Abstract
Background
We longitudinally assessed Down syndrome individuals at the age of risk of developing dementia to measure changes in brain anatomy and their relationship to cognitive impairment progression.
Methods
Forty-two Down syndrome individuals were initially included, of whom 27 (mean age 46.8 years) were evaluable on the basis of completing the 2-year follow-up and success in obtaining good quality MRI exams. Voxel-based morphometry was used to estimate regional brain volumes at baseline and follow-up on 3D anatomical images. Longitudinal volume changes for the group and their relationship with change in general cognitive status and specific cognitive domains were mapped.
Results
As a group, significant volume reduction was identified in the substantia innominata region of the basal forebrain, hippocampus, lateral temporal cortex and left arcuate fasciculus. Volume reduction in the substantia innominata and hippocampus was more prominent in individuals whose clinical status changed from cognitively stable to mild cognitive impairment or dementia during the follow-up. Relevantly, longitudinal memory score change was specifically associated with volume change in the hippocampus, prospective memory with prefrontal lobe and verbal comprehension with language-related brain areas.
Conclusions
Results are notably concordant with the well-established anatomical changes signaling the progression to dementia in Alzheimer's disease, despite the dense baseline pathology that developmentally accumulates in Down syndrome. This commonality supports the potential value of Down syndrome as a genetic model of Alzheimer's neurodegeneration and may serve to further support the view that Down syndrome patients are best candidates to benefit from treatment research in Alzheimer's disease.
Keywords: Brain aging, Alzheimer's, Cognitive aging
Highlights
-
•
Longitudinal changes in brain anatomy were identified in Down syndrome individuals.
-
•
Basal forebrain and hippocampal volume reductions paralleled clinical progression.
-
•
The overall anatomical pattern identified resembled Alzheimer's neurodegeneration.
1. Introduction
Down syndrome (DS) or chromosome 21 trisomy is the most common genetic cause of intellectual disability (Ballard et al., 2016). In addition to interference with brain development, aging is also disturbed in DS with an early presence of neurodegenerative changes (in virtually all DS individuals aged 40 or over) and clinical dementia in up to 70% of cases by the age of 60 (Dekker et al., 2015; Wiseman et al., 2015). The brain in older DS individuals displays many of the neuropathological features found in Alzheimer's disease (Head et al., 2016). This commonality is of capital importance in the research context, as it indicates a direct link between a genetic anomaly and neurodegeneration that may potentially contribute to elucidating the pathogenesis of Alzheimer's disease (Wiseman et al., 2015).
Previous neuroimaging research is prominent in indicating that demented DS patients do indeed show brain alterations in systems with typical degeneration in Alzheimer's disease (Emerson et al., 1995; Teipel and Hampel, 2006; RJ1 et al., 2008; Beacher et al., 2009; Powell et al., 2014; Sabbagh et al., 2015; Rafii et al., 2015; Lin et al., 2016). Nevertheless, existing cross-sectional studies are still not conclusive in distinguishing baseline DS dense brain pathology established during brain development from ongoing degenerative changes when progressing towards dementia. We present a longitudinal study on DS patients at the age of risk to developing dementia aimed to measure changes in brain anatomy and their relationship to cognitive deterioration.
2. Methods
2.1. Participants
Forty-two DS individuals were initially included in the study. Candidates were recruited from the community via parent organizations and were selected on the basis of age (40 years old upwards), DS confirmed by karyotype, capability to understand and follow MRI instructions, and also optimal attitude and willingness (participants and parents) to participate. Individuals with non-stable medical conditions were not considered eligible. Eight participants were excluded due to head motion during baseline MRI, 3 participants were lost in the follow-up period (follow-up cognitive testing was obtained, but they refused to be re-scanned) and 4 more were ruled out due to head motion during follow-up MRI exams. No subject was excluded on the basis of test performance. The final evaluable sample for MRI analysis included 27 DS individuals (15 females, 12 males) with genotype-confirmed trisomy 21 and a mean ± SD age of 46.8 ± 5.6 years, range 40–63 years (Table 1). The included (n = 27) and excluded (n = 15) participant subgroups did not significantly differ in terms of age, sex distribution, performance IQ and study-specific neuropsychological testing.
Table 1.
Characteristics of study participants and cognitive testing.
| Primary sample (n = 42) | Final sample (n = 27) | |
|---|---|---|
| Age (mean, SD years) | 46.0 (5.3) | 46.8 (5.6) |
| Gender (male/female) | 21/21 | 12/15 |
| Medical background (%) | ||
| Cardiovascular | 28.6% | 37.0% |
| Respiratory | 11.9% | 11.1% |
| Metabolic/Endocrine | 57.1% | 48.1% |
| Ophthalmological | 71.4% | 70.4% |
| Otorhinolaryngological | 4.8% | 7.4% |
| Disability levels (%)DSM-IV-TR | ||
| Mild | 33.3% | 29.6% |
| Moderate | 66.7% | 70.4% |
| Severe | 0% | 0% |
| Profound | 0% | 0% |
| Performance IQ, K-BIT (mean, SD)a | 59.3 (9.2) | 60.7 (9.0) |
| Knowledge (%) | ||
| Illiterate | 33.3% | 29.6% |
| Read/Write | 66.7% | 70.4% |
| Primary sample (n = 42) |
Final sample (n = 27) |
|||
|---|---|---|---|---|
| Cognitive testing (mean (SD) range) | Baseline | Follow-up | Baseline | Follow-up |
| Memory-Word List Learning | 24.3 (7.7) 8–42 |
25.8 (8.8) 9–45 |
25.7 (7.2) 13–42 |
25.8 (8.5) 9–42 |
| Verbal Comprehension | 8.9 (2.4) 2–12 |
9.2 (2.4) 0–12 |
9.0 (2.6) 2–12 |
9.2 (2.0) 3–12 |
| Block Construction | 6.2 (2.3) 3–10 |
5.2 (1.9)⁎ 2–8 |
6.1 (2.3) 3–10 |
5.2 (1.9)⁎ 2–8 |
| Object Recognition | 3.3 (1.5) 1–6 |
3.8 (1.3) 2–6 |
3.2 (1.3) 1–6 |
3.8 (1.2) 2–6 |
| Prospective Memory | 3.0 (1.4) 0–6 |
2.6 (1.9) 0–6 |
3.2 (1.4) 0–6 |
2.7 (1.7) 0–6 |
SD, standard deviation.
K-BIT, Kaufman Brief Intelligence Test (2nd edition); matrices test.
Significant score reduction at p < 0.01.
Our study was conducted in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). The study protocol was approved by the Clinical Research Ethical Committee of the Parc de Salut Mar (Barcelona). Written informed consent was obtained from parents. Verbal or written assent was additionally obtained from Down syndrome individuals.
2.2. Clinical assessment
Each participant underwent comprehensive medical, neurological and psychiatric evaluations and subsequent tailored neuropsychological testing to clinically establish (or rule out) the diagnoses of mild cognitive impairment (MCI) and dementia in terms of cognitive deterioration overlapping with developmental cognitive deficits associated with DS. The diagnosis of MCI and dementia was based on expert clinical judgement as is recommended in DS (Sheehan et al., 2015; Krinsky-McHale and Silverman, 2013; Fenoll et al., 2017). Operatively, a diagnosis of MCI was established on the basis of (i) a report of cognitive impairment by the patient (confirmed by a reliable informant) or by a reliable informant implying a change from previous capacities and (ii) no clinically relevant deterioration in adaptive skills and general cognition. The diagnosis of dementia was established when the patient met MCI criteria (i) and showed a perceptible deterioration in adaptive skills associated with memory impairment and at least one of the following disorders: aphasia, apraxia, agnosia or disturbance in executive functioning. At baseline, 2 DS individuals met MCI criteria and none for dementia.
2.3. Specific neuropsychological testing
To establish the correlation between regional brain volume changes over time and cognitive decline, one neuropsychological test was selected for each major Alzheimer's disease domain: memory impairment, aphasia, apraxia, agnosia and disturbance in executive functioning.
2.3.1. Memory
A version of the Rey Auditory-Verbal Learning Test (Geffen et al., 1990) adapted for people with intellectual disability (Esteba-Castillo et al., 2017) was used. The test measures learning, delayed recall and recognition. Only performance on learning was used. Participants were read a list of 12 words and were asked to evoke as many words as they could remember. The same list was repeated over five trials. Word-list learning over trials was measured as the sum of recalled words in trials 1 to 5.
2.3.2. Verbal comprehension-verbal abstract reasoning
This test combines an adapted version of the conventional “similarities” subtest (4 items) used in many intelligence batteries (participants are given two words or concepts and have to describe how they are similar) with comprehension of verbal sentences (5 items) reflecting different social situations (Esteba-Castillo et al., 2017). Each response was rated as 0 (incorrect), 1 (partial) or 2 (correct) with total maximum score of 18.
2.3.3. 3D block construction
As a measurement of constructional apraxia, we used a variation of the cubes subtest of the Developmental Neuropsychological Assessment-NEPSY battery (Korkman et al., 1998) adapted for people with intellectual disability (Esteba-Castillo et al., 2017). The participants used hand movements to construct 3D block patterns with methacrylate cubes to match a model. A total of 10 models were consecutively presented and 1 point was given for each correct construction (maximum score = 10).
2.3.4. Object recognition
The recognition of objects (unusual views) subtest of the Cambridge Examination for Mental Disorders of Older People with Down's Syndrome and Others with Intellectual Disabilities-CAMDEX-DS (Ball et al., 2006), validated for the Spanish population, was used (Esteba-Castillo et al., 2013). The test involves the recognition of objects (6 items) on images taken from unusual angles. The number of correct answers was duly registered.
2.3.5. Prospective memory
This task requires the interaction of executive and mnemonic components to remember intentions. We used two prospective memory items from the Rivermead Behavioral Memory Test (RBMT) (Wilson et al., 1985) adapted for people with intellectual disability (Esteba-Castillo et al., 2017). In the first situation, the participant had to remember (following a 20-min interference) to ask for his or her next appointment when cued by the explorer. In the second situation, the participant, upon cueing, was required to ask for a previously hidden object. Scores were lower when the number of given cues in each situation were higher (i.e., higher scores indicated better performance). The sum of scores from both situations was used to indicate the overall test performance.
2.4. MRI acquisition
A 1.5-Tesla Signa Excite system (General Electric, Milwaukee, WI) equipped with an eight-channel phased array head coil and single-shot echoplanar imaging (EPI) software was used. High-resolution 3D anatomical images were obtained using an axial T1-weighted three-dimensional fast spoiled gradient inversion recovery prepared sequence. A total of 134 contiguous slices were acquired with inversion time 400 ms, repetition time 11.9 ms, echo time 4.2 ms, flip angle 15°, field of view 30 cm, 256 × 256 pixel matrix, and slice thickness 1.2 mm. Each participant was assigned an MRI practice session with a specifically designed mock scanner to allow for habituation and minimize the probability of head motion during actual MRI sessions.
2.5. Image pre-processing
All the anatomical images were visually inspected prior to analysis by a trained operator to detect artefacts and motion effect. Eight participants were discarded at baseline and 4 at follow-up as a result of poor image quality. Gray and white matter tissue volumes were estimated at a voxel level using Statistical Parametric Mapping (SPM). SPM voxel-based morphometry (VBM) DARTEL algorithms were used with the following processing steps: segmentation of anatomical images into gray and white matter tissue probability maps in their native space; estimation of the deformations that best align the images together by iteratively registering the segmented images with their average; and, finally, the generation of spatially normalized and smoothed segmentations (10x10x10 mm FWHM) using the deformations estimated in the previous step. The analyses were performed with scaling by Jacobian determinants (estimates of volume change during normalization) to consider tissue volume. Normalized images were finally transformed to the standard SPM template, resliced to 1.5 mm resolution in Montreal Neurological Institute (MNI) space. In addition, the VBM tool allowed us to obtain global measurements (in ml) for gray matter, white matter and global CSF spaces in each participant.
2.6. Image analysis
As in a previous longitudinal assessment of brain volume changes over time (Soriano-Mas et al., 2011), we generated difference images (baseline voxel values minus follow-up voxel values) for both gray matter and white matter segments that served to map regional volume change in the whole sample (using one-sample t-test) and to compare the degree of anatomical change between individuals with and without cognitive deterioration during follow-up (using two-sample t-test). In addition, we estimated voxel-wise the correlations between volumetric longitudinal changes (i.e., the generated difference images) and cognitive longitudinal changes (baseline cognitive scores minus follow-up cognitive scores) using linear regression in SPM. Paired t-test was used to assess group mean differences between baseline and follow-up for global brain volumes (gray matter segment plus white matter segment) and CSF spaces.
Results were considered significant with clusters of 1701 ml (504 voxels) at a height threshold of p < 0.005, which satisfied the FWE (family wise error) rate correction of PFWE < 0.05 according to Monte Carlo simulations. Results below this threshold are also reported (FWE small volume corrected) for the hippocampus, which is a primary interest anatomical structure with a narrow section diameter.
3. Results
3.1. Behavioral results
The study follow-up had a mean duration of 23 months (SD, 2 months). The clinical status of five participants changed from cognitively stable to MCI and 2 more from cognitively stable to dementia. Therefore, 26% of cases (n = 7) in our sample showed clinical evidence of cognitive impairment progression in a period of approximately 2 years. In the whole group, score change for the selected cognitive tests was not significant, except for constructional apraxia assessment with Block Construction (Table 1).
3.2. Imaging results
Global brain volumes were measured to assess potential changes indicating a general effect over time on brain tissue. The brain volume at baseline in the follow-up sample was (mean ± SD) 995 ± 88 ml and the CSF space volume 267 ± 49 ml. No significant change was identified for these measurements after the follow-up. At the end of the study, the mean brain volume was 1000 ± 81 ml (paired t = 0.6; p = 0.548) and the mean CSF volume 269 ± 44 ml (paired t = 0.3; p = 0.772). Such an absence of general effects confers more anatomical specificity to the findings obtained in the regional analysis below.
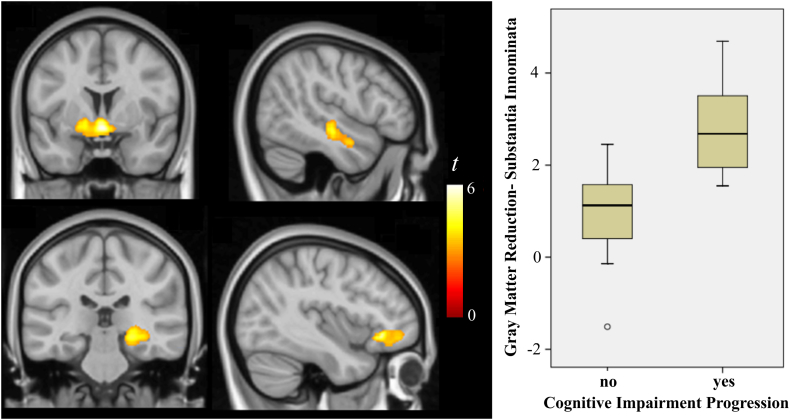
In the regional (VBM) analysis, significant gray matter volume reduction over time in the whole DS sample was identified in a region located at the basal forebrain and ventral aspect of the basal ganglia involving the substantia innominata, in the right orbitofrontal cortex and in the lateral aspect of the right temporal lobe (Fig. 1 and Table 2). Changes in the homologous temporal lobe region in the left hemisphere were not significant, although volume reduction was present at a lower cluster size threshold (Table 2). Significant white matter volume reduction was identified in a region implicating the right hippocampus and in the left arcuate fasciculus (Fig. 1 and Table 2).
Fig. 1.
Significant gray matter (top images) and white matter (bottom images) volume reduction over time in the whole DS sample. The box-plot corresponds to volume reduction (arbitrary units) for individuals with and without changes in their cognitive status at the brain coordinates showing the highest (peak) volume reduction in the whole sample. The right side of coronal views corresponds to the right hemisphere. The sagittal images correspond to the right (top) and left (bottom) hemispheres.
Table 2.
Regional brain volume change and correlation with change in cognitive scores.
| Number of voxels (ml) | x y z | t |
ta Adj. sex |
|
|---|---|---|---|---|
| Volume reduction Whole sample | ||||
| Substantia Innominata Region (gray matter) | 2358 (8.0) | 1.5 0–10.5 | 5.9 | 5.9 |
| R Lateral Temporal Cortex (gray matter) | 573 (1.9) | 49.5–18 −10.5 | 4.3 | 4.2 |
| L Lateral Temporal Cortex (gray matter) | 316 (1.1)b | −63 −18 −6 | 4.0 | 3.9 |
| R Orbitofrontal Cortex (gray matter) | 550 (1.9) | 19.5 37.5–21 | 5.4 | 5.3 |
| R Hippocampus Region (white matter) | 1375 (4.6) | 27–33 −6 | 5.0 | 4.9 |
| L Arcuate Fasciculus (white matter) | 1314 (4.4) | −43.5 30–7.5 | 5.8 | 5.8 |
| Volume reduction Cognitive impairment progression group > remaining sample | ||||
| Substantia Innominata Region (gray matter) | 907 (3.1) | 0 1.5–10.5 | 4.8 | 5.1 |
| L Hippocampus (gray matter) | 365 (1.2) | −30 − 25.5 −13.5 | 4.1 | 4.1 |
| Correlation between change in volume and change in cognitive score | ||||
| Memory-Word List Learning | ||||
| L Hippocampus-Amygdala (gray matter) | 444 (1.5) | −25.5 −25.5 −10.5 | 3.9 | 3.5 |
| R Thalamus (gray matter) | 665 (2.4) | 10.5–16.5 1.5 | −4.2 | −3.9 |
| Verbal Comprehension | ||||
| L Wernicke Area (gray matter) | 759 (2.6) | −49.5 −15 −10.5 | 4.0 | 4.0 |
| L Arcuate Fasciculus (white matter) | 1402 (4.7) | −36 0 30 | 4.0 | 3.5 |
| Object Recognition | ||||
| L Caudate Nucleus (adjacent white matter) | 1591 (5.4) | −12 18 1.5 | −4.7 | −4.4 |
| Executive Function-Prospective Memory | ||||
| L Prefrontal (gray matter) | 6578 (22.2) | −25.5 6 37.5 | 5.3 | 5.2 |
| R Prefrontal (gray matter) | 2773 (9.4) | 39 49.5 22.5 | 4.3 | 4.2 |
| Paracentral Lobule (gray matter) | 4850 (16.4) | 12–15 70.5 | 4.5 | 4.4 |
| L Prefrontal (white matter) | 5154 (17.4) | −19.5 16.5 24 | 6.5 | 6.3 |
| R Prefrontal (white matter) | 6478 (21.9) | 24 31.5 18 | 6.9 | 7.1 |
| L Inferior Temp/Hippocampus (white matter) | 1019 (3.4) | −28.5 −18 −13.5 | 3.8 | 3.7 |
| R Inferior Temp/Hippocampus (white matter) | 2079 (7.0) | 39–28.5 −21 | 3.9 | 3.9 |
x y z, coordinates (mm) given in Montreal Neurological Institute (MNI) space. Statistics at corrected threshold PFWE < 0.05 estimated using Monte Carlo simulations.
Adjusted by sex.
Subthreshold.
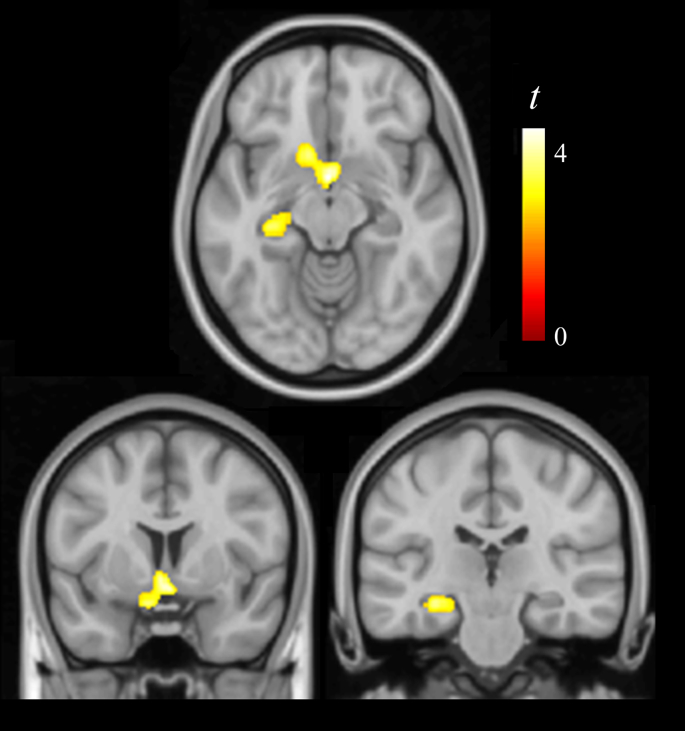
DS individuals with cognitive impairment progression showed significant gray matter volume reduction compared with the remaining sample also in the region of the substantia innominata, and in the left hippocampus (Fig. 2 and Table 2). Albeit less extensive, volume reduction in the substantia innominata region notably overlapped with changes in the whole sample. Fig. 1 shows a box-plot of volume reduction in individuals with and without cognitive impairment progression at the brain coordinate showing the largest (peak) volume reduction in the whole sample.
Fig. 2.
Regions showing significant gray matter volume reduction in DS individuals with clinical evidence of cognitive impairment progression compared with the remaining sample. The right side of coronal views corresponds to the right hemisphere.
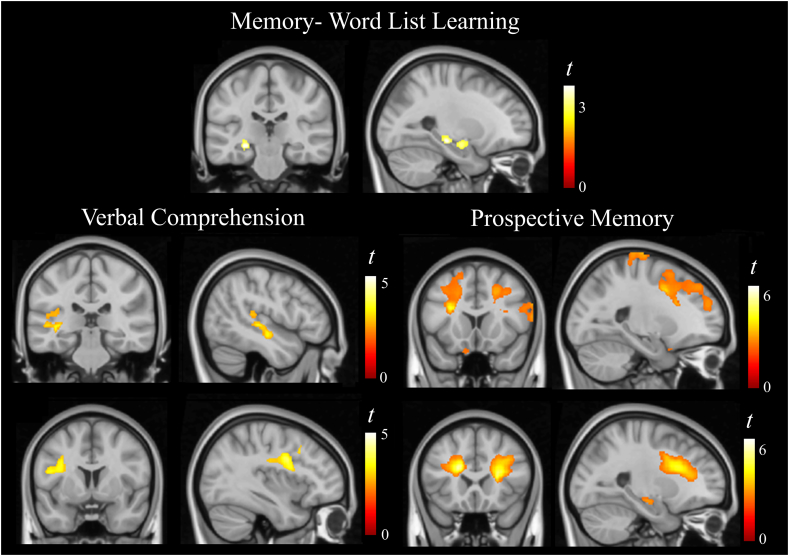
In the analysis of correlations between anatomical and cognitive longitudinal change, the following results were observed: a correlation between memory score reduction and gray matter volume reduction in a small portion of the left hippocampus and amygdala. A correlation between verbal comprehension score reduction and gray matter volume reduction within the Wernicke area (and related auditory cortex) and volume reduction in part of the left arcuate fasciculus. A correlation between prospective memory score reduction and both gray and white matter volume reduction in an extensive portion of the prefrontal lobe bilaterally, bilateral gray matter volume reduction in the paracentral lobule, and bilateral white matter volume reduction involving the inferior temporal cortex and the hippocampus (Fig. 3 and Table 2).
Fig. 3.
Correlations between anatomical and cognitive longitudinal changes. The right side of coronal views corresponds to the right hemisphere and all sagittal views correspond to the left hemisphere.
All these associations were observed in the expected positive direction (i.e., tissue volume reduction in parallel with cognitive score reduction). In addition, the analysis generated two results in the opposite direction involving a negative correlation between memory score change and volume change in the right thalamus, and between object recognition score change and volume change in white matter adjacent to the left caudate nucleus (Table 2). Finally, all the analyses were repeated adjusting by sex. We found no relevant sex effects (Table 2).
4. Discussion
Despite challenges inherent to longitudinal assessments particularly in individuals with a degree of intellectual disability, our study shows that the identification of brain anatomical changes preceding dementia in DS is feasible. Significant volume reduction after two years was identified in the substantia innominata region of the basal forebrain, the hippocampus, the lateral temporal cortex and the left arcuate fasciculus. Volume reduction in the substantia innominata and hippocampus was more prominent in the individuals showing variation in their general cognitive status during this period. Score change in different cognitive domains correlated with anatomical changes in specific brain systems. Of relevance were the associations of longitudinal change in the domain of memory with hippocampus, prospective memory with the prefrontal lobe and verbal comprehension with language-related brain areas.
Neurons of the cholinergic basal forebrain show neurofibrillary tangles in the earliest and presymptomatic stages of Alzheimer's disease (Theofilas et al., 2015; Mesulam et al., 2004; Mesulam, 2013), and both neurofibrillary tangle accumulation and neuronal loss are parallel to cognitive decline (Mesulam et al., 2004; Liu et al., 2015). The nucleus basalis of Meynert is the major element of the cholinergic complex located in the region of the substantia innominata below the anterior commissure and basal ganglia (Liu et al., 2015). In young people with DS, the nucleus basalis contains fewer neurons than in controls and the difference may accentuate in older individuals (Casanova et al., 1985; Mann et al., 1984) with combined developmental and degenerative changes. In our study, we have established a direct link between basal forebrain volume reduction and cognitive decline in the early stages of dementia in DS. Thus, basal forebrain degeneration would also seem to be an optimal marker of progression to dementia in DS.
Hippocampal volumetry is one of the most validated, accessible and widely used biomarkers in Alzheimer's disease, capable to reliably predicting time-to-progression from mild cognitive impairment to Alzheimer's dementia (Mak et al., 2017; Jack et al., 2010). In cross-sectional studies, the hippocampus volume is consistently smaller in young DS individuals compared with control subjects. However, volume alteration again is more important in older and demented individuals superimposed on developmental deficiency (Teipel and Hampel, 2006; Beacher et al., 2009; Aylward et al., 1999; Krasuski et al., 2002). Our results indicate that longitudinal assessment of hippocampal volume in DS may sufficiently distinguish the degenerative component from baseline alterations. We are aware of only one longitudinal assessment of brain volumetry published in DS, which precisely focused on the hippocampus. In the follow-up study by Aylward et al. (1999), changes in hippocampus volume over time were not statistically significant for either demented (n = 6) or non-demented (n = 13) DS subjects probably due to the small sample size.
A set of neocortical areas with relevant vulnerability to Alzheimer's disease neuropathology have collectively been called the cortical signature of the disease based on the fact that cortical thinning relates to symptom severity in the earliest stages (Dickerson et al., 2009) and during clinical progression (Verfaillie et al., 2016), and may even be detectable in asymptomatic individuals approximately a decade prior to dementia (Dickerson et al., 2011). All in all, the main elements of the cortical Alzheimer's disease signature were the superior prefrontal, inferior parietal and anterior temporal cortices. Previous research has shown that individuals with DS have a significantly greater age-related reduction in volume compared with normal control subjects in regions broadly corresponding to the vulnerable Alzheimer's disease cortical signature with a particularly large effect in the prefrontal cortex (Beacher et al., 2010). Our study shows a significant association between longitudinal volume change in an extensive portion of the superior aspect of the prefrontal lobe and prospective memory score change.
Prospective memory is a relatively complex task requiring the interaction of mnemonic and executive components to remember intentions after delay and interference. Neuroimaging studies in normal subjects have shown a consistent activation of the prefrontal lobe during prospective memory paradigms (Burgess et al., 2011). Our results suggest that prospective memory could reflect the functional status of the prefrontal lobe during the progression to dementia in DS, despite being one of the most developmentally affected brain structures (Fenoll et al., 2017; Pujol et al., 2015). Longitudinal behavioral studies also support the view that frontal lobe symptoms, in the form of disturbance in executive functioning, are early signs of dementia in DS (reviewed in Dekker et al., 2015).
All in all, cognitive score changes in the selected tests were small. The selection of relatively highly performing individuals may partially explain this event, probably added to some retest effects. Nevertheless, the correlations between cognitive score change and regional volume change showed notable neural system specificity. The only apparently non-consistent findings in our study were the negative correlations between volume change in the thalamus and memory score change, and between the white matter adjacent to the left caudate nucleus and object recognition. We consider that they may correspond to spurious associations, but, paradoxically, larger (Fortea et al., 2010) or relatively preserved (Benzinger et al., 2013) caudate nucleus volumes were also identified in mutation carriers in familial AD prior to dementia.
One challenge in MRI studies in populations with intellectual disability is to avoid excessive head motion during the acquisition. In this sample we used MRI practice sessions with a specifically designed mock scanner to minimize the problem. In addition, we decided to exclude cases with detectable image degradation, as no correction procedure is wholly efficient once the images have been acquired. Although accurate control of head motion effects may be a strength of the study, participant selection is also a limitation. In this context, our findings cannot generalize to all individuals with DS and conclusions should be limited to individuals with no severe or profound disability (Table 1). It is also relevant to note that our sample does not optimally represent the prevalence of dementia and MCI in the assessed age range (40 years old upwards). Both the selection of relatively highly performing individuals and the loss of participants entail a degree of limitation for the use of MRI to monitor Down syndrome individuals in clinical practice and clinical trials. A final limitation relates to using a 1.5-T system, as opposed to a 3-T system with higher MRI signal.
In conclusion, volume changes identified in our longitudinal assessment and their associations with cognitive impairment progression are, in general, notably consistent with well-established anatomical changes signaling the progression to dementia in Alzheimer's disease. Thus, brain involution in the older DS individuals would seem to resemble the degenerative process of Alzheimer's disease, despite the fact that it occurs in a complex situation with dense baseline pathology and a high potential for interactions between developmental and age-associated changes. Brain systems affected early in Alzheimer's disease such as the basal forebrain, hippocampus and the prefrontal lobe were selectively affected in DS preceding dementia in our study. Finally, we would also emphasize the bidirectional implications of our findings, by supporting both the potential value of DS as a genetic model of Alzheimer's neurodegeneration and the view that patients at risk of developing dementia in DS are best candidates to benefit from treatment research in Alzheimer's disease.
Acknowledgments
Acknowledgments
We thank the Agency of University and Research Funding Management of the Catalonia Government for their participation in the context of Research Groups SGR SGR2014-1673.
Funding
This work was supported in part by the Spanish Government (Grants PI12/02019, PSI-2014-53524-P). The funding source had no other role.
Potential conflicts of interest
We have no competing interests.
References
- Aylward E.H., Li Q., Honeycutt N.A. MRI volumes of the hippocampus and amygdala in adults with Down's syndrome with and without dementia. Am. J. Psychiatry. 1999;156(4):564–568. doi: 10.1176/ajp.156.4.564. [DOI] [PubMed] [Google Scholar]
- Ball S.L., Holland A.J., Huppert F., Treppner P., Dodd K. Cambridge University; Cambridge: 2006. The Cambridge Examination for Mental Disorders of Older People with Down's Syndrome and Others With Intellectual Disabilities (CAMDEX-DS) [Google Scholar]
- Ballard C., Mobley W., Hardy J., Williams G., Corbett A. Dementia in Down's syndrome. Lancet Neurol. 2016;15(6):622–636. doi: 10.1016/S1474-4422(16)00063-6. [DOI] [PubMed] [Google Scholar]
- Beacher F., Daly E., Simmons A. Alzheimer's disease and Down's syndrome: an in vivo MRI study. Psychol. Med. 2009;39(4):675–684. doi: 10.1017/S0033291708004054. [DOI] [PubMed] [Google Scholar]
- Beacher F., Daly E., Simmons A. Brain anatomy and ageing in non-demented adults with Down's syndrome: an in vivo MRI study. Psychol. Med. 2010;40(4):611–619. doi: 10.1017/S0033291709990985. [DOI] [PubMed] [Google Scholar]
- Benzinger T.L., Blazey T., Jack C.R., Jr. Regional variability of imaging biomarkers in autosomal dominant Alzheimer's disease. Proc. Natl. Acad. Sci. U. S. A. 2013;110(47):E4502–9. doi: 10.1073/pnas.1317918110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess P.W., Gonen-Yaacovi G., Volle E. Functional neuroimaging studies of prospective memory: what have we learnt so far? Neuropsychologia. 2011;49(8):2246–2257. doi: 10.1016/j.neuropsychologia.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Casanova M.F., Walker L.C., Whitehouse P.J., Price D.L. Abnormalities of the nucleus basalis in Down's syndrome. Ann. Neurol. 1985;18(3):310–313. doi: 10.1002/ana.410180306. [DOI] [PubMed] [Google Scholar]
- Dekker A.D., Strydom A., Coppus A.M. Behavioural and psychological symptoms of dementia in down syndrome: early indicators of clinical Alzheimer's disease? Cortex. 2015;73:36–61. doi: 10.1016/j.cortex.2015.07.032. [DOI] [PubMed] [Google Scholar]
- Dickerson B.C., Bakkour A., Salat D.H. The cortical signature of Alzheimer's disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb. Cortex. 2009;19(3):497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson B.C., Stoub T.R., Shah R.C. Alzheimer-signature MRI biomarker predicts AD dementia in cognitively normal adults. Neurology. 2011;76(16):1395–1402. doi: 10.1212/WNL.0b013e3182166e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson J.F., Kesslak J.P., Chen P.C., Lott I.T. Magnetic resonance imaging of the aging brain in down syndrome. Prog. Clin. Biol. Res. 1995;393:123–138. [PubMed] [Google Scholar]
- Esteba-Castillo S., Dalmau-Bueno A., Ribas-Vidal N., Vilà-Alsina M., Novell-Alsina R., García-Alba J. Adaptation and validation of CAMDEX-DS (Cambridge Examination for Mental Disorders of Older People with Down's syndrome and others with intellectual disabilities) in Spanish population with intellectual disabilities. Rev. Neurol. 2013;57(8):337–346. [PubMed] [Google Scholar]
- Esteba-Castillo S., Peña-Casanova J., García-Alba J. Barcelona test for intellectual disability: a new instrument for the neuropsychological assessment of adults with intellectual disability. Rev. Neurol. 2017;64:433–444. [PubMed] [Google Scholar]
- Fenoll R., Pujol J., Esteba-Castillo S. Anomalous white matter structure and the effect of age in Down Syndrome patients. J. Alzheimers Dis. 2017;57(1):61–70. doi: 10.3233/JAD-161112. [DOI] [PubMed] [Google Scholar]
- Fortea J., Sala-Llonch R., Bartrés-Faz D. Increased cortical thickness and caudate volume precede atrophy in PSEN1 mutation carriers. J. Alzheimers Dis. 2010;22(3):909–922. doi: 10.3233/JAD-2010-100678. [DOI] [PubMed] [Google Scholar]
- Geffen G., Moar K.J., O'Hanlon A.P., Clark C.R., Geffen L.B. Performance measures of 16- to 86-year old males and females on the auditory verbal learning test. Clin. Neuropsychol. 1990;4:45–63. doi: 10.1080/13854049008401496. [DOI] [PubMed] [Google Scholar]
- Head E., Lott I.T., Wilcock D.M., Lemere C.A. Aging in down syndrome and the development of Alzheimer's disease neuropathology. Curr. Alzheimer Res. 2016;13(1):18–29. doi: 10.2174/1567205012666151020114607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr, Wiste HJ, Vemuri P, et al. Brain beta-amyloid measures and magnetic resonance imaging atrophy both predict time-to-progression from mild cognitive impairment to Alzheimer's disease. Brain 2010;133(11):3336–3348. [DOI] [PMC free article] [PubMed]
- Korkman M., Kirk U., Kemp S. The Psychological Corporation; San Antonio, TX: 1998. NEPSY: A developmental neuropsychological assessment. [Google Scholar]
- Krasuski J.S., Alexander G.E., Horwitz B., Rapoport S.I., Schapiro M.B. Relation of medial temporal lobe volumes to age and memory function in nondemented adults with Down's syndrome: implications for the prodromal phase of Alzheimer's disease. Am. J. Psychiatry. 2002;159(1):74–81. doi: 10.1176/appi.ajp.159.1.74. [DOI] [PubMed] [Google Scholar]
- Krinsky-McHale S.J., Silverman W. Dementia and mild cognitive impairment in adults with intellectual disability: issues of diagnosis. Dev. Disabil. Res. Rev. 2013;18:31–42. doi: 10.1002/ddrr.1126. [DOI] [PubMed] [Google Scholar]
- Lin A.L., Powell D., Caban-Holt A., el et. (1)H-MRS metabolites in adults with down syndrome: effects of dementia. Neuroimage Clin. 2016;11:728–735. doi: 10.1016/j.nicl.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A.K., Chang R.C., Pearce R.K., Gentleman S.M. Nucleus basalis of Meynert revisited: anatomy, history and differential involvement in Alzheimer's and Parkinson's disease. Acta Neuropathol. 2015;129(4):527–540. doi: 10.1007/s00401-015-1392-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak E., Gabel S., Mirette H. Structural neuroimaging in preclinical dementia: From microstructural deficits and grey matter atrophy to macroscale connectomic changes. Ageing Res. Rev. 2017;35:250–264. doi: 10.1016/j.arr.2016.10.001. [DOI] [PubMed] [Google Scholar]
- Mann D.M., Yates P.O., Marcyniuk B. Alzheimer's presenile dementia, senile dementia of Alzheimer type and Down's syndrome in middle age form an age related continuum of pathological changes. Neuropathol. Appl. Neurobiol. 1984;10(3):185–207. doi: 10.1111/j.1365-2990.1984.tb00351.x. [DOI] [PubMed] [Google Scholar]
- Mesulam M.M. Cholinergic circuitry of the human nucleus basalis and its fate in Alzheimer's disease. J. Comp. Neurol. 2013;521(18):4124–4144. doi: 10.1002/cne.23415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M., Shaw P., Mash D., Weintraub S. Cholinergic nucleus basalis tauopathy emerges early in the aging-MCI-AD continuum. Ann. Neurol. 2004;55(6):815–828. doi: 10.1002/ana.20100. [DOI] [PubMed] [Google Scholar]
- Powell D., Caban-Holt A., Jicha G. Frontal white matter integrity in adults with Down syndrome with and without dementia. Neurobiol. Aging. 2014;35(7):1562–1569. doi: 10.1016/j.neurobiolaging.2014.01.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J., del Hoyo L., Blanco-Hinojo L. Anomalous brain functional connectivity contributing to poor adaptive behavior in Down syndrome. Cortex. 2015;64:148–156. doi: 10.1016/j.cortex.2014.10.012. [DOI] [PubMed] [Google Scholar]
- Rafii M.S., Wishnek H., Brewer J.B. The Down syndrome biomarker initiative (DSBI) pilot: proof of concept for deep phenotyping of Alzheimer's disease biomarkers in down syndrome. Front. Behav. Neurosci. 2015;9:239. doi: 10.3389/fnbeh.2015.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RJ1 Haier, Head K., Head E., Lott I.T. Neuroimaging of individuals with Down's syndrome at-risk for dementia: evidence for possible compensatory events. NeuroImage. 2008;39(3):1324–1332. doi: 10.1016/j.neuroimage.2007.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbagh M.N., Chen K., Rogers J. Florbetapir PET, FDG PET, and MRI in Down syndrome individuals with and without Alzheimer's dementia. Alzheimers Dement. 2015;11(8):994–1004. doi: 10.1016/j.jalz.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan R., Sinai A., Bass N. Dementia diagnostic criteria in Down syndrome. Int J Geriatr Psychiatry. 2015;30:857–863. doi: 10.1002/gps.4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano-Mas C., Hernández-Ribas R., Pujol J. Cross-sectional and longitudinal assessment of structural brain alterations in melancholic depression. Biol. Psychiatry. 2011;69(4):318–325. doi: 10.1016/j.biopsych.2010.07.029. [DOI] [PubMed] [Google Scholar]
- Teipel S.J., Hampel H. Neuroanatomy of down syndrome in vivo: a model of preclinical Alzheimer's disease. Behav. Genet. 2006;36(3):405–415. doi: 10.1007/s10519-006-9047-x. [DOI] [PubMed] [Google Scholar]
- Theofilas P., Dunlop S., Heinsen H., Grinberg L.T. Turning on the light within: subcortical nuclei of the Isodentritic Core and their role in Alzheimer's disease pathogenesis. J. Alzheimers Dis. 2015;46(1):17–34. doi: 10.3233/JAD-142682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verfaillie S.C., Tijms B., Versteeg A. Thinner temporal and parietal cortex is related to incident clinical progression to dementia in patients with subjective cognitive decline. Alzheimers Dement. 2016;5:43–52. doi: 10.1016/j.dadm.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson B.A., Cockburn J., Baddeley A.D. Bury St Edmunds, UK; Thames Valley Test Company: 1985. The Rivermead Behavioural Memory Test. [Google Scholar]
- Wiseman F.K., Al-Janabi T., Hardy J. A genetic cause of Alzheimer disease: mechanistic insights from down syndrome. Nat. Rev. Neurosci. 2015;16(9):564–574. doi: 10.1038/nrn3983. [DOI] [PMC free article] [PubMed] [Google Scholar]