Abstract
Complement was initially discovered as an assembly of plasma proteins “complementing” the cytolytic activity of antibodies. However, our current knowledge places this complex system of several plasma proteins, receptors, and regulators in the center of innate immunity as a bridge between the initial innate responses and adaptive immune reactions. Consequently, complement appears to be pivotal for elimination of pathogens, not only as an early response defense, but by directing the subsequent adaptive immune response. The discovery of functional intracellular complement and its roles in cellular metabolism opened novel avenues for research and potential therapeutic implications. The recent studies demonstrating immunoregulatory functions of complement in the tumor microenvironment and the premetastatic niche shifted the paradigm on our understanding of functions of the complement system in regulating immunity. Several complement proteins, through their interaction with cells in the tumor microenvironment and in metastasis-targeted organs, contribute to modulating tumor growth, antitumor immunity, angiogenesis, and therefore, the overall progression of malignancy and, perhaps, responsiveness of cancer to different therapies. Here, we focus on recent progress in our understanding of immunostimulatory vs. immunoregulatory functions of complement and potential applications of these findings to the design of novel therapies for cancer patients.
Introduction
The presence of cellular infiltrates composed of CD8+ (cytotoxic) effector T cells within malignant tissue, in several types of cancer (T-cell inflamed tumors), suggests efficient spontaneous priming of naïve CD8+ T cells against tumor-associated antigens [1, 2]. The type I interferon pathway seems to be pivotal for T cell priming in tumors [2]. In addition, in some patients, there are antibodies against tumor antigens [3]. Therefore, it appears that the human immune system can generate spontaneous adaptive immune responses against malignancy [2]. However, these responses are unable to eliminate tumors, likely, because of the intrinsic immunosuppressive properties of the tumor microenvironment [4]. This notion is further supported by the recent clinical success of the checkpoint inhibitors targeting T cell immunoregulatory mechanisms [5]. Therapeutic targeting of immunosuppressive mechanisms, operating in cancer patients, is more efficient in reducing or reversing cancer progression than attempts to induce de novo antitumor responses (cancer vaccines) [5]. Therefore, it is critical to understand immunoregulatory mechanisms, operating in primary cancer sites and metastasis-targeted organs to advance discovery of novel therapeutic targets or improve already existing forms of cancer immunotherapy. The improvement of checkpoint inhibitors’ efficacy is of the highest significance, given that only a fraction of cancer patients responds to this treatment and, in some patients, the clinical benefits are limited [5]. There is growing understanding and appreciation for the concept that only targeting several immunoregulatory mechanisms simultaneously can bring substantial clinical benefits for cancer patients.
The complement system has recently emerged as an important regulator of immunosuppressive mechanisms operating in primary tumor sites [6, 7] and metastasis-targeted organs [8, 9]. Although the role of complement in cancer remains understudied, several reports point to complement as a recruiter, inducer, and regulator of immunosuppressive cells in the tumor microenvironment and the premetastatic niche [7]. Recent work also demonstrated synergism between programmed cell-death 1 (PD-1) blockade and complement inhibition in reducing progression of tumors in a model of lung cancer [10]. These findings reveal a more practical avenue for ventures exploring the complement system as a target in a combined immunotherapy approach in concert with checkpoint inhibitors.
In contrast to T-cell inflamed tumors, in a subset of cancers, tumor tissue commonly lacks infiltrating T cells, suggesting immune exclusion. In these tumors, spontaneous priming of T cells does not occur, therefore, targeting T-cell checkpoints is unlikely to offer substantial clinical benefits [2]. Designing new immunotherapy for these patients seems to be especially challenging [11]. Given a key role of the complement system in regulating innate immunity in infection [12] and possible interconnections of early complement deficiencies with triggering the type I interferon pathway in systemic lupus erythematous (SLE) [13], it is tempting to speculate for a possible role for complement in preventing efficient priming of T cells in non-T cell inflamed tumors.
The complement proteins are abundant throughout the body and are produced in cells involved in immunity. In addition, complement regulates inflammation [14] and antitumor immunity [6, 7]. Therefore, it is conceivable that complement may play a central role in orchestrating immunosuppressive mechanisms that overwhelm antitumor immunity in cancer patients.
However, in the absence of malignancy, complement bridges initial innate immune responses with subsequent adaptive immunity by shaping and directing B and CD4+ T cells [15, 16], and is pivotal for induction of efficient immunity against pathogens [12, 17]. These seemingly contradictory functions of complement in regulating adaptive immunity require further studies and explaining the conflicting results will perhaps remain a challenge for some time in the field of complement biology. Here, we discuss essential functions of the complement system and its dichotomous role in regulating immunity in infection vs. cancer because this dichotomy needs to be cautiously considered when designing cancer immunotherapies targeting complement.
Components of the complement system
The complement system is composed of over 50 blood and lymph circulating, membrane-bound, and intracellular proteins [17]. The functions of some key complement components are summarized in the table 1. It is an integral part of innate immunity and constitutes the first line of immediate immune defense against invading pathogens. The complement proteins found in serum are mostly secreted by the liver and are comprised of pattern recognition receptors (PRR), involved in detection of pathogens, enzymes that are activated in a cascade like fashion, similar to the coagulation system, and effector molecules. However, the list of immune and non-immune cells that can produce complement proteins has been growing since the discovery of extrahepatic synthesis of complement proteins over three decades ago. For example, bone marrow C3 production accounts for over 9% of all C3 in circulation [18, 19]. Other components, such as factor D, are almost exclusively produced by adipocytes [20]. In fact, the majority of cells in the human body can produce at least one or more complement proteins [21]. The importance of this extrahepatic production is underlined by the observation that C4 produced by monocytes can rescue the humoral response against tumor-derived antigens in the absence of serum C4 [22].
Table 1.
Non-exhaustive list of complement proteins and their activation products.
| Complement protein/s | Receptor | Function | Reference |
|---|---|---|---|
| C3a | C3aR | Anaphylatoxin (can cause smooth muscle contraction, vasodilation and increased histamine release by mast cells), CD4+ T cell activation, monocyte activation (via NLRP3 inflammasome) cancer progression (direct or indirect effect) | [63, 65, 147] |
| C4a | PAR1, PAR4 | Anaphylatoxin | [169] |
| C5a | C5aR1, C5aR2 | Anaphylatoxin, NLRP3 inflammasome activation in CD4+ T cells and monocytes, regulates cancer progression | [66] |
| C3b | CD46, FH, CR1 | Opsonin (via its receptors), part of the C3 and C5 convertases, activates CD4 T cells in autocrine fashion | [61] |
| Factor H (FH) | None, binds to Factor I and C3b or cell surface | In complex with the serine protease factor I, cleave and inactivate C3b to iC3b | [170] |
| CD46 | None, binds to C3b, C4b and Factor I | In complex with the serine protease factor I, cleave and inactivate C3b (or C4b) to iC3b, potent costimulatory molecule on CD4+ T cell activation and metabolism | [61, 62, 73] |
| Complement receptor 1 (CR1, CD35) | None, binds to C3b, iC3b | cleaves iC3b to C3dg, mediates inhibitory signals in T cell proliferation | [171] |
| Complement receptor 2 (CR2, CD21) | None, binds the degradation products of C3b (iC3b, C3dg, C3d) | Potent costimulatory molecule in B cells. Also controls survival (CR1) of germinal center B cells during development | [172–174] |
| Complement receptor 3 (CR3 or CD11b/CD18, or Mac-1) | None, binds iC3b | Opsonizes iC3b coated bacteria | [28, 175] |
| Complement receptor 4 (CR4 or CD11c/CD18) | None, binds to different part of iC3b than CR3 | Opsonizes iC3b coated bacteria | [28, 175] |
Serum-derived complement can be activated via three pathways [17]. The alternative pathway is, evolutionarily, the oldest and is triggered either by bacterial surfaces or spontaneous fluid phase hydrolysis of the complement C3 thioester [23]. The lectin pathway is activated upon engagement of mannose binding lectin (MBL) or the ficolins (termed ficolin-1, ficolin-2, ficolin-3) to specific carbohydrates or N-acteyl residues respectively [24, 25]. The classical pathway, which was discovered first, is, evolutionarily, the youngest because it is initiated upon binding of C1q to the clusters of at least two IgG molecules (or one IgM), forming a complex with antigen [26]. All three activation pathways result in generation of complement C3 cleaving enzymes, termed C3 convertases (see [16] for more details). The C3 convertases cleave C3 to a small 10 kDa fragment - C3a and a large fragment - C3b. The C3b can attach to bacterial or other nearby surfaces and mark them for phagocytosis in a process known as opsonization [27, 28]. Upon the cleavage of approximately ten thousand C3 molecules, another C3b molecule associates with the C3 convertase to give rise of the C5 convertase. The C5 convertase cleaves C5 into a small fragment (C5a) and a large fragment (C5b). Complement proteins C3, C4 and C5 belong to the, evolutionarily, very old α2-macroglobulin family [17] and, as such, they have conserved sequences and structures in different species. The C3 and C5 contain active thioester moieties, which enable them to attach covalently to other molecules. While C4 does not contain active thioester moieties, it is also cleaved into a small fragment (C4a) and a large fragment (C4b), similar to C3 and C5.
The generation of the large fragments C3b and also C4b leads to opsonization of pathogens or cells and phagocytosis by monocytes or macrophages, via engagement of the receptors specific for C3 activation fragments. C3b bound to the cell surface is further processed via action of the soluble and membrane-bound complement regulators (see Table 1 for more details). The large fragment produced from C5 cleavage, C5b, attaches to a target surface (such as bacterial cell wall, or host cell) and forms the backbone for binding of C6, C7, C8 and finally C9 (Membrane Attack Complex-MAC). The C9 polymerizes to form a pore in the cell membrane, which can lead to cell lysis or cell activation, as it is in a case of cancer [29]. Due to the potential deleterious consequences of its cytolytic properties, complement activation is a highly controlled process through many soluble and membrane-bound complement regulatory proteins (CRPs) to avoid self-harm [30].
Elevated levels of C3a and C5a (anaphylatoxins) in serum have been reported in a number of diseases, including sepsis [31], SLE, age-related macular degeneration (AMD) [32], and rheumatoid arthritis [33]. The activity of anaphylatoxins is regulated by carboxypeptidases, including carboxypeptidase N [34], which cleaves a C-terminal arginine of C3a or C5a. The resulting peptides, termed C3a-desArg and C5-desArg, have different conformations and functions than C3a and C5a [35]. C3a-desArg (also known as acylation stimulating protein) loses its pro-inflammatory role but has reported roles in triglyceride metabolism and glucose uptake [36–38]. The C5a-desArg still retains approximately 1% of C5a anaphylactic and chemotactic activity, while the pro-inflammatory activity is reduced 10-fold [35]. The anaphylatoxins and C3 degradation products (C3b, iC3b) generally signal distress and/or danger and, thus, all immune and many non-immune cells in the body have receptors for them [39, 40]. It is, therefore, not surprising that in the last twenty years complement was found to play an important role in regulating adaptive immune responses.
The significance of the serum-derived complement system as a sentinel for pathogen invasion is underscored by recurrent severe infections in key complement component deficiencies [41, 42]. Similar to Toll like receptors (TLR) and the inflammasomes, serum complement PRRs not only recognize pathogen-associated molecular patterns (PAMPs) but also damage-associated molecular patterns (DAMPs). For example, the C1 complex (comprised of C1q, C1r, C1s) can bind to the surface blebs on apoptotic cells and mediate their clearance. Therefore, it is not surprising that C1 deficiencies or dysfunctions are associated with a high risk of SLE as a result of exposure of self-intracellular antigens, to immune recognition [43, 44]. C1q also prevents autoimmunity by skewing macrophage polarization and inhibiting Th1 and Th17 proliferation, as demonstrated in a mixed lymphocyte reaction [45]. Alternatively, DAMPs can also modulate the expression of complement proteins. For example, factor B (activator of the alternative pathway) was upregulated by exogenous DNA in mouse macrophages [46].
Complement in the interface between innate and adaptive immunity
Aside from its critical role in innate immunity, complement has well-established roles in regulating adaptive immunity. In fact, the receptors that recognize the fragments produced from complement activation transmit signals to B and T cells [39, 40]. The classical example for this is stimulation of CR2 through C3d-coated antigen, which reduces the threshold for B cell receptor (BCR) activation, thereby, providing important costimulation for optimal antibody production [47, 48]. This is consistent with the observation that serum C3-deficiency often causes common variable immunodeficiency [49]. In addition, CR1 and CR2 signaling in B cells and follicular dendritic cells contributes to induction of B cell memory and maintenance of B cell tolerance [50]. Furthermore, activation of the complement regulator CD46 on T cells is required for induction of T cell effector functions [12, 16].
In further support of a role of complement in adaptive immunity, C3 production in epithelial and endothelial cells was shown to be important in transplant rejection via regulation of T cell responses in vitro and in vivo [51]. The local (extrahepatic) complement production can be stimulated by various cytokines, which suggests that the complement system evolved to respond to local environmental signals [52, 53]. Interestingly, cytokine production can also be modulated by the proteins of the complement system [54, 55], suggesting a bidirectional feedback loop.
In the past nine years our understanding of the role of complement, produced and secreted by antigen presenting cells (APC), in T cell activation, has greatly increased. In particular, functions of C3aR and C5aR1 during activation and survival of CD4+ T cells have been well investigated [56–59]. The earlier studies attributed production of complement components to activated APCs and T cells that synthesize and secrete C3, C5, factor B, and factor D leading to alternative complement pathway activation. The C3 and C5 cleavage products, then, interact with their cognate receptors to induce Th1 responses manifested by IFN-γ production [56, 58, 60]. More recent studies specifically highlight the role of autocrine complement signaling in CD4+ T cell activation. In vitro experiments revealed that T cells can generate complement components, such as C3a and C3b that bind to their receptors C3aR and CD46, respectively, on the surface of the same cell, to costimulate the induction of Th1 responses and initiate the self-regulatory phase [61]. CD46, which is also known as membrane cofactor protein (MCP) and binds to C3b, seems to be particularly important in this process [62]. The important role of locally-produced complement was also recently documented in other cell types. For example, C3aR stimulation leads to inflammasome activation in monocytes and IL-1β secretion, which enhances generation of Th17 cells [63]. Inversely, complement also has immunoregulatory functions. For example, C3 degradation product iC3b promotes the development of myeloid-derived suppressor cells (MDSC) in vitro [64].
Recent discoveries demonstrated that complement activation fragments, generated by T cells, can also function inside these cells and are required for T cell-mediated immunity and cell survival. In resting human CD4+ T cells, activation of C3 occurs continuously within the cell through cleavage via the protease cathepsin L (CTSL), into bioactive C3a and C3b fragments. C3a activates C3aR located on the lysosomal surface that is required for survival of T cells via activation of the mechanistic target of rapamycin (mTOR) [65]. Activation of the T cell receptor (TCR) induces rapid translocation of intracellular C3a and C3b to the cell surface, where they both engage the surface C3aR and CD46, respectively. These regulatory events are crucial for IFN-γ production and human Th1 induction (of note, serum-derived C3 cannot drive this response). Indeed, individuals lacking CD46 expression or C3 secretion by T cells have diminished Th1 immunity (but normal Th2 and Th17 responses) and suffer from recurrent infections. Conversely, uncontrolled intracellular C3 activation leads to dysregulation of CD46-mediated co-activation of human T cells and contributes to pathologically hyperactive Th1 responses in rheumatoid arthritis [17, 61]. Human CD4+ T cells also contain the intracellular C5 activation system, although the C5 activating enzyme is still unknown. The engagement of intracellular C5aR1, upon TCR stimulation, is required for production of reactive oxygen species and NLRP3 inflammasome-driven IL-1β secretion by the T cell, which support sustained Th1 induction during T cell migration into tissues [66]. In mice, which do not express CD46 in the somatic tissue [67], activation of C3aR and C5aR1, expressed in T cells, seem to be critical for induction of Th1, Th17, and regulatory T cells (Tregs) [54]. However, a role for intracellular complement activation in mice has not yet been investigated.
After successful CD46 costimulation-driven Th1 expansion, CD46 engages in a, still undefined, crosstalk with the IL-2 receptor, and induces IL-10 production in Th1 cells, which drives these cells into a self-regulatory contraction phase [59, 61]. The deregulation of this crosstalk prevents normal IL-10 expression and contraction of the Th1 cell population, contributing to chronic diseases such as multiple sclerosis [68, 69] and rheumatoid arthritis [61]. Ni Choileain and colleagues recently proposed that CD46 recruitment to the immunological synapse was driven by O-glycosylation of the CD46 serine- threonine- proline-rich region, which appeared to be impaired in multiple sclerosis patients. Therefore, these patients cannot downregulate CD46 expression in their T cells, which is required for IL-10 expression and T cell contraction [69]. In addition, autocrine surface engagement of C5aR2 via, intracellularly generated, and then surface-shuttled C5a/C5a-desArg, negatively regulates intracellular C5aR1 stimulation and, thus, also aids in Th1 contraction [66]. Although a role of intracellular complement activation is currently best studied in human CD4+ T cells, it has been observed in many cell types suggesting broad biological relevance [65].
Intracellular C3 in CD4+ T cell metabolism
The discovery of intracellular complement C3 substantiated the idea that complement may also play important roles in regulating basic cellular processes. It is well appreciated that differentiation of immune cells in response to danger requires cell-specific reprogramming of metabolic pathways. The distinct metabolic signatures are key features of the different phases of the immune response, starting from a resting state, and progressing through an effector response, and, then, a memory phase [70–72]. The resting T cells are postulated to maintain a status of low glycolytic activity and reduced nutrient intake, using oxidative phosphorylation (OXPHOS) and fatty acid oxidation as an energy source, while activated T cells increase both glycolysis and OXPHOS as well as nutrient influx and, upon contraction, reduce levels of glycolysis and return to a state of low-level OXPHOS [70]. Intracellular complement activity was associated with T cell metabolic reprogramming of two of the metabolic states. First, intracellular C3a generated by CTSL-mediated processing of C3, within resting T cells, drives C3aR signaling on the lysosomal surface and via the low-level of mTOR activity needed for T cell survival [65]. Second, during CD4+ T cell activation, C3b, generated by CTSL on the cell surface, engages CD46. The CD46 costimulation is critically required for heightened expression of the glucose transporter GLUT1 (SLC2A1) and the amino acid (AA) transporter LAT1 (SLC7A5), which results in an increased influx of glucose and AAs, ‘fueling’ T cell activation and Th1 induction [73]. In addition to active regulation of nutrient transport, CD46 also regulates expression of the Late Endosomal-Lysosomal Adaptor, MAPK and mTOR Activator 5 (LAMTOR5). LAMTOR5 is a component of the Ragulator complex, which is essential for AA sensing by mTORC1[73]. In agreement with these observations, T cells from CD46-deficient patients failed to up-regulate GLUT1, LAT1 and LAMTOR5 expression upon activation and showed decreased mTORC1 activity and, thus, impaired Th1 responses [73]. Conversely, highly elevated intracellular C3 processing by CSTL and increased CD46 signaling occurring in CD4+ T cells, isolated from the inflamed joints of patients with juvenile idiopathic arthritis, leads to exaggerated mTOR activity and IFN-γ secretion [65]. For more details on recent advances in intracellular roles of complement in metabolic processes please refer to the comprehensive reviews [17, 71]. The role of intracellular complement in cancers has not been yet studied, however, a recent report showed the cleavage of tumor cell-derived C3 by CTSL (and Cathepsin B) [74] in CaCO2 cell line suggesting that it might be operative in some capacity in cancers as well.
Sources of Complement Proteins in Tumors and Metastasis Targeted Organs
The complement proteins are abundant in plasma and body fluids (https://emedicine.medscape.com/article/2086931-overview) [21]. Therefore, complement inactive proteins are readily available for activating enzymatic cleavages everywhere in the body without the need for upregulating complement genes. However, in cancer patients and mice with experimental tumors, increased production of complement proteins, reflected by increased concentrations of complement components in plasma and other body fluids, was observed [8, 75, 76]. These increased concentrations of complement components may result from increased production of complement by the liver, however, local upregulation of complement genes in primary tumor sites and metastasis-targeted organs is expected, given the well-established extrahepatic production of complement proteins, mainly by endothelial cells and cells participating in immune responses [21, 77]. These cells constitute an integral component of the tumor microenvironment [4, 78] and the premetastatic niche [79], thus, they are a potential source of various complement components within tumors and metastatic sites. For example, in a syngeneic mouse model of metastatic breast cancer, which spontaneously metastasizes to the lungs, concentrations of C3 in plasma increase over time [8]. Interestingly, in the same study increasing concentrations of C3 were also found in bronchoalvolar lavage and these increases were associated with upregulation of C3 and C5 genes in the lungs [8, 9]. Cytotoxic CD8+T cells were reported to produce C3, which regulated, after cleavage to C3a, IL-10 expression in these cells in tumors [80]. In contrast, some studies showed decrease in serum C3 levels. For example, in patients with squamous cell carcinomas [81] and colorectal cancer [82] downregulated C3 (or C3a) expression was observed.
Importantly, in addition to host cells, tumor cells can also produce complement proteins. In a syngeneic model of ovarian carcinoma (ID-8VEGF) and human ovarian carcinoma cell lines tumor cells were shown to produce C3 and complement activation was demonstrated in mouse cancer model even when tumor cells were implanted to C3-deficient mice [83]. Riihila and colleagues showed the expression of C3, factor B and factor I in squamous cell carcinoma cells, which supported growth and expansion of tumors [84, 85]. Very recently, Boire and colleagues, using four different human cancer cell lines, showed that C3 produced and secreted by cancer cells might aid their survival and leptomeningeal metastasis [86].
The local production and activation of complement may have significant implications for regulating tumor microenvironment, as complement effectors such as C3a and C5a are readily available for interactions with their reciprocal receptors on tumor or host cells, perhaps, avoiding rapid inactivation by carboxypeptidases.
Complement Activation in Cancer
It has long been known that complement activation occurs in cancers (Fig. 1) because deposition of activated complement fragments within tumor tissue can be detected [87]. The deposition of complement proteins including the C5b-9 terminal complement complex was demonstrated in breast cancer [88] and in papillary carcinoma of thyroid gland [89, 90]. Complement activation through the lectin pathway was shown in colorectal carcinoma [91, 92]. In ovarian cancer patients, complement fragments were detected in ascites [93]. The activation of complement in cancer patients was also confirmed by the presence of C5a in plasma of patients with non-small cell lung carcinoma [76]. In addition, the significance of plasma C4 cleavage fragment C4d as a prognostic biomarker was demonstrated for lung cancer [94] and malignant pleural mesothelioma [95]. The evidence for complement activation was shown for several other human malignancies [6], however, the particular complement activation pathway that plays a pivotal role in human tumors remains unclear.
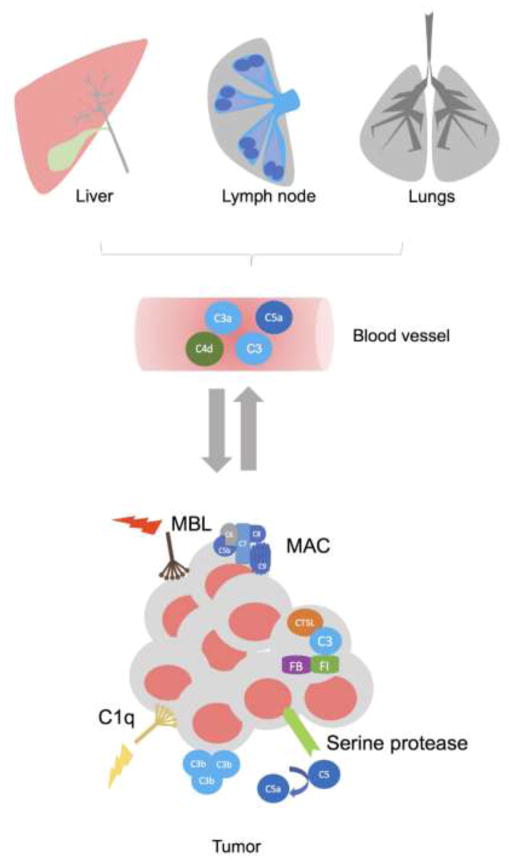
Figure 1. The sources of complement proteins and mechanisms of complement activation in cancer.
The complement proteins are abundant in plasma and other body fluids. Therefore, in cancer patients and mouse models of cancer, the large quantities of complement proteins are available for activation, virtually in every location in the body, including primary tumors and metastasis-targeted organs, without a need of additional complement protein synthesis. However, several studies demonstrated that the increased quantities of complement are produced in the liver, lymph nodes, metastasis-targeted organs, and tumors. The activation of complement was demonstrated in several malignancies because deposition of complement fragments (C1q, MBL, C5b-9, C3b) was detected in tumors and complement effector molecules (C3a and C5a) and complement cleavage fragments (C4d) were present in plasma, bronchoalveolar lavage or tumor homogenate. In addition to the three well characterized pathways of complement activation, C5a can be generated through C5 cleavage by serine proteases expressed in tumors, and C3 can be cleaved inside CD4+T cells and CaCO2 cell line through cathepsin L-mediated (CTSL) mechanism. This latter mechanism is pivotal to T cell homeostasis.
The studies in mouse models point to the classical pathway of complement activation because the deposition of C3 cleavage fragments and C1q but not MBL was shown within or in the vicinity of tumor blood vessels in a syngeneic model of Human Papilloma Virus (HPV)-induced cancer [96]. In addition, mice deficient in C4, which is required for both the classical and lectin pathways, had smaller tumors when compared to the littermate wild-type controls. In contrast, deficiency in factor B, participating in the alternative complement pathway, did not impact tumor growth in the same mouse model, indicating that the alternative pathway is not essential for tumor growth in this experimental setting [96]. In a transgenic ovarian cancer model, C3 cleavage products were found on the surface of tumor cells [97]. Importantly, in a syngeneic mouse model of aggressive metastatic breast cancer (4T1), in which tumor cells are injected into the mammary fat pad, and breast tumors subsequently metastasize to distant sites, similar to human breast cancer [98], complement C3 cleavage fragments were deposited in the lungs prior to metastasis, indicating participation of the complement system in the lung premetastatic niche [8]. The deposition of C3 cleavage fragments in the lung parenchyma was associated with increasing concentrations of C5a in mouse plasma over time [8].
Interestingly, inactive complement fragments can be cleaved and activated through various proteolytic enzymes that do not contribute to the three well-characterized (traditional) complement activation pathways. Collectively, this mechanism was termed the extrinsic complement pathway [14] and, in the context of the tumor microenvironment, includes cleavage of C5 by tumor cell membrane-bound serine proteases [99]. Perhaps one of the best documented examples of C5 cleavage outside the three traditional complement pathways is the cleavage by thrombin in the absence of C3 [100]. The ability of coagulation factors to interfere with complement activation points to the interconnections between complement and coagulation cascade that are well-document in several disease models [101], and may have significant implications in cancer, given multidirectional links between coagulation and tumor progression [102]. In addition, hypoxia present in virtually all solid tumors and subsequent cell death appear to be significant contributors to complement activation, at least, in mouse models [103].
Thus, it appears that complement is activated through different mechanisms and complement effectors are generated in several human malignancies and mouse models of cancer. These processes lead to increased concentrations of complement activation fragments within tumors, the premetastatic niche, and plasma. Therefore, in patients and mice with tumors, complement effector molecules are widely accessible to their receptors on tumor and host cells, virtually in every anatomical site of the body (Fig. 1). The mechanisms that drive complement activation or cleavage of individual complement components likely depend on the type of malignancy and composition of the tumor microenvironment. Given high complexity and heterogeneity of malignant tissues, it is conceivable that several modes of activation, via traditional and non-traditional mechanisms, contribute to generation of complement effector molecules in cancer patients.
Interactions of complement with components of the tumor microenvironment
Growing evidence demonstrates that tumor growth and progression of malignancy is highly dependent on the support of several components of the tumor microenvironment including resident and infiltrating host cells. The work in the last decade demonstrated critical contributions of complement to regulating this microenvironment (Fig. 2 for summary).
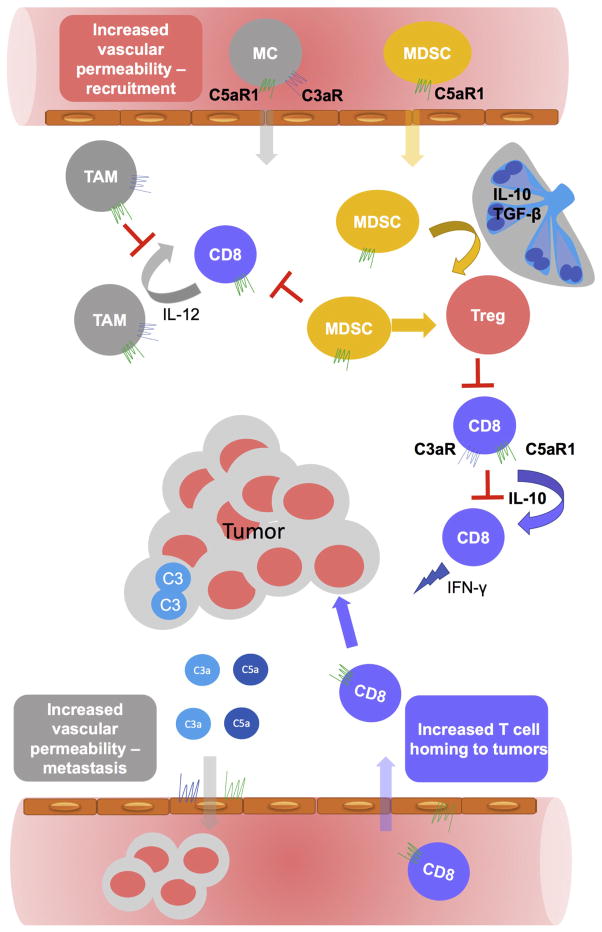
Figure 2. The diverse role of complement in regulating tumor microenvironment.
The complement system suppresses antitumor immunity by activating and attracting to tumors myeloid-derived suppressor cells (MDSC) and tumor-associated macrophages (TAM) that originate from recruited from blood myeloid precursors or monocytes (MC). Mechanistically signaling through C5aR1 is critical for regulating MDSC whereas both anaphylatoxin receptors C5aR1 and C3aR are involved in regulating TAMs. C5aR1 signaling controls IL-10 and TGF-β1 produced in tumor draining lymph nodes and, therefore, Tregs generation. In addition, C5aR1 regulated MDSC contributes to this process in tumors. C3 generated in CD8+T cells, through subsequent generation of C3a and C5a and their interaction with the reciprocal receptors, expressed in CD8+ T cells, inhibits IL-10 production in these cells and, therefore, their cytolytic antitumor function. C3 produced in tumors contributes to generation of anaphylatoxins that can directly promote growth and survival of tumor cells through their reciprocal receptors. The C3a and C5a-mediated changes in vascular permeability may facilitate entry of tumor cells into the blood vessels and metastasis. On the other hand, C5aR1-mediated changes in endothelium facilitate homing to tumors adoptively transferred CD8+T cells, thereby, limiting tumor growth.
Tumor associated macrophages
Tumor-associated macrophages (TAMs) are perhaps one of the best studied components of the tumor microenvironment that, since the late seventies, are known to promote tumor growth [104]. The presence of tumor infiltrates composed of TAMs is associated with poor prognosis in several cancers [104]. The tumor promoting properties of TAMs are very diverse and include: regulation of tumor cells motility and invasiveness, remodeling of extracellular matrix, promotion of angiogenesis, and immunoregulatory functions that contribute to inhibition of antitumor immunity [105]. Interestingly functionality of TAMs in tumors is reflected by the localization of these cells at the invasive tumor edge, where they significantly contribute to increased invasiveness of tumors [106]. The immunoregulatory function of TAMs seems to be less clear because of phenotypic and functional plasticity of these cells in regards to regulation of antitumor immunity [107]. It appears that several phenotypically and functionally different subtypes of these cells can be present in tumors, ranging from immunosuppressive cells, traditionally described as alternatively activated macrophages (M2), to antitumor, classically activated (M1) macrophages [105]. The macrophage plasticity is critically important because reversing immunosuppressive and protumor M2 to antitumor M1 macrophages is a promising therapeutic option [4, 108].
The studies on complement regulating TAMs are surprisingly scarce, given the importance of the complement anaphylatoxins for regulating macrophages in inflammation [14]. However, C5a-mediated regulation of macrophage plasticity was demonstrated in vitro and in vivo in a model of Leishmania infection [109]. According to this work, C5a inhibits TLR4 (LPS)-induced production of IL-12 in macrophages, a hallmark of classically-activated M1 macrophage [107]. Importantly, macrophage polarization skewed toward the M2 phenotype via C5aR1 signaling corresponded to inhibition of Th1 responses that are essential for triggering efficient antitumor cytotoxic immunity [110]. It is, therefore, likely that similar regulation of TAMs by C5aR1 in the tumor microenvironment may contribute to tumor promoting and immunoregulatory properties of these cells. In addition, C1q, coating apoptotic cells, was demonstrated to sequentially induce expression of type I IFNs, IL-27, and IL-10 in LPS stimulated human monocyte-derived macrophages [111], suggesting that C1q induces regulatory macrophages independent from the M1/M2 dichotomy [107]. Recent work from the same group showed that these C1q-induced changes in macrophage activation were also associated with upregulation of PD-L1 and PD-L2 in these cells. These functional macrophage changes led to reduced proliferation of allogenic or autologous Th1 and Th17 cells in a mixed lymphocyte reaction and showed a trend toward increasing Tregs proliferation [45].
The role of complement in recruiting TAMs and regulating their proangiogenic properties was demonstrated in the recent work exploring antitumor properties of pentraxin 3 (PTX3) [112]. The PTX3-deficiency increased incidence and growth of tumors in two mouse models of chemically induced carcinogenesis. The enhanced tumor growth was associated with increased activation of complement, demonstrated by deposition of C3 cleavage fragments in the tumor stroma and increased concentrations of C5a in tumor tissue homogenates. The enhanced complement activity led to increased infiltration of tumors by CD11b+F4/80+ macrophages and CD11b+Ly6Chigh monocytic myeloid cells that were likely a source for increased concentrations of vascular endothelial growth factor (VEGF). Backcrossing PTX3-deficient to C3-deficienct mice annulled this phenotype and reduced TAMs, proving complement-dependent actions of PTX3 in the tumor microenvironment. The effect of PTX3 on complement activation was factor H-dependent [112]. The specific contributions of C3aR to recruitment of F4/80 macrophages to tumors was also recently observed in a mouse model of melanoma through use of C3aR-deficient mice [113].
Myeloid-derived suppressor cells
MDSC initially described in in the peripheral blood of cancer patients [114] are potent suppressors of antitumor immunity [115] that affect antigen presenting capacity of dendritic cells [116], T cell responses [116–118], macrophage polarization [119], and NK cell cytotoxicity [120]. Similar to TAMs, they also promote tumor angiogenesis [121]. The link between MDSC and complement was first described in the original report on the tumor promoting functions of the complement system [96]. This work demonstrated that deficiencies in several complement components were associated with impaired tumor growth in a model of HPV-induced cancer. Importantly, C5aR1-deficiency and pharmacological blockade of C5aR1 by selective C5aR1 antagonist (PMX53) [122] impaired tumor growth, pointing to the C5a/C5aR1 signaling axis as an effector mechanism of complement-mediated tumor-promoting functions. Of note, tumor cells used for these studies did not express C5aR1, suggesting involvement of host cells in the complement-mediated effect. The reduced tumor growth in the absence of C5aR1 signaling correlated with increased infiltration of tumors by cytotoxic CD8+ T cells that are pivotal for immune responses to tumors [78]. The depletion of these T cells abrogated impact of C5aR1 deficiency on tumor growth, clearly indicating immunoregulatory properties of C5aR1. The mechanisms responsible for C5a/C5aR1 mediated suppression of antitumor T cell responses included activation of monocytic and recruitment of granulocytic MDSC, which expressed C5aR1, to tumors. Attracting MDSC to tumors was, perhaps, linked to the well-established chemotactic properties of C5a. However, it also involved changes in the expression of integrins of the surface of granulocytic MDSC. It was also shown that C5a/C5aR1 signaling regulates synthesis of reactive oxygen (ROS) and nitrate species (RNS) in monocytic MDSC. Since both ROS and RNS are known to inhibit antigen-specific responses of CD8+ T cells [123], this C5aR1-mediated effect appear to be crucial in impairing T cell ability to recognize tumor antigens. In addition, C5aR1 increased expression of arginase-1 in tumors, which regulates L-arginine metabolism, contributing to ROS and RNS generation, and seems to be pivotal for MDSC immunosuppressive functions in tumors [124]. These original observations [96] were corroborated by follow-up studies demonstrating involvement of C5a in regulating MDSC in a model of lung cancer [76]. In addition, blockade of C5aR1 resulted in reduced tumor expression of genes involved in regulating antitumor immunity including Arg1, Il-6, Il-10, Ctla4, Lag3 and Cd234 (PDL1) [76].
Regulatory T cells
At homeostasis, Tregs are essential for maintaining tolerance to self-antigens. Deficiency or alterations in their function leads to severe autoimmune reactions [125]. However, in cancer, Tregs are hijacked by tumors to inhibit antitumor T cell responses [126]. Tregs suppress tumor antigen presentation and impair CD8+ T cell-mediated cytotoxicity by inhibiting release of cytolytic granules [127]. Consistent with these findings, increased numbers of Tregs in human tumors correlated with reduced survival [128, 129]. In contrast, in some malignancies, the presence of Tregs is associated with improved prognosis [130]. Importantly, MDSC were implicated in generation of Tregs in tumors [131], therefore, given the connection between C5aR1 and MDSC, enhancement of Treg generation by complement would be expected.
Surprisingly, in contrast to this notion, recent work demonstrated that C3a and C5a via their reciprocal receptors expressed on naïve T cells, inhibit generation of induced Tregs via a TGF-β1-dependent mechanism [59]. However, the conclusions included in this work were based mostly on ex vivo mechanistic studies that were performed in a tumor-independent context.
Conversely, in vivo experiments demonstrated reduced numbers of Tregs in blood and the lungs of C5aR1-deficient mice in a model of metastatic breast cancer [8]. The impaired generation of Tregs in these mice was linked to reduced expression of TGF-β1 and IL-10 in myeloid cells infiltrating lungs [8]. Both of these cytokines have diverse immunosuppressive functions and were implicated in generation of Tregs in tumors [131]. The reduced numbers of Tregs in lungs correlated with the reduced lung metastatic burden [8]. In a transgenic model of breast cancer driven by Her2/neu overexpression blockade of C5aR1 with a specific C5aR1 inhibitor (PMX53) led to reduction of CD4+CD25+FoxP3+ Tregs in tumors and spleens. The reduced generation of Tregs in the absence of C5aR1 signaling was associated with reduced production of TGF-β1 and increased expression of IL-6 in myeloid cells in tumor infiltrating lymph nodes and remarkably reduced levels of TGF-β1 in mouse plasma [103]. Given the importance of TGF-β1 and IL-6 in controlling generation of various T cell effectors [132], it appears that C5aR1 signaling impacts generation of Tregs in tumor-bearing host by controlling the cytokine milieu in tumor draining lymph nodes and metastasis-targeted organs.
Given that complement is activated in several types of tumors [87] and this activation leads to generation of multiple C3 cleavage fragments that may interact with CD46 on T cells, it is possible the tumor-microenvironment might be ‘tipping the balance towards’ IL-10 production and limiting immune responses.
Dendritic cells
The dendritic cells (DCs) are pivotal in regulating spontaneous T cell responses to tumor antigens. DCs are traditionally divided into two major categories plasmacytoid DCs (pDCs) and conventional DCs (cDCs) [133]. pDCs present in tumors have beneficial effects as demonstrated by their efficacy in priming of T cells in vitro [134] and capacity to generate effective immune responses to tumors in vivo [135]. However, some studies have demonstrated the tolerogenic properties of these cells [136] and linked pDCs presence in tumors to poor clinical outcomes [137]. The regulatory properties of DCs are likely the result of immunosuppressive factors present in the tumor microenvironment that lead to reduced production of type I IFNs, decreased expression of costimulatory molecules and upregulation of indoleamine 2,3-dioxygenase (IDO) and PD-L1 [138–140].
Within cDCs, the CD8α+Baft3-dependent subtype seems to be most efficient in phagocytizing dead cells and in cross presenting class I MHC-restricted antigens to CD8+ T cells [141]. The particular efficacy of this subtype of cDCs in generating antitumor T cell responses is associated with the expression of the receptors recognizing dying or dead cells or fragments derived from these cells (DAMPs) and production of type I IFNs by DCs upon stimulation with DAMPs [78]. The cytosolic-DNA sensing pathway seems to be pivotal for upregulating type I IFNs by DCs in response to release of ligands from dying cells [142]. In brief, necrotic or apoptotic cells release free DNA or DNA in a vesicle-protected form that stimulates innate immunity and leads to subsequent activation of antitumor T cell responses [143].
Interestingly, similar mechanisms including upregulation of type I IFNs are involved in stimulation of immunity in inflammatory and autoimmune disorders [143] that are tightly linked to complement deficiencies. In fact, the homozygous deficiencies of early components of the classical pathway, including C1q, C1r, C1s, C4, and C2, are the strongest susceptibility factors for the development of SLE that have been identified in humans thus far [14]. Several hypotheses were proposed to explain the particular susceptibility of these individuals to SLE including a role of complement in clearing necrotic or apoptotic cells. Under physiological conditions complement plays a critical function in removal of waste material from dying cells including DNA [14]. Therefore, it is tempting to speculate that in tumors with intense complement activation, accessibility of material derived from dying cells for recognition by DCs can be limited as a result of complement activity, and, therefore, DCs cannot be properly activated to stimulate antitumor T cell responses.
T cells
The infiltration of human tumors by CD8+ T cells is an important prognostic factor in several malignancies and perhaps the most important predictive biomarker for success of immunotherapy targeting T-cell checkpoints [78]. The cytotoxic CD8+ T cells appear to be the most efficient immune effector, which is capable of recognizing and destroying tumor cells, when immunosuppressive mechanisms operating in tumors are alleviated [4]. Tumors lacking infiltrating CD8+ T cells are unlikely to respond to the currently-approved immunotherapies [11]. Therefore, it is perhaps of the highest priority to discover strategies that will “bring” T cells to tumors.
In regard to these efforts, a pharmacological blockade of C5aR1 significantly increased infiltration of tumors by CD8+ T cells in a syngeneic HPV-induced cancer model [96]. The significance of these T cells for controlling tumor growth was demonstrated by a positive correlation between the number of these cells and the tumor volume. Furthermore, depletion of these cells by neutralizing antibody entirely eliminated the beneficial effect of C5aR1-deficiency on tumor growth [96]. A similar effect was observed in a Her2/neu-induced transgenic model of breast cancer [103]. The pharmacological blockade of C5aR1 increased infiltration of tumors by tumor specific CD8+ T cells that expressed high levels of perforin, critical for these cells’ cytotoxic activity, and produced high amounts of IFN-γ [103]. These results were obtained in a model, in which mouse T cells are tolerant to rat Her2/neu, which is a self-antigen in this mouse. Thus, this model is suitable for testing a role of tolerance in antitumor immunity [144]. The C5aR1-controlled inhibition of the lung infiltration by CD8+ T cells was also demonstrated to be important for facilitating lung metastasis in a model of metastatic breast cancer [8], and, similar to primary tumors, the lack of C5aR1 improved antitumor function of T cells infiltrating the lungs, as demonstrated by increased IFN-γ production in these cells [8]. In a model of ovarian carcinoma (ID8-VEGF), inhibiting synthesis of C3 in tumor cells increased up to ten-fold infiltration of tumors by cytotoxic T cells, however, in contrast to other studies, these T cells appeared not to be critical for controlling tumor growth [83].
A recent study demonstrated the significant impact of C3aR and C5aR1 signaling on the IL-10-mediated antitumor properties of tumor infiltrating CD8+ T cells in melanoma (B16) and breast cancer (E0771) models [80]. According to this work, autocrine-signaling by C3 produced in CD8+ T cells inhibits IL-10 expression, an essential cytokine for the cytotoxic properties of these cells. The mechanisms regulating IL-10 seem to be associated with C3aR and C5aR1 signaling in CD8+ T cells. Despite the well-established immunosuppressive properties of IL-10 in the tumor microenvironment [145], there are early studies suggesting a role for this cytokine in T cell expansion and enhancing the cytolytic activity of CD8+ T cells [146]. In a recent study, it was suggested that the pleotropic effects of IL-10 on antitumor immunity are context/cell-dependent. It appears that IL-10 produced by tumor-infiltrating lymphocytes through autocrine regulation of these cells is mandatory for maintaining their antitumor properties [80].
CD4+ T cells have more complex functions in shaping antitumor immunity than CD8+ T cells. CD4+ can be divided into several subsets, including the Th1 and Th2 lineages that have different functions in antitumor immunity. Th1 cells, which secrete IFN-γ, are known to have antitumor properties, whereas IL-4 secreting Th2 cells have immunoregulatory and protumor properties. Therefore, the ratio of Th1/Th2 cells is a prognostic factor that correlates with tumor grade and stage in some malignancies [4]. The impact of complement on T cell polarization (Th1 vs. Th2) was demonstrated in two models of breast cancer [8, 103]. In both studies, the effect on T cell fate was dependent on C5aR1 signaling in MDSC. The mechanisms behind this effect involved regulation of the cytokine milieu in the lungs [8] and tumor-draining lymph nodes [103]. The protumor effect of the C3a-C3aR signaling axis has also been demonstrated in mouse lung cancer models. Kwak and colleagues showed that C3a, via C3aR expressed on the surface of CD4+T cells, down modulated their antitumor responses [147].
Endothelial cells
The paradigm shifting hypothesis by Judah Folkman that all tumors are angiogenesis-dependent [148] was perhaps one of the most important concepts emphasizing the high-dependence of, seemingly autonomous, malignancies on the host microenvironment. It is currently broadly accepted that angiogenesis and proliferating endothelial cells are hallmarks of cancer [149]. The role of complement in tumor associated angiogenesis has not yet thoroughly been investigated. However, indirect evidence supporting the possible impact of complement on regulation of angiogenesis in tumors exists. MDSC and TAMs, which are recruited to tumors through C5aR1 and C3aR signaling, secrete a multitude of proangiogenic factors [4]. Therefore, the complement anaphylatoxins may indirectly contribute to angiogenesis via regulating these components in the tumor microenvironment. In fact, a study in a transgenic model of ovarian carcinoma demonstrated that complement C3 and C5aR1 deficiencies were associated with impaired development of poorly vascularized tumors [97]. In addition, complement is implicated in angiogenesis in disease states and models other than cancer [150, 151].
Importantly, complement and, in particular, complement anaphylatoxins are potent inflammatory mediators responsible for orchestrating changes in vascular permeability and leukocyte extravasation during acute inflammation [14]. Increased vascular permeability is typical for tumor vasculature and likely facilitates both metastasis and infiltration of leukocytes into the tumor. Therefore, the contributions of complement to regulating vascular permeability in tumors are predictable. Although increased vascular permeability in tumors may be perceived as a factor facilitating metastasis and infiltration of the tumor by tumor-promoting leukocytes, increasing vascular permeability for infiltrating T cells may have beneficial therapeutic effects in the context of adoptive T cell therapies. Recent work by Facciabene and colleagues supports this notion, as they identified C5a/C5aR1 signaling as an important contributor to increased vascular permeability and homing of adoptively transfer T cells to tumors. Genetic deletion of C3 or C5aR1, and pharmacological blockade of C5aR1, impaired the ability of adoptively transfer T cells to cross the endothelial barrier, infiltrate tumors, and control tumor progression in vivo. Further, genetic chimera mice demonstrated that C3 and C5aR1 expression in the endothelium, but not in leukocytes, governs T cell homing [152].
Novel complement ligands at the interface between tumor and host cells
C5aR1 is the most studied complement receptor in the context of complement contributions to tumor growth. There are several papers convincingly demonstrating tumor promoting functions of this receptor either through signaling in tumor cells or regulating the immunosuppressive tumor microenvironment [6]. All these studies link functions of C5aR1 to its well-established ligand C5a, however, in addition to C5a, other proteins interact with C5aR1. The ribosomal protein S19 (RPS19) is the only additional endogenous ligand of C5aR1 identified in eukaryotes [153, 154]. Although RPS19 is an intracellular protein involved in ribosome biogenesis, it also has extracellular functions. RPS19, released from apoptotic cells, acts as a chemoattractant for leukocytes during inflammation through its interaction with C5aR1 [155]. It selectively enhances recruitment of monocytes that give rise to macrophages, involved in clearance of dying cells, and, simultaneously, halts migration of neutrophils to sites of inflammation [156]. Because cell death resulting from hypoxia is present in virtually all malignancies, even during the early stages of tumor growth [157], RPS19 is likely to be present outside cells in tumors and interact the ubiquitously-expressed C5aR1. Indeed, this notion was confirmed by a recent work demonstrating upregulation of RPS19 in human breast and ovarian cancer cell lines and presence of large quantities of extracellular RPS19 in malignant tissue from breast cancer patients and in murine models of breast cancer [103]. RPS19, released from apoptotic tumor cells, interacted with C5aR1 within the tumor microenvironment in both human and mice and competed for binding with C5a in vitro. RPS19 appeared to be critical for recruitment of MDSC to tumors and reducing tumor infiltrating CD8+ T cells. In addition, RPS19 regulated the production of TGF-β1 and IL-10 in myeloid cells within tumor-draining lymph nodes, promoting generation of Tregs and Th2 effectors. Reducing RPS19 in tumor cells, through a shRNA approach or blocking the C5aR1-RPS19 interaction, decreased RPS19-mediated immunosuppression, impaired tumor growth, and delayed development of tumors in a transgenic model of breast cancer [103]. These findings identified RPS19 as a novel player in tumor-associated immunoregulation, which acts at the interface between tumor and immune cells. The delayed development of tumors in a transgenic model of breast cancer, resulting from interruption of RPS19-C5aR1 signaling [103], suggests contributions of this regulatory axis to immune escape that occurs in a preclinical phase of tumor growth. Thus, the findings point to these mechanisms as early events initiating immunosuppression. Importantly, this work demonstrates that engagement of complement receptors for tumor progression does not require complement activation, further emphasizing high redundancy of mechanisms fueling tumor growth. This recent work provides initial preclinical evidence for targeting RPS19 for anticancer therapy enhancing antitumor T cell responses.
Complement in regulating metastasis
In the last decade, research on the contribution of complement to cancer development and progression focused on immunoregulation or tumor cell signaling in primary tumor sites. However, recent developments indicate a significant role of complement in controlling cancer metastasis, specifically, the premetastatic niche [8, 9]. This niche can be defined as a set of alterations that occur in metastasis-targeted organs, preceding arrival of tumor cells and facilitating seeding of these organs by tumor cells [79]. Although this concept was introduced more than a century ago by Stephen Paget, experimental evidence supporting this theory was lacking, until seminal papers by Hiratsuka and colleagues [158] and Kaplan and colleagues [159]. They demonstrated contributions of VEGFR1 and bone marrow derived cells to this step of metastatic progression. Less than a decade later MDSC, known for their immunoregulatory roles in primary tumors, were implicated in the premetastatic niche [160, 161]. Surprisingly, in these initial studies, their functions in the premetastatic niche were not firmly linked to their immunoregulatory potential.
Since the C5aR1 contributions to recruitment of MDSC to primary tumors were firmly established [76, 96], it was hypothesized that similar mechanisms operate in the premetastatic niches, and C5aR1 expressed in MDSCs provide the signaling necessary to attract these cells to different metastasis-targeted organs, prior to arrival of tumor cells. This hypothesis was confirmed by studies utilizing a syngeneic mouse model of metastatic breast cancer [8]. C5aR1-deficient or wild-type mice treated with a specific C5aR1 antagonist (PMX-53) had less metastases in their lungs and livers comparing to control mice. It appeared that C5aR1 regulated specifically metastatic process in this model because C5aR1-deficiency did not impact growth of primary breast tumors, thus, differences in metastasis were likely independent from processes occurring in a primary site. The differences in metastatic burden correlated with infiltration of the lungs and livers by MDSC. These MDSC were likely to be attracted to these premetastatic sites by C5a because circulating blood MDSC expressed C5aR1 and complement activation, demonstrated by deposition of C3 cleavage fragments, was observed in the premetastatic niche. In accordance with the mouse model, C3 cleavage fragments and MDSC were found in tumor-draining lymph nodes from breast cancer patients and C3 fragments’ deposition and local C3 production appeared to be increased in lymph nodes with metastases [8]. The reduced metastatic burden in the absence of C5aR1 signaling was caused by improved antitumor immunity, as demonstrated by increased infiltration of the lungs by CD8+ and CD4+ T cells, higher number of these T cell subsets in peripheral blood, skewed toward Th1 response CD4+ T cell polarization, and reduced Tregs in blood and the lungs. Both T cell subsets (CD4 and CD8) retrieved from the lungs also produced higher amounts of IFN-γ in C5aR1-deficient mice. The depletion of cytotoxic CD8+ T cells entirely wiped out the beneficial effect of C5aR1-deficiency on metastasis, confirming antitumor immunity as the primary mechanism protecting against metastases in the absence of C5aR1 signaling, and pointing to immunoregulatory roles of MDSC in the premetastatic niche [8].
C3 was also shown to play roles in cancer metastasis. Boire and colleagues showed that leptomeningeal metastases synthesize and secrete C3 [86]. C3a via C3aR ‘permeabilized’ the choroidal blood cerebrospinal fluid barrier facilitating crossing this barrier by tumor cells [86].
In contrast to MDSC and TAMs, functions of tissue resident macrophages, including pulmonary alveolar macrophages, in cancer remain unclear, with conflicting results suggesting both tumor-promoting vs. antitumor properties of these cells [162]. Recent studies demonstrate that these cells have significantly different biology from other macrophages. Therefore, their role in cancer should be revisited. In contrast to inflammatory macrophages and TAMs, which are recruited from bone marrow to inflammatory sites or tumors, respectively, tissue resident macrophages populate different organs during embryonic development, independently from hematopoiesis, and self-renew thereafter [163]. Therefore, pulmonary alveolar macrophages that have well-established immunoregulatory roles in healthy lungs [164] appear to be well-positioned to contribute to preconditioning this organ for metastasis through inhibition of antitumor immunity. The contribution of these cells to the premetastatic niche was established by recent work in a mouse model of metastatic breast cancer [9]. Alveolar macrophages were shown to accumulate in premetastatic lungs through C5aR1-mediated proliferation but not through recruitment from blood. In tumor-bearing mice, they inhibited Th1 and favored Th2 responses. In addition, alveolar macrophages reduced the number and antigen-presenting capacity of lung cDCs by regulating TGF-β1. The specific depletion of alveolar macrophages by clodronate-liposomes, administered to the airways, reversed immunosuppression and reduced lung metastatic burden. Depleting these cells synergized with C5aR1 inhibition leading to complete protection of the lungs from metastasis [9]. This work establishes C5aR1-regulated alveolar macrophages as important contributors to the lung premetastatic niche and offers a plausible explanation of lung susceptibility to hematogenous metastasis.
Perspectives for therapy
The recent successful introduction of immunotherapies targeting T cell checkpoints to clinical practice indicates that treatments targeting immunoregulatory mechanisms are, perhaps, the most promising approaches currently available for cancer patients. The discussion of studies on a role of complement in cancer, included in this review, clearly places complement in the midst of the tumor microenvironment as an important contributor to immunoregulatory mechanisms operating in primary tumors and metastasis-targeted organs. Therefore, complement is a potential target for immunotherapies that aim to enable tumor infiltrating T cells to kill tumor cells in primary and metastatic sites and targeting complement may help to overcome immune exclusion in non-T cell inflamed tumors. However, therapeutic efficacy of inhibiting complement pathways as a monotherapy appears to be modest comparing to other therapeutic interventions currently in preclinical or clinical development. Therefore, the combination of complement inhibition with other therapeutic interventions is more likely to bring substantial benefits for cancer patients.
The limited or lack of response to T cell checkpoint inhibitors, in some patients, and some type of cancers, has encouraged efforts to improve efficacy of this approach by combining T cell checkpoint inhibitors with other therapies, in particular those targeting additional immunoregulatory pathways. With its diverse immunoregulatory functions, complement seems to be a promising target for this strategy. Recent work has demonstrated the feasibility of this approach in preclinical models of lung carcinoma [10]. C5aR1 blockade and PD-1 inhibition were shown to synergize in reducing tumor growth, metastasis and improving antitumor immunity [10].
Several complement components were shown to promote tumor growth through different mechanisms and many of these complement fragments/effectors are generated through activation of the complement cascade and interact with their reciprocal receptor on tumor, immunoregulatory, or T cells. Therefore, inhibiting complement activation upstream of generation of complement effectors may appear to be reasonable approach, which will shut down several immunoregulatory or protumor pathways simultaneously. However, identification of novel ligands for complement receptors [103] and generation of complement effectors through extrinsic mechanisms [14, 99], not involving the traditional complement activation pathways, makes this strategy problematic. Perhaps, specific targeting of complement receptors through already existing inhibitors may turn out to be a more efficient and safe therapeutic approach.
Interestingly, targeting of the complement regulator CD46 for cancer immunotherapy has recently been explored. Sherbenou and colleagues tested anti-CD46 drug conjugates in a mouse xenograft model of multiple myeloma. CD46 antibody killed tumor cells via apoptosis, while sparing benign cells [165].
Targeting cellular metabolism has already been explored for cancer immunotherapy. For example, metabolic reprogramming of chimeric antigenic receptor (CAR) transduced T cells improves their effector function [166]. Given the role of complement in regulating T cell metabolism, targeting intracellular complement activation pathways to modulate antitumor T cell responses is an interesting option for future therapeutic strategies. It is important to note that many mouse studies report the immunosuppressive effect of C3 on tumor infiltrating T cells, which is not necessarily the case for patients, given the differences between the mouse and human complement systems.
In summary, recent studies demonstrated that functions of complement in the tumor microenvironment are cell and context-dependent and not necessarily harmful [80, 152]. Based on these studies, C3aR and C5aR1 signaling in myeloid-origin and T cells enhances immunosuppression in mice. However, in the context of adoptive T cell therapies, C5aR1 on endothelial cells seems to be pivotal for homing of T cells to tumors. Therefore, although the net effect of inhibiting complement is beneficial in terms of limiting tumor growth, metastasis, and improving antitumor immunity, specific targeting of complement receptors on myeloid and lymphoid cells but not on endothelium may offer more benefits for cancer patients. Further studies, utilizing cell-specific knockouts are perhaps required to support this notion. However, as useful as mouse cancer models are, one should be cautious when translating the relevance of mouse data to human disease. For example, mice lack CD46 which has been shown to be a key regulator of human CD4+ T cells. This picture is even further complicated by the importance of complement-mediated cytotoxicity (CDC) as a mechanism contributing to killing of tumor cells by therapeutic monoclonal antibodies that were proven to be efficient for some malignancies [167]. When these therapeutics antibodies are used, inhibiting complement activation, which appears to be useful in limiting complement-mediated immunosuppression, may actually reduce killing of tumor cells by these antibodies because less MAC will be formed due to this inhibition. In the context of therapies utilizing tumor cell-killing monoclonal antibodies, enhancing complement activity and neutralizing CRPs appears to be the optimal strategy [168]. These contributions of complement to killing of tumor cells by therapeutic antibodies have been extensively discussed in several recent review articles [6, 7] therefore, here, we limited our discussion on this topic.
This review presents a complex and multifaceted role of complement proteins in immune regulation and cancer. Many factors must be considered for designing new therapies, including the differences between the mouse and human complement system. In addition, different malignancies are perhaps differentially dependent on complement-mediated immune regulation. The discovery of intracellular complement activation and its intimate relation with metabolism in CD4+ T cells adds to complexity of complement-mediated regulation of cancer, as it is well known that tumors and activated T cells share similar metabolic traits [72]. It is for future studies to determine if complement plays a role in metabolism of cancer cells and if this relation can be therapeutically exploited. However, since C3 cleavage by CTSL has been already reported [74], this doesn’t seem too far-fetched.
Acknowledgments
This work was supported by the National Institute of Health (the grant R01CA190209 to M.M.M.) and Laura Bush Institute for Women’s Health (the seed grant to M.M.M.). We thank the Development Corporation of Abilene for continues financial support and Drs. Laurence Wood and Dawn Kochanek for critically revising this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boon T, Gajewski TF, Coulie PG. From defined human tumor antigens to effective immunization? Immunol Today. 1995;16(7):334–6. doi: 10.1016/0167-5699(95)80149-9. [DOI] [PubMed] [Google Scholar]
- 2.Woo SR, Corrales L, Gajewski TF. Innate immune recognition of cancer. Annu Rev Immunol. 2015;33:445–74. doi: 10.1146/annurev-immunol-032414-112043. [DOI] [PubMed] [Google Scholar]
- 3.Wang X, et al. Autoantibody signatures in prostate cancer. N Engl J Med. 2005;353(12):1224–35. doi: 10.1056/NEJMoa051931. [DOI] [PubMed] [Google Scholar]
- 4.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19(11):1423–37. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farkona S, Diamandis EP, Blasutig IM. Cancer immunotherapy: the beginning of the end of cancer? BMC Med. 2016;14:73. doi: 10.1186/s12916-016-0623-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reis ES, et al. Complement in cancer: untangling an intricate relationship. Nat Rev Immunol. 2017 doi: 10.1038/nri.2017.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Afshar-Kharghan V. The role of the complement system in cancer. J Clin Invest. 2017;127(3):780–789. doi: 10.1172/JCI90962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vadrevu SK, et al. Complement c5a receptor facilitates cancer metastasis by altering T-cell responses in the metastatic niche. Cancer Res. 2014;74(13):3454–65. doi: 10.1158/0008-5472.CAN-14-0157. [DOI] [PubMed] [Google Scholar]
- 9.Sharma SK, et al. Pulmonary alveolar macrophages contribute to the premetastatic niche by suppressing antitumor T cell responses in the lungs. J Immunol. 2015;194(11):5529–38. doi: 10.4049/jimmunol.1403215. [DOI] [PubMed] [Google Scholar]
- 10.Ajona D, et al. A Combined PD-1/C5a Blockade Synergistically Protects Against Lung Cancer Growth and Metastasis. Cancer Discov. 2017 doi: 10.1158/2159-8290.CD-16-1184. [DOI] [PubMed] [Google Scholar]
- 11.Gajewski TF. The Next Hurdle in Cancer Immunotherapy: Overcoming the Non-T-Cell-Inflamed Tumor Microenvironment. Semin Oncol. 2015;42(4):663–71. doi: 10.1053/j.seminoncol.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ricklin D, et al. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11(9):785–97. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Truedsson L, Bengtsson AA, Sturfelt G. Complement deficiencies and systemic lupus erythematosus. Autoimmunity. 2007;40(8):560–6. doi: 10.1080/08916930701510673. [DOI] [PubMed] [Google Scholar]
- 14.Markiewski MM, Lambris JD. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am J Pathol. 2007;171(3):715–27. doi: 10.2353/ajpath.2007.070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carroll MC. The complement system in regulation of adaptive immunity. Nat Immunol. 2004;5(10):981–6. doi: 10.1038/ni1113. [DOI] [PubMed] [Google Scholar]
- 16.Kolev M, Friec GL, Kemper C. Complement - tapping into new sites and effector systems. Nat Rev Immunol. 2014;14(12):811–20. doi: 10.1038/nri3761. [DOI] [PubMed] [Google Scholar]
- 17.Kolev M, Kemper C. Keeping It All Going-Complement Meets Metabolism. Front Immunol. 2017;8:1. doi: 10.3389/fimmu.2017.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naughton MA, et al. Extrahepatic secreted complement C3 contributes to circulating C3 levels in humans. J Immunol. 1996;156(8):3051–6. [PubMed] [Google Scholar]
- 19.Tang S, et al. Contribution of renal secreted complement C3 to the circulating pool in humans. J Immunol. 1999;162(7):4336–41. [PubMed] [Google Scholar]
- 20.White RT, et al. Human adipsin is identical to complement factor D and is expressed at high levels in adipose tissue. J Biol Chem. 1992;267(13):9210–3. [PubMed] [Google Scholar]
- 21.Morgan B, Gasque P. Extrahepatic complement biosynthesis: where, when and why? Clin Exp Immunol. 1997;107(1):1–7. doi: 10.1046/j.1365-2249.1997.d01-890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gadjeva M, et al. Macrophage-derived complement component C4 can restore humoral immunity in C4-deficient mice. J Immunol. 2002;169(10):5489–95. doi: 10.4049/jimmunol.169.10.5489. [DOI] [PubMed] [Google Scholar]
- 23.Rodríguez de Córdoba S, et al. Lessons from functional and structural analyses of disease-associated genetic variants in the complement alternative pathway. Biochim Biophys Acta. 2011;1812(1):12–22. doi: 10.1016/j.bbadis.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Bajic G, et al. Complement activation, regulation, and molecular basis for complement-related diseases. EMBO J. 2015;34(22):2735–57. doi: 10.15252/embj.201591881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hein E, Garred P. The Lectin Pathway of Complement and Biocompatibility. Adv Exp Med Biol. 2015;865:77–92. doi: 10.1007/978-3-319-18603-0_5. [DOI] [PubMed] [Google Scholar]
- 26.Merle NS, et al. Complement System Part I - Molecular Mechanisms of Activation and Regulation. Front Immunol. 2015;6:262. doi: 10.3389/fimmu.2015.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elvington M, Liszewski MK, Atkinson JP. Evolution of the complement system: from defense of the single cell to guardian of the intravascular space. Immunol Rev. 2016;274(1):9–15. doi: 10.1111/imr.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lukacsi S, et al. The role of CR3 (CD11b/CD18) and CR4 (CD11c/CD18) in complement-mediated phagocytosis and podosome formation by human phagocytes. Immunol Lett. 2017;189:64–72. doi: 10.1016/j.imlet.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 29.Towner LD, et al. Complement Membrane Attack and Tumorigenesis: A SYSTEMS BIOLOGY APPROACH. J Biol Chem. 2016;291(29):14927–38. doi: 10.1074/jbc.M115.708446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zipfel P, Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol. 2009;9(10):729–40. doi: 10.1038/nri2620. [DOI] [PubMed] [Google Scholar]
- 31.Stöve S, et al. Circulating complement proteins in patients with sepsis or systemic inflammatory response syndrome. Clin Diagn Lab Immunol. 1996;3(2):175–83. doi: 10.1128/cdli.3.2.175-183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Machalińska A, et al. Elevated plasma levels of C3a complement compound in the exudative form of age-related macular degeneration. Ophthalmic Res. 2009;42(1):54–9. doi: 10.1159/000219686. [DOI] [PubMed] [Google Scholar]
- 33.Hartmann K, et al. C3a and C5a stimulate chemotaxis of human mast cells. Blood. 1997;89(8):2863–70. [PubMed] [Google Scholar]
- 34.Matthews KW, Mueller-Ortiz SL, Wetsel RA. Carboxypeptidase N: a pleiotropic regulator of inflammation. Mol Immunol. 2004;40(11):785–93. doi: 10.1016/j.molimm.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 35.Bajic G, et al. Human C3a and C3a desArg anaphylatoxins have conserved structures, in contrast to C5a and C5a desArg. Protein Sci. 2013;22(2):204–12. doi: 10.1002/pro.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cui W, et al. Acylation-stimulating protein/C5L2-neutralizing antibodies alter triglyceride metabolism in vitro and in vivo. Am J Physiol Endocrinol Metab. 2007;293(6):E1482–91. doi: 10.1152/ajpendo.00565.2006. [DOI] [PubMed] [Google Scholar]
- 37.Paglialunga S, et al. Acylation-stimulating protein deficiency and altered adipose tissue in alternative complement pathway knockout mice. Am J Physiol Endocrinol Metab. 2008;294(3):E521–9. doi: 10.1152/ajpendo.00590.2007. [DOI] [PubMed] [Google Scholar]
- 38.Roy C, et al. Shift in metabolic fuel in acylation-stimulating protein-deficient mice following a high-fat diet. Am J Physiol Endocrinol Metab. 2008;294(6):E1051–9. doi: 10.1152/ajpendo.00689.2007. [DOI] [PubMed] [Google Scholar]
- 39.Kemper C, Atkinson JP. T-cell regulation: with complements from innate immunity. Nat Rev Immunol. 2007;7(1):9–18. doi: 10.1038/nri1994. [DOI] [PubMed] [Google Scholar]
- 40.Laumonnier Y, Karsten CM, Köhl J. Novel insights into the expression pattern of anaphylatoxin receptors in mice and men. Mol Immunol. 2017;89:44–58. doi: 10.1016/j.molimm.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 41.Couzi L, et al. Inherited deficiency of membrane cofactor protein expression and varying manifestations of recurrent atypical hemolytic uremic syndrome in a sibling pair. Am J Kidney Dis. 2008;52(2):e5–9. doi: 10.1053/j.ajkd.2008.02.359. [DOI] [PubMed] [Google Scholar]
- 42.Okura Y, et al. Clinical characteristics and genotype-phenotype correlations in C3 deficiency. J Allergy Clin Immunol. 2016;137(2):640–644.e1. doi: 10.1016/j.jaci.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 43.Abdulahad DA, et al. HMGB1 in systemic lupus Erythematosus: Its role in cutaneous lesions development. Autoimmun Rev. 2010;9(10):661–5. doi: 10.1016/j.autrev.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 44.Mayilyan KR. Complement genetics, deficiencies, and disease associations. Protein Cell. 2012;3(7):487–96. doi: 10.1007/s13238-012-2924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clarke EV, et al. Complement protein C1q bound to apoptotic cells suppresses human macrophage and dendritic cell-mediated Th17 and Th1 T cell subset proliferation. J Leukoc Biol. 2015;97(1):147–60. doi: 10.1189/jlb.3A0614-278R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaczorowski DJ, et al. Mammalian DNA is an endogenous danger signal that stimulates local synthesis and release of complement factor B. Mol Med. 2012;18:851–60. doi: 10.2119/molmed.2012.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dempsey PW, et al. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science. 1996;271(5247):348–50. doi: 10.1126/science.271.5247.348. [DOI] [PubMed] [Google Scholar]
- 48.Carroll MC, Isenman DE. Regulation of humoral immunity by complement. Immunity. 2012;37(2):199–207. doi: 10.1016/j.immuni.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ghannam A, et al. Human C3 deficiency associated with impairments in dendritic cell differentiation, memory B cells, and regulatory T cells. J Immunol. 2008;181(7):5158–66. doi: 10.4049/jimmunol.181.7.5158. [DOI] [PubMed] [Google Scholar]
- 50.Heesters BA, et al. Endocytosis and recycling of immune complexes by follicular dendritic cells enhances B cell antigen binding and activation. Immunity. 2013;38(6):1164–75. doi: 10.1016/j.immuni.2013.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pratt JR, Basheer SA, Sacks SH. Local synthesis of complement component C3 regulates acute renal transplant rejection. Nat Med. 2002;8(6):582–7. doi: 10.1038/nm0602-582. [DOI] [PubMed] [Google Scholar]
- 52.Bialas AR, Stevens B. TGF-β signaling regulates neuronal C1q expression and developmental synaptic refinement. Nat Neurosci. 2013;16(12):1773–82. doi: 10.1038/nn.3560. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 53.Shavva VS, et al. Hepatic nuclear factor 4α positively regulates complement C3 expression and does not interfere with TNFα-mediated stimulation of C3 expression in HepG2 cells. Gene. 2013;524(2):187–92. doi: 10.1016/j.gene.2013.04.036. [DOI] [PubMed] [Google Scholar]
- 54.Grailer JJ, Bosmann M, Ward PA. Regulatory effects of C5a on IL-17A, IL-17F, and IL-23. Front Immunol. 2012;3:387. doi: 10.3389/fimmu.2012.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Le Friec G, Kohl J, Kemper C. A complement a day keeps the Fox(p3) away. Nat Immunol. 2013;14(2):110–2. doi: 10.1038/ni.2515. [DOI] [PubMed] [Google Scholar]
- 56.Strainic MG, et al. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity. 2008;28(3):425–35. doi: 10.1016/j.immuni.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pavlov V, et al. Donor deficiency of decay-accelerating factor accelerates murine T cell-mediated cardiac allograft rejection. J Immunol. 2008;181(7):4580–9. doi: 10.4049/jimmunol.181.7.4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lalli PN, et al. Locally produced C5a binds to T cell-expressed C5aR to enhance effector T-cell expansion by limiting antigen-induced apoptosis. Blood. 2008;112(5):1759–66. doi: 10.1182/blood-2008-04-151068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Strainic MG, et al. Absence of signaling into CD4(+) cells via C3aR and C5aR enables autoinductive TGF-beta1 signaling and induction of Foxp3(+) regulatory T cells. Nat Immunol. 2013;14(2):162–71. doi: 10.1038/ni.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heeger P, et al. Decay-accelerating factor modulates induction of T cell immunity. J Exp Med. 2005;201(10):1523–30. doi: 10.1084/jem.20041967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cardone J, et al. Complement regulator CD46 temporally regulates cytokine production by conventional and unconventional T cells. Nat Immunol. 2010;11(9):862–71. doi: 10.1038/ni.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Le Friec G, et al. The CD46-Jagged1 interaction is critical for human TH1 immunity. Nat Immunol. 2012;13(12):1213–21. doi: 10.1038/ni.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Asgari E, et al. C3a modulates IL-1β secretion in human monocytes by regulating ATP efflux and subsequent NLRP3 inflammasome activation. Blood. 2013;122(20):3473–81. doi: 10.1182/blood-2013-05-502229. [DOI] [PubMed] [Google Scholar]
- 64.Hsieh CC, et al. The role of complement component 3 (C3) in differentiation of myeloid-derived suppressor cells. Blood. 2013;121(10):1760–8. doi: 10.1182/blood-2012-06-440214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liszewski MK, et al. Intracellular complement activation sustains T cell homeostasis and mediates effector differentiation. Immunity. 2013;39(6):1143–57. doi: 10.1016/j.immuni.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arbore G, et al. T helper 1 immunity requires complement-driven NLRP3 inflammasome activity in CD4+T cells. Science. 2016;352(6292):aad1210. doi: 10.1126/science.aad1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tsujimura A, et al. Molecular cloning of a murine homologue of membrane cofactor protein (CD46): preferential expression in testicular germ cells. Biochem J. 1998;330(Pt 1):163–8. doi: 10.1042/bj3300163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Astier AL, et al. Alterations in CD46-mediated Tr1 regulatory T cells in patients with multiple sclerosis. J Clin Invest. 2006;116(12):3252–7. doi: 10.1172/JCI29251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ni Choileain S, et al. TCR-stimulated changes in cell surface CD46 expression generate type 1 regulatory T cells. Sci Signal. 2017;10(502) doi: 10.1126/scisignal.aah6163. [DOI] [PubMed] [Google Scholar]
- 70.Buck MD, O’Sullivan D, Pearce EL. T cell metabolism drives immunity. J Exp Med. 2015;212(9):1345–60. doi: 10.1084/jem.20151159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hess C, Kemper C. Complement-Mediated Regulation of Metabolism and Basic Cellular Processes. Immunity. 2016;45(2):240–54. doi: 10.1016/j.immuni.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Newton R, Priyadharshini B, Turka LA. Immunometabolism of regulatory T cells. Nat Immunol. 2016;17(6):618–25. doi: 10.1038/ni.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kolev M, et al. Complement Regulates Nutrient Influx and Metabolic Reprogramming during Th1 Cell Responses. Immunity. 2015;42(6):1033–47. doi: 10.1016/j.immuni.2015.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Satyam A, et al. Intracellular Activation of Complement 3 Is Responsible for Intestinal Tissue Damage during Mesenteric Ischemia. J Immunol. 2017;198(2):788–797. doi: 10.4049/jimmunol.1502287. [DOI] [PubMed] [Google Scholar]
- 75.Gminski J, et al. Immunoglobulins and complement components levels in patients with lung cancer. Rom J Intern Med. 1992;30(1):39–44. [PubMed] [Google Scholar]
- 76.Corrales L, et al. Anaphylatoxin C5a creates a favorable microenvironment for lung cancer progression. J Immunol. 2012;189(9):4674–83. doi: 10.4049/jimmunol.1201654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lubbers R, et al. Production of complement components by cells of the immune system. Clin Exp Immunol. 2017;188(2):183–194. doi: 10.1111/cei.12952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14(10):1014–22. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sceneay J, Smyth MJ, Moller A. The pre-metastatic niche: finding common ground. Cancer and Metastasis Reviews. 2013 doi: 10.1007/s10555-013-9420-1. [DOI] [PubMed] [Google Scholar]
- 80.Wang Y, et al. Autocrine Complement Inhibits IL10-Dependent T-cell-Mediated Antitumor Immunity to Promote Tumor Progression. Cancer Discov. 2016;6(9):1022–35. doi: 10.1158/2159-8290.CD-15-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ornellas P, et al. Experimental validation of the complement protein C3a down expression in the plasma of patients with squamous cell carcinoma of the penis. Urol Oncol. 2017;35(9):545.e13–545.e18. doi: 10.1016/j.urolonc.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 82.Wang H, et al. Serum peptidome profiling for the diagnosis of colorectal cancer: discovery and validation in two independent cohorts. Oncotarget. 2017;8(35):59376–59386. doi: 10.18632/oncotarget.19587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cho MS, et al. Autocrine effects of tumor-derived complement. Cell Rep. 2014;6(6):1085–95. doi: 10.1016/j.celrep.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Riihilä P, et al. Complement Component C3 and Complement Factor B Promote Growth of Cutaneous Squamous Cell Carcinoma. Am J Pathol. 2017;187(5):1186–1197. doi: 10.1016/j.ajpath.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 85.Riihilä P, et al. Complement factor I promotes progression of cutaneous squamous cell carcinoma. J Invest Dermatol. 2015;135(2):579–588. doi: 10.1038/jid.2014.376. [DOI] [PubMed] [Google Scholar]
- 86.Boire A, et al. Complement Component 3 Adapts the Cerebrospinal Fluid for Leptomeningeal Metastasis. Cell. 2017;168(6):1101–1113.e13. doi: 10.1016/j.cell.2017.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Markiewski MM, Lambris JD. Is complement good or bad for cancer patients? A new perspective on an old dilemma. Trends Immunol. 2009;30(6):286–92. doi: 10.1016/j.it.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Niculescu F, et al. Persistent complement activation on tumor cells in breast cancer. Am J Pathol. 1992;140(5):1039–43. [PMC free article] [PubMed] [Google Scholar]
- 89.Yamakawa M, et al. Protection of thyroid cancer cells by complement-regulatory factors. Cancer. 1994;73(11):2808–17. doi: 10.1002/1097-0142(19940601)73:11<2808::aid-cncr2820731125>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 90.Lucas SD, et al. Tumor-specific deposition of immunoglobulin G and complement in papillary thyroid carcinoma. Hum Pathol. 1996;27(12):1329–35. doi: 10.1016/s0046-8177(96)90346-9. [DOI] [PubMed] [Google Scholar]
- 91.Baatrup G, et al. Activity and activation of the complement system in patients being operated on for cancer of the colon. Eur J Surg. 1994;160(9):503–10. [PubMed] [Google Scholar]
- 92.Ytting H, et al. Increased activity of the mannan-binding lectin complement activation pathway in patients with colorectal cancer. Scand J Gastroenterol. 2004;39(7):674–9. doi: 10.1080/00365520410005603. [DOI] [PubMed] [Google Scholar]
- 93.Bjorge L, et al. Ascitic complement system in ovarian cancer. Br J Cancer. 2005;92(5):895–905. doi: 10.1038/sj.bjc.6602334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ajona D, et al. Investigation of complement activation product c4d as a diagnostic and prognostic biomarker for lung cancer. J Natl Cancer Inst. 2013;105(18):1385–93. doi: 10.1093/jnci/djt205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Klikovits T, et al. Circulating complement component 4d (C4d) correlates with tumor volume, chemotherapeutic response and survival in patients with malignant pleural mesothelioma. Sci Rep. 2017;7(1):16456. doi: 10.1038/s41598-017-16551-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Markiewski MM, et al. Modulation of the antitumor immune response by complement. Nat Immunol. 2008;9(11):1225–35. doi: 10.1038/ni.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nunez-Cruz S, et al. Genetic and pharmacologic inhibition of complement impairs endothelial cell function and ablates ovarian cancer neovascularization. Neoplasia. 2012;14(11):994–1004. doi: 10.1593/neo.121262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pulaski BA, Ostrand-Rosenberg S. Mouse 4T1 breast tumor model. Curr Protoc Immunol. 2001;Chapter 20(Unit 20):2. doi: 10.1002/0471142735.im2002s39. [DOI] [PubMed] [Google Scholar]
- 99.Nitta H, et al. Cancer cells release anaphylatoxin C5a from C5 by serine protease to enhance invasiveness. Oncol Rep. 2014;32(4):1715–9. doi: 10.3892/or.2014.3341. [DOI] [PubMed] [Google Scholar]
- 100.Huber-Lang M, et al. Generation of C5a in the absence of C3: a new complement activation pathway. Nat Med. 2006;12(6):682–7. doi: 10.1038/nm1419. [DOI] [PubMed] [Google Scholar]
- 101.Markiewski MM, et al. Complement and coagulation: strangers or partners in crime? Trends Immunol. 2007;28(4):184–92. doi: 10.1016/j.it.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 102.Falanga A, Marchetti M, Vignoli A. Coagulation and cancer: biological and clinical aspects. J Thromb Haemost. 2013;11(2):223–33. doi: 10.1111/jth.12075. [DOI] [PubMed] [Google Scholar]
- 103.Markiewski MM, et al. The Ribosomal Protein S19 Suppresses Antitumor Immune Responses via the Complement C5a Receptor 1. J Immunol. 2017;198(7):2989–2999. doi: 10.4049/jimmunol.1602057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mantovani A, et al. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev. 2006;25(3):315–22. doi: 10.1007/s10555-006-9001-7. [DOI] [PubMed] [Google Scholar]
- 105.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22(2):231–7. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 106.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124(2):263–6. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 107.Murray PJ, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41(1):14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hagemann T, et al. “Re-educating” tumor-associated macrophages by targeting NF-kappaB. J Exp Med. 2008;205(6):1261–8. doi: 10.1084/jem.20080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hawlisch H, et al. C5a negatively regulates toll-like receptor 4-induced immune responses. Immunity. 2005;22(4):415–26. doi: 10.1016/j.immuni.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 110.Kennedy R, Celis E. Multiple roles for CD4+ T cells in anti-tumor immune responses. Immunol Rev. 2008;222:129–44. doi: 10.1111/j.1600-065X.2008.00616.x. [DOI] [PubMed] [Google Scholar]
- 111.Benoit ME, et al. Complement protein C1q directs macrophage polarization and limits inflammasome activity during the uptake of apoptotic cells. J Immunol. 2012;188(11):5682–93. doi: 10.4049/jimmunol.1103760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bonavita E, et al. PTX3 is an extrinsic oncosuppressor regulating complement-dependent inflammation in cancer. Cell. 2015;160(4):700–14. doi: 10.1016/j.cell.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 113.Nabizadeh JA, et al. The Complement C3a Receptor Contributes to Melanoma Tumorigenesis by Inhibiting Neutrophil and CD4+ T Cell Responses. J Immunol. 2016;196(11):4783–92. doi: 10.4049/jimmunol.1600210. [DOI] [PubMed] [Google Scholar]
- 114.Almand B, et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166(1):678–89. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 115.Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nat Rev Cancer. 2013;13(10):739–52. doi: 10.1038/nrc3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12(4):253–68. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mazzoni A, et al. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J Immunol. 2002;168(2):689–95. doi: 10.4049/jimmunol.168.2.689. [DOI] [PubMed] [Google Scholar]
- 118.Gabrilovich DI, et al. Mechanism of immune dysfunction in cancer mediated by immature Gr-1+ myeloid cells. J Immunol. 2001;166(9):5398–406. doi: 10.4049/jimmunol.166.9.5398. [DOI] [PubMed] [Google Scholar]
- 119.Sinha P, et al. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179(2):977–83. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- 120.Liu C, et al. Expansion of spleen myeloid suppressor cells represses NK cell cytotoxicity in tumor-bearing host. Blood. 2007;109(10):4336–42. doi: 10.1182/blood-2006-09-046201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yang L, et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6(4):409–21. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 122.Monk PN, et al. Function, structure and therapeutic potential of complement C5a receptors. Br J Pharmacol. 2007;152(4):429–48. doi: 10.1038/sj.bjp.0707332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kusmartsev S, et al. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol. 2004;172(2):989–99. doi: 10.4049/jimmunol.172.2.989. [DOI] [PubMed] [Google Scholar]
- 124.Marx J. Cancer immunology. Cancer’s bulwark against immune attack: MDS cells. Science. 2008;319(5860):154–6. doi: 10.1126/science.319.5860.154. [DOI] [PubMed] [Google Scholar]
- 125.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11(1):7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 126.Whiteside TL, Schuler P, Schilling B. Induced and natural regulatory T cells in human cancer. Expert Opin Biol Ther. 2012;12(10):1383–97. doi: 10.1517/14712598.2012.707184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.von Boehmer H, Daniel C. Therapeutic opportunities for manipulating T(Reg) cells in autoimmunity and cancer. Nat Rev Drug Discov. 2013;12(1):51–63. doi: 10.1038/nrd3683. [DOI] [PubMed] [Google Scholar]
- 128.Bates GJ, et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24(34):5373–80. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- 129.Fu J, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132(7):2328–39. doi: 10.1053/j.gastro.2007.03.102. [DOI] [PubMed] [Google Scholar]
- 130.Frey DM, et al. High frequency of tumor-infiltrating FOXP3(+) regulatory T cells predicts improved survival in mismatch repair-proficient colorectal cancer patients. Int J Cancer. 2010;126(11):2635–43. doi: 10.1002/ijc.24989. [DOI] [PubMed] [Google Scholar]
- 131.Ostrand-Rosenberg S, et al. Cross-talk between myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells enhances tumor-induced immune suppression. Seminars in Cancer Biology. 2012;22(4):275–81. doi: 10.1016/j.semcancer.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 133.Villadangos JA, Schnorrer P. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat Rev Immunol. 2007;7(7):543–55. doi: 10.1038/nri2103. [DOI] [PubMed] [Google Scholar]
- 134.Lou Y, et al. Plasmacytoid dendritic cells synergize with myeloid dendritic cells in the induction of antigen-specific antitumor immune responses. J Immunol. 2007;178(3):1534–41. doi: 10.4049/jimmunol.178.3.1534. [DOI] [PubMed] [Google Scholar]
- 135.Liu C, et al. Plasmacytoid dendritic cells induce NK cell-dependent, tumor antigen-specific T cell cross-priming and tumor regression in mice. J Clin Invest. 2008;118(3):1165–75. doi: 10.1172/JCI33583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wei S, et al. Plasmacytoid dendritic cells induce CD8+ regulatory T cells in human ovarian carcinoma. Cancer Res. 2005;65(12):5020–6. doi: 10.1158/0008-5472.CAN-04-4043. [DOI] [PubMed] [Google Scholar]
- 137.Labidi-Galy SI, et al. Plasmacytoid dendritic cells infiltrating ovarian cancer are associated with poor prognosis. Oncoimmunology. 2012;1(3):380–382. doi: 10.4161/onci.18801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Demoulin S, et al. Tumor microenvironment converts plasmacytoid dendritic cells into immunosuppressive/tolerogenic cells: insight into the molecular mechanisms. J Leukoc Biol. 2013;93(3):343–52. doi: 10.1189/jlb.0812397. [DOI] [PubMed] [Google Scholar]
- 139.Sisirak V, et al. Impaired IFN-alpha production by plasmacytoid dendritic cells favors regulatory T-cell expansion that may contribute to breast cancer progression. Cancer Res. 2012;72(20):5188–97. doi: 10.1158/0008-5472.CAN-11-3468. [DOI] [PubMed] [Google Scholar]
- 140.Chen W, et al. The indoleamine 2,3-dioxygenase pathway is essential for human plasmacytoid dendritic cell-induced adaptive T regulatory cell generation. J Immunol. 2008;181(8):5396–404. doi: 10.4049/jimmunol.181.8.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.den Haan JM, Lehar SM, Bevan MJ. CD8(+) but not CD8(-) dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med. 2000;192(12):1685–96. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Woo SR, et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. 2014;41(5):830–42. doi: 10.1016/j.immuni.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Crow MK. Type I interferon in systemic lupus erythematosus. Curr Top Microbiol Immunol. 2007;316:359–86. doi: 10.1007/978-3-540-71329-6_17. [DOI] [PubMed] [Google Scholar]
- 144.Singh R, Paterson Y. In the FVB/N HER-2/neu transgenic mouse both peripheral and central tolerance limit the immune response targeting HER-2/neu induced by Listeria monocytogenes-based vaccines. Cancer Immunol Immunother. 2007;56(6):927–38. doi: 10.1007/s00262-006-0237-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ouyang W, et al. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 146.Chen WF, Zlotnik A. IL-10: a novel cytotoxic T cell differentiation factor. J Immunol. 1991;147(2):528–34. [PubMed] [Google Scholar]
- 147.Kwak JW, et al. Complement activation via a C3a receptor pathway alters CD4+ T lymphocytes and mediates lung cancer progression. Cancer Res. 2017 doi: 10.1158/0008-5472.CAN-17-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 149.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 150.Bossi F, et al. C1q as a unique player in angiogenesis with therapeutic implication in wound healing. Proc Natl Acad Sci U S A. 2014;111(11):4209–14. doi: 10.1073/pnas.1311968111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Langer HF, et al. Complement-mediated inhibition of neovascularization reveals a point of convergence between innate immunity and angiogenesis. Blood. 2010;116(22):4395–403. doi: 10.1182/blood-2010-01-261503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Facciabene A, et al. Local endothelial complement activation reverses endothelial quiescence, enabling t-cell homing, and tumor control during t-cell immunotherapy. Oncoimmunology. 2017;6(9):e1326442. doi: 10.1080/2162402X.2017.1326442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nishiura H, Shibuya Y, Yamamoto T. S19 ribosomal protein cross-linked dimer causes monocyte-predominant infiltration by means of molecular mimicry to complement C5a. Lab Invest. 1998;78(12):1615–23. [PubMed] [Google Scholar]
- 154.Nishiura H, et al. Monocyte chemotactic factor in rheumatoid arthritis synovial tissue. Probably a cross-linked derivative of S19 ribosomal protein. J Biol Chem. 1996;271(2):878–82. doi: 10.1074/jbc.271.2.878. [DOI] [PubMed] [Google Scholar]
- 155.Nishimura T, et al. Apoptotic cells of an epithelial cell line, AsPC-1, release monocyte chemotactic S19 ribosomal protein dimer. J Biochem. 2001;129(3):445–54. doi: 10.1093/oxfordjournals.jbchem.a002876. [DOI] [PubMed] [Google Scholar]
- 156.Yamamoto T. Roles of the ribosomal protein S19 dimer and the C5a receptor in pathophysiological functions of phagocytic leukocytes. Pathology International. 2007;57(1):1–11. doi: 10.1111/j.1440-1827.2007.02049.x. [DOI] [PubMed] [Google Scholar]
- 157.Vaupel P, Harrison L. Tumor hypoxia: causative factors, compensatory mechanisms, and cellular response. Oncologist. 2004;9(Suppl 5):4–9. doi: 10.1634/theoncologist.9-90005-4. [DOI] [PubMed] [Google Scholar]
- 158.Hiratsuka S, et al. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell. 2002;2(4):289–300. doi: 10.1016/s1535-6108(02)00153-8. [DOI] [PubMed] [Google Scholar]
- 159.Kaplan RN, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438(7069):820–7. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yan HH, et al. Gr-1+CD11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Res. 2010;70(15):6139–49. doi: 10.1158/0008-5472.CAN-10-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gao D, et al. Myeloid progenitor cells in the premetastatic lung promote metastases by inducing mesenchymal to epithelial transition. Cancer Res. 2012;72(6):1384–94. doi: 10.1158/0008-5472.CAN-11-2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Davies LC, et al. Tissue-resident macrophages. Nat Immunol. 2013;14(10):986–95. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Holt PG, et al. Regulation of immunological homeostasis in the respiratory tract. Nat Rev Immunol. 2008;8(2):142–52. doi: 10.1038/nri2236. [DOI] [PubMed] [Google Scholar]
- 165.Sherbenou DW, et al. Antibody-drug conjugate targeting CD46 eliminates multiple myeloma cells. J Clin Invest. 2016;126(12):4640–4653. doi: 10.1172/JCI85856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Beezhold K, Byersdorfer CA. Targeting immuno-metabolism to improve anti-cancer therapies. Cancer Lett. 2017 doi: 10.1016/j.canlet.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Macor P, Tedesco F. Complement as effector system in cancer immunotherapy. Immunol Lett. 2007;111(1):6–13. doi: 10.1016/j.imlet.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 168.Fishelson Z, et al. Obstacles to cancer immunotherapy: expression of membrane complement regulatory proteins (mCRPs) in tumors. Mol Immunol. 2003;40(2–4):109–23. doi: 10.1016/s0161-5890(03)00112-3. [DOI] [PubMed] [Google Scholar]
- 169.Wang H, Ricklin D, Lambris JD. Complement-activation fragment C4a mediates effector functions by binding as untethered agonist to protease-activated receptors 1 and 4. Proc Natl Acad Sci U S A. 2017;114(41):10948–10953. doi: 10.1073/pnas.1707364114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Heurich M, et al. Common polymorphisms in C3, factor B, and factor H collaborate to determine systemic complement activity and disease risk. Proc Natl Acad Sci U S A. 2011;108(21):8761–6. doi: 10.1073/pnas.1019338108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wagner C, et al. The complement receptor 1, CR1 (CD35), mediates inhibitory signals in human T-lymphocytes. Mol Immunol. 2006;43(6):643–51. doi: 10.1016/j.molimm.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 172.Carter RH, et al. Synergistic interaction between complement receptor type 2 and membrane IgM on B lymphocytes. J Immunol. 1988;141(2):457–63. [PubMed] [Google Scholar]
- 173.Fischer MB, et al. Dependence of germinal center B cells on expression of CD21/CD35 for survival. Science. 1998;280(5363):582–5. doi: 10.1126/science.280.5363.582. [DOI] [PubMed] [Google Scholar]
- 174.Barrington RA, et al. CD21/CD19 coreceptor signaling promotes B cell survival during primary immune responses. J Immunol. 2005;175(5):2859–67. doi: 10.4049/jimmunol.175.5.2859. [DOI] [PubMed] [Google Scholar]
- 175.Bajic G, et al. Structural insight on the recognition of surface-bound opsonins by the integrin I domain of complement receptor 3. Proc Natl Acad Sci U S A. 2013;110(41):16426–31. doi: 10.1073/pnas.1311261110. [DOI] [PMC free article] [PubMed] [Google Scholar]