Summary
IDH mutation is of central importance in the diagnosis and treatment of gliomas. Fourier-transform infrared spectroscopy, in combination with a supervised machine-learning approach, can be used to detect metabolic alterations induced by IDH1 mutations in a fraction of the time of conventional techniques.
In this issue of Clinical Cancer Research, Uckermann and colleagues report on the detection of isocitrate dehydrogenase-1 (IDH1) mutational status in glioma using an optical technique called Fourier-transform infrared spectroscopy (FT-IR).(1) IDH mutations are essential for glioma diagnosis and hold major prognostic significance for glioma patients. On a cellular level, IDH mutations result in a cascade of metabolic alterations. Employing an FT-IR spectrometer, Uckermann et al. compared the biochemical properties of IDH1 mutant glioma cell lines, human cryosections, and fresh brain tumor specimens with their IDH1 wild-type counterparts. To evaluate subtle spectral differences, the authors employ quadratic discriminate analysis, a supervised machine-learning method, to achieve an IDH classification accuracy of 92.2% in glioma cells lines, 88% in human cryosections, and 86% in fresh brain tumor specimens.
On a biochemical level, one of the most significant metabolic aberrations induced by IDH mutation is the production of the oncometabolite, 2-hydroxyglutarate (2-HG), resulting from gain of function mutation, typically at the 132nd amino acid residue of IDH. The presence of 2-HG results in multiple, presumably oncogenic, alterations to cellular metabolism, altering levels of amino acids, glutathione metabolites, choline derivatives, and phospholipids (Fig. 1) (2) Interestingly, while detectable with FT-IR in IDH mutant cell lines, spectral evidence of 2-HG accumulation was not obvious in IDH mutant glioma tissue, in part due to the variability of 2-HG levels among IDH mutant tumors. In contrast, other FT-IR spectral differences between IDH mutant and wild-type tumors were detected, including C-O stretching vibrations characteristic of carbohydrates, CH2 bending characteristics of lipids and amide-bond specific vibrations seen in proteins. Uckermann et al. conclude these findings are consistent with expected downstream metabolic effects of IDH mutation in glioma induced by 2-HG. Indeed, the observed spectral evidence suggesting alterations in protein and lipid levels induced by IDH mutation is harmonious with detailed metabolomic analysis performed on IDH mutant human oligodenroglial cells utilizing multiple mass spectrometry platforms.(2) Further mechanistic studies, possibly in conjunction with mass spectrometry, which provides unparalleled chemical resolution, would be helpful in understanding the specific chemical species that underlie the FT-IR differences between IDH mutant and wild-type cell lines and tumors.
Figure 1.
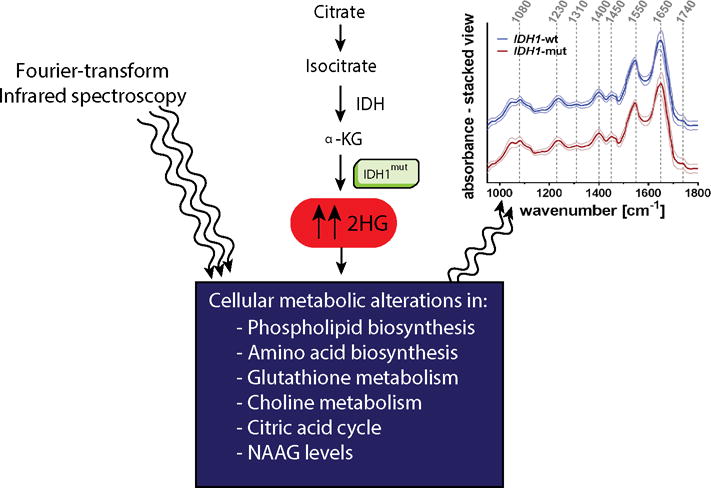
Molecular and metabolomics aberrations in IDH mutant cell lines and human tissue detected by FT-IR. IDH1 mutations lead to increased levels of 2-HG. Multiple downstream metabolic changes provide the substrate for machine-learning-based classification of IDH mutational status using spectral data from FT-IR.
Regardless of the specific biochemical alterations detected, FT-IR holds promise for intraoperative detection of IDH mutation since spectra can be rapidly acquired (~1 minute). In contrast, current methods for determining IDH status require intensive laboratory testing (DNA sequencing, PCR, and/or immunohistochemistry), precluding access to IDH genotyping during surgery. While several methods have been developed to establish preoperative or intraoperative molecular diagnosis, including magnetic resonance spectroscopy, rapid glioma genotyping assay(3), and desorption electrospray ionization mass spectrometry(4), they are limited by issues related to specimen preparation and highly specialized intraoperative instrumentation. Notably, Uckermann et al. utilize a commercially available FT-IR instrument that has promise for further validation in a manner that would create the possibility of wide clinical use.
Beyond its diagnostic value in detecting IDH mutation, FT-IR could conceivably be used as a tool by surgeons to ensure optimal surgical results: maximal tumor removal with minimal damage to adjacent healthy brain. Consequently, the value of FT-IR as a surgical tool is highly dependent on its ability to detect tumor infiltration by IDH mutant glioma cells, especially at the periphery of a resection cavity where the margins may be indistinct. In its current implementation, FT-IR is prone to classification errors between IDH mutant and wild-type tissues. The majority of the classification errors observed in fresh brain tumor specimens studied by Uckermann et al. occurred in tissue with infiltrating or recurrent tumor. This indicates that the classification accuracy may be sensitive to the degree of tumor infiltration, presumably working best where tumor cells are most concentrated. Understanding the degree of tumor infiltration required for detection of IDH mutation by FT-IR will be essential in determining the clinical value of this technique for surgical guidance.
While further clinical studies are required to establish a consensus on the role of IDH mutation in surgical planning and intraoperative decision-making, several studies highlight the translational potential of intraoperative detection of IDH mutation. Recent evidence demonstrates that patients with IDH1 mutant malignant gliomas (World Health Organization (WHO) grades III and IV) display better overall survival from maximal resection of both enhancing and non-enhancing tumor (median survival 9.75 y for >5 cc residual versus not reached for <5 cc).(5) This effect was not seen in patients with IDH1 wildtype malignant gliomas, where only resection of enhancing tumor provided a survival benefit. In a subsequent study investigating low-grade gliomas (WHO grade II), IDH wild-type tumors demonstrated prolonged time to malignant transformation and overall survival with greater volumetric extent of resection; however, these findings were not reproduced in the IDH mutant group.(6) We anticipate larger and more comprehensive clinical studies to evaluate the role of IDH status on intraoperative decision-making and how this impacts clinical outcome.
In summary, rapid detection of key cancer-driver mutations like IDH creates the possibility of improving the accuracy of preliminary diagnosis and the quality of surgical intervention for glioma patients. While Uckermann et al. focus on detection of IDH mutation, FT-IR and related optical techniques may ultimately be applied to the detection of other essential cancer-specific genetic abnormalities that alter cellular metabolism. As our understanding of the genetic basis of cancer deepens, so will the potential for a molecularly tailored approach to surgical intervention. In the future, rapid molecular diagnostic techniques may serve to link our growing understanding of cancer genetics with the safest, most accurate surgical treatment of cancer patients.
Acknowledgments
D.A. Orringer is supported by the National Institute of Biomedical Imaging and Bioengineering (R01EB017254).
Footnotes
Conflicts of Interests: D.A.O. is an advisor and shareholder of Invenio Imaging, Inc., a company focused on the commercialization of stimulated Raman histology.
References
- 1.Uckermann O, Juratli TA, Galli R, Conde M, Wiedemuth R, Krex D, et al. Optical analysis of glioma: Fourier-transform infrared spectroscopy reveals the IDH1 mutation status. Clin Cancer Res. 2017 Dec;:19. doi: 10.1158/1078-0432.CCR-17-1795. [DOI] [PubMed] [Google Scholar]
- 2.Reitman ZJ, Jin G, Karoly ED, Spasojevic I, Yang J, Kinzler KW, et al. Profiling the effects of isocitrate dehydrogenase 1 and 2 mutations on the cellular metabolome. Proc Natl Acad Sci U S A. 2011;108:3270–5. doi: 10.1073/pnas.1019393108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shankar GM, Francis JM, Rinne ML, Ramkissoon SH, Huang FW, Venteicher AS, et al. Rapid intraoperative molecular characterization of glioma. JAMA Oncol. 2015;1:662–7. doi: 10.1001/jamaoncol.2015.0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santagata S, Eberlin LS, Norton I, Calligaris D, Feldman DR, Ide JL, et al. Intraoperative mass spectrometry mapping of an onco-metabolite to guide brain tumor surgery. Proc Natl Acad Sci U S A. 2014;111:11121–6. doi: 10.1073/pnas.1404724111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beiko J, Suki D, Hess KR, Fox BD, Cheung V, Cabral M, et al. IDH1 mutant malignant astrocytomas are more amenable to surgical resection and have a survival benefit associated with maximal surgical resection. Neuro Oncol. 2014;16:81–91. doi: 10.1093/neuonc/not159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel T, Bander ED, Venn RA, Powell T, Cederquist GY, Schaefer PM, et al. The role of extent of resection in IDH1 wild-type or mutant low-grade gliomas. Neurosurgery. 2017 doi: 10.1093/neuros/nyx265. [DOI] [PMC free article] [PubMed] [Google Scholar]


