Abstract
Passiflora suberosa L. belonging to the family Passifloraceae is an important medicinal plant used in traditional medicinal system in Sri Lanka to treat diabetes, hypertension and skin diseases. We extracted P. suberosa leaves under reflux conditions using different solvents (hexane, chloroform, methanol and water), then subjected to phytochemical screening. Alkaloids, flavonoids and saponins and saponins and anthraquinones were present in hexane and chloroform extracts. Alkaloids, unsaturated sterols, triterpenes, saponins, flavonoids and tannins were observed in both methanol and aqueous extracts. Proanthocyanidins were observed only in the aqueous extract. Hence, aqueous and methanol extracts with most classes of phytochemicals present were subjected to antimicrobial, antioxidant, antihaemolytic activities and Brine shrimp lethality studies. Antibacterial activity and minimum inhibition concentrations were evaluated using three Gram-positive (Bacillus subtilis, Staphylococcus aureus and Enterococcus faecium) and three Gram-negative bacteria (Pseudumonas aeruginosa, Salmonella typhimuriam and Escherichia coli). The results indicated that only the methanol extract of P. suberosa exhibited antibacterial activities against all the strains of Gram-negative and Gram-positive bacterial with stronger activity against Gram-negative bacteria. DPHH (2,2-diphenyl-1-picrylhydrazy) scavenging assay was adopted to evaluate antioxidant properties while antihaemolytic and toxic activities were studied respectively using cow blood and Brine shrimp lethality assay. The IC50 values of the aqueous extract in both antioxidant and antihaemolytic assays were significantly lower than the standard ascorbic acid. Similar results were observed in the Brine shrimp lethality assay. In conclusion both aqueous and methanol extracts of P. suberosa leaves showed the presence of majority of phytochemicals including proanthocyanidins. Antibacterial activity was obtained only for methanol extract with better activity against Gram-negative bacteria. The aqueous extract showed better antioxidant, antihaemolytic and toxic activities than the methanol extract and their respective standards. Further investigations on the chemical composition and possible isolation of active ingredients is warranted.
Keywords: Passiflora suberosa, Antibacterial, Antioxidant, Toxic effects
Introduction
Being rich bio-resource of drugs, for centuries plants derived from drugs have become an essential source of traditional medicine specially in the developing countries (Atanasov et al., 2015). Even today, the majority of the world’s populations still rely on complementary medicine owing to their ability to combat various human diseases (Rangika, Dayananda & Peiris, 2015; Peiris, Dhanushka & Jayatilleka, 2015). The active principle of plants are the phytochemicals, mainly secondary metabolites including tannins, flavonoids, terpenoids, saponins, cyanogenic glycosides, nitrogen compounds (alkaloids, amines, betalanines), phenols, and phenolic glycosides (Abdelwahab et al., 2010), which play a vital role in reinforcement of plant tissue and plant survival (Anwer et al., 2013) against free radicals, cell proliferation and pathogenic microorganisms (Obeidat et al., 2012).
The increasing resistance of bacteria to antibiotics poses a significant challenge when combating infectious diseases caused by bacteria. This is a major risk for the world population; for example, currently in the United States alone people are adversely infected with pathogenic bacterial strains demonstrating resistant against antibiotics prescribed to treat infections. Furthermore, about 23,000 deaths are recorded each year due to antibiotic resistant infections (Center for Disease Control and Prevention, 2013). Drug resistant microbes can fast spread between people and animal, and from person to person. Gram-positive bacteria including Staphylococcus aureus (causing a range of infectious diseases from skin and wound infections to phenumonia); Enterococcus faecium, which is a leading cause of multi-drug resistant bacteria responsible for infections due to central lines, urinary drainage catheters, and ventilators. Gram-negative bacteria including Salmonella typhi (causing typhoid fever); Pseudomonas aeruginosa, a multidrug resistant pathogen responsible for triggering healthcare-associated infections including pneumonia, bloodstream infections, urinary tract infections, and surgical site infections are some examples for drug resistant bacteria (Center for Disease Control and Prevention, 2016). The alarming rise of drug resistant bacteria are problematic to control with the current available antibiotics on the market. Discovery of new antibiotics via de novo synthesis are time-consuming and expensive. Hence, the search of new remedies is urgently required (Center for Disease Control and Prevention, 2013). Recently, novel antibacterial antidotes from natural derivatives have been described and thus plants are receiving extensive consideration as possible antibiotic agents.
Living cells in the human body continuously produce reactive oxygen species and free radicals and are controlled by body enzymes. Over production of either free radicals, reactive oxygen species or failure in the defense mechanisms result in severe damage to cells and tissues leading to non communicable chronic diseases, specifically cancer. Antioxidant compounds have the ability to scavenge these free radicals or reactive oxygen species, which contribute to the various etiology of chronic health problems. Laboratory research conducted with rats have shown that the artificial antioxidants in the market could cause internal and external bleeding (Poljsak, Suput & Milisav, 2013). Antioxidant activity can also be determined using erythrocyte antioxidant proficiency. Mammalian erythrocytes have their own morphological and biological characteristics. Hemoglobin molecules and accumulation of polyunsaturated fatty acids make erythrocytes more vulnerable for peroxidation. Mammalian erythrocytes are continuously exposed to high oxygen tension leading to oxidative damages (Babu, Shylesh & Padikkala, 2001). Further exposure to toxicants can result in generation of free radicals causing membrane damages and hemolysis (Chakraborty & Verma, 2010). Hence, attention have given to use of naturally occurring antioxidants such as flavonoids, polyphenolic compounds to scavenge free radicals to protect erythrocyte membranes (Kaur, Kapoor & Kaur, 2011).
Passiflora suberosa of the family Passifloraceae is a native plant to tropical America and have been introduced and naturalized throughout the tropics (Space & Flynn, 2000). Of the genus Passiflora, the species suberosa is a relatively unexploited plant compared to other species. This has promoted us to investigate its antioxidant and antibacterial potentials. This plant species is known vernacularly as wild passion fruit, devil’s pumpkin or indigo berry and has been traditionally used by physicians for hypertension, skin diseases (Vianna et al., 2012; Dhawan, Dhawan & Sharma, 2004) and as a sedative and antihypertensive remedy in Mexican folk medicine (Edgar et al., 2013). In previous studies, Sudasinghe & Peiris (2018), demonstrated hypoglycemic activities of aqueous leaf extract of P. suberosa in mice. Leaves from the plant is eaten as a green vegetable. In the present study, phytochemical screening was conducted for several extractions. Since the aqueous and methanol extracts showed promising results for the phytochemical screening, these two extractions were extended to investigate the antioxidant, cytotoxic and antibacterial potential using gram-positive and -negative bacterial strains.
Materials & Methods
Chemicals
All chemicals, and reagents were of AR grade and obtained from Sigma-Aldrich, Germany. Water when used was distilled using GFL distillation apparatus.
Plant material and extraction
Fresh tender leaves of P. suberosa were hand-picked between 8.00 a.m.–9.00 a.m. from healthy plants from Nugegoda (6.4200°N, 80.0000°E), Sri Lanka. The plant was authenticated by Ms. S. A. H. P. Sudasinghe at Royal Botanical Garden, Peradeniya and a herbarium specimen (no: PS/01) was deposited. Leaves were washed with tap water, wiped and air-dried in the shade for 3 weeks. Dried leaves were cut into pieces, ground and stored in the freezer at −80 °C in sealed polythene bags until additional use.
Ground powder of P. suberosa leaves (100 g) were refluxed using 300 mL of hexane, chloroform, methanol and water separately for two hours. The resulting crude extracts were cooled to room temperature, filtered and made up to a final volume of 200 mL. Preliminary phytochemical screening was conducted to extract. Remaining portion of each extract was freeze dried for bioactivity testing. Freeze dried extracts were stored in the freezer −80 °C until further use.
Phytochemical analysis
Secondary compounds present in hexane, chloroform, methanol and aqueous extracts of P. suberosa leaves were determined. To determine the presence of alkaloids, sterols, triterpenes, saponins, flavonoids, proanthocyanidins and anthraquinones, methods described by Harborne (1998) was adopted. Since flavonoids and saponins could interfere with results of each other, thin layer chromatography was conducted for both saponins and flavonoids. To confirm presence of flavonoids in both aqueous and methanol P. suberosa leaf extracts, 6.8:0.2:2.8:0.2 (ethyl acetate: formic acid: dichloromethane: methanol) solvent system was used. Spot development was observed under the UV light subsequent to the spraying of visualizing chemicals. Raw leaf powder was dissolved in distilled water and shaken for 30 s to observe froth formation indicating the presence of saponins. The color intensity or the precipitate formation was used as analytical responses to these tests.
Antibacterial activity
Three Gram-positive bacteria species including Bacillus subtilis, S. aureus (ATCC25923) and Enterococcus faecium and 3 Gram-negative bacteria species including Pseudumonas aeruginosa (ATCC27853), Salmonella typhimuriam and Escherichia coli (ATCC 25922) was used in the study. The pure cultures of all the bacterial strains, which were selected on the basis of their importance as human pathogens except for the B. subtilis, which a model organism, was a gift from the Department of Microbiology, Faculty of Medical Sciences, University of Sri Jayewardenepura.
Antibacterial activity by agar well diffusion assay
Muller-Hinton agar media were made and under aseptic condition, laid in sterilized disposable petri dishes. Over night culturing of bacteria was conducted at 37 °C in Muller-Hinton agar. Homogenized bacterial cell suspensions was adjusted to 0.5 McFarland standards (5 × 108 CFU/mL). A disc impregnated with gentamycin (10 µg/disc) was used as a standard, while the respective solvents were used as the negative controls. The agar Petri dishes were incubated for 24 h at 37 °C. The antibacterial activity was evaluated by measuring the diameters of the growth inhibition zones (mm) for the tested pathogenic bacteria compared to the standards (Valgas et al., 2007). the measurement of the inhibition zones was conducted using three sample replications.
Minimum inhibitory concentration for antibacterial activity
Strains exhibited inhibition zones were subjected to minimum inhibitory concentration test. The minimum concentrations of a plant extract are the concentration where no growth was observed in a solid medium. To determine the MICs, the plant extracts were serially diluted ranging from 6.25 to 800 µg/mL (Samie et al., 2010). Microplates prepares with respective medium was inoculate with 0.5 Mcfarland standard (108 CFU/mL). Dilution series was prepared using broth micro-dilution procedure (1:100). Upon incubation at 37 °C for 1 day, the MIC was recorded (Otang, Grierson & Ndip, 2012).
Antioxidant screening
Free radical scavenging capability of freeze dried methanolic extract of P. suberosa leaves was determined using the DPPH assay according to a previously published method upon modification (Blois, 1958). A concentration series (800–6.25 µg/mL) of the freeze dried methanolic extract was prepared in methanol and DPPH was prepared by dissolving 10 mg of DPPH in 250 mL of methanol. Sample solutions at various concentrations (1.5 mL) were mixed with DPPH solution separately and the resulting mixtures were allowed to stand at room temperature for 30 min. Extent of discoloration of mixtures were determined at 517 nm using the UV-Visible spectrophotometer. All measurements were carried out in triplicate. For the control and the blank, DPPH solution in methanol and methanol was used respectively. Ascorbic acid was used as the standard. DPPH scavenging activity of the extracts were expressed as IC50 values, and was calculated using the equation given below.
The same procedure was repeated for the aqueous extract of P. suberosa leaves.
Antihaemolytic activity
The antihaemolytic activity of methanol and aqueous leaf extracts of P. suberosa was quantitatively analyzed using cow blood. Blood was collected from a slaughter house in Colombo and was centrifuged for 10 min at 1,500 rpm (Model: Minor 35, UK). Erythrocytes was separated from the plasma and washed three times with sterile phosphate buffer saline, centrifuged for 5 min at 1500 rpm. Separated erythrocytes were diluted with phosphate buffered saline (0.2M PBS, pH 7.4) to yield 4% suspension. To 2 mL of the suspension, different concentration (1 mL; 800–6.25 µg/mL) of the plant extracts were mixed and the final volume was adjusted to 5 mL using PBS and incubated at 37 °C for 5 min. Upon incubation, 0.5 mL of 3% H2O2 solution was added to induce the oxidative degradation of red blood cell membrane. The tubes were mixed gently and incubated (Model: R0001000920, UK) at 37 °C for 4 h with intermittent shaking. Subsequently, the tubes were centrifuged for 10 min at 1,500 rpm and the color density of the supernatant was determined using a spectrometer at 540 nm wavelength (Naim et al., 1976). Ascorbic acid and absence of a plant extracts was considered respectively as positive and negative controls. Antihaemolysis percentage of the extracts was calculated against the control was calculated as follows:
Where, Abcontrol was the absorbance of the control (water and erythrocytes without the extracts) and Absample was the absorbance in the presence of extracts.
Brine shrimp lethality assay
Brine shrimp lethality assay was conducted using Artemia salina. Artemia cysts was purchased from fish aquarium (Lanka aqua, Homagama) and was housed in a well aerated 1L capacity glass flask containing artificial seawater (prepared using 19 g of washed sea salt dissolving in 500 mL of water and adjusted to pH 8.5. After 48 h of incubation at the room temperatures (28 °C–30 °C) with constant exposure to florescence lamp to obtain free-swimming nauplii. Since cyst capsules floated on the surface, collecting nauplii from the bottom to ensure pure harvest. These freshly hatched nauplii were used for the bioassay.
Half dilution concentration series; 800, 400. 200, 100, 50, 25, 12.5, 6.25 µg/mL of both aqueous and methanol extraction of P. subsersoa were prepared in artificial seawater containing 2% Dimethyl sulfoxide DMSO (v/v). The assay was prepared using 20 mL of filtered seawater containing different concentrations of P. suberosa extracts and 1% of yeast extract (to feed nauplii) in well aerated (sufficient aeration was ensured) 25 mL of small beakers. Thirty nauplii were transferred to each beaker. The set up was kept for 24 h, under constant illumination of florescent lamp. After 24 h, umber of survived nauplii was counted under a dissecting microscope. Three replications were prepared for each concentration. Concentration series (6.25–800 µg/mL) of the standard potassium dichromate solution were used as the positive control while pure seawater with DMSO was used as the negative control. The death percentage (LC50) was determined using statistical analysis (Baravalia, Vaghasiya & Chanda, 2012). Percentage of death was calculated using the following equation (Sahgal et al., 2010).
Statistical analysis
Statistical comparison of the data was conducted using Minitab 14 for Windows software. Parametric one-way analysis of variance (ANOVA) followed by the Student T test was adopted to determine the significant differences. Data were expressed as the means ± standard error of the mean (SEM). The significant value was set at p ≤ 0.05. Linear regression analysis was used for the calculation of the toxic effect at different concentrations.
Results
Phytochemical analysis
Leaves were extracted using hexane, chloroform, methanol and water. The crude extracts thus obtained were subjected to qualitative phytochemical screening studies. Alkaloids, flavonoids and saponins were present in the hexane extract and saponins and anthraquinones were present in chloroform extract. Alkaloids, unsaturated sterols, triterpenes, saponins, flavonoids and tannins were observed in both methanol and water extracts. Proanthocyanidins were observed only in the aqueous extract. The phytochemical screening results are given in Table 1.
Table 1. Phytochemical components of chloroform, n-hexane, methanol and aqueous crude leaf extracts of P. suberosa leaves.
| Extraction method | ||||
|---|---|---|---|---|
| Class of compounds | Chloroform | n-hexane | Methanol | Aqueous |
| Alkaloids | − | + | + | + |
| Sterols | − | − | + | + |
| Titerpenes | − | − | + | + |
| Saponins | + | + | + | + |
| Flavonoids | − | + | + | + |
| Proanthocyanidin | − | − | − | + |
| Anthraquinones | + | − | − | − |
| Tannins | − | − | + | + |
Notes.
+, Presence of constituent; −, Absence of constituent.
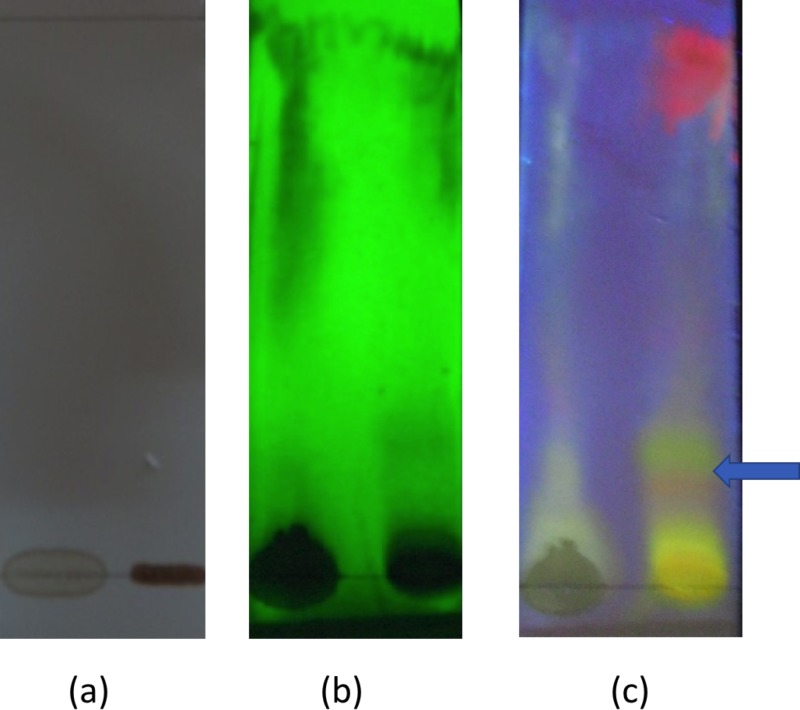
Both flavonoids and proanthocyanidins are present in the aqueous extract. Proanthocyandins present in the aqueous extract can give a false-positive result under acidic conditions of the flavonoid test. Thus, the presence of flavonoids in the aqueous extract was confirmed by thing layer chromatographic studies using NPR as the visualizing agent. Observed fluorescence quenching at UV-254 mm and orange and yellow colour spots under UV-364 mm without and with spraying the NPR reagent respectively confirmed the presence of flavonoids in the aqueous extract (Fig. 1).
Figure 1. Thin layer chromatography of aqueous leaf extract of Passiflora suberosa.
Thin layer chromatography observed under UV light (A) before spraying natural product visualizing reagent (NPR); (B) after spraying NPR, observed under UV-254 mm; and (C) after sparing NPR and observed under UV-364 mm. Orange and yellow spot appeared at UV-364 (indicated by an arrow) confirmed the presence of flavonoid.
Antibacterial screening
Antibacterial activity was evaluated in both water and methanol extracts of P. suberosa against three Gram-negative and three Gram-positive bacteria and the results are presented in Table 2. No activity was exhibited by the aqueous extract, while the methanol extract exhibited stronger activities against all tested bacterial strains. The methanol extract of P. suberosa exhibited inhibition zones ranging from 10–13 mm for Gram-negative bacteria with the greatest inhibition of zone (13 mm) produced against the clinical strain E. coli. Similarly, the leaf extract exhibited inhibition zones between 11–12.7 mm against Gram-positive bacteria and the maximum inhibition of 12.7 mm was observed against the pathogenic strain S. aureus. The positive control tested (gentamycin) exhibited an inhibition zone of 14 mm.
Table 2. IC50 values of methanol and aqueous leaf extracts from P. suberosa.
| Zone of inhibition (mm) | MIC (µg/mL) | |||
|---|---|---|---|---|
| Bacterial strains | Aq | MeoH | Aq | MeoH |
| Gram-positive bacteria | ||||
| B. subtilis | – | 10.0 ± 1.0 | na | 12.5 |
| S. aureus | – | 12.7 ± 0.6 | na | 25 |
| E. faecium | – | 11.3 ± 0.6 | na | 25 |
| Gram-negative bacteria | ||||
| P. aeruginosa | – | 12.3 ± 0.3 | na | 6.5 |
| S. typhimuriam | – | 12.0 ± 0 | na | 6.5 |
| E. coli | – | 13.0 ± 0.3 | na | 6.5 |
Notes.
Data presented as the mean ± SEM (n = 3). (−), no inhibition; na, not applicable; Aq, aqueous extract; MeoH, methanol extract.
The MIC assay of the methanol extract of P. suberosa exhibited strong activity against all 6 bacterial strains, with MIC values ranging from 6.25–25 µg/mL for different strains (Table 2). The strongest activity was exhibited against the clinical strains of Gram-negative bacteria (E. coli, P. aeruginosa, and S. typhimuriam) with the MIC value of 6.25 µg/mL. For the Gram-positive bacteria, the most communal MIC value was 25 µg/mL for S. aureus and E. faecium strains. Against the pathogenic strain B. subtilis, the MIC value detected was 12.5 µg/mL
Antioxidant screening
The free radical- scavenging activity of DPPH was increased in a linear manner for methanol, aqueous extracts, and ascorbic acid solvent (Table 3). Aqueous extraction of leaves displays lower IC50 value (74.33 ± 0.88 µg/mL), than methanol extraction (418.67 ± 2.73 µg/mL), Both extracts showed significant difference (p < 0.05) when compared with DPPH scavenging activity value of ascorbic acid was 166.17 ± 0.60 µg/ml. Low IC50 value indicates a high antioxidant activity thus indicating higher antioxidant activity of the water extract than the methanol extract.
Table 3. The antibacterial activities of different concentrations of methanol leaf extract of P. suberosa against Gram positive and Gram-negative bacteria.
| Experiment | Test samples | IC50 (µg/mL) |
|---|---|---|
| DPPH antioxidant activity | Methanol extract | 418.67 ± 2.73 |
| Aqueous extract | 74.33 ± 0.88 | |
| Ascorbic acid | 166.17 ± 0.60 | |
| Antihaemolytic activity | Methanol extract | 610.25 ± 0.15 |
| Aqueous extract | 80.08 ± 0.01 | |
| Ascorbic acid | 220 ± 0.01 |
Notes.
Data represented as the mean ± SEM (n = 9). *p < 0.05 compared with the control.
Antihemolytic activity
Antihemolytic activities of both extracts are presented in Table 3. Both aqueous and methanol extracts of P. suberosa significantly (p < 0.05) inhibited the haemolysis of cow blood compared to positive control (ascorbic acid) in a concentration dependent manner. High antihemolytic activity was observed with the aqueous extract (IC50: 80.08 ± 0.01) with an inhibition value 660% greater than the methanol extract (610.25 ± 0.15).
Brine shrimp lethality assay
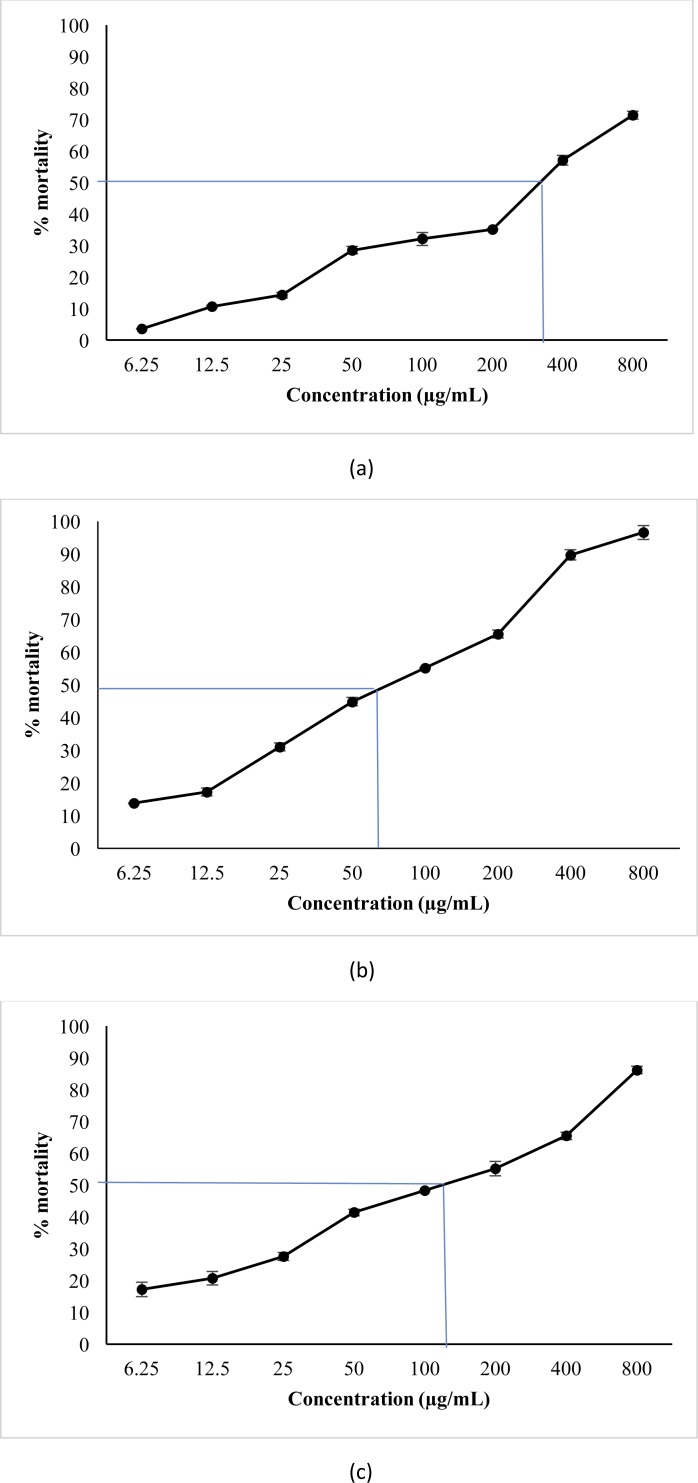
The mortality rate of brine shrimps was increased with the increasing concentrations of the both extracts (Fig. 2). This suggests a direct proportional relationship between concentrations and mortality. The highest mortality was observed in the aqueous extract. The toxic effects of both methanol and aqueous extracts showed a significant (p < 0.05) difference compared to the control. The IC50 value obtained for the water extract (60.26 ± 0.80 µg/mL) was significantly (p < 0.05) lower than both methanol extract (309.02 ± 0.003 µg/mL) and the positive control (96.31 ± 2.64 µg/mL) thus indicating strong activity of the aqueous extract as a toxic agent.
Figure 2. Percentage mortality of A. salina larvae induced by aqueous and methanol extracts of P. suberosa leaf at different concentrations.
Methanol extract (A): IC50 = 309.02 ± 0.003 µg/mL; aqueous extract of P. suberosa leaf; (B) IC50 = 60.26 ± 0.80 µg/mL; potassium dichromate, positive control (C): IC50 = 96.31 ± 2.64 µg/mL. Data presented as the mean ± SEM (n = 9).
Discussion
The results of our assays confirmed that P. suberosa is traditionally used in traditional medicinal practices to treat different disease conditions. This is the first report of in vitro antioxidant, cytotoxicity and antibacterial effects of P. suberosa. Though other species in the genus Passiflora are well investigated, existing knowledge of species suberosa is very limited.
The zones of inhibition for the methanol extraction ranged from 10–13 mm. MIC for the extract ranged from 6.25–25 µg/mL. Amongst the tested bacterial strains, E. coli found to be the most sensitive. Activity of crude extracts is considered to be significant if MIC values are below 100 µg/mL, moderate when 100 < MIC < 625 µg/mL or low when MIC > 625 µg/mL (Kuete, 2010). The MIC values obtained for Gram-positive and negative bacteria were equal or less than 25 µg/mL, thus indicating that the antibacterial efficacy of the methanol extract of P. suberosa is rather high and could be used as a potential antibacterial agent.
The data also indicated that Gram-negative bacteria were more sensitive than Gram-positive bacteria. In contrast to the previous findings of Passiflora species (P. quadrangularis, P. maliformis, and P. edulis) leaves extract with methanol, which hardly exhibited susceptible to gram negative bacteria (Ramaiya, Bujang & Zakaria, 2014), our results indicated that Gram-negative bacteria were more susceptible towards the P. suberosa leaf extract. The morphological difference of cell walls of Gram-positive and Gram-negative bacteria may be one of the most acceptable reason for such observation (Delcour, 2009). However, further studies on mechanisms of actions is warranted.
It has been established that tannins, saponins, phenolic compounds, and flavonoids are responsible for antibacterial potency of plants (Tiwari et al., 2011). According to phytochemical results, though both extracts of P. suberosa contain tannins, saponins and flavonoids, only the methanol extract showed antibacterial activities. The MIC values of conventional antibiotics are in the range of 15–107 µg/mL (Cermak et al., 2017) and observed MIC values for methanol extract of P. suberosa ranged from 10–13 µg/mL indicating high antibacterial activity of the plant extract. Gram-negative bacteria pose a huge challenge in modern days due to emerging drug resistance of these pathogens. The methanol extract of P. suberosa could be used as a potential antibacterial chemotherapy specially against Gram-negative bacteria.
Formation of reactive oxygen species (ROS) such as hydrogen peroxide, hydroxyl radical, lipid peroxides and superoxide anions occur in the body due to increased production and/or decreased elimination of ROS. The ROS production is increased during at pathophysiological condition resulting in membrane lipids, nucleic acids, carbohydrates and protein damages (Sharma et al., 2012). In the present study, the antioxidant assays conducted for both extracts resulted in a concentration-dependent protective effect against free radical generated by DPPH, which has been used as a model to investigate oxidative stress generated in vivo by anion radicals. Drugs such as desferoxamine inhibit generation of ROS and are capable of reducing pathological cell damages. The presence of anthocyanins, sponins and polyphenolic compounds such as flavonoids (Pandey & Rizvi, 2009) are responsible for radical scavenging by transferring protons to free radical generated by DPPH. The phytochemical screening confirms the presence of flavonoids in both extracts. The IC50 values obtained for the aqueous extract is six times lower than the methanol extract. Furthermore, the antioxidant capacity of the aqueous extract of P. suberosa species was stronger than the positive control (ascorbic acid) and antioxidant activities reported against extracts from other Passiflora species including P. alata, P. edulis, P. qudraungularis, and P. maliformis (Ramaiya, Bujang & Zakaria, 2014). Hence, the capacity of antioxidant efficacy of the aqueous leaf extract of P. suberosa leaves can be classified as strong. Only the aqueous extract showed the presence of proanthocyanidine, which may be the reason for the difference observed in the extracts.
Erythrocytes are considered as a prime target of free radical attack because they possess high amount of polyunsaturated fatty acids. Further, oxygen transport is associated with haemoglobin molecules, which are potent promoters of reactive oxygen species (Chakraborty & Shah, 2011). Both aqueous and methanol leaf extracts of P. suberosa were able to inhibit haemolysis of cow erythrocytes in a dose dependent manner. Presence of phenolic compounds such as flavonoids, and tannin in the extracts can be considered to be responsible for inhibition of free radicals formed as a result of lipid peroxidation (Medini et al., 2014). Antihaemolytic activity exhibited by the aqueous extract of P. suberosa is stronger than both methanol extract of P. suberosa and the positive control (ascorbic acid). Anthocyanin has been reported to reduce their haemolysis of erythrocytes due to their antioxidant efficacy (Hebbel et al., 1982). The phytochemical screening showed the presence of anthocyanin in the aqueous extract of P. suberosa thus leading to potent exhibited antihaemolytic activity. Furthermore, antihaemolytic activity found to be an important feature of antisickling agents. Sickle cell anemia modifies the membrane flexibility of erythrocytes making them more sensitive and fragile against free radicals. Any compound with potent antioxidant efficacy such as the aqueous extract of P. suberosa has the ability to prevent haemoglobin from oxidizing and thus preventing generation of free radicals (Mpiana et al., 2013) and therefore, could be used as an antisickling drug.
Brine shrimp lethality test exhibited a linear correlation between brine shrimp toxicity and human nasopharyngeal carcinoma and with some human solid tumors (Ghosh & Chatterjee, 2013). The test revealed that both methanol and aqueous extracts were capable of exhibiting mortality and toxic effects on brine shrimps Aqueous and methanol extracts of P. suberosa found be toxic towards Brine shrimp and the concentrations required for 50% death were 60.3 and 309 µg / mL respectively. According to the standard Brine shrimp lethality stipulation, if IC50 value < 100 µg/mL is considered as bioactive in toxicity evaluation of plant extracts (Meyer et al., 1982). Based on the standard benchmark, the IC50 value of both studied extracts showed IC50 value less than 400 µg/mL indicating their toxic activity. Brine shrimp toxic activity exhibited by the water extract of P. suberosa was about five and half times more powerful than the methanol extract. Moreover, the extracts exhibiting IC50 less than 100 µg/mL on Brine shrimp test is categorized as a potent toxic substance (Meyer et al., 1982). The IC50 value for the aqueous extract is significantly less than 100 µg/mL, thus indicating that the toxic efficacy of the aqueous extract of P. suberosa is quite high and could be used as a possible anticancer agent.
The Brine shrimp lethality could be related to antioxidant activity of the extracts since anthocyanins, saponins, tannins, flavonoids and polyphenols act not only as free radical scavengers but also as proliferative cell inhibitor and apoptosis inducer (Spavieri et al., 2010). The aqueous extract exhibited both high antioxidant and toxic activities when compared to the methanol extract and the respective positive controls indicating that the extract may contain high amount of phytochemicals. Therefore, the aqueous extract could have classified as a potent cytotoxic agent. The antioxidant activity of water extract of P. suberosa was the highest among the genus Passiflora (Ramaiya, Bujang & Zakaria, 2014).
Conclusions
For the first time, we report the antibacterial and antioxidant activities of aqueous and methanol leaf extractions. It was discovered that the methanol extract of P. suberosa is a stronger antibacterial agent against Gram-negative bacteria of P. suberosa. We discovered a progress in the clinical development of antibacterial drug that target infectious caused by Gram-negative bacteria. The aqueous extract of P. suberosa exhibited strong antioxidant, antihaemolytic, and toxic activities than the methanol extract and the respective standards. Therefore, investigations with cancer cell lines are warranted to clarify anticancer properties. Isolating and examining individual bioactive compounds present in P. suberosa may consequently be a good candidate for the development of new drug leads against sickle cell anemia, and infectious diseases caused by Gram-negative bacteria.
Supplemental Information
Photograph showing mature leaves, and fruits. Photo credit—Ms Hasani Sudasinghe.
Acknowledgments
The authors would like to acknowledge the Departments of Chemistry, Faculty of Applied Sciences and Microbiology, Faculty of Medical Sciences for providing assistance throughout the research project.
Funding Statement
This work was supported by the University of Sri Jayewardenepura (No: ASP/01/RE/SCI/ 2016/26). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Kumudu R.V. Bandara performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Chayanika Padumadasa prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft, her expertise on chemistry.
Dinithi C. Peiris conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The raw data are provided in the Supplemental File.
References
- Abdelwahab et al. (2010).Abdelwahab SI, Abdul AB, Elhassan MM, Mohan S, Mariod AA. Phenolic content and antioxidant activities of Goniothalamus umbrosus extracts. International Journal Natural Products and Pharmaceutical Sciences. 2010;1:1–6. [Google Scholar]
- Anwer et al. (2013).Anwer N, Waqar MA, Iqbal M, Mushtaq M, Sobia A. Phytochemical analysis, free radical scavenging capacity and antimicrobial properties of impatiens bicolor plant. International Food Research Journal. 2013;20:99–103. [Google Scholar]
- Atanasov et al. (2015).Atanasov AG, Waltenberger B, Pferschy-Wenzig EM, Linder T, Wawrosch C, Uhrin P, Temmi V, Wang L, Schwaiger S, Heiss EH, Rollinger JM, Schuster D, Breuss JM, Bochkov V, Mihovllovic MD, Kopp B, Bauer R, Drisch VM, Stuppner H. Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnology Advances. 2015;33:1582–1614. doi: 10.1016/j.biotechadv.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu, Shylesh & Padikkala (2001).Babu BH, Shylesh BS, Padikkala J. Antioxidant and hepatoprotective effect of Alanthus icicifocus. Fitoterapia. 2001;72:272–277. doi: 10.1016/S0367-326X(00)00300-2. [DOI] [PubMed] [Google Scholar]
- Baravalia, Vaghasiya & Chanda (2012).Baravalia Y, Vaghasiya Y, Chanda S. Brine Shrimp cytotoxicity, anti-inflammatory and analgesic properties of Woodfordia fruticosa Kurz Flowers. Iranian Journal of Pharmaceutical Research. 2012;11:851–856. [PMC free article] [PubMed] [Google Scholar]
- Blois (1958).Blois MS. Antioxidant determination by the use of a stable free radical. Nature. 1958;181:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- Center for Disease Control and Prevention (2013).Center for Disease Control and Prevention (CDC) National Center for Health Statistics. Health, United States: With Special Feature on Prescription Drugs. 2013. https://www.cdc.gov/nchs/data/hus/hus13.pdf. [16 September 2017]. https://www.cdc.gov/nchs/data/hus/hus13.pdf
- Center for Disease Control and Prevention (2016).Center for Disease Control and Prevention (CDC) Antibiotic/antimicrobial resistance. 2016. https://www.cdc.gov/drugresistance/biggest_threats.html. [12 April 2018]. https://www.cdc.gov/drugresistance/biggest_threats.html
- Cermak et al. (2017).Cermak P, Olsovska J, Mikyska A, Dusek M, Kadleckova Z, Vanicek J, Nyc O, Sigler K, Bostikova V, Bostik P. Strong antimicrobial activity of xanthohumol and other derivatives from hops (Humulus lupulus L.) on gut anaerobic bacteria. APMIS Journal of Pathology, Microbiology and Immunology. 2017;125:1033–1038. doi: 10.1111/apm.12747. [DOI] [PubMed] [Google Scholar]
- Chakraborty & Shah (2011).Chakraborty D, Shah B. Antimicrobial, antioxidative and antihemolytic Activity of Piper betel leaf extracts. International Journal of Pharmacy and Pharmaceutical Sciences. 2011;3:192–199. [Google Scholar]
- Chakraborty & Verma (2010).Chakraborty D, Verma RJ. Ameliorative effect of Emblica officinalis aqueous extract against ochratoxin—induced lipid peroxidation in the kidney and liver of mice. International Journal of Occupational Medicine & Environmental Health. 2010;23:1–11. doi: 10.2478/v10001-010-0009-4. [DOI] [PubMed] [Google Scholar]
- Delcour (2009).Delcour AH. Outer membrane permeability and antibiotic resistance. Biochimica Biophysica Acta. 2009;1794:808–816. doi: 10.1016/j.bbapap.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawan, Dhawan & Sharma (2004).Dhawan K, Dhawan S, Sharma A. Passiflora: a review update. Journal of Ethnopharmacology. 2004;94:1–23. doi: 10.1016/j.jep.2004.02.023. [DOI] [PubMed] [Google Scholar]
- Edgar et al. (2013).Edgar EG, Cisneros RN, Molina AS, Garciglia RS. Plants used in Mexican folk medicine with antidiabetic and antihypertensive properties. Pharmacology. 2013;2:15–23. [Google Scholar]
- Ghosh & Chatterjee (2013).Ghosh A, Chatterjee P. Brine shrimp cytotoxic activity of 50% alcoholic extract of croton bonplandianum baill. Asian Journal of Pharmaceutical and Clinical Research. 2013;6:215–218. [Google Scholar]
- Harborne (1998).Harborne JB. Phytochemical methods a guide to modern techniques of plant analysis. Chapman & Hall; London: 1998. [Google Scholar]
- Hebbel et al. (1982).Hebbel RP, Eaton JW, Balasingam M, Steinberg MH. Spontaneous oxygen radical generation by sickle cell erythrocytes. The Journal of Clinical Investigation. 1982;70:1253–1259. doi: 10.1172/JCI110724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur, Kapoor & Kaur (2011).Kaur R, Kapoor K, Kaur H. Plants as a source of anticancer agents. Journal of Natural Products and Plant Resources. 2011;1:119–124. [Google Scholar]
- Kuete (2010).Kuete V. Potential of Cameroonian plants and derived products against microbial infections: a review. Planta Medica. 2010;76:1479–1791. doi: 10.1055/s-0030-1250027. [DOI] [PubMed] [Google Scholar]
- Medini et al. (2014).Medini F, Fellah H, Ksouri R, Abdelly C. Total phenolic, flavonoid and tannin contents and antioxidant and antimicrobial activities of organic extracts of shoots of the plant Limonium delicatulum. Journal of Taibah University of Science. 2014;8:216–224. doi: 10.1016/j.jtusci.2014.01.003. [DOI] [Google Scholar]
- Meyer et al. (1982).Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, McLaughlin JL. Brine shrimp: a convenient general bioassay for active plant constituents. Planta Medica. 1982;45:31–34. doi: 10.1055/s-2007-971236. [DOI] [PubMed] [Google Scholar]
- Mpiana et al. (2013).Mpiana P, Lombe B, Ombeni A, Ngbolua K, Tshibangu D, Wimba L, Tshilanda D, Mushagalusa F, Muyisa S. In vitro sickling inhibitory effects and anti-sickle erythrocytes hemolysis of Dicliptera colorata CB Clarke, Euphorbia hirta L. and Sorghum bicolor (L.) Moench. Open Journal of Blood Diseases. 2013;3:43–48. doi: 10.4236/ojbd.2013.31009. [DOI] [Google Scholar]
- Naim et al. (1976).Naim M, Gestetner B, Bondi A, Birk Y. Antioxidative and antihaemolytic activities of soybean isoflavones. Journal of Agricultural and Food Chemistry. 1976;24:1174–1177. doi: 10.1021/jf60208a029. [DOI] [PubMed] [Google Scholar]
- Obeidat et al. (2012).Obeidat M, Shatnawi M, Al-alawi M, Al-Zu‘bi E, Al-Dmoor H, Al-Qudah M, El-Qudah J, Otri I. Antimicrobial activity of crude extracts of some plant leaves. Research Journal of Microbiology. 2012;7:59–67. doi: 10.3923/jm.2012.59.67. [DOI] [Google Scholar]
- Otang, Grierson & Ndip (2012).Otang WM, Grierson DS, Ndip RN. Antifungal activity of Arctotis arctotoides (L) O. Hoffm. and Gasteria bicolor Haw. against opportunistic fungi associated with human immunodeficiency virus/acquired immunodeficiency syndrome. Pharmacognocy Magazine. 2012;8:135–140. doi: 10.4103/0973-1296.96564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey & Rizvi (2009).Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Medicine and Cellular Longevity. 2009;2:270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris, Dhanushka & Jayatilleka (2015).Peiris LDC, Dhanushka MAT, Jayatilleka TAHDG. Evaluation of aqueous leaf extract of Cardiospermum halicacabum (L.) on fertility of male rats. Hindawi Bio Med Research International. 2015;2015:175726. doi: 10.1155/2015/175726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poljsak, Suput & Milisav (2013).Poljsak B, Suput D, Milisav I. Achieving the balance between ROS and antioxidants: when to use the synthetic antioxidants. Oxidative Medicine and Cellular Longevity. 2013;2013:1–11. doi: 10.1155/2013/956792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaiya, Bujang & Zakaria (2014).Ramaiya SD, Bujang JS, Zakaria MH. Assessment of total phenolic, antioxidant and antibacterial activities of Passiflora species. The Scientific World Journal. 2014;2014:167309. doi: 10.1155/2014/167309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangika, Dayananda & Peiris (2015).Rangika BS, Dayananda PD, Peiris DC. Hypoglycemic and hypolipidemic activities of aqueous extract of flowers from Nyctanthes arbor-tristis L. in male mice. BMC Complementary and Alternative Medicine. 2015;15:289. doi: 10.1186/s12906-015-0807-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahgal et al. (2010).Sahgal G, Ramanathan S, Sasidharan S, Mordi MN, Ismail S, Mansor SM. Brine shrimp lethality and acute oral toxicity studies on Swietenia mahagoni (L) Jacq. seed methanolic extract. Pharmacognosy Research. 2010;2:215–220. doi: 10.4103/0974-8490.69107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samie et al. (2010).Samie A, Tambani T, Harshfield E, Green E, Ramalivhana JE, Bessong PO. Antifungal activities of selected Venda medicinal plants against Candida albicans, Candida krusei and Cryptococcus neoformans isolated from South African AIDS patients. Arican Journal of Biotechnology. 2010;9:2965–2966. [Google Scholar]
- Sharma et al. (2012).Sharma P, Jha AB, Dubey RS, Pessarakli M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. Journal of Botany. 2012;2012 doi: 10.1155/2012/217037. Article 217037. [DOI] [Google Scholar]
- Space & Flynn (2000).Space JC, Flynn T. USDA Forest Service, Pacific Southwest Research Station, Institute of Pacific Islands Forestry Honolulu, Hawai’i, USAhttp://www.hear.org/pier/pdf/niue_report.pdf Report to the Government of Niue on invasive plant species of environmental concern. 2000
- Spavieri et al. (2010).Spavieri J, Allmendinger A, Kaiser M, Casey R, Hingley-Wilson S, Lalvani A, Guiry MD, Blunden G, Tasdemir D. Antimycobacterial, antiprotozoal and cytotoxic potential of twenty-one brown algae (Phaeophyceae) from British and Irish waters. Phototherapy Research. 2010;24:1724–1729. doi: 10.1002/ptr.3208. [DOI] [PubMed] [Google Scholar]
- Sudasinghe & Peiris (2018).Sudasinghe SAHP, Peiris LDC. Hypoglycemic and hypolipidemic activity of aqueous leaf extract of Passiflora suberosa L. PeerJ. 2018;6:e4389. doi: 10.7717/peerj.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari et al. (2011).Tiwari P, Kumar B, Kaur M, Kaur G, Kaur H. Phytochemical screening and extraction: a review. Internationale Pharmaseutica Sciencia. 2011;1:98–106. [Google Scholar]
- Valgas et al. (2007).Valgas C, De Souza SM, Smania EFA, Smania JA. Screening methods to determine antibacterial activity of natural products. Brazilian Journal of Microbiology. 2007;38:369–380. doi: 10.1590/S1517-83822007000200034. [DOI] [Google Scholar]
- Vianna et al. (2012).Vianna MG, Ferreira AL, Garcia RO, Falcao E, Pacheco G, Mansur E. Comparison of verification-based techniques in the efficacy of cryopreservation of Passiflora suberosa L. and P. foetida L. shoot tips. Cryobiology. 2012;65:346–352. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Photograph showing mature leaves, and fruits. Photo credit—Ms Hasani Sudasinghe.
Data Availability Statement
The following information was supplied regarding data availability:
The raw data are provided in the Supplemental File.