Abstract
Cancer progression and recurrence are linked to a rare population of cancer stem cells (CSC). Here we hypothesized that interactions with the extracellular matrix drive CSC proliferation and tumor-initiating capacity and investigated the functions of scaffold protein tissue transglutaminase (TG2) in ovarian CSC. Complexes formed by TG2, fibronectin (FN), and integrin β1 were enriched in ovarian CSC and detectable in tumors. A function-inhibiting antibody against the TG2 FN-binding domain suppressed complex formation, CSC proliferation as spheroids, tumor-initiating capacity, and stemness-associated Wnt/β-catenin signaling. Disruption of the interaction between TG2 and FN also blocked spheroid formation and the response to Wnt ligands. TG2 and the Wnt receptor Frizzled 7 (Fzd7) form a complex in cancer cells and tumors, leading to Wnt pathway activation. Protein docking and peptide inhibition demonstrate that the interaction between TG2 and Fzd7 overlaps with the FN binding domain of TG2. These results support a new function of TG2 in ovarian CSC, linked to spheroid proliferation and tumor-initiating capacity and mediated through direct interactions with Fzd7. We propose this complex as a new stem cell target.
Keywords: cancer stem cells, tissue transglutaminase, Frizzled 7, fibronectin, integrin β1, β-catenin, Wnt signaling, ovarian cancer
Introduction
Ovarian cancer (OC) is the most lethal gynecological cancer (1), characterized by rapid growth, dissemination in the peritoneal space and universal development of chemo-resistance, leading to fatal tumor recurrence after primary treatment (2). The accumulation of malignant ascites in the peritoneal cavity provides a favorable tumor microenvironment (TME) enriched in secreted inflammatory cytokines (3), growth factors (4) and extracellular macromolecules (collagen, fibronectin (FN), and laminin) (5) which regulates oncogenic signaling. In this milieu, cancer cells form multicellular spheroids enriched in stem/progenitor cells. The goal of this study was to mechanistically dissect signaling pathways engaged by the extracellular matrix (ECM), which regulate ovarian cancer stem cells (OCSCs) proliferation in the peritoneal environment.
Tissue transglutaminase (TG2) is a multi-functional protein with protein crosslinking, GTPase and scaffold functions. Its activities are regulated by environmental factors and cellular localization. High intracellular GTP and low Ca2+ concentrations inhibit TG2’s enzymatic function, while in the extracellular domain, in the presence of Ca2+, the protein exerts transglutaminase activity, crosslinking glutamine rich ECM proteins. Resolution of the three-dimensional (3D) structure of TG2 provided important insight into the complex regulation of its functions (6). In addition to the catalytic triad, the protein has a FN-binding domain within its N-terminus. Previous studies identified the β hairpin loop as the FN-binding region (amino acids 88–106) (7). However, more recent analyses using hydrogen/deuterium exchange and mass spectrometry point to residues K30, R116, and H134 in the N-terminus as being critical to forming a complex with the 42 kDa gelatin-binding domain of FN (8). On the surface of cells, the complex between TG2 and FN is further supported and stabilized by direct interactions of both proteins with integrins, the major receptors involved in cellular adhesion to the ECM. Due to strong noncovalent association with both proteins, TG2 significantly enhances the interaction of cells with the matrix, serving as a bridge between integrins and FN (9). While little is known about specifics of the integrin-TG2 complexes, the complementary TG2-FN binding sites have been delineated, and disruption of this interaction appears a promising approach for interfering with cell-ECM interaction (9). Here we study the functions of this complex in regulation of OCSC-signaling and functions.
TG2 was identified as an overexpressed transcript in OC cells and tumors (10), and its aberrant expression was correlated with peritoneal dissemination (10,11) and spheroid proliferation (12). TG2 expression was induced by TGF-β in the TME via NF-κB activation and linked to the rare, but highly tumorigenic OCSC population (12). The link between TG2 and CSCs was recorded in other solid tumors. For example, TG2 upregulation was also recorded in the highly tumorigenic subpopulation of CD44+/CD24− breast CSCs characterized by self-renewal and mammosphere-forming capacity (13), in epidermal and glioblastoma CSCs, where it promoted spheroid proliferation and tumor formation (14,15). The connection between TG2 and chemotherapy or radiation-resistance in cancer cells (16) was attributed to activation of the NF-κB survival pathway (17,18) and of “outside-in” signaling (19), being consistent with the chemo-resistant phenotype of CSCs. However, the mechanism by which TG2 regulates the functions of CSCs remains unclear.
Here we show that not only TG2, but also the other members of the ECM adhesion complex (FN and integrin β1) are enriched in OCSCs and contribute to activation of the stemness-associated Wnt pathway. Disruption of this complex by a function-inhibiting antibody disrupted sphere proliferation, stem cell characteristics, and tumor initiation capacity (TIC). We demonstrated that the anti-TG2 antibody or TG2 knock down inhibited ovarian CSCs through blockade of Wnt signaling. This pathway was engaged by TG2 via direct interaction with the receptor Fzd7, which was identified here as a new TG2 partner. In all, our findings propose a new CSC target and point to a novel protein-protein interaction (PPI), which is critical to the maintenance of the CSC phenotype.
MATERIALS AND METHODS
Chemicals and reagents
Unless stated otherwise, chemicals and reagents were from Sigma-Aldrich (St Louis, MO, USA). Monoclonal FN was from BD Biosciences (San Jose, CA, USA); monoclonal TG2 (clone 4G3), integrin β1 (MAB1959, clone P5D2) and integrin β1 (MAB2251, clone B3B11) were from EMD Millipore Corporation (Temecula, CA, USA), monoclonal TG2 (CUB 7402) and polyclonal TG2 (Ab-4) were from Thermo Scientific (Fremont, CA, USA); polyclonal Fzd1 and Fzd7 antibodies were from Abcam (Cambridge, MA, USA); and GAPDH from Biodesign International (Saco, ME). Secondary HRP-conjugated antibodies were from Amersham Biosciences (San Francisco, CA) and Santa Cruz Biotechnology Inc (Santa Cruz, CA). The ALDEFLUOR kit assay was from StemCell Technologies (Vancouver, BC Canada). Lentiviral particles containing shRNA targeting Fzd7 and scrambled shRNA were purchased from Sigma-Aldrich. Recombinant human Wnt7A and 3A were purchased from R&D Systems (Minneapolis, MN). The TG2 peptide was synthesized by Biosynthesis (Lewisville, Texas, USA). Recombinant full length and mutant TG2 were expressed and purified, as previously described (20).
Cell lines
Human OC SKOV3 and HEY cell lines were from the American Type Culture Collection (Rockville, MD). OVCA432, COV362 and OVCAR5 cells were provided by Drs. Robert Bigsby and Kenneth Nephew, respectively (Indiana University). All cell lines were authenticated by Short Tandem Repeat (STR) analysis (IDEXX BioResearch, Columbia, MO), and tested to be mycoplasma negative by the Skin Tissue Engineering Core at Northwestern University. For the experimental procedures, OC cell lines were used between 5–10 passages after thawing. For cell culture method specifications see SM.
Primary human cells
De-identified malignant ascites fluid specimens from OC patients (n = 4) were obtained at the Indiana University Simon Cancer Center (IUSCC) under an IRB approved protocol (IUCRO#505). The study was conducted in accordance with the International Ethical Guidelines for Biomedical Research Involving Human Subjects, and represented minimal risk to subjects. All subjects had stage III or IV high-grade serous OC or primary peritoneal carcinomatosis. For primary cells isolation from human specimens (OC ascites and primary tumors) and culture method specifications see SM.
Sphere formation assay, ALDEFLUOR assay and Fluorescence-Activated Cell Sorting, Co-immunoprecipitation (Co-IP), Immunofluorescence (IF), Quantitative RT-PCR, Annexin V/7-AAD staining, Immunohistochemistry (IHC), Transduction, Gene Reporter Assay, and Western blot analysis were performed as previously described (11,12,21,22); for details see Supplementary Material (SM). The primers used are in Supplementary Table S1.
In vivo xenograft studies
All animal experiments were conducted following protocols approved by the IU Animal Care and Use Committee. An equal number of IgG control and 4G3 treated spheres were injected subcutaneously into the left and right flank of 7–8 week-old female athymic nude mice, respectively, with 6 mice randomly assigned to each group. At the end of the study (e.g., when at least one tumor reached 2,000 mm3), mice were euthanized, tumors were harvested, measured and weighed. For detailed information see SM.
In situ proximity ligation assay (PLA)
Interaction between TG2 and integrin β1 or Fzd7 was measured in OC paraffin-embedded tissue sections by PLA (23) using Duolink reagents (Millipore-Sigma) and following the manufacturer recommendations. De-identified human OC specimens and on a tissue microarray were obtained from Pantomics Inc. (Richmond, CA; #OVC1021, n = 93). Normal fallopian tube control was obtained from Pantomics Inc. Details are included in SM. Percentage of cells staining and intensity of the staining was graded from 0 (no staining) to 3+ (strong staining). An H score was calculated as the product between intensity and percentage of stained cells and tumors were classified as positive or negative if H score was > or < median score, respectively.
Homology Model of human Fzd7
As the full length crystal structure of Fzd7 has not yet been solved, we built a robust homology model using the Prime 3.1 software implemented in Schrodinger platform (24). For details see SM.
Statistical Analysis
The mRNA microarray (Agilent 244K Custom Gene Expression G4502A-07 and Affymetrix Human Exon 1.0 ST Array) and RNASeqv2 level 3 data for the TCGA ovarian cancer cohort were retrieved from Broad GDAC Firehose http://gdac.broadinstitute.org/. The clinical information associated with these samples was obtained from cBioPortal (http://www.cbioportal.org). Survival analysis was performed in R (version 3.2.5) (http:///www.r-project.org/) and the statistical significance was defined as p < 0.05 (see SM). One-way ANOVA and the Student’s t test were used for other comparisons between groups.
Results
OCSCs express high levels of TG2, FN1 and Integrin β1
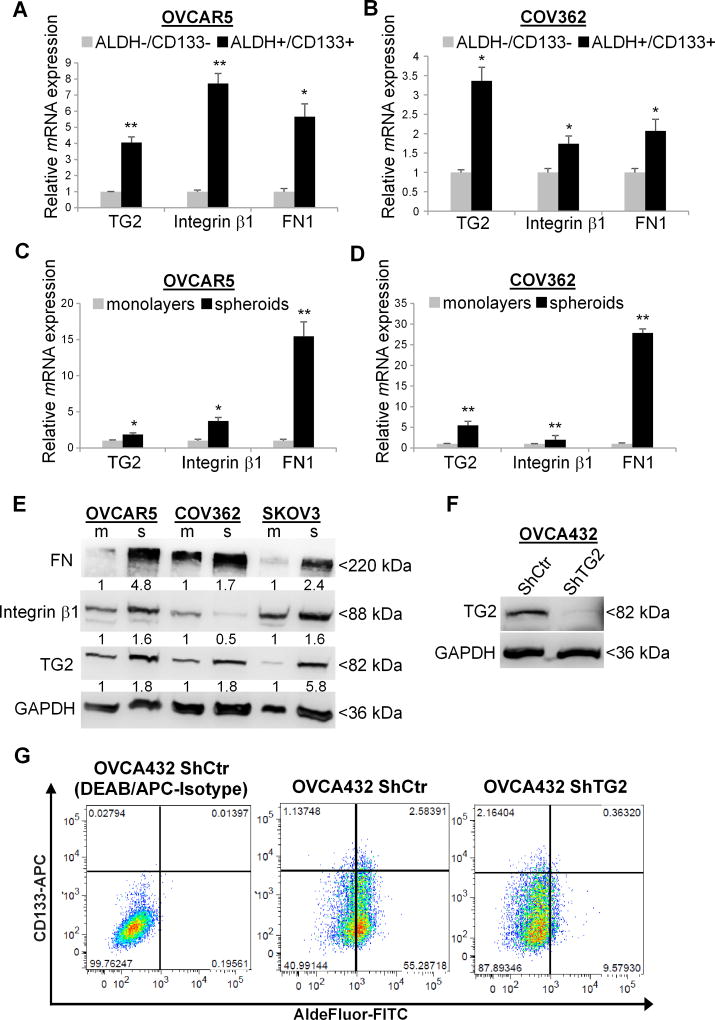
To determine TG2 expression in OCSCs, we used Q-RT-PCR and compared ALDH+/CD133+ and ALDH−/CD133− cells FACS sorted from OVCAR5 and COV362, two representative HGSOC cell lines (25,26). TG2 mRNA expression was significantly increased in OCSCs compared to non-CSC (ALDH−/CD133− Fig. 1A–B). Additionally, integrin β1 and FN expression levels were increased in CSCs vs. non-CSCs. (Fig. 1A–B). ALDH+/CD133+ were also highly enriched in CSC specific transcription factors (Sox2, Nanog, Oct-4) and ALDH1A1 expression (Supplementary Fig. S1A–S1B).
Figure 1. Ovarian CSCs express high levels of TG2, Integrin β1, and FN1.
A–B. TG2, integrin β1 (ITGB1), and FN1 mRNA levels measured by quantitative real-time PCR in ALDH+/CD133+ vs. ALDH/CD133 isolated from human ovarian cancer OVCAR5 and COV362 cells. (N ≥ 3; * P < 0.05, ** P < 0.01). C–D. Expression of TG2, integrin β1 (ITGB1), and FN1 in OVCAR5 and COV362 cells grown as monolayers or spheroids under low adherence conditions. (N ≥ 3; * P < 0.05, ** P < 0.01). E. Western blotting shows TG2, integrin β1, and FN expression levels in OVCAR5, COV362, and SKOV3 grown as monolayers (m) and spheroids (s). Densitometry quantifies TG2, integrin β1, and FN expression levels normalized for GAPDH. F. Western blot measured TG2 expression levels in OVCA432 cells stably transduced with non-targeting ShRNA vector control (Sh-Ctr) compared to pool of ShRNA targeting TG2 (Sh-TG2). G. Flow cytometry measures ALDEFLUOR-FITC+/CD133-APC+ cells in OVCA432 cells transduced with ShCtr empty vector compared with TG2 knock down (Sh-TG2). DEAB/APC-Isotype treated cells serve as negative controls. Measurements were performed in three replicates.
Given that cells growing as spheroids under non-differentiating conditions are enriched in CSCs compared to cells growing as monolayers (21,27), TG2, integrin β1 (ITGB1) and FN expression levels were also quantified in these models and found to be significantly upregulated in OC spheroids versus monolayers across several HGSOC cell lines and primary OC cells derived from malignant ascites at the mRNA (Fig. 1C–D and Supplementary Fig. S1C–S1D) and protein levels (Fig. 1E). Consistent with a stem cell enriched phenotype, expression of stemness-associated transcription factors (Oct-4, Nanog, and Sox2) and of ALDH1A1 was increased in OC spheroids vs. monolayers (Supplementary Fig. S1E–S1F).
To further establish the significance of TG2 to the CSC phenotype, we used OC cells in which TG2 was knocked down by stable transduction of a pool of shRNA or of an antisense construct targeting TG2 (11,12). Western blotting confirmed decreased TG2 protein levels in transduced OVCA432, HEY and SKOV3 cells (Fig. 1F and Supplementary Fig. 1G). The ALDH+ and the ALDH+/CD133+ cell populations were significantly decreased in all three OC cell lines (Fig. 1G and Supplementary Fig. S1H), supporting the critical role of TG2 in the maintenance of ovarian CSCs.
TG2, FN, and Integrin β1 form a complex in ovarian CSCs
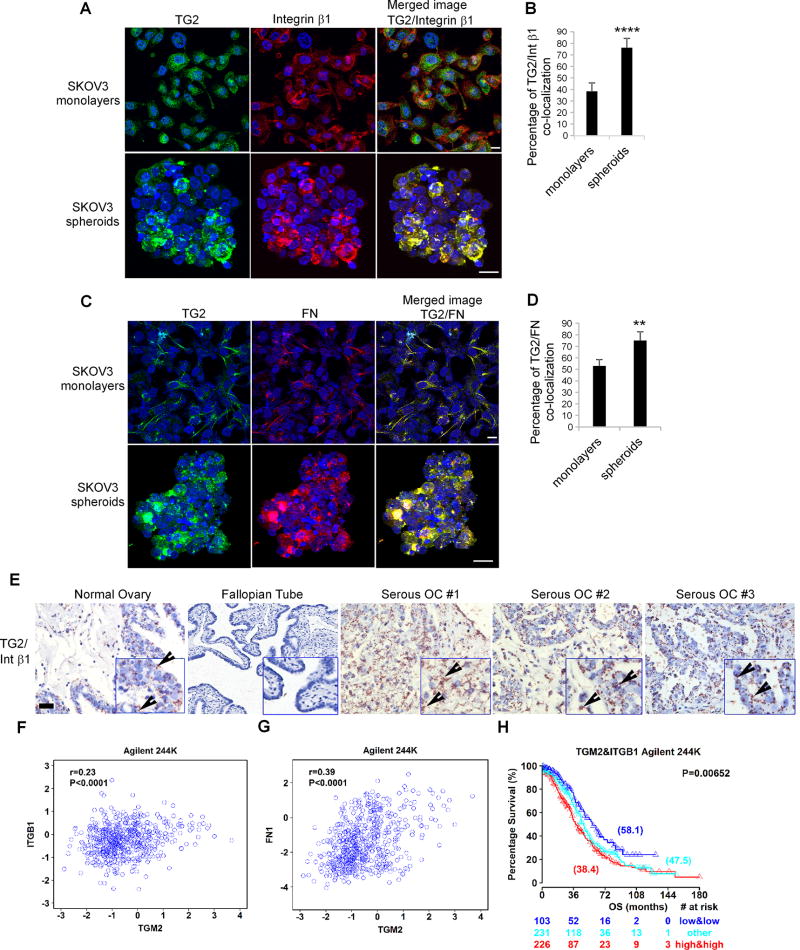
By binding to integrin β1 and FN, TG2 stabilizes a ternary complex at the plasma membrane, which propagates “outside-in” signaling (9). TG2/integrin β1 co-localization by IF staining and quantified by Metamorph was increased in CSCs-rich spheroids compared to monolayers (Fig. 2A–B, n ≥ 5; p < 0.0001). Likewise, TG2 co-localized with FN in both monolayer and spheroid cultures (Fig. 2C) and significant enrichment of TG2-FN clusters was noted in CSC-rich 3D culture systems compared to monolayers (Fig. 2D, n ≥ 5; p < 0.01). Collectively, these data support the enrichment in TG2/FN/integrin β1 complexes in spheroids and suggest a functional role in ovarian CSCs.
Figure 2. TG2/FN/Integrin β1 form a complex in OC spheroids.
A. IF staining for TG2 (Alexa Fluor 488, green) and integrin β1 (Alexa Fluor 568, red) in OC spheroids and monolayers. Protein co-localization is identified by yellow spectra on merged images. B. Quantification of co-localized proteins was calculated by volume area of green over red spectra by using Metamorph software (N ≥ 3; ****P<0.0001). C. IF staining for TG2 (Alexa Fluor 488, green) and FN (Alexa Fluor 568, red) in OC monolayers and spheroids. D. Quantification of co-localized proteins was calculated by volume area of green over red spectra (N ≥ 3; **P<0.01). E. TG2/integrin β1 co-localization detected by PLA in human ovarian tumors, normal surface ovarian and fallopian tube epithelium, included on a multi-tissue array. Representative images are shown (×200 magnification). Bar corresponds to 10µm. F–G. Correlation between TG2 and integrin β1 (ITGB1) mRNA expression levels (R=0.23; P<0.0001) and between TG2 and FN1 mRNA expression levels (R=0.39; P<0.0001) in the ovarian cancer Agilent 244K TCGA database. H. Overall survival curves generated using the Kaplan-Meier plot for tumors expressing high levels of TG2 and of ITGB1 vs. those expressing low levels of TG2 and of ITGB1 vs. all others in the Agilent 244K TCGA database (P=0.00652). The median survival time for each group is presented in brackets. The numbers of patients at risk in low, high and others mRNA expression groups at different time points are presented at the bottom of the graph.
To determine whether this PPI is detectable in human tumors, we used PLA, a technique capable of identifying proteins localized within 40 nm distance in tissue. TG2 and integrin β1 expression and co-localization was measured on a tissue multi-array including 93 ovarian tumors. Complex formation was detectable in 85 of 93 ovarian malignant tumors, of which 43 displayed intense staining, but not in normal surface ovarian or fallopian tube epithelium (n=7; Fig. 2E and Supplementary Table S2), supporting a functional interaction between the two proteins in malignant tissue in vivo.
Further, we explored the TCGA ovarian cancer database which houses gene expression data of 560 clinically annotated HGSOC tumors. TG2 expression was strongly correlated with ITGB1 (R=0.23, p<0.0001) (Fig. 2F) and with FN1 (R=0.39, p<0.0001) (Fig. 2G and Supplementary Fig. S2A–B). Furthermore, TG2 and ITGB1 were independent markers of survival (not shown) and were therefore included in a final multivariable analysis of overall survival in both Agilent 244K (Fig. 2H) and Affymetrix Human Exon 1.0 ST (Supplementary Fig. S2C), along with tumor stage. Patients with high TG2 and ITGB1 expression levels had an increased estimated risk of death when compared to those with low TG2 and ITGB1 expression levels (Fig. 2H). The difference in median survival time between the groups associated with high levels of ITGB1 and TG2 was significantly larger than the difference between the groups classified into high and low based on the expression of TG2 or ITGB1 alone. Similar results were obtained by exploring Agilent 244K (Fig. 2F–H and Supplementary Tables S3–S6). These data support the significance of TG2 at the interface with the ECM in human ovarian tumors impacting clinical outcomes.
TG2-FN blockade suppresses sphere formation and the tumor initiating capacity of ovarian CSCs
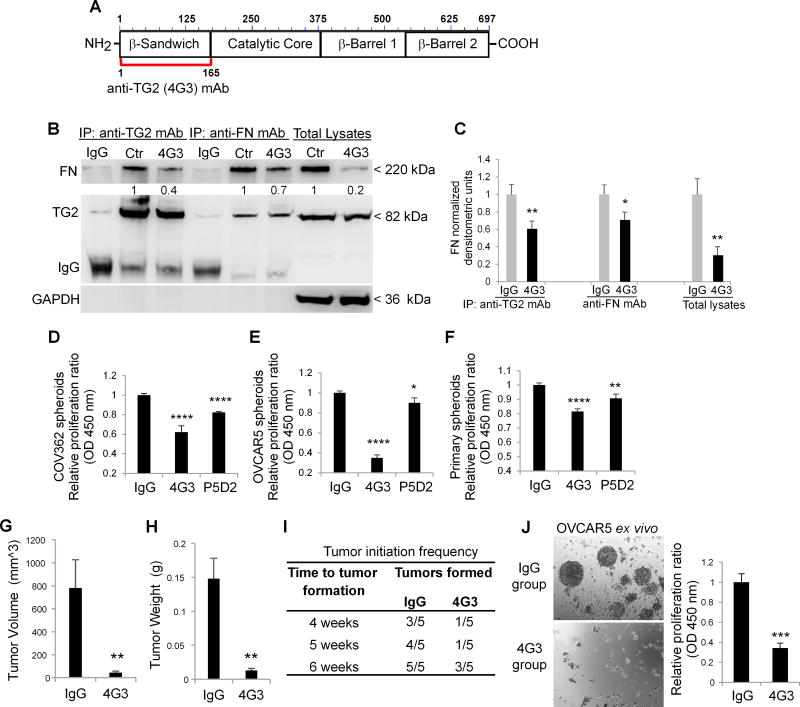
When secreted in the ECM, TG2 binds to the I6II1,2I7–9 modules forming the 42 kDa gelatin-binding domain of FN with high affinity (Kd ~8–10 nM), regulating matrix assembly (28). Hence, we used an inhibitory anti-TG2 monoclonal antibody (mAb) 4G3 against the N-terminal domain of TG2 (aa1–165) (29), responsible for FN binding (Fig. 3A). Co-IP with anti-TG2 and anti-FN antibodies demonstrated that 4G3 disrupts the TG2/FN interaction in ALDH+/CD133+ cells grown as spheres (Fig. 3B–C). In addition, the FN protein level was decreased in cell lysates from 4G3-treated compared to control cells (Fig. 3B–C).
Figure 3. TG2/FN/Integrin β1 complex regulates spheroids proliferation and tumor initiating capacity.
A. Graphical representation of the epitope targeted by the 4G3 mAb overlapping with the FN-binding domain of TG2 (amino acids 1-165). B. Co-IP with anti-TG2 and anti-FN mAbs of cell lysates from OVCAR5 spheroids treated with 4G3 (10µg/ml) for 6 days. Western blotting was performed by using anti-TG2 and FN monoclonal antibodies. C. Densitometric analysis results are shown as means ± SEM. (N= 3; *P<0.05, **P<0.01). D–F. CCK-8 assay quantifies proliferation of spheroids derived from OC cell lines and primary cells treated with inhibitory mAbs directed against the FN-binding domain of TG2 (4G3), and integrin β1 (clone P5D2) (N= 8; *P< 0.05, **P< 0.01, ****P< 0.0001). G–H. Tumor weights and volumes derived from ALDH+/CD133+ sorted from OVCAR5 cells and treated with 4G3 or IgG control and injected sq in nude mice, as described (N= 5; **P< 0.01). I. Time to tumor formation for 10,000 ALDH+/CD133+ cells pre-treated with IgG or 4G3, grown as spheroids for 6 days, and injected sq in nude mice. J. Spheroid morphology (left panel) and proliferation assay (right panel) of cells isolated from xenografts and grown ex vivo. (N= 3; ***P< 0.001).
Next, we assessed the effects of disrupting the TG2/FN/integrin β1 complex in ovarian CSCs. The anti-TG2 antibody significantly suppressed spheroids’ proliferation compared to IgG isotype control in COV362 (n=8, p<0.0001) and OVCAR5 cells (n=8, p<0.0001) (Fig. 3D–E). By comparison, the function-blocking antibody P5D2 directed against integrin β1, which had been reported to inhibit stemness in other models (30), also blocked sphere proliferation (n=8, p<0.001), but less efficiently than 4G3 (Fig. 3D–E, p<0.05). Similar results were observed in primary CSCs flow sorted from human OC ascites (Fig. 3F, n=4 specimens tested). Importantly, 4G3 did not induce apoptosis or necrosis in OVCAR5, SKOV3 and COV362 spheroids, as measured by Annexin V/7-AAD staining (Supplementary Fig. S3A). In addition, the TG2/FN and TG2/integrin β1 complexes were disrupted by 4G3, as shown by IF staining of ovarian CSCs derived from ascites specimens (Supplementary Fig. S3B–C). These data support that disruption of this complex at the interface with the ECM inhibits CSCs proliferation.
The rare population of ovarian CSCs is responsible for initiating tumor formation in vivo when injected in small numbers in nu/nu mice (31). Ten thousand ALDH+/CD133+ cells sorted from OVCAR5 cells were cultured under stem cell conditions and treated ex vivo with 4G3 or IgG isotype control for 6 days before subcutaneous inoculation into the flanks of female nu/nu mice. Tumor volumes (means ± SEM) were reduced from 781.9 ± 246.2 mm3 (control) to 45.9 ± 12.9 mm3 (4G3-treated ovarian CSCs; n= 5, p < 0.01; Fig. 3G). Tumor weights (means ± SEM) were also reduced from 0.148 ± 0.032 gms (control) to 0.013 ± 0.003 gms (4G3 treated cells, n= 5, p < 0.01) (Fig. 3H). Tumor initiation frequency was also significantly delayed and inhibited by 4G3 (Fig. 3I). To verify whether the reduced TIC was due to a depletion of CSCs maintained in vivo, cells dissociated from 4G3 and IgG pre-treated tumors were cultured ex vivo in anchorage-independent conditions. Single cells derived from 4G3 treated tumors were not able to form spheroids compared to cells derived from control tumors (n = 3, p < 0.001, Fig. 3J). Altogether, these observations demonstrate that targeting the TG2-FN-integrin β1 complex disrupts OC spheroid formation, proliferation, and TIC.
TG2-FN complex disruption blocks CSC-signaling
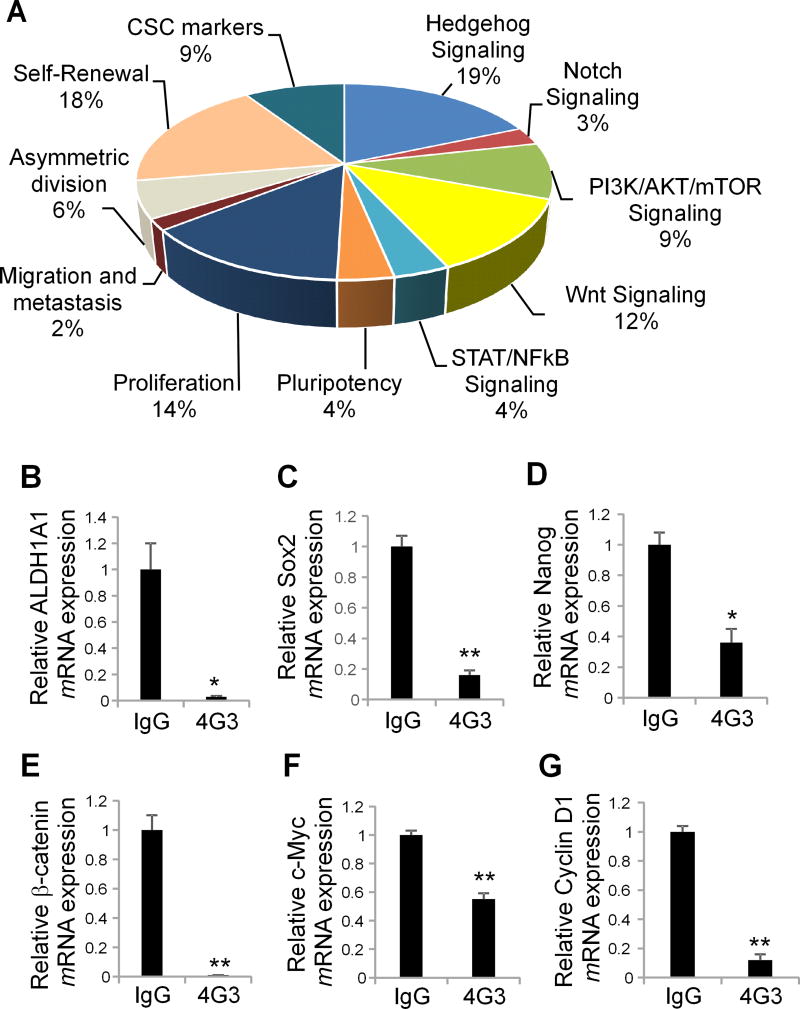
To identify key oncogenic pathways regulated by the TG2/FN complexes and linked to the stem cell phenotype, differential gene expression was evaluated by Q-RT-PCR using a human CSC-focused gene array in 4G3 and IgG treated spheres derived from ALDH+/CD133+ flow sorted OC cells. Genes related to CSC-maintenance, self-renewal, and proliferation were downregulated (p < 0.05 and fold change >2, Fig. 4A) and validated through Q-RT-PCR in 4G3-treated cells compared to controls. ALDH1A1, Nanog, Oct-4, and Sox2 mRNA expression levels were significantly decreased in 4G3-treated OVCAR5 and COV362 cells compared to controls (n ≥ 3, p < 0.05, and p < 0.01, respectively, Fig. 4B–D and Supplementary Fig. S4A). Hedgehog and Wnt signaling, two key developmental pathways also linked to cancer stemness, were among the most significantly downregulated mechanisms after TG2/FN complex disruption by 4G3 in OC spheroids. mRNA expression levels of β-catenin and of target genes c-Myc and cyclin D1 were significantly decreased in 4G3-treated OVCAR5 and COV362 cells compared to controls (n ≥ 3, p < 0.01; Fig. 4E–G and Supplementary Fig. S4B).
Figure 4. TG2/FN/Integrin β1 complex regulates β-catenin activation.
A. Expression levels of stemness associated genes in 4G3 compared to control (IgG) treated OVCAR5 cells grown as spheroids were quantified by RT2 Profiler PCR array. Pie chart analysis illustrates the fold-changes (≥2.0) of downregulated genes (% of total) for each represented pathway in control vs treated spheres. B–D. Quantitative RT-PCR for ALDH1A1, Sox2, and Nanog mRNA expression levels in 4G3 compared to control (IgG) treated OVCAR5 spheroids. E–G. Quantitative RT-PCR for β-catenin, c-Myc, and cyclin D1 in 4G3 compared to control (IgG) treated OVCAR5 spheroids (N ≥ 6; *P< 0.05, **P< 0.01).
TG2-FN complex disruption inhibits Wnt signaling
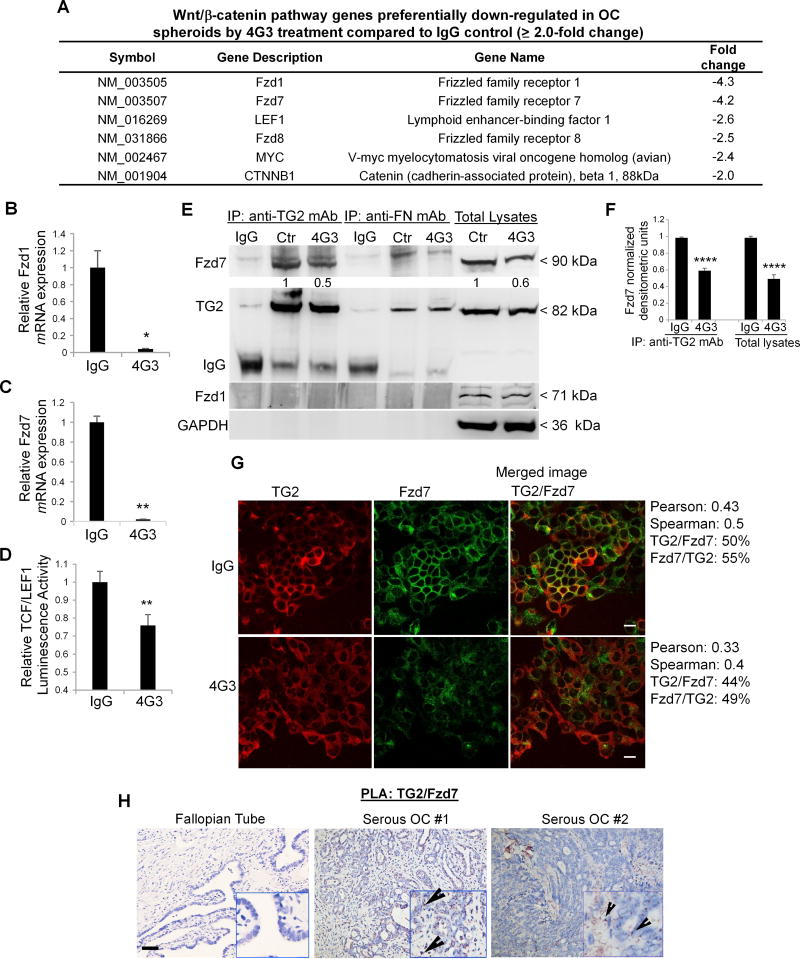
Having previously demonstrated that TG2 activates β-catenin in OC cells by altering cell adhesion to the matrix (32) and the critical role of Wnt/β-catenin in the maintenance of OC spheroids by direct transcriptional regulation of ALDH1A1 (21), we further explored the mechanism by which the TG2-FN-integrin β1 complex regulates Wnt/β-catenin and cancer stemness. By using a Q-RT-PCR array focused on Wnt/β-catenin signaling we observed an overall global downregulation of genes in this pathway in 4G3 treated spheroids compared to the IgG control group. Specifically, 32 Wnt-related transcripts, of which 24 are known positive regulators of the pathway, were downregulated > 2.0-fold (P<0.05) in 4G3 treated cells compared to controls (Fig. 5A). Reversely, Wnt inhibitors were upregulated, suggesting a role of the TG2/FN complex in fine-tuning Wnt signaling. Among the Wnt pathway elements affected by disruption of the TG2/FN complex, the Frizzled (Fzd) receptors 1 and 7 were significantly downregulated (> 4.0 fold), along with β-catenin, its co-transcriptional regulator LEF1, and the target gene c-Myc (> 2.0 fold change). Q-RT-PCR confirmed Fzd1 and Fzd7 mRNA expression level downregulation in 4G3 treated ovarian CSCs from OVCAR5 and COV362 cells compared to controls (n ≥ 3, P<0.05 and P<0.01, respectively) (Fig. 5B–C and Supplementary Fig. S5A). 4G3-mediated disruption of TG2-FN complex significantly reduced β-catenin/TCF transcriptional activity as measured by TCF/LEF1 reporter assay in OVCAR5 and COV362 cells (n ≥ 3, p < 0.01) (Fig. 5D and Supplementary Fig. S5B). This inhibition was maintained in vivo as determined by decreased β-catenin staining in xenografts derived from ovarian CSCs pre-treated with 4G3 vs. IgG (Supplementary Fig. S5C).
Figure 5. TG2 and Fzd7 form a complex in OC cells.
A. List of Wnt/β-catenin pathway genes down-regulated in OVCAR5 spheroids by 4G3 treatment compared to IgG control (≥2.0-fold change). B–C. Quantitative RT-PCR for Fzd1 and Fzd7 in OVCAR5 spheroids (N ≥ 6; *P< 0.05, **P< 0.01). D. OVCAR5 cells were co-transfected with TCF/LEF1 luciferase reporter and Renilla control plasmid, prior to treatment with 4G3 or IgG (control) and plated as spheroids. Luciferase signal relative to Renilla activity is expressed as fold increase (N ≥ 6; **P< 0.01). E. Co-IP with anti-TG2 and anti-FN mAbs of cell lysates from OVCAR5 spheroids treated with 4G3. Western blotting was performed by using anti-Fzd7, Fzd1, and GAPDH antibodies. F. Densitometric analysis results are shown as means ± SEM. (N=3; ****P<0.0001). G. IF staining for TG2 (red) and Fzd7 (green) in control and 4G3 treated OC cell lines (×400 magnification). Quantification of co-localized proteins was calculated by volume area of green over red spectra in IgG control (N ≥ 3; Spearman’s rank correlation= 0.5) vs 4G3 treated cells (N ≥ 3; Spearman’s rank correlation= 0.40). H. TG2/Fzd7 co-localization detected by PLA in human ovarian tumors included on a multi-tissue array and in normal fallopian tube epithelium. Representative images are shown (×200 magnification). Bar corresponds to 10µm.
To investigate the mechanism by which the TG2/FN complex alters Wnt signaling, we tested the possibility that components of the complex directly interact with the Fzd receptors. Fzd7, but not Fzd1, was detected in endogenous protein lysates from CSCs pulled down with an anti-TG2 antibody (Fig. 5E). The TG2-Fzd7 complex was disrupted by 4G3, suggesting that the potential area of interaction between TG2 and Fzd7 is located in the N-terminal domain of TG2 (amino acids 1–165), which is targeted by 4G3 (Fig. 5E–F). In contrast, no interaction between FN and Fzd7 or between FN and Fzd1 was observed, as measured by co-IP with an anti-FN antibody. In addition, immunoblotting of total lysates showed a downregulation of Fzd7 expression levels in 4G3 treated ovarian CSCs compared to control-treated cells, consistent with mRNA results.
IF and confocal microscopy confirmed co-localization of TG2 and Fzd7 at the plasma membrane (Spearman’s r= 0.5, Pearson r = 0.43; Fig. 5G). 4G3 induced a 10% reduction in the overlap between TG2 and Fzd7 compared to IgG (Spearman’s r= 0.4, Pearson r = 0.3). In addition, integrin β1 co-localized with Fzd7 (Spearman’s r= 0.42, Pearson r = 0.57, Supplementary Fig. S5D) and FN co-localized with Fzd7 (Spearman’s r= 0.58 and Pearson r= 0.3, Supplementary Fig. S5E). The anti-TG2 antibody 4G3 decreased both integrin β1/Fzd7 (~60% to 30%) and FN/Fzd7 co-localization (50 to 25%). PLA demonstrated TG2/Fzd7 co-localization in ovarian tumors, but not in normal fallopian tube epithelium (Fig. 5H). However, the signal was less intense compared to the co-localization signal for TG2- integrin β1 and was detectable in only 22 of 93 tumors stained, presumably due to the restricted expression of Fzd7 in ovarian tumors. These data suggest that the TG2/FN complex through its interactions with Fzd7 may act as a direct Wnt/β-catenin co-activator.
TG2 forms a complex with FZD7
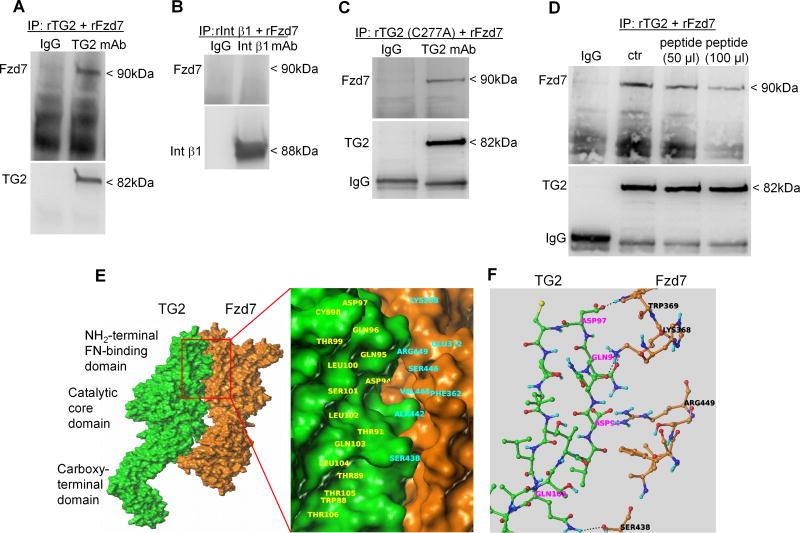
To further define the interaction between TG2 and Fzd7, we used full-length recombinant TG2, integrin β1, and Fzd7 proteins. Co-IP with anti-TG2 antibody demonstrates direct interaction between TG2 and Fzd7 (Fig. 6A). In contrast, recombinant integrin β1 and Fzd7 did not co-IP when an anti-integrin β1 antibody was used (Fig. 6B). In the ECM, TG2 can act as active enzyme or as a scaffold protein non-covalently interacting with other ECM components (9,33). To determine whether the TG2/Fzd7 interaction depends on its enzymatic function, we used recombinant TG2 carrying a C277A inactivating mutation (34). Co-IP with anti-TG2 antibody demonstrated that Fzd7 was found in a complex with C277A–TG2, suggesting independence of the protein’s catalytic function (Fig. 6C). To further delineate the TG2/Fzd7 interaction we used the 81DAVEEGDWTATVVDQQDCTLSLQLTTPANA110 peptide overlapping with part of the FN binding domain (7). The peptide disrupted partially the TG2-Fzd7 complex in a dose dependent manner, as shown by co-IP experiments with anti-TG2 antibody (Fig. 6D), suggesting that the TG2 region binding Fzd7 may be located near the FN interacting N-terminal domain.
Fig 6. TG2 forms a complex with Fzd7 in OC spheroids.
A. Co-IP with anti-TG2 mAb and western blotting for TG2 and Fzd7 using full length recombinant TG2 and Fzd7. B. Co-IP with anti- integrin β1 mAb and western blotting for integrin β1 and Fzd7 using recombinant integrin β1 and Fzd7. C. Co-IP with anti-TG2 mAb and western blotting for TG2 and Fzd7 using recombinant mutant TG2 (C277A) and Fzd7. D. Co-IP with anti-TG2 mAb and western blotting for TG2 and Fzd7 using recombinant TG2 and Fzd7 in the presence of different concentrations of a synthetic peptide 81DAVEEGDWTATVVDQQDCTLSLQLTTPANA110. E. Putative poses for protein-protein interaction were identified by using the crystal structure of TG2 (2Q3Z.pdb) available in the protein database and the virtual structure of Fzd7 obtained through homology modeling. F. Proposed interacting amino acids residues for TG2 and Fzd7.
Protein-Protein Docking
To further study the interaction between TG2 and FZD7, we considered the crystal structure of TG2 (2Q3Z.pdb) available in protein database and the model structure of Fzd7 obtained earlier through homology modeling. The protein-protein docking panel available in Schrodinger suite (35), which has an interface to the Piper program (36), was employed, considering TG2 as the receptor and Fzd7 as the ligand. The ligand was rotated 70,000 times varying every 5° in the space of Euler angles and each of the orientations of the ligand were translated to find the best docking score with respect to the receptor. Based on the docking scores and the energetics of the interaction, we selected 32 distinct potential docking poses of the two proteins. Out of the 32 poses, two illustrated that the most probable region of interactions with Fzd7 overlaps with W88-T106 residues of TG2. We performed energy minimization and compared the energetics of the two poses and found the PPI pose shown in Fig. 6E as the most likely interacting position. By analyzing the proposed PPIs, we found that the TG2 residue Gln96 can form at least two potential strong hydrogen bonds with the protonated Lys368 of Fzd7. Furthermore, Gln103 of TG2 can form a hydrogen bond with the side chain of Ser438 of Fzd7 and Asp97 of TG2 with Trp369 of Fzd7. In addition to these potential hydrogen bonds, a few electrostatic and Van der Waals interactions between the two proteins are shown in Fig. 6F.
TG2/Fzd7 interaction regulates Wnt signaling and ovarian spheroid proliferation
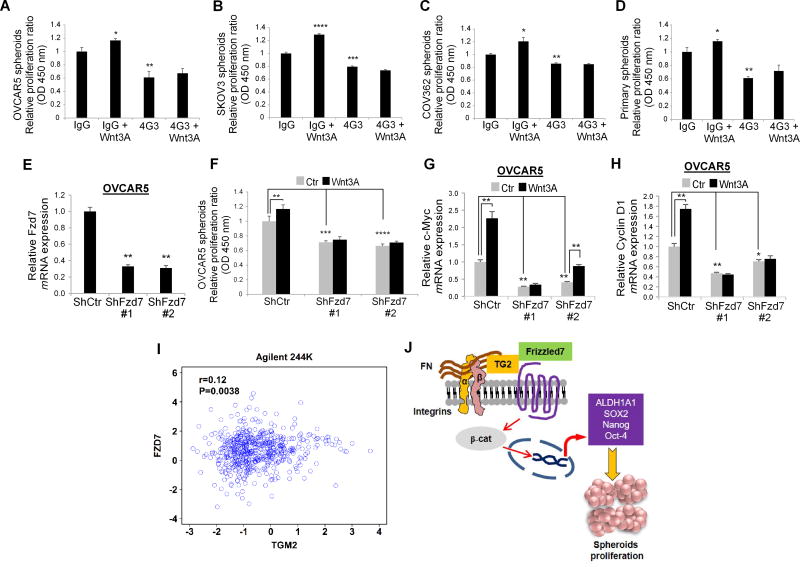
Next, we tested how TG2 and Fzd7 impact Wnt signaling in ovarian CSCs. TG2 inhibition by 4G3 blocked OVCAR5, COV362, SKOV3 and primary OC derived CSCs’ proliferation as spheres in basal conditions and after treatment with Wnt3A (Fig. 7A–D) or Wnt 7A (Supplementary Fig. S6A–D). To determine the function of Fzd7 in this pathway, the receptor was stably knocked down by using two ShRNA sequences in OVCAR5 (Fig. 7E) and SKOV3 cells (Supplementary Fig. S7A). Fzd7 knock down blocked OVCAR5 and SKOV3 derived CSCs’ proliferation as spheres in basal conditions and after treatment with Wnt3A and Wnt7A (Fig. 7F, Supplementary Fig. S7B–C) and expression of target genes in response to Wnt3A and Wnt7A (Fig. 7G–H, Supplementary Fig. SD-I).
Figure 7. TG2/Fzd7 direct interaction regulates spheroid proliferation.
A–D. Proliferation assay measured the number of cells growing as spheroids derived from OC cell lines (OVCAR5, COV362, and SKOV3) and from primary OC cells derived from malignant ascites after treatment with 4G3 and/or Wnt3A for 6 days compared with IgG controls. (N ≥ 3; *P< 0.05, **P< 0.01, ***P<0.001, ****P< 0.0001). E. Q-RT-PCR for Fzd7 in OVCAR5 cells stably transduced with scrambled- or Fzd7-targeting ShRNA (N ≥ 3). F. Spheroids proliferation assay in OVCAR5 cells stably transduced with scrambled- or Fzd7-targeting ShRNA and treated with Wnt3A (150ng/mL) or control. (N ≥ 3). G–H. Q-RT-PCR for c-Myc and cyclin D1 in OVCAR5 cells transduced with scrambled vs. Fzd7-targeting ShRNA and treated with Wnt3A (150ng/mL) or control. (N ≥ 3). I. Correlation between TG2 and Fzd7, mRNA expression levels (R= 0.12, P= 0.0038) in the ovarian cancer Agilent 244K TCGA database. J. Proposed mechanism by which TG2/FN complex interacts with Fzd7 and promotes Wnt/β-catenin-mediated cancer stemness.
Interestingly, TG2 expression was also strongly correlated with Fzd7 (R=0.12, p= 0.0038) in the ovarian TCGA’s Agilent 244K (Fig. 7I) and Affymetrix Human Exon 1.0 ST platforms (Supplementary Fig. S7J). Collectively, these results strongly support the role of TG2 promoting CSC signaling and proliferation by direct interaction with Fzd7 and activation of Wnt/β-catenin signaling (Fig. 7J).
Discussion
Our results demonstrate that TG2 and its partner proteins, FN1 and integrin β1, are upregulated and form a functional complex in OCSCs co-expressing ALDH+/CD133+ (22,37) compared to non-CSCs (ALDH−/CD133−). This complex is enriched in cancer cells grown as spheroids in ultra-low adherence conditions compared to cells grown under differentiating conditions as monolayers. We show that targeting the TG2/FN1/integrin β1 complex by using a blocking antibody disrupts the stem cell phenotype by inhibiting Wnt/β-catenin signaling. Importantly, we found Fzd7, a Wnt ligand receptor, as a new TG2-interacting protein in OCSCs required for transduction of Wnt ligand signals. Our findings have several implications.
First, we reaffirm the significance of TG2 to the CSC phenotype and point to a new mechanism by which the protein promotes stem cell survival and tumor initiation capacity. The observations that TG2 expression is enriched in ovarian CSCs are consistent with our previous findings (12) as well as with recent reports in other solid tumors (13–15). Importantly, here we show that TG2 directly modulates the interaction between OCSCs and the ECM by forming a complex with integrin β1. The ternary complex formed and stabilized by TG2 with integrin β1 and FN has been described in normal fibroblasts (7). Here, we demonstrate that the TG2/integrin β1 and TG2/FN complexes are significantly enriched in ALDH+/CD133+ OCSCs grown as spheroids compared to non-CSCs and cells grown as monolayers. The involvement of integrins in cancer stemness has been recognized in other contexts (38,39), yet our results provide a new understanding of the mechanisms by which integrins anchoring CSCs in the matrix engage stemness pathways. The TG2/integrin β1 complexes were also detectable in human ovarian tumors by PLA, supporting their functional role in vivo. Furthermore, the expression of each of the proteins in this complex, individually, as well as together, correlated with clinical outcomes, establishing clinical relevance of our findings.
To understand the role of TG2/FN/integrin β1 complexes promoting the OCSC phenotype and spheroids proliferation, we used an inhibitory antibody for the TG2/FN interaction. The anti-TG2 antibody (29) was more effective than anti-integrin β1 mAb decreasing ALDH+/CD133+ survival and spheroids proliferation. Anti-TG2 mAbs have been shown to inhibit the migration of monocytic cells in inflamed tissue rich in FN matrices (29). Possible explanations of this phenomenon include the specific and high affinity binding of TG2 to the 42 kDa gelatin-binding domain of FN (28), and with the critical importance of TG2/FN organization in modulating β integrins expression and function in a model of tumor growth and dissemination (10). Additionally, TG2 knock down diminished the OCSC population.
One of the hallmarks of CSCs is to recapitulate hierarchically organized human tumors when injected in immunodeficient mice (31). Here we show that 4G3-mediated TG2-FN blockade inhibited the tumor initiating capacity of ALDH+/CD133+ cells in nude mice and cells isolated from 4G3-treated tumors and cultured ex vivo were unable to form spheroids. Unbiased expression profiling analysis demonstrated that 4G3-mediated TG2/FN complex disruption downregulated genes related to CSC-maintenance, self-renewal and proliferation, linking TG2-mediated ECM components assembly with the expression of ALDH1A1, Nanog, Oct-4, and Sox2, well known CSC markers.
Second, our results point to the Wnt/β-catenin pathway as the main regulator of stemness disrupted by inhibition of TG2/FN/integrin β1 complexes. β-catenin activation has been correlated with progression of human cancers (40) and the functions of CSCs in lung adenocarcinoma (41) and colorectal cancer (42). We previously showed that β-catenin directly regulates the expression of the stem cell marker ALDH1A1 in OC, thus being involved in maintenance of the OCSC phenotype (21). We have previously demonstrated that the TG2/FN complex regulates β-catenin activation through a c-Src dependent mechanism (32). Extracellular TG2 was shown to activate canonical β-catenin signaling in vascular smooth muscle cells by directly binding to LRP5/6 (43,44). Here we identify the Fzd7 receptor as a new TG2 substrate, linking the adhesion complex mediated by TG2 to stemness-associated β-catenin pathway. Although interactions between TG2 and all Wnt receptors were not fully explored, the interaction between TG2 and Fzd7 appears to be specific and direct. Additionally, the other components of the complex (integrin β1 or FN) did not directly interact with Fzd7. To better delineate this PPI, we used both chemical and virtual docking strategies. Both the full length and the C277A mutant TG2 formed a complex with Fzd7, excluding the possibility that the enzymatic function of TG2 plays a role in this interaction. However, a synthetic peptide, comprising the amino acid residues corresponding to the FN binding sequence of TG2, blocked complex formation in a dose dependent manner, suggesting that the interacting domain is near the TG2’s N-terminus. Virtual docking using the available TG2 structure and a reconstituted Fzd7 structure predicted that the interaction sites were in the FN-binding domain of TG2 (W88-T106) and involved amino acids (>358–379) and (>445–470) of Fzd7.
The functions of Fzd7 in cancer are incompletely elucidated. Fzd7 was found to be up-regulated in OC compared to normal surface ovarian epithelium (45) and was linked to the canonical Wnt pathway in colorectal cancer (46), breast (47), and hepatocellular carcinoma (48). Our data demonstrate a role for the receptor in a complex with TG2, transducing Wnt signals and promoting OCSCs’ proliferation as spheres. Co-localization of the receptor with TG2 was demonstrated in ovarian tumors for this first time here, supporting future investigation of its functions in this context.
Finally, we propose a positive feedback loop mechanism to explain the overall decrease in Wnt-related genes after 4G3 treatment in OCSCs. TG2/FN complex formation acting as Fzd7 coactivator increases Wnt/β-catenin signal transduction that in turn fuels a positive feedback loop, mediating the transcriptional regulation and membrane distribution of frizzled receptors and activation of other positive Wnt/β-catenin key regulatory genes. Several Wnt/β-catenin signaling-related genes such as LEF1 and Fzd7 contain putative TCF/LEF1 binding sites within their promoter regions, suggesting they may be self-regulated through Wnt/β-catenin canonical signaling.
In conclusion, our results provide strong evidence supporting a key function of TG2/FN/integrin β1 complexes in OCSCs. By demonstrating that TG2 directly binds the Fzd7 receptor, we identify a novel function of TG2 and a new mechanism promoting CSCs’ proliferation and tumorigenicity. These results point to TG2/FN/integrin clusters or the newly discovered co-receptor complex TG2/Fzd7 as potential new therapeutic CSC targets.
Supplementary Material
Significance.
Findings reveal a new mechanism by which ovarian cancer stem cells interact with the tumor microenvironment, promoting cell proliferation and tumor initiation.
Acknowledgments
This work was made possible by funding from the US Department of Veterans Affairs (I01 BX000792-06), the Diana Princess of Wales endowed Professorship from the Lurie Cancer Center (to DM) and Friends of Prentice award (to SC). We acknowledge the technical support provided by the Imaging Core Facility and Flow Cytometry Core supported by the Lurie Cancer Center and the NCI-P30 (CA 060553) award.
Footnotes
Conflict of Interest: None
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Lengyel E. Ovarian cancer development and metastasis. Am J Pathol. 2010;177:1053–64. doi: 10.2353/ajpath.2010.100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matte I, Lane D, Laplante C, Rancourt C, Piche A. Profiling of cytokines in human epithelial ovarian cancer ascites. Am J Cancer Res. 2012;2:566–80. [PMC free article] [PubMed] [Google Scholar]
- 4.Mills GB, May C, Hill M, Campbell S, Shaw P, Marks A. Ascitic fluid from human ovarian cancer patients contains growth factors necessary for intraperitoneal growth of human ovarian adenocarcinoma cells. J Clin Invest. 1990;86:851–5. doi: 10.1172/JCI114784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burleson KM, Casey RC, Skubitz KM, Pambuccian SE, Oegema TR, Jr, Skubitz AP. Ovarian carcinoma ascites spheroids adhere to extracellular matrix components and mesothelial cell monolayers. Gynecol Oncol. 2004;93:170–81. doi: 10.1016/j.ygyno.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 6.Pinkas DM, Strop P, Brunger AT, Khosla C. Transglutaminase 2 undergoes a large conformational change upon activation. PLoS Biol. 2007;5:e327. doi: 10.1371/journal.pbio.0050327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hang J, Zemskov EA, Lorand L, Belkin AM. Identification of a novel recognition sequence for fibronectin within the NH2-terminal beta-sandwich domain of tissue transglutaminase. J Biol Chem. 2005;280:23675–83. doi: 10.1074/jbc.M503323200. [DOI] [PubMed] [Google Scholar]
- 8.Cardoso I, Osterlund EC, Stamnaes J, Iversen R, Andersen JT, Jorgensen TJ, et al. Dissecting the interaction between transglutaminase 2 and fibronectin. Amino Acids. 2017;49:489–500. doi: 10.1007/s00726-016-2296-y. [DOI] [PubMed] [Google Scholar]
- 9.Akimov SS, Krylov D, Fleischman LF, Belkin AM. Tissue transglutaminase is an integrin-binding adhesion coreceptor for fibronectin. J Cell Biol. 2000;148:825–38. doi: 10.1083/jcb.148.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satpathy M, Cao L, Pincheira R, Emerson R, Bigsby R, Nakshatri H, et al. Enhanced peritoneal ovarian tumor dissemination by tissue transglutaminase. Cancer Res. 2007;67:7194–202. doi: 10.1158/0008-5472.CAN-07-0307. [DOI] [PubMed] [Google Scholar]
- 11.Shao M, Cao L, Shen C, Satpathy M, Chelladurai B, Bigsby RM, et al. Epithelial-to-mesenchymal transition and ovarian tumor progression induced by tissue transglutaminase. Cancer Res. 2009;69:9192–201. doi: 10.1158/0008-5472.CAN-09-1257. [DOI] [PubMed] [Google Scholar]
- 12.Cao L, Shao M, Schilder J, Guise T, Mohammad KS, Matei D. Tissue transglutaminase links TGF-beta, epithelial to mesenchymal transition and a stem cell phenotype in ovarian cancer. Oncogene. 2012;31:2521–34. doi: 10.1038/onc.2011.429. [DOI] [PubMed] [Google Scholar]
- 13.Kumar A, Gao H, Xu J, Reuben J, Yu D, Mehta K. Evidence that aberrant expression of tissue transglutaminase promotes stem cell characteristics in mammary epithelial cells. PLoS One. 2011;6:e20701. doi: 10.1371/journal.pone.0020701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kerr C, Szmacinski H, Fisher ML, Nance B, Lakowicz JR, Akbar A, et al. Transamidase site-targeted agents alter the conformation of the transglutaminase cancer stem cell survival protein to reduce GTP binding activity and cancer stem cell survival. Oncogene. 2017;36:2981–90. doi: 10.1038/onc.2016.452. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 15.Sullivan KE, Rojas K, Cerione RA, Nakano I, Wilson KF. The stem cell/cancer stem cell marker ALDH1A3 regulates the expression of the survival factor tissue transglutaminase, in mesenchymal glioma stem cells. Oncotarget. 2017;8:22325–43. doi: 10.18632/oncotarget.16479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehta K, Fok J, Miller FR, Koul D, Sahin AA. Prognostic significance of tissue transglutaminase in drug resistant and metastatic breast cancer. Clin Cancer Res. 2004;10:8068–76. doi: 10.1158/1078-0432.CCR-04-1107. [DOI] [PubMed] [Google Scholar]
- 17.Mann AP, Verma A, Sethi G, Manavathi B, Wang H, Fok JY, et al. Overexpression of tissue transglutaminase leads to constitutive activation of nuclear factor-kappaB in cancer cells: delineation of a novel pathway. Cancer Res. 2006;66:8788–95. doi: 10.1158/0008-5472.CAN-06-1457. [DOI] [PubMed] [Google Scholar]
- 18.Cao L, Petrusca DN, Satpathy M, Nakshatri H, Petrache I, Matei D. Tissue transglutaminase protects epithelial ovarian cancer cells from cisplatin-induced apoptosis by promoting cell survival signaling. Carcinogenesis. 2008;29:1893–900. doi: 10.1093/carcin/bgn158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verma A, Wang H, Manavathi B, Fok JY, Mann AP, Kumar R, et al. Increased expression of tissue transglutaminase in pancreatic ductal adenocarcinoma and its implications in drug resistance and metastasis. Cancer Res. 2006;66:10525–33. doi: 10.1158/0008-5472.CAN-06-2387. [DOI] [PubMed] [Google Scholar]
- 20.Yakubov B, Chen L, Belkin AM, Zhang S, Chelladurai B, Zhang ZY, et al. Small molecule inhibitors target the tissue transglutaminase and fibronectin interaction. PLoS One. 2014;9:e89285. doi: 10.1371/journal.pone.0089285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Condello S, Morgan CA, Nagdas S, Cao L, Turek J, Hurley TD, et al. beta-Catenin-regulated ALDH1A1 is a target in ovarian cancer spheroids. Oncogene. 2015;34:2297–308. doi: 10.1038/onc.2014.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, Condello S, Thomes-Pepin J, Ma X, Xia Y, Hurley TD, et al. Lipid Desaturation Is a Metabolic Marker and Therapeutic Target of Ovarian Cancer Stem Cells. Cell Stem Cell. 2017;20:303–14. doi: 10.1016/j.stem.2016.11.004. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soderberg O, Gullberg M, Jarvius M, Ridderstrale K, Leuchowius KJ, Jarvius J, et al. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat Methods. 2006;3:995–1000. doi: 10.1038/nmeth947. [DOI] [PubMed] [Google Scholar]
- 24.Jacobson MP, Pincus DL, Rapp CS, Day TJ, Honig B, Shaw DE, et al. A hierarchical approach to all-atom protein loop prediction. Proteins. 2004;55:351–67. doi: 10.1002/prot.10613. [DOI] [PubMed] [Google Scholar]
- 25.Mitra AK, Davis DA, Tomar S, Roy L, Gurler H, Xie J, et al. In vivo tumor growth of high-grade serous ovarian cancer cell lines. Gynecol Oncol. 2015;138:372–7. doi: 10.1016/j.ygyno.2015.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Domcke S, Sinha R, Levine DA, Sander C, Schultz N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat Commun. 2013;4:2126. doi: 10.1038/ncomms3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawrenson K, Notaridou M, Lee N, Benjamin E, Jacobs IJ, Jones C, et al. In vitro three-dimensional modeling of fallopian tube secretory epithelial cells. BMC cell biology. 2013;14:43. doi: 10.1186/1471-2121-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lorand L, Dailey JE, Turner PM. Fibronectin as a carrier for the transglutaminase from human erythrocytes. Proc Natl Acad Sci U S A. 1988;85:1057–9. doi: 10.1073/pnas.85.4.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akimov SS, Belkin AM. Cell surface tissue transglutaminase is involved in adhesion and migration of monocytic cells on fibronectin. Blood. 2001;98:1567–76. doi: 10.1182/blood.v98.5.1567. [DOI] [PubMed] [Google Scholar]
- 30.Lu Z, Roohani-Esfahani SI, Li J, Zreiqat H. Synergistic effect of nanomaterials and BMP-2 signalling in inducing osteogenic differentiation of adipose tissue-derived mesenchymal stem cells. Nanomedicine. 2015;11:219–28. doi: 10.1016/j.nano.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 31.Zhang S, Balch C, Chan MW, Lai HC, Matei D, Schilder JM, et al. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68:4311–20. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Condello S, Cao L, Matei D. Tissue transglutaminase regulates beta-catenin signaling through a c-Src-dependent mechanism. FASEB J. 2013;27:3100–12. doi: 10.1096/fj.12-222620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Telci D, Wang Z, Li X, Verderio EA, Humphries MJ, Baccarini M, et al. Fibronectin-tissue transglutaminase matrix rescues RGD-impaired cell adhesion through syndecan-4 and beta1 integrin co-signaling. J Biol Chem. 2008;283:20937–47. doi: 10.1074/jbc.M801763200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu S, Cerione RA, Clardy J. Structural basis for the guanine nucleotide-binding activity of tissue transglutaminase and its regulation of transamidation activity. Proc Natl Acad Sci U S A. 2002;99:2743–7. doi: 10.1073/pnas.042454899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schroedinger. Small-molecule drug discovery Suite 2017-1. LLC; New York: 2017. [Google Scholar]
- 36.Chuang GY, Kozakov D, Brenke R, Comeau SR, Vajda S. DARS (Decoys As the Reference State) potentials for protein-protein docking. Biophys J. 2008;95:4217–27. doi: 10.1529/biophysj.108.135814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silva IA, Bai S, McLean K, Yang K, Griffith K, Thomas D, et al. Aldehyde dehydrogenase in combination with CD133 defines angiogenic ovarian cancer stem cells that portend poor patient survival. Cancer Res. 2011;71:3991–4001. doi: 10.1158/0008-5472.CAN-10-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goel HL, Gritsko T, Pursell B, Chang C, Shultz LD, Greiner DL, et al. Regulated splicing of the alpha6 integrin cytoplasmic domain determines the fate of breast cancer stem cells. Cell Rep. 2014;7:747–61. doi: 10.1016/j.celrep.2014.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Q, Eades G, Yao Y, Zhang Y, Zhou Q. Characterization of a stem-like subpopulation in basal-like ductal carcinoma in situ (DCIS) lesions. J Biol Chem. 2014;289:1303–12. doi: 10.1074/jbc.M113.502278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–50. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 41.Tammela T, Sanchez-Rivera FJ, Cetinbas NM, Wu K, Joshi NS, Helenius K, et al. A Wnt-producing niche drives proliferative potential and progression in lung adenocarcinoma. Nature. 2017;545:355–9. doi: 10.1038/nature22334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li J, Yu B, Deng P, Cheng Y, Yu Y, Kevork K, et al. KDM3 epigenetically controls tumorigenic potentials of human colorectal cancer stem cells through Wnt/beta-catenin signalling. Nat Commun. 2017;8:15146. doi: 10.1038/ncomms15146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Faverman L, Mikhaylova L, Malmquist J, Nurminskaya M. Extracellular transglutaminase 2 activates beta-catenin signaling in calcifying vascular smooth muscle cells. FEBS Lett. 2008;582:1552–7. doi: 10.1016/j.febslet.2008.03.053. [DOI] [PubMed] [Google Scholar]
- 44.Deasey S, Nurminsky D, Shanmugasundaram S, Lima F, Nurminskaya M. Transglutaminase 2 as a novel activator of LRP6/beta-catenin signaling. Cell Signal. 2013;25:2646–51. doi: 10.1016/j.cellsig.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matei D, Graeber TG, Baldwin RL, Karlan BY, Rao J, Chang DD. Gene expression in epithelial ovarian carcinoma. Oncogene. 2002;21:6289–98. doi: 10.1038/sj.onc.1205785. [DOI] [PubMed] [Google Scholar]
- 46.Ueno K, Hiura M, Suehiro Y, Hazama S, Hirata H, Oka M, et al. Frizzled-7 as a potential therapeutic target in colorectal cancer. Neoplasia. 2008;10:697–705. doi: 10.1593/neo.08320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang L, Wu X, Wang Y, Zhang K, Wu J, Yuan YC, et al. FZD7 has a critical role in cell proliferation in triple negative breast cancer. Oncogene. 2011;30:4437–46. doi: 10.1038/onc.2011.145. [DOI] [PubMed] [Google Scholar]
- 48.Merle P, Kim M, Herrmann M, Gupte A, Lefrancois L, Califano S, et al. Oncogenic role of the frizzled-7/beta-catenin pathway in hepatocellular carcinoma. J Hepatol. 2005;43:854–62. doi: 10.1016/j.jhep.2005.05.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.