Abstract
Background: Childhood metabolic syndrome (MetS) is associated with insulin resistance and increased risk for later development of type 2 diabetes (T2DM) and cardiovascular disease (CVD). In using MetS severity z-scores, our objective was to assess longitudinal associations in MetS severity, fasting insulin levels as a sign of insulin resistance and risk for T2DM, and uric acid levels as a biomarker of oxidative stress leading to CVD.
Methods: We used linear regression to analyze longitudinal data from 285 white and black participants from the Bogalusa Heart Study evaluated at baseline at ages 5–19 and as young adults after a mean of 12.0 years follow-up. We assessed correlations between childhood MetS severity and young-adult MetS severity, fasting insulin, and uric acid levels, both overall and by sex- and racial subgroups.
Results: Overall, childhood MetS z-scores were positively associated with young-adult MetS z-scores (r = 0.52), insulin (r = 0.34), and uric acid (r = 0.28) (all P < 0.001). These associations were consistent across all sex- and racial subgroups, except for young adult uric acid in white males in which childhood MetS-z was not associated (r = 0.15, P = 0.243). There was a strong cross-sectional association of young-adult MetS z-scores with insulin (r = 0.70) and uric acid (r = 0.57) (both P < 0.001), which was consistent for all sex- and racial subgroups.
Conclusions: These positive longitudinal correlations between childhood MetS z-scores and markers of later insulin resistance and oxidative stress suggest long-term durability of risk for CVD and T2DM. This suggests potential for MetS severity to serve as an indicator to monitor for future risk of T2DM and CVD.
Keywords: : metabolic syndrome, cardiovascular disease, type 2 diabetes, insulin resistance, oxidative stress, risk
Introduction
The childhood obesity epidemic is widespread, with the World Health Organization (WHO) reporting that in 2013 more than 42 million children were overweight and obese around the world.1 Obesity, particularly central or abdominal obesity, is a major risk factor for the metabolic syndrome (MetS), which is associated with insulin resistance and results in increased risk of cardiovascular disease (CVD) and type 2 diabetes (T2DM).2–4 MetS appears to have underpinnings of cellular dysfunction, systemic inflammation, and oxidative stress.5,6
MetS is classically defined in criteria such as those of the National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP-III), by the presence of three or more of the following abnormalities: increased waist circumference, elevated blood pressures, elevated triglyceride levels, low HDL, and increased fasting glucose levels.7 These criteria have been adapted for use in pediatrics by replacing waist circumference with BMI z-score8 and adjusting cutoff values for the other components to be more appropriate for levels seen among adolescents.9 However, these traditional MetS criteria have limitations both in known racial/ethnic discrepancies10–12 and in providing only a binary estimate of the presence of MetS.
We developed a MetS-severity z-score using confirmatory factor analysis on cross-sectional data from adolescents from the National Health and Nutrition Examination Survey (NHANES) to give differential weighting to the various components and additionally address variations in rates of MetS between different sexes and races/ethnicities.13 As a marker of disease risk, adolescent MetS severity z-scores were associated with later CVD14,15 and diabetes15,16 as adults. In cross-sectional studies, this adolescent MetS z-score was correlated with markers of CVD and diabetes risk,13,17–19 as well as with markers associated with the processes underlying MetS, including C-reactive protein as a marker of inflammation and uric acid as a marker of oxidative stress.13 However, prior assessments have been limited in assessing these longitudinal relationships by sex- and racial/ethnic subgroup and in assessment of longitudinal measures of MetS and its underlying pathophysiology. Our goal in the current study was to assess longitudinal associations between childhood MetS severity z-scores and future young-adult levels of MetS severity (as an assessment of the score's durability over time) and levels of fasting insulin and uric acid (as markers of insulin resistance and underlying oxidative stress), both overall and on a sex- and race-specific basis. We hypothesized that we would see temporal associations of childhood MetS with later markers and that these relations would be similar in subpopulations of sex and race.
Methods & Statistical Analysis
Participants were evaluated as part of the Bogalusa Heart Study, a longitudinal cohort of individuals in the area surrounding Bogalusa, Louisiana. Assessments occurred between September 1973 and December 1996, with seven cross-sectional surveys of children and adolescents (aged 5–19 years) and a follow-up survey as young adults (aged 18–38 years), who had participated earlier as children and remained accessible. Enrollment was performed on a rolling basis, and for the current study, the baseline visit was defined as being the first in which all MetS components were present. Participants with missing data regarding MetS components, either in childhood/adolescence (defined for this analysis as age 5–19 years) or young adulthood, were excluded from the analysis.
Before assessment, participants were instructed to fast for 12 hrs before the visit, with compliance ascertained the morning of the examination. Height and weight were measured twice to within 0.1 cm and within 0.1 kg, respectively, and the mean values were used to calculate BMI. Replicate blood pressure measurements were obtained on the right arm of the participants in a relaxed sitting position. Arm measurements, length and circumference, were made during the examination to ensure proper cuff size. Systolic and diastolic blood pressure levels were obtained on the right arm of the participants in a relaxed sitting position using mercury sphygmomanometers. Blood pressure levels were reported as the mean of six replicate readings, taken by each of two randomly assigned and trained observers blinded to each other's readings. Lipid and glucose measurements were assessed from frozen serum samples in childhood and young adulthood,20 and insulin21 and uric acid22 were assessed in young adults. We obtained data through the BioLINC of the National Heart, Lung and Blood Institute (https://biolincc.nhlbi.nih.gov/home).
MetS z-scores were calculated using sex and race-specific algorithms using participants' systolic blood pressure, triglycerides, HDL, fasting glucose, and a measure of adiposity, BMI z-score, and waist circumference among adolescents and young adults, respectively. Methods for derivation of the scores were previously described (http://mets.health-outcomes-policy.ufl.edu/calculator/).10,13 These formulae were derived from US nationally-representative data from non-Hispanic white, non-Hispanic black, and Hispanic adolescents aged 12–19 years and adults aged 20–64 years participating in NHANES 1999–2010, using confirmatory factor analysis on sex- and racial/ethnic subgroups to produce differential weights for the five MetS components based on how MetS was manifest in each group. Separate formulae were derived among adolescents and adults. The presence of MetS as a dichotomous variable was defined for adults by the National Cholesterol Education Program's Adult Treatment Panel III (NCEP ATP-III)7 criteria and for adolescents as adaptation of criteria.9 Participants were classified as having ATP-III MetS if they had ≥3 of the following abnormalities: BMI z-score ≥1.645, fasting glucose ≥100 mg/dL, fasting triglycerides ≥110 mg/dL, HDL ≤40 mg/dL for men or ≤50 mg/dL for women, and systolic or diastolic blood pressure exceeding the 90th percentile for height, age, and sex.23 Diabetes was determined by fasting glucose >125 mg/dL.
Statistics
Fasting insulin levels were adjusted on a natural log scale to normalize the values for comparison. Homeostasis model of insulin resistance (HOMA-IR) was calculated as HOMA-IR = (fasting insulin x fasting glucose)/405.24 Pearson's r correlations were calculated to assess the degree of linear association between childhood and young-adult values. Linear associations between childhood MetS z-scores and young-adult MetS z-scores, natural log of fasting insulin levels [ln(insulin)], and serum uric acid levels were similarly assessed. Correlations were also calculated to evaluate linear associations between young adult MetS z-scores and ln(insulin), as well as uric acid levels. Statistical significance was set to α = 0.05. Data were also analyzed by sex and race/ethnicity, organized into subpopulations of white males, white females, black males, and black females. As a sensitivity analysis we evaluated correlations between factors among those with follow-up ≥5 years.
Results
We analyzed data from 285 participants of the Bogalusa Heart Study with MetS z-scores calculated at baseline and follow-up (Table 1). Of these, 188 individuals were white, and the other 97 were black. One hundred eleven participants, 38.9%, were men. Participants' ages ranged from 5 to 19 years with a mean of 13.3 ± 2.9 years. Sixty eight participants were ages 5–11 years at baseline, while the other 217 participants were ages 12–19 years. Ages at follow-up ranged from 20 to 37 years with a mean of 25.3 ± 4.9 years. The number of years to follow-up time ranged from 2 to 21 years with a mean of 12.0 years. No children or adolescents had diabetes at presentation, while 5 had developed diabetes by follow-up as young adults.
Table 1.
Participant Characteristics (N = 285)
| Child visit | Adult follow-up visit | |
|---|---|---|
| Age | 13.3 ± 2.9 | 25.3 ± 4.9 |
| Male | 111 (38.9%) | 111 (38.9%) |
| White | 188 (66.0%) | 188 (66.0%) |
| ATP-III MetS | 9 (3.2%) | 40 (14.0%) |
| Income | ||
| <$15,000 | — | 111 (38.9%) |
| $15,001–$30,000 | — | 71 (24.9%) |
| >$30,000 | — | 68 (23.9%) |
| Unknown | — | 35 (12.3%) |
| MetS z-score | −0.3 ± 0.7 | −0.6 ± 0.9 |
| Glucose | 86.9 ± 7.8 | 79.6 ± 11.9 |
| HDL | 50.0 ± 11.2 | 47.3 ± 11.9 |
| (ln) Triglycerides | 4.2 ± 0.4 | 4.5 ± 0.6 |
| SBP | 105.4 ± 10.5 | 111.2 ± 10.0 |
| BMI-Z | 0.4 ± 1.2 | — |
| Waist circumference | — | 86.4 ± 17.2 |
| Uric acid | — | 4.6 ± 1.5 n = 280 |
| Insulin | — | 13.9 ± 14.1 n = 274 |
| HOMA-IR | 2.9 ± 3.3 n = 274 |
|
MetS by traditional criteria over time
We evaluated the durability of MetS over time according to traditional ATP-III criteria by comparing the prevalence of MetS at baseline in childhood versus presence at follow-up (Table 2). The prevalence of ATP-III MetS increased between childhood and adulthood, being present in only 9 participants at baseline and 40 at follow-up. There was notable lack of durability, in that three of the nine with MetS at baseline no longer had MetS at follow-up. Overall in our study of 285 patients, the sensitivity of baseline ATP-III MetS in predicting early young adult MetS is 15.0%, while the specificity was 98.8%.
Table 2.
ATP-III MetS Prevalence Between Childhood and Young Adults
| Young adult | Childhood | |||
|---|---|---|---|---|
| ATP-III MetSastatus | n (%) | MetS z-scores Mean (95% CI) | ATP-III MetS status | n (%) |
| No | 245 (86.0) | −0.42 (−0.50 to −0.33) | No | 242 (98.8b) |
| Yes | 3 (1.2) | |||
| Yes | 40 (14.0) | 0.35 (0.15–0.55) | No | 34 (85.0) |
| Yes | 6 (15.0c) | |||
Presence of ATP-III MetS according to presence of ≥3 of the following abnormalities: BMI z-score ≥1.645, fasting glucose ≥100 mg/dL, fasting triglycerides ≥110 mg/dL, HDL ≤40 mg/dL for males and ≤50 for females, and systolic or diastolic blood pressure exceeding the 90th percentile for height, age, and sex.
Specificity of childhood ATP-III at predicting young adult ATP-III.
Sensitivity of childhood ATP-III at predicting young adult ATP-III.
MetS severity over time
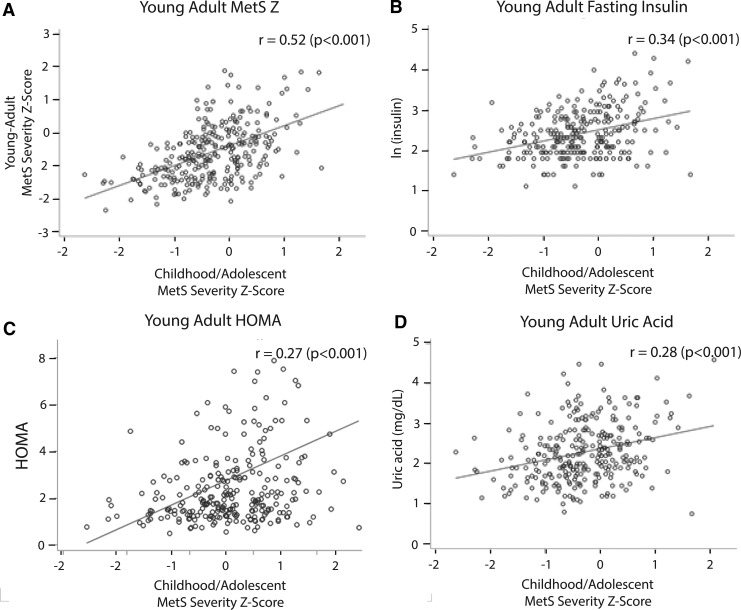
MetS severity in childhood was low, with a mean of −0.3 ± 0.7 (Table 1). MetS severity in young adulthood was very low −0.6 ± 0.9. These low MetS z-scores in young adulthood are likely due to the participants' relatively young age of 20–37, whereas the z-score itself is based on US adults age of 20–65 years, including older individuals with age-related increases in metabolic abnormalities. Childhood MetS z-scores were positively correlated with MetS z-scores as young adults (r = 0.522, P < 0.001) (Fig. 1). This was true for all sex- and racial subgroups, which ranged in r's from 0.404 for black females to 0.726 for black males, all P < 0.01 (Table 3). Because these MetS z-scores were originally derived among adolescents aged 12–19 years, whereas the analytic cohort is composed of children aged 5–19 years, we also evaluated correlation between childhood MetS Z and young adult MetS Z for children aged 5–11 years to assess the validity of these scores in this age group. This r was 0.537, P < 0.0001, demonstrating long-term validity, even in this younger age group.
FIG. 1.
Long-term associations of childhood MetS severity. Scatterplots of childhood MetS z-scores with measures as young adults (mean 12.0 years later) of (A) MetS severity, (B) natural-log fasting insulin, (C) homeostasis model of insulin resistance (HOMA-IR), and (D) uric acid.
Table 3.
Childhood and Young Adult Correlations with Young Adult: MetS, Insulin, and Uric Acid by Sex and Race
| Overall | White males | White females | Black males | Black females | |
|---|---|---|---|---|---|
| Childhood MetS z-scorea | |||||
| MetS z-score | N = 285 | N = 68 | N = 120 | N = 43 | N = 54 |
| 0.522 | 0.436 | 0.541 | 0.726 | 0.404 | |
| P < 0.0001 | P = 0.0002 | P < 0.0001 | P < 0.0001 | P = 0.0024 | |
| ln(Insulin) | N = 274 | N = 66 | N = 114 | N = 42 | N = 52 |
| 0.338 | 0.356 | 0.331 | 0.334 | 0.381 | |
| P < 0.0001 | P = 0.0033 | P = 0.0003 | P = 0.0304 | P = 0.0053 | |
| HOMA-IR | N = 274 | N = 66 | N = 114 | N = 42 | N = 52 |
| 0.268 | 0.453 | 0.349 | 0.304 | 0.183 | |
| P < 0.0001 | P = 0.0001 | P = 0.0001 | P = 0.0500 | P = 0.1950 | |
| Uric acid | N = 280 | N = 67 | N = 119 | N = 40 | N = 54 |
| 0.283 | 0.145 | 0.351 | 0.544 | 0.312 | |
| P < 0.0001 | P = 0.2432 | P < 0.0001 | P = 0.0003 | P = 0.0215 | |
| Young adult MetS z-scoreb | |||||
| ln(Insulin) | N = 274 | N = 66 | N = 114 | N = 42 | N = 52 |
| 0.7000 | 0.741 | 0.770 | 0.715 | 0.729 | |
| P < 0.0001 | P < 0.0001 | P < 0.0001 | P < 0.0001 | P < 0.0001 | |
| HOMA-IR | N = 274 | N = 66 | N = 114 | N = 42 | N = 52 |
| 0.557 | 0.665 | 0.723 | 0.686 | 0.558 | |
| P < 0.0001 | P < 0.0001 | P < 0.0001 | P < 0.0001 | P < 0.0001 | |
| Uric acid | N = 280 | N = 67 | N = 119 | N = 40 | N = 54 |
| 0.565 | 0.428 | 0.646 | 0.708 | 0.5000 | |
| P < 0.0001 | P = 0.0003 | P < 0.0001 | P < 0.0001 | P = 0.0001 | |
Longitudinal correlations between childhood MetS z-scores and young adult z-scores, natural-log-transformation of fasting insulin levels, and uric acid levels with results shown in r values by subgroups of sex and race/ethnicity.
Cross-sectional correlations between young adult z-scores and natural-log-transformation of fasting insulin levels, as well as uric acid levels with results shown in r values by subgroups of sex and race/ethnicity.
HOMA-IR, homeostasis model of insulin resistance.
MetS severity versus insulin and uric acid
Childhood MetS severity z-scores correlated positively with natural-log-transformed levels of young adult fasting insulin (r = 0.338, P < 0.0001) (Fig. 1). This relationship was very consistent across sex- and racial subgroups; correlations ranged from 0.331 to 0.381 (all P < 0.05) (Table 3). Not surprisingly, cross-sectional association of young adult MetS with young adult insulin was much higher than for childhood MetS with young adult insulin (0.700, P < 0.0001). This association between young adult MetS and insulin was very consistent across sex- and racial subgroups, with correlations ranging from 0.715 to 0.770 (all P < 0.0001). Results were similar in evaluating HOMA-IR (Fig. 1 and Table 3).
Childhood MetS z-scores were significantly correlated with young adult levels of uric acid (r = 0.283; P < 0.0001) (Fig. 1). In comparing subpopulations organized by sex and race, the same relationship held true that childhood MetS z-scores were positively correlated with young adult uric acid levels, and these correlations were statistically significant for all groups except for white males (Table 3). As expected, cross-sectional correlation between young adult MetS severity and uric acid was relatively higher (r = 0.565, P < 0.0001) compared to longitudinal correlation; this correlation was weakest in white males (r = 0.428, P = 0.0003) and strongest in black males (r = 0.708, P < 0.0001) (Table 3).
In sensitivity analyses, none of the long-term associations between MetS severity (childhood or young-adult) and young adult levels of insulin, HOMA-IR, or uric acid was affected by restricting the analysis to those with ≥5 years follow-up interval (data not shown).
Discussion
While childhood MetS as assessed using classical criteria has been established to be correlated with future CVD and T2DM,3,8 we found, as has been previously reported,25,26 that there was overall low durability of ATP-III MetS between childhood and young adulthood, with 33% of individuals with childhood MetS (n = 9) reverting to no-MetS (n = 3) over an interval of 12 years. By contrast, we found a relatively high correlation of a MetS severity z-score between childhood and young adulthood in this biracial cohort—as had been suggested previously for individual components27 and ATP-III MetS criteria.8 Furthermore, MetS z-scores were correlated with future levels of surrogate markers of processes associated with MetS, including fasting levels of insulin as a surrogate of insulin resistance and uric acid as a biomarker of oxidative stress. This study was particularly unique in demonstrating (1) long-term associations between childhood MetS and later oxidative stress, (2) long-term associations between childhood MetS z-scores among children aged 5–11 years, for which the MetS z-score was not previously validated, and (3) the strength of associations by sex- and racial subgroup. These long-term connections suggest either shared persistent underpinnings of obesity and unhealthy lifestyle practices and/or genetic tendencies toward insulin resistance, oxidative stress, and MetS. Whichever be the cause of these connections, we noticed not surprisingly that adult MetS severity, in cross-sectional analysis, was even more closely linked. Overall, these data support a durable state of elevated MetS severity and other pathophysiological processes between childhood and young adulthood, suggesting utility in screening for MetS severity during childhood to trigger earlier intervention and prevention of CVD and T2DM.
In considering the strength of associations between factors, it is perhaps not surprising that the strongest association was between childhood MetS severity and young adult MetS severity, suggesting that whatever processes drive the abnormalities in MetS may be similar within individuals and persist over time.8 The next strongest longitudinal association was between childhood MetS and future fasting insulin, as we had noted in a prior cohort.15 Since its original description, MetS has been associated with insulin resistance.28 This concept is further confirmed by the strong cross-sectional association between young adult MetS severity scores and insulin. The longitudinal associations between childhood MetS severity and young adult uric acid were the weakest as were the young adult cross-sectional association with uric acid,29 potentially underscoring either that not all elevations in uric acid are related to oxidative stress or that oxidative stress is one of the multiple processes that contribute to MetS.17
The MetS severity z-scores that we used were derived by sex and racial subgroup because of prior data suggesting differences in how MetS manifests in various populations.30,31 In particular, non-Hispanic black males are classified as having MetS at a lower rate compared to other subgroups32 despite having high risk for T2DM33 and death from CVD34—two outcomes heavily associated with MetS. In analyzing the data by sex and race in the current study, childhood MetS z-scores were significantly correlated with young adult MetS in each of the sex- and racial subgroups, with the highest degree of association interestingly being among black males. Among the four sex- and racial subgroups, there was a remarkable homogeneity of strength of association of longitudinal associations of childhood MetS severity and later insulin (all groups with r's around 0.35) and of cross-sectional associations of young adult MetS severity and insulin (all groups with r's around 0.72). This supports the theory that the MetS severity score operates similarly as an estimate of underlying insulin resistance for each group.
We did notice a difference by sex- and racial subgroup in correlations between MetS severity and uric acid with significant longitudinal associations among white females, black males, and black females. By contrast, among white males, uric acid levels were poorly correlated with childhood MetS z-scores and less associated with adult MetS z-scores compared to those of the other subgroups. This was in some ways surprising because in a prior cross-sectional study using NHANES data, we found that while white male adolescents had the highest levels of uric acid of all sex- and racial subgroups, they also had the tightest link between ATP-III MetS and uric acid.17 Another study showed a relative lower degree of correlation between uric acid levels and MetS-associated variables for non-Hispanic white females compared to that of non-Hispanic white males.29 However, we had not previously evaluated linear associations between MetS severity z-scores on a sex- and race/ethnicity basis and will need to evaluate this more closely in other cohorts to assess whether this lower association among white males and uric acid levels is seen elsewhere.
Limitations to these analyses include study exclusion criteria. We lacked childhood HDL cholesterol levels among a large majority of Bogalusa Heart Study participants, limiting our ability to assess childhood MetS severity to a relatively small analytic sample. However, we did not note overall differences in associations with adult MetS severity scores, insulin levels, or uric acid levels among those with and without childhood HDL measures, suggesting that the lack of HDL measures did not affect these relationships. In addition, we lacked data regarding use of medications that may have affected fasting insulin, lipids, or inflammatory processes at the time of collecting blood samples during follow-up and may have influenced the relationships that we noted. Finally, we were unable to determine whether incident diabetes between childhood and adulthood (n = 5) reflected Type 1 or Type 2 diabetes. While we assume these diabetes cases to have been predominantly Type 2 in nature, we note that any cases of Type 1 diabetes by adulthood may bias us against finding associations between young childhood MetS z-scores and adult MetS z-scores, as we would not have necessarily expected high childhood MetS z-scores among individuals who later developed autoimmune Type 1 diabetes. However, this study also had several strengths, including longitudinal follow-up with a mean of 12.0 years of a biracial cohort with data on current metabolic states.
In conclusion, we found persistent longitudinal correlations between childhood MetS severity and later measures of insulin resistance and oxidative stress. These data and others support the potential for childhood MetS z-scores to be used as an indicator for future risk of atherosclerotic disease and T2DM. Future longitudinal studies should include determination of thresholds of metabolic risk based on childhood MetS severity and assessment of whether lifestyle changes and/or medication use among individuals with higher childhood MetS z-scores alter their long-term CVD and T2DM risk profile.
Acknowledgment
This work was supported by National Institutes of Health grant 1R01HL120960 (MJG and MDD).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.World_Health_Organization. Interim report of the Commission on Ending Childhood Obesity. Geneva, Switzerland: World Health Organization; 2015 [Google Scholar]
- 2.Tzou WS, Douglas PS, Srinivasan SR, et al. Increased subclinical atherosclerosis in young adults with metabolic syndrome: The Bogalusa Heart Study. J Am Coll Cardiol 2005;46:457–463 [DOI] [PubMed] [Google Scholar]
- 3.Morrison JA, Friedman LA, Gray-McGuire C. Metabolic syndrome in childhood predicts adult cardiovascular disease 25 years later: The Princeton Lipid Research Clinics follow-up study. Pediatrics 2007;120:340–345 [DOI] [PubMed] [Google Scholar]
- 4.Bao W, Srinivasan SR, Wattigney WA, et al. Persistence of multiple cardiovascular risk clustering related to syndrome X from childhood to young adulthood. The Bogalusa Heart Study. Arch Intern Med 1994;154:1842–1847 [PubMed] [Google Scholar]
- 5.DeBoer MD. Obesity, systemic inflammation, and increased risk for cardiovascular disease and diabetes among adolescents: A need for screening tools to target interventions. Nutrition 2013;29:379–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Ferranti S, Mozaffarian D. The perfect storm: Obesity, adipocyte dysfunction, and metabolic consequences. Clin Chem 2008;54:945–955 [DOI] [PubMed] [Google Scholar]
- 7.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome - An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005;112:2735–2752 [DOI] [PubMed] [Google Scholar]
- 8.Morrison JA, Friedman LA, Wang P, et al. Metabolic syndrome in childhood predicts adult metabolic syndrome and type 2 diabetes mellitus 25 to 30 years later. J Pediatr 2008;152:201–206 [DOI] [PubMed] [Google Scholar]
- 9.Ford ES, Li C, Cook S, et al. Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation 2007;115:2526–2532 [DOI] [PubMed] [Google Scholar]
- 10.Gurka MJ, Lilly CL, Oliver MN, et al. An examination of sex and racial/ethnic differences in the metabolic syndrome among adults: A confirmatory factor analysis and a resulting continuous severity score. Metab Clin Exp 2014;63:218–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeBoer MD, Gurka MJ. Low sensitivity for the metabolic syndrome to detect uric acid elevations in females and non-Hispanic-black male adolescents: An analysis of NHANES 1999–2006. Atherosclerosis 2012;220:575–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeBoer MD. Underdiagnosis of metabolic syndrome in non-hispanic black adolescents: A call for ethnic-specific criteria. Curr Cardiovasc Risk Rep 2010;4:302–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gurka MJ, Ice CL, Sun SS, et al. A confirmatory factor analysis of the metabolic syndrome in adolescents: An examination of sex and racial/ethnic differences. Cardiovasc Diabetol 2012;11:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol 2010;56:1113–1132 [DOI] [PubMed] [Google Scholar]
- 15.DeBoer MD, Gurka MJ, Morrison JA, et al. Inter-relationships between the severity of metabolic syndrome, insulin and adiponectin and their relationship to future type 2 diabetes and cardiovascular disease. Int J Obes (Lond) 2016;40:1353–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeBoer MD, Gurka MJ, Woo JG, et al. Severity of the metabolic syndrome as a predictor of type 2 diabetes between childhood and adulthood: The Princeton Lipid Research Cohort Study. Diabetologia 2015;58:2745–2752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee AM, Gurka MJ, DeBoer MD. Correlation of metabolic syndrome severity with cardiovascular health markers in adolescents. Metabolism 2017;69:87–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee AM, Fermin CR, Filipp SL, et al. Examining trends in prediabetes and its relationship with the metabolic syndrome in US adolescents, 1999–2014. Acta Diabetol 2017;54:373–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fermin CR, Lee AM, Filipp SL, et al. Serum alanine aminotransferase trends and their relationship with obesity and metabolic syndrome in United States Adolescents, 1999–2014. Metab Syndr Relat Disord 2017;15:276–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li S, Chen W, Srinivasan SR, et al. Childhood cardiovascular risk factors and carotid vascular changes in adulthood: The Bogalusa Heart Study. JAMA 2003;290:2271–2276 [DOI] [PubMed] [Google Scholar]
- 21.Jiang X, Srinivasan SR, Webber LS, et al. Association of fasting insulin level with serum lipid and lipoprotein levels in children, adolescents, and young adults: The Bogalusa Heart Study. Arch Intern Med 1995;155:190–196 [PubMed] [Google Scholar]
- 22.Agamah ES, Srinivasan SR, Webber LS, et al. Serum uric acid and its relation to cardiovascular disease risk factors in children and young adults from a biracial community: The Bogalusa Heart Study. J Lab Clin Med 1991;118:241–249 [PubMed] [Google Scholar]
- 23.Lee AM, Gurka MJ, DeBoer MD. Trends in metabolic syndrome severity and lifestyle factors among adolescents. Pediatrics 2016;137:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 25.Gustafson JK, Yanoff LB, Easter BD, et al. The stability of metabolic syndrome in children and adolescents. J Clin Endocrinol Metab 2009;94:4828–4834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li C, Ford ES, Huang TTK, et al. Patterns of change in cardiometabolic risk factors associated with the metabolic syndrome among children and adolescents: The Fels Longitudinal Study. J Pediatr 2009;155:S5.e9-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frontini MG, Srinivasan SR, Berenson GS. Longitudinal changes in risk variables underlying metabolic Syndrome X from childhood to young adulthood in female subjects with a history of early menarche: The Bogalusa Heart Study. Int J Obes Relat Metab Disord 2003;27:1398–1404 [DOI] [PubMed] [Google Scholar]
- 28.Wilson PW, D'Agostino RB, Parise H, et al. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation 2005;112:3066–3072 [DOI] [PubMed] [Google Scholar]
- 29.DeBoer MD, Dong L, Gurka MJ. Racial/ethnic and sex differences in the relationship between uric acid and metabolic syndrome in adolescents: An analysis of National Health and Nutrition Survey 1999–2006. Metabolism 2012;61:554–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker SE, Gurka MJ, Oliver MN, et al. Racial/ethnic discrepancies in the metabolic syndrome begin in childhood and persist after adjustment for environmental factors. Nutr Metab Cardiovasc Dis 2012;22:141–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeBoer MD, Gurka MJ, Sumner AE. Diagnosis of the metabolic syndrome is associated with disproportionately high levels of high-sensitivity C-reactive protein in non-hispanic black adolescents: An analysis of NHANES 1999–2008. Diabetes Care 2011;34:734–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park YW, Zhu S, Palaniappan L, et al. The metabolic syndrome: Prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med 2003;163:427–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Narayan KM, Boyle JP, Thompson TJ, et al. Lifetime risk for diabetes mellitus in the United States. JAMA 2003;290:1884–1890 [DOI] [PubMed] [Google Scholar]
- 34.Yusuf S, Reddy S, Ounpuu S, et al. Global burden of cardiovascular diseases: Part II: Variations in cardiovascular disease by specific ethnic groups and geographic regions and prevention strategies. Circulation 2001;104:2855–2864 [DOI] [PubMed] [Google Scholar]