Abstract
Arginine is one of the most versatile semi-essential amino acids. Further to the primary role in protein biosynthesis, arginine is involved in the urea cycle, and it is a precursor of nitric oxide. Arginine deficiency is associated with neurodegenerative diseases such as Parkinson’s, Huntington’s and Alzheimer’s diseases (AD). In this study, we administer arginine intracerebroventricularly in a murine model of AD and evaluate cognitive functions in a set of behavioral tests. In addition, the effect of arginine on synaptic plasticity was tested electrophysiologically by assessment of the hippocampal long-term potentiation (LTP). The effect of arginine on β amyloidosis was tested immunohistochemically. A role of arginine in the prevention of cytotoxicity and apoptosis was evaluated in vitro on PC-12 cells. The results indicate that intracerebroventricular administration of arginine improves spatial memory acquisition in 3xTg-AD mice, however, without significantly reducing intraneuronal β amyloidosis. Arginine shows little or no impact on LTP and does not rescue LTP deterioration induced by Aβ. Nevertheless, arginine possesses neuroprotective and antiapoptotic properties.
Keywords: Alzheimer’s disease, L-arginine, spatial memory, amyloid beta, cytotoxicity, apoptosis
Introduction
Alzheimer’s disease is a slowly progressive neurodegenerative disorder with prevalence among elderly people and women (Hebert et al. 2013). The AD is characterized morphologically by diffuse neuritic plaques containing Aβ peptide and neurofibrillary tangles, which are aggregates of hyperphosphorylated tau protein (Schaeffer et al. 2011). The exact cause of AD is unknown, and several studies have proposed that the pathogenesis of AD, at least its sporadic form, is related to inflammatory processes (Heneka and O’Banion 2007), atherosclerosis, redox stress (Sultana and Butterfield 2010), and abnormal brain glucose metabolism (Calsolaro and Edison 2016). Some studies support the hypothesis that AD is correlated with dysfunction of metabolic pathways that are translated into neurological symptoms (Cai et al. 2012), (Craft 2009). Targeting these various metabolic pathways could be used to treat AD.
Current treatment strategies are focusing upon manipulating with cholinergic and glutamatergic neurotransmission (Weinstock et al. 2001). All of them demonstrate a modest clinical success, and, in fact, no effective treatment is available to target the principal mechanism of the AD. We believe that new strategies combining several approaches and targets for the treatment of AD have to be proposed. Such approaches could include an auxiliary intervention into the metabolic pathways and personalized correction of disbalances.
Arginine as a therapeutic agent
L-Arginine has been used for the treatment of various diseases. L-arginine has been shown to stimulate immune responses and promote wound healing (Barbul A, Lazarou SA, Efron DT, Wasserkrug HL 1990). In particular, L-arginine stimulates wound healing and immune function in the elderly (Kirk et al. 1993). Oral supplementation with 17 g doses of arginine for two weeks in the elderly significantly improves positive nitrogen balance. Remarkably, arginine significantly reduced the levels of total serum cholesterol and low-density lipoprotein. No adverse effects were observed at the dosage of 17 g/day of arginine (Hurson et al. 1995). Kirk et al. showed that elderly patients could tolerate an even greater arginine dose of 30 g/day (Kirk et al. 1993).
The L-arginine dietary supplement also improves performance in elderly male cyclists and enhances their exercise capacity (S. Chen et al. 2010). L-arginine dietary supplement attenuates the increased platelet reactivity in hypercholesterolemic patients (Wolf et al. 1997) and prevents atherogenesis (Cooke et al. 1992). Finally, L-arginine dietary supplement reduces restenosis after experimental angioplasty in rabbits (Tarry and Makhoul 1994).
Arginine and its derivatives have also been used for the treatment of neurological disorders. L-arginine administration within 30 minutes of a stroke significantly decreases frequency and severity of stroke-like symptoms (Koga et al. 2005). Remarkably, 1.6 g of L-Arginine supplemented daily for three months in the diet of patients with senile dementia increased cognitive function by about 40% (Ohtsuka and Nakaya 2000). Remarkably, the effect of the drug did not last after the end of the treatment.
Arginine metabolism as a target for treatment of AD
Arginine is a conditionally essential α-amino acid that is used in the biosynthesis of proteins. Arginine possesses a broad spectrum of regulatory functions, which are predicated upon its chemical structure and activity. An overwhelming review of the possible physiological effects of the amino acid upon the development of AD has been done by Yi et al (Yi et al. 2009).
Quite a lot of vital metabolites, (i.e., NO, urea) are derived from it, and their cytoprotective and antioxidant properties are presently well known. The well-marked cationic properties of the guanidine group of arginine contribute to its ability to undergo protonation. It is well established that arginine derivatives and the amino acid itself can regulate peroxidation processes in membranes (Milyutina 1990). Evidently, arginine reacts directly with the superoxide anion-radical that may be a basis for its protective effects under extreme conditions. Additionally, arginine regulates cell division and release of hormones, plays a role in the wounds’ healing and removing of ammonia, and possesses various immune functions (Böger and Bode-Böger 2001), (Tapiero et al. 2002).
Epidemiological studies indicate that daily dietary arginine intake inversely correlates with AD morbidity. The mean intake of arginine among men is >50% greater than within women in accordance with the lower prevalence of AD in men (Mielke, Vemuri, and Rocca 2014) and the finding that about two-thirds of the individuals diagnosed with the AD are women (Hebert et al. 2013). Also, the elderly consume ~30% less arginine compared to 20-40-year-olds (King, Mainous, and Geesey 2008). Additionally, a moderate decrease of arginine level was detected in CSF and plasma of AD patients in some recent studies (Ibanez et al. 2012), (Fonteh et al. 2007).
In the healthy individuals, L-Arginine is transported from the circulating blood into the brain via Na+-independent cationic amino acid transporter (CAT1) expressed at the BBB (O’Kane et al. 2006). It was established that the L-arginine influx transport at the rat BBB is saturable with a Michaelis-Menten constant (Km) value of 56 μM. The physiological serum concentration of L-arginine is significantly higher in the rodents (about 170 μM) and humans (about 100 μM ) (Stoll, Wadhwani, and Smith 1993). Therefore, since L-arginine in mammals is derived mostly from renal de novo synthesis and dietary intake, CAT1 at the BBB functions as a sole supply pathway for L-arginine to the brain (Tachikawa and Hosoya 2011).
Despite the capability of arginine to pass the BBB, the capacity of its transporter is limited (Shin et al. 1985). The limit makes oral administration of arginine insufficient to show all of its possible effects. In the present research, we check the direct effect of arginine upon cognitive functions in a murine model of AD. We bypass the BBB by intraventricular administration of the amino acid and eliminate the effect of the arginine derivatives, which also possess neuroprotective qualities and might be generated in substantial quantities as a reaction to general administration of arginine.
In the present research, we check the direct effect of arginine upon cognitive functions in a murine model of AD. We bypass the BBB by intraventricular administration of the amino acid and eliminate the effect of the arginine derivatives, which also possess neuroprotective qualities and might be generated in substantial quantities as a reaction to general administration of arginine.
Materials and Methods
Mouse strains
There are several animal models of the AD which were created to study the disease. A triple-transgenic mice model of AD (3xTg-AD) exhibits synaptic deficiency with both plaque and tangle pathology (Oddo et al. 2003).
3xTg-AD mice were purchased from the Jackson Laboratory® and bred in our animal facility. Twenty-four age-matched 6.5 months old female mice were used for all experiments.
All experimental protocols were performed in accordance with the instructions of the Israeli Ministry of Health’s Council for Experimentation on Animals and with Bar Ilan University guidelines for the use and care of laboratory animals in research, supervised by the institutional animal care and use committee. The experimental protocol was approved by the Committee on the Ethics of Animal Experiments of the Bar Ilan University (Permit Number: 32 - 08 – 2012).
Before and during the experiment, mice were socially housed in standard plastic cages in humidity (30%) and temperature (22°C) controlled room with a 12-h reverse light/dark cycle. The animals were provided with water and food ad libitum.
The animals were randomly divided into two equal groups (a control group with artificial CSF administration, and an experimental group with L-arginine solution in ACSF).
Drug administration and surgical procedure
L-arginine and saline control solutions were directly administered into the brain lateral ventricles using osmotic minipumps and cannulae. The animals were anesthetized with 2% isoflurane and placed in a stereotaxic apparatus. Anesthesia was maintained with 1% isoflurane for the duration of the surgery. First, a subcutaneous pocket for the Alzet® pump capsule was made with a pair of scissors. The skull of the mice was drilled stereotactically according to the mouse brain atlas with the coordinates: -0.2 mm caudal, 0.9 mm lateral to bregma. Then, a small bent cannula (Alzet®) was slowly lowered stereotactically into the hole and cemented to the skull with Loctite 454 (Alzet®). Once in place, the cannula reaches 2.5 mm in the dorsoventral direction (Sanchez-Mendoza et al. 2016).
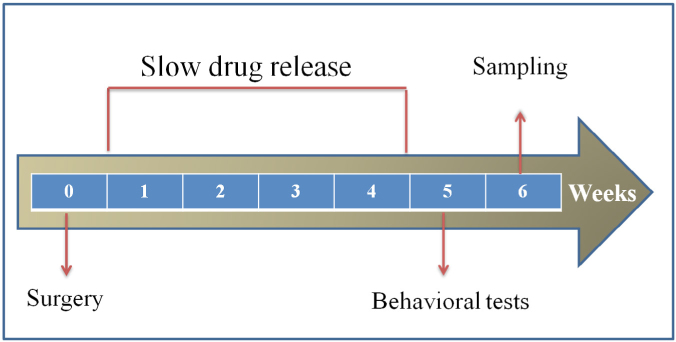
An activated during 24 hours in DDW osmotic minipumps (Alzet, model 1004, 28 days delivery) prefilled with 100 μl of L-arginine (1.148 M, pH 7.3) in ACSF (Ecocyte Bioscience) or ACSF at pH 7.3 have been connected to the cannula via a vinyl catheter tube. The pumps were placed in a spinal subcutaneous pocket prepared at the earlier stage of the operation and stapled (Alzet AutoClip). The timeline of the experiment including treatments and behavioral tests presented in figure 1.
Figure 1.
The timeline of treatments and tests.
Cell culture
Rat pheochromocytoma-derived PC12 cells have similar characteristics of nerve cells and are widely used in various studies neurotoxicity and apoptosis assays (Jung et al. 2008). It has been commonly used in numerous investigations of potential neuroprotective agents to treat neurodegenerative diseases, such as Huntington’s disease, Parkinson’s disease, and AD (Scotter et al. 2010), (Wan et al. 2010).
The cell line (#88022401) was obtained from Sigma®. The cells were maintained in DMEM medium supplemented with 10% fetal bovine serum, 5% horse serum, 100 U/mL penicillin and 100 μg/mL streptomycin at 37 °C in a humidified 95% air/5% CO2 incubator. All cells were cultured in collagen-coated culture dishes. The medium was changed every other day, and cells were plated at an appropriate density according to each experimental scale.
Apoptotic morphology observation by Hoechst 33342 staining
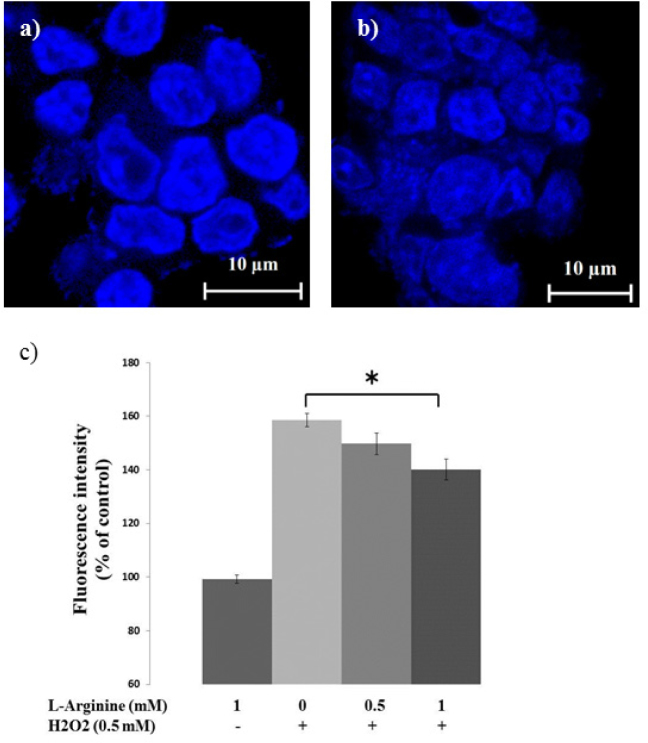
Chromosomal condensation and morphological changes in the nucleus were observed by using the chromatin dye, Hoechst 33342 (Roche®). Cells with homogeneously stained nuclei were considered to be viable. Chromatin condensation or fragmentation indicated apoptosis. Briefly, PC12 cells (1×106) were plated in 6 well plates and cultured on cover slips (pretreated with poly-D-lysine) with three mL of medium in each well. After 24 hours the cells were exposed to 500 μM of H2O2 with various concentrations of L-arginine for additional 24 h. After treatments, the cells were fixed with 4% formaldehyde in PBS for 20 min at room temperature and stained with one μg/mL Hoechst 33342 for 20 min. Cells were photographed under a fluorescent microscope (Olympus, Tokyo, Japan) and quantified by ImageJ 1.51 software. In each image, identical rectangular regions of interest with the selection tool of ImageJ were selected randomly, and the average pixel intensity was measured by the software.
Cell viability assays
Cell viability was assessed by measuring formazan produced by the reduction of MTT. The MTT assay is a sensitive measurement of the normal metabolic status of cells, particularly those of mitochondria, which reflects early cellular redox changes. Therefore, the amount of formazan produced is proportional to the number of viable cells. Briefly, the cells were plated in 96 well poly-d-lysine-coated culture plates at the density of 1 × 104 cells/well at 37 °C for 24 hours with media containing various concentrations of L-arginine and were treated with pre-aggregated during 24 hours 50 μM Aβ(25-35) or 0.5 mM of H2O2. These concentrations of Aβ and H2O2 have been used previously in various studies to check the viability of PC-12 cells (Yao, Drieu, and Papadopoulos 2001), (Heo and Lee 2005). Subsequently, MTT reagent (final concentration, 0.5 mg/mL) was added to each of the wells, and the plate was incubated for three hours at 37 °C. At the end of the incubation, the medium with MTT was removed and 100 μL DMSO was added to each well. The formazan reduction product was measured by reading absorbance at 570 nm in a microplate reader (Infinite® M1000). Cell viability was presented as a percentage of the control culture.
Additionally, trypan blue dye exclusion assay was used to determine the number of viable cells present in a cell suspension. Briefly, PC-12 cells were treated with varying concentrations (0-1 mM) of L-Arginine for 24 hours, and after that, the cells were removed, centrifuged and resuspended in Dulbecco's phosphate buffer saline with trypan blue (1 volume per 4 volumes of the medium (Sigma Chemical Co.)). The viable and nonviable cells were counted on a hemocytometer using an inverted microscope (Olympus, Tokyo, Japan).
Behavioral tests
Morris water maze (MWM)
The maze consisted of a black circular tank with of 120 cm diameter and height of 40 cm. Water was maintained at a temperature of 25°C and rendered opaque by adding skim milk powder (Sigma). A platform submerged 1 cm below the surface was placed in a quadrant of the maze and kept in the same position during all trials. Several visual cues were positioned around the pool. A video camera in conjunction with the EthoVision XT 10 video tracking software (Noldus) was used for the measurement of the time taken to escape to the platform. Four locations around the pool were labeled as north, south, east, and west for a test start position.
The mice were acclimatized to the test room conditions for 30 min before the experiment. Each animal was placed in the pool at one of four locations. The mice were allowed to find the platform within 60 seconds and stay on it for 10 seconds. If a mouse could not escape during the time limit, it was directed gently towards the platform. Each animal received two learning sessions per day with a 15 minutes interval between trials during five successive days.
The probe test without the platform was performed on the sixth day. The mice were subjected to a single trial with free swimming during 60 seconds. The relative time spent in the area that was defined by the software as two platform’s diameters and the number of times the animals crossed the platform zone have been analyzed.
Spontaneous alternation Y-Maze
Spontaneous alternation behavior reflects spatial working memory, which is a form of short-term memory. It was assessed by spontaneous alternation behavior during a single session in the Y-maze. The Y-maze used in this study consisted of three arms (each of 35 cm long, 25 cm high, and 10 cm wide). For all animals, the maze was placed in the same position during the procedure. The mice were placed at the center of the maze and allowed to move freely for 5 min. An arm entry was counted when the hind paws of the mouse were completely within the arm. The maze was cleaned with a 10% aqueous ethanol solution between each trial. Spontaneous alternation behavior was defined as the entry into all three arms on consecutive choices. The number of maximum spontaneous alternation behaviors was calculated as the total number of arms entered minus 2. The percentage of spontaneous alternation was calculated as [actual alternations] / [total number of alternations] × 100. A video camera in conjunction with the EthoVision XT 10 video tracking software (Noldus) was used for recording the behavior.
Immunostaining
Five animals from each group were sacrificed and perfused with ice-cold 4% PFA. The brain was taken and fixated in 4% PFA for immunohistochemical analysis. Then they were sliced on a sliding microtome to produce 30-micron floating sections. These sections were blocked for one hour in blocking solution containing 10% horse serum, 0.3% triton and undiluted phosphate-buffered saline (PBS). The sections were then incubated overnight with primary antibodies 6E10 (1:150 ENCO), at room temperature, followed by washing and incubation for an hour with secondary antibodies Alexa488 (1:200, Thermo Fisher Scientific), and Hoechst (1:5000, Sigma). Anti-Aβ antibody (6E10) was purchased from ENCO.
Imaging and quantification
The number of Aβ deposits throughout the hippocampi (3-6 sections examined per mouse) was quantified. Imaging was done using a Nikon Eclipse E600 microscope equipped with a 40× objective and the acquisition software NIS-Elements AR (Nikon Instruments). Microscopy images were analyzed using the open source software ImageJ (http://imagej.nih.gov/ij/) with the plugin from Wright Cell Imaging Facility. The average of the mean intensity of the staining was obtained for each hippocampal subfield, and the background was subtracted before calculating the reciprocal values.
Antibody microarray analyses
The evaluation of “hit” proteins’ expression or phosphorylation of specific residues on these proteins was performed by the use of the Kinex KAM-880 Antibody Microarray (Kinexus Bioinformatics Corp., Vancouver, B.C.), in accordance with the manufacturer specification. The analyses were done with hippocampal lysates of mice treated with arginine and ASCF as described on Kinexus’ web page (www.kinexus.ca). Briefly, lysate protein from each sample (100 μg) was labeled covalently with a fluorescent dye combination. Free dye molecules have been then removed via gel filtration. After blocking non-specific binding sites on the array, an incubation chamber was mounted onto the microarray to permit the loading of 2 samples (one arginine treated, and one ACSF treated). After the incubation, unbound proteins were washed away. Two 16-bit images have been captured by a ScanArray Reader (Perkin-Elmer) for each array. Then, microarray data were analyzed using a web server for functional interpretation of gene lists (http://biit.cs.ut.ee/gprofiler), and a list of priority genes was generated. Finally, Cytoscape software was applied for the topological analysis and network visualization of the priority genes.
Electrophysiology
Male six months old C57BL/6 mice were anesthetized with isoflurane and decapitated. Brains were quickly removed and submerged in ice-cold dissection solution (concentrations in mM: 124 NaCl, 3 KCl, 1.25 NaH2PO4, 26 NaHCO3, 1.3 CaCl2, 7 MgCl2, and 10 D-glucose, pH equilibrated, with 95% O2 – 5% CO2). Transverse hippocampal slices (350μm thick) were prepared using a vibratome (Leica VT1000S, Germany) and immediately transferred to a recording solution (composition as above, except the CaCl2 and MgCl2 concentrations, were adjusted to 2.5 and 1.3, respectively). Slices were heated to 36°C in water bath for 40 min and then kept at room temperature. Amyloid β peptide (25-35) was dissolved in mQ water to 1 mM stock solution and frozen. 24 h before the experiment, the Aβ peptide was dissolved to 50 nM in ACSF and incubated at +4°C for oligomerization. Slices were incubated for 1 hour with control ACSF, one mM or 100 μM of L-arginine or mixture of 50 nM Aβ (25-35) and L-arginine before transferring to the recording chamber. During the experiments, slices were perfused by a continuously flowing (appr. 4 ml/min) recording solution at 32-33°C. Electrophysiological recordings were carried out using SliceMaster system (Scientifica, UK). Field excitatory postsynaptic potentials (fEPSP) were recorded from stratum radiatum in area CA1 using glass microelectrodes (1-2 MΩ) filled with the recording solution. Baseline synaptic responses were evoked by paired-pulse stimulation with 50 ms interval of the Schaffer collaterals at 0.033 Hz with a bipolar electrode. Test stimulation intensity was adjusted to evoke fEPSP with amplitude 50% of maximal and was kept constant throughout the experiment. LTP was induced with four 100-Hz trains spaced 5 min apart. The data were recorded and analyzed by Spyke2 and SigmaPlot. For statistical analysis, the latest 5 minutes (116-120 min after LTP induction) were used. For baseline responses analysis fiber volley amplitudes and appropriate fEPSP slopes during test stimulation were evaluated. PPF ratio was calculated as PPF (S2EPSP/S1EPSP), where S1EPSP and S2EPSP are the slopes of EPSP in response to the first and the second stimuli with different intervals, respectively. PPF measures were carried out just before and after LTP recordings.
Statistical analysis
Statistical analyses were conducted using SPSS 22.0 for Windows. All results are presented as mean with standard error. Escape latency during the training was determined by repeated-measures ANOVA with the session as a within-subject factor and treatment as a between-subjects factor. In the probe trial, Student’s t-test for latency and two-way ANOVA with treatment-time in quadrants as between-subject factors were applied. One-way ANOVA was used to determine the significant differences between the groups followed by a Dunnett’s t-test for multiple comparisons. The results are considered significant when p < 0.05. All values are expressed as means ± SEM.
Each in vitro experiment consisted of three separate plates from the same culture which were averaged. Each test was replicated for two more times and the results presented as means ± SEM of three independent experiments. The statistical analyses were done using oneway ANOVA followed by Student–Newman–Keuls multiple comparison tests. Statistical significance was drawn at p < 0.05.
Results
Administration of L-arginine during one month significantly improved memory acquisition in 3xTg-AD mice
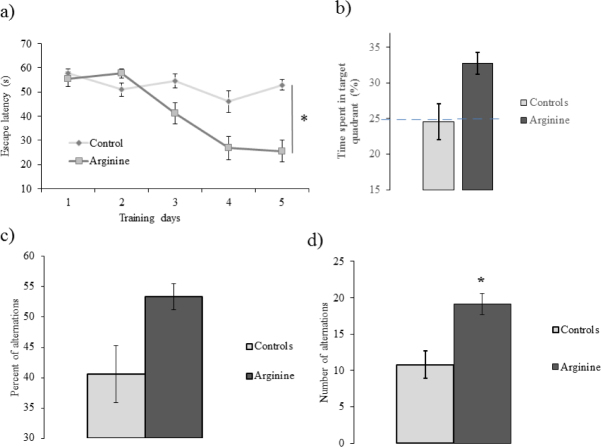
The learning curve of the mice from the experimental group reflects gradually declining escape latency pattern across the trials (Figure 2a). On the day with probe tests, the animals from experimental group spent about 33 % of the time in the target quadrant, which was significantly better than results shown by the controls (Figure 2b). The mice which were injected with ACSF did not demonstrate the results above chance (i.e., 25%), which accords with the current literature (Niikura et al. 2011). Figures 2 (c, d) depict spontaneous alternation results in Y-maze. Both number and percentage of alternations indicate that mice treated with L-arginine show significantly better spatial working memory than control animals.
Figure 2.
Assessment of long and short-term memory acquisition. (a) MWM test learning curves are showing escape latencies during five testing days (n=12 for each group). (b) The percent time spent in the target quadrant on the probe trial day. The mice injected with arginine spent significantly more time in the learning phase target quadrant than the controls. Spontaneous alternation in Y-maze. (c) A total number of alternations. (d) Percentage of alternation. Values are shown as the mean ± s.e.m. ∗ p < 0.05 (n=12 for each group).
Administration of L-arginine has no significant impact on the rate of Aβ deposition
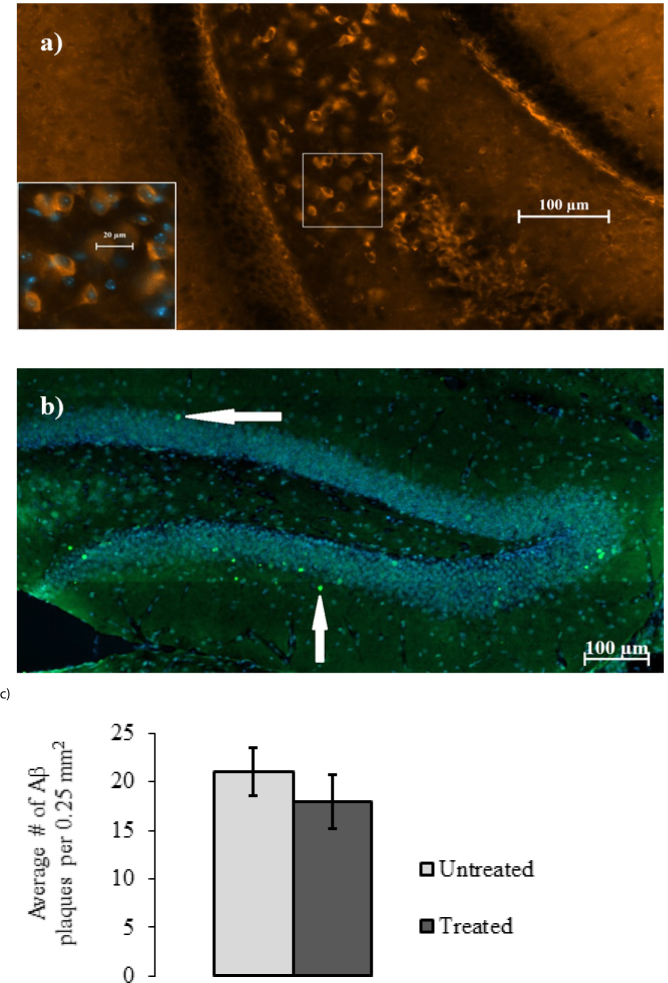
Immunohistochemistry was employed to detect deposits of intra- and extraneuronal Aβ in the hippocampi of brain slices from treated (n=5) and untreated (n=5) 3xTg-AD mice. Multiple intracellular deposits of amyloid beta and scarce plaques have been detected in the cortices, amygdala, and hippocampi. There were no significant differences in the amount of amyloid beta deposition between two groups (figure 3).
Figure 3.
Immunofluorescence and quantification of senile amyloid plaques in the hippocampal areas. a) A typical 20 x magnification image of the dentate gyrus from treated with L-arginine mouse with visible Aβ deposits. The inset shows a high magnification (40 x) merged with DAPI view with Aβ deposits. b) A typical 10x magnification image of the hippocampus from treated with L-arginine mouse. Scarce extracellular Aβ plaques are visible (arrows show the amyloid plaques in the brain section). No differences are seen between treated and untreated mice as shown by quantification of the hilus intraneuronal Aβ loads. c) Quantification of Aβ levels is presented with bar graphs of mean pixel counts per dentate gyrus ±SEM.
L-arginine treatment induces cellular pathways involved in neuroprotection including oxidative stress protection, and defense response
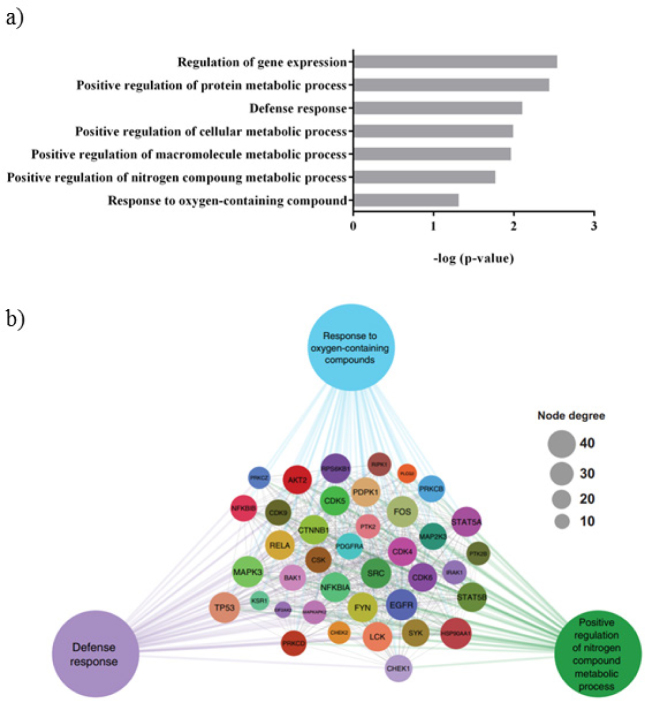
KAM 880 antibody microarray analysis reveals that treatment of 3xTG-AD mice with L-arginine leads to upregulation of several critical biological processes, including stress defense, response to oxygen-containing compounds, and metabolism of nitrogen compounds (figure 4a).
Figure 4.
Highlighted enriched terms and associated differentially expressed proteins. a) L-Arginine treatment induces multiple metabolic pathways using DAVID .b) STRING was used to build a physical and functional protein-protein association network in Cytoscape for differentially expressed proteins involved in a subset of the highlighted terms. Node degrees represent the amount of interactions per protein.
There are 65 regulatory proteins which demonstrate significant changes in L-Arginine treated animals compared to ACSF treated mice. Among them, 19 are considered as priority leads with ≥90% change from control (ACSF treated) and 46 as possible leads with ≥60% change (supplementary table 1).
In order to visualize molecular interaction networks and biological pathways, Cytoscape was applied (http://www.cytoscape.org). To link the differential changes in protein expression and the changes in network connectivity to biological pathways we built a functional map (figure 4b).
L-Arginine mitigates hydrogen peroxide-induced apoptosis in cultured PC-12 cells
Morphological nuclear changes and rate of apoptosis in PC12 cells treated with 500 μM of H2O2 and L-arginine at different concentrations were assessed by use of Hoechst 33258 staining. Alterations of nuclear morphology characterized by condensed and fragmented nuclei were considered to be markers of apoptosis (figure 5). Treatment of PC12 with H2O2 leads to severe nuclear damage and alterations of the structure. Chromatin of the cells cultured with no arginine (figure 5a) appears to be much more condensed compared to the cells cultured with one mM of L-arginine(figure 5b). Cells being treated with H2O2 for 24 h exhibited nuclear chromatin aggregation and apoptotic bodies. Arginine mitigates these morphological changes of the nuclei, which was consistent with the MTT results in Fig. 6.
Figure 5.
L-arginine protected PC12 cells against H2O2-induced apoptosis (apoptotic morphology observation by Hoechst 33342). Morphological analysis of nuclear chromatin in a) no arginine, b) 1 mM of L-arginine. The figures are representatives of a set of identical experiments repeated three times. c) Quantification of DAPI fluorescence intensity.
Figure 6.
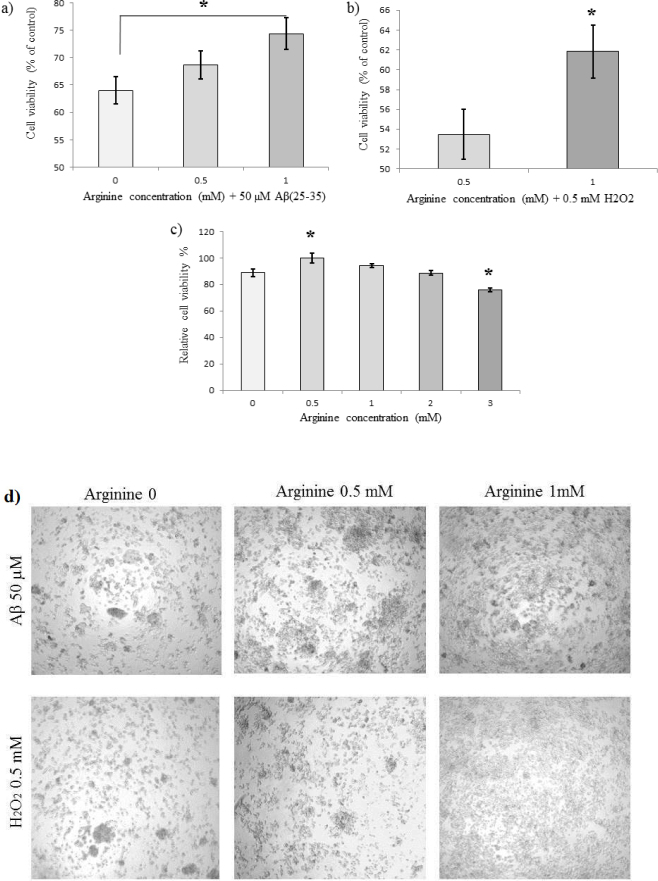
Effects of different concentrations of L-arginine in the medium on the viability and morphology of PC12 cells treated with 50 μM of Aβ(25-35) or 0.5 mM of H2O2. (a) Effect of Aβ(25-35) on the viability of PC12 cells (exposure to 24 h). (b) Effect of 0.5 mM H2O2 on the viability of PC12 cells (exposure to 24 h). MTT assay demonstrates that arginine protects cells against Aβ(25-35) and H2O2 toxicity in a concentration-dependent manner; (c) Cytoprotective and cytotoxic effects of various concentrations of L-Arginine (trypan blue test)(d) treatments of PC-12 cells with Aβ(25-35) and H2O2 in the media containing different concentrations of L-arginine induced morphological alteration, which seem to be dose-dependent; ∗ p < 0.05, ∗∗ p < 0.01, ∗∗∗ p < 0.001.
Figure 7.
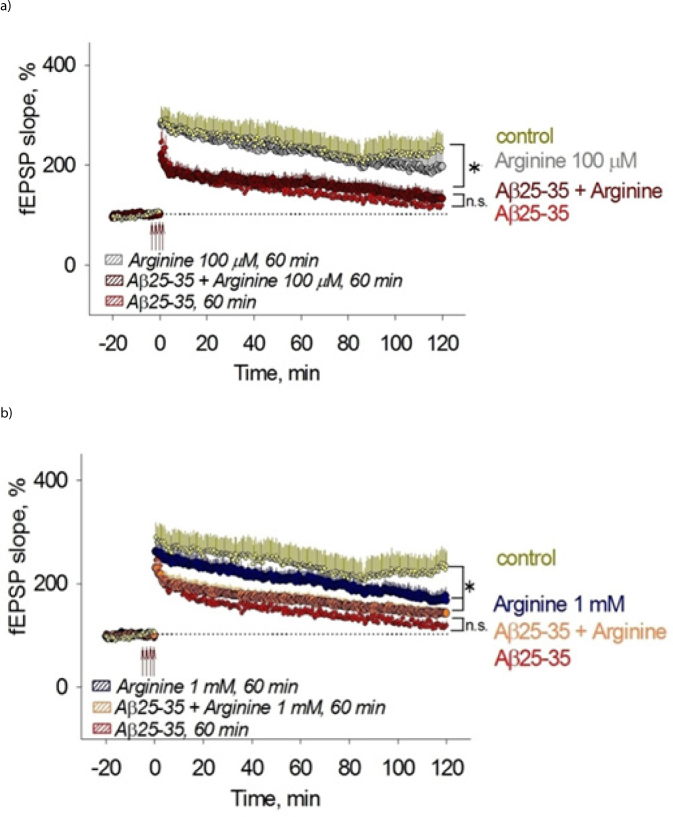
Effect of a) 100 μM and b) 1mM of L-arginine and Aα(25-35) on LTP induction.
The rate of cell apoptosis in the plates with no arginine was significantly higher than that in the experimental plates.
L-arginine protects cells against Aβ(25-35) and Hydrogen peroxide-induced toxicity in a concentration-dependent manner
To study the effect of L-arginine on cell viability in the Aβ(25-35) or H2O2-stressed cultured PC12 cells, the cells were incubated with various concentrations of arginine and 50 μM of Aβ(25-35) or 0.5 mM H2O2. The treatments induced morphological dose-dependent alteration which can be observed under the light microscope (Figure 6d). The relative rate of induced cell death was evaluated by MTT and trypan blue assays. As shown in Figure 6b, PC12 cells exposed for 24 hours to 50 μM of aged Aβ(25-35) in the media containing L-arginine exhibit significantly enhanced viability. Incubation of the cells with 0.5 mM H2O2 for 24 hours leads to deterioration of viability rate, which can be reversed by an increase in arginine concentration up to one mM. Remarkably, arginine itself demonstrates cytotoxic properties in concentrations greater than one mM (figure 6c), which accords with the current literature (Todoroki et al. 1998). It was demonstrated that high concentrations (more than one mM) of L-Arginine induce severe DNA damage and arrest at the GI phase. Moreover, it was approved that ROS are produced in cells exposed to high (about two mM) of L-arginine. Our data are in agreement with these results. Consequently, we show that L-arginine reduces the cytotoxic effect of 50 μM Aβ(25-35) and 0.5 mM H2O2 in a dose-dependent manner.
L-Arginine does not rescue impaired by Aβ hippocampal long-term potentiation
Our results demonstrate that high concentrations (about one mM) of L-arginine significantly deteriorate hippocampal LTP (figure 6b). Medium containing 50 nM of Aβ suppresses generation of LTP (figure 6 a,b). Administration of aggregated Aβ (25-35) significantly decreased fEPSP slope and PS amplitude in Aβ group compared to the control group; though, did not affect baseline activity of the neurons.
Inhibition of LTP induction by aged amyloid beta and particularly Aβ (25--35) without affecting the basal synaptic transmission and post-tetanic potentiation was demonstrated previously in various studies (Q. S. Chen et al. 2000).
We evidence that the presence of low concentrations of L-arginine in the medium containing Aβ or without it does not influence significantly fEPSP slope and PS amplitude; however, high concentrations of L-arginine have a severe deteriorative effect upon LTP induction.
Discussion
Amino acids play an essential role in neuronal signaling and energy supply. The correct balance of amino acids is critical for normal neuronal functioning. Therefore, deviations in their metabolism may influence the neurodegenerative processes. Postmortem brains of AD patients demonstrate various alterations in the level of amino acids, and a moderate decrease of arginine level was detected in the CSF and plasma (Ibanez et al. 2012). Therefore, we hypothesize that arginine plays a role in the pathogenesis of the AD and may be used to treat the disease.
Neurons are strongly dependent upon oxidative phosphorylation as an energy source compared to other cells and highly vulnerable to oxidative stress (Shetty, Galeffi, and Turner 2012). As a general rule, the oxidative stress increases during aging (Finkel and Holbrook 2000). In the course of the progression of age-related neurodegeneration and, especially, with the progression of AD, the capacity of neurons to maintain the redox balance severely declines, which leads to the mitochondrial dysfunction, accumulation of free radicals, and neuronal injury (X. Chen, Guo, and Kong 2012). Moreover, it was shown in transgenic mouse models of AD and in ex vivo experiments using postmortem brain tissue taken from AD patients that Aβ deposits directly associated with free-radical generation (Mclellan et al. 2003). Therefore, it was hypothesized that increased oxidative damage is a primary cause of AD pathogenesis (Perry et al. 2002).
There is a consensus that L-arginine can protect neurons against oxidative stress via exerting its antioxidant potentials. Furthermore, Kan et al. recently have shown on a novel rodent model that the development of AD symptoms is associated with a significant reduction of global arginine bioavailability and an intervention into arginine metabolism via inhibition of ornithine decarboxylase protects the animals from AD-like pathology and improves cognitive functions in mice (Kan et al. 2015). It was demonstrated on different models that L-arginine enhances resistance against oxidative stress. The substance extends the lifespan of C. elegans under both oxidative and heat stress and possesses free radical scavenging ability (Ma et al. 2016). Arginine is the immediate precursor of nitric oxide (NO). It was shown by way of different cell cultures that NO may serve as an antioxidant agent, which protects cells from damage caused by ROS. There are suggestions that the mechanism for protection by NO is the interception of ROS and metallo-oxo species generated by NO (Wink et al. 1993).
Our primary intent in the current research was to study the biochemical roles of L-arginine in the development of AD. The use of arginine was motivated by its involvement in diverse physiological and pathological processes, including cellular redox metabolism, inflammation, regulation of cerebral blood flow, and neuroplasticity.
Despite the capability of L-arginine to pass the BBB, the capacity of its transporter is limited (Shin et al. 1985). In order to circumvent the BBB and eliminate possible effects of arginine derivatives by peripheral metabolism, the substance was administered intraventricularly. So, we test the direct cognitive effects of arginine and its metabolites in the brain of 3xTg mice.
Our results elucidate some controversies of the “arginine paradox,” the term, that has been used to denote the phenomenon in which L-arginine administration drives NOS activity and improves NO-mediated functions in vivo even when L-arginine is excessively available (Kurz and Harrison 1997). The baseline plasma concentration of arginine is about 25-fold higher than the Michaelis-Menten constant (Km) of the isolated eNOS in vitro (Bode-Böger, Scalera, and Ignarro 2007), however, L-arginine supplement does affect NOS activity. The intracellular physiological concentration of arginine is about several hundred micromoles per liter, which far exceeds the 5 micromoles Km for eNOS, nevertheless, the exogenous arginine still escalates NO production (Dioguardi 2011).
Arginine metabolism is a multifarious and extremely interregulated physiological process, which is highly sensitive to the bioavailability of the amino acid. Exogenous arginine supplementation has been confirmed to improve status in a long list of diseases, particularly, among the elderly people. Our results validate a significant improvement of behavioral function in the 3xTg-AD mouse model, without altering the amyloid aspects of AD neuropathology. To our knowledge, we provide the very first demonstration that chronic intraventricular administration of L-arginine can improve short and long-term memory acquisition.
We prove, that the cognitive effect of L-arginine administration is not related to the reduction of amyloid plaques formation or facilitation of neuroplasticity (LTP), but associated with cytoprotective and antiapoptotic potentials of arginine. Application of antibody microarray reveals various cellular pathways involved in neuroprotection that were induced by L-arginine treatment. Amplified response to oxygen-containing compounds, positive regulation of nitrogen compound metabolic pathways and defense response are the most critical, in our opinion, amongst them.
We have tested our hypothesis in vitro on the PC-12 cellular model. We evidence the neuroprotective effect of arginine against H2O2 and Aβ(25-35) induced toxicity on PC12 cells and demonstrate that arginine itself in high doses possesses cytotoxic properties.
The results support the conclusion that L-arginine protects neurons against Aβ cytotoxicity and reduces the rate of apoptosis. Our work confirms an intriguing therapeutic role of L-arginine in the development of AD as a potent metabolic agent interfering with redox system and reducing apoptosis. We believe that our research should aid in the rational development of therapeutic agents for the intervention in the course of various relevant human diseases.
Author contributions
Gennadiy Fonar and Baruh Polis were involved in all the aspects of the work. Tomer Meirson performed the bioinformatic analysis. Alexander Maltsev performed the electrophysiology experiments. Evan Elliott supervised parts of the experiments. Avraham O. Samson conceived and designed the experiments.
Statements
All experimental protocols were approved by the Faculty of Medicine, Bar Ilan University ethics committee, ethics’ protocol number 32 - 08 – 2012. All methods were carried out in accordance with relevant guidelines and regulations.
Study Funding
This research was supported by a Marie Curie CIG grant 322113, a Leir foundation grant, a Ginzburg family foundation grant, and a Katz foundation grant to AOS. Electrophysiological experiments were supported by Russian Science Foundation (RSF; grant no. 14-25-00072).
References
- [1].Barbul A, Lazarou SA, Efron DT, Wasserkrug HL, Efron G.. “Arginine Enhances Wound Healing and Lymphocyte Immune Response in Humans.”. Surgery. 1990;108(2):336–37. [PubMed] [Google Scholar]
- [2].Bode-Böger Stefanie M., Fortunato Scalera, Louis J. Ignarro. “The L-Arginine Paradox: Importance of the L-Arginine/asymmetrical Dimethylarginine Ratio.”. Pharmacology and Therapeutics. 2007;114(3):295–306. doi: 10.1016/j.pharmthera.2007.03.002. [DOI] [PubMed] [Google Scholar]
- [3].Böger Rainer H, Stefanie M Bode-Böger. “The Clinical Pharmacology of L-Arginine.”. Annu. Rev. Pharmacol. Toxicol. 2001;41:79–99. doi: 10.1146/annurev.pharmtox.41.1.79. [DOI] [PubMed] [Google Scholar]
- [4].Cai Huan, Wei-na Cong, Sunggoan Ji, Sarah Rothman, Stuart Maudsley, Bronwen Martin. “Metabolic Dysfunction in Alzheimer’s Disease and Related Neurodegenerative Disorders.”. Current Alzheimer Research. 2012;9(1):5–17. doi: 10.2174/156720512799015064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Calsolaro Valeria, Paul Edison. “Alterations in Glucose Metabolism in Alzheimer’s Disease.”. Recent Patents on Endocrine, Metabolic & Immune Drug Discovery. 2016;10(1):31–39. doi: 10.2174/1872214810666160615102809. [DOI] [PubMed] [Google Scholar]
- [6].Chen Q S, Kagan B L, Hirakura Y, Xie C W. “Impairment of Hippocampal Long-Term Potentiation by Alzheimer Amyloid Beta-Peptides.”. Journal of Neuroscience Research. 2000;60(1):65–72. doi: 10.1002/(SICI)1097-4547(20000401)60:1<65::AID-JNR7>3.0.CO;2-Q[pii]. [DOI] [PubMed] [Google Scholar]
- [7].Chen Steve, Woosong Kim, Susanne M Henning, Catherine L Carpenter, Zhaoping Li.. “Arginine and Antioxidant Supplement on Performance in Elderly Male Cyclists: A Randomized Controlled Trial.”. Journal of the International Society of Sports Nutrition. 2010;7(1):13. doi: 10.1186/1550-2783-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chen Xueping, Chunyan Guo, Jiming Kong. “Oxidative Stress in Neurodegenerative Diseases.”. Neural Regeneration Research. 2012 doi: 10.3969/j.issn.1673-5374.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cooke John P., Alan H. Singer, Philip Tsao, Pauline Zera, Reed A. Rowan, Margaret E. Billingham. “Antiatherogenic Effects of L-Arginine in the Hypercholesterolemic Rabbit.”. Journal of Clinical Investigation. 1992;90(3):1168–72. doi: 10.1172/JCI115937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Craft S.. “The Role of Metabolic Disorders in Alzheimer Disease and Vascular Dementia: Two Roads Converged.”. Arch Neurol. 2009;66(3):300–305. doi: 10.1001/archneurol.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dioguardi Francesco Saverio. “To Give or Not to Give? Lessons from the Arginine Paradox.”. Journal of Nutrigenetics and Nutrigenomics. 2011 doi: 10.1159/000327777. [DOI] [PubMed] [Google Scholar]
- [12].Finkel T, Holbrook N J. “Oxidants, Oxidative Stress and the Biology of Ageing.”. Nature. 2000;408(6809):239–47. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- [13].Fonteh A. N., Harrington R. J., Tsai A., Liao P., Harrington M. G.. “Free Amino Acid and Dipeptide Changes in the Body Fluids from Alzheimer’s Disease Subjects.”. Amino Acids. 2007;32(2):213–24. doi: 10.1007/s00726-006-0409-8. [DOI] [PubMed] [Google Scholar]
- [14].Hebert L E, Weuve P A Scherr, Evans D A. “Alzheimer Disease in the United States (2010-2050) Estimated Using the 2010 Census.”. Neurology. 2013;80(19):1778–83. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Heneka Michael T., Kerry O’Banion M.. “Inflammatory Processes in Alzheimer’s Disease.”. Journal of Neuroimmunology. 2007 doi: 10.1016/j.jneuroim.2006.11.017. [DOI] [PubMed] [Google Scholar]
- [16].Heo H J, Lee C Y. “Strawberry and Its Anthocyanins Reduce Oxidative Stress-Induced Apoptosis in PC12 Cells.”. J Agric Food Chem. 2005;53(6):1984–89. doi: 10.1021/jf048616l. [DOI] [PubMed] [Google Scholar]
- [17].Hurson M, Regan M., Kirk S., Wasserkrug H., Barbul A. “Metabolic Effects of Arginine in a Healthy Elderly Population.”. Journal of Parenteral and Enteral Nutrition. 1995;19(3):227–30. doi: 10.1177/0148607195019003227. [DOI] [PubMed] [Google Scholar]
- [18].Ibanez C, Simo C, Martin-Alvarez P J, Kivipelto M, Winblad B, Cedazo-Minguez A, Cifuentes A. “Toward a Predictive Model of Alzheimer’s Disease Progression Using Capillary Electrophoresis-Mass Spectrometry Metabolomics.”. Anal.Chem. 2012;84(20):8532–40. doi: 10.1021/ac301243k. [DOI] [PubMed] [Google Scholar]
- [19].Jung Ji Yeon, Kwang Hoon Roh, Yeon Jin Jeong, Sun Hun Kim, Eun Ju Lee, Min Seok Kim, Won Mann Oh, Hee Kyun Oh, Won Jae Kim. “Estradiol Protects PC12 Cells against CoCl2-Induced Apoptosis.”. Brain Research Bulletin. 2008;76(6):579–85. doi: 10.1016/j.brainresbull.2008.04.006. [DOI] [PubMed] [Google Scholar]
- [20].Kan Matthew J., Jennifer E. Lee, Joan G. Wilson, Angela L. Everhart, Candice M. Brown, Andrew N. Hoofnagle, Marilyn Jansen, Michael P. Vitek, Michael D. Gunn, Carol A. Colton. “Arginine Deprivation and Immune Suppression in a Mouse Model of Alzheimer’s Disease.”. The Journal of Neuroscience. 2015;35(15):5969–82. doi: 10.1523/JNEUROSCI.4668-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].King Dana E., Arch G. Mainous, Mark E. Geesey. “Variation in L-Arginine Intake Follow Demographics and Lifestyle Factors That May Impact Cardiovascular Disease Risk.”. Nutrition Research. 2008;28(1):21–24. doi: 10.1016/j.nutres.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kirk S J, Hurson M, Regan M C, Holt D R, Wasserkrug H L, Barbul a. “Arginine Stimulates Wound Healing and Immune Function in Elderly Human Beings.”. Surgery. 1993;114(2):155–159. doi: 10.1177/0148607194018006559. discussion 160. [DOI] [PubMed] [Google Scholar]
- [23].Koga Y., Akita Y., Nishioka J., Yatsuga S., Povalko N., Tanabe Y., Fujimoto S., Matsuishi T.. “L-Arginine Improves the Symptoms of Strokelike Episodes in MELAS.”. Neurology. 2005;64(4):710–12. doi: 10.1212/01.WNL.0000151976.60624.01. [DOI] [PubMed] [Google Scholar]
- [24].Kurz S., Harrison D. G.. “Insulin and the Arginine Paradox.”. Journal of Clinical Investigation. 1997 doi: 10.1172/JCI119166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ma Heran, Yudan Ma, Zhixian Zhang, Ziyuan Zhao, Ran Lin, Jinming Zhu, Yi Guo, Li Xu.. “L-Arginine Enhances Resistance against Oxidative Stress and Heat Stress in Caenorhabditis Elegans.”. International Journal of Environmental Research and Public Health. 2016;13(10) doi: 10.3390/ijerph13100969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mclellan Megan E, Stephen T Kajdasz, Bradley T Hyman, Brian J Bacskai. “In Vivo Imaging of Reactive Oxygen Species Specifically Associated with Thioflavine S-Positive Amyloid Plaques by Multiphoton Microscopy.”. Journal of Neuroscience. 2003;23(6):2212–17. doi: 10.1523/JNEUROSCI.23-06-02212.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mielke Michelle M., Prashanthi Vemuri, Walter A. Rocca. “Clinical Epidemiology of Alzheimer’s Disease: Assessing Sex and Gender Differences.”. Clinical Epidemiology. 2014 doi: 10.2147/CLEP.S37929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Milyutina NP.. “Antiradical and Antioxidant Effect of Arginine and Its Action on Lipid Peroxidation in Hypoxia.”. Bulletin of Experimental Biology and Medicine. 1990;110(9):263–65. [PubMed] [Google Scholar]
- [29].Niikura Takako, Elkhansa Sidahmed, Chiho Hirata-Fukae, Paul S. Aisen, Yasuji Matsuoka. “A Humanin Derivative Reduces Amyloid Beta Accumulation and Ameliorates Memory Deficit in Triple Transgenic Mice.”. PLoS ONE. 2011;6(1) doi: 10.1371/journal.pone.0016259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Oddo Salvatore, Antonella Caccamo, Jason D. Shepherd, Paul Murphy M., Todd E. Golde, Rakez Kayed, Raju Metherate, Mark P. Mattson, Yama Akbari, Frank M. LaFerla. “Triple-Transgenic Model of Alzheimer’s Disease with Plaques and Tangles: Intracellular Aβ and Synaptic Dysfunction.”. Neuron. 2003;39(3):409–21. doi: 10.1016/S0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- [31].Ohtsuka Y, Nakaya J. “Effect of Oral Administration of L-Arginine on Senile Dementia.”. American Journal of Medicine. 2000;108(5):2000. doi: 10.1016/s0002-9343(99)00396-4. [DOI] [PubMed] [Google Scholar]
- [32].Perry George, Marta a Taddeo, Akihiko Nunomura, Xiongwei Zhu, Tania Zenteno-Savin, Kelly L Drew, Shun Shimohama, Jesús Avila, Rudolph J Castellani, Mark a Smith. “Comparative Biology and Pathology of Oxidative Stress in Alzheimer and Other Neurodegenerative Diseases: Beyond Damage and Response.”. Comparative Biochemistry and Physiology. Toxicology & Pharmacology: CBP. 2002;133(4):507–13. doi: 10.1016/s1532-0456(02)00119-9. S1532045602001199. [pii] [DOI] [PubMed] [Google Scholar]
- [33].Sanchez-Mendoza Eduardo H., Jeismar Carballo, Marines Longart, Dirk M. Hermann, Thorsten R. Doeppner. “Implantation of Miniosmotic Pumps and Delivery of Tract Tracers to Study Brain Reorganization in Pathophysiological Conditions.”. Journal of Visualized Experiments. 2016;107:1–9. doi: 10.3791/52932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Schaeffer Evelin L, Micheli Figueiró, Wagner I, Gattaz F. “Insights into Alzheimer Disease Pathogenesis from Studies in Transgenic Animal Models.”. Clinics. 2011;66(S1):45–54. doi: 10.1590/S1807-59322011001300006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Scotter E L, Goodfellow C E, Graham E S, Dragunow M, Glass M. “Neuroprotective Potential of CB1 Receptor Agonists in an in Vitro Model of Huntington’s Disease.”. British Journal of Pharmacology. 2010;160(3):747–61. doi: 10.1111/j.1476-5381.2010.00773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Shetty Pavan K., Francesca Galeffi, Dennis A. Turner. “Cellular Links between Neuronal Activity and Energy Homeostasis.”. Frontiers in Pharmacology. 2012 3 Mar. doi: 10.3389/fphar.2012.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Shin W., Fong W., Pang S., Wong P.. “Limited Blood Brain Barrier Transport of Polyamines.”. Journal of Neurochemistry. 1985;44(4):1056–59. doi: 10.1111/j.1471-4159.1985.tb08724.x. [DOI] [PubMed] [Google Scholar]
- [38].Sultana Rukhsana, Allan Butterfield D. “Role of Oxidative Stress in the Progression of Alzheimer’s Disease.”. Journal of Alzheimer’s Disease : JAD. 2010;19(1):341–53. doi: 10.3233/JAD-2010-1222. [DOI] [PubMed] [Google Scholar]
- [39].Tapiero H, Mathé G, Couvreur P, Tew K D. “I. Arginine.”. Biomedicine & Pharmacotherapy = Biomédecine & Pharmacothérapie. 2002;56(9):439–45. doi: 10.1016/S0753-3322(02)00284-6. [DOI] [PubMed] [Google Scholar]
- [40].Tarry Wallace C, Raymond G Makhoul. “L-Arginine Improves Endothelium-Dependent Vasorelaxation and Reduces Intimal Hyperplasia After Balloon Angioplasty.”. Arteriosclerosis, Thrombosis, and Vascular Biology. 1994;14(6):938–43. doi: 10.1161/01.atv.14.6.938. [DOI] [PubMed] [Google Scholar]
- [41].Todoroki S, Goto S, Urata Y, Komatsu K, Sumikawa K, Ogura T, Matsuda I, Kondo T. “High Concentration of L-Arginine Suppresses Nitric Oxide Synthase Activity and Produces Reactive Oxygen Species in NB9 Human Neuroblastoma Cells.”. Molecular Medicine (Cambridge, Mass.) 1998;4(8):515–24. [PMC free article] [PubMed] [Google Scholar]
- [42].Wan J., Fu A. K. Y., Ip F. C. F., Ng H. K., Hugon J., Page G., Wang J. H., Lai K. O., Wu Z., Ip N. Y.. “Tyk2/STAT3 Signaling Mediates -Amyloid-Induced Neuronal Cell Death: Implications in Alzheimer’s Disease.”. Journal of Neuroscience. 2010;30(20):6873–81. doi: 10.1523/JNEUROSCI.0519-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Weinstock M, Kirschbaum-Slager N, Lazarovici P, Bejar C, Youdim M B, Shoham S. “Neuroprotective Effects of Novel Cholinesterase Inhibitors Derived from Rasagiline as Potential Anti-Alzheimer Drugs.”. Annals of the New York Academy of Sciences. 2001;939:148–61. doi: 10.1111/j.1749-6632.2001.tb03622.x. http://www.ncbi.nlm.nih.gov/pubmed/11462767 [DOI] [PubMed] [Google Scholar]
- [44].Wink D a, Hanbauer I, Krishna M C, DeGraff W, Gamson J, Mitchell J B. “Nitric Oxide Protects against Cellular Damage and Cytotoxicity from Reactive Oxygen Species.”. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(21):9813–17. doi: 10.1073/pnas.90.21.9813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wolf Andreas, Christoff Zalpour, Gregor Theilmeier, Bing Yin Wang, Adrian Ma, Barbara Anderson, Philip S. Tsao, John P. Cooke. “Dietary L-Arginine Supplementation Normalizes Platelet Aggregation in Hypercholesterolemic Humans.”. Journal of the American College of Cardiology. 1997;29(3):479–85. doi: 10.1016/S0735-1097(97)00523-8. [DOI] [PubMed] [Google Scholar]
- [46].Yao Z, Drieu K, Papadopoulos V. “The Ginkgo Biloba Extract EGb 761 Rescues the PC12 Neuronal Cells from B-Amyloid-Induced Cell Death by Inhibiting the Formation of B-Amyloid-Derived Diffusible Neurotoxic Ligands.”. Brain Res. 2001;889:181–90. doi: 10.1016/s0006-8993(00)03131-0. [DOI] [PubMed] [Google Scholar]
- [47].Yi Jing, Laura L. Horky, Avi L. Friedlich, Ying Shi, Jack T. Rogers, Xudeng Huang. “L-Arginine and Alzheimer’s Disase.”. International Journal of Clinical and Experimental Pathology. 2009;2(3):211–38. [PMC free article] [PubMed] [Google Scholar]