Abstract
Significance: Under physiological conditions, neurons and glia are in a healthy, redox-balanced environment; when injury perturbs this equilibrium, a neuroinflammatory state is established by activated microglia that triggers pro-inflammatory responses and alters the oxidant/antioxidant balance, thus leading to neuronal loss and neurodegeneration. In neurodegenerative diseases (such as Alzheimer's disease, Parkinson's disease, amyothrophic lateral sclerosis, and multiple sclerosis), the brain is in a constitutively self-sustaining cycle of inflammation and oxidative stress that prompts and amplifies brain damage.
Recent Advances: Recently, an increasing amount of scientific data highlight the ability of specific nutrients to cross the blood-brain barrier, and to modulate inflammatory and oxidative pathways. Therefore, nutritional approaches may contribute to restore the lost equilibrium among factors accounting for neurodegeneration.
Critical Issues: Herein, we critically examine how essential lipids (including fatty acids, liposoluble vitamins and phytosterols) might contribute to accelerate or prevent the onset and progression of such pathologies. In particular, we highlight that experimental and clinical findings, although promising, are still inadequate to draw definitive conclusions.
Future Directions: More research is warranted in order to establish the real impact of lipid intake on brain health, especially when redox balance and inflammatory responses have been already compromised. In the future, it would be hoped to gain a detailed knowledge of chemical modifications and dynamic properties of such nutrients, before planning to exploit them as potential therapeutics. Antioxid. Redox Signal. 29, 37–60.
Keywords: : fatty acids, liposoluble vitamins, neuroinflammation, nutrition, phytosterols
Introduction
In the past decades, an ever-growing body of evidence has emphasized the existence of an intricate cross-talk between neurons and immune cells, which maintains and guarantees brain homeostasis (26). When injury perturbs this delicate equilibrium, inflammatory responses that originated in the central nervous system (CNS) give rise to the so-called “neuroinflammation.” In this context, glia (non-neuronal cells, including microglia and astrocytes) represent the main source of neurotoxic molecules [i.e., inflammatory mediators and reactive oxygen (nitrogen) species], responsible for progressive loss of the structure and function of neuronal cells, thus leading to functional and mental impairment (32, 205). Neuroinflammation is, indeed, continuously emerging as a critical factor in the pathogenesis and progression of neurodegenerative disorders, including Alzheimer's disease (AD), Parkinson's disease (PD), amyotrophic lateral sclerosis (ALS), and multiple sclerosis (MS) (63, 72, 73, 76, 88, 186, 221). The self-sustaining cycle of neuroinflammation and neurodegeneration, characterized by dying neurons that activate microglia that, in turn, release factors killing additional neurons, suggests that the neuroinflammatory cascade might have a causal role in disease pathogenesis.
During the past decades, many epidemiological studies have increasingly pointed out the role of diet as one of the major modifiable determinants of Western diet-associated diseases, such as obesity, diabetes, cardiovascular disease, hypertension, stroke, and some types of cancer. Food, indeed, due to the coexistence of specific components, appears to have significant effects (both positive and negative) on well-being throughout life. Importantly, dietary adjustments and the so-called “personalized nutrition” may not only influence existing health but also determine whether or not an individual will eventually develop such diseases (167).
Some of the specific dietary components that increase or decrease the probability of occurrence of neurodegenerative diseases in any subject, and interventions to modify their impact, have been identified (1, 146, 193). Among them, bioactive lipids have been intensely studied, as modulators of redox state and inflammation in the CNS. Along with a structural function, indeed, lipids may also contribute to neuronal processes by influencing various signaling pathways directing neuronal survival or death. For instance, different lipids have been shown to act as both pro- and anti-apoptotic second messengers, to modulate innate and adaptive immunity, and to trigger or resolve inflammation (8, 38, 40).
Extensive studies have revealed that these bioactive lipids mainly act at the level of lipid rafts, which are privileged platforms for lipid–lipid and lipid–protein interactions at the plasma membrane. These dynamic membrane domains (enriched in certain neutral and acidic glycosphingolipids, sphingomyelin, and sterols) modulate signaling receptors and ion channels, thus regulating the cross-talk between the extracellular and intracellular milieu (102). Dysfunction of lipid rafts leads to abnormal neuronal and glial functions, so that it represents a pathogenic factor for several neurodegenerative diseases (8). The relevance of bioactive lipids in neurodegeneration is further supported by the finding that lipid changes occur in early stages of neuronal pathologies: In an experimental model of PD, for instance, 19 lipid species (from phosphatidylcholine and lysophosphotidylcholine lipid classes) were found to be downregulated, whereas lysophosphatidylcholine (16:0) and lysophosphatidylcholine (18:1), both important for neuroinflammatory signaling, appeared to be upregulated within the substantia nigra (61).
In this study, we provide an overview of the role in neuroinflammatory responses of some of the most widely studied dietary lipids. For the sake of clarity, we have chosen to focus on essential lipids with antioxidant and anti-inflammatory properties, which are polyunsaturated fatty acids (PUFAs) and lipophilic vitamins. After a brief description of the main hallmarks (misfolding, redox stress, and neuroinflammation) shared by the mentioned pathologies, the potential effects exerted by dietary lipids on microglia and astrocyte functions, referring to the most relevant in vitro, in vivo, and clinical studies, are discussed. Although not essential nutrients, phytosterols are also mentioned, as they represent the most powerful exogenous cholesterol-lowering agents in the CNS, for prevention or delay of most late-onset CNS diseases.
Hallmarks of Neurodegeneration
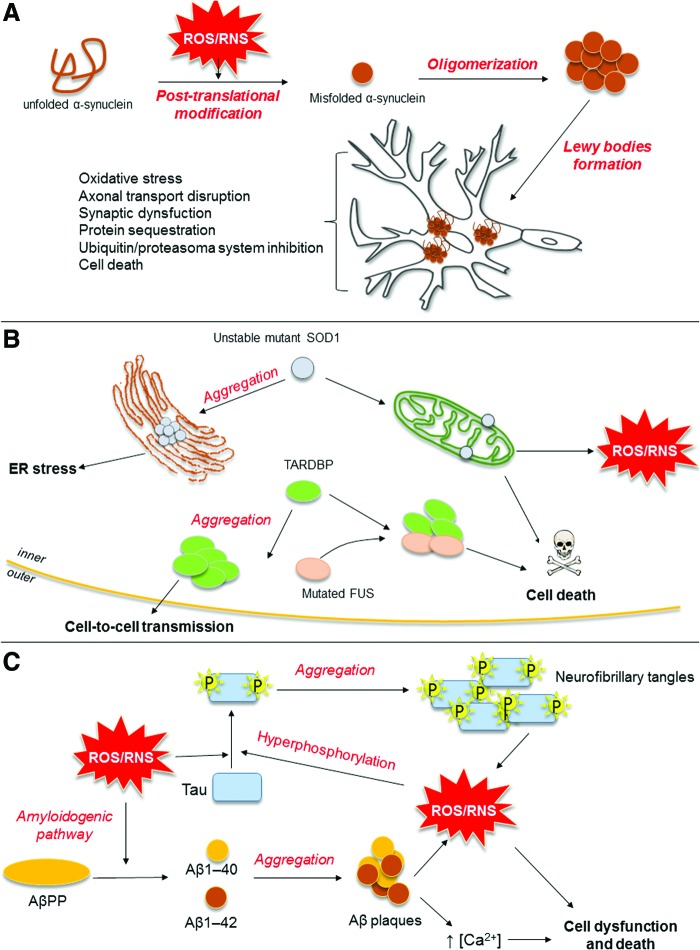
Even though each neurodegenerative disease has its own clinical features, common hallmarks can be identified (Fig. 1) (103, 127). First, all of them share the accumulation of abnormally folded, disease-specific proteins that damage and kill neurons. Protein misfolding occurs in PD, where α-synuclein (αSyn) forms proteinaceous cytoplasmic inclusions (named Lewy bodies), whose accumulation leads to death of dopaminergic neurons in the substantia nigra of the basal ganglia. Similarly, several ALS-associated proteins [superoxide dismutase 1 (SOD1), TAR DNA binding protein, or Fused-in-Sarcoma protein] are embodied in inclusions present in motor neurons, as a result of defects in protein degradation. Finally, in AD, abnormally folded β-amyloid (Aβ) peptide deposits outside neurons in formations known as senile plaques, and hyper-phosphorylated Tau protein aggregates in intracellular neurofibrillary tangles (103, 127).
FIG. 1.
Schematic representation of three common protein misfolding diseases. (A) Causal relationship between oxidative/nitrosative stress and misfolded αSyn in Parkinson's disease. Oxidative and nitrosative stress is mainly responsible for the misfolding process of αSyn. ROS and RSN, indeed, induce αSyn post-translational modifications that promote its oligomerization. αSyn oligomers, in turn, form cytoplasmic inclusions, termed Lewy bodies, whose accumulation leads to dysfunction and death of dopaminergic neurons in the substantia nigra of the basal ganglia. (B) Potential mechanisms triggered by mutated SOD1, TARDBP, and FUS proteins in amyotrophic lateral sclerosis. Unstable mutant SOD1 proteins form misfolded protein aggregates, which abnormally locate in ER (where they trigger ER stress), as well as in the mitochondrial outer membrane, where they cause mitochondrial dysfunction and activate apoptotic cascades. Cytosolic aggregation of mutated or dysregulated TARDBP (normally localized in the nucleus, where it modulates RNA metabolism) may have cell-to-cell transmitting ability. TARDBP can also form a toxic complex with FUS (another RNA processing protein), thus sequestering essential components for motoneuron survival. (C) Relationship among Aβ deposition, hyper-phosphorylated Tau aggregation, and neurotoxic mechanisms involved in the pathogenesis of Alzheimer's disease. Oxidative and nitrosative stress triggers accumulation of potentially neurotoxic Aβ peptide by activating the β-secretase-dependent amyloidogenic process of AβPP and secretion of Aβ1-40 and Aβ1-42. Aβ oligomers formed by toxic misfolded Aβ peptides and ROS production exacerbate oxidative stress and mitochondrial dysfunction, thus triggering Tau protein hyperphosphorylation and its aggregation into neurofibrillary tangles. All these events converge to dysfunction and death of neuronal cells. αSyn, α-synuclein; Aβ, β-amyloid; AβPP, amyloid β protein precursor; ER, endoplasmic reticulum; FUS, Fused-in-Sarcoma; RNS, reactive nitrogen species; ROS, reactive oxygen species; SOD1, superoxide dismutase 1; TARDBP, TAR DNA binding protein. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Misfolded proteins are strictly linked to two other common features of neurodegenerative diseases, which are oxidative (nitrosative) stress and inflammation. Under normal conditions, reactive oxygen species (ROS) and reactive nitrogen species (RNS) regulate physiological functions of the CNS, including the process of learning and memory; however, when redox balance is impaired, oxidative (nitrosative) stress elicits neurotoxicity by altering redox-sensitive signaling cascades and triggering oxidation and misfolding of key proteins (103). Just as an example, ROS and RNS increase the phosphorylation of Tau and the activity of β-secretase, thus stimulating the amyloidogenic process; whereas in PD, oxidation and nitration of αSyn promote its oligomerization and, in the meanwhile, reduce the clearance of this aberrant protein by proteasome and autophagy (77, 103). The scenario is further worsened by the fact that misfolded proteins can lead, by themselves, to ROS and RNS accumulation. For instance, by activating different pathways and interacting with metal ions, Aβ stimulates the production of superoxide and hydrogen peroxide (11, 25, 103, 110). Interestingly, common hallmarks of neurodegenerative diseases have been found to target mitochondria, thus triggering mitochondrial dysfunction and subsequent ROS generation. This is of particular relevance if one keeps in mind that neurons, because of high oxygen consumption, can accumulate more ROS than any other cell and, therefore, are more sensitive to oxidant unbalance (103). Aging is another contributor to oxidative stress, and unsurprisingly neurodegenerative diseases occur in aged individuals: When getting older, loss of endogenous antioxidant defenses [including SOD, heme oxygenase-1 (HO-1), and γ-glutamyl cysteinyl synthetase] occurs, leading to increased free radical production (13). Weakened cellular antioxidant defenses, together with increased ROS and RNS generation, activate redox-sensitive transcription factors, including nuclear factor-κB (NF-κB) and activator protein-1, both of which upregulate the expression of many genes involved in neurodegeneration, including pro-inflammatory mediators.
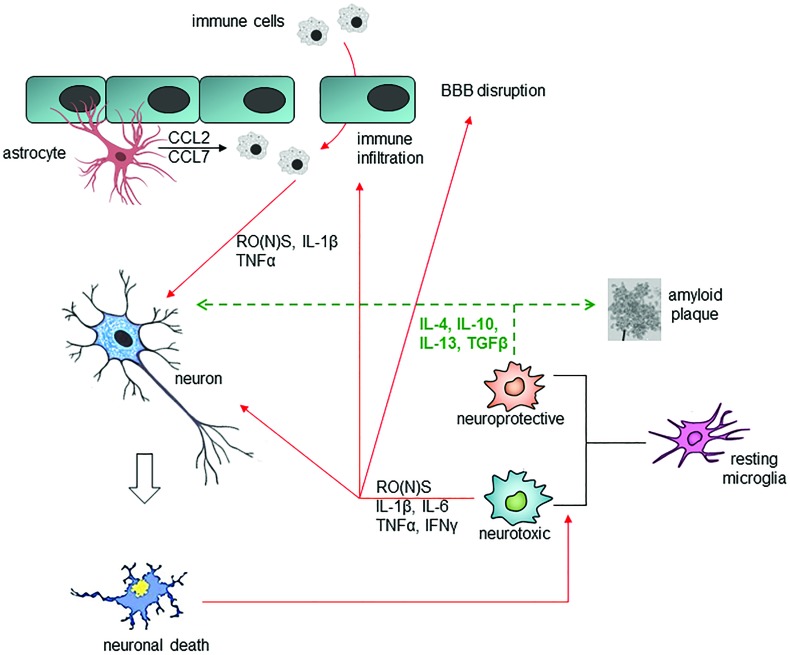
Progressive deposition of misfolded proteins and oxidative (nitrosative) stress converge into a common signaling pathway, which leads to chronic activation of immune cells (77, 108, 161). In this context, microglia, CNS-resident immune cells, play a pivotal role (Fig. 2), as they are involved in the defense of the brain against pathogens and cellular debris, as well as in providing factors that support tissue maintenance and plasticity of neuronal circuits (20, 107). Once activated by pathological stimuli (e.g., neuronal death or protein aggregation), microglial cells migrate to the site of injury and trigger pro-inflammatory responses that lead to damage and loss of neurons, through: (1) release within the brain of pro-inflammatory cytokines [interleukin (IL)-1β, IL-6, tumor necrosis factor alpha (TNFα), and interferon gamma (IFNγ)]; (2) recruitment of peripheral immune cells; (3) increase in free radicals and eicosanoids; (4) impairment of blood–brain barrier (BBB) permeability; and (5) leukocyte invasion (45, 121, 176). Besides this neurotoxic potential (M1 phenotype), microglia might also acquire a neuroprotective M2 phenotype, which is characterized by the ability of releasing anti-inflammatory cytokines [including IL-10, IL-4, IL-13, and transforming growth factor beta (TGFβ)] and increased phagocytic capacity, thus favoring angiogenesis, remodeling, and tissue repair (72). The balance between M1 and M2 phenotypes is finely tuned and sensitive to type and duration of stimulus, state of disease, involved brain regions, and interaction with other cells.
FIG. 2.
Cross-talk between glial cells and neurons under physiological and pathological conditions. Neurotoxic pathways are depicted with red lines; neuroprotective pathways are depicted with green dashed lines. Misfolded proteins and oxidative (nitrosative) stress lead to chronic activation of microglia: once activated, microglial cells and astrocytes release pro-inflammatory cytokines and free radical species, triggering neuronal death, impairment of blood-brain barrier permeability and leukocyte invasion. Conversely, microglia might also acquire a neuroprotective phenotype, characterized by the ability of releasing anti-inflammatory cytokines and increased phagocytic capacity. BBB, blood–brain barrier; CCL, chemokine (C-C motif) ligand; IFN-γ, interferon gamma; IL, interleukin; TGFβ, transforming growth factor beta; TNFα, tumor necrosis factor alpha. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Astrocytes are also responsible for brain health, representing a source of different molecules that orchestrate a multitude of central events such as growth of axons, synaptogenesis, formation and maintenance of BBB, and neurotransmission (205, 211). In addition, astrocytes actively participate in inflammatory responses, due to: (1) the presence on their membrane surface of a wide range of receptors for pro-inflammatory cytokines (especially IL-1β and TNFα), chemokines, and damage signals [including toll-like receptor (TLR) ligands]; (2) the ability to release pro- and anti-inflammatory cytokines; and (3) the ability to produce ROS from the mitochondrial respiratory chain (60).
From the information already provided, it seems apparent that at least three factors (proteostasis, redox stress, and inflammation) contribute toward maintaining the brain in a constitutively reactive, unhealthy state. A vicious circle is, therefore, established whereby single contributors influence each other, exacerbating the pathological signs. This phenomen is evident during AD pathogenesis, where activated microglial cells are responsible for clearance of Aβ and Tau aggregates (216), but on the other hand the prolonged release of pro-inflammatory cytokines further induces Tau hyper-phosphorylation and neuronal loss, thus worsening the disease (209, 210).
It remains to be clarified as to what the impact of each factor is during the onset and progression of neurodegenerative diseases, and whether one is more prevalent than others during the different stages. Indeed, chronic treatment of humans with low levels of cyclooxygenase (COX) inhibitors (nonsteroidal anti-inflammatory drugs) has been shown to reduce the risk for AD (207), but clinical trials with celecoxib or naproxen failed to give positive results in AD patients (3, 4, 48). Further, although blockade of IL-12/IL-23 signaling attenuates amyloid burden and cognitive defects (208), anti-inflammatory strategies targeting IL-10 signaling have recently been shown to have negative effects on Aβ proteostasis, thus exacerbating AD-related pathology (33, 69).
Finally, it should be stressed that redox stressors are listed among factors that induce glial senescence. Oxidative stress and mitochondrial dysfunction trigger all markers of cellular senescence, such as over-expression of cell-cycle inhibitors and of the lysosomal enzyme β-galactosidase, loss of proliferation, formation of DNA damage foci, chromatin remodeling, and, more importantly in the case of glia, induction of a secretory pro-inflammatory phenotype (209).
Essential Dietary Bioactive Lipids
Epidemiological studies have shown that the Mediterranean diet may be useful for the prevention of cognitive decline and dementia in later life (179). The beneficial effects appear to be related to the presence, at least in part, of different bioactive lipids, including PUFAs and lipophilic vitamins. These belong to the class of “essential nutrients,” as they cannot be synthesized by mammals (including humans) and, therefore, must be supplied with diet.
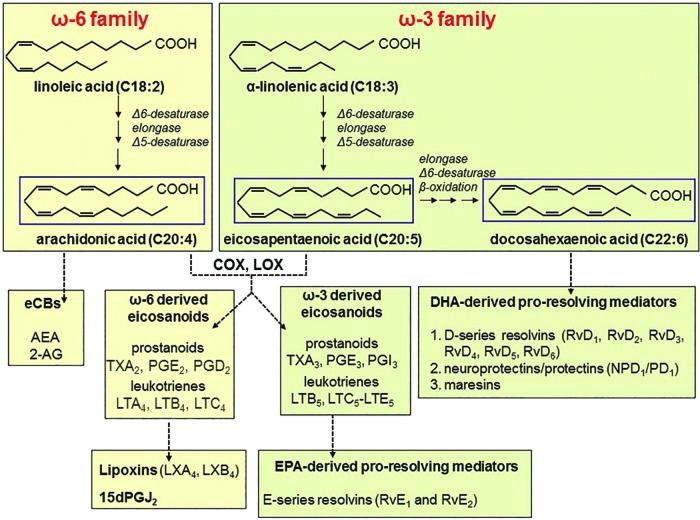
Linoleic acid (LA; 18:2 Δ9,12) and α-linolenic acid (ALA; 18:3 Δ9,12,15) are the precursors of all members of the two non-convertible families, that is, ω6- and ω3-PUFAs. Both LA and ALA can be elongated and desaturated by human cells to more highly unsaturated members, mainly the ω6 arachidonic acid (AA; 20:4 Δ5,8,11,14), and the ω3 eicosapentaenoic acid (EPA; 20:5 Δ5,8,11,14,17) and docosahexaenoic acid (DHA; 22:6 Δ4,7,10,13,16,19) (Fig. 3, upper panel).
FIG. 3.
PUFAs and their derivatives. Upper panels: ω3 and ω6 families. Lower panels: oxidative and non-oxidative derivatives of ω3 and ω6 series. After the action of LOX and COX enzymes, AA gives rise to pro-inflammatory leukotrienes (LTB4 and LTC4) and prostaglandins (PGE2 and PGD2), respectively. AA can also be responsible for originating anti-inflammatory and pro-resolving lipid mediators, including lipoxins LXA4 and LXB4 (derived from LTA4 and LTB4 by the sequential action of 5-LOX and 15-LOX, respectively), and 15-deoxy-prostaglandin J2 (15dPGJ2; formed from PGD2 by the action of prostaglandin D2 synthase). In addition to ω3-derived eicosanoids, EPA and DHA lead to the formation of anti-inflammatory, pro-resolving mediators. This class encompasses the E-series resolvins (RvE1 and RvE2) derived from EPA, and the D-series resolvins (RvD1, RvD2, RvD3, RvD4, RvD5, RvD6), the neuroprotectins/protectins (NPD1/PD1), and the maresins originated from DHA. 2-AG, 2-arachidonoylglycerol; AA, arachidonic acid; AEA, N-arachidonoyl-ethanolamine; COX, cyclooxygenase; DHA, docosahexaenoic acid; eCBs, endocannabinoids; EPA, eicosapentaenoic acid; LOX, lipoxygenase; LX, lipoxin; PUFA, polyunsaturated fatty acid; Rv, resolvin. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
LA is found in the seeds of most vegetables, including corn, soybean, canola, sunflower, and safflower, whereas AA is present in most meats and products from animals fed corn-based diets that are high in ω6-PUFAs. Green leafy vegetables, such as purslane and spinach, and seeds of flax, linseed, walnuts, and others are the predominant sources of ALA, whereas fish is the main source of EPA and DHA (183). Once taken up by cells, PUFAs are incorporated into membrane phospholipids, from where they can be released by the action of phospholipase A2 (PLA2) enzymes, on specific stimuli; nonetheless, up to 90% of AA, DHA, and EPA are rapidly re-esterified to phospholipids, and only <10% act as cell mediators or are enzymatically converted into pro- or anti-inflammatory products (10). Indeed, through the action of distinct lipoxygenase (LOX) and COX isozymes (Fig. 3, lower panel), DHA and EPA may undergo oxidative metabolism, leading to generation of anti-inflammatory (ω3-derived eicosanoids) and pro-resolving mediators (resolvins, neuroprotectins, and maresins) (173); whereas oxidative metabolism of AA, except for generation of lipoxins (LXs), leads to production of pro-inflammatory mediators [leukotrienies and prostaglandins (PGs)] (154). Finally, it should be recalled that non-oxidative metabolism of AA gives rise to another class of compounds, collectively known as endocannabinoids (eCBs), whose main representative members are N-arachidonoyl-ethanolamine (AEA) and 2-arachidonoylglycerol (2-AG) (111).
Among lipophilic vitamins, A and E are the most relevant for prevention of inflammation and neurodegeneration, due to their potent antioxidant activity. In this context, the action of vitamin A (be it retinal, retinol, and retinoic acid) is fundamental for immune system functionality, as its deficiency mainly correlates with defects in adaptive immunity (168). This vitamin is obtained from eggs, liver, bottled milk, or fortified cereals, as well as from foods enriched in retinyl esters, which represent ∼75% of vitamin A. In addition, vegetables (such as tomato, carrot, or spinach) represent the most abundant source of carotenoids, which are vitamin A precursors. This group of pigments, widely distributed in nature, confers distinct colors to fruits and flowers, and it plays critical roles in plant growth and development (134). More than 700 carotenoids have been identified, and they are divided into two groups: carotenes, which are polyunsaturated hydrocarbons (e.g., α- and β-carotene, lycopene), and xanthophylls, which are oxygenated forms of carotenoids (e.g., lutein, β-cryptoxanthin, astaxanthin).
Vitamin E is the collective term for two natural groups of lipid-soluble bioactive compounds: saturated tocopherols and poly-unsaturated tocotrienols, which are also known as tocols. Corn, soybean, and olive oil are the primary dietary source of tocopherols, whereas rice bran, palm, and barley oils are particularly rich in tocotrienols; the latter substances are, however, much less prevalent than tocopherols (84). Vitamin E is recognized as the major chain-breaking antioxidant in the body, which is able to protect the integrity of membranes by inhibiting both lipid peroxidation (101) and low-density lipoprotein oxidation (195).
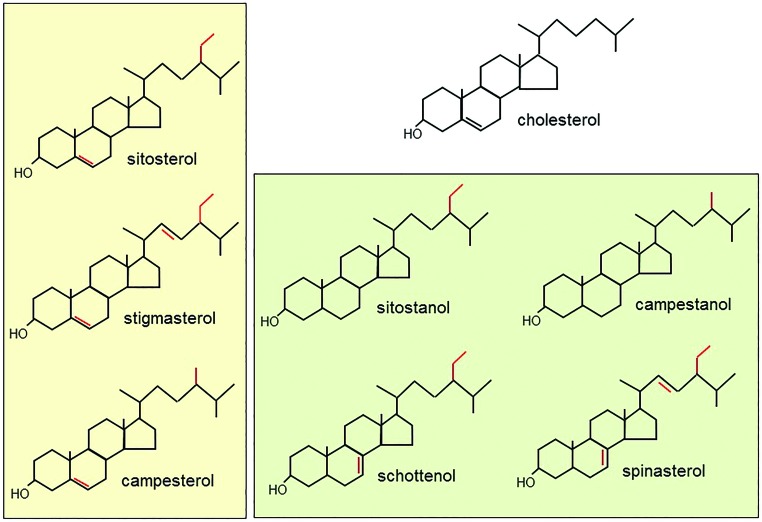
On a final note, phytosterols deserve to be mentioned, although they are not essential nutrients. Indeed, these compounds are often used as cholesterol-lowering agents in the CNS, which represents the most cholesterol-rich organ by weight, able to accumulate ∼23% of total free cholesterol (56, 80). Phytosterols are a heterogeneous class of natural compounds, including plant sterols (β-sitosterol, stigmasterol, campesterol, fucosterol) and their saturated stanol derivatives (spinasterol, schottenol, sitostanol), whose biochemical structure resembles cholesterol in mammals (Fig. 4). These substances are abundantly present in vegetables, fruits, nuts, and cereals; a daily non-vegetarian diet contains ∼250 mg of phytosterols, whereas a vegetarian diet contains more than 500 mg (188).
FIG. 4.
Chemical structure of plant sterols and stanols. The structure of cholesterol is also shown for comparison. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
PUFAs in Neuroinflammation
As already described, PUFAs and their metabolic products play important roles in coordinating immunity and inflammation; their direct engagement in neuroinflammation is currently receiving a great deal of attention, especially in the light of evidence that the brain contains significantly high amounts of both ω6- and ω3-PUFAs. Igarashi et al. (80) found that, in the human brain, ω6- and ω3-PUFAs are 16.9% and 12.1% of total FA content, respectively, with AA representing 8.6% and DHA representing 11.5% of total FAs. EPA is maintained at a much lower brain concentration (0.4% of total FAs), most likely because of a very rapid breakdown by β-oxidation. Noticeably, the endogenous synthesis of AA, EPA, and DHA is low within the brain if compared with the uptake of unesterified FAs from the plasma pool (52, 53). In the brain, PUFA uptake is mainly mediated by fatty acid transport proteins 1 and 4 and fatty acid translocase/CD36, concomitantly with passive diffusion across the BBB (34, 156). The uptake does not appear to be selective, as the different PUFAs show a very similar chemical structure, and they accumulate to a similar extent. Once taken up, PUFAs are either committed toward β-oxidation or stored into brain membranes, where they compete for the incorporation of phospholipids at the same sn-2 position (34, 156). Altogether, these findings suggest that the brain maintains PUFA levels mainly via dietary uptake, and, therefore, PUFA content mainly depends on intrinsic and extrinsic factors, including ω3/ω6 PUFA ratio in food, PUFA bioavailability, and quality and quantity of different lipoproteins.
EPA, DHA, and their metabolites have emerged as major players in resolution of inflammation. This finding, together with the fact that DHA is the most abundant ω3-PUFA in the brain, calls for a better understanding of ω3-PUFA potential in preventing and/or counteracting CNS inflammatory states. Despite available data being often contradictory, it seems fair to state that both in vitro and in vivo findings appear to be rather promising (Table 1). In primary and immortalized microglial cells, both DHA and EPA have been shown to reduce the expression of inducible nitric oxide synthase (iNOS) [via upregulation of phosphoinositide 3 kinase (PI3K)/protein kinase B (Akt) signaling, and subsequent HO-1 activity], COX-2, IL-6, and TNFα, otherwise activated by different stimuli such as IFNγ, myelin, Aβ, and lipopolysaccharide (LPS) (35, 89, 106, 120). EPA and DHA counteract the cellular inflammatory state through different, yet complementary, mechanisms: DHA attenuates the pro-inflammatory response by inhibiting nitric oxide (NO) and IL-6, whereas EPA increases the anti-inflammatory response by enhancing IL-10 production (97).
Table 1.
Relevant In Vitro Studies on ω3-Polyunsaturated Fatty Acid Effects in Neuroinflammation-Related Diseases
| Experimental protocol | Main findings | References | |
|---|---|---|---|
| Neuroinflammation | Murine BV2 or rat primary microglia treated with 30 μM DHA for 6 h | ↑ HO-1 activity | Lu et al.(106) |
| Murine primary microglia pre-treated with 20 μM EPA or DHA for 24 h before 2.5 ng/mL LPS for 24 h | ↓ M1 microglia activation | Chen et al.(35) | |
| ↑ M2 microglia activation | |||
| Murine primary microglia pre-treated with 20 μM EPA alone or combined with other nutrients for 24 h before 50 ng/mL LPS for 24 h | ↑ IL-10 | Kurtys et al.(97) | |
| Murine primary microglia pre-treated with 20 μM DHA (6.67 μM when combined with other nutrients) for 24 h before 50 ng/mL LPS for 24 h | ↓ NO and IL-6 | ||
| Murine BV2 microglia pre-treated with 10 nM RvD1 or RvE1 for 30 min before 1 μg/mL LPS for 6 and 24 h | ↓ IL-1β, TNFα, IL-6 | Rey et al.(157) | |
| Neonatal rat microglia pre-treated with RvD2 for 1 h before 0.1 ng/mL LPS for 24 h | ↓ IL-18, IL-6, NO, TNFα, IL-1β | Tian et al.(194) | |
| ↓ iNOS | |||
| ↓ ROS levels | |||
| AD | Human CHME3 microglia treated with 1 μg/mL Aβ plus 0.1–1 μM EPA or DHA up to 24 h | ↓ M1 microglia activation | Hjorth et al.(74) |
| ↑ Aβ phagocytosis | |||
| Human CHME3 microglia treated with 1 μg/mL Aβ plus 0.1–1 μM DHA up to 24 h | ↑ M2 microglia activation | ||
| Human neuronal-glial cells treated with 5 μM Aβ plus 50 nM NPD1 for 48 h | ↓ Apoptosis | Zhao et al.(227) | |
| ↓ COX-2, B-94, TNFα | |||
| Human neuronal-glial cells overexpressing APP treated with 500 nM NPD1 | ↓ β-secretase-1 | ||
| ↑ α-secretase ADAM10 | |||
| MS | Primary murine immature dendritic cells pre-treated with 50 μM DHA for 24 h before 0.1 μM LPS for 24 h | ↓ Dentritic cell maturation and cytokine production | Kong et al.(96) |
| ↓ Dendritic cell-dependent CD4+ T cell activation | |||
| ↓ T cell differentiation into Th1/Th17 subsets | |||
| Murine primary microglia pre-treated with 20 μM EPA or DHA for 24 h before 5 ng/mL INFγ plus 1–10 μg/mL myelin for 24 h | ↓ NO and TNFα | Chen et al.(35) | |
| Murine primary microglia pre-treated with 20 μM EPA or DHA for 24 h before labeled myelin | ↑ Myelin phagocytosis | ||
| PD | Rat primary mesencephalic neurons pre-treated with 100 nM NPD1 for 5 min before 100 nM rotenone, 100 μM MPP+, or 100 μM MPTP up to 48 h | ↑ Cell survival | Calandria et al.(29) |
| ↓ Dendrite retraction | |||
| Microglial cells from neonatal rats pre-treated with RvD2 for 60 min before 100 ng/mL LPS for 24 h | ↑ Neuronal survival in SNpc | Tian et al.(194) | |
| ↓ Glial cell activation |
↓ decrease; ↑ increase; Aβ, β-amyloid; AD, Alzheimer's disease; APP, amyloid precursor protein; COX-2, cyclooxygenase-2; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; HO-1, heme oxygenase-1; IL, interleukin; INFγ, interferon gamma; iNOS, inducible nitric oxide synthase; LPS, lipopolysaccharide; MPP+, 1-methyl-4-phenylpyridinium ion; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; MS, multiple sclerosis; NO, nitric oxide; NPD1, neuroprotectin D1; PD, Parkinson's disease; ROS, reactive oxygen species; Rv, resolvin; SNpc, substantia nigra pars compacta; TNFα, tumor necrosis factor alpha.
Accumulating evidence attributes to resolvins and neuroprotectins the pro-resolution action of their ω3-PUFAs precursors, in both central and peripheral inflamed tissues. For instance, BV-2 cells express receptors for resolvin (Rv)D1 and RvE1 (i.e., LXA4 receptor/formyl peptide receptor 2 for RvD1 and chemokine-like receptor 1 for RvE1, respectively), as well as the enzymes responsible for their synthesis (i.e., 5- and 15-LOX) (157). On LPS stimulation, the expression of receptors and enzymes in BV-2 cells increases, so that accumulation of RvD1 and RvE1 reduces the expression of the pro-inflammatory cytokines IL-1β, TNFα, and IL-6 (157). In a recent study, RvD2 was found to reduce microglial inflammation via inhibition of LPS-triggered expression of NF-κB, TNFα, IL-1, IL-18, IL-6, IL-1β, and iNOS (194). In addition, in human neuronal-glial cells and in 3xTg-AD mice (a triple transgenic model of AD), neuroprotectin D1 (NPD1) downregulates Aβ42-triggered expression of COX-2 and of the TNFα-inducible pro-inflammatory element B-94. Moreover, it promotes the amyloid precursor protein (APP) non-amyloidogenic pathway, by downregulating β-secretase-1 and activating the α-secretase ADAM10 (227). Finally, NPD1 preserves the integrity of the dendritic arbor and reverts the dendrite retraction induced in dopaminergic neurons by 1-methyl-4-phenylpyridinium ion (MPP+), an inhibitor of mitochondrial complex I that is widely used to elicit a severe PD-like syndrome (29).
As a result of their anti-inflammatory action, ω3-PUFAs powerfully modulate the M1/M2 microglial balance. Both EPA and DHA decrease pro-inflammatory M1 markers CD40 and CD86, and they enhance Aβ phagocytosis by human microglia. In particular, DHA also shows a stimulatory effect on the anti-inflammatory M2 marker CD206 (74). Moreover, RvD1 has been found to enhance M2 polarization in BV-2 microglial cells, through a mechanism that is possibly related to activation of signal transducer and activator of transcription 6 (STAT6) and peroxisome proliferator-activated receptor (PPAR)γ signaling pathways (104). By the same mechanism (i.e., a shift in microglial polarization toward the beneficial M2 phenotype), ω3-PUFAs enhance myelin phagocytosis and improve motor and cognitive functions in the cuprizone mouse model of MS (35).
As yet, in vivo data on the impact of ω3-PUFA supplementation on neurodegenerative diseases are still scant and often unclear (22), although they seem to speak in favor of a beneficial role in alleviating disease symptoms (Table 2). By employing 6-hydroxydopamine-treated rats, which develop a PD-like syndrome, Delattre et al. (50) reported that prolonged (from the post-weaning period until adulthood) ω3-PUFA ingestion efficiently reduced lipid peroxidation and dyskinesia; however, it did not ameliorate motor deficits, much likely through stimulation of dopaminergic turnover in the remaining terminals of the lesioned striatum. By using another experimental model of neurodegeneration, the same authors have recently reported that dopaminergic damage could also be reverted via maternal ω3-PUFA supplementation of prenatally LPS-exposed offspring; the positive effect was not linked to inhibition of apoptotic death in dopaminergic cells, but rather to maintainance of neurochemical integrity in remaining neurons, thus ameliorating learning and memory deficits (49). In 3xTg-AD mice, chronic supplementation with DHA-rich fish oil improves cognition and prevents dysfunction of entorhinal neurons in the cortex, one of the brain regions that develop early neurofibrillary tangles and Aβ plaques in AD patients (7). In another transgenic APP/presenilin 1 rat model of AD, DHA supplementation can modulate Aβ aggregation, as it reduces hippocampal Aβ plaque density by increasing the less toxic fibrillar Aβ oligomers and decreasing the more toxic prefibrillar Aβ oligomers (192). In aged rats, EPA treatment can attenuate the Aβ-induced impairment in long-term potentiation, by decreasing hippocampal concentrations of IFNγ, which are known to trigger microglial activation and amyloidogenic processing of APP (109, 223). In experimental autoimmune encephalomyelitis (EAE) mice, an animal model of MS, DHA improves clinical symptoms by acting on dendritic cell-dependent T-lymphocyte activation (96). In addition, in mice fed with cuprizone-enriched food, both DHA and EPA attenuate the copper chelator-mediated demyelination, via a series of anti-inflammatory responses, among which microglial phenotype switching is the most evident (35). Since M1 to M2 phenotype shift usually occurs at the beginning of remyelination (122), ω3-PUFAs may not only reduce demyelination but also favor the remyelination process in MS. Clearly, further studies are deemed necessary to investigate the therapeutic potential of these FAs for MS patients.
Table 2.
Relevant In Vivo Studies on ω3-Polyunsaturated Fatty Acid Effects in Neuroinflammation-Related Diseases
| Experimental protocol | Main findings | References | |
|---|---|---|---|
| AD | 3xTg-AD mice | ↓ Hippocampal NPD1 and DHA | Zhao et al.(227) |
| 3xTg-AD mice fed with fish oil microcapsule containing DHA:EPA (4:1; 0.6% DHA w/w) | ↑ Cell capacitance | Arsenault et al.(7) | |
| Improvement of cognition | |||
| Prevention of dysfunction of entorhinal cortical neurons | |||
| APP/PS1 rats fed for 4 months with safflower oil-based diet supplemented with DHA 0.6% (w/w) from algae | ↓ Hippocampal Aβ plaque density | Teng et al.(192) | |
| ↑ Fibrillar Aβ oligomerization | |||
| ↓ Prefibrillar Aβ oligomerization | |||
| Improvements on behavioral testing | |||
| AD/aging | Aged rats fed for 4 weeks with EPA (125 mg/rat/die) before intracerebroventricular injection of 20 μM Aβ | ↓ Long-term potentiation impairment | Lynch et al.(109) |
| ↓ Hippocampal IFNγ and IL-1β | |||
| MS | Mice fed with DHA-enriched diet (0.22%, DHA w/w) for 5 weeks before EAE induction | ↔ EAE onset | Kong et al.(96) |
| ↓ EAE severity and mortality | |||
| ↓ Th1/Th17 cells | |||
| Mice fed with a regular diet containing 0.2% cuprizone plus 15 μg/kg DHA and EPA for 5 weeks | ↑ Myelin phagocytosis | Chen et al.(35) | |
| ↓ M1 microglia activation | |||
| ↑ M2 microglia activation | |||
| Improvement of motor and cognitive functions | |||
| PD | Rats fed from 21 to 90 days of life with fish oil before injection of 4 μg 6-OHDA in medial forebrain bundle | ↓ Lipid peroxidation | Delattre et al.(50) |
| ↑ Dopaminergic neuron turnover | |||
| ↔ Motor deficits | |||
| ↓ Dyskinesia | |||
| Maternal supplementation with 3.0 g/kg of fish oil (18% EPA +12% DHA) during pregnancy: (1) Rat offspring prenatally (at gestation day 11) exposed to 1 mg/kg LPS (2) Stereotaxic re-exposure of rat offspring (at day 90) to 1 μg LPS |
↓ Dopaminergic neuron damage | Delattre et al.(49) | |
| ↔ Dopaminergic neuron apoptosis | |||
| ↓ Glial cell activation | |||
| Amelioration of learning and memory deficits | |||
| Rats pre-treated with 25–100 ng/kg RvD2 for 3 days before and after LPS injection (30 days in total) | ↓ Numbers of apo-morphine-induced rotational cycles | Tian et al.(194) | |
| ALS | G93A-SOD1 mice | ↓ DHA-phosphatidylcholine in spinal cord at terminal stage | Arima et al.(6) |
| G93A-SOD1 mice fed with: (1) EPA-enriched diet (300 mg/kg/die) for 7 days at pre-symptomatic stage or (2) EPA-enriched diet (300 mg/kg/die) for 7 days at disease onset |
(1) ↑ Lipid peroxidation; ↑ Disease progression | Yip et al.(225) | |
| (2) ↑ Lipid peroxidation; ↔ Disease onset, progression, and survival |
↓ decrease; ↑ increase; ↔ no change; 6-OHDA, 6-hydroxydopamine; ALS, amyotrophic lateral sclerosis; EAE, experimental autoimmune encephalomyelitis; PS1, presenilin 1.
Detrimental rather than beneficial effects of dietary ω3-PUFAs on glial activation, and thus on disease progression and survival of G93A-SOD1 mice, a mouse model of ALS, have been reported (225). EPA-enriched diet does not affect either ALS onset and survival or the course of motor deficit, when administered at disease onset, whereas it accelerates disease progression if used at a pre-symptomatic stage. The main mechanism accounting for this negative action lies in lipid peroxidation that damages tissue vacuolization. Incidentally, the spinal cord of ALS patients shows reduced DHA levels (81), and G93A-SOD1 mice have low amounts of DHA-containing phosphatidylcholines in their spinal cords at terminal stages of the disease, but not in the pre-symptomatic or early stages (6).
Likewise, both positive and negative effects have been reported in human studies (Table 3). A cross-sectional population-based study among 1613 subjects, ranging from 45 to 70 years, documented that fatty fish consumption inversely correlated with the risk of impaired overall cognitive function (87). Consistently, a multi-center incident case–control study reported a significant decrease in first clinical diagnosis of CNS demyelination risk, in subjects with high ω3-PUFA intake, particularly from fish (75), and five large prospective cohort studies suggest that ALA and marine ω3-PUFAs intake reduces risk of developing ALS (64). A recent systematic review, summarizing data from population-based longitudinal observational studies, demonstrated that fish consumption significantly reduces cognitive decline rates and incidence of AD (139). Moreover, several epidemiological studies suggest that ω3-PUFA supplementation is also positively associated with clinical outcomes in MS patients (83). Finally, an interventional study that enrolled relapsing-remitting MS patients showed that chronic ω3-PUFA supplementation (12 months of fish oil) significantly reduced serum levels of TNFα, IL-1β, and IL-6 (155), without any effect on the glutathione redox system (182). Nonetheless, other studies have emphasized the positive effect of ω3-PUFA supplementation on cognitive decline in healthy, but not in diseased people. Indeed, a 24-week supplementation with 900 mg/die DHA improved age-related cognitive decline in 485 healthy adults, suggesting potential benefits of purified DHA to treat cognitive decline in the normal elderly (226). A 24-week ω3-PUFA supplementation ameliorated mild cognitive impairment (MCI), but it failed to improve cognitive performance in mild AD patients (36). Correspondingly, a randomized, double-blind, placebo-controlled trial showed that DHA supplementation (2 g/die for 18 months) did not slow the rate of cognitive and functional decline in individuals with mild-to-moderate AD (151). Finally, the OmegAD study reported beneficial effects in mild, but not in advanced AD patients after 6 months of ω3-PUFA treatment (65). Taken together, these findings suggest that high ω-3 PUFAs intake may be useful for preventing or delaying cognitive decline and/or dementia, but it seems uneffective in the late stages of neurodegeneration. A possible explanation can derive from the observation that, due to the presence of double bonds, DHA and EPA are the most susceptible PUFAs to free radical attack, so that they can generate neurotoxic reactive products (e.g., trans-4-hydroxy-2-hexenal and F4-neuroprostanes from DHA, and F3-isoprostanes from EPA), whose levels are raised during neurodegenerative diseases (66, 126, 130, 169, 175). Therefore, if on the one hand ω3-PUFAs are beneficial for the diseased brain by counteracting neuroinflammation, on the other hand they might represent a harmful pro-oxidative stimulus that can exacerbate disease progression.
Table 3.
Relevant Human Studies on ω3-Polyunsaturated Fatty Acid Effects in Neuroinflammation-Related Diseases
| Experimental protocol | Main findings | References | |
|---|---|---|---|
| Cognitive functions | 1613 middle-aged subjects self-administered food-frequency questionnaire | Risk of impaired cognitive function inversely correlated with fish consumption | Kalmijn et al.(87) |
| AD | 23 AD and 23 MCI patients orally receiving 1.8 mg/die ω3-PUFA for 24 weeks | ↔ Cognitive performance in AD patients | Chiu et al.(36) |
| ↑ Cognitive performance in MCI patients | |||
| 204 AD patients orally receiving 1.7 g/die DHA and 0.6 g/die EPA for 6 or 12 months | ↔ Cognitive performance in advanced AD patients | Freund-Levi et al.(65) | |
| ↑ Beneficial effects in mild AD patients only after 6 months | |||
| DHA supplementation (2 g/die) in 171 individuals with mild-to-moderate AD for 18 months | ↔ Cognitive and functional decline in AD patients | Quinn et al.(151) | |
| Age-related cognitive decline | 485 healthy older adults with mild memory complaints orally receiving 900 mg/die DHA for 24 months | ↑ DHA plasma levels | Yurko-Mauro et al.(226) |
| Improvement of learning and memory function | |||
| MS | 1493 relapsing-remitting MS patients self-administered food frequency questionnaire | Quality of life directly correlated with frequent consumption of fish and/or ω3-PUFA supplementation. Disease activity and disability inversely correlated | Jelinek et al.(83) |
| 50 relapsing-remitting MS patients orally receiving 4 g/die of fish oil (0.8 g EPA and 1.6 g DHA) for 12 months | ↓ Serum levels of TNFα, IL-1 β, IL-6, ↓ NO metabolites | Ramirez-Ramirez et al.(155) | |
| ↑ ω3-PUFA | Sorto-Gomez et al.(182) | ||
| ↓ ω6-PUFA | |||
| ↔ Glutathione reductase activity | |||
| ↔ GSH/GSSG ratio | |||
| ALS | 995 definite or probable patients (age 18–81 years) self-administered food frequency questionnaire about ω3-PUFA daily intake | ALS risk inversely correlated with ω3-PUFA intake | Fitzgerald et al.(64) |
↓ decrease; ↑ increase; ↔ no change; GSH/GSSG, reduced GSH/oxidized GSH; MCI, mild cognitive impairment.
As for ω-3 PUFAs, AA (derived either from dietary intake or from endogenous synthesis from its LA precursor) represents the second most abundant PUFA after DHA (80), and it has been recognized as a risk factor for age-associated neurodegenerative diseases. Indeed, AA can be hydrolyzed from brain membrane phospholipids by specific PLA2 isozymes, and then it can be converted into pro-inflammatory mediators by the action of selected COX or LOX isozymes, which produce PGs and thromboxanes or leukotrienes, respectively (10). In neurodegenerative diseases, increased activation of these enzymes usually occurs, leading to increased production of eicosanoids and activation of microglia and astrocytes. Consistently, the use of synthetic PLA2 inhibitors, as well as of phytochemical-based inhibitors such as curcumin or Ginkgo biloba extracts, has been shown to be useful for the treatment of oxidative stress and neuroinflammation associated with cognitive impairment (137, 228).
Less clear is the therapeutical efficacy of blocking AA oxidative metabolism. As already mentioned, the beneficial effects of COX inhibitors observed in vitro have not been confirmed in human studies (3, 4, 48, 207). Nonetheless, dual inhibitors that are able to block the action of COXs and 5-LOX that are over-expressed in experimental MS animal models and human patients (92, 217) have recently been investigated. Such an inhibitor, flavocoxid, exerts protective effects by reducing pro-inflammatory cytokine release in MS and AD mice, as well as Aβ plaques, learning and memory loss in AD models (19, 95).
It should be recalled that AA can also generate, through an alternative 5-LOX pathway, protective compounds such as LXA4, which acts as a “stop signal” of inflammatory processes (172). In Tg2576 transgenic AD mice, aspirin-triggered LXA4 has been shown to reduce Aβ and phosphorylated Tau levels, as well as microglial and astrocyte reactivity, while promoting clearance of Aβ aggregates and enhancing cognitive performance (57, 117, 172). Also orally administered, mushroom-derived LXA4 was able to upregulate LX levels in cortex and hippocampus, and to activate vitagenes (encoding for members of the heat shock protein family, HO-1, the thioredoxin/thioredoxin reductase system, and LXA4 itself) involved in the cellular stress response triggered by proteotoxic stresses and accumulation of misfolded proteins (196, 197). The vitagene system, therefore, is emerging as an integrated system, cooperating with the proteostasis network to counteract aggregation of misfolded proteins and to promote cellular stress tolerance. These data, together with the finding that LXA4 levels are reduced with age and in postmortem brain tissue and cerebrospinal fluid samples from AD patients, may suggest that activation of LXA4 signaling and modulation of vitagene proteins are promising targets for novel cytoprotective interventions against oxidative stress-driven neuroinflammation and neurodegenerative diseases (28, 57, 117, 215, 196, 197).
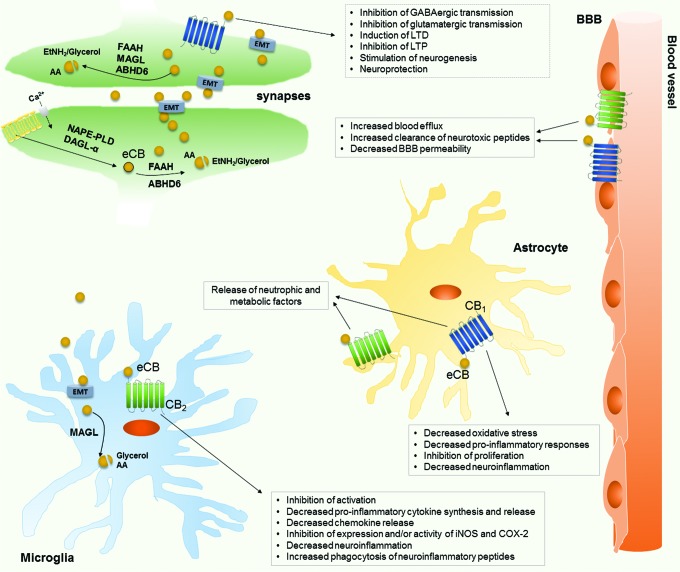
Finally, non-oxidative products of AA such as the eCBs AEA and 2-AG are emerging as new players in neurodegeneration (Fig. 5). eCBs, whose levels are regulated by the activity of metabolic enzymes, as well as by AA availability, bind to and functionally activate G protein-coupled type-1 and type-2 cannabinoid (CB1 and CB2) receptors (15). Within the CNS, eCBs reduce excitotoxicity and modulate adult neurogenesis and oligodendrocyte formation, through activation of CB1 (111). Instead, by acting at CB2, they regulate the reactivity of glial cells, stimulate cell migration, and inhibit release of pro-inflammatory cytokines (40, 62). Moreover, eCBs provide trophic and metabolic support to neurons, by astroglial CB1/CB2 activation, or limit oxidative stress and inflammatory responses through interactions with nuclear factor (erythroid-derived)-like 2 (Nrf-2), NF-κB, and PPARs (40, 62).
FIG. 5.
Schematic representation of eCB actions in the CNS, in physiological conditions and during neuroinflammation. Anandamide (AEA) and 2-AG are produced on demand from AA-containing phospholipids, via multiple biosynthetic pathways requiring (among others) the catalytic activity of NAPE-PLD (especially present in presinaptical terminals) for AEA, and of the sn-1-specific DAGL-α (in dendritic spines of excitatory synapses) for 2-AG. In CNS, eCBs are post-sinaptically produced and exported through the EMT; once in the synaptic space, eCBs (mainly 2-AG) bind the CB1 receptor to inhibit, in a retrograde manner, neurotransmitter release. The eCB biological action is stopped by hydrolysis catalyzed by either FAAH (for AEA) and MAGL (for 2-AG) or ABHD6 that selectively recognizes 2-AG. In microglia and astrocytes, eCBs activate CB1 and CB2 receptors, playing distinct and often ovarlapping effects; in neuroinflammatory conditions, they exert neuroprotective effects by inhibiting pro-inflammatory cytokine release and increasing the production of anti-inflammatory mediators. Also, the effect of eCBs on BBB is reported here, by highlighting their protective effect on BBB integrity and functionality. ABHD6, alpha/beta-hydrolase domain containing 6; CNS, central nervous system; DAGL-α, diacylglycerol lipase-α; EMT, endocannabinoid membrane transporter; etNH2, ethanolamine; FAAH, fatty acid amide hydrolase; iNOS, inducible nitric oxide synthase; LTD, long-term depression; LTP, long-term potentiation; MAGL, monoacylglycerol lipase; NAPE-PLD, N-acyl-phosphatidylethanolamines-hydrolyzing phospholipase D. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The time- and brain region-specific alterations of eCB signaling during neuroinflammation and neurodegeneration have been investigated in detail (16, 38, 39, 79, 204). Changes in both AEA and 2-AG levels have been found in several disorders, together with negative or positive modulation of their biosynthetic and degradative enzymes. For example, in amyloidosis and AD animal models, eCB concentrations are modulated during disease progression, so that pharmacological or genetic regulation of metabolic (mainly degradative) enzymes can be viewed as a novel therapeutical target for reducing disease hallmarks (113, 200, 204). The protective role of eCB upregulation seems to be particularly relevant in MS (31, 218), with protective pathways ranging from downregulation of IL-23 and IL-12 release by microglial cells (42) to inhibition of cytokine production by myeloid, but not plasmacytoid, dendritic cells in MS patients (37). More recently, in monocytes obtained from both relapsing-remitting MS patients and healthy controls, AEA has been shown to suppress the viral TLR activation-mediated production of IL-12p40 and IL-6 (38).
From the data already summarized, it can be hypothesized that tissue concentrations, and biological activity thereof, of AA derivatives might be modulated by dietary AA content. Indeed, several studies have highlighted that FAs, and even more the ω3-/ω6-PUFA ratio, might be diet modulators of AA-derivative levels. Overall, it appears that PUFAs show immunomodulatory properties in the brain, with ω3-PUFAs being mainly anti-inflammatory and ω6-PUFAs being more prone to enhance inflammation. Depending on which PUFA is present in the diet, neuroinflammation will be reduced or enhanced, and subsequently, well-being in the elderly will be either improved or worsened. Both AA and DHA are incorporated into brain membranes and, therefore, their abundance may define which inflammatory mediator will be produced. Indeed, DHA competes with AA as a substrate of COXs and LOXs, so that the synthesis of inflammatory eicosanoids is counteracted by the production of anti-inflammatory metabolites (30, 98). In Western diet, the ω3/ω6 PUFA ratio is heavily biased (usually 1 to 10–20) toward ω6-PUFAs (177), so that the excess of ω6 precursors promotes the formation of AA that, in turn, increases the risk for chronic inflammatory diseases (98). In this context, ω3-enriched diets may be recommended to reduce neuroinflammation. For instance, a high intake of DHA or EPA has been shown to reduce AA concentrations in the brain, and especially in glial cells, thus exerting neuroprotective effects (98). These results were confirmed through genetic engineering, whereby mice genetically modified to produce high amounts of endogenous ω3-PUFAs were shown to respond to an immune challenge better than their wild-type counterparts, in terms of anti-inflammatory phenotype of microglia and cognitive performances (51).
On a final note, when considering personalized nutrition, it should be always kept in mind that several studies have demonstrated that ω3-PUFA intake, and especially that of DHA supplements, may improve cognitive development only during infancy, whereas it does not increase cognitive performance in children, adults, elderly, nor in AD subjects (85, 145). Therefore, any nutritional intervention should be planned at a very early point in anybody's life.
Vitamin A and Carotenoids
An intriguing aspect of the potential benefits of vitamin A and carotenoids in certain CNS illnesses has been underlined by several studies on the ability of these bioactive lipids to modulate oxidative stress and inflammatory reactions in astrocytes and microglia. In murine astrocyte primary cultures, retinoic acid efficaciously inibited the release of chemokines, including chemokine (C-C motif) ligand (CCL)2, CCL3, CCL5, and chemokine (C-X-C motif) ligand (CXCL)1 and CXCL2, which attract and activate monocytes and neutrophils across the BBB (201). In primary microglia cells, vitamin A, in combination with ω3-PUFAs and vitamin D, has been shown to inhibit LPS-induced release of inflammatory mediators (such as NO and IL-6); this convergence between different nutrients is encouraging for the design of a dietary intervention that is aimed at reducing neuroinflammation, for prophylactic or therapeutic purposes (97). Tomato-derived lycopene (a carotenoid without pro-vitamin A activity) has been proved to drive glial cells toward a neuroprotective M2 phenotype, by in vitro suppression of the expression of COX-2 and inflammatory mediators, via the adenosine monophosphate-activated protein kinase-α1/HO-1 pathways. This finding was confirmed in vivo, as lycopene administration mitigated microglial activation and motor coordination dysfunction caused by an intraperitoneal LPS injection (105). More recently, Wu et al. (220) have highlighted the neuroprotective effects of astaxanthin (naturally occurring in algae, crustaceans, shellfish, and various plants) also in neurons: By stimulating HO-1 expression, this xanthophyll has, indeed, been shown to protect neuronal cultures against Aβ- or MPP+-induced cytotoxicity (212, 224).
The beneficial effects on AD and PD have been further supported by in vivo studies. In haloperidol-treated rats, a model of tardive dyskinesia, lycopene supplementation leads to a dose-dependent: (i) decrease of lipid peroxidation and nitrite levels; (ii) elevation of glutathione content; and (iii) attenuation of the oxidative stress-dependent release of neuroinflammatory mediators (such as TNFα, IL-1β, and IL-6) in the striatum; all these effects were accompanied by significant amelioration of motor activity (47). The latter study is in agreement with other reports documenting similar protective effects of lycopene (as well as of the other carotenoid lutein) on dopaminergic neurons against cytotoxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Indeed, by enhancing antioxidant defense and diminishing mitochondrial dysfunction and apoptotic death, these carotenoids improve behavioral deficits and motor abnormalities that are induced by the neurotoxin (133, 149, 178). Lycopene has also been suggested as an adjuvant in AD therapy, since in vivo studies demonstrated a dose-dependent improvement of Aβ-induced spatial learning and memory impairment, maybe linked to the ability of lycopene to downregulate the expression of pro-inflammatory cytokines (namely TNFα, TGFβ, and IL-1β), to prevent mitochondrial dysfunction and ROS generation, and to decrease the apoptotic rate (150, 163).
Finally, epidemiological, observational, and supplementation studies have underlined the ability of both vitamin A and carotenoids to cross the BBB and to counteract the oxidative-inflammatory state within the brain, thereby reducing the risk of neuropathology. A healthy human cohort study (10 men and 28 women, average age 53 ± 20 years) has revealed a correlation between plasma carotenoid levels and markers of inflammation and oxidative stress, as well as NAD+ stores; in particular, an inverse, age- and sex-dependent correlation has been found between carotenoid concentrations and plasma and cerebrospinal fluid IL-6 levels (68). A positive association has also been found between α and β carotenoid levels and plasma total antioxidant capacity, as well as between α carotenoids and cerebrospinal fluid TNFα levels (68). In the latter case, TNFα levels found in the cerebrospinal fluid were consistent with the finding that, when present below the physiological range, this multifunctional, pleiotropic cytokine exerts positive neuromodulatory effects, rather than acting as a pro-inflammatory and neurotoxic agent (142, 206). Therefore, it is conceivable that α carotenoids may exert a positive role against neuroinflammation by promoting the healthy neuromodulatory function of TNFα. Finally, the positive association between lycopene and central NAD+ stores (68), together with the ability of carotenoids to quench ROS and restore poly(adenosine diphosphate-ribose) polymerase-1 activity (82), supports the hypothesis that these phytonutrients may be beneficial in reducing DNA damage.
Defects in retinoid metabolism may predispose to degeneration of motor neurons, thus leading to muscle weakness and atrophy, whereas activation of different retinoid receptors (RAR and RXR isoforms) has been proved to slow down loss of motor neurons at both pre-symptomatic and early symptomatic stages of the disease (41, 94, 159). It is not surprising, therefore, that vitamin A deficiency correlates with dysregulation of immune tolerance and pathogenic immune cell production in MS. Coherently, several observational studies documented vitamin A deficiency in MS patients, whereas high concentrations of plasma retinol correlate with outcome improvement in MS patients (158). In this context, the regulation of different T cell subsets and BBB function deserves mention. Retinoic acid modulates the balance between T helper (Th)1/Th2 and Th17/T regulatory (Treg) cells, as well as dendritic and B cell functions, thus re-establishing the balance between pathogenic and immunoprotective cells as well as increasing tolerance of autoimmunity (2, 158). In addition, retinoic acid can be listed among the astrocyte-derived factors halting MS lesion progression, as it is able to limit inflammatory damage and loss of BBB integrity (124). Several vitamin A supplementation studies further support the protective role of this vitamin in MS progression. A controlled, randomized clinical trial (enrolling 101 patients, aged 20–45 years) reported that 1-year retinyl palmitate supplementation significantly improved clinical progression and psychiatric signs (including fatigue and depression) of MS, although no changes were seen for number of relapses and brain lesions (17, 18). In a clinical trial of 36 relapsing-remitting MS patients who received 25,000 IU retinyl palmitate, over a 6-month period, vitamin supplementation was proven to upregulate TGFβ and Forkhead box P3 gene expression in peripheral blood cells, thus re-establishing Th17/Treg balance (162). More recently, another randomized, double-blind controlled trial, where 39 relapsing-remitting MS patients (aged 20–45 years) were supplemented with retinyl palmitate for 6 months, showed that treatment significantly reduced the expression of transcription factor T-box expressed in T cells, the key transcription factor committing CD4+ T cells to the Th1 lineage (189) and, thus, inhibiting the expression of Th1-secreted IFNγ (125).
Vitamin E, Tocopherols, and Tocotrienols
Although all tocols are active vitamers, for a long time α-tocopherol has been recognized as the most important and active form of the vitamin, mostly due to its abundance (it is presently at a 1:1000 ratio in cell membrane lipids) and to mechanisms of transport and inactivation (23, 99). α-Tocopherol, indeed, has higher binding affinity for the α-tocopherol-binding protein (responsible for preferential retention of α-tocopherol and discrimination against other vitamers) and lower propensity to be metabolized by cytochrome P450 and β-oxidation pathways (84, 140, 181). Recent in vitro and in vivo studies, combined with epidemiological studies, have changed this concept, showing how the other vitamers may have biological properties that are distinct from α-tocopherol. Tocotrienols appear to be more powerful agents than tocopherols, by virtue of their cholesterol-lowering property and better antioxidant activities (129, 152, 153). Structural and dynamic studies have shown that tocotrienols are more mobile within biological membranes and are recycled by other antioxidants more efficiently than tocopherols, thereby more powerfully scavenging free radicals (187); a more efficient cellular uptake for tocotrienols with respect to tocopherols has also been reported, as is the case of astrocytes that intracellularly transport γ-tocotrienol more efficiently than α-tocopherol (171).
Several experimental and clinical studies highlight the central role of vitamin E in preserving neurological structure and function, under both physiological and pathological conditions: (i) α-tocotrienol and α-tocopherol protect primary neuronal cells against toxicity induced by glutamate (as well as by a number of other toxins) (138, 170); (ii) orally supplemented vitamin E isomers reach the cerebrospinal fluid and the brain (203), with tocotrienols being more bioavailable than tocopherols (their unsaturated hydrocarbon tails allow for better penetration into fatty tissues) (91); (iii) primary vitamin E deficiency is associated with certain neurological diseases (136, 198); (iv) γ-tocopherols reduce the concentration of nitrogen dioxide that is involved in several neurological diseases (86), as well as they reduce NO release by LPS-stimulated microglia (191); and (v) an inverse correlation between incidence of dementia/AD and plasma levels or dietary intake of all vitamers has been reported (21, 54, 112, 114, 128, 148, 160, 222).
Despite these findings, the role of vitamin E in neuroinflammation remains somewhat controversial and not well defined. Some in vitro and in vivo models of PD have shown that low concentrations of δ-tocotrienol protected neurons against MPTP-induced cytotoxicity, via a mechanism that was dependent on the estrogen receptor β-PI3K/Akt pathway (131, 132). Nonetheless, several authors reported negative outcomes, maybe linked to alternative pathways that are activated by vitamin E at supra-physiological doses (>400 IU/die). For example, Miyamoto et al. (123) reported that a very high dose of α-tocopherol activated microglia in spontaneously hypertensive rats, and it significantly increased the expression of glial fibrillary acidic protein, a hallmark of astroglial activation. Khanna et al. (90) showed that, in mice fed with α-tocopherol-enriched diet (used at the equivalent human dose of 1680 IU/die), α-tocopherol exacerbated microglial activation and oxidative stress, as well as the neuroinflammatory signaling observed after stroke-induced brain injury. Similarly, high concentrations of γ-tocotrienol, but not of α-tocopherol, were cytotoxic for primary astrocytes in culture (115). Accordingly, healthy human volunteers who received a dose of 596 IU/die of α-tocopherol for 4 weeks showed increased levels of lysophosphatidycholine (219), representing both a general marker of inflammation (213) and a pro-inflammatory mediator that is able to trigger processing and secretion of mature IL-1β in activated brain microglia (185). Finally, several randomized, double-blind controlled clinical trials provided strong evidence that vitamin E does not slow the progression of cognitive deterioration in aging people. In particular, vitamin E supplementation (1000 IU twice daily) for 3 years does not improve cognition or function in Down Syndrome patients older than 50 years of age (166), nor does it delay clinical progression to AD in 769 subjects with amnestic MCI (143).
Discrepancy among different studies and lack of definitive conclusions can be explained by considering that vitamin E is a general antioxidant, whose molecular mechanisms are not yet fully elucidated. In addition, vitamin E seems to have a hormetic profile, showing a typical inverted U-shaped dose–response curve, characterized by a low-dose protective effect and a high-dose harmful effect (27). Hormesis is an endogenous protective mechanism accounting for adaptability and plasticity of biological systems; the typical biphasic dose–response curve displayed by hormetic compounds may explain the limits experienced with numerous nutritional (including vitamin E supplementation studies) and pharmacological interventions (27). Finally, most of the studies underlining the vitamin E-dependent modulation of microglia neuroinflammatory cascades employed stabilized rice bran extract (RBE) as a vitamin E-enriched food (14, 70, 71, 180). It should, however, be recalled that, in addition to tocols, RBE contains other antioxidant compounds, including carotenoids, γ-oryzanol (a mixture of ferulic acid esters, triterpene alcohols, and phytosterols), and polyphenols (180); therefore, it is conceivable that the potential value of RBE as a nutraceutical for prevention of neuroinflammatory diseases may result from the synergistic interaction of multiple bioactive agents, rather than from the exclusive action of vitamin E.
Phytosterols
In the CNS, cholesterol mainly localizes in myelin sheaths surrounding the axons (∼70% of total cholesterol) and only a minority (the remaining 30%) resides within the plasma membrane of neurons and glial cells (55, 184); by modulating membrane fluidity and functionality, cholesterol plays a crucial role in cell-to-cell cross-talk, intracellular signaling, and synapse formation (144). The finding that impairment in cholesterol homeostasis is involved in neuroinflammatory disorders, together with the ability of plant sterols to cross the BBB, has powered research on the role of phytosterols in healthy and diseased CNS.
Sterols with a lower molecular side-chain complexity (e.g., campesterol) can easily cross the endothelial barrier and, then, are incorporated within lipid rafts of CNS parenchymal cells (202). By lowering the cholesterol content in lipid rafts, plant sterols (i) counteract oxidative stress (via estrogen receptor, PI3K, and glycogen synthase kinase-3β), by stimulating the expression of antioxidant molecules, such as glutathione transferase, glutathione peroxidase, and glutamyl cysteine ligase (174); (ii) prevent the proteolytic processing of APP, by inhibiting β- and γ-secretases, by decreasing β-site amyloid precursor protein cleaving enzyme 1 internalization to endosomal compartments, and by promoting migration of APP toward non-raft regions (24, 214).
The therapeutic potential during disease-related cognitive impairment can also be ascribed to the anti-inflammatory action, as reported in MS animal models, where plant sterol (60% sitosterol, 25% campesterol, and 15% stigmasterol) intake upregulates the expression of anti-inflammatory IL-10 and inhibits the expression of pro-inflammatory factors (CCL2, TNFα, IL-6, and IFNγ) (199). Phytosterols (including sitosterol, stigmasterol, schottenol, and fucosterol) inhibit inflammatory pathways in CNS infiltrating and resident immune cells, by activating liver X receptors (LXRs) that suppress inflammatory responses and, moreover, promote (together with activation of RXRγ) CNS repair processes and remyelination (59, 78, 100, 147). Impairment of LXR signaling has been shown to lead to progressive degeneration of motor neurons: At 7 months of age, LXRβ knock-out mice develop severe motor impairment closely resembling ALS (5). The strict link among LXR signaling, phytosterol/cholesterol metabolism, and neuroinflammatory diseases is also underlined by the finding that frequency of sporadic ALS seems to be higher among statin users with respect to non-users (58). Likely, statins impair LXR signaling, which, in turn, regulates the Niemann-Pick C1-like 1 protein (responsible for both cholesterol and phytosterol intestinal absorption) and adenosine triphosphate-binding cassette type 5 and 8 (responsible for efflux of cholesterol and phytosterols from enterocytes to the gut lumen), in an opposite manner (12). Therefore, LXR-dependent perturbations of cholesterol/phytosterol metabolism in the CNS will occur, and this might explain the increased risk of ALS observed in statin-treated patients (44, 119). Based on these data, a phytosterol-mediated activation of LXRs might be an interesting therapeutic tool for neurodegenerative and neuroinflammatory diseases, as natural sterols do not elicit the severe side-effects (i.e., hepatic hypertriglyceridemia and liver steatosis) that are usually observed with synthetic LXR agonists (67). However, more research is needed since the transfer of different plant sterols to the CNS is limited.
It should be recalled, however, that some phytosterols (in particular, β-sitosterol) may shift the balance between neuroprotective and neurotoxic effects, after structural modifications. For example, β-sitosterol protects neurons and glial cells from oxidative stress and lipid peroxidation (174), but its oxidative or glycosylated derivatives are neurotoxic, via glutamate excitotoxicity, ROS generation, and cell death (118, 141, 190). In particular, glycosylation hampers the binding and activation of LXRs, thereby masking the neuroprotective effect of phytosterols. In line with this hypothesis, administration of β-sitosterol worsened the ALS-like phenotype in LXRβ−/− mice (93) and excessive dietary intake of β-sitosterol-β-D-glucoside present in cycad seeds (Cycas circinalis) was implicated as the cause of ALS/parkinsonism-dementia complex observed in specific geographic areas (116, 190).
Conclusions
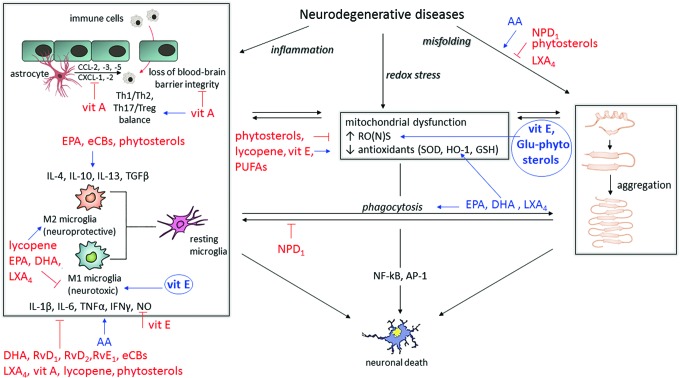
A wealth of literature data highlight the crucial role played by essential lipids in maintaining brain health, as they modulate both neuronal plasticity and immune activity of glial cells, so that dysfunction in lipid metabolism contributes to the onset and progression of neurodegenerative diseases that are characterized by neuroinflammatory pathways (Fig. 6). For example, epidemiological data indicate a higher risk of cognitive decline in people who have lower intake or blood levels of ω3-PUFAs (46), and an inverse association between plasma EPA and lower gray matter atrophy and slower cognitive decline has been reported in aged people (164, 165).
FIG. 6.
Main effects of dietary bioactive lipids on hallmarks of neurodegeneration. The three major culprits of neurodegenerative diseases (inflammation, redox stress, and protein misfolding) are depicted in black boxes. Stimulating effects of different lipids are shown in blue; inhibitory effects of different lipids are shown in red. Circles represent potential harmful effects that are derived from either elevated concentrations or chemical modifications. AP-1, activator protein-1; CXCL, chemokine (C-X-C motif) ligand; Glu-phytosterols, glycosylated phytosterols; GSH, glutathione; HO-1, heme oxygenase-1; NF-κB, nuclear factor-κB; vit, vitamin. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Based on these findings, different studies have suggested that a nutritional approach might be useful to prevent and/or treat such pathologies. Nonetheless, available data are somewhat controversial and far from being conclusive. Regarding ω3-PUFAs (which are the best investigated), traditionally there has been a lack of discrimination between different compounds, so that effects have been broadly attributed to this group of molecules as a whole. This is the case for the majority of clinical trials enrolling healthy older adults, individuals with MCI or AD: In these trials, mixed EPA and DHA preparations and at different ratios have usually been used (85). Though this approach does not allow one to discriminate among different compounds, one has to take into account that dietetic sources contain a mix of all PUFAs; therefore, to formulate a nutritional approach for preventing/curing neuroinflammatory diseases, it may not be central to understand what is doing what, also because a synergistic action between the different compounds may actually do the job. In addition, many questions about the ideal ω3/ω6-PUFA ratio remain to be addressed before drawing conclusions: What is the real link between AA and DHA? May bioavailability of different FAs account for selective incorporation into brain phospholipids? As they show somewhat overlapping effects, may an increase of dietary DHA limit the AA-induced neurodegenerative effects? Answering these questions is deemed necessary to fully understand the involvement of ω3/ω6-PUFAs in the onset and progression of neurodegeneration.
In addition, some useful effects of antioxidant vitamins and phytosterols need to be better clarified, before translating in vivo data to clinical practice. In the first case, the positive activation of the RXR receptor has led to a consideration of its synthetic agonist bexarotene, already approved by the U.S. Food and Drug Administration for cancer treatment, as a candidate for AD clinical trials. Although bexarotene possesses documented beneficial effects in AD animal models (where it facilitates intracellular Aβ clearance, reduces neuron loss, upregulates markers of synaptic integrity, and improves cognition), multiple failures have been reported, particularly in the context of apolipoprotein E genotype representing one of the most potent risk factors for AD (9, 43, 135). These negative data highlight, once again, the complexity of molecular mechanisms underlying neurodegenerative diseases, so that the synergistic action of dietary antioxidant vitamins should be more effective than direct activation of a single target with a synthetic drug. With respect to plant sterols, another caveat should be kept in mind, that is, detailed knowledge of its chemical structure and dynamic properties before planning to exploit it as a potential therapeutic.
Although there is no doubt that a nutritional strategy would be advisable to ameliorate the quality of life of patients, other factors are involved in the pathogenesis of neuroinflammatory diseases. The overall picture is, therefore, more complex than expected, and nutritional intervention should simply accompany pharmacological therapies, to obtain beneficial effects in diseased people.
Abbreviations Used
- αSyn
α-synuclein
- 2-AG
2-arachidonoylglycerol
- 6-OHDA
6-hydroxydopamine
- AA
arachidonic acid
- Aβ
β-amyloid
- AβPP
amyloid β protein precursor
- ABHD6
alpha/beta-hydrolase domain containing 6
- AD
Alzheimer's disease
- AEA
N-arachidonoyl-ethanolamine
- Akt
protein kinase B
- ALA
α-linolenic acid
- ALS
amyotrophic lateral sclerosis
- AP-1
activator protein-1
- APP
amyloid precursor protein
- BBB
blood–brain barrier
- CB
cannabinoid
- CCL
chemokine (C-C motif) ligand
- CNS
central nervous system
- COX
cyclooxygenase
- CXCL
chemokine (C-X-C motif) ligand
- DAGL-α
diacylglycerol lipase-α
- DHA
docosahexaenoic acid
- EAE
autoimmune encephalomyelitis
- eCBs
endocannabinoids
- EMT
endocannabinoid membrane transporter
- EPA
eicosapentaenoic acid
- ER
endoplasmic reticulum
- etNH2
ethanolamine
- FAAH
fatty acid amide hydrolase
- FUS
Fused-in-Sarcoma
- GSH/GSSG
reduced GSH/oxidized GSH
- HO-1
heme oxygenase-1
- IFN-γ
interferon gamma
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- LA
linoleic acid
- LOX
lipoxygenase
- LPS
lipopolysaccharide
- LTD
long-term depression
- LTP
long-term potentiation
- LX
lipoxin
- LXR
liver X receptor
- MAGL
monoacylglycerol lipase
- MCI
mild cognitive impairment
- MPP+
1-methyl-4-phenylpyridinium ion
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MS
multiple sclerosis
- NAPE-PLD
N-acyl-phosphatidylethanolamines-hydrolyzing phospholipase D
- NF-κB
nuclear factor-κB
- NO
nitric oxide
- NPD1
neuroprotectin D1
- PD
Parkinson's disease
- PG
prostaglandin
- PI3K
phosphoinositide 3 kinase
- PLA2
phospholipase A2
- PPAR
peroxisome proliferator-activated receptor
- PS1
presenilin 1
- PUFA
polyunsaturated fatty acid
- RBE
rice bran extract
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- RXR
retinoid X receptor
- SNpc
substantia nigra pars compacta
- SOD1
superoxide dismutase 1
- TARDBP
TAR DNA binding protein
- TGFβ
transforming growth factor beta
- Th
T helper
- TLR
toll-like receptor
- TNFα
tumor necrosis factor alpha
- Treg
T regulatory
Acknowledgment
This investigation was partly supported by the Italian Ministry of Education, University and Research (MIUR), under PRIN 2015 competitive grant to T.B. (local PI) and M.M. (project coordinator).
References
- 1.Abate G, Marziano M, Rungratanawanich W, Memo M, and Uberti D. Nutrition and AGE-ing: focusing on Alzheimer's disease. Oxid Med Cell Longev 2017: 7039816, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdolahi M, Yavari P, Honarvar NM, Bitarafan S, Mahmoudi M, and Saboor-Yaraghi AA. Molecular mechanisms of the action of vitamin A in Th17/Treg axis in multiple sclerosis. J Mol Neurosci 57: 605–613, 2015 [DOI] [PubMed] [Google Scholar]
- 3.ADAPT Research Group, Martin BK, Szekely C, Brandt J, Piantadosi S, Breitner JC, Craft S, Evans D, Green R, and Mullan M. Cognitive function over time in the Alzheimer's disease Anti-inflammatory Prevention Trial (ADAPT): results of a randomized, controlled trial of naproxen and celecoxib. Arch Neurol 65: 896–905, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alzheimer's Disease Anti-inflammatory Prevention Trial Research Group. Results of a follow-up study to the randomized Alzheimer's Disease Anti-inflammatory Prevention Trial (ADAPT). Alzheimers Dement 9: 714–723, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersson S, Gustafsson N, Warner M, and Gustafsson JA. Inactivation of liver X receptor beta leads to adult-onset motor neuron degeneration in male mice. Proc Natl Acad Sci U S A 102: 3857–3862, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arima H, Omura T, Hayasaka T, Masaki N, Hanada M, Xu D, Banno T, Kobayashi K, Takeuchi H, Kadomatsu K, Matsuyama Y, and Setou M. Reductions of docosahexaenoic acid-containing phosphatidylcholine levels in the anterior horn of an ALS mouse model. Neuroscience 297: 127–136, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Arsenault D, Julien C, Tremblay C, and Calon F. DHA improves cognition and prevents dysfunction of entorhinal cortex neurons in 3xTg-AD mice. PLoS One 6: e17397, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asakura K, Ueda A, and Mutoh T. Lipid rafts and their possible involvements in neuroimmunological disorders. Front Biosci 20: 303–313, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Balducci C, Paladini A, Micotti E, Tolomeo D, La Vitola P, Grigoli E, Richardson JC, and Forloni G. The continuing failure of bexarotene in Alzheimer's disease mice. J Alzheimers Dis 46: 471–482, 2015 [DOI] [PubMed] [Google Scholar]
- 10.Bazinet RP. and Laye S. Polyunsaturated fatty acids and their metabolites in brain function and disease Nat Rev Neurosci 15: 771–785, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Behl C, Davies JB, Lesley R, and Schubert D. Hydrogen peroxide mediates amyloid protein toxicity. Cell 77: 817–827, 1994 [DOI] [PubMed] [Google Scholar]
- 12.Bełtowski J. Statins and ALS: the possible role of impaired LXR signalling. Med Sci Monit 16: RA73–RA78, 2010 [PubMed] [Google Scholar]
- 13.Benek O, Aitken L, Hroch L, Kuca K, Gunn-Moore F, and Musilek K. A direct interaction between mitochondrial proteins and amyloid-β peptide and its significance for the progression and treatment of Alzheimer's disease. Curr Med Chem 22: 1056–1085, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Bhatia HS, Baron J, Hagl S, Eckert GP, and Fiebich BL. Rice bran derivatives alleviate microglia activation: possible involvement of MAPK pathway. J Neuroinflammation 13: 148–163, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bisogno T. and Maccarrone M. Endocannabinoid signaling and its regulation by nutrients. Biofactors 40: 373–380, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Bisogno T, Oddi S, Piccoli A, Fazio D, and Maccarrone M. Type-2 cannabinoid receptors in neurodegeneration. Pharmacol Res 111: 721–730, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Bitarafan S, Saboor-Yaraghi A, Sahraian MA, Nafissi S, Togha M, Beladi Moghadam N, Roostaei T, Siassi F, Eshraghian MR, Ghanaati H, Jafarirad S, Rafiei B, and Harirchian MH. Impact of Vitamin A supplementation on disease progression in patients with multiple sclerosis. Arch Iran Med 18: 435–440, 2015 [PubMed] [Google Scholar]
- 18.Bitarafan S, Saboor-Yaraghi A, Sahraian MA, Soltani D, Nafissi S, Togha M, Beladi Moghadam N, Roostaei T, Mohammadzadeh Honarvar N, and Harirchian MH. Effect of vitamin A supplementation on fatigue and depression in multiple sclerosis patients: a double-blind placebo-controlled clinical trial. Iran J Allergy Asthma Immunol 15: 13–19, 2016 [PubMed] [Google Scholar]
- 19.Bitto A, Giuliani D, Pallio G, Irrera N, Vandini E, Canalini F, Zaffe D, Ottani A, Minutoli L, Rinaldi M, Guarini S, Squadrito F, and Altavilla D. Effects of COX1-2/5-LOX blockade in Alzheimer transgenic 3xTg-AD mice. Inflamm Res 66: 389–398, 2017 [DOI] [PubMed] [Google Scholar]
- 20.Boche D, Perry VH, and Nicoll JA. Review: activation patterns of microglia and their identification in the human brain. Neuropath Appl Neuro 39: 3–18, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Bourdel-Marchasson I, Delmas-Beauvieux MC, Peuchant E, Richard-Harston S, Decamps A, Reignier B, Emeriau JP, and Rainfray M. Antioxidant defences and oxidative stress markers in erythrocytes and plasma from normally nourished elderly Alzheimer patients. Age Ageing 30: 235–241, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Bousquet M, Calon F, and Cicchetti F. Impact of ω-3 fatty acids in Parkinson's disease. Ageing Res Rev 10: 453–463, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Brigelius-Flohe R. and Traber MG. Vitamin E: function and metabolism. FASEB J 13: 1145–1155, 1999 [PubMed] [Google Scholar]
- 24.Burg VK, Grimm HS, Rothhaar TL, Grosgen S, Hundsdorfer B, Haupenthal VJ, Zimmer VC, Mett J, Weingärtner O, Laufs U, Broersen LM, Tanila H, Vanmierlo T, Lütjohann D, Hartmann T, and Grimm MO. Plant sterols the better cholesterol in Alzheimer's disease? A mechanistical study. J Neurosci 33: 16072–16087, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bush AI, Masters CL, and Tanzi RE. Copper, beta-amyloid, and Alzheimer's disease: tapping a sensitive connection. Proc Natl Acad Sci U S A 100: 11193–11194, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butterworth RF. Altered glial–neuronal crosstalk: cornerstone in the pathogenesis of hepatic encephalopathy. Neurochem Int 57: 383–388, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Calabrese EJ, Dhawan G, Kapoor R, Iavicoli I, and Calabrese V. What is hormesis and its relevance to healthy aging and longevity? Biogerontology 16: 693–707, 2015 [DOI] [PubMed] [Google Scholar]
- 28.Calabrese V, Cornelius C, Dinkova-Kostova AT, Calabrese EJ, and Mattson MP. Cellular stress responses, the hormesis paradigm, and vitagenes: novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid Redox Signal 13: 1763–1811, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calandria JM, Sharp MW, and Bazan NG. The docosanoid neuroprotectin D1 induces TH-positive neuronal survival in a cellular model of Parkinson's disease. Cell Mol Neurobiol 35: 1127–1136, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calder PC. Fatty acids and inflammation: the cutting edge between food and pharma. Eur J Pharmacol 668: S50–S58, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Centonze D, Bari M, Rossi S, Prosperetti C, Furlan R, Fezza F, De Chiara V, Battistini L, Bernardi G, Bernardini S, Martino G, and Maccarrone M. The endocannabinoid system is dysregulated in multiple sclerosis and in experimental autoimmune encephalomyelitis. Brain 130: 2543–2553, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Chaboub LS. and Deneen B. Astrocyte form and function in the developing central nervous system. Semin Pediatr Neurol 20: 230–235, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chakrabarty P, Li A, Ceballos-Diaz C, Eddy JA, Funk CC, Moore B, DiNunno N, Rosario AM, Cruz PE, Verbeeck C, Sacino A, Nix S, Janus C, Price ND, Das P, and Golde TE. IL-10 alters immunoproteostasis in APP mice, increasing plaque burden and worsening cognitive behavior. Neuron 85: 519–533, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen CT, Green JT, Orr SK, and Bazinet RP. Regulation of brain polyunsaturated fatty acid uptake and turnover. Prostaglandins Leukot Essent Fatty Acids 79: 85–91, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Chen S, Zhang H, Pu H, Wang G, Li W, Leak RK, Chen J, Liou AK, and Hu X. n-3 PUFA supplementation benefits microglial responses to myelin pathology. Sci Rep 4: 7458, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiu CC, Su KP, Cheng TC, Liu HC, Chang CJ, Dewey ME, Stewart R, and Huang SY. The effects of omega-3 fatty acids monotherapy in Alzheimer's disease and mild cognitive impairment: a preliminary randomized double-blind placebo-controlled study. Prog Neuropsychopharmacol Biol Psychiatry 32: 1538–1544, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Chiurchiù V, Cencioni MT, Bisicchia E, De Bardi M, Gasperini C, Borsellino G, Centonze D, Battistini L, and Maccarrone M. Distinct modulation of human myeloid and plasmacytoid dendritic cells by anandamide in multiple sclerosis. Ann Neurol 73: 626–636, 2013 [DOI] [PubMed] [Google Scholar]
- 38.Chiurchiù V, Leuti A, Cencioni MT, Albanese M, De Bardi M, Bisogno T, Centonze D, Battistini L, and Maccarrone M. Modulation of monocytes by bioactive lipid anandamide in multiple sclerosis involves distinct toll-like receptors. Pharmacol Res 113: 313–319, 2016 [DOI] [PubMed] [Google Scholar]
- 39.Chiurchiù V, Leuti A, and Maccarrone M. Cannabinoid signaling and neuroinflammatory diseases: a melting pot for the regulation of brain immune responses. J Neuroimmune Pharmacol 10: 268–280, 2015 [DOI] [PubMed] [Google Scholar]
- 40.Chiurchiù V. and Maccarrone M. Bioactive lipids as modulators of immunity, inflammation and emotions. Curr Opin Pharmacol 29: 54–62, 2016 [DOI] [PubMed] [Google Scholar]
- 41.Corcoran J, So PL, and Maden M. Absence of retinoids can induce motoneuron disease in the adult rat and a retinoid defect is present in motoneuron disease patients. J Cell Sci 115: 4735–4741, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Correa F, Hernangómez-Herrero M, Mestre L, Loría F, Docagne F, and Guaza C. The endocannabinoid anandamide downregulates IL-23 and IL-12 subunits in a viral model of multiple sclerosis: evidence for a cross-talk between IL-12p70/IL-23 axis and IL-10 in microglial cells. Brain Behav Immun 25: 736–749, 2011 [DOI] [PubMed] [Google Scholar]
- 43.Cummings JL, Zhong K, Kinney JW, Heaney C, Moll-Tudla J, Joshi A, Pontecorvo M, Devous M, Tang A, and Bena J. Double-blind, placebo-controlled, proof-of-concept trial of bexarotene Xin moderate Alzheimer's disease. Alzheimers Res Ther 8: 4, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cutler RG, Pedersen WA, Camandola S. Rothstein JD, and Mattson MP. Evidence that accumulation of ceramides and cholesterol esters mediates oxidative stress-induced death of motor neurons in amyotrophic lateral sclerosis. Ann Neurol 52: 448–457, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Czeh M, Gressens P, and Kaindl AM. The yin and yang of microglia. Dev Neurosci 33: 199–209, 2011 [DOI] [PubMed] [Google Scholar]
- 46.Dacks PA, Shineman DW, and Fillit HM. Current evidence for the clinical use of long-chain polyunsaturated n-3 fatty acids to prevent age-related cognitive decline and Alzheimer's disease. J Nutr Health Aging 17: 240–251, 2013 [DOI] [PubMed] [Google Scholar]
- 47.Datta S, Jamwal S, Deshmukh R, and Kumar P. Beneficial effects of lycopene against haloperidol induced orofacial dyskinesia in rats: possible neurotransmitters and neuroinflammation modulation. Eur J Pharmacol 771: 229–235, 2016 [DOI] [PubMed] [Google Scholar]
- 48.Deardorff WJ. and Grossberg GT. Targeting neuroinflammation in Alzheimer's disease: evidence for NSAIDs and novel therapeutics. Expert Rev Neurother 17: 17–32, 2017 [DOI] [PubMed] [Google Scholar]
- 49.Delattre AM, Carabelli B, Mori MA, Kempe PG, Rizzo de Souza LE, Zanata SM, Machado RB, Suchecki D, Andrade da Costa BL, Lima MM, and Ferraz AC. Maternal omega-3 supplement improves dopaminergic system in pre- and postnatal inflammation-induced neurotoxicity in Parkinson's disease model. Mol Neurobiol 54: 2090–2106, 2016 [DOI] [PubMed] [Google Scholar]
- 50.Delattre AM, Kiss A, Szawka RE, Anselmo-Franci JA, Bagatini PB, Xavier LL, Rigon P, Achaval M, Iagher F, de David C, Marroni NA, and Ferraz AC. Evaluation of chronic omega-3 fatty acids supplementation on behavioral and neurochemical alterations in 6-hydroxydopamine-lesion model of Parkinson's disease. Neurosci Res 66: 256–264, 2010 [DOI] [PubMed] [Google Scholar]
- 51.Delpech JC, Madore C, Joffre C, Aubert A, Kang JX, Nadjar A, and Layé S. Transgenic increase in n-3/n-6 fatty acid ratio protects against cognitive deficits induced by an immune challenge through decrease of neuroinflammation. Neuropsychopharmacology 40: 525–536, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Demar JC, Jr, Lee H-J, Ma K, Chang L, Bell JM, Rapoport SI, and Bazinet RP. Brain elongation of linoleic acid is a negligible source of the arachidonate in brain phospholipids of adult rats. Biochim Biophys Acta 1761: 1050–1059, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Demar JC, Jr, Ma K, Chang L, Bell JM, and Rapoport SI. α-Linolenic acid does not contribute appreciably to docosahexaenoic acid within brain phospholipids of adult rats fed a diet enriched in docosahexaenoic acid. J Neurochem 94: 1063–1076, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Devore EE, Grodstein F, van Rooij FJ, Hofman A, Stampfer MJ, Witteman JC, and Breteler MM. Dietary antioxidants and longterm risk of dementia. Arch Neurol 67: 819–825, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dietschy JM. Central nervous system: cholesterol turnover, brain development and neurodegeneration. Biol Chem 390: 287–293, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dietschy JM. and Turley SD. Thematic review series: brain lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J Lipid Res 45: 1375–1397, 2004 [DOI] [PubMed] [Google Scholar]
- 57.Dunn HC, Ager RR, Baglietto-Vargas D, Cheng D, Kitazawa M, Cribbs DH, and Medeiros R. Restoration of lipoxin A4 signaling reduces Alzheimer's disease-like pathology in the 3xTg-AD mouse model. J Alzheimers Dis 43: 893–903, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Edwards IR, Star K, and Kiuru A. Statins, neuromuscular degenerative disease and an amyotrophic lateral sclerosis-like syndrome: an analysis of individual case safety reports from vigibase. Drug Safety 30: 515–525, 2007 [DOI] [PubMed] [Google Scholar]
- 59.El Kharrassi Y, Samadi M, Lopez T, Nury T, El Kebbaj R, Andreoletti P, El Hajj HI, Vamecq J, Moustaid K, Latruffe N, El Kebbaj MS, Masson D, Lizard G, Nasser B, and Cherkaoui-Malki M. Biological activities of schottenol and spinasterol, two natural phytosterols present in argan oil and in cactus pear seed oil, on murine microglial BV2 cells. Biochem Biophys Res Commun 446: 798–804, 2014 [DOI] [PubMed] [Google Scholar]
- 60.Farina C, Aloisi F, and Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol 28: 138–145, 2007 [DOI] [PubMed] [Google Scholar]
- 61.Farmer K, Smith CA, Hayley S, and Smith J. Major alterations of phosphatidylcholine and lysophosphotidylcholine lipids in the substantia nigra using an early stage model of Parkinson's disease. Int J Mol Sci 16: 18865–18877, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fernández-Ruiz J, Romero J, and Ramos JA. Endocannabinoids and neurodegenerative disorders: Parkinson's disease, Huntington's Chorea, Alzheimer's disease, and others. Handb Exp Pharmacol 231: 233–259, 2015 [DOI] [PubMed] [Google Scholar]
- 63.Ferrari CC, Pott Godoy MC, Tarelli R, Chertoff M, Depino AM, and Pitossi FJ. Progressive neurodegeneration and motor disabilities induced by chronic expression of IL-1beta in the substantia nigra. Neurobiol Dis 24: 183–193, 2006 [DOI] [PubMed] [Google Scholar]
- 64.Fitzgerald KC, O'Reilly ÉJ, Falcone GJ, McCullough ML, Park Y, Kolonel LN, and Ascherio A. Dietary ω-3 polyunsaturated fatty acid intake and risk for amyotrophic lateral sclerosis. JAMA Neurol 71: 1102–1110, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Freund-Levi Y, Eriksdotter-Jönhagen M, Cederholm T, Basun H, Faxén-Irving G, Garlind A, Vedin I, Vessby B, Wahlund LO, and Palmblad J. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: a randomized double-blind trial. Arch Neurol 63: 1402–1408, 2006 [DOI] [PubMed] [Google Scholar]
- 66.Galano JM, Lee JC, Gladine C, Comte B, Le Guennec JY, Oger C, and Durand T. Non-enzymatic cyclic oxygenated metabolites of adrenic, docosahexaenoic, eicosapentaenoic and α-linolenic acids; bioactivities and potential use as biomarkers. Biochim Biophys Acta 1851: 446–455, 2015 [DOI] [PubMed] [Google Scholar]
- 67.Grefhorst A, Elzinga BM, Voshol PJ, Plosch T, Kok T, Bloks VW, van der Sluijs FH, Havekes LM, Romijn JA, Verkade HJ, and Kuipers F. Stimulation of lipogenesis by pharmacological activation of the liver X receptor leads to production of large, triglyceride-rich very low density lipoprotein particles. J Biol Chem 277: 34182–34190, 2002 [DOI] [PubMed] [Google Scholar]
- 68.Guest J, Grant R, Garg M, Mori TA, Croft KD, and Bilgin A. Cerebrospinal fluid levels of inflammation, oxidative stress and NAD+ are linked to differences in plasma carotenoid concentrations. J Neuroinflammation 11: 117–127, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guillot-Sestier MV, Doty KR, Gate D, Rodriguez J, Jr, Leung BP, Rezai-Zadeh K, and Town T. Il10 deficiency rebalances innate immunity to mitigate Alzheimer-like pathology. Neuron 85: 534–548, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hagl S, Berressem D, Grewal R, Sus N, Frank J, and Eckert GP. Rice bran extract improves mitochondrial dysfunction in brains of aged NMRI mice. Nutr Neurosci 19: 1–10, 2015 [DOI] [PubMed] [Google Scholar]
- 71.Hagl S, Grewal R, Ciobanu I, Helal A, Khayyal MT, Muller WE, and Eckert GP. Rice bran extract compensates mitochondrial dysfunction in a cellular model of early Alzheimer's disease. J Alzheimers Dis 43: 927–938, 2015 [DOI] [PubMed] [Google Scholar]
- 72.Heneka MT, Carson MJ, and Khoury JE. Neuroinflammation in Alzheimer's disease. Lancet Neurol 14: 388–405, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hirsch EC, Vyas S, and Hunot S. Neuroinflammation in Parkinson's disease. Parkinsonism Relat Disord 18: S210–S212, 2012 [DOI] [PubMed] [Google Scholar]
- 74.Hjorth E, Zhu M, Toro VC, Vedin I, Palmblad J, Cederholm T, Freund-Levi Y, Faxen-Irving G, Wahlund LO, Basun H, Eriksdotter M, and Schultzberg M. Omega-3 fatty acids enhance phagocytosis of Alzheimer's disease-related amyloid-β42 by human microglia and decrease inflammatory markers. J Alzheimers Dis 35: 697–713, 2013 [DOI] [PubMed] [Google Scholar]
- 75.Hoare S, Lithander F, van der Mei I, Ponsonby AL, and Lucas R; Ausimmune Investigator Group. Higher intake of omega-3 polyunsaturated fatty acids is associated with a decreased risk of a first clinical diagnosis of central nervous system demyelination: results from the ausimmune study. Mult Scler 22: 884–892, 2016 [DOI] [PubMed] [Google Scholar]
- 76.Hsiao HY, Chen YC, Chen HM, Tu PH, and Chern YA. Critical role of astrocyte-mediated nuclear factor-κB-dependent inflammation in Huntington's disease. Hum Mol Genet 22: 1826–1842, 2013 [DOI] [PubMed] [Google Scholar]
- 77.Hsieh HL. and Yang CM. Role of redox signaling in neuroinflammation and neurodegenerative diseases. Biomed Res Int 2013: 484613, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang JK, Jarjour AA, Nait Oumesmar B, Kerninon C, Williams A, Krezel W, Kagechika H, Bauer J, Zhao C, Baron-Van Evercooren A, Chambon P, Ffrench-Constant C, and Franklin RJ. Retinoid X receptor gamma signaling accelerates CNS remyelination. Nat Neurosci 14: 45–53, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Iannotti FA, Di Marzo V, and Petrosino S. Endocannabinoids and endocannabinoid-related mediators: targets, metabolism and role in neurological disorders. Prog Lipid Res 62: 107–128, 2016 [DOI] [PubMed] [Google Scholar]
- 80.Igarashi M, Ma K, Gao F, Kim HW, Greenstein D, Rapoport SI, and Rao JS. Brain lipid concentrations in bipolar disorder. J Psychiat Res 44: 177–182, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ilieva EV, Ayala V, Jove M, Dalfo E, Cacabelos D, Povedano M, Bellmunt MJ, Ferrer I, Pamplona R, and Portero-Otin M. Oxidative and endoplasmic reticulum stress interplay in sporadic amyotrophic lateral sclerosis Brain 130: 3111–3123, 2007 [DOI] [PubMed] [Google Scholar]
- 82.Ismail MF. and Mohamed HM. Modulatory effect of lycopene on deltamethrininduced testicular injury in rats. Cell Biochem Biophys 65: 425–432, 2013 [DOI] [PubMed] [Google Scholar]
- 83.Jelinek GA, Hadgkiss EJ, Weiland TJ, Pereira NG, Marck CH, and van der Meer DM. Association of fish consumption and Omega 3 supplementation with quality of life, disability and disease activity in an international cohort of people with multiple sclerosis. Int J Neurosci 123: 792–800, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jiang Q. Natural forms of vitamin E: metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic Biol Med 72: 76–90, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jiao J, Li Q, Chu J, Zeng W, Yang M, and Zhu S. Effect of n-3 PUFA supplementation on cognitive function throughout the life span from infancy to old age: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr 100: 1422–1436, 2014 [DOI] [PubMed] [Google Scholar]
- 86.Ju J, Picinich SC, Yang ZH, Zhao Y, Suh N, Kong AN, and Yang CS. Cancer-preventive activities of tocopherols and tocotrienols. Carcinogenesis 31: 533–542, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kalmijn S, van Boxtel MP, Ocké M, Verschuren WM, Kromhout D, and Launer LJ. Dietary intake of fatty acids and fish in relation to cognitive performance at middle age. Neurology 62: 275–280, 2004 [DOI] [PubMed] [Google Scholar]
- 88.Kassa RM, Mariotti R, Bonaconsa M, Bertini G, and Bentivoglio M. Gene, cell and axon changes in the familial amyotrophic lateral sclerosis mouse sensorimotor cortex. J Neuropathol Exp Neurol 68: 59–72, 2009 [DOI] [PubMed] [Google Scholar]
- 89.Kawashima A, Harada T, Imada K, Yano T, and Mizuguchi K. Eicosapentaenoic acid inhibits interleukin-6 production in interleukin-1beta-stimulated C6 glioma cells through peroxisome proliferator-activated receptor-gamma. Prostaglandins Leukot Essent Fatty Acids 79: 59–65, 2008 [DOI] [PubMed] [Google Scholar]
- 90.Khanna S, Heigel M, Weist J, Gnyawali S, Teplitsky S, Roy S, Sen CK, and Rink C. Excessive α-tocopherol exacerbates microglial activation and brain injury caused by acute ischemic stroke. FASEB J 29: 828–836, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Khanna S, Patel V, Rink C, Roy S, and Sen CK. Delivery of orally supplemented alpha-tocotrienol to vital organs of rats and tocopherol-transport protein deficient mice. Free Radic Biol Med 39: 1310–1319, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kihara Y, Matsushita T, Kita Y, Uematsu S, Akira S, Kira J, Ishii S, and Shimizu T. Targeted lipidomics reveals mPGES-1-PGE2 as a therapeutic target for multiple sclerosis. Proc Natl Acad Sci U S A 106: 21807–21812, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim HJ, Fan X, Gabbi C. Yakimchuk K, Parini P, Warner M, and Gustafsson JA. Liver X receptor beta (LXRb): a link between beta-sitosterol and amyotrophic lateral sclerosis-Parkinson's dementia. Proc Natl Acad Sci U S A 105: 2094–2099, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kolarcik CL. and Bowser R. Retinoid signaling alterations in amyotrophic lateral sclerosis. Am J Neurodegener Dis 1: 130–145, 2012 [PMC free article] [PubMed] [Google Scholar]
- 95.Kong W, Hooper KM, and Ganea D. The natural dual cyclooxygenase and 5-lipoxygenase inhibitor flavocoxid is protective in EAE through effects on Th1/Th17 differentiation and macrophage/microglia activation. Brain Behav Immun 53: 59–71, 2016 [DOI] [PubMed] [Google Scholar]
- 96.Kong W, Yen JH, and Ganea D. Docosahexaenoic acid prevents dendritic cell maturation, inhibits antigen-specific Th1/Th17 differentiation and suppresses experimental autoimmune encephalomyelitis. Brain Behav Immun 25: 872–882, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kurtys E, Eisel ULM, Verkuyl JM, Broersen LM, Dierckx RAJO, and de Vries EFJ. The combination of vitamins and omega-3 fatty acids has an enhanced anti-inflammatory effect on microglia. Neurochem Int 99: 206–214, 2016 [DOI] [PubMed] [Google Scholar]
- 98.Laye S. Polyunsaturated fatty acids, neuroinflammation and well being. Prostaglandins Leukot Essent Fatty Acids 82: 295–303, 2010 [DOI] [PubMed] [Google Scholar]
- 99.Lee J, Koo N, and Min DB. Reactive oxygen species, aging, and antioxidative nutraceuticals. Compr Rev Food Sci Food Saf 3: 21–33, 2004 [DOI] [PubMed] [Google Scholar]
- 100.Lee JH, Park SM, Kim OS, Lee CS, Woo JH, Park SJ, Joe EH, and Jou I. Differential SUMOylation of LXRalpha and LXRbeta mediates transrepression of STAT1 inflammatory signaling in IFN-gamma-stimulated brain astrocytes. Mol Cell 35: 806–817, 2009 [DOI] [PubMed] [Google Scholar]
- 101.Leng X, Kinnun JJ, Marquardt D, Ghefli M, Kučerka N, Katsaras J, Atkinson J, Harroun TA, Feller SE, and Wassall SR. α-Tocopherol is well designed to protect polyunsaturated phospholipids: MD simulations. Biophys J 109: 1608–1618, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Levental I. and Veatch SL. The continuing mystery of lipid rafts. J Mol Biol 428: 4749–4764, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li J, O W, Li W, Jiang ZG, and Ghanbari HA. Oxidative stress and neurodegenerative disorders. Int J Mol Sci 14: 24438–24475, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li L, Wu Y, Wang Y, Wu J, Song L, Xian W, Yuan S, Pei L, and Shang Y. Resolvin D1 promotes the interleukin-4-induced alternative activation in BV-2 microglial cells. J Neuroinflammation 11: 72, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lin HY, Huang BR, Yeh WL, Lee CH, Huang SS, Lai CH, Lin H, and Lu DY. Antineuroinflammatory effects of lycopene via activation of adenosine monophosphate-activated protein kinase-α1/heme oxygenase-1 pathways. Neurobiol Aging 35: 191–202, 2014 [DOI] [PubMed] [Google Scholar]
- 106.Lu DY, Tsao YY, Leung YM, and Su KP. Docosahexaenoic acid suppresses neuroinflammatory responses and induces heme oxygenase-1 expression in BV-2 microglia: implications of antidepressant effects for omega-3 fatty acids. Neuropsychopharmacology 35: 2238–2248, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lucin KM. and Wyss-Coray T. Immune activation in brain aging and neurodegeneration: too much or too little? Neuron 64: 110–122, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lugrin J, Rosenblatt-Velin N, Parapanov R, and Liaudet L. The role of oxidative stress during inflammatory processes. Biol Chem 395: 203–230, 2014 [DOI] [PubMed] [Google Scholar]
- 109.Lynch AM, Loane DJ, Minogue AM, Clarke RM, Kilroy D, Nally RE, Roche OJ, O'Connell F, and Lynch MA. Eicosapentaenoic acid confers neuroprotection in the amyloid-beta challenged aged hippocampus. Neurobiol Aging 28: 845–855, 2007 [DOI] [PubMed] [Google Scholar]
- 110.Lynch T, Cherny RA, and Bush AI. Oxidative processes in Alzheimer's disease: the role of abeta-metal interactions. Exp Gerontol 35: 445–451, 2000 [DOI] [PubMed] [Google Scholar]
- 111.Maccarrone M, Guzmán M, Mackie K, Doherty P, and Harkany T. Programming of neural cells by (endo)cannabinoids: from physiological rules to emerging therapies. Nat Rev Neurosci 15: 786–801, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mangialasche F, Kivipelto M, Mecocci P, Rizzuto D, Palmer K, Winblad B, and Fratiglioni L. High plasma levels of vitamin E forms and reduced Alzheimer's disease risk in advanced age. J Alzheimers Dis 20: 1029–1037, 2010 [DOI] [PubMed] [Google Scholar]
- 113.Maroof N, Ravipati S, Pardon MC, Barrett DA, and Kendall DA. Reductions in endocannabinoid levels and enhanced coupling of cannabinoid receptors in the striatum are accompanied by cognitive impairments in the AβPPswe/PS1ΔE9 mouse model of Alzheimer's disease. J Alzheimers Dis 42: 227–245, 2014 [DOI] [PubMed] [Google Scholar]
- 114.Martin A, Youdim K, Szprengiel A, Shukitt-Hale B, and Joseph J. Roles of vitamins E and C on neurodegenerative diseases and cognitive performance. Nutr Rev 60: 308–326, 2002 [DOI] [PubMed] [Google Scholar]
- 115.Mazlan M, Sue Mian T, Mat Top G, and Zurinah Wan Ngah W. Comparative effects of alpha-tocopherol and gamma-tocotrienol against hydrogen peroxide induced apoptosis on primary-cultured astrocytes. J Neurol Sci 243: 5–12, 2006 [DOI] [PubMed] [Google Scholar]
- 116.McGeer PL. and Steele JC. The ALS/PDC syndrome of Guam: potential biomarkers for an enigmatic disorder. Prog Neurobiol 95: 663–669, 2011 [DOI] [PubMed] [Google Scholar]
- 117.Medeiros R, Kitazawa M, Passos GF, Baglietto-Vargas D, Cheng D, Cribbs DH, and LaFerla FM. Aspirin-triggered lipoxin A4 stimulates alternative activation of microglia and reduces Alzheimer disease-like pathology in mice. Am J Pathol 182: 1780–1789, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mehta A, Prabhakar M, Kumar P, Deshmukh R, and Sharma PL. Excitotoxicity: bridge to various triggers in neurodegenerative disorders. Eur J Pharmacol 698: 6–18, 2013 [DOI] [PubMed] [Google Scholar]
- 119.Miettinen TA, Gylling H, Lindbohm N. Miettinen TE, Rajaratnam RA, and Relas H. Finnish treat-to-target study investigators. Serum noncholesterol sterols during inhibition of cholesterol synthesis by statins. J Lab Clin Med 141: 131–137, 2003 [DOI] [PubMed] [Google Scholar]
- 120.Minogue AM, Lynch AM, Loane DJ, Herron CE, and Lynch MA. Modulation of amyloid-beta-induced and age-associated changes in rat hippocampus by eicosapentaenoic acid. J Neurochem 103: 914–926, 2007 [DOI] [PubMed] [Google Scholar]
- 121.Minter MR, Moore Z, Zhang M, Brody KM, Jones NC, Shultz SR, Taylor JM, and Crack PJ. Deletion of the type-1 interferon receptor in APPSWE/PS1ΔE9 mice preserves cognitive function and alters glial phenotype. Acta Neuropathol Commun 4: 72, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL, van Wijngaarden P, Wagers AJ, Williams A, Franklin RJ, and ffrench-Constant C. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci 16: 1211–1218, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Miyamoto K, Shiozaki M, Shibata M, Koike M, Uchiyama Y, and Gotow T. Very-high-dose alpha-tocopherol supplementation increases blood pressure and causes possible adverse central nervous system effects in stroke-prone spontaneously hypertensive rats. J Neurosci Res 87: 556–566, 2009 [DOI] [PubMed] [Google Scholar]
- 124.Mizee MR, Nijland PG, van der Pol SM, Drexhage JA, van Het Hof B, Mebius R, van der Valk P, van Horssen J, Reijerkerk A, and de Vries HE. Astrocyte-derived retinoic acid: a novel regulator of blood–brain barrier function in multiple sclerosis. Acta Neuropathol 128: 691–703, 2014 [DOI] [PubMed] [Google Scholar]
- 125.Mohammadzadeh Honarvar N, Harirchian MH, Abdolahi M, Abedi E, Bitarafan S, Koohdani F, Siassi F, Sahraian MA, Chahardoli R, Zareei M, Salehi E, Geranmehr M, and Saboor-Yaraghi AA. Retinyl palmitate supplementation modulates T-bet and interferon gamma gene expression in multiple sclerosis patients. J Mol Neurosci 59: 360–365, 2016 [DOI] [PubMed] [Google Scholar]
- 126.Montine KS, Quinn JF, Zhang J, Fessel JP, Roberts LJ, 2nd, Morrow JD, and Montine TJ. Isoprostanes and related products of lipid peroxidation in neurodegenerative diseases. Chem Phys Lipids 128: 117–124, 2004 [DOI] [PubMed] [Google Scholar]
- 127.Morales I, Farías GA, Cortes N, and Maccioni RB. Neuroinflammation and Neurodegeneration. In: Update on Dementia, edited by Moretti DV. Rijeka, Croatia: InTech, 2016 [Google Scholar]
- 128.Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS, Aggarwal NT, and Scherr PA. Relation of the tocopherol forms to incident Alzheimer disease and to cognitive change. Am J Clin Nutr 81: 508–514, 2005 [DOI] [PubMed] [Google Scholar]
- 129.Muid S, Ali AM, Yusoff K, and Nawawi H. Optimal antioxidant activity with moderate concentrations of tocotrienol rich fraction (TRF) in vitro assays. Int Food Res J 20: 687–694, 2013 [Google Scholar]
- 130.Musiek ES, Cha JK, Yin H, Zackert WE, Terry ES, Porter NA, Montine TJ, and Morrow JD. Quantification of F-ring isoprostane-like compounds (F4-neuroprostanes) derived from docosahexaenoic acid in vivo in humans by a stable isotope dilution mass spectrometric assay. J Chromatogr B Analyt Technol Biomed Life Sci 799: 95–102, 2004 [DOI] [PubMed] [Google Scholar]
- 131.Nakaso K, Horikoshi Y, Takahashi T, Hanaki T, Nakasone M, Kitagawa Y, Koike T, and Matsura T. Estrogen receptor-mediated effect of δ-tocotrienol prevents neurotoxicity and motor deficit in the MPTP mouse model of Parkinson's disease. Neurosci Lett 610: 117–122, 2016 [DOI] [PubMed] [Google Scholar]
- 132.Nakaso K, Tajima N, Horikosh Y, Nakasone M, Hanaki T, Kamizaki K, and Matsura T. The estrogen receptor-β-PI3K/Akt pathway mediates the cytoprotective effects of tocotrienol in a cellular Parkinson's disease model. Biochem Biophys Acta Mol Bas Dis 1842: 1303–1312, 2014 [DOI] [PubMed] [Google Scholar]
- 133.Nataraj J, Manivasagam T, Thenmozhi AJ, and Essa MM. Lutein protects dopaminergic neurons against MPTP-induced apoptotic death and motor dysfunction by ameliorating mitochondrial disruption and oxidative stress. Nutr Neurosci 19: 237–246, 2016 [DOI] [PubMed] [Google Scholar]
- 134.Nisar N, Li L, Lu S, Khin NC, and Pogson BJ. Carotenoid metabolism in plants. Mol Plant 8: 68–82, 2015 [DOI] [PubMed] [Google Scholar]
- 135.O'Hare E, Jeggo R, Kim EM, Barbour B, Walczak JS, Palmer P, Lyons T, Page D, Hanna D, Meara JR, Spanswick D, Guo JP, McGeer EG, McGeer PL, and Hobson P. Lack of support for bexarotene as a treatment for Alzheimer's disease. Neuropharmacology 100: 124–130, 2016 [DOI] [PubMed] [Google Scholar]
- 136.Ohshima Y, Mizuno T, Yamada K, Matsumoto S, Nagakane Y, Kondo M, Kuriyama N, Miyazaki T, Takeda K, Nishimura T, Nakagawa M, Ozasa K, and Watanabe Y. Low vitamin and carotenoid levels are related to cerebral white matter lesions. J Nutr Health Aging 17: 456–460, 2013 [DOI] [PubMed] [Google Scholar]
- 137.Ong W-Y, Farooqui T, Kokotos G, and Farooqui AA. Synthetic and natural inhibitors of phospholipases A2: their importance for understanding and treatment of neurological disorders. ACS Chem Neurosci 6: 814–831, 2015 [DOI] [PubMed] [Google Scholar]
- 138.Osakada F, Hashino A, Kume T, Katsuki H, Kaneko S, and Akaike A. Alpha-tocotrienol provides the most potent neuroprotection among vitamin E analogs on cultured striatal neurons. Neuropharmacology 47: 904–915, 2004 [DOI] [PubMed] [Google Scholar]
- 139.Otaegui-Arrazola A, Amiano P, Elbusto A, Urdaneta E, and Martínez-Lage P. Diet, cognition, and Alzheimer's disease: food for thought. Eur J Nutr 53: 1–23, 2014 [DOI] [PubMed] [Google Scholar]
- 140.Ouahchi K, Arita M, Kayden H, Hentati F, Ben Hamida M, Sokol R, Arai H, Inoue K, Mandel JL, and Koenig M. Ataxia with isolated vitamin E deficiency is caused by mutations in the alpha-tocopherol transfer protein. Nat Genet 9: 141–145, 1995 [DOI] [PubMed] [Google Scholar]
- 141.Panov A, Kubalik N, Brooks BR, and Shaw CA. In vitro effects of cholesterol beta-Dglucoside, cholesterol and cycad phytosterol glucosides on respiration and reactive oxygen species generation in brain mitochondria. J Membrane Biol 237: 71–77, 2010 [DOI] [PubMed] [Google Scholar]
- 142.Perry SW, Dewhurst S, Bellizzi MJ, and Gelbard HA. Tumor necrosis factor-alpha in normal and diseased brain: conflicting effects via intraneuronal receptor crosstalk? J Neurovirol 8: 611–624, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, Galasko D, Jin S, Kaye J, Levey A, Pfeiffer E, Sano M, van Dyck CH, and Thal LJ; Alzheimer's Disease Cooperative Study Group. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med 352: 2379–2388, 2005 [DOI] [PubMed] [Google Scholar]
- 144.Petrov AM, Kasimov MR, and Zefirov AL. Brain cholesterol metabolism and its defects: linkage to neurodegenerative diseases and synaptic dysfunction. Acta Naturae 8: 58–73, 2016 [PMC free article] [PubMed] [Google Scholar]
- 145.Phillips MA, Childs CE, Calder PC, and Rogers PJ. No effect of omega-3 fatty acid supplementation on cognition and mood in individuals with cognitive impairment and probable Alzheimer's Disease: a randomised controlled trial. Int J Mol Sci 16: 24600–24613, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Piemontese L. Plant food supplements with antioxidant properties for the treatment of chronic and neurodegenerative diseases: benefits or risks? J Diet Suppl 14: 478–484, 2017 [DOI] [PubMed] [Google Scholar]
- 147.Plat J, Nichols JA, and Mensink RP. Plant sterols and stanols: effects on mixed micellar composition and LXR (target gene) activation. J Lipid Res 46: 2468–2476, 2005 [DOI] [PubMed] [Google Scholar]
- 148.Praticò D. and Sung S. Lipid peroxidation and oxidative imbalance: early functional events in Alzheimer's disease. J Alzheimers Dis 6: 171–175, 2004 [DOI] [PubMed] [Google Scholar]
- 149.Prema A, Janakiraman U, Manivasagam T, and Thenmozhi AJ. Neuroprotective effect of lycopene against MPTP induced experimental Parkinson's disease in mice. Neurosci Lett 599: 12–19, 2015 [DOI] [PubMed] [Google Scholar]
- 150.Qu M, Li L, Chen C, Li M, Pei L, Chu F, Yang J, Yu Z, Wang D, and Zhou Z. Protective effects of lycopene against amyloid β-induced neurotoxicity in cultured rat cortical neurons. Neurosci Lett 505: 286–290, 2011 [DOI] [PubMed] [Google Scholar]
- 151.Quinn JF, Raman R, Thomas RG, Yurko-Mauro K, Nelson EB, Van Dyck C, Galvin JE, Emond J, Jack CR, Jr, Weiner M, Shinto L, and Aisen PS. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: a randomized trial. JAMA 304: 1903–1911, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Qureshi AA, Burger WC, Peterson DM, and Elson CE. The structure of an inhibitor of cholesterol biosysnthesis isolated from barley. J Biol Chem 261: 10544–10550, 1986 [PubMed] [Google Scholar]
- 153.Qureshi AA, Sami SA, Salser WA, and Khan FA. Dose-dependent suppression of serum cholesterol by tocotrienol-rich fraction (TRF25) of rice bran in hypercholesterolemic humans. Atherosclerosis 161: 199–207, 2002 [DOI] [PubMed] [Google Scholar]
- 154.Rajakariar R, Hilliard M, Lawrence T, Trivedi S, Colville-Nash P, Bellingan G, Fitzgerald D, Yaqoob MM, and Gilroy DW. Hematopoietic prostaglandin D2 synthase controls the onset and resolution of acute inflammation through PGD2 and 15-deoxyΔ12–14 PGJ2. Proc Natl Acad Sci U S A 104: 20979–20984, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ramirez-Ramirez V, Macias-Islas MA, Ortiz GG, Pacheco-Moises F, Torres-Sanchez ED, Sorto-Gomez TE, Cruz-Ramos JA, Orozco-Aviña G, and Celis de la Rosa AJ. Efficacy of fish oil on serum of TNFα, IL-1β, and IL-6 oxidative stress markers in multiple sclerosis treated with interferon beta-1b. Oxid Med Cell Longev 2013: 709493, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rapoport SI, Chang MC, and Spector AA. Delivery and turnover of plasma-derived essential PUFAs in mammalian brain. J Lipid Res 42: 678–685, 2001 [PubMed] [Google Scholar]
- 157.Rey C, Nadjar A, Buaud B, Vaysse C, Aubert A, Pallet V, Layé S, and Joffre C. Resolvin D1 and E1 promote resolution of inflammation in microglial cells in vitro. Brain Behav Immun 55: 249–259, 2016 [DOI] [PubMed] [Google Scholar]
- 158.Reza Dorosty-Motlagh A, Mohammadzadeh Honarvar N, Sedighiyan M, and Abdolahi M. The molecular mechanisms of vitamin A deficiency in multiple sclerosis. J Mol Neurosci 60: 82–90, 2016 [DOI] [PubMed] [Google Scholar]
- 159.Riancho J, Berciano MT, Ruiz-Soto M, Berciano J, Landreth G, and Lafarga M. Retinoids and motor neuron disease: potential role in amyotrophic lateral sclerosis. J Neurol Sci 360: 115–120, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rinaldi P, Polidori MC, Metastasio A, Mariani E, Mattioli P, Cherubini A, Catani M, Cecchetti R, Senin U, and Mecocci P. Plasma antioxidants are similarly depleted in mild cognitive impairment and in Alzheimer's disease. Neurobiol Aging 24: 915–919, 2003 [DOI] [PubMed] [Google Scholar]
- 161.Rojo AI, McBean G, Cindric M, Egea J, López MG, Rada P, Zarkovic N, and Cuadrado A. Redox control of microglial function: molecular mechanisms and functional significance. Antioxid Redox Signal 21: 1766–1801, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Saboor-Yaraghi AA, Harirchian MH, Mohammadzadeh Honarvar N, Bitarafan S, Abdolahi M, Siassi F, Salehi E, Sahraian MA, Eshraghian MR, Roostaei T, and Koohdani F. The effect of vitamin A supplementation on FoxP3 and TGFβ gene expression in avonex-treated multiple sclerosis patients. J Mol Neurosci 56: 608–612, 2015 [DOI] [PubMed] [Google Scholar]
- 163.Sachdeva AK. and Chopra K. Lycopene abrogates Aβ(1–42)-mediated neuroinflammatory cascade in an experimental model of Alzheimer's disease. J Nutr Biochem 26: 736–744, 2015 [DOI] [PubMed] [Google Scholar]
- 164.Samieri C, Féart C, Proust-Lima C, Peuchant E, Dartigues JF, Amieva H, and Barberger-Gateau P. ω-3 fatty acids and cognitive decline: modulation by ApoEɛ4 allele and depression. Neurobiol Aging 32: 2317.e13–2317.e22, 2011 [DOI] [PubMed] [Google Scholar]
- 165.Samieri C, Maillard P, Crivello F, Proust-Lima C, Peuchant E, Helmer C, Amieva H, Allard M, Dartigues JF, Cunnane SC, Mazoyer BM, and Barberger-Gateau P. Plasma long-chain omega-3 fatty acids and atrophy of the medial temporal lobe. Neurology 79: 642–650, 2012 [DOI] [PubMed] [Google Scholar]
- 166.Sano M, Aisen PS, Andrews HF, Tsai WY, Lai F, and Dalton AJ; International Down Syndrome and Alzheimer's Disease Consortium. Vitamin E in aging persons with down syndrome: a randomized, placebo-controlled clinical trial. Neurology 86: 2071–2076, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Savini I, Gasperi V, and Catani MV. Nutrigenetics. in eLS. John Wiley & Sons Ltd, Chichester; DOI: 10.1002/9780470015902.a0021028 http://www.els.net [DOI] [Google Scholar]
- 168.Schaible UE. and Kaufmann SH. Malnutrition and infection: complex mechanisms and global impacts. PLoS Med 4: e115, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Seet RC, Lee CY, Lim EC, Tan JJ, Quek AM, Chong WL, Looi WF, Huang SH, Wang H, Chan YH, and Halliwell B. Oxidative damage in Parkinson disease: measurement using accurate biomarkers. Free Radic Biol Med 48: 560–566, 2010 [DOI] [PubMed] [Google Scholar]
- 170.Selvaraju TR, Khaza'ai H, Vidyadaran S, Abd Mutalib MS, and Vasudevan R. The neuroprotective effects of tocotrienol rich fraction and alpha tocopherol against glutamate injury in astrocytes. Bosn J Basic Med Sci 14: 195–204, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sen CK, Khanna S, Roy S, and Packer L. Molecular basis of vitamin E action. Tocotrienol potently inhibits glutamate-induced pp60(c-Src) kinase activation and death of HT4 neuronal cells. J Biol Chem 275: 13049–13055, 2000 [DOI] [PubMed] [Google Scholar]
- 172.Serhan CN. Lipoxins and aspirin-triggered 15-epi-lipoxins are the first lipid mediators of endogenous anti-inflammation and resolution. Prostaglandins Leukot Essent Fatty Acids 73: 141–162, 2005 [DOI] [PubMed] [Google Scholar]
- 173.Serhan CN. and Petasis NA. Resolvins and protectins in inflammation-resolution. Chem Rev 111: 5922–5943, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Shi C, Wu F, Zhu XC, and Xu J. Incorporation of beta-sitosterol into the membrane increases resistance to oxidative stress and lipid peroxidation via estrogen receptor-mediated PI3K/GSK3beta signaling. Biochim Biophys Acta 1830: 2538–2544, 2013 [DOI] [PubMed] [Google Scholar]
- 175.Shibata N, Yamada S, Uchida K, Hirano A, Sakoda S, Fujimura H, Sasaki S, Iwata M, Toi S, Kawaguchi M, Yamamoto T, and Kobayashi M. Accumulation of protein-bound 4-hydroxy-2-hexenal in spinal cords from patients with sporadic amyotrophic lateral sclerosis. Brain Res 1019: 170–177, 2004 [DOI] [PubMed] [Google Scholar]
- 176.Sica A. and Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122: 787–795, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Simopoulos AP. Evolutionary aspects of diet: the omega-6/omega-3 ratio and the brain. Mol Neurobiol 44: 203–215, 2011 [DOI] [PubMed] [Google Scholar]
- 178.Smeyne RJ. and Jackson-Lewis V. The MPTP model of Parkinson's disease. Brain Res Mol Brain Res 134: 57–66, 2005 [DOI] [PubMed] [Google Scholar]
- 179.Smith PJ. and Blumenthal JA. Dietary factors and cognitive decline. Prev Alzheimers Dis 3: 53–64, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sohail M, Rakha A, Butt MS, Iqbal MJ, and Rashid S. Rice bran nutraceutics: a comprehensive review. Crit Rev Food Sci Nutr 57: 3771–3780, 2016 [DOI] [PubMed] [Google Scholar]
- 181.Sontag TJ. and Parker RS. Influence of major structural features of tocopherols and tocotrienols on their omega-oxidation by tocopherol-omega-hydroxylase. J Lipid Res 48: 1090–1098, 2007 [DOI] [PubMed] [Google Scholar]
- 182.Sorto-Gomez TE, Ortiz GG, Pacheco-Moises FP, Torres-Sanchez ED, Ramirez-Ramirez V, Macias-Islas MA, de la Rosa AC, and Velázquez-Brizuela IE. Effect of fish oil on glutathione redox system in multiple sclerosis. Am J Neurodegener Dis 5: 145–151, 2016 [PMC free article] [PubMed] [Google Scholar]
- 183.Souci SW, Fachmann W, and Kraut H. (Eds). Food Composition and Nutrition Tables. Munchen, Germany: Deutsche Forschungsanstalt für Lebensmittelchemie, 1989/1990 [Google Scholar]
- 184.Spohn M. and Davison AN. Cholesterol metabolism in myelin and other subcellular fractions of rat brain. J Lipid Res 13: 563–570, 1972 [PubMed] [Google Scholar]
- 185.Stock C, Schilling T, Schwab A, and Eder C. Lysophosphatidylcholine stimulates IL-1beta release from microglia via a P2X7 receptor-independent mechanism. J Immunol 177: 8560–8568, 2006 [DOI] [PubMed] [Google Scholar]
- 186.Sugama S, Takenouchi T, Cho BP, Joh TH, Hashimoto M, and Kitani H. Possible roles of microglial cells for neurotoxicity in clinical neurodegenerative diseases and experimental animal models. Inflamm Allergy Drug Targets 8: 277–284, 2009 [DOI] [PubMed] [Google Scholar]
- 187.Suzuki YJ, Tsuchiya M, Wassall SR, Choo YM, Govil G, Kagan VE, and Packer L. Structural and dynamic membrane properties of alpha-tocopherol and alpha-tocotrienol: implication to the molecular mechanism of their antioxidant potency. Biochemistry 32: 10692–10699, 1993 [DOI] [PubMed] [Google Scholar]
- 188.Swanson JE. Bioactive food components. In: Encycl Food Cult, edited by Katz SH. New York, NY: Scribner C's Sons, 2003, pp. 201–205 [Google Scholar]
- 189.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, and Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 100: 655–669, 2000 [DOI] [PubMed] [Google Scholar]
- 190.Tabata RC, Wilson JM, Ly P, Zwiegers P, Kwok D, Van Kampen JM, Cashman N, and Shaw CA. Chronic exposure to dietary sterol glucosides is neurotoxic to motor neurons and induces an ALS-PDC phenotype. Neuromolecular Med 10: 24–39, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tan SW, Ramasamy R, Abdullah M, and Vidyadaran S. Inhibitory effects of palm α-,γ- and δ-tocotrienol on lipopolysaccharide-induced nitric oxide production in BV2 microglia. Cell Immunol 271: 205–209, 2011 [DOI] [PubMed] [Google Scholar]
- 192.Teng E, Taylor K, Bilousova T, Weiland D, Pham T, Zuo X, Yang F, Chen PP, Glabe CG, Takacs A, Hoffman DR, Frautschy SA, and Cole GM. Dietary DHA supplementation in an APP/PS1 transgenic rat model of AD reduces behavioral and Aβ pathology and modulates Aβ oligomerization. Neurobiol Dis 82: 552–560, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Thomas MH, Pelleieux S, Vitale N, and Olivier JL. Dietary arachidonic acid as a risk factor for age-associated neurodegenerative diseases: potential mechanisms. Biochimie 130: 168–177, 2016 [DOI] [PubMed] [Google Scholar]
- 194.Tian Y, Zhang Y, Zhang R, Qiao S, and Fan J. Resolvin D2 recovers neural injury by suppressing inflammatory mediators expression in lipopolysaccharide-induced Parkinson's disease rat model. Biochem Biophys Res Commun 460: 799–805, 2015 [DOI] [PubMed] [Google Scholar]
- 195.Traber M. and Packer L. Vitamin E: beyond antioxidant function. Am J Clin Nutr 62: S1501–S1509, 1995 [DOI] [PubMed] [Google Scholar]
- 196.Trovato A, Siracusa R, Di Paola R, Scuto M, Fronte V, Koverech G, Luca M, Serra A, Toscano MA, Petralia A, Cuzzocrea S, and Calabrese V. Redox modulation of cellular stress response and lipoxin A4 expression by Coriolus versicolor in rat brain: relevance to Alzheimer's disease pathogenesis. Neurotoxicology 53: 350–358, 2016 [DOI] [PubMed] [Google Scholar]
- 197.Trovato A, Siracusa R, Di Paola R, Scuto M, Ontario ML, Bua O, Di Mauro P, Toscano MA, Petralia CCT, Maiolino L, Serra A, Cuzzocrea S, and Calabrese V. Redox modulation of cellular stress response and lipoxin A4 expression by Hericium erinaceus in rat brain: relevance to Alzheimer's disease pathogenesis. Immun Ageing 13: 23–34, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ulatowski LM. and Manor D. Vitamin E and neurodegeneration. Neurobiol Dis 84: 78–83, 2015 [DOI] [PubMed] [Google Scholar]
- 199.Valerio M, Liu HB, Heffner R, Zivadinov R, Ramanathan M, Weinstock-Guttman B, and Awad AB. Phytosterols ameliorate clinical manifestations and inflammation in experimental autoimmune encephalomyelitis. Inflamm Res 60: 457–465, 2011 [DOI] [PubMed] [Google Scholar]
- 200.van der Stelt M, Mazzola C, Esposito G, Matias I, Petrosino S, De Filippis D, Micale V, Steardo L, Drago F, Iuvone T, and Di Marzo V. Endocannabinoids and beta-amyloid-induced neurotoxicity in vivo: effect of pharmacological elevation of endocannabinoid levels. Cell Mol Life Sci 63: 1410–1424, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.van Neerven S, Regen T, Wolf D, Nemes A, Johann S, Beyer C, Hanisch UK, and Mey J. Inflammatory chemokine release of astrocytes in vitro is reduced by all-trans retinoic acid. J Neurochem 114: 1511–1526, 2010 [DOI] [PubMed] [Google Scholar]
- 202.Vanmierlo T, Weingartner O, van der Pol S, Husche C, Kerksiek A, Friedrichs S, Sijbrands E, Steinbusch H, Grimm M, Hartmann T, Laufs U, Böhm M, de Vries HE, Mulder M, and Lütjohann D. Dietary intake of plant sterols stably increases plant sterol levels in the murine brain. J Lipid Res 53: 726–735, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Vatassery GT, Fahn S, and Kuskowski MA. alpha-Tocopherol in CSF of subjects taking high-dose vitamin E in the DATATOP study: Parkinson Study Group. Neurology 50: 1900–1902, 1998 [DOI] [PubMed] [Google Scholar]
- 204.Vázquez C, Tolón RM, Grande MT, Caraza M, Moreno M, Koester EC, Villaescusa B, Ruiz-Valdepeñas L, Fernández-Sánchez FJ, Cravatt BF, Hillard CJ, and Romero J. Endocannabinoid regulation of amyloid-induced neuroinflammation. Neurobiol Aging 36: 3008–3019, 2015 [DOI] [PubMed] [Google Scholar]
- 205.Verkhratsky A, Nedergaard M, and Hertz L. Why are astrocytes important? Neurochem Res 40: 389–401, 2015 [DOI] [PubMed] [Google Scholar]
- 206.Vitkovic L, Bockaert J, and Jacque C: “Inflammatory” cytokines: neuromodulators in normal brain? J Neurochem 74: 457–471, 2000 [DOI] [PubMed] [Google Scholar]
- 207.Vlad SC, Miller DR, Kowall NW, and Felson DT. Protective effects of NSAIDs on the development of Alzheimer disease. Neurology 70: 1672–1677, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Vom Berg J, Prokop S, Miller KR, Obst J, Kalin RE, Lopategui-Cabezas I, Wegner A, Mair F, Schipke CG, Peters O Peters O, Winter Y, Becher B, and Heppner FL. Inhibition of IL-12/IL-23 signaling reduces Alzheimer's disease-like pathology and cognitive decline. Nat Med 18: 1812–1819, 2012 [DOI] [PubMed] [Google Scholar]
- 209.von Bernhardi R, Eugenín-von Bernhardi L, and Eugenín J. Microglial cell dysregulation in brain aging and neurodegeneration. Front Aging Neurosci 7: 124, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.von Bernhardi R, Tichauer JE, and Eugenín J. Aging dependent changes of microglial cells and their relevance for neurodegenerative disorders. J Neurochem 112: 1099–1114, 2010 [DOI] [PubMed] [Google Scholar]
- 211.Wang F, Yuan T, Pereira A, Jr, Verkhratsky A, and Huang JH. Glial cells and synaptic plasticity. Neural Plast 2016: 5042902, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Wang HQ, Sun XB, Xu YX, Zhao H, Zhu QY, and Zhu CQ. Astaxanthin upregulates heme oxygenase-1 expression through ERK1/2 pathway and its protective effect against beta-amyloid-induced cytotoxicity in SH-SY5Y cells. Brain Res 1360: 159–167, 2010 [DOI] [PubMed] [Google Scholar]
- 213.Wang HY, Liu CB, Wu HW, and Kuo JS. Direct profiling of phospholipids and lysophospholipids in rat brain sections after ischemic stroke. Rapid Commun Mass Spectrom 24: 2057–2064, 2010 [DOI] [PubMed] [Google Scholar]
- 214.Wang J, Wu F, and Shi C. Substitution of membrane cholesterol with betasitosterol promotes nonamyloidogenic cleavage of endogenous amyloid precursor protein. Neuroscience 247: 227–233, 2013 [DOI] [PubMed] [Google Scholar]
- 215.Wang X, Hjorth E, Vedin I, Eriksdotter M, Freund-Levi Y, Wahlund LO, Cederholm T, Palmblad J, and Schultzberg M. Effects of n-3 FA supplementation on the release of proresolving lipid mediators by blood mononuclear cells: the OmegAD study. J Lipid Res 56: 674–681, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wang X, Zhu M, Hjorth E, Cortés-Toro V, Eyjolfsdottir H, Graff C, Nennesmo I, Palmblad J, Eriksdotter M, Sambamurti K, Fitzgerald JM, Serhan CN, Granholm AC, and Schultzberg M. Resolution of inflammation is altered in Alzheimer's disease. Alzheimers Dement 11: 40–50, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Whitney LW, Ludwin SK, McFarland HF, and Biddison WE. Microarray analysis of gene expression in multiple sclerosis and EAE identifies 5-lipoxygenase as a component of inflammatory lesions. J Neuroimmunol 121: 40–48, 2001 [DOI] [PubMed] [Google Scholar]
- 218.Witting A, Weydt P, Hong S, Kliot M, Moller T, and Stella N. Endocannabinoids accumulate in spinal cord of SOD1 G93A transgenic mice. J Neurochem 89: 1555–1557, 2004 [DOI] [PubMed] [Google Scholar]
- 219.Wong M. and Lodge JK. A metabolomic investigation of the effects of vitamin E supplementation in humans. Nutr Metab 9: 110, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Wu H, Niu H, Shao A, Wu C, Dixon BJ, Zhang J, Yang S, and Wang Y. Astaxanthin as a potential neuroprotective agent for neurological diseases. Mar Drugs 13: 5750–5766, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wyss-Coray T. and Rogers J. Inflammation in Alzheimer disease-a brief review of the basic science and clinical literature. Cold Spring Harb Perspect Med 2: a006346, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Xia W. and Mo H. Potential of tocotrienols in the prevention and therapy of Alzheimer's disease. J Nutr Biochem 31: 1–9, 2016 [DOI] [PubMed] [Google Scholar]
- 223.Yamamoto M, Kiyota T, Horiba M, Buescher JL, Walsh SM, Gendelman HE, Ikezu T. Interferon-gamma and tumor necrosis factor-alpha regulate amyloid-beta plaque deposition and beta-secretase expression in Swedish mutant APP transgenic mice. Am J Pathol 170: 680–692, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ye Q, Huang B, Zhang X, Zhu Y, and Chen X. Astaxanthin protects against MPP(+)-induced oxidative stress in PC12 cells via the HO-1/NOX2 axis. BMC Neurosci 13: 156, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Yip PK, Pizzasegola C, Gladman S, Biggio ML, Marino M, Jayasinghe M, Ullah F, Dyall SC, Malaspina A, Bendotti C, and Michael-Titus A. The omega-3 fatty acid eicosapentaenoic acid accelerates disease progression in a model of amyotrophic lateral sclerosis. PLoS One 8: e61626, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Yurko-Mauro K, McCarthy D, Rom D, Nelson EB, Ryan AS, Blackwell A, Salem N, Jr, Stedman M; and MIDAS Investigators. Beneficial effects of docosahexaenoic acid on cognition in age-related cognitive decline. Alzheimers Dement 6: 456–464, 2010 [DOI] [PubMed] [Google Scholar]
- 227.Zhao Y, Calon F, Julien C, Winkler JW, Petasis NA, Lukiw WJ, and Bazan NG. Docosahexaenoic acid-derived neuroprotectin D1 induces neuronal survival via secretase- and PPARγ-mediated mechanisms in Alzheimer's disease models. PLoS One 6: e15816, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Zhao Z, Liu N, Huang J, Lu PH, and Xu XM. Inhibition of cPLA2 activation by Ginkgo biloba extract protects spinal cord neurons from glutamate excitotoxicity and oxidative stress-induced cell death. J Neurochem 116: 1057–1065, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]