Abstract
Several actinomycetes strains were isolated from different marine sponges collected from the Red Sea shore in Egypt. The efficiency of their crude extracts to inhibit histone deacetylase (HDAC) enzyme was investigated in the nuclear extract of Hela cell line. The crude extract corresponding to Streptomyces sp. SP9 isolated from the marine sponge Pseudoceratina arabica showed a promising HDAC inhibitory activity with 64 and 81% at 50 and 100 µg/ml, respectively. The strain was identified as Streptomyces sp. by phylogenetic analyses based on its 16S rRNA gene sequence. The major compounds of Streptomyces sp. SP9 were isolated and purified by different chromatographic methods. The chemical structure of the isolated compounds was identified on the basis of their spectroscopic data including mass, 1H and 13C NMR, and by comparison with those of authenticated samples. Structures of compounds 1 and 2 were established as heliomycin and tetracenomycin D, respectively. These compounds exhibited HDAC inhibitory activities with IC50 values of 29.8 ± 0.04 µg/ml for heliomycin (1) and 10.9 ± 0.02 µg/ml for tetracenomycin D (2). A computational docking study for compounds 1 and 2 against HDAC1, HDAC2, and HDAC3 was performed to formulate a hypothetical mechanism by which the tested compounds inhibit HDAC. Tetracenomycin D (2) showed a good binding interactions with HDAC2 (− 5.230 kcal/mol) and HDAC3 (− 6.361 kcal/mol).
Electronic supplementary material
The online version of this article (10.1007/s13205-018-1304-1) contains supplementary material, which is available to authorized users.
Keywords: Histone deacetylase, Actinomycetes, Sponges, Red Sea
Introduction
Genetic defects such as chromosomal abnormalities, gene mutations, insertions, and deletions are considered as the main leading cause of cancer (Bolden et al. 2006). However, epigenetic alterations of gene expression/regulation due to modifications of histone proteins were also found to have a significant contribution to the onset and progression of cancer (Konstantinopoulos et al. 2007). This mechanism is controlled by the process of chromatin condensation as a result of post-translational modifications of lysine residues in the N-terminal tail of histone (La Thangue 2004). Lysine modifications in histone protein are mainly represented in both acetylation and methylation, which influence the structural integrity of chromatin and the transcriptional activity as well (Jenuwein and Allis 2001). Acetylation/deacetylation process of both histone and non-histone proteins is suggested to be governed by two groups of enzymes, histone acetyltransferase (HAT), and histone deacetylase (HDAC). The balance between acetylation and deacetylation of histone was reported to regulate gene expression via chromatin modifications (Marks et al. 2000; Roth et al. 2001). Acetylation of histone by HAT plays a central role in the relaxation of chromatin structure and subsequent increase of transcriptional activity, whereas histone deacetylation by HDAC results in more compacted chromatin, thus repressing gene transcription. Expression of apoptotic factors, proteins that regulate cell differentiation and cell cycle progression, was found to be assigned to histone acetylation. However, an increased level of histone deacetylation is linked to the development of cancer via activation of tumor regulatory genes (Mottamal et al. 2015). As per literature, inhibition of HDAC activity is considered as a promising drug target in the field of cancer therapy (Yan et al. 2016).
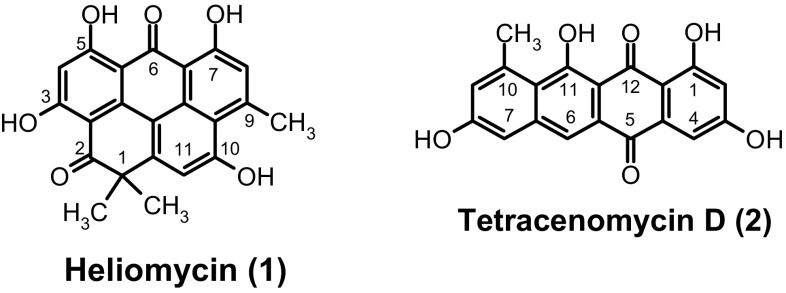
Marine environment remains unexplored source of natural products with unique structures (Blunt et al. 2017). The development of bioactive secondary metabolites from marine origin, particularly from marine invertebrates like sponges-associated microorganisms, gains the attention of several research groups (Belarbi et al. 2003; Piel et al. 2004; Abdelmohsen et al. 2014). Actinomycetes commonly exist as symbiotic microorganisms, particularly associated with marine sponges (Radwan et al. 2010). They are good sources for new lead or seed compounds in drug discovery (Abdelfattah et al. 2012, 2016a, b; Yixizhuoma et al. 2015, 2017). The in vitro anti-cancer effect of various natural compounds from different marine sponge-associated actinomycetes was investigated against several cancer cell lines (Izumikawa et al. 2010; Li et al. 2011). In our searching for bioactive secondary metabolites from marine microorganisms (Elmallah et al. 2017), we report two potent HDAC inhibitors (HDACIs), heliomycin (1) and tetracenomycin D (2) (Fig. 1) isolated from marine sponge Pseudoceratina arabica-associated Streptomyces sp. SP9.
Fig. 1.
Structure of compounds 1 and 2
Materials and methods
Collection of sponge samples
Four sponge samples were collected by diving in Ras Mohammed, South of Sinai, Egypt. The samples were identified as Pseudoceratina arabica, Erylus lendenfeldi sollas, Spheciospongia mastoidea, and Hyrtios erectus by Prof. Rob. W. M. van Soest (University of Amsterdam, Netherlands). The fresh samples were preserved in ice-cold sterile Ziploc bag containing seawater and then transported to our laboratory.
Isolation of actinomycetes
To isolate the sponge-associated actinomycetes, the sponge samples were rinsed several times with sterilized seawater to remove unwanted debris. Each sample was cut into small pieces and then aseptically homogenized using sterile sea water. 100 µl of the diluted homogenates (10− 1–10− 3) were spread on two different actinomycete-selective M1 and ISP2 media (Mincer et al. 2005). Cultivation media were prepared with 50% sterilized sea water, supplemented with Nalidixic acid (50 µg/ml) and cycloheximide (75 µg/ml) as anti-bacterial and anti-fungal agents, respectively. Plates were incubated at 28 °C for 7–30 days until the colonies appeared. The colonies of representative actinomycetes were picked up and subsequently spread on ISP2 media. Each strain was purified by streaking method and took a voucher number. The isolated strains were identified based on the morphological appearance (Kieser et al. 2000).
Preparation of crude extracts
The isolated strains were cultivated in 250 ml Erlenmeyer flasks containing 100 ml of Waksman medium (glucose: 2.0 g/100 ml, meat extract: 0.5 g/100 ml, peptone: 0.5 g/100 ml, dried yeast: 0.3 g/100 ml, NaCl: 0.5 g/100 ml, and CaCO3: 0.3 g/100 ml) in 50% sea water. The liquid cultures were grown at 28 °C for 3–5 days with continuous shaking at 200 r.p.m. The culture broths were subjected to a consecutive extraction with ethyl acetate. The residual cell pellets were extracted with acetone. Afterwards, the solvents of both culture broth and mycelia were evaporated and stored in small vial at − 20 °C for further use. Each vial took a serial number corresponding to the identification number of its strain. Each crude extract was dissolved in dimethyl sulfoxide (DMSO) for further investigation.
HDAC inhibition screen
The HDAC inhibition activity of the crude extracts was measured with HDAC colorimetric assay kit (BioVision, Linda Vista Avenue, Mountain View, CA; Catalog #K331-100). Positive control was referred to HDAC in the nuclear extract of Hela cell line. The HDAC inhibitor of trichostatin A (TSA) 100 ng/ml was used to demonstrate the specificity of deacetylase activities. In 96 well plate, the crude extracts of the isolated strains at concentrations of 50 and 100 µg/ml were incubated with HeLa nuclear extract, l0 µl of the 10X HDAC assay buffer, and 5 µl of the HDAC colorimetric substrate at 37 °C for 1 h. To stop the reaction, 10 µl of lysine developer was added. Finally, the plate was incubated at 37 °C for 30 min and the developed color was measured at 405 nm using ELISA plate reader.
Upscaling, extraction, and isolation of compounds from of Streptomyces sp. SP9
A small piece of a well grown agar strain was used to inoculate 100 ml of Waksman media in 250 ml Erlenmeyer flasks. The culture was incubated at 28 °C for 3 days. Afterwards, 5 ml of the inoculum was added to 100 ml of a freshly prepared liquid Waksman media. The inoculated media were further incubated at 28 °C with continuous shaking at 120 rpm for 5 days. The crude extract of the tested strains was prepared by centrifugation of the liquid culture at 4000 rpm for 15 min followed by successive solvent extraction with ethyl acetate. The mycelial fraction of each strain was extracted with acetone. Following removal of acetone, the aqueous solution was extracted with ethyl acetate. The extracts from water phase and biomass were combined under reduced pressure. The crude extract (2.33 g) was fractionated using silica gel PSQ100B column chromatography with a gradient of 0–100% MeOH/CH2Cl2 to yield four fractions. Sephadex LH-20 column was used to purify fraction II (123.3 mg). Compounds were finally purified by preparative thin layer chromatography (PTLC) to give heliomycin (1, 5.1 mg) and tetracenomycin D (2, 4.6 mg).
Heliomycin (1): Orange solid; (-)-ESI-MS m/z 375 ([M-H]−; (-)-HRESI-MS m/z 375.0859 (calcd. for C22H15O6, 335.0556); 1HNMR ([D6] DMSO, 600 MHz): H = 14.55 (s, 1H, 7-OH), 14.36 (s, 1H, 3-OH), 14.07 (s, 1H, 5-OH), 11.40 (br. s, 1H, 10-OH), 7.23 (s, 1H, 11-H), 7.01 (s, 1H, 8-H), 6.34 (s, 1H, 4-H), 2.90 (s, 3H, 9-CH3), 1.56 (s, 6H, 1-CH3); 13CNMR ([D6] DMSO, 125 MHz): δC = 204.9 (C-2), 183.5 (C-6), 170.7 (C-3), 170.5 (C-5), 167.6 (C-7), 162.1 (C-10), 152.7 (C-11a), 152.1 (C-9), 142.2 (C-11c), 139.1 (C-9b), 128.5 (C-8), 128.4 (C-9a), 118.2 (C-11), 114.2 (C-11b), 107.1 (C-6a), 105.9 (C-5a), 102.1 (C-2a), 99.4 (C-4), 46.1 (C-1), 28.9 (2Me-1), 25.5 (Me-9).
Tetracenomycin D (2): Red solid, (-)-ESI-MS: m/z (%) = 335 ([M-H]−, 100); 1HNMR ([D6] DMSO, 600 MHz): H 14.62 (s br,1H,11-OH), 12.17 (s br,1H,1-OH), 7.68 (s, 1H, 6-H), 7.08 (d, 3J = 1.9 Hz, 1H, 9-H), 6.99 (d, 3J = 2.2 Hz, 1H, 4-H), 6.86 (d, 3J = 1.9 Hz, 1H, 7-H), 6.41 (d, 3J = 2.2 Hz, 1H, 2-H), 2.74 (s, 3H, 10-CH3); 13CNMR ([D6] DMSO, 125 MHz): C 187.4 (C-12), 180.8 (C-5), 166.3 (C-1), 165.9 (C-3), 164.2 (C-8), 159.5 (C-11), 141.0 (C-6a), 139.5 (C-10), 135.3 (C-4a), 127.7 (C-5a), 123.0 (C-6), 120.9 (C-9), 119.4 (C-10a), 111.3 (C-4), 108.7 (C-12a), 108.6 (C-2), 107.8 (C-7), 106.3 (C-11a), 24.2 (Me-10).
Docking experiment
Crystal structures of histone deacetylases (HDAC) were obtained from the Protein Data Bank with PDB codes: 5ICN (HDAC1), 4LXZ (HDAC2), and 4A69 (HDAC3). Docking of heliomycin (1) and tetracenomycin D (2) was carried out using Schrodinger 16.4 software Glide’s Extra Precision (XP) (Friesner et al. 2006). The best Docking Score is obtained as the most negative value for the active ligands. Heliomycin (1) and tetracenomycin D (2) were assembled using the Maestro 9.2 and LigPrep 2.4 software. Parameters of the molecular docking were set to the default hard potential function. The active positions were set within 10 Å radius around the ligand found in histone deacetylases (HDAC) structures. The ligands were docked with the active site using the ‘extra precision’ glide docking (Glide XP) which docks ligands flexibly. The size of grid box for each protein was set to 20 Å by default. The binding site residues of each protein are summarized in Table 1.
Table 1.
Binding site residues of heliomycin (1) and tetracenomycin D (2) with HDAC1, HDAC2, and HDAC 3
| Pdb (Protein) | Binding site residues |
|---|---|
| 5ICN (HDAC1) | Asp 334 (A), Gln 339 (A), Lys 331 (A), Thr 332 (A), Thr 333 (A), Tyr 336 (A), Arg 270 (B), Arg 306 (B), Glu 335 (B) Gly 338 (B), phe 341 (B) and Tyr 336 (B) |
| 4LXZ (HDAC2) | Gln 358 (A), Gly 207 (A), Glu 208 (A), Lys 205 (A), Tyr 206 (A), Asp 337 (A), Arg 41 (B), Hid 38 (B), Glu 340 (B) and Tyr 338 (B) |
| 4A69 (HDAC3) | Arg 345 (A), Gln 71 (B), Gly 99 (B), Glu 102 (B), Leu 70 (B), Phe 101 (B), Pro 75 (B), Pro 98 (B), Ser 74 (B), Thr 76 (B), Val 73 (B), Ala 455 (D), Gly 452 (D), Ser 456 (D) and Val 463 (D) |
Results and discussion
The Red Sea is a rich and diverse ecosystem. It has various kinds of marine habitats such as sea-grass beds, mangroves, coral reefs, numerous fish species, sponges, and different microbial communities (Alkershi and Menon 2011; Mustafa et al. 2014). However, little investigations have been conducted to explore the actinobacterial communities from the Red Sea sponges (Abdelmohsen et al. 2014).
Isolation of actinomycetes and HDAC screening
Fifteen actinomycete-associated sponges were isolated from four sponge samples collected from Ras Mohammed Protectorate in Sharm el-Sheikh, South of Sinai, Egypt. Actinomycetes had been identified by their morphological characters (Kieser et al. 2000). The highest number of actinomycetes was obtained from Pseudoceratina arabica (i.e., seven strains) followed by Erylus lendenfeldi sollas (i.e., four strains) and Spheciospongia mastoidea (i.e., three strains). The sponge Hyrtios erectus also produces one actinomycete. The isolated strains were cultivated in 250 ml Erlenmeyer flasks and each has 100 mL of Waksman medium at 28 °C for 5 days (Waksman 1961). After extraction with ethyl acetate and methanol, the crude extracts were produced and took a number similar to the number of its own actinomycete strain. As we interested in bioactive natural products from actinomycetes (Abdelfattah et al. 2016a, b), the effect of crude extracts (SP1–SP15) on histone deacetylase (HDAC) inhibitory activity was examined using HeLa nuclear. All crude extracts were prepared at the final concentrations of 50 and 100 µg/ml (Fig. 2). Five extracts corresponding to the actinomycetes SP1, SP3, SP8, SP9, and SP11 significantly inhibited HDAC activity as compared to the trichostatin A (100 ng/ml). The crude extract of SP9 isolated from Pseudoceratina arabica exhibited the most potent HDAC inhibition of 64 and 81% at 50 and 100 µg/ml, respectively. From the literature, several actinomycetes crude extracts exhibited potent HDAC activity (Varghese et al. 2015).
Fig. 2.
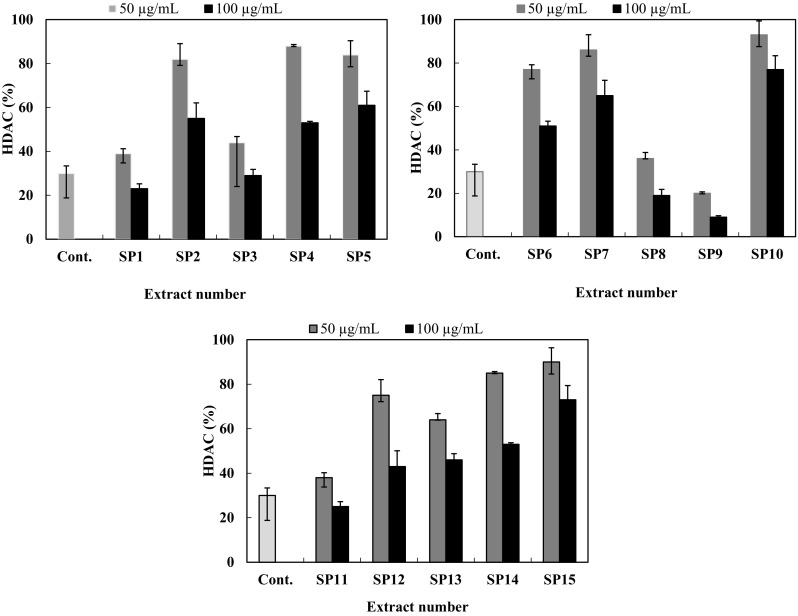
HDAC inhibitory activity of different bacterial crude extracts (SP1–SP15). The concentration of each sample in the reaction mixture was 50 and 100 µg/ml. Trichostatin A (100 ng/ml) was used as a positive control
The promising bacterial strain SP9 was characterized based on partial 16S rRNA gene sequencing (Kisser et al. 2000; Abdelfattah et al. 2016a, b). The 16S rRNA gene sequence (1123 bp) of the strain SP9 was obtained and submitted to GenBank with the number KU182929. The partial 16S rRNA gene sequence analysis showed that SP9 to be most similar to those of Streptomyces flavoviridis and Streptomyces heliomycini, with sequence identities of 100 and 99%, respectively. A maximum-likelihood tree (Radwan et al. 2010) was constructed for the isolate SP9 to show the relationship between the strain and some other related actinomycetes species (Fig. S1). Bootstrap analysis was used to assess the tree topology by performing 1000 resembling (Piel et al. 2005). From the tree, the high similarity and high bootstrap values proposed that the bacterial strain SP9 belonged to the genus Streptomyces sp.
Production, purification and structure assignment of secondary metabolites
To isolate and identify compounds responsible for the histone deacetylase inhibitory activity (HDAC) in the crude extract of SP9, the strain was subjected to upscale fermentation. The Streptomyces sp. SP9 was cultivated on 6 L Waksman medium for 5 days at 28 °C. The liquid culture was centrifuged and extracted to give a brown biomass. The crude extract was applied to silica gel column chromatography to give four fractions. Working up of the fractions resulted in the isolation of the heliomycin (1) and tetracenomycin D (2). The molecular weight of 1 was determined to be 376 Daltons by electrospray ionisation mass spectrometry (ESI-MS). The low-resolution ESI-MS (Fig. S4) in the negative ion mode showed a molecular ion at m/z 375 [M-H]−. The molecular formula of 1 was determined to be C22H15O6 by HRESI-MS data (m/z 375.0859 [M-H]−). The 1H NMR spectrum of 1 (Fig. S2) revealed four chelated hydroxyl groups (δH 14.55, 14.36, 14.07, and 11.72) and three singlets aromatic protons (δH 7.21, 7.01, and 6.31). The spectrum also showed one methyl signal (δH 2.90) and two magnetically equivalent methyl groups singlet (δH 1.56). The 13C NMR spectrum of 1 (Fig. S3) showed signals for 2 carbonyl groups (δC 204.9 and 183.5), 4 aromatic carbons bearing oxygen (δC 170.7, 170.5, 167.6, and 162.1), and 12 additional aromatic carbons (δC 152.7–99.4). Moreover, one aliphatic carbon (δC 46.1), two methyl groups (δC 28.9), and one aromatic methyl (δC 25.5) were observed. Compound 1 (Fig. 1) was identified as heliomycin (1) by searching in SciFinder and comparing the NMR data with literature values (Kock et al. 2005). Compound 2 was obtained as a red solid and gave a red color after spraying with 2N sodium hydroxide solution. Using anisaldehyde/sulphuric acid and heating, color of compound 2 was turned to blue. The UV range of 2 (maxima 213, 278, 338 and 487 nm) looks like the UV spectra of different natural anthraquinones (Abdelfattah et al. 2003). The (-)-ESI mass spectrum (Fig. S7) of 2 depicted peaks at m/z 335 [M-H]− which led to a molecular weight of 336 Daltons. The 1H NMR spectrum of 2 (Fig. S5) showed the presence of two hydroxyl groups (δH 14.62 and 12.17), five aromatic protons of which two sets of meta-coupled aromatic signals [δH 6.99 (d, J = 2.2 Hz) and 6.41 (d, J = 2.2 Hz); 7.08 (d, J = 1.9 Hz) and 6.86 (d, J = 1.9 Hz)] were observed. The aromatic proton at δH 7.68 can be assigned at peri-position to the carbonyl due to its lower field. The aliphatic pattern revealed one methyl signal (δH 2.74). The 13C NMR spectrum revealed two carbonyl groups (δC 187.4, and 180.8), four sp2 carbons connected to oxygen atoms (δC 166.3, 165.9, 164.2, and 159.5), five sp2 methine carbons (δC 123.0, 120.9, 111.3, 108.7, and 107.8), and another seven sp2 quaternary carbons. In the aliphatic reign, one methyl group (δC 24.2) was observed. A search in SciFinder, with the NMR data of 2, gave one compound designed as tetracenomycin D (Fig. 1). The structure of 2 was affirmed by comparison of the NMR data with those in the literature (Rohr et al. 1988).
HDAC assay and docking study
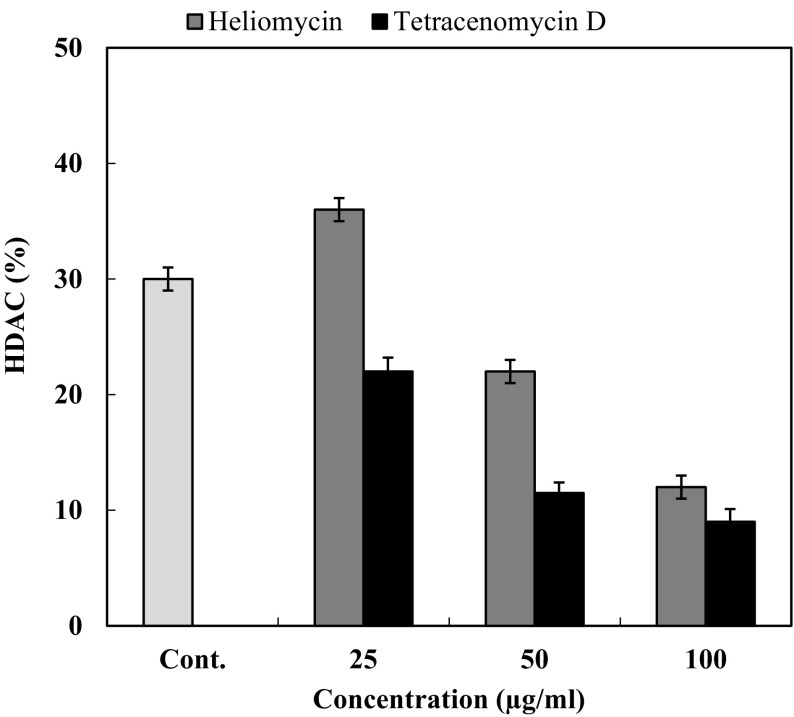
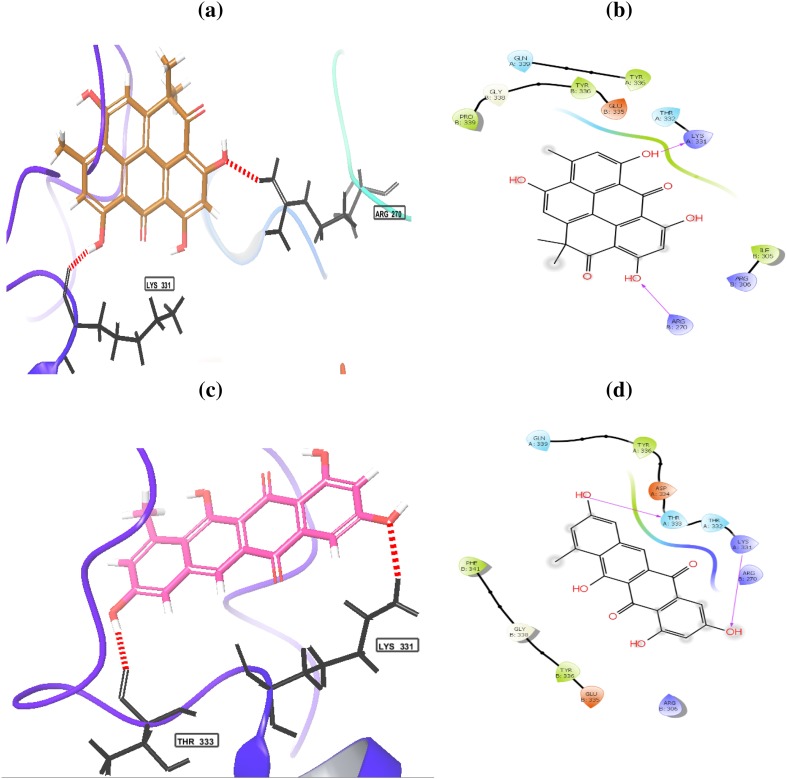
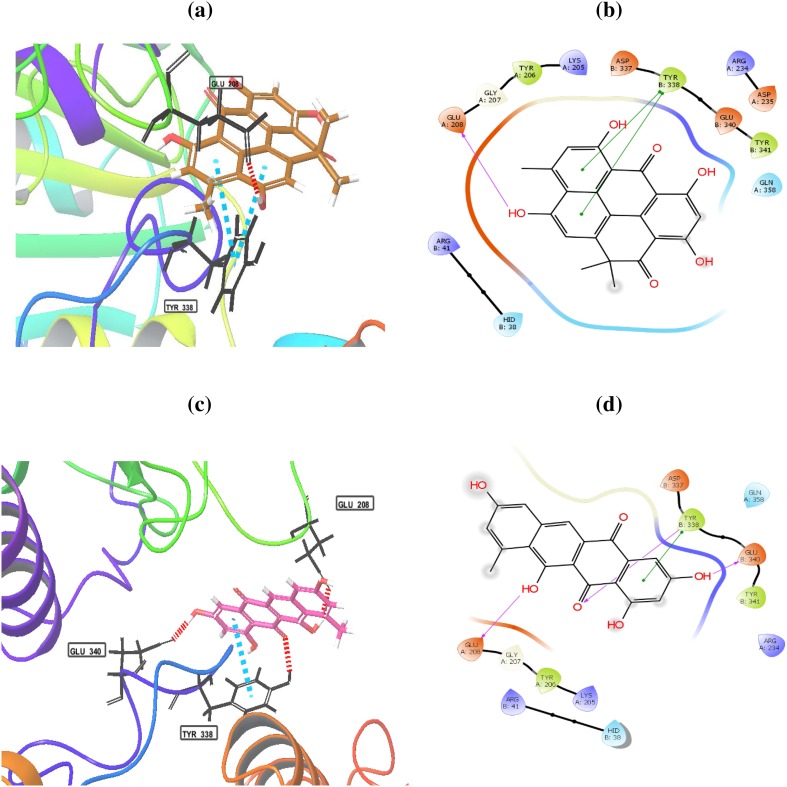
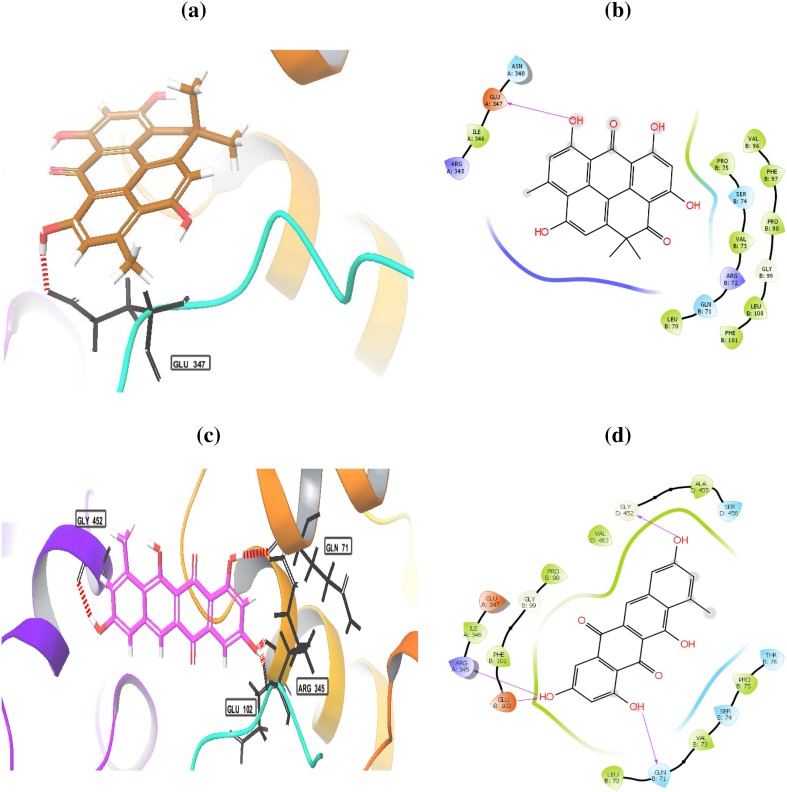
The histone deacetylase inhibition activity of heliomycin (1) and tetracenomycin D (2) was performed, as shown in Fig. 3. Three different concentrations (25, 50, and 100 µg/ml) of compounds were tested. Both compounds possessed a marked inhibition effect toward HDAC activity at the depicted concentrations. The results showed that percentage of inhibition for heliomycin (1) was determined to be 64, 78.1, and 88% at 25, 50, and 100 µg, respectively. The IC50 value for heliomycin (1) was found to 29.8 ± 0.04 µg/ml. Tetracenomycin D (2) exhibited more inhibition effect toward HDAC activity than heliomycin (1) with IC50 value of 10.9 ± 0.02 µg/ml. Heliomycin (1) and tetracenomycin D (2) were previously isolated from different actinomycetes (Rohr et al. 1988; Kock et al. 2005). Their anti-proliferative effects were detected against different cancer cells (Lee et al. 1993; Martin et al. 2001; Gorajana et al 2007; Vijayabharathi et al. 2011), whereas the effect of these compounds on the HDAC activity has not yet been reported. To expect the binding mode of heliomycin (1) and tetracenomycin D (2) in the active sites of the human histone deacetylases HDAC1, HDAC2, and HDAC3, molecular docking was carried using the Glide software. The HDAC1 can bind with heliomycin (1) through two hydrogen bonds with Lys331 (A) and Arg279 (B) (Fig. 4; Table 2). Tetracenomycin D (2) can form two hydrogen bonds with Lys331 (A) and Thr333 (A). The docking scores of HDAC1 with compounds 1 and 2 were − 3.974 and − 4.678 kcal/mol, respectively. The binding mode of heliomycin (1) with HDAC2 (Fig. 5; Table 2) showed it can form one hydrogen bond with Glu 208(A) and two π−π interactions with Tyr 338(B). It has a calculated docking score of − 4.977 kcal/mol. Based on the Glide docking score of compound 2 (− 4.977 kcal/mol), it can form hydrogen bonds with Glu 208(A), Glu 340(B) and Tyr 338(B), and π−π interactions with Tyr 338(B). The docking results also showed that tetracenomycin D (2) having the highest docking score of − 6.361 kcal/mol and maximum inhibitory activity with HDAC3 (Fig. 6; Table 2). It forms hydrogen bonds with Arg 345(A), Gln 71 (B), Glu 102 (B) and Gly 452(D). On the other hand, interaction of heliomycin (1) with HDAC3 showed a docking score of − 5.1831 kcal/mol and one hydrogen bond formation with Glu 347(A). Based on the in silico and in vitro data, heliomycin (1) and tetracenomycin D (2) seem to exhibit pronounced inhibitory activity against histone deacetylase (HDAC). This research work reveals that sponge-associated actinomycetes may be a valuable source of natural products with promising biological activity.
Fig. 3.
HDAC inhibitory activity of heliomycin (1) and tetracenomycin D (2). The concentration of each sample in the reaction mixture was 25, 50, and 100 µg/ml. Trichostatin A (100 ng/ml) was used as a positive control
Fig. 4.
Binding modes of heliomycin (a, b) and tetracenomycin D (c, d) with the histone deacetylase 1 (HDAC1)
Table 2.
In silico docking study of heliomycin (1) and tetracenomycin D (2) with HDAC1, HDAC2, and HDAC 3
| HDAC member | Compound | Docking score kcal/mol | Hydrogen bond interactions |
|---|---|---|---|
| HDAC1 | Heliomycin | − 3.974 | Lys331(A) and Arg279(B) |
| Tetracenomycin D | − 4.678 | Lys331(A) and Thr333(A) | |
| HDAC2 | Heliomycin | − 4.977 | Glu 208(A) |
| Tetracenomycin D | − 5.230 | Glu 208(A), Glu 340(B) and Tyr 338(B) | |
| HDAC3 | Heliomycin | − 5.183 | Glu 347(A) |
| Tetracenomycin D | − 6.361 | Arg 345(A), Gln 71 (B), Glu 102 (B) and Gly 452(D) |
Fig. 5.
Binding modes of heliomycin (a, b) and tetracenomycin D (c, d) with the histone deacetylase 2 (HDAC2)
Fig. 6.
Binding modes of heliomycin (a, b) and tetracenomycin D (c, d) with the histone deacetylase 3 (HDAC3)
Conclusion
In conclusion, 15 actinomycetes were isolated from 4 different sponge samples collected from the Red Sea. The crude extract obtained from the Streptomyces sp. Sp9 isolated from the Red Sea sponge Pseudoceratina arabica had the most potent HDAC inhibitory activity. The main constituents of the extract were heliomycin (1) and tetracenomycin D (2). These compounds are groups of widely distributed microbial natural compounds with diverse pharmacological activities. In this way, we introduce heliomycin (1) and tetracenomycin D (2) as promising natural products with histone deacetylase inhibitory activity.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are grateful for the financial support from Science and Technology Development Fund (STDF) in Egypt (Grant no. 6884). We thank Professor Rob van Soest (University of Amsterdam, Netherlands) for taxonomic identification of the sponge.
Compliance with ethical standards
Conflict of interest
No conflict of interest was declared.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s13205-018-1304-1) contains supplementary material, which is available to authorized users.
References
- Abdelfattah MS, Maskey RP, Asolkar RN, Grün-Wollny I, Laatsch H. Seitomycin: isolation, structure elucidation and biological activity of a new angucycline antibiotic from a terrestrial Streptomycete. J Antibiot. 2003;56:539–542. doi: 10.7164/antibiotics.56.539. [DOI] [PubMed] [Google Scholar]
- Abdelfattah MS, Toume K, Ishibashi M. Yoropyrazone, a new naphthopyridazone alkaloid isolated from Streptomyces sp. IFM 11307 and evaluation of its TRAIL resistance-overcoming activity. J Antibiot. 2012;65:245–248. doi: 10.1038/ja.2012.11. [DOI] [PubMed] [Google Scholar]
- Abdelfattah MS, Ishikawa N, Utpal K, Ishibashi M. Sulfotanone, a new alkyl sulfonic acid derivative from Streptomyces sp. IFM 11694 with TRAIL resistance-overcoming activity. J Nat Med. 2016;70:266–270. doi: 10.1007/s11418-015-0951-3. [DOI] [PubMed] [Google Scholar]
- Abdelfattah MS, Elmallah MIY, Hawas UW, Abou El-Kassema LT, Eid MAG. Isolation and characterization of marine-derived actinomycetes with cytotoxic activity from the Red Sea coast. Asian Pac J Trop Biomed. 2016;6:651–657. doi: 10.1016/j.apjtb.2016.06.004. [DOI] [Google Scholar]
- Abdelmohsen UR, Yang C, Horn H, Hajjar D, Ravasi T, Hentschel U. Actinomycetes from Red Sea sponges: Sources for chemical and phylogenetic diversity. Mar Drugs. 2014;12:2771–2789. doi: 10.3390/md12052771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkershi A, Menon N. Phytoplankton in polluted waters of the Red Sea coast of Yemen. J Mar Biol Assoc India. 2011;53:1–6. [Google Scholar]
- Belarbi EH, Gómez AC, Chisti Y, Camacho FG, Grima EM. Producing drug from marine sponges. Biotechnol Advan. 2003;21:585–598. doi: 10.1016/S0734-9750(03)00100-9. [DOI] [PubMed] [Google Scholar]
- Blunt JW, Copp BR, Keyzers RA, Munro MH, Prinsep MR. Marine natural products. Nat Prod Rep. 2017;34:235–294. doi: 10.1039/C6NP00124F. [DOI] [PubMed] [Google Scholar]
- Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5:769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- Elmallah MIY, Micheau O, Eid MAG, Hebishy AMS, Abdelfattah MS. Marine actinomycetes crude extracts with potent TRAIL-resistance overcoming activity against breast cancer. Oncol Rep. 2017;37:3635-–3642. doi: 10.3892/or.2017.5595. [DOI] [PubMed] [Google Scholar]
- Friesner RA, Murphy RB, Repasky MP, Frye LL, Greenwood JR, Halgren TA, Sanschagrin PC, Mainz DT. Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein—ligand complexes. J Med Chem. 2006;49:6177–6196. doi: 10.1021/jm051256o. [DOI] [PubMed] [Google Scholar]
- Gorajana A, Venkatesan M, Vinjamuri S, Kurada BV, Peela S, Jangam P, Poluri E, Zeeck A. Resistoflavine, cytotoxic compound from a marine actinomycete, Streptomyces chibaensis AUBN1/7. Microbiol Res. 2007;162:322–327. doi: 10.1016/j.micres.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Izumikawa M, Khan ST, Takagi M, Shin-Ya K. Sponge-derived Streptomyces producing isoprenoids via the mevalonate pathway. J Nat Prod. 2010;73:208–212. doi: 10.1021/np900747t. [DOI] [PubMed] [Google Scholar]
- Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- Kieser T, Bibb MJ, Buttner MJ, Chater KF, Hopwood DA. Practical streptomyces genetics. 2. Norwich: The John Innes Center Foundation; 2000. [Google Scholar]
- Kock I, Maskey RP, Biabani MA, Helmke E, Laatsch H. 1-Hydroxy-1-norresistomycin and resistoflavin methyl ether: new antibiotics from marine-derived Streptomycetes. J Antibiot. 2005;58:53–534. doi: 10.1038/ja.2005.73. [DOI] [PubMed] [Google Scholar]
- Konstantinopoulos PA, Karamouzis MV, Papavassiliou AG. Focus on acetylation: the role of histone deacetylase inhibitors in cancer therapy and beyond. Expert Opin Investig Drugs. 2007;5:569–571. doi: 10.1517/13543784.16.5.569. [DOI] [PubMed] [Google Scholar]
- La Thangue NB. Histone deacetylase inhibitors and cancer therapy. J Chemother. 2004;4:64–67. doi: 10.1179/joc.2004.16.Supplement-1.64. [DOI] [PubMed] [Google Scholar]
- Lee SW, Kim SE, Kim YH, Kim HS, Bang HJ, Kim HM, Lee JJ. Antitumoral compound, MCS-202, an effector on proliferation and morphology of human breast tumor cell line, MCF-7. Sanop Misaengmu Hakhoechi. 1993;21:594–599. [Google Scholar]
- Li K, Li QL, Ji NY, Liu B, Zhang W, Cao XP. Deoxyuridines from the marine sponge associated actinomycete Streptomyces microflavus. Mar Drugs. 2011;9:690–695. doi: 10.3390/md9050690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks PA, Richon VM, Rifkind RA. Histone deacetylase inhibitors: Inducers of differentiation or apoptosis of transformed cells. J Natl Cancer Inst. 2000;92:1210–1216. doi: 10.1093/jnci/92.15.1210. [DOI] [PubMed] [Google Scholar]
- Martin P, Rodier S, Mondon M, Renoux B, Pfeiffer B, Renard P, Pierre A, Gesson JP. Synthesis and cytotoxic activity of tetracenomycin D and of saintopin analogues. Bioorg Med Chem. 2001;10:253–260. doi: 10.1016/S0968-0896(01)00273-5. [DOI] [PubMed] [Google Scholar]
- Mincer TJ, Fenical W, Jensen PR. Culture-dependent and culture-independent diversity within the obligate marine actinomycete genus Salinispora. Appl Environ Microbiol. 2005;71:7019–7028. doi: 10.1128/AEM.71.11.7019-7028.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottamal M, Zheng S, Huang TL, Wang G. Histone Deacetylase inhibitors in clinical studies as templates for new anticancer agents. Molecules. 2015;20:3898–3941. doi: 10.3390/molecules20033898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa GA, Abd-Elgawad A, Abdel-Haleem AM, Siam R. Egypt’s Red Sea coast: phylogenetic analysis of cultured microbial consortia in industrialized sites. Front Microbiol. 2014;5:363. doi: 10.3389/fmicb.2014.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piel J, Hui D, Wen G, Butzke D, Platzer M, Fusetani N, Matsunaga S. Antitumor polyketide biosynthesis by an uncultivated bacterial symbiont of the marine sponge Theonella swinhoei. Proc Natl Acad Sci USA. 2004;101:16222–16227. doi: 10.1073/pnas.0405976101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piel J, Butzke D, Fusetani N, Hui D, Platzer M, Wen G, Matsunaga S. Exploring the chemistry of uncultivated bacterial symbionts: antitumor polyketides of the Pederin family. J Nat Prod. 2005;68:472–479. doi: 10.1021/np049612d. [DOI] [PubMed] [Google Scholar]
- Radwan M, Hanora A, Zan J, Mohamed NM, Abo-Elmatty DM, Abou-El-Ela SH, Hill RT. Bacterial community analyses of two red sea sponges. Mar Biotechnol. 2010;12:350–360. doi: 10.1007/s10126-009-9239-5. [DOI] [PubMed] [Google Scholar]
- Rohr J, Eick S, Zeek A, Reuschenbach P, Zähner H, Fiedler HP. Metabolic products of microorganisms. 249. Tetracenomycins B3 and D3, key intermediates of the elloramycin and tetracenomycin C biosynthesis. J Antibiot. 1988;41:1066–1073. doi: 10.7164/antibiotics.41.1066. [DOI] [PubMed] [Google Scholar]
- Roth SY, Denu JM, Allis CD. Histone acetyltransferases. Annu Rev Biochem. 2001;70:81–120. doi: 10.1146/annurev.biochem.70.1.81. [DOI] [PubMed] [Google Scholar]
- Varghese TA, Jayasri MA, Suthindhiran K. Marine Actinomycetes as potential source for histone deacetylase inhibitors and epigenetic modulation. Lett Appl Microbiol. 2015;61:69–76. doi: 10.1111/lam.12430. [DOI] [PubMed] [Google Scholar]
- Vijayabharathi R, Bruheim P, Andreassen T, Raja DS, Devi PB, Sathyabama S, Priyadarisini VB. Assessment of resistomycin, as an anticancer compound isolated and characterized from Streptomyces aurantiacus AAA5. J Microbiol. 2011;49:920–926. doi: 10.1007/s12275-011-1260-5. [DOI] [PubMed] [Google Scholar]
- Waksman SA. The actinomycetes: classification, identification, and descriptions of genera and species. Baltimore: Williams & Wilkins Co.; 1961. pp. 61–292. [Google Scholar]
- Yan M, Qian YM, Yue CF, Wang ZF, Wang BC, Zhang W, Zheng FM, Liu Q. Inhibition of histone deacetylases induces formation of multipolar spindles and subsequent p53-dependent apoptosis in nasopharyngeal carcinoma cells. Oncotarget. 2016;7:44171–44184. doi: 10.18632/oncotarget.9922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yixizhuoma, Tsukahara K, Kazufumi T, Ishikawa N, Abdelfattah MS, Ishibashi M. Novel cytotoxic isobenzofuran derivatives from Streptomyces sp. IFM 11490. Tetrahedron Lett. 2015;56:6345–6347. doi: 10.1016/j.tetlet.2015.09.116. [DOI] [Google Scholar]
- Yixizhuoma, Ishikawa N, Abdelfattah MS, Ishibashi M. Elmenols C-H, new angucycline derivatives isolated from a culture of Streptomyces sp. IFM 11490. J Antibiot. 2017;70:601–606. doi: 10.1038/ja.2016.158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.