Abstract
Here, we provide methods for assembly of mitotic spindles and interphase asters in Xenopus egg extract, and compare them to spindles and asters in the egg and zygote. Classic cycled spindles which can segregate chromosomes are made by adding sperm nuclei to interphase extract to promote nucleus formation and DNA replication. After 90 minutes, interphase nuclei are converted to cycled CSF spindles by addition of CSF extract. These spindles can segregate chromosomes. CSF spindles are made by addition of sperm nuclei to CSF extract. They lack functional kinetochores but suffice for some biochemical applications. Large interphase asters are prepared by addition of AurkA bead artificial centrosomes or sperm nuclei to actin-intact egg extract. These asters grow rapidly to hundreds of microns in radius by branching microtubule nucleation at the periphery, so the aster as a whole is a network of short, dynamic microtubules. They resemble the sperm aster after fertilization, and the asters that grow out of the poles of the mitotic spindle at anaphase. When interphase asters grow into each other they interact and assemble aster interaction zones at the shared boundary. These zones consist of a line (in extract) or disc (in zygotes) of antiparallel microtubule bundles coated with Chromosome Passenger Complex (CPC), Centralspindlin and other cytokinesis midzone proteins. Interaction zones function to block interpenetration of microtubules from the two asters, and to signal to the cortex to induce cleavage furrows. By reconstituting them in extract we can dissect the biophysics of spatially regulated cytokinesis signaling.
Materials
This Protocol describes only methods for assembly and imaging of spindles and interphase asters. To assemble these structures additional reagents are required: Xenopus egg extract, microtubule organizing centers (MTOC) and fluorescent probes. For protocols for these reagents see references below.
Reagents
Actin-intact CSF extract for aster assembly (Field et al., 2017).
CSF extract containing Cytochalasin D for spindle assembly see (Good and Heald et al article in this volume)(Desai et al., 1999)(Maresca and Heald, 2006).
Fluorescently labeled tubulin: Bovine brain tubulin is labeled on lysines with an ALEXA dye NHS ester as described (Hyman et al., 1991) and added at ~200 nM final. High quality labeled tubulin can also be purchased from Cytoskeleton Inc.
Microtubule Organizing centers (MTOCs): two types
AurkA beads (Aurora Kinase A antibody on magnetic beads.) These nucleate large symmetric asters, (Tsai and Zeng, 2005) (Ishihara et al., 2014a) (Nguyen et al., 2014)(Ishihara et al., 2016).
Sperm nuclei at (1-5 × 107/ml stock) for both spindle and aster assembly (Gatlin et al., this volume). Sperm nuclei contain a centrosome as well as DNA. Chromatin can nucleate microtubules (Heald et al., 1996), and it also sends an asymmetric signal via Aurora kinase B (AurkB) activity. As a result, sperm plus centrosomes nucleate asters that are polarized in their structure and activity early in the cell cycle (Field et al., 2015).
Protein probes: We use several labeling methods: pure GFP fusion proteins, pure proteins labeled with reactive dyes, directly labeled antibodies to visualize endogenous proteins see (Field et al., 2017)
Buffers/solutions
100 × Calcium: (40 mM) prepared in water or 1× sperm dilution buffer.
Extract Fix: 60% (v/v) glycerol, 1× MMR: 5 mM Na-Hepes (pH 7.8), 100 μM EDTA, 100 mM NaCl, 2 mM KCl, 1 mM MgCl2 and 2 mM CaCl2 with 1 μg/ml Hoechst (Sigma 33342/333258) and 10% formaldehyde. Prepared fresh. Note: MMR can be prepared as a 10× stock, autoclaved and store at RT.
Hoechst: For live imaging of DNA, 1 mg/ml solution (Sigma 33342) this catalogue # is required for live imaging ~10 ng/ml should be sufficient.
Sperm dilution buffer (1×): 10 mM Hepes (pH 7.7), 1 mM MgCl2, 100 mM KCl, 150 mM sucrose. Can be prepared as 5× stock and stored at −20°C.
Equipment
Coverslips: 22 × 22 and 18 × 18 mm, 1.5mm thick. Plain glass or passivated (see method below).
Metal support slides (See method below)
1.5 ml microfuge tubes
Microscope slides
Wide bore pipet tips
VALAP: 1:1:1 mixture of wax, Vaseline, and lanolin
20°C water bath. We generally make our own with an ice bucket, water and ice.
Cycled Spindle Assembly Procedure
Method
Add 40 μl of CSF extract to a 1.5 μl microfuge tube on ice.
Add sperm nuclei to a final concentration of 100- 300 sperm nuclei/μl of extract.
Add 0.4 μl of 40 mM CaCl2 to a final concentration of 0.4 mM.
Immediately flick the tube ~8 times carefully. It is important to mix in the calcium rapidly and completely. This releases the CSF arrest and the extract starts going into interphase.
Incubate at 20°C for 90 min, flicking occasionally.
Monitor progression of the reaction use a Squash Fixation: Pipet a 1 μl aliquot of the reaction on a microscope slide, overlay with 3 μl of Extract Fix and cover gently with an 18 × 18 coverslip and view by fluorescence microscopy. At 90 min nuclei (visualized by Hoechst) should be large and round indicating successful interphase nuclear envelope assembly, which is essential for DNA replication. Microtubules (visualized by fluorescent tubulin) are typically disorganized. (Figure 1A)
Add 60 μl of CSF extract. Flick the tube ~8 times carefully. This forces the extract back into into CSF arrest.
Incubate at 20°C for 60 min, flicking occasionally.
Monitor reaction progression using a Squash Fix (above). At 30 min after CSF add back (i.e. at 120 min total) nuclei should be broken down with chromatin starting to condense into individual aggregates. At 60 min after CSF add back the extract should be dominated by robust bipolar spindles with chromatin at the metaphase plate. In a typical extract, 40-60% of the total structures are bipolar spindles with the remainder either monopolar or large aggregates. (Figure 1A)
Fluorescent probes can be added at any time in the reaction, since most spindle components turn over rapidly. We typically assemble spindles containing no probes, and add probes to smaller aliquots of the reaction then incubate for a few minutes to allow incorporation. In this way, multiple team members imaging different proteins can share the reaction. We typically initiate new assembly reactions every hour for ~6hrs.
For live imaging see Imaging of Spindles and Asters Procedure below.
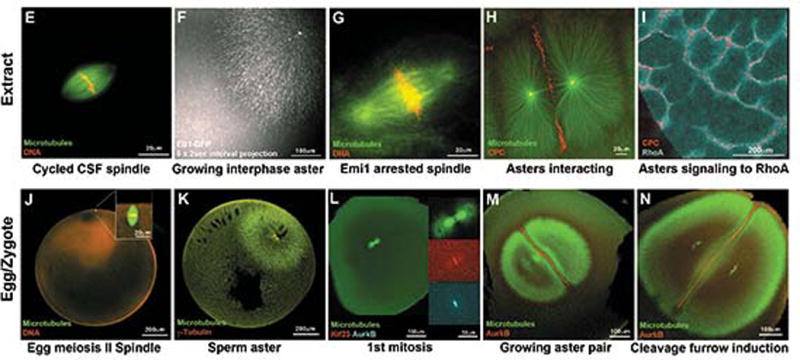
Figure 1. Spindles and asters in egg extract and fixed eggs.
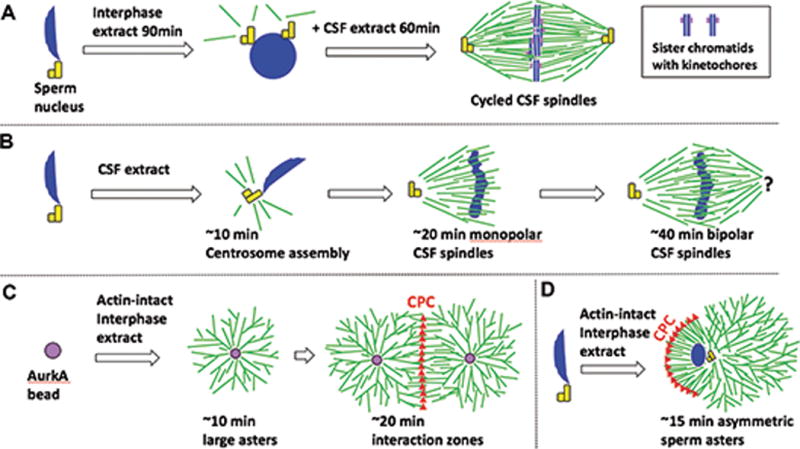
(A-D) Schematic of spindle and asters assembly in egg extract. Microtubules green, DNA blue, centrosomes yellow, AurkA beads purple, CPC red.
(E-I) Spindles and interphase asters in egg extract by live widefield (E-H) or TIRF(I).
(J-N) Spindles and interphase asters in fixed, cleared eggs by confocal immunofluorescence.
A) Cycled CSF spindles with replicated sister chromatids and functional kinetochores.
B) CSF spindles which lack functional kinetochores.
C) Interphase bead asters grow rapidly by branching nucleation, and form CPC-positive interaction zones when they meet.
D) Interphase sperm asters are similar to C), but they grow asymmetrically due to CPC recruitment on the chromatin-proximal boundary (Field et al 2015).
E) Cycled CSF spindle. Note lack of asters at poles indicating meiosis-II morphology.
F) Growing interphase bead aster. This image shows a projection of 5 sequential EB1-GFP images for plus end tracking (Ishihara et al., 2014).
G) Cycled spindle arrested with Emi1 to model zygote mitotic spindles. Note asters at poles (Wühr et al., 2008).
H) Interphase bead asters interacting and recruiting CPC between them.
I) Signaling from aster interaction zones between bead asters to a RhoA.GTP reporter on a supported lipid bilayer (Nguyen et al., 2014).
J) Unfertilized egg with meiosis-II spindle.
K) Sperm aster during pronuclear migration.
L) 1st mitosis, early anaphase. Note asters at spindle poles.
M) Expanding sister asters after 1st anaphase. Note CPC-positive interaction zone.
N) Sister asters just before furrow induction. The furrow forms where the interaction zone touches the cortex.
CSF Spindle Assembly Procedure
Method
Add 40 μl of CSF extract to a 1.5 μl microfuge tube.
Add fluorescent tubulin and other probes if desired.
Add sperm nuclei to a final concentration of 100- 300 sperm nuclei/μl of extract.
Gently mix by flicking.
Incubate at 20°C. Continue to flick occasionally during incubation.
At 15, 30, 45 and 60 min take 1 μl samples to test the progress of the reaction via Squash Fixation (above). At 10 min, a small microtubule aster emanates from the sperm centrosome. By 20-30 min the chromatin has migrated away from the centrosome, and microtubules are polarized toward the chromatin (these structures are termed half spindles). They achieve bipolarity either by fusion (Sawin and Mitchison, 1991) or spontaneous bipolarization (Mitchison et al., 2004). In a typical extract, 40-60% of the total structures are bipolar spindles by 60 min. This number can vary from <10% in very poor extracts to >90% in the best extracts. (Figure 1B)
For live imaging see Imaging Spindles and Asters Procedure below.
Interphase Aster and Aster Interaction Zone Assembly Procedure
Method
Add 25-100 μl of actin-intact CSF extract to a 1.5 ml microfuge tube.
Add probes (as above) and mix well on ice. To observe aster interaction zone formation the probes should include one for imaging the CPC. For this we use GFP-DasraA, Alexa labeled anti-AurkB or anti-INCENP (Field et al., 2015). The antibodies tend to promote CPC activation, and should be added at the minimal concentration needed for visualization.
Add MTOCs (sperm nuclei or AurkA beads) and mix on ice. The final concentration must be experimentally determined so the asters are spaced as desired.
Add CaCl2 to a final concentration of 0.4mM from 100× stock.
Gently pipet the entire solution up and down 3-5 times to ensure mixing using a wide bore tip, or flick the tube ~8 times. It is important to mix well after calcium addition. This converts the reaction to interphase.
Incubate at 20 °C for 2-10 min.
Incubate on ice for at least two minutes. Flick several times to break up any aggregates caused by gelation contraction.
The aster assembly reaction is initiated by squashing between two passivated coverslips and warming of the preparation to RT. Aster interaction zones are formed when asters grow large enough to interact and recruit microtubule signaling complexes CPC and Centralspindlin. (Figure 1 C,D)
For live imaging see Imaging Spindles and Asters Procedure below.
Imaging Spindles and Asters Procedure
For live imaging experiments, we typically squash small volumes of extract between two coverslips to provide a 5-40 μm thick preparation. Cleaning and preparation of the glass surface is important. Untreated glass tends to bind motor proteins such as dynein that influence microtubule organization, and it also prevents Chromosomal Passenger Complex (CPC) recruitment to zones between asters. Many PEG passivation schemes have been described in the literature, reviewed in (Field et al., 2017). Here we include our current favorite which is simple and fast. Cycled spindles are extremely robust, and not much affected by squashing under uncoated glass surfaces. Pre-2010, work from the Mitchison lab on cycled spindles generally used squashes between uncoated slides and coverslips. Passivation is much more important for interphase asters, which can be torn up by dynein bound to uncoated glass surfaces. Figure 1 E-I shows examples of microtubule assemblies in Xenopus egg extract. Figure 1 J-N shows the corresponding assemblies in fixed Xenopus eggs or zygotes. Egg and zygote fixation protocol in Field et al., 2015.
Materials
Reagents
Ethanol 70%
1 M Hepes buffer pH 7.4
Hoechst: For live imaging of DNA (Sigma 33342) ~10 ng/ml should be sufficient.
Milli-Q distilled water
Poly-L-lysine-g-polyethylene glycol (PLL-g-PEG) from (SuSoS PLL(20)-g[3.5]-PEG(2))
Equipment
Bunsen burner
Glass coverslips, No. 1.5 thickness, 22 × 22 and 18 × 18 mm
Metal support slides: We machined stainless steel or aluminum slides with 18 mm circular holes in the center. Slides are 2 mm thick. A single hole slide is 24 × 75mm and a 4 hole slide is 50 × 75 mm and 2mm thick. These are assembled into imaging chambers (below) that can be viewed using both upright and inverted microscopes. See (Field, et al 2017).
Nitrogen or house air
Parafilm
Teflon rack for coverslips (Life Technologies C14784)
VALAP, 1:1:1 mixture of wax, Vaseline, and lanolin
Passivation of glass surfaces procedure
Method
All steps performed at room temperature.
Coverslips (18 mm and 22 mm) are individually rinsed in 70% ethanol for a few seconds, removed, flamed with a Bunsen burner, cooled in air for a few seconds.
Cooled coverslips are placed onto droplets of 0.1 mg/mL PLL- g-PEG in 10 μM HEPES pH 7.4 on a piece of Parafilm. Coverslips 22 × 22 mm in size require 110 μL of PEG solution, 18 × 18 mm coverslips require 90 μL. Allow coverslips to incubate for 15-30 min.
Wash coverslips 3× in Milli-Q water. For the first two washes place each coverslip, PEG side down in a droplet of Milli-Q water. Allow them to incubate for 5 min. For the last wash dip each coverslip into a beaker of Milli-Q water.
Coverslips are placed in slots in a Teflon rack so they do not contact one another. (Keep track of PEG treated surface).
Dry coverslips with a jet of nitrogen gas, to prevent residue from water droplets. Use the same day, but can be stored for up to a week at room temperature in the dark (Field et al., 2017).
Mounting the sample for imaging procedure
Method
Assemble imaging chamber. Warm a metal slide to 70°C on a hot plate. Dab the slide near the opening with a small amount of molten VALAP. Place a 22 × 22 mm square passivated coverslip on top of the metal slide, covering the opening. The passivated side is upward—facing away from the slide. Cool to RT.
Pipet 4-12 μL of extract onto the center of the 22mm2 coverslip.
Immediately and gently place an 18 × 18 mm coverslip on top with passivated side towards the extract. Try to avoid air bubbles. A few air bubbles will not hurt, but they may move and destroy nearby asters or spindles. They also slow depletion of oxygen in a zone ~300 μm wide around each bubble.
After the droplet spreads out fully (~5 sec) seal edges with VALAP so the sample does not dry out. 7.5 μL extract gives a squash nominally 23 μm in thickness.
Timing: Cycled and CSF spindles are at steady state in structure and dynamics, time is not critical. However, it takes a few minutes for the mitochondria in extract to deplete oxygen, longer if bubbles are present. Photo-bleaching of chemical chromofluors will be fast until oxygen depletion has occurred. Note that ATP supply is not affected by deoxygenation (Niethammer et al., 2008). Interphase asters grow rapidly after warming to RT, so it is important to initiate imaging as soon as possible. To image early steps in aster growth we prepare unsealed squashes with the slide resting on a metal block in an ice bucket, to keep the squash cold until imaging. Condensed water vapor on the coverslip can be removed with a stream of dry air or nitrogen.
Temperature: The ideal temperature for preparing the squashes and imaging is 16-18°C. Adequate results can be obtained at temperatures up to ~25°C, though spindles are more likely to enter anaphase spontaneously at higher temperatures.
Trouble shooting spindle and aster assembly reactions
Problem
Occasionally an extract prep will be non-functional for either the interphase or mitotic reaction. Typical problems include fragmentation of chromatin, which may be due to induction of apoptosis, or an extract that gets stuck in interphase or mitosis.
Solution
If this occurs in the first reaction it is wise to terminate the experiment. After more than 4hrs in the ice bucket even initially good extract may stop working for further assembly reactions. Probably the most important variables for a functional extract are the health of the frogs, care taken over “gardening” the eggs, i.e. removing dead or aberrant eggs prior to extract preparation and the initial egg packing spin which removes excess buffer before crushing the eggs. Excess buffer dilutes the extract.
Problem
The extract does not release from CSF arrest and enter interphase. This is a common problem for novices and can be detected via microscopy. Microtubules appear as spindle-like structures rather than asters.
Solution
The ER rapidly uptakes calcium, decreasing the cytosolic concentration, so immediate and thorough mixing after calcium addition is crucial to release CSF arrest. Larger volumes may cause problems with mixing and gas exchange. Mix reactions in 1.5 ml tubes and keep reaction volumes to 100 μl at most.
Discussion
General considerations for working with extracts
Xenopus extracts are a powerful tool but their use can be frustrating because of high variability. The extracts are sensitive to physical perturbations and must be treated gently to ensure best results. Avoid vortexing or pipetting up and down vigorously. Use wide bore pipet tips (these can be purchased or created but cutting) — an approximately 2 mm diameter is optimal. Use a fresh tip for each aliquot, extracts are viscous.
It is very important is to avoid diluting extracts more that 10% of original volume. Keep in mind that some buffers and reagents can inhibit spindle assembly. When adding small molecules, we have found that the EC50 for drug action is often 10-1000 fold higher in extract than in tissue culture cells, although mechanism and specificity are maintained. We believe there are two reasons: (1) tissue culture cells are surrounded by a large reservoir of drug in the medium, and can accumulate drugs to a higher internal concentration; (2) hydrophobic drugs partition into lipid reservoirs and/or acidic compartments present in extract. For these reasons, EC50 for drug action in extract must be determined empirically, and we aim to use them at ~5× the EC50 to maximize specificity. If drugs are added from a DMSO stock, it is important to keep the total percent DMSO at <0.5%. Drugs stocks are often prepared as concentrated stocks in DMSO, e.g. 1000×. These should be diluted in small volumes of egg extract to give 10-50× final stocks. Dilution in aqueous buffer can cause hydrophobic drugs to precipitate, in which case the final concentration is unreliable.
Acknowledgments
This work was supported by NIH grant GM39565 (T.J.M.), MBL fellowships from the Evans Foundation, MBL Associates, and the Colwin Fund (T.J.M. and C.M.F.). Authors thank the Nikon Imaging Center at Harvard Medical School for microscopy support; and the National Xenopus Resource at MBL for Xenopus animals and care.
References
- Desai A, Murray A, Mitchison TJ, Walczak CE. The use of Xenopus egg extracts to study mitotic spindle assembly and function in vitro. Methods Cell Biol. 1999;61:385–412. doi: 10.1016/s0091-679x(08)61991-3. [DOI] [PubMed] [Google Scholar]
- Field CM, Groen AC, Nguyen PA, Mitchison TJ. Spindle-to-cortex communication in cleaving, polyspermic Xenopus eggs. Mol Biol Cell. 2015;26:3628–3640. doi: 10.1091/mbc.E15-04-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field CM, Pelletier JF, Mitchison TJ. Xenopus extract approaches to studying microtubule organization and signaling in cytokinesis. Methods Cell Biol. 2017;137:395–435. doi: 10.1016/bs.mcb.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heald R, Tournebize R, Blank T, Sandaltzopoulos R, Becker P, Hyman A, Karsenti E. Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature. 1996;382:420–425. doi: 10.1038/382420a0. [DOI] [PubMed] [Google Scholar]
- Hyman A, Drechsel D, Kellogg D, Salser S, Sawin K, Steffen P, Wordeman L, Mitchison T. Preparation of modified tubulins. Meth Enzymol. 1991;196:478–485. doi: 10.1016/0076-6879(91)96041-o. [DOI] [PubMed] [Google Scholar]
- Ishihara K, Nguyen PA, Groen AC, Field CM, Mitchison TJ. Microtubule nucleation remote from centrosomes may explain how asters span large cells. Proc Natl Acad Sci USA. 2014a;111:17715–17722. doi: 10.1073/pnas.1418796111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara K, Nguyen PA, Groen AC, Field CM, Mitchison TJ. Microtubule nucleation remote from centrosomes may explain how asters span large cells. Proc Natl Acad Sci USA. 2014b;111:17715–17722. doi: 10.1073/pnas.1418796111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara K, Korolev KS, Mitchison TJ. Physical basis of large microtubule aster growth. Elife. 2016;5 doi: 10.7554/eLife.19145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresca TJ, Heald R. Methods for studying spindle assembly and chromosome condensation in Xenopus egg extracts. Methods Mol Biol. 2006;322:459–474. doi: 10.1007/978-1-59745-000-3_33. [DOI] [PubMed] [Google Scholar]
- Mitchison TJ, Maddox P, Groen A, Cameron L, Perlman Z, Ohi R, Desai A, Salmon ED, Kapoor TM. Bipolarization and poleward flux correlate during Xenopus extract spindle assembly. Mol Biol Cell. 2004;15:5603–5615. doi: 10.1091/mbc.E04-05-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen PA, Groen AC, Loose M, Ishihara K, Wühr M, Field CM, Mitchison TJ. Spatial organization of cytokinesis signaling reconstituted in a cell-free system. Science. 2014;346:244–247. doi: 10.1126/science.1256773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niethammer P, Kueh HY, Mitchison TJ. Spatial patterning of metabolism by mitochondria, oxygen, and energy sinks in a model cytoplasm. Curr Biol. 2008;18:586–591. doi: 10.1016/j.cub.2008.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin KE, Mitchison TJ. Mitotic spindle assembly by two different pathways in vitro. J Cell Biol. 1991;112:925–940. doi: 10.1083/jcb.112.5.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wühr M, Chen Y, Dumont S, Groen AC, Needleman DJ, Salic A, Mitchison TJ. Evidence for an upper limit to mitotic spindle length. Curr Biol. 2008;18:1256–1261. doi: 10.1016/j.cub.2008.07.092. [DOI] [PMC free article] [PubMed] [Google Scholar]


