and internal capsule, cerebralcortex, brainstem and cerebellum respectively. The incidence of upper limb spasticity varies in different infarction sites (P < 0.05).
Conclusion
The post-stroke upper limb spasticity rates were different according to the different cerebral infarction site. Patients with the ganglia and internal capsule infarctions had the highest risk of developing post-stroke upper limb spasticity.
Keywords: Post-stroke upper limb spasticity, Cerebral infarction site, Upper limb pain
1. Introduction
Spastic paralysis is common sequelae of stroke, and approximately 17% to 40% of patients experienced poststroke upper limb spasticity [1, 2]. If this condition is not treated timely and appropriately, it can cause pain in the limb, limb muscle atrophy, joint contracture, deformation and consequently lead to limited joint mobility and difficulty in undergoing rehabilitation training [2,3,4]. As a result, the recovery of patient’s normal activities is adversely affected or the state of illness is aggravated. Studies have rarely reported the correlation between poststroke upper limb spasticity and cerebral infarction site [5, 6]. To elucidate the post-stroke upper limb spasticity rate and its correlation with and cerebral infarction site, we conducted this study to further evaluate their relations and discuss discus its potential mechanism in order to provide more useful information for clinical health providers.
2. Material and methods
2.1. Patient’s inclusion
A total of 498 inpatient and outpatient cases are included in our present study. The participants include 279 males and 219 females aged 18–75 years (average age = 65.27 ± 16.48 years). Patients inclusion criteria: Stroke was diagnosed according to the brain CT or MRI; Patients satisfy the diagnostic criteria for cerebral infarction of the 2008 Chinese Medical Association “Clinical Diagnosis and Treatment Guidelines-Neurological Volume”; The patients were fist first-onset of stroke. The patient’s exclusion criteria: Patients with previous stroke, other central nervous system diseases, mental diseases, and serious complications or those who cannot be examined because of disturbance of consciousness are excluded.
Ethical approval: The research related to human use has been complied with all the relevant national regulations, institutional policies and in accordance the tenets of the Helsinki Declaration, and has been approved by the authors’ institutional review board or equivalent committee.
Informed consent: Informed consent has been obtained from all individuals included in this study.
2.2. Research tools
The following relevant records of the patients hospitalized for 2 weeks, 1 month, 2 months, 3 months, 6 months, and 12 months are collected: (1) visual analog scale pain scoring; (2) brain injury site (CT or nuclear magnetic resonance images checked after admission, lesions, such as those in the cortex (frontal lobe, temporal lobe, occipital lobe, and parietal lobe), cortical area (basal ganglia, capsule), cortex and subcortical mixed area, brain stem, and cerebellum, area of cerebral infarction (Adams classification) recorded according to the maximum low-density shadow area corresponding to the symptoms and signs revealed by CT or MRI films, that is, the length and width of the maximum diameter of the lesions were multiplied to obtain the relative area of cerebral infarction; and (3) questionnaires on general characteristics, including gender, age, occupation, education, marital status, and past medical history (hypertension, diabetes, coronary heart disease, and hyperlipidemia).
2.3. Research methods
After 12 months, the cases are grouped on the basis of muscle tension of the hemiplegic limb assessed with Ashworth scale: (1) spasm group whose score is greater than 1 and (2) non-spasm group whose score is less than 1 or is equivalent to 1. The following patients are excluded: those who receive sedative drugs or muscle pineal agents, those who continue medicine intake after 1 month of wash-out period, those who experience myodystonia caused by peripheral neuropathy and other central nervous system lesions, and those who suffer from the recurrence of acute cerebrovascular diseases.
2.4. Statistical analysis
A dual-computer and double-person entry in the questionnaire is carried out in Epidata3.1, and data are statistically analyzed in SPSS22.0. The measurement data were expressed with X ± S and the comparison between groups was made based on the t-test of the sample mean. The enumeration data were expressed with a relative number, and the comparison between groups was made based on the χ2 test. P<0.05 was considered statistical different.
3. Results
3.1. Incidence and characteristics of poststroke upper limb spasticity
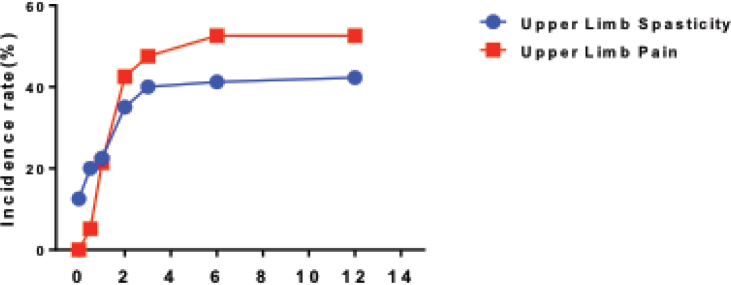
The total incidence of upper limb spasticity is 34.5%, which includes 68 cases (39.52%) of Ashworth grade 2 patients and 101 cases (58.7%) of Ashworth grade 3 patients. The incidences of upper extremity spasms are 12.5%, 20%, 22.5%, 35%, 40%, and 42.5% in 2 weeks, 1 month, 2 months, 3 months, 6 months, and 12 months, respectively. The incidence of upper extremity spasms increases with time (Figure. 1).
Figure 1.
The incidence rate of spasticity and pain in the upper limb
3.2. Correlation between the occurrence of post-stroke upper limb spasticity and pain
After the patients were admitted, the incidences of upper limb pain are 2%, 5%, 20%, 42.5%, 47.5%, 52.5%, and 52.5% in 2 weeks, 1 month, 2 months, 3 months, 6 months, and 12 months, respectively. The incidence of upper limb spasticity increases with time (Figure 1).
3.3. Relationship between upper limb spasticity and cerebral infarction site
The incidences of upper limb spasticity are 12.1%, 63.3%, 58.5%, 9.4% and 8.3% for the cerebral infarction site of the cortical and subcortical mixed areas, basal ganglia and internal capsule, cerebral cortex, brainstem and cerebellum respectively, Figure 2. The incidence of upper limb spasticity varies in different infarction sites (P <0.05). The incidence of upper limb spasticity is the highest in basal ganglia and internal capsule infarctions and is the lowest in epencephal infarction (Table 1).
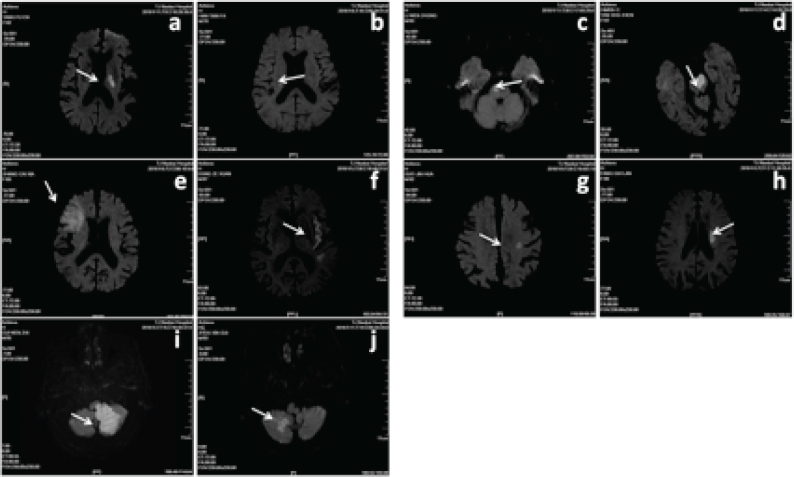
Figure 2.
The MRI diagram of the infarct site of the included patients (a,b: Basal ganglia infarction; c,d: Brainstem infarction; e,f: Cerebral cortical infarction; g,h: Subcutaneous infarction; i,j: Cerebellar infarction)
Table 1.
Relationship between upper limb spasticity and cerebral infarction site
| Infarction site | Incidence (%) | 95%CI of incidence (%) |
|---|---|---|
| Cortical and subcortical mixed areas (n=9l) | 11(12.1) | (4.49, 17.73) |
| Basal ganglia and internal capsule (n=169) | 107(63.3) | (55.43, 69.34) |
| Cerebral cortex (n=82) | 48(58.5) | (49.55, 70.44) |
| Brainstem (n=53) | 5(9.4) | (1.39, 18.61) |
| Cerebellum (n=12) | 1(8.3) | (1.10, 18.90) |
4. Discussions
Post-stroke upper limb spasticity is a challenging condition because physicians experience difficulty in identifying and treating. Epidemiological studies on post-stroke upper limb spasticity in China have been rarely performed. In most studies [1], the incidence of post-stroke upper limb spasticity is 17%–40%. Keng H. Kong et al.[7] conducted a 12-month observation of 163 patients who suffered from cerebral infarction and found that 33%, 43%, and 49% experienced upper limb spasticity in the 3rd, 6th, and 12th months, respectively. They performed another 6-month observation of 328 patients with cerebral infarction and observed that 16% and 43% of these patients suffered from upper limb spasticity in the 3rd and 6th months, respectively [8]. Our results demonstrate that the cumulative incidence of upper limb spasticity after a year of cerebral infarction is 34.5%, which includes 68 patients (39.52%) with Ashworth grade 2, 101 patients (58.7%) with Ashworth grade 3, and patients with Ashworth grade 4 but without severe spasm. The incidence in China is slightly higher than those in other countries possibly because of different sample sizes and observation periods.
The pathogenesis of post-stroke upper limb spasticity is extremely complex, and its occurrence is associated with many factors. Factors related to post-stroke upper limb spasticity have been extensively investigated, and retrospective studies have suggested that the occurrence of upper limb spasticity exhibits a complex relationship with the location and area of lesion, course of disease, degree of neurological deficits, and medical history. These factors constitute the pathophysiological basis of spastic paralysis [9,10,11]. In our present study, we evaluated the post-stroke upper limb spasticity rate of different cerebral infarction site and found that the total incidence of upper limb spasticity is 34.5%. The incidences of upper limb spasticity are 12.1%, 63.3%, 58.5%, 9.4% and 8.3% when cerebral infarction occurs in the cortical and subcortical mixed areas, basal ganglia and internal capsule, cerebral cortex, brainstem and cerebellum respectively. The results indicated that the incidence of upper limb spasticity varies in different infarction sites. The incidence of upper limb spasticity is the highest in basal ganglia and internal capsule infarctions and is the lowest in epencephal infarction. Therefore, patients with basal ganglia and internal capsule infarctions had significant elevated risk of developing post-stroke upper limb spasticity.
Approximately 80% of cases of post-stroke hemiplegia originate from the cortex or internal capsule and basal ganglia damage, and 20% are caused by brain stem damage. This study demonstrates that the occurrence of post-stroke upper limb spasticity is closely related to the site of cerebral infarction but is slightly associated with the area of cerebral infarction. The incidence of upper limb spasticity is 63.3% when cerebral infarction occurs in the basal ganglia, internal capsule, and cortex. Fátima et al. [14] also found that the cerebral infarction in the basal ganglia and internal capsule is closely linked with the occurrence of upper limb spasticity. The incidence of spasm in cortical infarction is lower than that in basal ganglia and internal capsule infarction but is higher than that in cortex and subcortical mixed infarction. Therefore, if infarction arises in the basal ganglia and internal capsule, then the lesion area is small and the recovery of its functional outcome may be fatal.
In summary, this study presents a preliminary understanding on the prevalence of post-stroke upper limb spasticity and its incidence of different cerebral infarction site. Patients with the ganglia and internal capsule infarctions had the highest risk of developing post-stroke upper limb spasticity.
Footnotes
Conflicts of interest: The authors have no conflicts of interest to declare.
References
- [1].Brainin M, Norrving B, Sunnerhagen KS, Goldstein LB, Cramer SC, Donnan GA, Duncan PW, Francisco G, Good D, Graham G, Kissela BM, Olver J, Ward A, Wissel J, Zorowitz R. International PSSDSG. Poststroke chronic disease management: towards improved identification and interventions for poststroke spasticity-related complications. Int J Stroke. 2011;6:42–46. doi: 10.1111/j.1747-4949.2010.00539.x. [DOI] [PubMed] [Google Scholar]
- [2].Wissel J, Schelosky LD, Scott J, Christe W, Faiss JH, Mueller J.. Early development of spasticity following stroke: a prospective, observational trial. J Neurol. 2010;257:1067–1072. doi: 10.1007/s00415-010-5463-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wissel J, Verrier M, Simpson DM, Charles D, Guinto P, Papapetropoulos S, Sunnerhagen KS.. Post-stroke spasticity: predictors of early development and considerations for therapeutic intervention. PM R. 2015;7:60–67. doi: 10.1016/j.pmrj.2014.08.946. [DOI] [PubMed] [Google Scholar]
- [4].Yablon SA, Brin MF, VanDenburgh AM, Zhou J, Garabedian-Ruffalo SM, Abu-Shakra S, Beddingfield FC. Dose response with onabotulinumtoxinA for post-stroke spasticity: a pooled data analysis. Mov Disord. 2011;26:209–215. doi: 10.1002/mds.23426. 3rd. [DOI] [PubMed] [Google Scholar]
- [5].Li S, Francisco GE.. New insights into the pathophysiology of post-stroke spasticity. Front Hum Neurosci. 2015;9:192. doi: 10.3389/fnhum.2015.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ward AB.. A literature review of the pathophysiology and onset of post-stroke spasticity. Eur J Neurol. 2012;19:21–27. doi: 10.1111/j.1468-1331.2011.03448.x. [DOI] [PubMed] [Google Scholar]
- [7].Kong KH, Lee J, Chua KS.. Occurrence and temporal evolution of upper limb spasticity in stroke patients admitted to a rehabilitation unit. Arch Phys Med Rehabil. 2012;93:143–148. doi: 10.1016/j.apmr.2011.06.027. [DOI] [PubMed] [Google Scholar]
- [8].Lundstrom E, Terent A, Borg J.. Prevalence of disabling spasticity 1 year after first-ever stroke. Eur J Neurol. 2008;15:533–539. doi: 10.1111/j.1468-1331.2008.02114.x. [DOI] [PubMed] [Google Scholar]
- [9].Francisco GE, McGuire JR.. Poststroke spasticity management. Stroke. 2012;43:3132–3136. doi: 10.1161/STROKEAHA.111.639831. [DOI] [PubMed] [Google Scholar]
- [10].Urban PP, Wolf T, Uebele M, Marx JJ, Vogt T, Stoeter P, Bauermann T, Weibrich C, Vucurevic GD, Schneider A, Wissel J.. Occurence and clinical predictors of spasticity after ischemic stroke. Stroke. 2010;41:2016–2020. doi: 10.1161/STROKEAHA.110.581991. [DOI] [PubMed] [Google Scholar]
- [11].Lundstrom E, Smits A, Terent A, Borg J.. Time-course and determinants of spasticity during the first six months following first-ever stroke. J Rehabil Med. 2010;42:296–301. doi: 10.2340/16501977-0509. [DOI] [PubMed] [Google Scholar]
- [12].Shelton FN, Reding MJ.. Effect of lesion location on upper limb motor recovery after stroke. Stroke. 2001;32:107–112. doi: 10.1161/01.str.32.1.107. [DOI] [PubMed] [Google Scholar]