Abstract
Background
Steroid receptor coactivator-interacting protein (SIP) inhibits the activation of nuclear factor-kappa B (NF-κB) by interacting with p65. The occurrence of acute pancreatitis (AP) is closely associated with pro-inflammatory response. The present study aimed to investigate the role of SIP on myocardial injury caused by AP.
Material/Methods
Rat pancreatic acinar tumor cell line AR42J cells were treated with caerulein to establish AP cell models. The levels of TNF-α, IL-6, cTnI, CK-MB, and LDH1 were detected by ELISA assay. The mRNA and protein expression levels of SIP, p-p65, and p65 were detected by qRT-PCR and western blot analysis, respectively. Next, the AP cell models were non-transfected or transfected with SIP plasmids or SIP siRNA. ELISA assay was also performed to test the levels of TNF-α, IL-6, cTnI, CK-MB, and LDH1. Moreover, qRT-PCR and western blot analysis were performed to measure the mRNA and protein expression levels of SIP, p-p65, and p65, respectively.
Results
Caerulein upregulated the levels of TNF-α, IL-6, cTnI, CK-MB, and LDH1. These upregulations were reduced by SIP plasmids and promoted by SIP siRNA, respectively. Caerulein also increased the mRNA and protein expression levels of p-p65. However, the increases were attenuated by SIP plasmids and enhanced by SIP siRNA, respectively.
Conclusions
In conclusion, the results suggested that SIP may inhibit the inflammatory response by deactivating p65, thus reducing the myocardial damage caused by AP.
MeSH Keywords: Nuclear Receptor Coactivator 2; Pancreatitis, Acute Necrotizing; Receptor Activator of Nuclear Factor-kappa B
Background
Acute pancreatitis (AP) is a common inflammatory disorder in the pancreas, with high mortality. The incidence of AP has increased significantly in recent years [1,2]. AP may be caused by generalized inflammation due to sepsis [3,4]. There are various risk factors for AP, such as alcohol abuse, gallstones, drugs, surgical procedures, infectious agents, and lipid metabolic disorders. AP is one of the leading cause of hospital stays [2]. However, the exact mechanisms underlying the progression of AP and its complications are not fully clear. AP usually causes systemic inflammation response syndrome and multiple-organ injuries, and AP-induced myocardial injury is one of the leading causes of mortality [5,6]. The mechanism and pathogenesis of AP-induced myocardial injury are of great importance in clinical treatments. Moreover, cardiac troponin I (cTnI) [7], creatine kinase-KB isoenzyme (CK-MB) [8], and lactate dehydrogenase 1 (LDH1) [9] are early makers of myocardial injury.
A recent study reported that the occurrence of AP is closely related with pro-inflammatory response [10]. Tumor necrosis factor (TNF)-α and interleukin (IL)-6 are important pro-inflammatory mediators, which causing a generalized inflammatory response [11]. Nuclear factor-kappa B (NF-κB) p65 is a major factor in gene transcription, its activation upregulates the level of inflammatory factors, thus promoting the occurrence and development of gastrointestinal diseases [12].
The steroid receptor coactivator (SRC) family consists of three homologous members (SRC-1, SRC-2, and SRC-3), which are important regulators for steroid hormones and mitogenic activity [13]. SRC-interacting protein (SIP) interacts with SRC coactivators in the cytoplasm and regulates the transactivation activity of SRC in the nucleus [14].
In the present study, we established AP cell models with caerulein. First, the levels of cTnI, CK-MB, and LDH1 were measured for the rapid diagnosis of acute myocardial injury. Then the cells were non-transfected or transfected with SIP plasmids or SIP siRNA. ELISA assay, qRT-PCR, and western blot analysis were performed to detect the expression levels of pro-inflammatory mediators (TNF-α and IL-6) with the aim to investigate the influence of SIP on inflammatory reaction and myocardial injury in AP cell models.
Material and Methods
Cell culture and treatment
The rat pancreatic acinar tumor cell line AR42J was purchased from the American Type Culture Collection (ATCC, Rockville, MD, USA). Cells were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS) at 37°C in a 5% CO2, 95% air atmosphere. Subsequently, the cells were treated with 100 nM caerulein to establish cell models for AP [15], while cells in the normal control (NC) group were cultured with DMEM containing 10% FCS.
Transfection
After treatment, AR42J cells were non-transfected or transfected with SIP plasmids or SIP siRNA using Lipofectamine™ 2000 (Life Technologies, Rockville, MD, USA) following manufacturer’s instructions. Con plasmids or Con siRNA was used as the negative control.
ELISA
The levels of TNF-α, IL-6, cTnI, CK-MB, and LDH1 in cell cultures of different groups were measured with TNF-α, IL-6, cTnI, CK-MB, and LDH1 ELISA kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to manufacturer’s instructions.
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and reverse-transcribed into complementary DNA (cDNA) by using PrimeScript™ RT reagent Kit (Perfect Real Time) (Takara Biomedical Technology (Beijing) Co., Ltd., Beijing, China) according to manufacturer’s instructions, respectively. Then, qRT-PCR was performed using One Step SYBR® PrimeScript™ RT-PCR Kit (Perfect Real Time) (Takara Biomedical Technology (Beijing) Co., Ltd., Beijing, China) following manufacturer’s instructions. The expression levels of SIP and p65 mRNA were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Relative quantification of SIP and p65 mRNA expression was performed using the 2−ΔΔCt calculations. The amplification conditions were as follows: Initial activation at 95°C for 10 minutes, followed by 40 amplification cycles of denaturation at 95°C for 30 seconds, annealing at 55°C for 30 seconds, and extension at 72°C for 30 seconds. The following primers were used:
SIP, 5′-CCCAGAAGTTCCTGCCGAAT-3′,
5′-GACTCTTGGGAGGCTGCTTG-3′;
p65, 5′-CATACGCTGACCCTAGCCTG-3′,
5′-TTTCTTCAATCCGGTGGCGA-3′;
GAPDH, 5′-TTCACCACCATGGAGAAGGC -3′,
5′-TTCTGAGTGGCAGTGATGGC -3′.
Western blot analysis
Total protein was lysed in PIPA lysis buffer (Thermo Scientific) on ice for 30 minutes. The lysates were centrifuged at 12,000 rpm for 30 minutes at 4°C. Protein concentration was determined by using a BCA protein assay kit (Beyotime, Shanghai, China). Equal amounts of protein (20 μg) were separated in a 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore). Then the membranes were blocked with 5% bovine serum albumin (BSA) at room temperature for one hour and incubated with primary antibodies (SIP, 1: 1,000, ab167084; p65, 1: 1,000, ab16502; p-p65,1: 1,000, ab6503; GAPDH, 1: 1,000, ab9485; Abcam, USA) at 4°C overnight. Afterwards, the membranes were washed with TBST and incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies at room temperature for one hour. The bands were detected by an enhanced chemiluminescence (ECL) kit (Beyotime, Shanghai, China). Grey levels of the bands were quantified with Quantity One software (Bio-Rad).
Statistical analysis
All data were expressed as means ±SD and analyzed by one-way analysis of variance (ANOVA) and Student’s t-tests. Statistical analysis was performed by SPSS 20.0. A value of p<0.05 was considered significant.
Results
Establishment of AP cell models
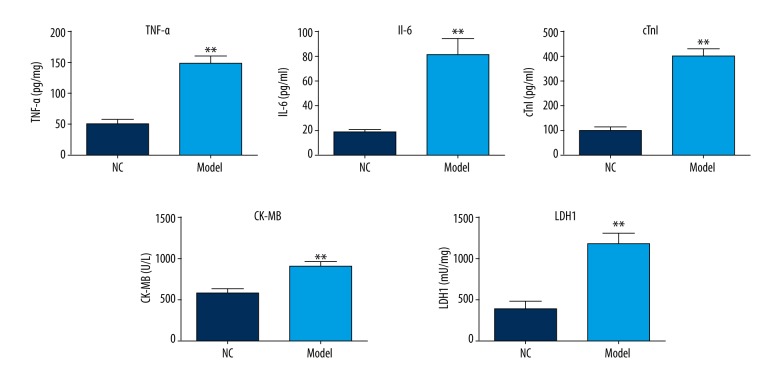
To establish the AP cell models, AR42J cells were treated with caerulein. After treatment, the levels of TNF-α, IL-6, cTnI, CK-MB, and LDH1 in cell cultures were determined by ELISA assay. As shown in Figure 1, the levels of TNF-α, IL-6, cTnI, CK-MB, and LDH1 were significantly increased in the AP cell models compared with the NC group.
Figure 1.
Effects of caerulein on the levels of TNF-α, IL-6, cTn1, CK-MB, and LDH1. AR42J cells were treated with 100 nM caerulein. ELISA assay was performed to detect the levels of TNF-α, IL-6, cTn1, CK-MB, and LDH1. ** p<0.01.
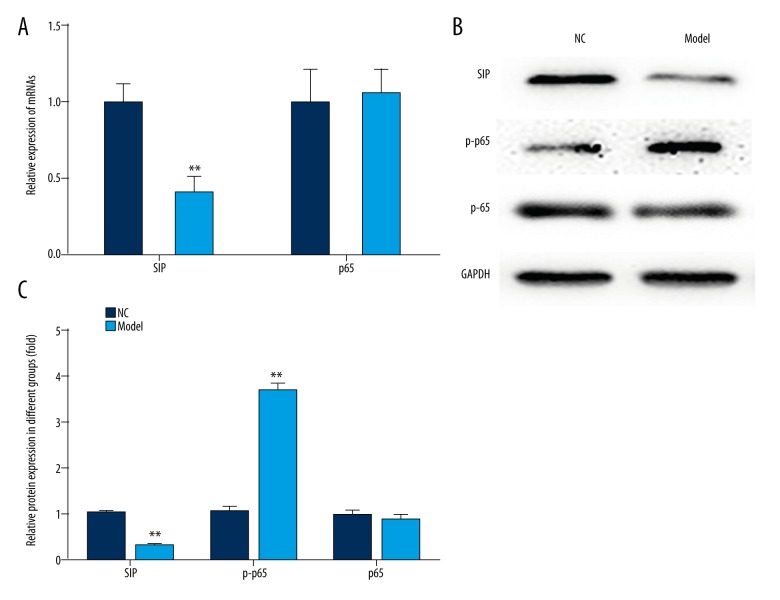
Meanwhile, the expression of SIP, p-p65, and p65 were measured by qRT-PCR and western blot analysis, respectively. The results of qRT-PCR showed that the expression of SIP mRNA in cells was upregulated after treatment with caerulein (Figure 2A). The results of western blot analysis showed that the expression of SIP, p-p65 protein in cells was upregulated after treatment with caerulein (Figure 2B, 2C). However, there was no significant difference in the expression of p65 after treatment (Figure 2).
Figure 2.
Effects of caerulein on the expression of SIP, p-p65, and p65. AR42J cells were treated with 100 nM caerulein. (A) qRT-PCR was performed to measure the expression of SIP and p65. (B, C) Western blot analysis was performed to measure the expression of SIP, p-p65, and p65. ** p<0.01.
These results indicated that the AP cell models were successfully established.
SIP plasmid represses myocardial injury caused by AP
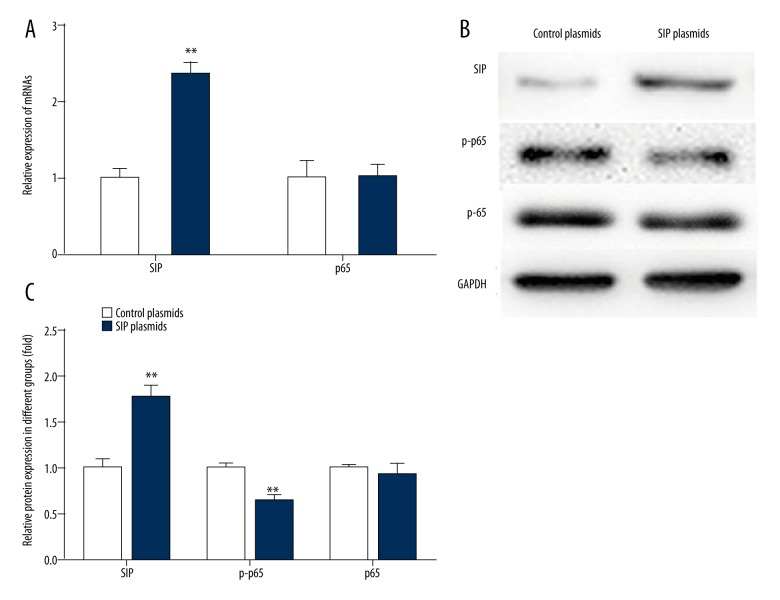
The AP cell models were non-transfected or transfected with SIP plasmid. As shown in Figure 3, the mRNA and protein expression levels of SIP were significantly higher after transfection. Overexpression of SIP attenuated the expression of p-p65 protein in the AP cell models (Figure 3B, 3C). There was no significant difference in the expression of p65 after transfection (Figure 3).
Figure 3.
Effects of SIP plasmids on the expression of SIP, p-p65, and p65. AP cell models were transfected with SIP plasmids. (A) qRT-PCR was performed to measure the expression of SIP and p65. ** p<0.01. (B, C) Western blot analysis was performed to measure the expression of SIP, p-p65, and p65. ** p<0.01.
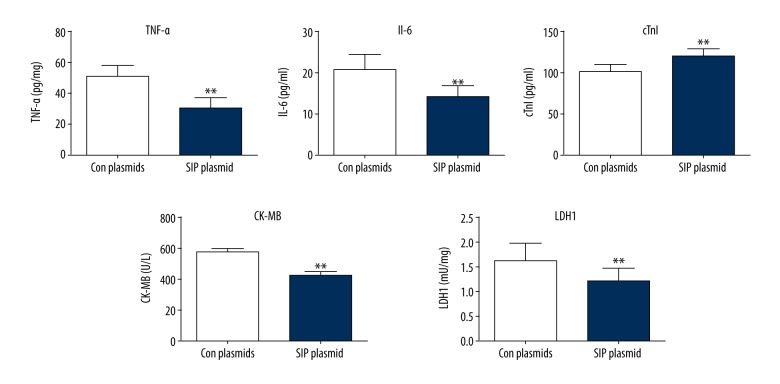
After transfection, the levels of TNF-α, IL-6, cTnI, CK-MB, and LDH1 in cell cultures were determined by ELISA assay. As shown in Figure 4, SIP overexpression significantly decreased the levels of TNF-α, IL-6, cTnI, CK-MB, and LDH1 in the AP cell models.
Figure 4.
Effects of SIP plasmids on the levels of TNF-α, IL-6, cTn1, CK-MB, and LDH1. AP cell models were transfected with SIP plasmids. ELISA assay was performed to detect the levels of TNF-α, IL-6, cTn1, CK-MB, and LDH1. ** p<0.01.
These results showed that SIP plasmid decreased the levels of TNF-α, IL-6, cTnI, CK-MB, LDH1, and p-p65. These data indicated that SIP may repress myocardial injury caused by AP through interacting with p65.
SIP siRNA aggravates myocardial injury caused by AP
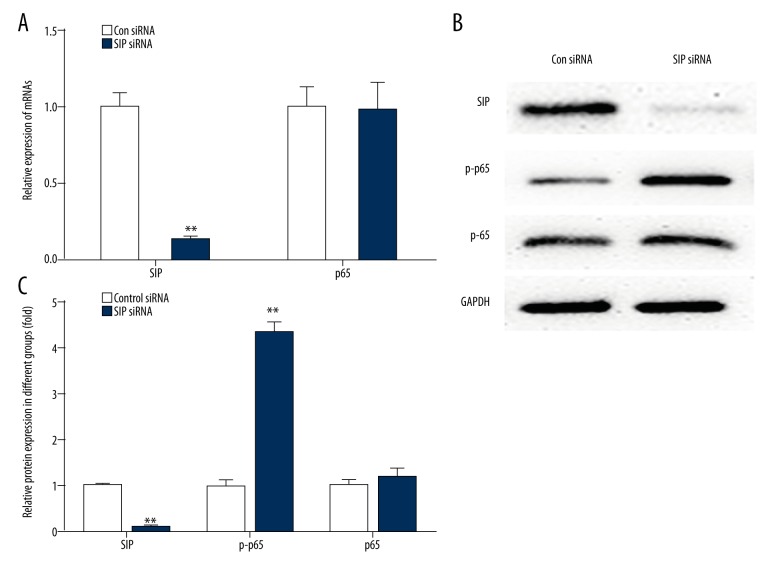
The AP cell models were non-transfected or transfected with SIP siRNA. As shown in Figure 5, the mRNA and protein expression levels of SIP were significantly lower after transfection. Inhibition of SIP increased the expression of p-p65 protein in the AP cell models (Figure 5B, 5C). However, there was no significant difference in the expression of p65 after transfection (Figure 5).
Figure 5.
Effects of SIP siRNA on the expression of SIP, p-p65, and p65. AP cell models were transfected with SIP siRNA. (A) qRT-PCR was performed to measure the expression of SIP and p65. ** p<0.01. (B, C) Western blot analysis was performed to measure the expression of SIP, p-p65, and p65. ** p<0.01.
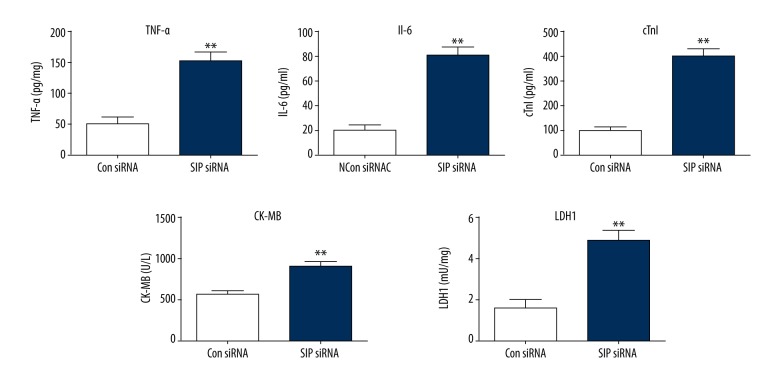
After transfection, the levels of TNF-α, IL-6, cTnI, CK-MB, and LDH1 in cell cultures were determined by ELISA assay. As shown in Figure 6, inhibition of SIP significantly increased the levels of TNF-α, IL-6, cTnI, CK-MB, and LDH1 in the AP cell models.
Figure 6.
Effects of SIP siRNA on the levels of TNF-α, IL-6, cTn1, CK-MB, and LDH1. AP cell models were transfected with SIP siRNA. ELISA assay was performed to detect the levels of TNF-α, IL-6, cTn1, CK-MB and LDH1. ** p<0.01.
These results showed that SIP plasmid increased the levels of TNF-α, IL-6, cTnI, CK-MB, LDH1, and p-p65. These data confirmed that SIP can repress myocardial injury caused by AP through interacting with p65.
Discussion
AP is an acute inflammatory process in the pancreas that frequently leads to local and systemic complications. The mortality of AP is due to multiple systemic organ failure and death triggered by the associated systemic inflammatory response [2]. Myocardial injury induced by AP is one of the leading causes of mortality [6]. In this study, we treated cells with caerulein to establish the AP cell models. Specific markers for the diagnosis of myocardial injury are cTnI, CK-MB, and LDH1 [16,17]. The results of our ELISA assay showed that the levels of cTnI, CK-MB, and LDH1 were significantly increased in the AP cell models. These data indicated that the AP cell models induced myocardial injury, thus, the models were successfully established.
Inflammatory mediators (e.g., TNF-α and IL-6) play important roles in the development and progression of AP and are closely related to complications [18]. TNF-α is mainly secreted by activated mononuclear macrophages, as a promoter of cytokine cascades with multiple biological effects [19]. Intestinal diseases can promote the expression of proinflammatory cytokines, and TNF-α is an important inflammatory transmitter that plays a very important role in the initiation and maintenance of intestinal inflammation [20]. TNF-α can induce the production and release of other proinflammatory cytokines such as IL-1 and IL-8, leading to cascade reactions and further amplification of the initial inflammatory signal [21]. IL-6 is secreted by T cells and macrophages, acts as both a pro-inflammatory cytokine and an anti-inflammatory myokine [22]. In our study, the levels of TNF-α and IL-6 in the AP cell models were significantly upregulated compared to those in the NC group. These results confirmed that TNF-α and IL-6 may be associated with the development of AP.
The regulation of TNF-α is closely associated with NF-κB. NF-κB is active by extracellular signals, enhancing the gene transcription of TNF-α and promoting the expression of TNF-α; subsequently TNF-α actives NF-κB by positive feedback mechanism, further increasing the secretion of TNF-α [21]. NF-κB is a protein complex that regulates DNA transcription, cytokine production, and cell survival. There are five proteins in the mammalian NF-κB family: NF-κB1, NF-κB2, RelA (p65), RelB, and c-Rel [23]. SIP is a steroid receptor coactivator that interacts with p65 to inhibit p65 activation. In the AP cell models, the mRNA and protein expression levels of SIP were markedly reduced, meanwhile the protein expression levels of p-p65 were significantly increased. The AP cell models were non-transfected or transfected with SIP plasmids or SIP siRNA in order to investigate the role of SIP on AP-induced myocardial injury through interactions with p65.
In the present study, overexpression of SIP inhibited the protein expression levels of p-p65, however, inhibition of SIP promoted the protein expression levels of p-p65. These results proved that SIP may inhibit the activation of p65 in cells. On the other hand, overexpression of SIP reduced the levels of TNF-α, IL-6, cTnI, CK-MB, and LDH1. These results indicated that SIP may inhibit the inflammatory reaction and relieve myocardial injury induced by AP. Furthermore, SIP siRNA upregulated the levels of TNF-α, IL-6, cTnI, CK-MB, and LDH1. These results showed that SIP may inhibit the inflammatory reaction and relieve myocardial injury induced by AP.
Conclusions
In conclusion, our study results suggested that SIP interacts with p65 to inhibit the activation of p65 in order to inhibit the inflammatory response and to reduce the myocardial damage in the AP cell models.
Acknowledgments
Dr. Bin Zhu was supported by the Program of Health Top-notch Talent of Changzhou City and Youth Medical Talent of Jiangsu Province.
Footnotes
Conflicts of interest
No conflicts of interest exit in this study.
Source of support: This work was supported by the Program of Bureau of Science and Technology Foundation of Changzhou (CJ20159022), Major Science and Technology Project of Changzhou Municipal Commission of Health and Family Planning (ZD201505) and “333 Project” (BRA2016122) of Jiangsu Province
References
- 1.Lankisch PG, Apte M, Banks PA. Acute pancreatitis. Lancet. 2015;386(9988):85–96. doi: 10.1016/S0140-6736(14)60649-8. [DOI] [PubMed] [Google Scholar]
- 2.Pandol SJ, Saluja AK, Imrie CW, Banks PA. Acute pancreatitis: Bench to the bedside. Gastroenterology. 2007;132(3):1127–51. doi: 10.1053/j.gastro.2007.01.055. [DOI] [PubMed] [Google Scholar]
- 3.Isik A, Firat D. Bilateral intra-areolar polythelia. BreastJ. 2017 doi: 10.1111/tbj.12838. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Isik A, Idiz O, Firat D. Novel approaches in pilonidal sinus treatment. Prague Med Rep. 2016;117(4):145–52. doi: 10.14712/23362936.2016.15. [DOI] [PubMed] [Google Scholar]
- 5.Calleja GA, Barkin JS. Acute pancreatitis. Me Clin North Am. 1993;77(5):1037–56. doi: 10.1016/s0025-7125(16)30209-7. [DOI] [PubMed] [Google Scholar]
- 6.Robertson CM, Coopersmith CM. The systemic inflammatory response syndrome. Microbes Infect. 2006;8(5):1382–89. doi: 10.1016/j.micinf.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 7.Ilva T, Lund J, Porela P, et al. Early markers of myocardial injury: cTnI is enough. Clin Chim Acta. 2009;400(1–2):82–85. doi: 10.1016/j.cca.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Kontos MC, Anderson FP, Hanbury CM, et al. Use of the combination of myoglobin and CK-MB mass for the rapid diagnosis of acute myocardial infarction. Am J Emerg Med. 1997;15(1):14–19. doi: 10.1016/s0735-6757(97)90040-1. [DOI] [PubMed] [Google Scholar]
- 9.Kutsal A, Saydam GS, Yucel D, Balk M. Changes in the serum levels of CK-MB, LDH, LDH1, SGOT and myoglobin due to cardiac surgery. J Cardiovasc Surg. 1991;32(4):516–22. [PubMed] [Google Scholar]
- 10.Lima PR, de Melo TS, Carvalho KM, et al. 1,8-cineole (eucalyptol) ameliorates cerulein-induced acute pancreatitis via modulation of cytokines, oxidative stress and NF-kappaB activity in mice. Life Sci. 2013;92(24–26):1195–201. doi: 10.1016/j.lfs.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Minkov GA, Halacheva KS, Yovtchev YP, Gulubova MV. Pathophysiological mechanisms of acute pancreatitis define inflammatory markers of clinical prognosis. Pancreas. 2015;44(5):713–17. doi: 10.1097/MPA.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 12.Schmid RM, Adler G. NF-kappaB/rel/IkappaB: Implications in gastrointestinal diseases. Gastroenterology. 2000;118(6):1208–28. doi: 10.1016/s0016-5085(00)70374-x. [DOI] [PubMed] [Google Scholar]
- 13.York B, O’Malley BW. Steroid receptor coactivator (SRC) family: Masters of systems biology. J Biol Chem. 2010;285(50):38743–50. doi: 10.1074/jbc.R110.193367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Zhang H, Liang J, et al. SIP, a novel ankyrin repeat containing protein, sequesters steroid receptor coactivators in the cytoplasm. EMBO J. 2007;26(11):2645–57. doi: 10.1038/sj.emboj.7601710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen J, Chen J, Wang X, et al. Ligustrazine alleviates acute pancreatitis by accelerating acinar cell apoptosis at early phase via the suppression of p38 and Erk MAPK pathways. Biomed Pharmacother. 2016;82:1–7. doi: 10.1016/j.biopha.2016.04.048. [DOI] [PubMed] [Google Scholar]
- 16.Martins JT, Li DJ, Baskin LB, et al. Comparison of cardiac troponin I and lactate dehydrogenase isoenzymes for the late diagnosis of myocardial injury. Am J Clin Pathol. 1996;106(6):705–8. doi: 10.1093/ajcp/106.6.705. [DOI] [PubMed] [Google Scholar]
- 17.Collinson PO, Stubbs PJ, Kessler AC Multicentre Evaluation Of Routine Immunoassay Of Troponin T Study. Multicentre evaluation of the diagnostic value of cardiac troponin T, CK-MB mass, and myoglobin for assessing patients with suspected acute coronary syndromes in routine clinical practice. Heart. 2003;89(3):280–86. doi: 10.1136/heart.89.3.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang LZ, Luo MY, Zhang JS, et al. Effect of ulinastatin on serum inflammatory factors in Asian patients with acute pancreatitis before and after treatment: A meta-analysis. Int J Clin Pharmacol Ther. 2016;54(11):890–98. doi: 10.5414/CP202454. [DOI] [PubMed] [Google Scholar]
- 19.Shalaby MR, Pennica D, Palladino MA., Jr An overview of the history and biologic properties of tumor necrosis factors. Springer Semin Immunopathol. 1986;9(1):33–37. doi: 10.1007/BF00201903. [DOI] [PubMed] [Google Scholar]
- 20.Su C, Salzberg BA, Lewis JD, et al. Efficacy of anti-tumor necrosis factor therapy in patients with ulcerative colitis. Am J Gastroenterol. 2002;97(10):2577–84. doi: 10.1111/j.1572-0241.2002.06026.x. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Huang Q, Ong CN, et al. Chrysin sensitizes tumor necrosis factor-alpha-induced apoptosis in human tumor cells via suppression of nuclear factor-kappaB. Cancer Lett. 2010;293(1):109–16. doi: 10.1016/j.canlet.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Heinrich PC, Behrmann I, Haan S, et al. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374(Pt 1):1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilmore TD. Introduction to NF-kappaB: Players, pathways, perspectives. Oncogene. 2006;25(51):6680–84. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]