Abstract
Objective
To assess clinical outcomes including imaging findings on computed tomography (CT), pulmonary function testing (PFT), and glucocorticoid use in patients with the antisynthetase syndrome (AS) and interstitial lung disease (ILD) treated with rituximab (RTX).
Methods
We retrospectively identified all patients at two institutions with AS-ILD who were treated with RTX. Baseline demographics, PFTs and chest CTs were assessed before and after RTX. Two radiologists independently evaluated CTs using a standardized scoring system.
Results
Twenty-five subjects at the Brigham and Women’s Hospital (n=13) and University of Pittsburgh Medical Center (n=12) were included. Antisynthetase antibodies were identified in all patients (16 Jo-1, 6 PL-12, 3 PL-7). In 21 cases (84%), the principal indication for RTX use was recurrent or progressive ILD due to failure of other agents. Comparing pre- and post-RTX pulmonary parameters at 12 months, CT score and FVC% were stable or improved in 88% and 79% of subjects, respectively. TLC% increased from 56±13 to 64±13 and glucocorticoid dose decreased from 18±9 to 12±12mg/day. Although DLCO% declined slightly at 1 year, it increased from 42±17 to 70±20 at 3 years. The most common imaging patterns on CT were NSIP (n=13) and UIP/fibrotic NSIP (n=5), of which 5 had concurrent elements of COP.
Conclusions
Stability or improvement in pulmonary function or severity of ILD on CT was seen in most patients. Use of RTX was well tolerated in the majority of patients. RTX may play a therapeutic role in patients with AS-ILD and further clinical investigation is warranted.
Key indexing terms: Interstitial lung disease, Antisynthetase syndrome, Rituximab
Introduction
The antisynthetase syndrome (AS) is rare and characterized by myositis associated with antisynthetase antibodies, including, but not limited to, anti-Jo-1, -PL-7, -PL-12, -OJ, -EJ as well as additional clinical features including polyarthritis, fever, Raynaud phenomenon, mechanic’s hands, and interstitial lung disease (ILD).[1,2] ILD is the most serious complication of AS (AS-ILD), occurring in 70–89% of patients with an increase in morbidity and mortality in affected individuals.[3–8] Some variability in ILD incidence, phenotype and mortality has been noted based on antibody subtype.[7–9] It is imperative to establish effective treatment options for this serious complication. To date, many therapies have been reported for ILD with glucocorticoids remaining as the first line of treatment.[3,5,10] Due to the lack of prospective trials, specific use of additional immunosuppressive agents is empiric.
Rituximab (RTX) is a chimeric monoclonal antibody against CD 20+ B-cells, which results in B-cell depletion.[11,12] Evidence for the effectiveness of B cell depletion exists in a number of immune-mediated conditions, including rheumatoid arthritis, anti-neutrophil cytoplasmic antibody-associated vasculitis, and immune thrombocytopenic purpura.[13–18] RTX has also been specifically studied in myositis[19–24] and scleroderma-ILD[25,26]. Of note, a retrospective analysis of 50 patients with severe ILD of varying etiologies, excluding IPF, demonstrated an improvement in pulmonary function tests (PFTs) at 6 and 12 months post RTX.[27] The only randomized trial of RTX therapy in the AS was nested within a double-blind, placebo-phase trial of RTX in 200 refractory adult and juvenile dermatomyositis and adult polymyositis by Oddis, et. al., in which 17% of subjects possessed antisynthetase autoantibodies (primarily anti-Jo-1).[19] In this prospective trial, 83% of subjects met the pre-determined definition of improvement after RTX, but no subgroup analysis was performed and lung function and CT changes were not assessed as an outcome in the synthetase (+) subjects. There is limited data regarding the use of RTX in AS-ILD, mostly derived from retrospective case series/case reports, in which improvements in pulmonary function and high resolution computed tomography (HRCT) scans were noted.[20,21,28–36] Adverse events mainly included infectious complications, such as P. jirovecii pneumonia, which were occasionally fatal, as well as rash, arrhythmia, and serum sickness.[21,30,31,33,34,37]
Our objective was to assess clinical outcomes including pulmonary function, severity of ILD on HRCT, and concurrent glucocorticoid dosing in a cohort of patients with AS-ILD treated with RTX at 2 institutions.
Materials and Methods
Study design and population
We retrospectively identified all patients at the Brigham and Women’s Hospital (BWH), Boston, MA and University of Pittsburgh Medical Center (UPMC), Pittsburgh, PA, with antisynthetase autoantibodies who presented with ILD and were treated with RTX since 2007 (BWH) and 2005 (UPMC). Inclusion criteria included the presence of antisynthetase autoantibodies, a diagnosis of ILD, treatment with RTX, and at least one PFT and/or CT scan at baseline and again between 1–3 years after treatment with RTX. Exclusion criteria included lack of adequate follow-up or lung transplantation prior to 1 year after administration of RTX. Demographic characteristics, antisynthetase autoantibodies, clinical symptoms, laboratory abnormalities, concomitant glucocorticoid (i.e. prednisone dose) and other immunosuppressive use, PFTs and HRCT chest imaging findings were extracted from the electronic medical record through April, 2016. At BWH, RTX was routinely dosed every 6 months after the initial administration of RTX, whereas at UPMC, RTX was given initially with variable intervals of subsequent administration in the AS-ILD subjects.
Antisynthetase antibody detection
The majority of antisynthetase antibodies were detected using the Myositis Profile available through the Oklahoma Medical Research Foundation (OMRF) Clinical Immunology Laboratory (n=21), which includes testing for 12 myositis-specific and myositis associated antibodies using RNA immunoprecipitation. Testing on the other 4 subjects was performed through a variety of other labs. Anti-SSA was measured using standard CLIA certified laboratories at both institutions.
Pulmonary function testing
Serial PFTs completed primarily for clinical indications were reviewed. For consistency, only pre-bronchodilator values were used for the analyses since bronchodilator therapy was not routinely given. Reference values for spirometry were derived from the 3rd National Health and Nutrition Examination Survey (NHANES III) in the United States;[38] whereas lung volumes were standardized using predicted equations based on Crapo, et. al.,[39] and DLCO predicted equations were based on Cotes, et. al.[40]
HRCT analysis
Chest CT scans were done at the two study sites using standard institutional CT protocols (including axial HRCT images) as clinically indicated. Two radiologists (RM and FC, with 8 and 2 years experience as a thoracic radiologist) independently evaluated axial 3–5 mm chest CT scans and 1mm HRCT scans recording their subjective assessment of the ILD pattern as usual interstitial pneumonia (UIP), nonspecific interstitial pneumonia (NSIP) or cryptogenic organizing pneumonia (COP). Co-existence of more than one CT pattern was possible and was also recorded. This was followed by a more detailed quantitative assessment and calculation of a CT severity score [Table S1]. The thin-section CT findings were graded on a scale of 1–6 as shown in Table S1. Presence of each of the six different types of imaging patterns was assessed independently in three (upper, middle, and lower) zones of each lung. The upper zone was defined as the area above the level of the carina, the middle zone as the area between the level of the carina and the level of inferior pulmonary vein, and the lower zone as the area below the level of inferior pulmonary vein. The extent of each abnormality was determined by visually estimating the percentage (to the nearest 5%) of the affected lung parenchyma in each zone. The abnormality score for each zone was calculated by multiplying the percentage area by the point value (the score of 1–6).[41,42] The score for each zone was calculated by multiplying the percentage of the area to the nearest 5% by the grading scale score. The six zone scores were averaged to determine the total score for each patient. The scores of the two observers were averaged.
Statistical analysis
Chest CT scan scores, PFTs, and prednisone dose were assessed before and after RTX. Average values were calculated at baseline for all subjects with follow-up values at any time point (1, 2 or 3 years), and average values at subsequent time points were calculated only for subjects with baseline values for comparison. An improvement in CT severity scores was defined as a ≥10% decrease while improvement in PFTs was defined as a ≥10% increase in FVC. Stability in CT score was defined as a ≤10% increase while PFT stability was defined as a ≤10% decrease in FVC.[43] Paired univariate analyses were conducted with Wilcoxon signed rank tests where appropriate. Subgroup analyses were based on antisynthetase autoantibodies, ILD pattern, baseline mean HRCT score (194), PFT parameters indicating severe disease (FVC% < 50%, TLC% < 50%, and DLCO < 35%), some of which have been associated with increased ILD mortality,[44,45] use of RTX as initial or rescue therapy, and the presence of concurrent immunosuppression at time of starting RTX. A p-value of <0.05 was considered significant. All analyses were performed using Statistical Analysis Software version 9.3 (SAS Institute, Cary, NC). This project was granted IRB approval by the Partners Human Research Committee at the BWH (protocol number 2014P000110) and UPMC (IRB0409097).
Results
At BWH, 16 consecutive patients treated with RTX for AS-ILD since 2007 were identified. One proceeded to lung transplant 2 months after RTX and was excluded while 2 additional patients were excluded due to lack of PFT or HRCT follow-up. There were no deaths recorded in the medical record. At UPMC, 21 consecutive AS-ILD patients treated with RTX since 2005 were identified. Eight were excluded due to lack of follow-up PFT and/or HRCT data, 1 of whom died from respiratory failure 5 months after receiving RTX, and 1 additional subject proceeded to lung transplant 10 months after RTX and was also excluded. Two UPMC subjects included in this study also died; 1 died from respiratory failure secondary to progression of underlying ILD 2 years after receiving RTX (Table S2 UPMC Subject 4) and 1 died from anoxic brain injury approximately 10 years after receiving RTX (Table S2 UPMC Subject 6). Available baseline and follow-up HRCT and PFTs for each BWH and UPMC subject are detailed in Table 1.
Table 1.
Available baseline and follow-up CT and PFTs for each BWH and UPMC subject.
| Subject | Baseline CT | 1 year f/u CT | 2 year f/u CT | 3 year f/u CT | Baseline PFTs | 1 year f/u PFTs | 2 year f/u PFTs | 3 year f/u PFTs |
|---|---|---|---|---|---|---|---|---|
| BWH | ||||||||
| 1 | • | • | • | • | • | |||
| 2 | • | • | • | • | • | • | ||
| 3 | • | • | • | • | • | |||
| 4 | • | • | • | • | • | |||
| 5 | • | • | • | • | • | • | ||
| 6 | • | • | • | • | ||||
| 7 | • | • | • | • | • | • | ||
| 8 | • | • | • | • | • | • | ||
| 9 | • | • | • | |||||
| 10 | • | • | • | • | ||||
| 11 | • | • | • | • | ||||
| 12 | • | • | • | • | ||||
| 13 | • | • | • | |||||
| UPMC | ||||||||
| 1 | • | • | ||||||
| 2 | • | • | • | • | ||||
| 3 | • | • | • | |||||
| 4 | • | • | • | |||||
| 5 | • | • | • | • | ||||
| 6 | • | • | • | |||||
| 7 | • | • | • | • | ||||
| 8 | • | • | • | • | • | |||
| 9 | • | • | • | |||||
| 10 | • | • | • | |||||
| 11 | • | • | ||||||
| 12 | • | • | • |
Abbreviations: CT – Computed tomography, PFTs - Pulmonary function tests, BWH – Brigham and Women’s Hospital, UPMC – University of Pittsburgh Medical Center
Of the 25 subjects included in our cohort, the mean age was 49, and 20 (80%) were female [Table 2]. Eight patients (32%) had a smoking history and 2 patients (8%) were current smokers. Antisynthetase antibodies were identified in all patients (16 Jo-1, 6 PL-12, 3 PL-7). Four subjects were anti-SSA positive, 15 were negative, and 6 were unavailable. The most common clinical features noted at any time in the clinical course included myositis (n=19) and Raynaud phenomenon (n=14), followed by mechanic’s hands/rash/arthritis (n=12), and fever (n=6). Baseline HRCTs (n=21) revealed the following patterns: 9 NSIP, 4 UIP/fibrotic NSIP, 4 NSIP/COP, 1 COP, 1 fibrotic NSIP/COP, 1 COP/bronchiolitis obliterans (BO) and 1 acute interstitial pneumonitis (AIP)/diffuse alveolar damage (DAD) [Figure 1]. Abnormalities of the esophagus identified on HRCT included a patulous esophagus (n=7) and hiatal hernia (n=1). The Spearman’s rank correlation coefficient for quantitative CT scoring interobserver agreement between the two radiologists was 0.95.
Table 2.
Baseline Demographics of AS Subjects
| Variable | Number (%) or Mean ± SD where appropriate | ||
|---|---|---|---|
| BWH Cohort (n=13) |
UPMC Cohort (n=12) |
Total AS Subjects (n=25) |
|
| Age (years) at time of RTX | 51 ± 10 | 47 ± 14 | 49 ± 12 |
| Gender (female) | 12 (92%) | 8 (67%) | 20 (80%) |
| Race (white) | 4 (31%) | 6 (50%) | 10 (40%) |
| BMI | 27.5 ± 9.6 | 31.4 ± 7.6 | 29.1 ± 8.9 |
| Ever smoker | 4 (31%) | 4 (33%) | 8 (32%) |
| Current smoker | 0 (0%) | 2 (17%) | 2 (8%) |
| Antisynthetase Ab | |||
| Jo-1 | 6 (46%) | 10 (83%) | 16 (64%) |
| PL-12 | 4 (31%) | 2 (17%) | 6 (24%) |
| PL-7 | 3 (23%) | 0 (0%) | 3 (12%) |
| Anti-SSA | 1 (8%) | 3 (25%) | 4 (16%) |
| Symptoms | |||
| Myositis | 12 (92%) | 7 (58%) | 19 (76%) |
| Arthritis | 9 (69%) | 3 (25%) | 12 (48%) |
| Mechanic’s hands | 9 (69%) | 3 (25%) | 12 (48%) |
| Skin rashes | 8 (62%) | 4 (33%) | 12 (48%) |
| Raynaud’s | 7 (54%) | 7 (58%) | 14 (56%) |
| Fever | 4 (31%) | 2 (17%) | 6 (24%) |
| Medications (Prior to RTX/Concurrent with RTX initiation) | |||
| Azathioprine | 9 (69%)/1 (8%) | 7 (58%)/0 (0%) | 16 (64%)/1 (4%) |
| Mycophenolate Mofetil | 8 (62%)/4 (31%) | 8 (67%)/4 (33%) | 16 (64%)/8 (32%) |
| Cyclophosphamide | 6 (46%)/0 (0%) | 5 (42%)/1 (8%) | 11 (44%)/1 (4%) |
| Tacrolimus | 2 (15%)/0 (0%) | 7 (58%)/1 (8%) | 9 (36%)/1 (4%) |
| Methotrexate | 4 (31%)/0 (0%) | 7 (58%)/1 (8%) | 11 (44%)/1 (4%) |
| IVIG | 4 (31%)/0 (0%) | 3 (25%)/1 (8%) | 6 (24%)/1 (4%) |
| Leflunomide | 3 (23%)/0 (0%) | 0 (0%)/0 (0%) | 3 (12%)/0 (0%) |
| Etanercept | 1 (8%)/0 (0%) | 0 (0%)/0 (0%) | 1 (4%)/0 (0%) |
| Baseline laboratory values | |||
| CK | 872 ± 1537 | 3744 ± 7273 | 2308 ± 5337 |
| Aldolase | 18 ± 16 | 54 ± 72 | 29 ± 42 |
| ESR | 24 ± 17 | 36 ± 41 | 28 ± 28 |
| CRP | 6.2 ± 4.7 | 30.8 ± 36.2 | 10.1 ± 14.6 |
Number missing: Race (n=3), BMI (n=3), Ever smoke (n=1), Anti-SSA (n=6), Myositis (n=2), Mechanics (n=7), Raynaud (n=6), Fever (n=9), Arthritis (n=4), Rash (n=9), CK (n=2), Aldolase (n11), ESR (n=8), CRP (n=11)
Abbreviations: AS – Antisynthetase syndrome, SD – Standard deviation, RTX – Rituximab, BWH - Brigham and Women’s Hospital, UPMC – University of Pittsburgh Medical Center, CK – Creatinine kinase, ESR - Erythrocyte sedimentation rate, CRP – C-reactive protein
Figure 1. CT scan before and after treatment with Rituximab for BWH Subject 2.
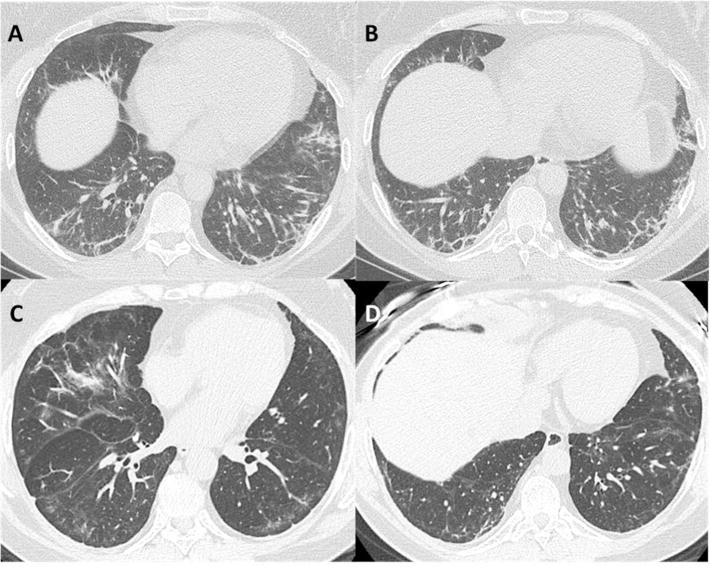
Pre-Rituximab (CT score 125.1) – Axial HRCT images in lung windows (A + B) demonstrate subpleural reticulation, coarse linear bands, groundglass abnormality and patchy consolidation within the lower lobes, lingula and right middle lobe in a predominantly peripheral, subpleural distribution. Affected areas of lung demonstrate traction bronchiectasis and architectural distortion. No honeycombing was present, and lung volumes were mildly reduced. Pattern of interstitial pneumonitis has overlapping features of both non-specific interstitial pneumonia (NSIP) and cryptogenic organizing pneumonia (COP).
>3-years Post-Rituximab (CT score 113.7) – Axial HRCT images (C + D) in lung windows demonstrates definite improvement in bilateral subpleural reticulation, consolidation and architectural distortion. Residual groundglass opacity is present with subpleural sparing, imaging features are consistent with a milder non-specific interstitial pneumonia (NSIP) pattern.
The diagnosis of AS followed the diagnosis of ILD in 18 patients (72%) by a mean of 2.5 years. The mean time to initiation of RTX after ILD identification was 4.4 years. In 21 cases (84%), the principal indication for RTX use was recurrent or progressive ILD due to failure of other immunosuppressive agents. In 4 subjects (16%), RTX was used as the first glucocorticoid-sparing agent. Medications used prior to switching to RTX are detailed in Table 1. Twenty-three subjects were on prednisone at the time of starting RTX (92%) and 12 subjects (48%) were on additional concurrent immunosuppressive agents at the time of starting RTX: mycophenolate mofetil (n=7), tacrolimus and mycophenolate mofetil (n=1), azathioprine (n=1), cyclophosphamide (n=1), IVIG (n=1) and methotrexate (n=1). Three patients had documented adverse events to the RTX after the initial dosing: 1 anaphylaxis and 2 serious gastrointestinal complications requiring surgery who later resumed RTX. Infectious complications while on RTX included pneumonia (n=1), influenza (n=1), bronchitis (n=1), clostridium difficile colitis/UTI (n=1), diverticulitis (n=1), varicella zoster (n=2), cellulitis (n=1), and sinus infection (n=1).
We compared the baseline and 1-year post RTX findings in the 21 subjects with either PFTs and/or HRCT imaging at 1 year of follow-up and then analyzed subjects with up to 3-year PFT (n=7) and/or HRCT (n=4) follow-up [Tables 3 and S2 and Figure 2]. The average CT scan score at 1 year (n=8) was stable or improved in 88% of subjects while the FVC% (n=19) was similarly stable or improved in 79%. The average TLC% in all subjects (n=7) increased from 56±13 to 64±13 at 1 year with a statistically significant average individual TLC% increase of 8.3% (p=0.016). Similar trends were seen at 24 months. Based on very limited 36 month follow-up data for only 7 subjects, there was a statistically significant average individual increase in FVC% at 3 years of 21% (p=0.016). Although the average DLCO% in all subjects declined slightly from 42±17 to 36±16 at the 1-year time-point, there was an increase at 2 years to an average of 53±26. The glucocorticoid dose was stable or decreased in 88% of subjects at 1 year with an average drop of 6mg and a statistically significant average individual decrease of 6.2mg (p=0.041). When comparing subjects who received repeat RTX dosing (n=17) with those who received a one-time dose (n=9), those with repeat RTX dosing showed improvement in all parameters, including a significant average increase in FVC% and TLC% of 9.3% (p=0.0077) and 8.5% (p=0.031) respectively [Table 4], despite starting with a lower average FVC%, TLC%, and DLCO%, although a comparison of baseline values between the two groups did not reach statistical significance. Those who received a single dose of RTX had a decline in most parameters including a worsening CT score, decrease in FVC, and decrease in DLCO, although the numbers were too small to reach statistical significance. Overall, 100% of subjects (5/5) who had repeat RTX dosing had treatment success (defined as stabilization or improvement) with regards to CT score and 93% (13/14) had treatment success with regards to FVC%. In the single RTX group, those numbers declined to 67% (2/3) for CT score and 40% (2/5) for FVC% [Table 4].
Table 3.
Comparison of pre and post-RTX CT scan score, PFT measurements, and prednisone dose for all subjects with follow-up at 1–3 years (n=25). Mean ± SD presented.
| Baseline | 1 Year Follow-up | 2 Year Follow-up | 3 Year Follow-up | |
|---|---|---|---|---|
| CT Scan | ||||
| Average score | 194 ± 58 n=15 |
195 ± 55 n=8 |
187 ± 52 n=5 |
161 ± 55 n=4 |
|
Paired average Δ (p-value)* |
N/A | −2.0 (0.84) |
−0.9 (0.88) |
−38.9 (0.63) |
| Improved/Stable** | N/A | 2/5 (88%) | 0/4 (80%) | 1/2 (75%) |
| PFTs | ||||
| FVC% | 57 ± 17 n=22 |
60 ± 20 n=19 |
65 ± 22 n=12 |
82 ± 20 n=7 |
|
Paired average Δ (p-value) |
N/A | +3.7 (0.18) |
+7.7 (0.15) |
+21.1 (0.016) |
| Improved/Stable | N/A | 8/7 (79%) | 6/4 (83%) | 7/0 (100%) |
| TLC% | 56 ± 13 n=7 |
64 ± 13 n=7 |
67 ± 24 n=5 |
75 ± 19 n=2 |
|
Paired average Δ (p-value) |
N/A | +8.3 (0.016) |
+8.6 (0.44) |
+23.5 (0.50) |
| DLCO% | 42 ± 17 n=19 |
36 ± 16 n=17 |
53 ± 26 n=9 |
70 ± 20 n=4 |
|
Paired average Δ(p-value) |
N/A | −3.34 (0.46) |
+9.6 (0.074) |
+14.8 (0.25) |
| Prednisone | ||||
| Average Dose | 18 ± 9 n=20 |
12 ± 12 n=17 |
10 ± 8 n=6 |
6 ± 6 n=4 |
|
Paired average Δ (p-value) |
N/A | −6.2 (0.041) |
−3.3 (0.63) |
−15.1 (0.13) |
| Improved/Stable | N/A | 11/4 (88%) | 2/2 (67%) | 4/0 (100%) |
Paired average Δ represents difference in the mean of paired observations with the p-value as calculated using the Wilcoxon signed rank test. A p-value of <0.05 was considered significant.
Improved is defined by >10% decrease in CT score or >10 improvement in FVC%. Stable is defined by <10% increase in CT score or <10 decline in FVC% (Pioped criteria). Values are compared to baseline time point.
Abbreviations: RTX – Rituximab, CT – Computed tomography, PFTs - Pulmonary function tests, SD – Standard deviation, FVC% - Forced vital capacity (% predicted), TLC% - Total lung capacity (% predicted), DLCO% - Diffusion capacity of carbon monoxide (% predicted)
Figure 2. Graph of CT score (A) and forced vital capacity percent predicted (FVC%) (B) by subject over 3 years of follow-up.
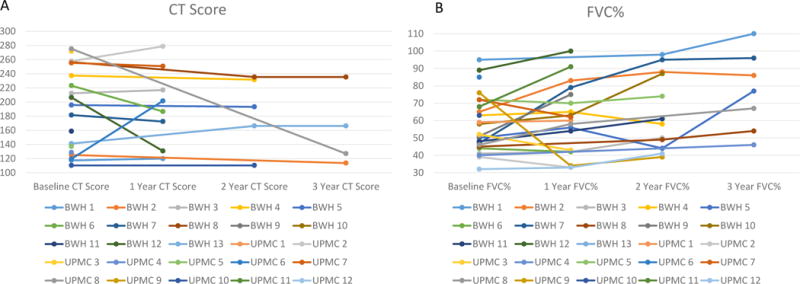
2A: Line graph of CT scores demonstrating stability or improvement (decrease in CT score) in ≥75% of subjects over the 3 years of follow-up.
2B: Line graph of FVC% demonstrating stability or improvement in ≥79% of subjects over the 3 years of follow-up.
Table 4.
Comparison of pre and post-RTX CT scan score and PFT measurements comparing averages at baseline and 1 year with the average individual change (p-value) in subjects with repeat RTX dosing vs. subjects with a one-time RTX dose. Mean ± SD presented.
| Repeat RTX Dosing n=17 |
One-Time RTX Dosing n=8 |
|||
|---|---|---|---|---|
| Baseline | 1 Year Follow-up | Baseline | 1 Year Follow-up | |
| CT Scan | n=5 | n=3 | ||
| Average score | 171 ± 42 | 152 ± 40 | 211± 79 | 244 ± 39 |
|
Paired average Δ (p-value)* |
−22.9 (0.31) |
32.8 (0.50) |
||
|
Improved/Stable** Failure** |
N=2/3 (100%) N=0 (0%) |
N=0/2 (67%) N=1(33%) |
||
| FVC | n=14 | n=5 | ||
| FVC Percent Predicted | 54 ± 14 | 63 ± 20 | 64 ± 15 | 52 ± 17 |
|
Paired average Δ (p-value) |
+9.3 (0.0077) |
−11.8 (0.13) |
||
|
Improved/Stable Failure |
N=8/5 (93%) N=1 (7%) |
N=0/2 (40%) N=3 (60%) |
||
| TLC | n=6 | n=1 | ||
| TLC Percent Predicted | 53 ± 13 | 62 ± 14 | 70 | 77 |
|
Paired average Δ (p-value) |
+8.5 (0.031) |
N/A | ||
| DLCO | n=12 | n=5 | ||
| DLCO Percent Predicted | 38 ± 12 | 39 ± 18 | 43 ± 16 | 29 ± 8 |
|
Paired average Δ (p-value) |
+1.2 (0.59) |
−14.2 (0.13) |
||
Paired average Δ represents difference in the mean of paired observations with the p-value as calculated using the Wilcoxon signed rank test. A p-value of <0.05 was considered significant.
Improved is defined by >10% decrease in CT score or >10 improvement in FVC%. Stable is defined by <10% increase in CT score or <10 decline in FVC% (Pioped criteria). Failure is defined by a ≥10% increase in CT score or a ≥10% decline in FVC%. Values are compared to baseline time point.
Abbreviations: RTX – Rituximab, CT – Computed tomography, PFTs - Pulmonary function tests, SD – Standard deviation, FVC% - Forced vital capacity (% predicted), TLC% - Total lung capacity (% predicted), DLCO% - Diffusion capacity of carbon monoxide (% predicted)
Although limited in their interpretability given the insufficient numbers, we did perform subgroup analyses by autoantibody status (Jo-1, PL-12, PL-7), radiographic pattern (NSIP, NSIP and COP, or fibrotic ILD), severity of lung disease (CT fibrosis score > or ≤ 194, FVC% < or ≥ 50%, TLC% < or ≥ 50%, DLCO% < or ≥ 35%), use of RTX as rescue therapy or initial therapy, and absence or presence of concurrent immunosuppression at the time of initiating RTX [Table S3]. Improvement with RTX was more apparant in individuals who had an NSIP pattern on CT scan, or had less severe lung disease based on the FVC or DLCO. Those in whom RTX was started due to failure of other immunosuppressive agents and those who were on concurrent immunosuppression also had a more notable response. However, small subject numbers limited the generalizability of the above findings and prohibited paired statistical analyses in many subgroups.
Discussion
In this study of 25 AS-ILD subjects treated with RTX at 2 academic referral centers, we observed stability or improvement in CT imaging and/or physiologic testing (FVC%, TLC%, and DLCO%) in most patients at 1 and 3 years of follow-up. Further, there was a significant steroid-sparing effect and RTX was generally well tolerated. Subgroup analyses demonstrated a benefit to repeat RTX dosing over a single RTX cycle, but in general, it seemed that the greatest benefit to RTX was seen at 3 years of follow-up, although the small numbers of patients with data at this endpoint limits the generalizability of the conclusions..
Previous studies addressing RTX for treatment of AS-ILD have also shown a favourable response,[20,21,28–34,36] although investigations are mostly limited to case reports and retrospective studies. In small case series of AS-ILD patients following treatment with RTX, objective improvement in PFTs (FVC%, TLC%, and DLCO%) have been noted,[20,21,28,30,33–36] as has improvement or resolution of ground glass opacities and stability or improvement of fibrosis on HRCT.[20,28,30,33,35] In one similar retrospective study by Andersson, et. al., ILD extent was found to be reduced or stabilized in 21/23 patients and FVC% increased by an average of 24% in 24 patients followed longitudinally for up to 60 months.[33] In another small study by Marie, et. al., of 7 AS subjects with refractory ILD treated with RTX at D0, D14, and M6, there was a statistically significant improvement in the median FVC/DLCO and an improvement (n=5) or stability (n=2) in HRCT at 1 year of follow-up, as well as a decrease or resolution of respiratory symptoms in all subjects. Further, these subjects had a significant decrease in the concurrent median daily prednisone dose from 20mg/day to 9mg/day.[35] However, unlike our study, this was a single-center study that did not assess individual change and had insufficient power to analyze subgroups. There has been 1 prospective trial by Allenbach, et. al., in which 10 antisynthetase (+) subjects who had failed conventional treatment received RTX at D0, D15, and M6.[31] This study demonstrated an improvement or stabilization of ILD as measured by FVC and DLCO in 9/10 subjects and either a >50% decrease in steroid dose or discontinuation of at least 1 other immunomodulatory/immunosuppressive drug in 6/10 patients. Only 1 subject had a reduction in interstitial infiltrates on CT scan and improvement in ILD (5/10 subjects) was defined as a 10% increase in FVC or 15% increase in DLCO, while stabilization (4/10 subjects) was defined as a <10% decrease in FVC or <15% decrease in DLCO at M12.
Although our study and previous case series and retrospective studies have reported favorable responses in AS-ILD treated with RTX, the mechanism of its therapeutic effect is uncertain. It is unclear if the B cell deleting mechanism attributed to RTX represents the mechanism of action leading to its effectiveness in AS-ILD. Studies in other immune-mediated diseases suggest that RTX may lead to normalization of auto-reactive T cells,[46,47] and the repopulation of the B-cells following RTX may be antigenically naïve, transitional B-cells, suggesting a possible ‘resetting’ of the immune system that contributes to a therapeutic effect.[48] A retrospective study of 50 systemic autoimmune rheumatic disease-related ILD patients with myositis improved their PFTs following B-cell depletion,[27] suggesting a key pathogenic role of T cells in myositis-ILD. Future mechanistic studies should address the pathogenic potential of specific antibodies in AS and whether B cell depletion after RTX is responsible for its favorable effect in AS-ILD. In addition, the future elucidation of antisynthetase autoantibody-specific differences or whether the presence of other biomarkers are useful in predicting a response to B cell depletion should be investigated to guide treatment algorithms in AS-ILD.
In our study, all patients in the BWH cohort received repeat RTX dosing on average every 6 months, whereas most patients in the UPMC cohort received only a one time dose (n=8) with only 4 receiving repeat dosing at variable intervals. A better objective response in FVC and TLC was noted in patients with repeat RTX dosing, with a trend towards improved HRCT scores. However, the sample size is too small to draw any significant conclusions and there may be inherent bias since the reason for redosing in the UPMC cohort was not able to be ascertained, raising the question as to whether those subjects who were redosed were the patients who responded favorably, while those who were not redosed did not respond to initial RTX dosing. In general, there is no standard of care regarding the frequency of RTX dosing in myositis given the paucity of data and the variability of 3rd party payer approval. One retrospective study demonstrated that in 11 AS patients with severe, progressive ILD refractory to other immunotherapy, RTX appeared to stabilize and/or improve ILD in 7 patients during the first 6 months only,[28] suggesting that frequent dosing vs. one-time RTX dosing was more effective. This observation, our findings, especially the significant improvement at 3 years, and the pathophysiologic mechanisms discussed above suggest that optimal RTX dosing may require additional dosing rather than an initial cycle of RTX and that RTX may take a long time to have an effect.
Our study has a few important limitations. (1) The small sample size limits the discovery of statistically significant findings, particularly regarding the interpretability of subgroup analyses. (2) The retrospective chart review design is limited in that not all subjects have data at all time points and there are very few subjects with 3 year follow-up, potentially introducing a source of bias. However, all subjects had at least 1 HRCT or PFT done >1 year from the time of initial RTX dosing. To address this limitation, the difference in the mean of paired observations was presented to allow for paired analyses. (3) There was variability in the dosing of RTX at the two independent centers, which complicates composite data analyses. (4) There is limited generalizability given that this study was conducted in 2 clinical centers. (5) There is an inherent difficulty in assessing radiographic outcomes in ILD associated with AS as there is no validated specific scoring system for autoimmune ILD. Although numerous HRCT severity scoring systems exist for evaluation of patients with IPF, no specific scoring system has yet been proposed for patients with ILD in the setting of AS. Given the presence of multiple radiologic patterns (UIP, NSIP and COP) in this subset of patients, we adapted a CT scoring system proposed by Ichikado and Muller, et. al.,[41,42] which has previously been used for quantitative assessment of HRCT abnormalities in a variety of clinical scenarios ranging from acute interstitial pneumonitis, acute respiratory distress syndrome, and idiopathic pulmonary fibrosis. (6) The exclusion of the UPMC subject who proceed to transplant after 10 months might have biased the data analysis in favour of RTX given they declined over the 10 months after RTX and subsequently received a lung transplant. Additional, prospective, randomized, multi-center trials should address these limitations.
In summary, RTX may play a therapeutic role in patients with AS-ILD. This study has added a significant body of data to the existing information on RTX in AS-ILD, and suggests that further clinical investigation into the effectiveness of RTX as a treatment for AS-ILD, pathophysiologic mechanisms of action, ideal dosing interval, and length of treatment is warranted.[4]
Supplementary Material
Acknowledgments
None.
Source of support: This work was supported by the National Institutes of Health [grant number 1 K23 HL119558 to TJD].
Footnotes
Departments and Institutions to which the work should be attributed: Departments of Medicine and Radiology, Brigham and Women’s Hospital; Departments of Medicine and Radiology, University of Pittsburgh School of Medicine
References
- 1.Katzap E, Barilla-LaBarca ML, Marder G. Antisynthetase syndrome. Curr Rheumatol Rep. 2011;13:175–181. doi: 10.1007/s11926-011-0176-8. [DOI] [PubMed] [Google Scholar]
- 2.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts) N Engl J Med. 1975;292:344–347. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 3.Connors GR, Christopher-Stine L, Oddis CV, Danoff SK. Interstitial lung disease associated with the idiopathic inflammatory myopathies: What progress has been made in the past 35 years? Chest. 2010;138:1464–1474. doi: 10.1378/chest.10-0180. [DOI] [PubMed] [Google Scholar]
- 4.Hallowell RW, Danoff SK. Interstitial lung disease associated with the idiopathic inflammatory myopathies and the antisynthetase syndrome: Recent advances. Curr Opin Rheumatol. 2014;26:684–689. doi: 10.1097/BOR.0000000000000104. [DOI] [PubMed] [Google Scholar]
- 5.Marie I, Hatron PY, Dominique S, Cherin P, Mouthon L, Menard JF. Short-term and long-term outcomes of interstitial lung disease in polymyositis and dermatomyositis: A series of 107 patients. Arthritis Rheum. 2011;63:3439–3447. doi: 10.1002/art.30513. [DOI] [PubMed] [Google Scholar]
- 6.Debray MP, Borie R, Revel MP, Naccache JM, Khalil A, Toper C, et al. Interstitial lung disease in anti-synthetase syndrome: Initial and follow-up ct findings. Eur J Radiol. 2015;84:516–523. doi: 10.1016/j.ejrad.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 7.Aggarwal R, Cassidy E, Fertig N, Koontz DC, Lucas M, Ascherman DP, et al. Patients with non-jo-1 anti-trna-synthetase autoantibodies have worse survival than jo-1 positive patients. Ann Rheum Dis. 2014;73:227–232. doi: 10.1136/annrheumdis-2012-201800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marie I, Josse S, Hatron PY, Dominique S, Hachulla E, Janvresse A, et al. Interstitial lung disease in anti-jo-1 patients with antisynthetase syndrome. Arthritis Care Res (Hoboken) 2013;65:800–808. doi: 10.1002/acr.21895. [DOI] [PubMed] [Google Scholar]
- 9.Tomonaga M, Sakamoto N, Ishimatsu Y, Kakugawa T, Harada T, Nakashima S, et al. Comparison of pulmonary involvement between patients expressing anti-pl-7 and anti-jo-1 antibodies. Lung. 2015;193:79–83. doi: 10.1007/s00408-014-9665-7. [DOI] [PubMed] [Google Scholar]
- 10.Aggarwal R, Oddis CV. Therapeutic approaches in myositis. Curr Rheumatol Rep. 2011;13:182–191. doi: 10.1007/s11926-011-0172-z. [DOI] [PubMed] [Google Scholar]
- 11.Perosa F, Prete M, Racanelli V, Dammacco F. Cd20-depleting therapy in autoimmune diseases: From basic research to the clinic. J Intern Med. 2010;267:260–277. doi: 10.1111/j.1365-2796.2009.02207.x. [DOI] [PubMed] [Google Scholar]
- 12.Leandro MJ, Cambridge G, Ehrenstein MR, Edwards JC. Reconstitution of peripheral blood b cells after depletion with rituximab in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:613–620. doi: 10.1002/art.21617. [DOI] [PubMed] [Google Scholar]
- 13.Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, et al. Efficacy of b-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350:2572–2581. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 14.Emery P, Fleischmann R, Filipowicz-Sosnowska A, Schechtman J, Szczepanski L, Kavanaugh A, et al. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: Results of a phase iib randomized, double-blind, placebo-controlled, dose-ranging trial. Arthritis Rheum. 2006;54:1390–1400. doi: 10.1002/art.21778. [DOI] [PubMed] [Google Scholar]
- 15.Cohen SB, Emery P, Greenwald MW, Dougados M, Furie RA, Genovese MC, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase iii trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54:2793–2806. doi: 10.1002/art.22025. [DOI] [PubMed] [Google Scholar]
- 16.Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, et al. Rituximab versus cyclophosphamide for anca-associated vasculitis. N Engl J Med. 2010;363:221–232. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnold DM, Dentali F, Crowther MA, Meyer RM, Cook RJ, Sigouin C, et al. Systematic review: Efficacy and safety of rituximab for adults with idiopathic thrombocytopenic purpura. Ann Intern Med. 2007;146:25–33. doi: 10.7326/0003-4819-146-1-200701020-00006. [DOI] [PubMed] [Google Scholar]
- 18.Gottenberg JE, Guillevin L, Lambotte O, Combe B, Allanore Y, Cantagrel A, et al. Tolerance and short term efficacy of rituximab in 43 patients with systemic autoimmune diseases. Ann Rheum Dis. 2005;64:913–920. doi: 10.1136/ard.2004.029694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oddis CV, Reed AM, Aggarwal R, Rider LG, Ascherman DP, Levesque MC, et al. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: A randomized, placebo-phase trial. Arthritis Rheum. 2013;65:314–324. doi: 10.1002/art.37754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nalotto L, Iaccarino L, Zen M, Gatto M, Borella E, Domenighetti M, et al. Rituximab in refractory idiopathic inflammatory myopathies and antisynthetase syndrome: Personal experience and review of the literature. Immunol Res. 2013;56:362–370. doi: 10.1007/s12026-013-8408-9. [DOI] [PubMed] [Google Scholar]
- 21.Unger L, Kampf S, Luthke K, Aringer M. Rituximab therapy in patients with refractory dermatomyositis or polymyositis: Differential effects in a real-life population. Rheumatology (Oxford) 2014;53:1630–1638. doi: 10.1093/rheumatology/keu024. [DOI] [PubMed] [Google Scholar]
- 22.Fasano S, Gordon P, Hajji R, Loyo E, Isenberg DA. Rituximab in the treatment of inflammatory myopathies: A review. Rheumatology (Oxford) 2017;56:26–36. doi: 10.1093/rheumatology/kew146. [DOI] [PubMed] [Google Scholar]
- 23.Aggarwal R, Bandos A, Reed AM, Ascherman DP, Barohn RJ, Feldman BM, et al. Predictors of clinical improvement in rituximab-treated refractory adult and juvenile dermatomyositis and adult polymyositis. Arthritis Rheumatol. 2014;66:740–749. doi: 10.1002/art.38270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahler EA, Blom M, Voermans NC, van Engelen BG, van Riel PL, Vonk MC. Rituximab treatment in patients with refractory inflammatory myopathies. Rheumatology (Oxford) 2011;50:2206–2213. doi: 10.1093/rheumatology/ker088. [DOI] [PubMed] [Google Scholar]
- 25.Jordan S, Distler JH, Maurer B, Huscher D, van Laar JM, Allanore Y, et al. Effects and safety of rituximab in systemic sclerosis: An analysis from the european scleroderma trial and research (eustar) group. Ann Rheum Dis. 2015;74:1188–1194. doi: 10.1136/annrheumdis-2013-204522. [DOI] [PubMed] [Google Scholar]
- 26.Daoussis D, Liossis SN, Tsamandas AC, Kalogeropoulou C, Kazantzi A, Korfiatis P, et al. Is there a role for b-cell depletion as therapy for scleroderma? A case report and review of the literature. Semin Arthritis Rheum. 2010;40:127–136. doi: 10.1016/j.semarthrit.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Keir GJ, Maher TM, Ming D, Abdullah R, de Lauretis A, Wickremasinghe M, et al. Rituximab in severe, treatment-refractory interstitial lung disease. Respirology. 2014;19:353–359. doi: 10.1111/resp.12214. [DOI] [PubMed] [Google Scholar]
- 28.Sem M, Molberg O, Lund MB, Gran JT. Rituximab treatment of the anti-synthetase syndrome: A retrospective case series. Rheumatology (Oxford) 2009;48:968–971. doi: 10.1093/rheumatology/kep157. [DOI] [PubMed] [Google Scholar]
- 29.Dasa O, Ruzieh M, Oraibi O. Successful treatment of life-threatening interstitial lung disease secondary to antisynthetase syndrome using rituximab: A case report and review of the literature. Am J Ther. 2016;23:e639–645. doi: 10.1097/MJT.0000000000000245. [DOI] [PubMed] [Google Scholar]
- 30.Bauhammer J, Blank N, Max R, Lorenz HM, Wagner U, Krause D, et al. Rituximab in the treatment of jo1 antibody-associated antisynthetase syndrome: Anti-ro52 positivity as a marker for severity and treatment response. J Rheumatol. 2016;43:1566–1574. doi: 10.3899/jrheum.150844. [DOI] [PubMed] [Google Scholar]
- 31.Allenbach Y, Guiguet M, Rigolet A, Marie I, Hachulla E, Drouot L, et al. Efficacy of rituximab in refractory inflammatory myopathies associated with anti-synthetase auto-antibodies: An open-label, phase ii trial. PLoS One. 2015;10:e0133702. doi: 10.1371/journal.pone.0133702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vandenbroucke E, Grutters JC, Altenburg J, Boersma WG, ter Borg EJ, van den Bosch JM. Rituximab in life threatening antisynthetase syndrome. Rheumatol Int. 2009;29:1499–1502. doi: 10.1007/s00296-009-0859-x. [DOI] [PubMed] [Google Scholar]
- 33.Andersson H, Sem M, Lund MB, Aalokken TM, Gunther A, Walle-Hansen R, et al. Long-term experience with rituximab in anti-synthetase syndrome-related interstitial lung disease. Rheumatology (Oxford) 2015;54:1420–1428. doi: 10.1093/rheumatology/kev004. [DOI] [PubMed] [Google Scholar]
- 34.Lepri G, Avouac J, Airo P, Anguita Santos F, Bellando-Randone S, Blagojevic J, et al. Effects of rituximab in connective tissue disorders related interstitial lung disease. Clin Exp Rheumatol. 2016;34(Suppl 100):181–185. [PubMed] [Google Scholar]
- 35.Marie I, Dominique S, Janvresse A, Levesque H, Menard JF. Rituximab therapy for refractory interstitial lung disease related to antisynthetase syndrome. Respir Med. 2012;106:581–587. doi: 10.1016/j.rmed.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 36.Sharp C, McCabe M, Dodds N, Edey A, Mayers L, Adamali H, et al. Rituximab in autoimmune connective tissue disease-associated interstitial lung disease. Rheumatology (Oxford) 2016;55:1318–1324. doi: 10.1093/rheumatology/kew195. [DOI] [PubMed] [Google Scholar]
- 37.Belhassen-Garcia M, Rabano-Gutierrez A, Velasco-Tirado V, Romero-Alegria A, Perez-Garcia ML, Martin-Oterino JA. Atypical progressive multifocal leukoencephalopathy in a patient with antisynthetase syndrome. Intern Med. 2015;54:519–524. doi: 10.2169/internalmedicine.54.2748. [DOI] [PubMed] [Google Scholar]
- 38.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general u.S. Population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 39.Crapo RO, Morris AH, Clayton PD, Nixon CR. Lung volumes in healthy nonsmoking adults. Bull Eur Physiopathol Respir. 1982;18:419–425. [PubMed] [Google Scholar]
- 40.Cotes JE, Chinn DJ, Quanjer PH, Roca J, Yernault JC. Standardization of the measurement of transfer factor (diffusing capacity). Report working party standardization of lung function tests, european community for steel and coal. Official statement of the european respiratory society. Eur Respir J Suppl. 1993;16:41–52. [PubMed] [Google Scholar]
- 41.Fujimoto K, Taniguchi H, Johkoh T, Kondoh Y, Ichikado K, Sumikawa H, et al. Acute exacerbation of idiopathic pulmonary fibrosis: High-resolution ct scores predict mortality. Eur Radiol. 2012;22:83–92. doi: 10.1007/s00330-011-2211-6. [DOI] [PubMed] [Google Scholar]
- 42.Ichikado K, Suga M, Muller NL, Taniguchi H, Kondoh Y, Akira M, et al. Acute interstitial pneumonia: Comparison of high-resolution computed tomography findings between survivors and nonsurvivors. Am J Respir Crit Care Med. 2002;165:1551–1556. doi: 10.1164/rccm.2106157. [DOI] [PubMed] [Google Scholar]
- 43.Collard HR, King TE, Jr, Bartelson BB, Vourlekis JS, Schwarz MI, Brown KK. Changes in clinical and physiologic variables predict survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2003;168:538–542. doi: 10.1164/rccm.200211-1311OC. [DOI] [PubMed] [Google Scholar]
- 44.Ryerson CJ, Vittinghoff E, Ley B, Lee JS, Mooney JJ, Jones KD, et al. Predicting survival across chronic interstitial lung disease: The ild-gap model. Chest. 2013 doi: 10.1378/chest.13-1474. [DOI] [PubMed] [Google Scholar]
- 45.Berry CE, Wise RA. Interpretation of pulmonary function test: Issues and controversies. Clin Rev Allergy Immunol. 2009;37:173–180. doi: 10.1007/s12016-009-8123-4. [DOI] [PubMed] [Google Scholar]
- 46.Stasi R, Del Poeta G, Stipa E, Evangelista ML, Trawinska MM, Cooper N, et al. Response to b-cell depleting therapy with rituximab reverts the abnormalities of t-cell subsets in patients with idiopathic thrombocytopenic purpura. Blood. 2007;110:2924–2930. doi: 10.1182/blood-2007-02-068999. [DOI] [PubMed] [Google Scholar]
- 47.Cutler C, Miklos D, Kim HT, Treister N, Woo SB, Bienfang D, et al. Rituximab for steroid-refractory chronic graft-versus-host disease. Blood. 2006;108:756–762. doi: 10.1182/blood-2006-01-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anolik JH, Barnard J, Owen T, Zheng B, Kemshetti S, Looney RJ, et al. Delayed memory b cell recovery in peripheral blood and lymphoid tissue in systemic lupus erythematosus after b cell depletion therapy. Arthritis Rheum. 2007;56:3044–3056. doi: 10.1002/art.22810. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


