Abstract
OBJECTIVE
We conducted a systematic review of the literature relating early life socioeconomic position (SEP) to breast cancer incidence and mortality from a critical period and life course trajectory perspective.
METHODS
PubMed, EMBASE and Web of Science were searched to identify cohort studies that evaluated the impact of early life SEP indicators on the incidence and/or mortality from breast cancer in adulthood.
RESULTS
Nine distinct studies evaluated the relationship between early life SEP and breast cancer between 1990 and 2016. Five reports assessed breast cancer incidence and five assessed breast cancer mortality as outcomes; one study assessed both incidence and mortality. While lower early life SEP was associated with reduced breast cancer incidence and increased breast cancer mortality in the US, studies conducted in Europe were unable to establish a consistent association.
CONCLUSIONS
We found moderate support for the association between early life SEP and incidence and mortality from breast cancer. The impact of early life SEP on breast cancer incidence and mortality appeared to vary between countries. We urge further investigation of the role of lifelong SEP trajectories in breast cancer outcomes.
Keywords: Socioeconomic position, breast cancer, incidence, mortality
INTRODUCTION
The life-course approach to chronic disease epidemiology emphasizes the importance of timing of exposures and postulates that early life exposures, including socioeconomic factors such as parental income, education and employment, may directly influence the etiology of chronic disease later in life (Ben-Shlomo and Kuh 2002; Wadhwa et al. 2009). According to the critical period model, exposures acting during a specific period have lasting effects on the structure and function of tissues, organs, and bodily systems (Ben-Shlomo and Kuh 2002). In addition, consistent with the chains of risk approach, social inequalities in cause-specific mortality may lie in socially determined exposures at different stages of life (Davey Smith et al. 2000). For example, adverse social circumstances in early life may influence the adoption and maintenance of adverse health behaviors that may persist into adulthood (van de Mheen et al. 1998) and may also affect cognitive development (Lovallo et al. 2012) and educational opportunity and attainment, which in turn may influence adult socioeconomic position (SEP) (Ben-Shlomo and Smith 1991), a predictor of adult chronic disease (Chan et al. 2004; Louwman et al. 2004; Mao et al. 2001; Webster et al. 2008). Notably, epidemiologic studies that adjust for adult factors are able to identify the independent influence of early life factors consistent with the critical period model. On the other hand, studies that do not adjust for these factors can only assess the cumulative risk of both early life SEP and subsequent exposures.
While ample epidemiologic evidence supports an association of adverse socioeconomic circumstances in early life with increased chronic disease incidence and mortality (Konig et al. 2003; Melchior et al. 2006; Naess et al. 2004; Pensola and Martikainen 2003; Power et al. 2005), particularly cardiovascular disease (Frankel et al. 1999; Galobardes et al. 2006; Smith et al. 2001), the extent to which early life SEP may affect cancer endpoints remains incompletely understood.
We systematically reviewed the published literature to determine the quality and strength of evidence relating SEP in early life with breast cancer incidence and mortality. Breast cancer remains the most prevalent cancer type among women globally (Ferlay et al. 2010), and studies have shown inconsistent associations between early life SEP and breast cancer(Okasha et al. 2002). Children in lower SEP families are more likely to be overweight (Thibault et al. 2010) and higher body mass index in childhood is associated with reduced breast cancer risk in adulthood (Ruder et al. 2008). However, early life dietary patterns resulting in childhood obesity have also been associated with higher circulating estrogen and earlier age at menarche, another independent risk factor for breast cancer (Maclure et al. 1991; MacMahon et al. 1973; Vihko and Apter 1989). In addition, reproductive factors place better-educated women at increased breast cancer risk due in part to an older age at first birth and lower parity (Heck and Pamuk 1997; Reeves et al. 2007), whereas higher educational attainment is associated with better survival (Bouchardy et al. 2006). Early life SEP is also a strong predictor of SEP in adulthood (Kuh et al. 1997), which is associated with increased risk of breast cancer (Chan et al. 2004; Webster et al. 2008). The early life period includes the prenatal environment, infancy, childhood and adolescence(Ben-Shlomo and Kuh 2002; Davey Smith et al. 2000), and we sought to evaluate whether the association of early life SEP with breast cancer may be consistent with a critical period approach.
METHODS
Three databases (PubMed, EMBASE and Web of Science) were searched to identify papers examining the association between early-life SEP and the incidence and/or mortality from breast cancer in adulthood. The evaluation of early-life SEP was based on measures of parental income, education, employment and/or occupation, and the early-life periods include childhood and adolescence.
The detailed search strategies are included in the Supplemental File. PubMed searches were conducted using a combination of Medical Subject Headings (MeSHs) and Keywords (in title or abstract.) EMBASE was searched using EMTREE and title/abstract search. Web of Science was searched using keywords. Each set of search results was limited by publication year from 1980 through May 2016 and further limited by study types (case control, cohort study, longitudinal study and prospective study.) Only articles in English were Included in this review.
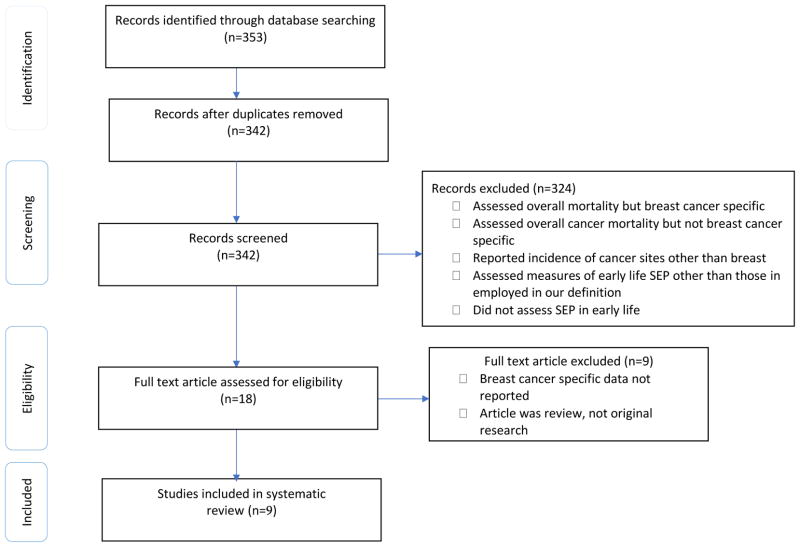
Our search retrieved a total of 353 articles from PubMed, EMBASE and Web of Science, and after manually removing 11 duplicates, we were left with a total of 342 unique references. Of the 342 screened articles, 324 studies - that did not report early life SEP, reported measures of SEP other than those included in our definition, did not report either breast cancer-specific incidence or mortality, or reported incidence of a cancer site other than breast- were excluded. We obtained full text for all 18 remaining articles, and after review, we additionally excluded 9 articles that did not report breast cancer-specific outcomes or were themselves systematic reviews. The remaining nine articles were included in this review. We followed the PRISMA guidelines [Transparent Report of Systematic Reviews and Meta-Analysis (Moher et al. 2009)] as a methodological template for this review (Figure 1).
FIGURE 1.
PRISMA Flow diagram of literature search for the association between early life socio-economic position and breast cancer incidence and mortality.
Inclusion and exclusion criteria
We included studies with the following characteristics: (i) reported on breast cancer incidence or breast cancer-specific mortality in adulthood; (ii) measured at least one SEP indicator in early life. We excluded studies that were published in languages other than English, those for which full text was not available, or that consisted of narrative reviews, comments or letters.
For each of the studies included in the review we abstracted information on setting, number of participants, number of incident breast cancers or breast cancer deaths, SEP indicator and the age at which it was assessed, study outcome and age at which it was assessed, reported measure of association between early life SEP and outcomes as well as corresponding confidence intervals (CI). When multiple measures of association were reported, we reported results from the fully adjusted model. We did not perform a formal meta-analysis for the studies included in this review, because the necessary conditions of comparability of exposures and outcomes, together with homogeneity of direction and strength of association are not met (Egger et al. 1998). In this study, we present a qualitative synthesis of the current scientific evidence regarding the effect of early life SEP on the incidence of breast cancer and mortality following this diagnosis.
RESULTS
We identified 9 unique studies that evaluated the relationship between early life SEP and breast cancer(de Kok et al. 2008; Dirx et al. 1999; Lawlor et al. 2006; Lope et al. 2016; Naess et al. 2007; Power et al. 2005; Pudrovska and Anikputa 2012; Strand and Kunst 2007; Titus-Ernstoff et al. 2002): two case-control (Lope et al. 2016; Titus-Ernstoff et al. 2002), and seven cohort studies (de Kok et al. 2008; Dirx et al. 1999; Lawlor et al. 2006; Naess et al. 2007; Power et al. 2005; Pudrovska and Anikputa 2012; Strand and Kunst 2007). One case-control study was conducted in the US (Titus-Ernstoff et al. 2002) and the second was conducted in Spain (Lope et al. 2016). The seven cohort studies were conducted in the US (Pudrovska and Anikputa 2012), Netherlands (de Kok et al. 2008; Dirx et al. 1999), Norway (Naess et al. 2007; Strand and Kunst 2007), Sweden (Lawlor et al. 2006), and the United Kingdom (Power et al. 2005).
Study Characteristics
Of the six studies included in this review that used father’s occupation as an early life SEP indicator (de Kok et al. 2008; Dirx et al. 1999; Naess et al. 2007; Power et al. 2005; Pudrovska and Anikputa 2012; Strand and Kunst 2007), two considered father’s education, mother’s education, father’s occupational prestige, and/or an indicator of whether the family income exceeded the median family income for the time period(Pudrovska and Anikputa 2012; Titus-Ernstoff et al. 2002). Another two studies considered childhood household income, father’s and mother’s education in addition to father’s occupation as indicators of early life SEP (Lope et al. 2016; Strand and Kunst 2007) (Table 1). Most of the included studies collected SEP information self-reported from adult participants. However, studies from Norway and Sweden utilized individual census data linked with cancer registry using personal identification numbers. One study combined occupational class of the head of the household and participant’s height, which is determined by childhood socioeconomic circumstances to classify early life SEP as manual versus non-manual (Lawlor et al. 2006). One study was conducted in 1999 (Dirx et al. 1999), one in 2002(Titus-Ernstoff et al. 2002), five between 2005 and 2008 (de Kok et al. 2008; Lawlor et al. 2006; Naess et al. 2007; Power et al. 2005; Strand and Kunst 2007), one in 2012 (Pudrovska and Anikputa 2012), and one in 2016 (Lope et al. 2016).
Table 1.
Early Life Socio-Economic Position and Breast Cancer Incidence and Mortality in Women in US and Europe, 1999–2016
| Author, Year | Setting | Total # of cases | Total # of women | Sample Description | SEP indicator | Outcome assessment | Analysis | Results Effect Estimate (95% CI) |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Incidence | ||||||||
| Dirx et al, 1999(Dirx et al. 1999) | Netherlands | 1009 | 62,573 | 55–69 years at baseline. Diet and early life factors assessed at baseline in 1986, follow up for 6.3 years | Father’s employment | Cancer registry | Age at diagnosis, age at menopause, parity, age at first birth, family history of breast cancer, benign breast disease, alcohol use, energy consumption, education | RR (95% CI): Father’s unemployment: 0.9 (0.7–1.2) |
| Titus-Ernstroff, et al. 2002(Titus- Ernstoff et al. 2002) | USA | 5659 | 11,587 | 50–75 years at diagnosis with invasive breast cancer and randomly selected controls matched with cases on age | Father’s education | Statewide cancer registries in Massachusetts, New Hampshire and Wisconsin | Adjusted for age at diagnosis and state |
OR (95% CI): Father’s education (years) 0–7 years: 0.95 (0.79 – 1.15) 8–11 years: 1.00 >=12 years: 1.22 (1.03 – 1.42) |
| de Kok, et al. 2008 (de Kok et al. 2008) | Netherlands | 209 | 12,978 | 15–75 years at baseline (April 1, 1991). SEP assessed retrospectively when participant was 12 years old or when father was last employed. Incidence follow-up, 13–14 years. | Father’s occupation | Questionnaire | Adjusted for age at diagnosis, gender, and marital status |
HR (95% CI): High professional: 1 Lower professional: 1.04 (0.56–1.92) Self-employed: 0.83 (0.45–1.53) High manual: 0.99 (0.55–1.77) Low manual: 1.09 (0.60–1.97) |
| Pudrovska, et al. 2012 (Pudrovska and Anikputa 2012) | USA | 275 | 4,275 | Participants were born in 1939. Early life SEP was self-reported in 1957 and 1975. Participant’s own education SEP information was reported in 1975–1977. | Father’s education Mother’s education Father’s occupation Father’s occupational prestige Family income above median |
Interviews | Adjusted for birth year, age at menarche, age at menopause, the presence and age of hysterectomy/oophorectomy, and family history of breast cancer. |
HR (SE), p-value: Mother high school graduate vs. mother not a high school graduate: 1.23 (0.147), p <0.05 Father high school graduate vs. father not a high school graduate: 0.991 (0.122), p >0.05 Family income above median vs. family income below median: 1.34 (0.162), p<0.05 |
| Lope et al. 2016(Lope et al. 2016) | Spain | 2713 | 5868 | 20–85 year old breast cancer cases and randomly selected population controls matched on age, sex and region | Parental income at birth | 23 hospital registries in 12 Spanish provinces | Adjusted for age at diagnosis, study level, BMI, age at first birth, previous biopsy, family history of breast cancer, age at menarche, menopausal status |
OR All women (95% CI): Low: 0.94 (0.80 – 1.11) Middle: 1.00 High: 0.45 (0.29 – 0.70) |
|
| ||||||||
| Mortality | ||||||||
|
| ||||||||
| Power, et al. 2005 (Power et al. 2005) | Britain | 266 | 11,855 | Participants were mothers of the members of the 1958 British birth cohort. Their median age in 1958 was 27 years (14–49). Childhood SEP was evaluted at the time they left school. Adult SEP was based on the occupation held by the male head of the household in 1958. Mortality was recorded between 1958 and December, 2003. | Father’s occupation | Questionnaire, interviews, medical records, NHS Central Registry | Adjusted for age at diagnosis, adult social class, smoking, and BMI. |
HR (95% CI): Professional/managerial: 1 Unskilled nonmanual: 0.98 (0.58–1.65) Skilled manual: 0.84 (0.60–1.18) Unskilled manual: 0.94 (0.65–1.35) |
| Lawlor, et al. 2006 (Lawlor et al. 2006) | Sweden | NA | 866,993 | Participants were born in 1944 – 1960 and were 0–16 when early life SEP was assessed. Participants’ later life social class was assesses in 1970, 1980, and 1990. Mortality was recorded between 1970 and December 31st, 2001. | Occupational social class of head of household & participant’s height were combined to create early life SEP as mannual versus nonmanual. | Swedish Cause of Death Register and the Swedish Population and Housing Census databases | Adjusted for age at diagnosis |
HR (95% CI): Nonmanual social class: 1 Manual social class: 1.06 (0.95–1.17) |
| Strand, et al. 2007 (Strand and Kunst 2007) | Norway | 292 | 3,297,476 PY | Participants were born in 1955–1965 and were 5–15 years old in 1970 when early life SEP was assessed. Adult SEP was assessed in 1990, when participants were 25–45. Mortality was recorded between November 3rd, 1990 and December 31st, 2001. | Father’s occupation, mother’s education, father’s education, childhood household income | Norwegian registry and census records | Adjusted for age at diagnosis, adult income, participant’s own education |
RII (95% CI): Comparing persons worst-off category to those in best-off; Father’s education: 0.91 (0.60, 1.43) Mother’s education: 1.14 (0.70, 1.84) Father’s occupation: 1.24 (0.80, 1.95) Household income: 1.08 (0.71, 1.64) |
| Naess, et al. 2007(Naess et al. 2007) | Norway | 1,137 | 491,363 | Participants were 0–20 in 1960, lived in private households of < 13 people with both parents and who survived until 1990. Participants’ adult SEP was recorded in 1990. Mortality was recorded between November 3rd, 1990 and December 31st, 2001 | Father’s occupational class | Tax register data, census data, death register (Statistics Norway) | Adjusted for age at diagnosis, adult SEP, and interaction between early life and adult SEP. |
RII (95% CI): Comparing the worst-off person with the best-off person in the SEP hierarchy: 0.91 (0.74, 1.12) |
| Pudrovska, et al. 2012 (Pudrovska and Anikputa 2012) | Wisconsin, USA | 275 | 4,275 | Participants were born in 1939. Early life SEP was self-reported in 1957 and 1975. Participant’s own education SEP information was reported in 1975–1977. | Father’s education Mother’s education Father’s occupation Father’s occupational prestige Family income above median |
Interviews | Adjusted for birth year, age at menarche, age at menopause, the presence and age of hysterectomy/oophorectomy, and family history of breast cancer. |
HR (SE), p-value: Mother high school graduate vs. mother not a high school graduate: 0.902 (0.272), p <0.05 Father high school graduate vs. father not a high school graduate: 0.125 (0.157) p <0.001 Family income above median vs. family income below median: 0.818 (0.253), p >0.05 |
PY: Person years. NA: Number was not reported.
SEP=Socio-Economic Position
HR: Hazard Ratio; OR: Odds Ratio: RII: Relative Index of Inequality; SE: Standard Error; CI: Confidence Interval
Studies of Breast Cancer Incidence
Five studies reported on breast cancer incidence in adulthood (de Kok et al. 2008; Dirx et al. 1999; Lope et al. 2016; Pudrovska and Anikputa 2012; Titus-Ernstoff et al. 2002) (Table 1). Two US studies observed a positive association between higher early life SEP and breast cancer incidence; one study from Spain observed a protective effect of higher early life SEP on breast cancer incidence, while two studies from the Netherlands reported no significant associations. Pudrovska et al. found a significant positive association between higher early life SEP for girls born in 1939 and breast cancer incidence in the late 1970’s in a US cohort (mother a high school graduate vs. not a graduate: hazard ratio [HR] =1.23, standard error [SE] =0.147, P <0.05; family income exceeding median vs. below median: HR=1.34, SE=0.162, P<0.05) after adjusting for birth year, age at menarche, age at menopause, history of hysterectomy/oophorectomy and age at which it occurred, and family history of breast cancer (Pudrovska and Anikputa 2012). Titus-Ernstoff et al. reported significant positive associations between father’s education and breast cancer diagnosis within the US, with 22% higher breast cancer odds for women whose fathers had at least 12 years of education compared to those whose fathers had fewer years of education (OR= 1.22, 95% CI: 1.03 – 1.45, P value=0.01)(Titus-Ernstoff et al. 2002). In contrast, Lope et al. reported an inverse association in Spain, with women of higher socioeconomic level at birth less likely to be diagnosed with breast cancer (OR: 0.45, 95% CI: 0.29 – 0.70)(Lope et al. 2016). DeKok et al. reported reduced breast cancer incidence in a population of Dutch women for some categories of lower early life SEP assessed in the early 1990s after adjusting for age at diagnosis, gender, and marital status; none of the associations reached statistical significance (high vs. low professional: HR=1.04, 95% CI 0.56–1.92; self-employed: HR=0.83, 95% CI 0.45–1.53; high manual: HR=0.99, 95% CI 0.55–1.77; low manual: HR=1.09, 95% CI 0.60–1.97) (de Kok et al. 2008). Dirx et al. also observed no significant association between having an unemployed father during the Depression period and breast cancer risk among women in the Netherlands (RR=0.9, 95% CI: 0.7–1.2)(Dirx et al. 1999).
Studies of Breast Cancer Mortality
Five studies reported on breast cancer mortality in adulthood (Lawlor et al. 2006; Naess et al. 2007; Power et al. 2005; Pudrovska and Anikputa 2012; Strand and Kunst 2007); one US study reported an inverse association between paternal SEP and breast cancer mortality, however the other studies did not observe statistically significant associations, although they were likely not sufficiently powered to detect small effect sizes (Table 1). A US study by Pudrovska et al. observed that higher early life SEP was associated with reduced risk of breast cancer mortality (father a high school graduate vs. not a high school graduate HR= 0.125, SE=0.157, P<0.0001) after adjusting for age at diagnosis and reproductive factors (Pudrovska and Anikputa 2012). Two studies, one British (Power et al. 2005) and one Norwegian (Naess et al. 2007), reported statistically insignificant associations of lower early life SEP with breast cancer mortality. Another Norwegian study reported reduced risk of breast cancer mortality with lower father’s education (< 9 vs. ≥13 years: relative index of inequality [RII] = 0.91, 95% CI 0.60–1.43), and increased risk with higher mother’s education (< 9 vs. ≥13 years: RRI = 1.14, 95% CI 0.70–1.84) (Strand and Kunst 2007). None of the associations across SEP measures reached statistical significance after adjusting for age at diagnosis and participant’s adult SEP (Naess et al. 2007; Power et al. 2005; Strand and Kunst 2007). A Swedish study also reported elevated but non-significant risk of breast cancer mortality in women with low early life SEP after adjusting for age at diagnosis (manual vs. non-manual social class: HR=1.06, 95%CI 0.95–1.17) (Lawlor et al. 2006).
DISCUSSION
In this systematic review, we identified five studies that focused on breast cancer incidence (de Kok et al. 2008; Dirx et al. 1999; Lope et al. 2016; Pudrovska and Anikputa 2012; Titus-Ernstoff et al. 2002) and five studies that evaluated breast cancer mortality (de Kok et al. 2008; Naess et al. 2007; Power et al. 2005; Pudrovska and Anikputa 2012; Strand and Kunst 2007) as a function of early life SEP. In contrast to a Dutch (de Kok et al. 2008) and a Spanish (Lope et al. 2016) study that found protective effects of higher early life SEP on breast cancer incidence, two US studies (Pudrovska and Anikputa 2012; Titus-Ernstoff et al. 2002) that evaluated a number of SEP indicators found that, consistent with the critical period hypothesis, high early life SEP was associated with increased breast cancer incidence. Whereas studies in Scandinavian (de Kok et al. 2008; Dirx et al. 1999; Naess et al. 2007; Strand and Kunst 2007) and European (Power et al. 2005) countries provided little evidence of an association between early life SEP and breast cancer mortality, one US study (Pudrovska and Anikputa 2012) supported a positive association.
The nature of the association between early life SEP and breast cancer endpoints remains unclear. A systematic review of the evidence linking prenatal and early life exposures to breast cancer risk reported no consistent pattern of association between markers of SEP, such as relative weight in childhood or adolescence, physical activity in early life, smoking at a young age, alcohol consumption and breast cancer risk (Okasha et al. 2003). However, most of the studies included in the review utilized a case-control design and may be vulnerable to bias, and did not consistently adjust for measures of adult SEP. Prenatal and early life markers of SEP that have been related to breast cancer risk include birth-weight (Michels et al. 1996), age at menarche (Kelsey et al. 1993), diet (Hislop et al. 1986), and weight in childhood (Whittemore et al. 1985). Braithwaite et al observed that although parental education was not a predictor of early menarche, there was a strong positive association between household income and early menarche among White girls, but an inverse association among Black girls(Braithwaite et al. 2009). Dietary patterns and childhood weight are also known to be strongly influenced by early life SEP(Hidaka et al. 2016), and these associations vary by geography and race(Kirkpatrick et al. 2012). Hence, the association between early life SEP and breast cancer risk may be modified by country-level (e.g. support programs for low-income parents) and/or individual (e.g. race/ethnicity) factors that are currently not well understood. The studies included in this review examining breast cancer incidence outcomes were all from high-income countries of the US and Europe. More studies from geographically and racially diverse populations, e.g. countries with lower childhood obesity rates and a wider range of early life SEP, will be needed to fully address this research question.
Another recent review of this topic observed that 9 out of 11 included studies did not report significant associations between childhood SEP and overall cancer mortality, although smoking-related cancers were significantly associated with childhood SEP(Vohra et al. 2016). This review updates the previous systematic review of this topic with more recent publications. While the studies conducted in Britain, Norway, and Sweden were unable to establish a consistent association between early life SEP and breast cancer mortality, a US study reported a significant reduction in the risk of breast cancer mortality in women with higher early life SEP (Pudrovska and Anikputa 2012). These findings suggest that the impact of early life SEP on breast cancer incidence and mortality could vary by contextual factors (Kunitz 2007). For example, the establishment of mammography screening programs resulted in an upsurge in breast cancer incidence due to the early detection in the US, but not in the Netherlands (Bray et al. 2004). Therefore, it is possible that a higher number of breast cancer cases in the US were diagnosed among women with higher SEP due to increased access to screening.
Evidence from earlier epidemiologic studies indicates that SEP influences adult cancer risk above and beyond the independent effects of income and education(Webster et al. 2008). Our findings provide additional evidence that it is also useful to differentiate between childhood and adulthood SEP rather than combining them in a single measure as each likely exerts independent effects on breast cancer risk via separate pathways (Pensola and Martikainen 2003). The association between high early life SEP and increased risk of breast cancer in adulthood observed in several studies may be partly explained by adult SEP, and operate via mediation by reproductive risk factors (e.g. contraceptive use, age at menarche for breast cancer) and behavioral risk factors such as smoking, alcohol consumption, and BMI, which are important risk factors for many cancers (Davey Smith et al. 2007; Louwman et al. 2004). On the other hand, higher SEP in early life and adulthood may lead to improved cancer outcomes such as reduced risk and mortality through other pathways. For instance, there may exist a common factor, such as cognitive ability, that may predict both good health and educational attainment (Strand and Kunst 2007), and educational accomplishments often lead to higher incomes that result in favorable living and working conditions (Strand and Kunst 2007) as well as improved access to healthcare, which likely results in timely and high quality cancer treatment, and hence reduced mortality. These biological pathways highlight areas in which targeted cancer control and prevention efforts may focus in the long term. For instance, if low early life SEP is associated with increased risk of obesity, an important breast cancer risk factor, then strategies focused on reducing overweight/obesity in childhood may help decrease the future risk of breast cancer in this subgroup. Such prevention strategies may focus on adolescent girls and young women with low early life SEP and potentially reduce the risk of breast cancer in adulthood. Attempts to better elucidate the socioeconomic distribution of adult health and risk of mortality must, therefore, consider the influence of socioeconomic trajectory across the entire life-course (Davey Smith et al. 1998; Melchior et al. 2006). This approach will warrant obtaining detailed and prospective assessment of SEP measures in early-life and adulthood and incorporating the measures in cancer registries and new prospective studies of cancer to identify risk factors for breast cancer and/or mortality during critical periods. This approach is part of current practice in several countries such as the Netherlands, Norway and Sweden where cancer registries incorporate data from individual records across the health system- from birth until death. While this may not be feasible in many countries presently, there is growing recognition that current cancer registry data (e.g. SEER) need to be linked with multiple other sources of epidemiologic data in order to fill critical gaps in the knowledge of risk factors across the life-course. In addition, prospective studies or birth cohorts may also benefit from including detailed measures of early life SES at baseline regardless of the primary study outcome, to ensure that future studies and pooling projects may further examine early life factors in adult health with minimal recall and/or selection bias.
Studies included in this review have a number of limitations, including the lack of comprehensive measures of early life SEP. The majority of the articles reviewed used only father’s occupation as a measure of childhood SEP, which despite offering some common ground for inter-study comparisons, provides a limited picture of early life SEP. For example, we were unable to account for parental age in these associations, which may be associated with lower SEP if younger parents are more likely to still be in educational or vocational training programs. A comparison of the association between different indicators and childhood SEP could provide insight into the mechanisms linking childhood SEP with adult health. Further, given the long period of time between childhood and ascertainment of cancer outcomes in adulthood, studies relying on self-reported early life SEP retrospectively may be susceptible to significant recall bias. However, the use of multiple measures of SEP, including parental education and employment helps to minimize serious recall bias since employment based measures are likely less susceptible to recall bias compared with education based measures. Furthermore, several of the included articles were prospective cohort studies, and evaluated childhood SEP at baseline, minimizing the length of time between childhood and cancer outcome ascertainment. The strengths of the studies reviewed include a cohort design in most studies, large sample sizes, long follow-up, and accurate incidence and mortality information obtained from national registries.
In conclusion, our systematic review provides some evidence that lower early life SEP may be associated with reduced breast cancer incidence and increased risk of breast cancer mortality in adulthood. We urge further studies on this topic with a more comprehensive assessment of early life SEP and adjustment for SEP in adulthood alongside other relevant covariates in the socio-demographic, behavioral and clinical domains. In addition to considering the individual effect of early life and/or adult SEP on adult chronic disease and mortality, it will be important for future studies to investigate the impact of lifelong trajectories of socioeconomic circumstances on these outcomes.
Supplementary Material
Acknowledgments
We are grateful to Felicia Widjaja and Grace Kim for assistance with data collection and management.
Funding: This research was supported in part by Grant# 121891-MRSG-12-007-01-CPHPS from the American Cancer Society.
Footnotes
Competing Interests: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The authors have no further financial relationship with the funders.
Contributors Statement: Min-Lin Fang conducted the literature search. Dr. Tomi Akinyemiju, Mr. Josh Demb and Ms. Monika Izano synthesized data from included studies and prepared the first draft of the manuscript. Drs. David Rehkopf and Robert Hiatt participated in the writing of the manuscript. Dr. Dejana Braithwaite oversaw concept design, analysis, and interpretation of the data. All authors approved the last draft of the manuscript.
References
- Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. International journal of epidemiology. 2002;31:285–293. [PubMed] [Google Scholar]
- Ben-Shlomo Y, Smith GD. Deprivation in infancy or in adult life: which is more important for mortality risk? Lancet. 1991;337:530–534. doi: 10.1016/0140-6736(91)91307-g. [DOI] [PubMed] [Google Scholar]
- Bouchardy C, Verkooijen HM, Fioretta G. Social class is an important and independent prognostic factor of breast cancer mortality. International journal of cancer Journal international du cancer. 2006;119:1145–1151. doi: 10.1002/ijc.21889. [DOI] [PubMed] [Google Scholar]
- Braithwaite D, et al. Socioeconomic status in relation to early menarche among black and white girls. Cancer Causes Control. 2009;20:713–720. doi: 10.1007/s10552-008-9284-9. [DOI] [PubMed] [Google Scholar]
- Bray F, McCarron P, Parkin DM. The changing global patterns of female breast cancer incidence and mortality. Breast cancer research: BCR. 2004;6:229–239. doi: 10.1186/bcr932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AT, et al. Azacitidine induces demethylation of the Epstein-Barr virus genome in tumors. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2004;22:1373–1381. doi: 10.1200/JCO.2004.04.185. [DOI] [PubMed] [Google Scholar]
- Davey Smith G, Gunnell D, Ben-Shlomo Y. Life-course approaches to socio-economic differentials in cause specific adult mortality. In: Leon DA, Walt G, editors. Poverty, Inequality and Health: An International Perspective. Oxford University Press; Oxford: 2000. pp. 88–124. [Google Scholar]
- Davey Smith G, et al. Education and occupational social class: which is the more important indicator of mortality risk? Journal of epidemiology and community health. 1998;52:153–160. doi: 10.1136/jech.52.3.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey Smith G, Hypponen E, Power C, Lawlor DA. Offspring birth weight and parental mortality: prospective observational study and meta-analysis. American journal of epidemiology. 2007;166:160–169. doi: 10.1093/aje/kwm054. [DOI] [PubMed] [Google Scholar]
- de Kok IM, van Lenthe FJ, Avendano M, Louwman M, Coebergh JW, Mackenbach JP. Childhood social class and cancer incidence: results of the globe study. Soc Sci Med. 2008;66:1131–1139. doi: 10.1016/j.socscimed.2007.11.035. [DOI] [PubMed] [Google Scholar]
- Dirx MJ, van den Brandt PA, Goldbohm RA, Lumey LH. Diet in adolescence and the risk of breast cancer: results of the Netherlands Cohort Study. Cancer Causes Control. 1999;10:189–199. doi: 10.1023/a:1008821524297. [DOI] [PubMed] [Google Scholar]
- Egger M, Schneider M, Davey Smith G. Spurious precision? Meta-analysis of observational studies. BMJ. 1998;316:140–144. doi: 10.1136/bmj.316.7125.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLOBOCAN 2008 v2.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet] International Agency for Research on Cancer; 2010. [Accessed 31/3/2013]. http://globocan.iarc.fr/factsheet.asp - BOTH. [Google Scholar]
- Frankel S, Smith GD, Gunnell D. Childhood socioeconomic position and adult cardiovascular mortality: the Boyd Orr Cohort. American journal of epidemiology. 1999;150:1081–1084. doi: 10.1093/oxfordjournals.aje.a009932. [DOI] [PubMed] [Google Scholar]
- Galobardes B, Smith GD, Lynch JW. Systematic review of the influence of childhood socioeconomic circumstances on risk for cardiovascular disease in adulthood. Annals of epidemiology. 2006;16:91–104. doi: 10.1016/j.annepidem.2005.06.053. [DOI] [PubMed] [Google Scholar]
- Heck KE, Pamuk ER. Explaining the relation between education and postmenopausal breast cancer. American journal of epidemiology. 1997;145:366–372. doi: 10.1093/oxfordjournals.aje.a009114. [DOI] [PubMed] [Google Scholar]
- Hidaka BH, Kerling EH, Thodosoff JM, Sullivan DK, Colombo J, Carlson SE. Dietary patterns of early childhood and maternal socioeconomic status in a unique prospective sample from a randomized controlled trial of Prenatal DHA Supplementation. BMC Pediatr. 2016;16:191. doi: 10.1186/s12887-016-0729-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hislop TG, Coldman AJ, Elwood JM, Brauer G, Kan L. Childhood and recent eating patterns and risk of breast cancer. Cancer detection and prevention. 1986;9:47–58. [PubMed] [Google Scholar]
- Kelsey JL, Gammon MD, John EM. Reproductive factors and breast cancer. Epidemiologic reviews. 1993;15:36–47. doi: 10.1093/oxfordjournals.epirev.a036115. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick SI, Dodd KW, Reedy J, Krebs-Smith SM. Income and race/ethnicity are associated with adherence to food-based dietary guidance among US adults and children. J Acad Nutr Diet. 2012;112:624–635. e626. doi: 10.1016/j.jand.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig CW, et al. Frequent embolization in peripheral angioplasty: detection with an embolism protection device (AngioGuard) and electron microscopy. Cardiovascular and interventional radiology. 2003;26:334–339. doi: 10.1007/s00270-003-2656-3. [DOI] [PubMed] [Google Scholar]
- Kuh D, Power C, Blane D, Bartley M. Social pathways between childhood and adult health. In: Kuh D, Ben-Shlomo Y, editors. A Life Course Approach to Chronic Disease and Epidemiology: tracing the origins of ill health from early to adult life. 1. Oxford University Press; Oxford: 1997. [Google Scholar]
- Kunitz SJ. The health of populations: general theories and particular realities. Oxford University Press; Oxford; New York: 2007. [Google Scholar]
- Lawlor DA, Sterne JA, Tynelius P, Davey Smith G, Rasmussen F. Association of childhood socioeconomic position with cause-specific mortality in a prospective record linkage study of 1,839,384 individuals. American journal of epidemiology. 2006;164:907–915. doi: 10.1093/aje/kwj319. [DOI] [PubMed] [Google Scholar]
- Lope V, et al. Perinatal and childhood factors and risk of breast cancer subtypes in adulthood. Cancer Epidemiol. 2016;40:22–30. doi: 10.1016/j.canep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- Louwman WJ, van Lenthe FJ, Coebergh JW, Mackenbach JP. Behaviour partly explains educational differences in cancer incidence in the south-eastern Netherlands: the longitudinal GLOBE study. Eur J Cancer Prev. 2004;13:119–125. doi: 10.1097/00008469-200404000-00005. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Farag NH, Sorocco KH, Acheson A, Cohoon AJ, Vincent AS. Early Life Adversity Contributes to Impaired Cognition and Impulsive Behavior: Studies from the Oklahoma Family Health Patterns Project. Alcoholism, clinical and experimental research. 2012 doi: 10.1111/acer.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclure M, Travis LB, Willett W, MacMahon B. A prospective cohort study of nutrient intake and age at menarche. The American journal of clinical nutrition. 1991;54:649–656. doi: 10.1093/ajcn/54.4.649. [DOI] [PubMed] [Google Scholar]
- MacMahon B, Cole P, Brown J. Etiology of human breast cancer: a review. Journal of the National Cancer Institute. 1973;50:21–42. doi: 10.1093/jnci/50.1.21. [DOI] [PubMed] [Google Scholar]
- Mao Y, Hu J, Ugnat AM, Semenciw R, Fincham S. Socioeconomic status and lung cancer risk in Canada. International journal of epidemiology. 2001;30:809–817. doi: 10.1093/ije/30.4.809. [DOI] [PubMed] [Google Scholar]
- Melchior M, Berkman LF, Kawachi I, Krieger N, Zins M, Bonenfant S, Goldberg M. Lifelong socioeconomic trajectory and premature mortality (35–65 years) in France: findings from the GAZEL Cohort Study. Journal of epidemiology and community health. 2006;60:937–944. doi: 10.1136/jech.2005.042440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels KB, et al. Birthweight as a risk factor for breast cancer. Lancet. 1996;348:1542–1546. doi: 10.1016/S0140-6736(96)03102-9. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of clinical epidemiology. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Naess O, Claussen B, Thelle DS, Davey Smith G. Cumulative deprivation and cause specific mortality. A census based study of life course influences over three decades. Journal of epidemiology and community health. 2004;58:599–603. doi: 10.1136/jech.2003.010207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naess O, Strand BH, Smith GD. Childhood and adulthood socioeconomic position across 20 causes of death: a prospective cohort study of 800,000 Norwegian men and women. Journal of epidemiology and community health. 2007;61:1004–1009. doi: 10.1136/jech.2006.052811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okasha M, Gunnell D, Holly J, Davey Smith G. Childhood growth and adult cancer. Best Pract Res Clin Endocrinol Metab. 2002;16:225–241. doi: 10.1053/beem.2002.0204. [DOI] [PubMed] [Google Scholar]
- Okasha M, McCarron P, Gunnell D, Smith GD. Exposures in childhood, adolescence and early adulthood and breast cancer risk: a systematic review of the literature. Breast cancer research and treatment. 2003;78:223–276. doi: 10.1023/a:1022988918755. [DOI] [PubMed] [Google Scholar]
- Pensola TH, Martikainen P. Effect of living conditions in the parental home and youth paths on the social class differences in mortality among women. Scandinavian journal of public health. 2003;31:428–438. doi: 10.1080/14034950310003980. [DOI] [PubMed] [Google Scholar]
- Power C, Hypponen E, Smith GD. Socioeconomic position in childhood and early adult life and risk of mortality: a prospective study of the mothers of the 1958 British birth cohort. American journal of public health. 2005;95:1396–1402. doi: 10.2105/AJPH.2004.047340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pudrovska T, Anikputa B. The role of early-life socioeconomic status in breast cancer incidence and mortality: unraveling life course mechanisms. Journal of aging and health. 2012;24:323–344. doi: 10.1177/0898264311422744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335:1134. doi: 10.1136/bmj.39367.495995.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruder EH, Dorgan JF, Kranz S, Kris-Etherton PM, Hartman TJ. Examining breast cancer growth and lifestyle risk factors: early life, childhood, and adolescence. Clinical breast cancer. 2008;8:334–342. doi: 10.3816/CBC.2008.n.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GD, McCarron P, Okasha M, McEwen J. Social circumstances in childhood and cardiovascular disease mortality: prospective observational study of Glasgow University students. Journal of epidemiology and community health. 2001;55:340–341. doi: 10.1136/jech.55.5.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand BH, Kunst A. Childhood socioeconomic position and cause-specific mortality in early adulthood. American journal of epidemiology. 2007;165:85–93. doi: 10.1093/aje/kwj352. [DOI] [PubMed] [Google Scholar]
- Thibault H, Contrand B, Saubusse E, Baine M, Maurice-Tison S. Risk factors for overweight and obesity in French adolescents: physical activity, sedentary behavior and parental characteristics. Nutrition. 2010;26:192–200. doi: 10.1016/j.nut.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Titus-Ernstoff L, et al. Early life factors in relation to breast cancer risk in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2002;11:207–210. [PubMed] [Google Scholar]
- van de Mheen H, Stronks K, Looman CW, Mackenbach JP. Does childhood socioeconomic status influence adult health through behavioural factors? International journal of epidemiology. 1998;27:431–437. doi: 10.1093/ije/27.3.431. [DOI] [PubMed] [Google Scholar]
- Vihko R, Apter D. Endogenous steroids in the pathophysiology of breast cancer. Critical reviews in oncology/hematology. 1989;9:1–16. doi: 10.1016/s1040-8428(89)80012-5. [DOI] [PubMed] [Google Scholar]
- Vohra J, Marmot MG, Bauld L, Hiatt RA. Socioeconomic position in childhood and cancer in adulthood: a rapid-review. Journal of epidemiology and community health. 2016;70:629–634. doi: 10.1136/jech-2015-206274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa PD, Buss C, Entringer S, Swanson JM. Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Semin Reprod Med. 2009;27:358–368. doi: 10.1055/s-0029-1237424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster TF, Hoffman K, Weinberg J, Vieira V, Aschengrau A. Community- and individual-level socioeconomic status and breast cancer risk: multilevel modeling on Cape Cod, Massachusetts. Environmental health perspectives. 2008;116:1125–1129. doi: 10.1289/ehp.10818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittemore AS, Paffenbarger RS, Jr, Anderson K, Lee JE. Early precursors of site-specific cancers in college men and women. Journal of the National Cancer Institute. 1985;74:43–51. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.