Abstract
Chemotherapy-induced autophagy is a proposed mechanism of chemoresistance and potential therapeutic target in osteosarcoma. We evaluated heat shock protein 27 (HSP27) and autophagy-related proteins as predictors of pathologic treatment response and prognostic markers among osteosarcoma patients who received standard chemotherapy. We analyzed 394 tumor specimens (pre-treatment, post-treatment, and metastases) from 260 osteosarcoma patients by immunohistochemistry for cytoplasmic light chain 3B (LC3B)-positive puncta, sequestosome 1 (SQSTM1), high mobility group box 1 (HMGB1), and HSP27 expression. The staining percentage and intensity for each marker were scored and the extent to which marker expression was correlated with pathologic response, relapse-free survival (RFS), and overall survival (OS) was assessed. LCB3+ puncta in post-treatment primary tumors (50%) and metastases (67%) was significantly higher than in pre-treatment biopsy specimens (30%; p=0.023 and <0.001). Among 215 patients with localized osteosarcoma, both pre-treatment (multivariate hazard ratio [HR]=26.7 [95% confidence interval=1.47–484], p=0.026) and post-treatment HSP27 expression (multivariate HR=1.85 [1.03–3.33], p=0.039) were associated with worse OS. Lack of LC3B+ puncta at resection was an independent poor prognostic marker in both univariate (HR=1.78 [1.05–3.03], p=0.034) and multivariate models (HR=1.75 [1.01–3.04], p=0.045). Patients with LC3B+/HSP27− tumors at resection had the best 10-year OS (75%) whereas patients with LC3B-/HSP27+ tumors had the worst 10-year survival (25%). Neither HSP27 expression nor the presence of LCB3+ puncta was correlated with pathologic treatment response. Our findings establish HSP27 expression and LC3B+ puncta as independent prognostic markers in osteosarcoma patients receiving standard chemotherapy and support further investigation into strategies targeting HSP27 or modulating autophagy in osteosarcoma treatment.
Keywords: Osteosarcoma, biomarkers, autophagy, light chain 3B, heat shock protein 27
Introduction
Overall survival of patients with osteosarcoma who have disease relapse after standard treatment has not improved significantly in more than 30 years (1,2). Standard therapy for localized osteosarcoma consists of neoadjuvant chemotherapy with cisplatin, doxorubicin, and high-dose methotrexate (MAP chemotherapy), followed by primary tumor resection with pathologic response assessment, followed by additional MAP chemotherapy. Compared with patients who have a good pathologic treatment response to MAP chemotherapy (≥90% tumor necrosis), patients with a poor response to MAP chemotherapy (<90% tumor necrosis) have significantly worse outcomes (3,4). Attempts to improve the survival of these poor responders by modifying or intensifying postoperative MAP chemotherapy have failed (5), underscoring the need to identify patients at high risk for metastatic relapse, as such patients could instead be considered for clinical trials that combine molecular biomarkers and targeted therapies in either the neoadjuvant or adjuvant setting. Thus, the identification of novel prognostic biomarkers that can be assessed either at diagnosis or at resection following neoadjuvant chemotherapy is an unmet need in osteosarcoma.
Induction of autophagy and overexpression of HSPs are 2 proposed mechanisms of chemoresistance to MAP and therefore warrant evaluation as biomarkers and potential therapeutic targets in osteosarcoma. Autophagy is a physiologic mechanism involving cellular catabolism to maintain homeostasis and cell survival under stress. In cancer, it can play a dual role either contributing to treatment resistance, cell survival, and relapse or serving as an alternate pathway leading to cell death (6,7). Multiple preclinical studies of autophagy in osteosarcoma have focused on chemotherapy-induced autophagy as a mechanism of chemoresistance (8). The components of MAP chemotherapy—cisplatin, doxorubicin, and methotrexate—have all been shown to induce autophagy in osteosarcoma cell lines, which then promotes tumor cell survival by decreasing sensitivity to chemotherapy (9–11). A second mechanism of chemoresistance, HSPs protect cells from stress-associated injury and are overexpressed in a wide range of human malignancies (12). HSP27 expression can be induced by cytotoxic drugs and protects cells from apoptosis. Its overexpression is a biomarker of poor prognosis in multiple cancers and has been shown to predict chemotherapy resistance (12). In a small series, HSPs were shown to have prognostic relevance in osteosarcoma (13,14).
In vitro studies have shown that MAP chemotherapy upregulates the protein high mobility group box 1 (HMGB1), thereby inducing autophagy and resulting in chemoresistance in osteosarcoma, indicating the protein’s potential as a therapeutic target in osteosarcoma (15,16). HMGB1 regulates autophagy and mitophagy through multiple mechanisms; in particular, nuclear HMGB1 regulates the expression of heat shock protein 27 (HSP27, also known as HSPB1), a cell cytoskeleton regulator that plays a critical role in the intracellular trafficking necessary for autophagy (17). Loss of either HMGB1 or HSP27 can result in a similar autophagy-deficient phenotype with decreased adenosine triphosphate synthesis suggesting that HSP27 is functionally necessary to maintain cytoprotective autophagy.
Previously we demonstrated that chemotherapy-induced autophagy has a dual role in osteosarcoma in vitro, leading to either cell survival or cell death, and that the overexpression of phosphorylated HSP27 (pHSP27) in osteosarcoma cells following chemotherapy treatment is correlated with chemoresistance and the cytoprotective role of autophagy (18,19). Therefore we hypothesized that induction of autophagy following chemotherapy and overexpression of HSP27/pHSP27 would be associated with poor response to chemotherapy and inferior survival in osteosarcoma patients. The purpose of the present study was to determine the clinical significance of two proposed mechanisms of chemoresistance in osteosarcoma, autophagy and HSP27. We used immunohistochemistry to evaluate the presence of and relationship between LC3B+ puncta and HSP27 expression in a clinically annotated tissue microarray osteosarcoma. We assessed pre-treatment biopsy specimens, post-treatment primary tumor resection specimens, and resected metastasis specimens for these markers and sought to determine the extent to which they are correlated with pathologic treatment response, relapse-free survival (RFS), and OS.
Patients and Methods
Patients
The study population included 260 pediatric and adult patients with primary appendicular skeletal osteosarcomas evaluated at The University of Texas MD Anderson Cancer Center between 1985 and 2012 whose 392 tissue specimens were included on an institutional tissue microarray. In all cases, an expert sarcoma pathologist had established the diagnosis according to the World Health Organization Classification of Tumours of Soft Tissue and Bone (20). Patients with extraskeletal osteosarcoma, considered a separate entity, were excluded from the study. Clinical data were collected by a retrospective chart review. The study was approved by MD Anderson’s Institutional Review Board and was exempt from obtaining patients’ written informed consent due to the low risk nature of the study. Specifically, the study utilized de-identified archival surgical pathology material. All patient information was maintained in a separate encrypted file with restricted access. All studies were conducted in accordance with the Declaration of Helsinki.
Construction of tissue microarrays
Decalcified formalin-fixed, paraffin-embedded (FFPE) blocks of osteosarcoma tissue from biopsy specimens obtained prior to neoadjuvant chemotherapy and surgical resection specimens (resected primary tumors and resected metastases) were retrieved from MD Anderson’s institutional tumor bank. All specimens were fixed for at least 8 hours in buffered formalin-fixed and decalcified using 10% formic acid. Two bone and sarcoma pathologists reviewed slides of hematoxylin and eosin–stained sections from each FFPE block to identify representative tumor areas. Two tissue cores (0.6-mm diameter) extracted from representative tumor areas of the FFPE blocks were used to construct tissue microarrays.
Immunohistochemical studies
Slides of 4-μm-thick unstained tissue sections were prepared from the tissue microarrays of decalcified FFPE human osteosarcoma specimens. Immunohistochemical studies were performed using a Bond III/Rx autostainer (Leica Biosystems, Buffalo Grove, IL) with monoclonoal antibodies against human HSP27 (1:1000; Thermo Fischer Scientific, clone MA3-15), LC3B (1:50; NanoTools/Axorra, clone 5F10 (21)), SQSTM1 (also known as p62; 1:25,000; Progen Biotechnik, clone GP62-C), and HMGB1 (1:700; Thermo Fischer Scientific, clone PA1-1692).
Immunohistochemical staining was independently scored by two trained sarcoma pathologists (WLW and JWT), both of whom were blinded to the clinical data at the time of assessment. The immunostaining percentage (0–100%) and intensity (0, negative; 1, weak; 2, moderate; 3, strong) were evaluated. LC3B expression was evaluated using previously validated immunohistochemical methods (22) that included assessments of granular cytoplasmic or punctate staining. To evaluate the presence of autophagic flux, we quantified the intensity of SQSTM1/p62 (sequestesome 1) staining in osteosarcoma cells in relation to LC3B. Both the nuclear and cytoplasmic staining of SQSTM1 and HMGB1 were assessed. Specimens were considered to have HSP27 or LC3B expression if ≥10% of their tumor cells stained positive for the protein. In addition, cut-points of the staining percentage (≥ median vs < median) and staining intensity (negative vs weak/moderate/strong) were assessed as prognostic factors.
Statistical analysis
OS was defined as the time from the date of diagnosis to the date of death or last contact. RFS was defined as the time from the date of surgery to the date of relapse or death, whichever occurred first, or to the date of last contact. Progression-free survival (PFS) was defined as the time from the date of diagnosis to the date of progression or death, whichever occurred first, or to the date of last contact amongst patients with primary metastatic disease. The distributions of OS, RFS, and PFS were estimated by the Kaplan-Meier method (23). The log-rank test was performed to assess differences in survival between groups (24). Regression analyses based on the Cox proportional hazards regression model were conducted on OS, RFS, and PFS (25). For the biomarker analysis in resection specimens and pathologic response to neoadjuvant chemotherapy (good defined as tumor necrosis ≥90% vs poor, tumor necrosis <90% as previously described (3)), the landmark analysis method was used; the date of surgery was the starting point for defining OS and RFS (26). The stepwise method was used to build a multivariate Cox proportional hazards regression model. Functional form, proportional hazard assumption, and multicollinearity were examined. The correlation between two continuous factors was measured by Spearman correlation (27). The chi-squared test and Fisher exact test were used to determine whether proportions of patients with factors of interest differed significantly between groups (28). McNemar’s test was used to assess the association in paired specimens. Logistic regression models using generalized estimating equations (GEE) were used to compare biomarker expressions (positive vs negative) between specimens obtained prior to treatment, at resection following neoadjuvant chemotherapy, and in resected metastasis considering the intra-patient correlations in paired specimens. A two-sided p-value of <0.05 was considered statistically significant. SAS version 9.4 was used to perform all analyses.
Results
Patients
The patients’ clinical and pathologic characteristics are summarized in Table 1. Of the 260 patients included in the study, 215 (83%) had localized disease and 45 (17%) had metastatic disease at diagnosis. Most patients were diagnosed with high-grade (253; 97%), conventional osteosarcomas (205; 79%) involving either the femur (140; 54%) or tibia (43; 17%). Almost all patients (241; 93%) received neoadjuvant chemotherapy followed by resection of the primary tumor and/or metastasis; of these patients, 108 (45%) had a good pathologic response to chemotherapy, and 133 (55%) had a poor response.
Table 1.
Patients’ clinical and pathological characteristics
| Factor | No. of patients (%) |
|---|---|
| Median age at diagnosis (range) | 18 (4–90) |
| Gender | |
| Male | 153 (59) |
| Female | 107 (41) |
| Stage at presentation | |
| Localized | 215 (83) |
| Metastatic | 45 (17) |
| Primary site | |
| Femur | 140 (54) |
| Tibia | 43 (17) |
| Fibula | 10 (4) |
| Humerus | 32 (12) |
| Radius/ulna | 3 (1) |
| Mandible | 1 (0) |
| Rib/chest wall | 7 (3) |
| Pelvis/acetabulum | 18 (7) |
| Other | 5 (2) |
| Histologic subtype | |
| Osteoblastic | 110 (42) |
| Chondroblastic | 49 (19) |
| Fibroblastic | 46 (18) |
| Telangiectatic | 23 (9) |
| Dedifferentiated parosteal | 12 (5) |
| Small cell | 6 (2) |
| High grade surface | 3 (1) |
| Other high grade | 6 (2) |
| Other intermediate/low grade | 5 (2) |
| Radiation-associated osteosarcoma | |
| No | 250 (97) |
| Yes | 8 (3) |
| Grade | |
| Low | 6 (2) |
| Intermediate | 1 (0) |
| High | 253 (97) |
| Pathologic responsea | |
| Good (≥90% necrosis) | 108 (45) |
| Poor (<90% necrosis) | 133 (55) |
Pathologic response was assessed in 241 patients who received preoperative chemotherapy only.
The 215 patients with localized disease at diagnosis had a median follow-up time of 11 years. During the follow-up period, 107 of these patients had disease relapse and 105 died, including 15 patients without a documented relapse. Among the patients with relapse, 17 (16%) were alive at the time of analysis. As expected, patients with radiation-associated osteosarcoma had worse RFS as compared to those with primary osteosarcoma (hazard ratio [HR]=2.85 [95% confidence interval (CI)=1.33–6.12], p=0.007). Higher age at diagnosis (HR=1.02 [1.00–1.03], p=0.0063) and radiation-associated osteosarcoma (HR=3.76 [1.74–8.12], p=0.001) were significantly associated with worse OS in the univariate model; these factors remained significant in the multivariate analysis (p<0.03 for all; Table 2). In the landmark analysis, patients with poor pathologic response to neoadjuvant chemotherapy had a trend towards worse OS (HR=1.40 [0.94–2.07], p=0.097).
Table 2.
Univariate and multivariate analysis for factors associated with overall survival in patients with localized osteosarcoma
| Factor | No. of deaths | Total no. of patients | Univariate analysis
|
Multivariate analysisa
|
||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |||
| Age at diagnosis, years | 105 | 215 | 1.02 (1.00, 1.03) | 0.0063 | 1.01 (1.00, 1.03) | 0.0294 |
| Sex | ||||||
| Female | 40 | 89 | Ref | |||
| Male | 65 | 126 | 1.11 (0.75, 1.64) | 0.615 | ||
| Radiation-associated osteosarcoma | ||||||
| No | 98 | 207 | Ref | Ref | ||
| Yes | 7 | 8 | 3.76 (1.74, 8.12) | 0.001 | 3.08 (1.38, 6.86) | 0.0061 |
| Primary site | ||||||
| Others | 48 | 103 | Ref | |||
| Femur | 57 | 112 | 1.10 (0.76, 1.65) | 0.556 | ||
| Grade | ||||||
| Low | 1 | 6 | Ref | |||
| High/Intermediate | 104 | 209 | 4.23 (0.59, 30.4) | 0.152 | ||
| Preoperative chemotherapy | ||||||
| No | 2 | 7 | Ref | |||
| Yes | 103 | 208 | 1.49 (0.37, 6.05) | 0.577 | ||
| Pathologic response b | ||||||
| Good | 43 | 92 | Ref | |||
| Poor | 59 | 113 | 1.40 (0.94, 2.07) | 0.097 | ||
| Pre-treatment biomarkers | ||||||
| LC3B+ | ||||||
| ≥10% | 3 | 16 | Ref | Ref | ||
| <10% | 9 | 34 | 1.38 (0.37, 5.12) | 0.631 | 1.40 (0.34, 5.82) | 0.646 |
| LC3B percent | ||||||
| >Median | 3 | 16 | Ref | Ref | ||
| ≤Median | 9 | 34 | 1.38 (0.37, 5.12) | 0.631 | 1.40 (0.34, 5.82) | 0.646 |
| HSP27+ | ||||||
| <10% | 1 | 8 | Ref | Ref | ||
| ≥10% | 16 | 42 | 3.78 (0.50, 28.6) | 0.198 | 26.7 (1.47, 484) | 0.0263 |
| Resection biomarkers | ||||||
| LC3B+ | ||||||
| ≥10% | 22 | 53 | Ref | Ref | ||
| <10% | 36 | 54 | 1.78 (1.05, 3.03) | 0.0337 | 1.75 (1.01, 3.04) | 0.0448 |
| LC3B percent | ||||||
| >Median | 18 | 48 | Ref | Ref | ||
| ≤Median | 40 | 59 | 2.12 (1.21, 3.70) | 0.0082 | 2.15 (1.21, 3.81) | 0.0089 |
| HSP27+ | ||||||
| <10% | 18 | 47 | Ref | Ref | ||
| ≥10% | 35 | 56 | 2.00 (1.12, 3.56) | 0.0197 | 1.85 (1.03, 3.33) | 0.0395 |
Abbreviations: HR, hazard ratio; CI, confidence interval.
Adjusted for age and radiation-associated osteosarcoma.
Landmark analysis.
The 45 patients with metastatic disease at diagnosis had a median follow-up time of 10.6 years. During the follow-up period, 32 of these patients (71%) died. The median OS duration was 2.47 years (95% CI, 1.70–3.51 years). Patients with poor response to neoadjuvant chemotherapy had worse PFS (HR=2.40 [1.11–5.22], p=0.027). While a failure to receive preoperative chemotherapy was associated with inferior OS in this subset, no other clinical characteristics were significantly associated with OS in the univariate analysis (Supplemental Table S1).
LC3B and HSP27 expression
The tissue microarray included 392 osteosarcoma specimens consisting of 114 pre-treatment biopsy specimens, 184 primary tumor resection specimens, and 94 metastasis resection specimens, which included 26 synchronous metastasis specimens (28%) and 66 metachronous metastasis specimens (72%). These specimens’ biomarker expression profiles are given in Table 3. Due to the fragility of specimens following the decalcification and staining process, not all specimens were evaluable for all biomarkers analyzed.
Table 3.
HSP27 and LC3B expression in osteosarcoma specimens
| No. of specimens (%) | ||||
|---|---|---|---|---|
|
| ||||
| Factor | All | Pre-treatment biopsy | Primary tumor resection | Metastasis resection |
| HSP27 intensity | ||||
| None | 41 (16) | 9 (16) | 28 (21) | 4 (6) |
| Weak | 144 (57) | 28 (48) | 74 (56) | 42 (67) |
| Moderate | 38 (15) | 16 (28) | 13 (10) | 9 (14) |
| Strong | 29 (12) | 5 (9) | 16 (12) | 8 (13) |
| HSP27 percent | ||||
| <10% | 85 (34) | 9 (15) | 63 (48) | 13 (21) |
| ≥10% | 168 (66) | 50 (85) | 68 (52) | 50 (79) |
| LC3B intensity | ||||
| None | 122 (47) | 38 (66) | 64 (47) | 20 (30) |
| Weak | 82 (31) | 7 (12) | 43 (31) | 32 (48) |
| Moderate | 54 (21) | 11 (19) | 29 (21) | 14 (21) |
| Strong | 3 (1) | 2 (3) | 1 (1) | |
| LC3B percent | ||||
| <10% | 128 (49) | 38 (66) | 68 (50) | 22 (33) |
| ≥10% | 133 (51) | 20 (34) | 69 (50) | 44 (67) |
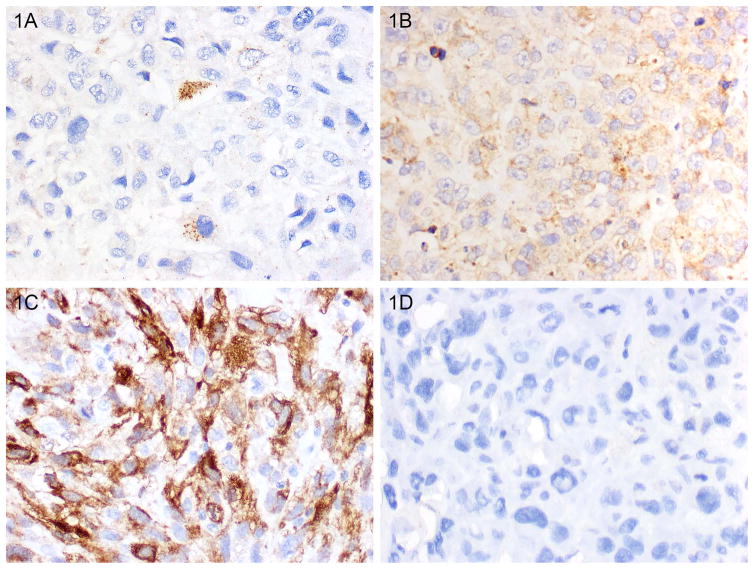
The percentages of osteosarcoma cells with clearly visible cytoplasmic LC3B+ puncta were quantified, and specimens with ≥10% of osteosarcoma cells with LC3B+ puncta were considered positive for LCB3 expression based on established cutoffs in other tumor types (29). The rates of LCB3 expression of the primary tumor resection specimens and metastasis resection specimens were significantly higher than that of pre-treatment biopsy specimens (50% and 67% respectively vs 34%, p=0.023 and p<0.001, GEE). The intensity of cytoplasmic LC3B staining was heterogeneous as well. A higher proportion of pre-treatment biopsy specimens were graded as 0 or negative (66%) as compared to resection (47%) or metastasis (30%, Table 3). Examples of LC3B staining are shown in Figure 1A–B.
Figure 1.
Representative LC3B and HSP27 labeling. Variable tumoral labeling and intensity was seen (see Table 2). Illustrated are some examples of staining patterns seen. Tumor with A. scattered cytoplasmic and punctate LC3B labeling, B. diffuse cytoplasmic and punctate LC3B labeling, C. Positive HSP27 labeling, and D. Negative HSP27 labeling. (Magnification 400x.)
Paired pre-treatment biopsy and post-treatment primary tumor resection specimens from 18 patients were assessed for the presence of LC3B+ puncta. Of the 9 patients whose biopsy specimens were negative for LC3B+ puncta, 5 (56%) had resection specimens positive for the autophagy marker. Conversely, of the 9 patients whose biopsy specimens were positive for LC3B+ puncta, 8 (89%) had resection specimens positive for the marker (p=n.s, supplemental Table S2). Similarly, of 6 patients without LC3B+ puncta in pre-treatment specimens, 3 subsequently had LC3B+ puncta in their metachronous metastasis specimens. Paired primary tumor resection and metastasis resection specimens were available for 26 patients. Among the 13 patients with primary tumors without LC3B+ puncta at resection, 9 (69%) had LC3B+ puncta in metachronous metastasis specimens (p=n.s, supplemental Table S2).
Assessment of the osteosarcoma specimens’ nuclear and cytoplasmic expressions of SQSTM1 showed limited staining with the majority of specimens negative for any expression. Only a few specimens demonstrated predominantly cytoplasmic labeling, whereas others demonstrated both nuclear and cytoplasmic labeling (Supplemental Figure S1A–B). SQSTM1 intensity (both nuclear and cytoplasmic) was higher in post-treatment resection and metastasis specimens as compared to pre-treatment, with 38% of metastasis demonstrating moderate or strong cytoplasmic staining (Supplemental Table S3). There was no statistically significant difference in SQSTM1 cytoplasmic intensity amongst LC3B+ vs LC3B− tumor specimens (p=0.083).
Evaluation of HMGB1 revealed predominant nuclear HGMB1 staining, with 41% of specimens showing moderate or strong staining intensity; a few specimens had both nuclear and cytoplasmic HGMB1 staining (Supplemental Figure S1C). Given a cutoff of >50% of tumor cells with nuclear HMGB1 expression, 55% of all specimens were considered positive for HMGB1. The proportions of HMGB1+ specimens did not differ significantly between pre-treatment and resection specimens (43% vs 52%, p=0.375, GEE) but was significantly higher in osteosarcoma metastasis as compared to pre-treatment (43% vs 66%, p=0.048, GEE) (Supplemental Table S3). Further, nuclear HMGB1 expression was not correlated with LC3B expression in pre-treatment specimens (Spearman correlation −0.091, p=0.73) or post-treatment primary resection specimens (Spearman correlation 0.092, p=0.323).
The percent and intensity of HSP27 expression varied, with some specimens having strong diffuse HSP27 expression in tumor cells and others having no appreciable HSP27 expression (Figure 1C–D). Two-thirds of all specimens were positive for HSP27 expression. The proportions of HSP27+ pre-treatment biopsy specimens and metastasis resection specimens were significantly higher than that of HSP27+ primary tumor resection specimens (85% and 79% respectively vs 52%, p<0.001 and p<0.001, GEE). The intensity of HSP27 staining varied across groups. HSP27 expression was not correlated with LC3B expression in pre-treatment biopsy specimens (Spearman correlation 0.006, p=0.945).
Prognostic significance of LC3B+ puncta and HSP27 expression in patients with localized osteosarcoma
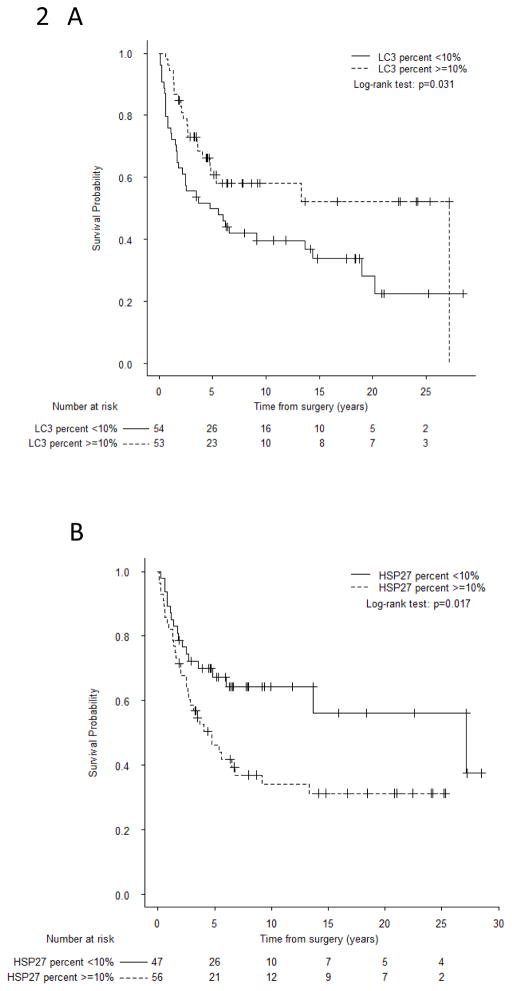
The presence of LC3B+ puncta in pre-treatment biopsy specimens was not associated with RFS or OS. Patients with localized osteosarcoma and LC3B+ puncta at resection (50% of cases) had better OS (p=0.031; Figure 2A). The lack of LCB3+ puncta at resection was associated with worse RFS in the univariate analysis (HR=1.81 [1.10–2.98], p=0.019; log-rank p=0.017, Supplemental Figure S2) but only maintained borderline significance in the multivariate analysis adjusted for radiation-associated disease (HR=1.65 [0.99–2.75], p=0.053). The lack of LC3B+ puncta in primary tumor resection specimens was significantly associated with worse OS in both the univariate analysis (HR=1.78 [1.05–3.03], p=0.034) and multivariate analysis adjusted for age and radiation-associated disease (HR=1.75 [1.01–3.04], p=0.045; Table 2). The use of LC3B staining intensity in primary tumor resection specimens for risk stratification of patients with localized disease was also investigated; negative expression vs weak/moderate/strong expression showed the greatest significance in stratifying risk groups (HR=1.78 [1.05–3.00], p=0.032). Taken together, our findings suggest that the presence of LC3B+ puncta is an independent prognostic biomarker of improved survival following neoadjuvant chemotherapy.
Figure 2.
Overall survival of patients with localized osteosarcoma based on LC3B+ puncta status or HSP27 expression status at resection. A. Stratified by cytoplasmic LC3B+ puncta status (≥10% [positive] vs < 10% [negative]). The presence of LC3B+ puncta at resection was associated with better OS. B. Stratified by HSP27 expression status (≥10% [positive] vs <10% [negative]). HSP27 expression was associated with worse OS. P values were calculated with a log-rank test.
The prognostic significance of nuclear HMGB1 expression in pre-treatment biopsy and primary tumor resection specimens was evaluated. Neither HMGB1 staining intensity nor HMGB1 expression (>50% nuclear expression) held prognostic significance in relation to RFS or OS at either time point. Similarly, the combination of LC3B+ puncta and nuclear HMGB1 expression was not associated with clinical outcomes.
Most pre-treatment osteosarcoma specimens had HSP27 expression. Although HSP27 expression in pre-treatment specimens was not a significant predictor of RFS or OS in the univariate analysis, in the multivariate analysis, it was associated with worse RFS (HR=12.5 [1.34–116], p=0.027) and OS (HR=26.7 [1.47–484], p=0.026; Table 2). Patients with HSP27+ osteosarcoma at resection had a significantly shorter OS duration than patients whose tumor lacked HSP27 expression (Figure 2B, p=0.017). In both the univariate and multivariate models, HSP27 expression in resection specimens was associated with worse RFS (univariate: HR=1.92 [1.13–3.28], p=0.016; multivariate: HR=1.76 [1.03–3.03], p=0.040; log-rank p=0.014, Supplemental Figure S3) as well as worse OS (univariate: HR=2.00 [1.12–3.56], p=0.020; multivariate: HR=1.85 [1.03–3.33], p=0.040; Table 2).
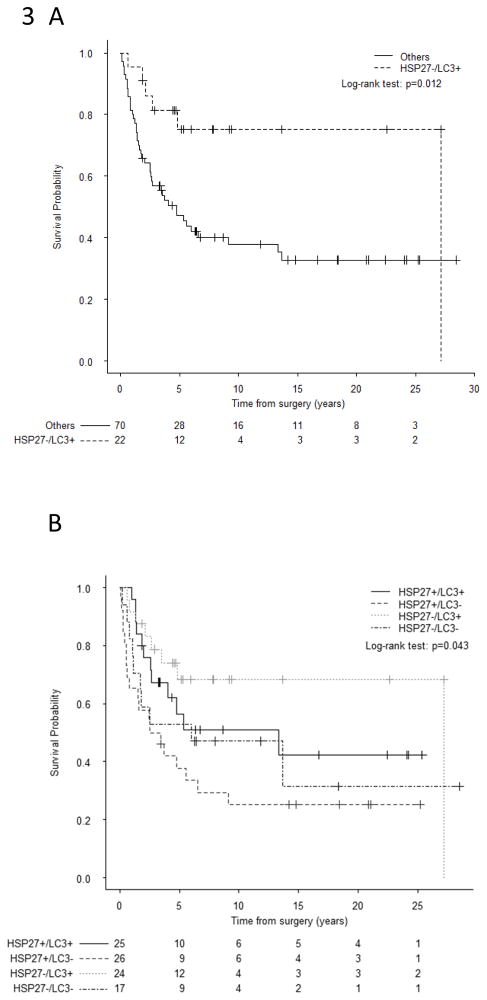
The combined assessment of HSP27 expression and LC3B+ puncta was evaluated for further risk stratification among patients with localized disease. In pre-treatment biopsy specimens from 36 evaluable patients, the HSP27+/LC3B− combination was associated with a trend towards worse OS (p=0.087). Among the 92 patients with localized disease for whom resection specimens with HSP27 and LC3B biomarker data were available, those with HSP27−/LC3B+ tumors had significantly better OS as compared to all others (p=0.012; Figure 3A). Among these 92 patients, those with HSP27−/LC3B+ tumors had favorable survival outcomes; those with HSP27+/LC3B+ or HSP27−/LC3B− tumors were at an intermediate risk of death; and those with HSP27+/LC3B− tumors had the worst survival outcomes (p=0.017, Figure 3B). The estimated 10-year OS rates for these groups were 75.4%, 55.4%, 37.2%, and 25%, respectively.
Figure 3.
Overall survival of patients with localized osteosarcoma based on analysis of the combination of LC3B+ puncta and HSP27 expression statuses at resection. A. Patients with LC3B+ puncta and negative HSP27 expression vs all other groups. B. Groups stratified according to the presence or absence of both HSP27 expression and LC3B+ puncta. The combination of negative HSP27 expression and the presence of LC3B+ puncta was associated with favorable OS. P values were calculated with a log-rank test.
Pathologic treatment response assessed by percent tumor necrosis following neoadjuvant chemotherapy is the most well established prognostic marker in patients with localized osteosarcoma. Neither the percentage of LC3B expression nor that of HSP27 expression in pre-treatment specimens was correlated with percent tumor necrosis following neoadjuvant chemotherapy (Spearman correlation, −0.12 and −0.063, respectively; p=0.384 and 0.655, respectively). Further, neither the presence of LC3B+ puncta nor HSP27 expression in pre-treatment specimens was associated with good pathologic response (p=0.391 and 1.00, respectively; Supplemental Table S4). In addition, neither marker in resection specimens was associated with good pathologic response (p=0.479 for LC3B+ and p=0.847 for HSP27). In a subset of patients with localized osteosarcoma that had poor response to neoadjuvant chemotherapy, the lack of LC3B+ puncta was associated with a trend towards worse OS (log-rank p=0.062; Cox HR=1.87 [0.96–3.64], p=0.067; Supplemental Figure S4).
Discussion
LC3B+ puncta, a surrogate marker of autophagy, and HSP27 have both been shown to have prognostic significance in various tumor types. Although preclinical data have suggested that autophagy and HSP27 are potential therapeutic targets in osteosarcoma, no studies have evaluated these markers in relation to RFS, OS, or treatment response. Here we show that both cytoplasmic LC3B+ puncta and HSP27 expression are relevant prognostic biomarkers in patients with localized osteosarcoma in the largest series to date. Patients whose tumors lack LC3B+ puncta at resection following neoadjuvant chemotherapy have a poor prognosis, as do patients whose tumors express HSP27 at resection. By combining these 2 markers at resection, we were able to identify a favorable risk group (HSP27−/LC3B+) and those with particularly high risk of death (HSP27+/LC3B−) when treated with standard therapy. As such, these markers may be valuable in risk stratification.
Several studies have examined the role of chemotherapy-induced autophagy in osteosarcoma cell lines. The majority of this preclinical data suggest that chemotherapy-induced autophagy is a mechanism of chemoresistance to standard MAP chemotherapy in osteosarcoma. Autophagy inhibition either via the knockdown of key autophagy genes such as the Beclin-1 gene, BECN1 (30), or pharmacologic inhibition with compounds such as 3-methyladenine (11), bafilomycin A1 (31), or chloroquine (10) results in a decrease in proliferation and an increase in apoptosis of osteosarcoma cells treated with doxorubicin or cisplatin. Conversely, Holloman et al found that autophagy-inhibition via ATG5 knockdown could have opposing effects and resulted in decreased chemotherapy-induced cell death in DLM8 cells (18). In addition, agents that enhance autophagy such as the cyclin-dependent kinase inhibitor roscovitine have shown synergistic cytotoxicity when combined with doxorubicin in vitro suggesting that autophagy may serve as an alternate cell death pathway in osteosarcoma (32). Given this duality, it is prudent to analyze the presence and prognostic significance of autophagy markers such as LC3B+ puncta, SQSTM1, and HMGB1 expression in osteosarcoma patients.
In our study, a limited number of patients’ pre-treatment specimens exhibited LC3B+ puncta suggestive of basal autophagy. However, the presence of LC3B+ puncta prior to treatment was not associated with RFS, OS, or pathologic response to neoadjuvant chemotherapy. Overall a significantly higher proportion of post-treatment specimens had LC3B+ puncta and in the limited subset of patients with paired samples, the majority of patients who were initially negative for LC3B+ puncta at diagnosis became positive for the marker at resection following chemotherapy (although this finding was not statistically significant). The presence of LC3B+ puncta at resection was associated with improved overall survival but did not correlate with pathologic treatment response. Taken together these findings suggest that LC3B+ puncta may be induced by standard chemotherapy in osteosarcoma and that the presence of LC3B+ puncta is an independent prognostic biomarker rather than a surrogate marker of pathologic treatment response. As there are multiple pathways to achieve autophagy, additional studies are needed to confirm the mechanistic significance of these findings.
The presence of LC3B puncta following chemotherapy was a positive prognostic marker in our series of osteosarcoma. A similar association has been shown in a large series of breast cancer patients where the presence of cytoplasmic LC3B was a favorable prognostic marker (29). Using the same methodology and cutoff (>10%) used in the present study, Laoire et al. found that the combination of LC3B+ puncta and nuclear HMGB1 expression in tumor cells was associated with prolonged metastasis-free and disease-specific survival in breast cancer patients. They also showed that the presence of LC3B+ puncta was correlated with a reduction in SQSTM1 expression, suggesting an increase in autophagic flux. In the current study, most osteosarcoma specimens had no or weak SQSTM1 expression. Neither nuclear HMGB1 expression alone or in combination with LC3B+ puncta was prognostically relevant.
The findings of our study are in agreement with those of prior studies showing that HSP27 overexpression in pre-treatment biopsy specimens is an independent factor for poor prognosis (14,33). In the prior two small series, the proportions of HSP27+ biopsy specimens (22% and 24%) and resection specimens (33% and 37%) were much lower than those in the present study (85% and 52%, respectively). Further, whereas one of these series found an association between HSP27 expression in resection specimens and poor response among 19 patients, we found that HSP27 expression was not correlated with pathologic treatment response. Our findings, in conjunction with prior preclinical studies (34–36), support further investigation into targeting HSP27 in osteosarcoma treatment.
Finally, we investigated the combination of HSP27 and LC3B on the basis of preclinical data suggesting an association among HSP27, HMGB1, and autophagy. Loss of HSP27 has been shown to decrease autophagy, and phosphorylation of HSP27 is required for the protein’s function as a cytoskeleton regulator in mitophagy and autophagy (17). In osteosarcoma cell lines, post-treatment pHSP27 overexpression is associated with a cytoprotective role of autophagy and chemoresistance. In the current study, we attempted to assess pHSP27 expression in osteosarcoma specimens; however, staining results were limited, possibly due to decalcification (representative pHSP27 labeling is available in supplemental figure S5; summary of pHSP27 expression supplemental table S5). We found no correlation between the presence of LC3B+ puncta and total HSP27 expression in osteosarcoma specimens.
HSP27 and LC3B can be considered as independent biomarkers in osteosarcoma; analyzed together, they enable improved risk stratification for patients with localized osteosarcoma following neoadjuvant chemotherapy. One of the limitations of this approach, however, is awaiting evaluation of these markers at resection following approximately 12 weeks of therapy. Unlike tumor necrosis which relies on adequate sampling and mapping, the assessment of these biomarkers may be easier to implement reliably and accurately in the clinic. Predictive biomarkers of response to conventional MAP chemotherapy are still needed. A prospective analysis of paired specimens may be beneficial in validating HSP27 and LC3B+ puncta as prognostic—and potentially predictive—biomarkers in osteosarcoma and would support further investigation into HSP27-targeted therapies or adding agents that promote autophagy to standard chemotherapy in high-risk osteosarcoma patients.
Conclusions
The presence of the autophagy-associated protein LCB+ puncta in osteosarcoma tumor cells following standard chemotherapy is associated with favorable outcomes. Conversely, HSP27 expression at diagnosis or after neoadjuvant chemotherapy is a negative prognostic marker in osteosarcoma. Patients whose tumors lack LC3B+ puncta and express HSP27 following neoadjuvant chemotherapy have a particularly high risk for disease relapse and death. These findings establish HSP27 and LC3B+ puncta as prognostic biomarkers in osteosarcoma and serve as a rationale for future studies examining the underlying mechanisms and potential clinical applications of targeting HSP27 and/or modulating autophagy in osteosarcoma treatment.
Supplementary Material
Acknowledgments
J.A. Livingston is supported by a Paul Calabresi Career Development Award for Clinical Oncology (K12 CA088084) and the 2016 Conquer Cancer Foundation QuadW Young Investigator Award in memory of Willie Tichenor. C.H. Leung and H.Y. Lin are supported in part by the Cancer Center Support Grant (NCI Grant P30 CA016672). The authors are also grateful to the financial support of the Triumph Over Kid Cancer Foundation and acknowledge the assistance of the Department of Scientific Publications in scientific editing and manuscript preparation.
Footnotes
Conflict of Interest: The authors have no conflicts of interest to declare.
References
- 1.Allison DC, Carney SC, Ahlmann ER, Hendifar A, Chawla S, Fedenko A, et al. A meta-analysis of osteosarcoma outcomes in the modern medical era. Sarcoma. 2012 doi: 10.1155/2012/704872. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004. Cancer. 2009;115(7):1531–43. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raymond A, Chawla S, Carrasco C, Ayala A, Fanning C, Grice B, et al. Osteosarcoma chemotherapy effect: a prognostic factor. 1987:212–36. [PubMed] [Google Scholar]
- 4.Rosen G, Caparros B, Huvos A, Kosloff C, Nirenberg A, Cacavio A, et al. Preoperative chemotherapy for osteogenic sarcoma. Cancer. 1982;49(122):1. doi: 10.1002/1097-0142(19820315)49:6<1221::aid-cncr2820490625>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 5.Marina NM, Smeland S, Bielack SS, Bernstein M, Jovic G, Krailo MD, et al. Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high-grade osteosarcoma (EURAMOS-1): an open-label, international, randomised controlled trial. The Lancet Oncology. 2016;17(10):1396–408. doi: 10.1016/S1470-2045(16)30214-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisenberg-Lerner A, Kimchi A. The paradox of autophagy and its implication in cancer etiology and therapy. Apoptosis : an international journal on programmed cell death. 2009;14(4):376–91. doi: 10.1007/s10495-008-0307-5. [DOI] [PubMed] [Google Scholar]
- 7.Kimmelman AC. The dynamic nature of autophagy in cancer. Genes & development. 2011;25(19):1999–2010. doi: 10.1101/gad.17558811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’farrill JGN. Autophagy in Osteosarcoma. In: Kleinerman ES, editor. Current Advances in Osteosarcoma. Springer; 2014. [Google Scholar]
- 9.Zhao D, Yuan H, Yi F, Meng C, Zhu Q. Autophagy prevents doxorubicin-induced apoptosis in osteosarcoma. Molecular medicine reports. 2014;9(5):1975–81. doi: 10.3892/mmr.2014.2055. [DOI] [PubMed] [Google Scholar]
- 10.Shen C, Wang W, Tao L, Liu B, Yang Z, Tao H. Chloroquine blocks the autophagic process in cisplatin-resistant osteosarcoma cells by regulating the expression of p62/SQSTM1. International journal of molecular medicine. 2013;32(2):448–56. doi: 10.3892/ijmm.2013.1399. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Z, Shao Z, Xiong L, Yang S. Inhibition of autophagy enhances cisplatin-induced apoptosis in the MG63 human osteosarcoma cell line. Oncology letters. 2015;10(5):2941–6. doi: 10.3892/ol.2015.3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell stress & chaperones. 2005;10(2):86. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moon A, Bacchini P, Bertoni F, Olvi LG, Santini-Arawo E, Kim YW, et al. Expression of heat shock proteins in osteosarcomas. Pathology. 2010;42(5):421–5. doi: 10.3109/00313025.2010.493866. [DOI] [PubMed] [Google Scholar]
- 14.Uozaki H, Horiuchi H, Ishida T, Iijima T, Imamura T, Machinami R. Overexpression of resistance-related proteins (metallothioneins, glutathione-S-transferase π, heat shock protein 27, and lung resistance-related protein) in osteosarcoma. Cancer. 1997;79(12):2336–44. doi: 10.1002/(sici)1097-0142(19970615)79:12<2336::aid-cncr7>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 15.Huang J, Ni J, Liu K, Yu Y, Xie M, Kang R, et al. HMGB1 promotes drug resistance in osteosarcoma. Cancer research. 2012;72(1):230–8. doi: 10.1158/0008-5472.can-11-2001. [DOI] [PubMed] [Google Scholar]
- 16.Huang J, Liu K, Yu Y, Xie M, Kang R, Vernon P, et al. Targeting HMGB1-mediated autophagy as a novel therapeutic strategy for osteosarcoma. Autophagy. 2012;8(2):275–7. doi: 10.4161/auto.8.2.18940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang R, Livesey KM, Zeh I, Herbert J, Loze MT, Tang D. Metabolic regulation by HMGB1-mediated autophagy and mitophagy. Autophagy. 2011;7(10):1256–8. doi: 10.4161/auto.7.10.16753. [DOI] [PubMed] [Google Scholar]
- 18.Hollomon MG, Gordon N, Santiago-O’Farrill JM, Kleinerman ES. Knockdown of autophagy-related protein 5, ATG5, decreases oxidative stress and has an opposing effect on camptothecin-induced cytotoxicity in osteosarcoma cells. BMC cancer. 2013;13(1):500. doi: 10.1186/1471-2407-13-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santiago-O’Farrill JM, Kleinerman ES, Hollomon MG, Livingston JA, Wang WL, Gordon N. Phosphorylated heat shock protein 27 as a potential biomarker to predict the role of chemotherapy-induced autophagy in osteosarcoma response to therapy. Oncotarget. doi: 10.18632/oncotarget.20308. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doyle LA. Sarcoma classification: an update based on the 2013 World Health Organization Classification of Tumors of Soft Tissue and Bone. Cancer. 2014;120(12):1763–74. doi: 10.1002/cncr.28657. [DOI] [PubMed] [Google Scholar]
- 21.Gupta A, Roy S, Lazar AJ, Wang W-L, McAuliffe JC, Reynoso D, et al. Autophagy inhibition and antimalarials promote cell death in gastrointestinal stromal tumor (GIST) Proceedings of the National Academy of Sciences. 2010;107(32):14333–8. doi: 10.1073/pnas.1000248107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ladoire S, Chaba K, Martins I, Sukkurwala AQ, Adjemian S, Michaud M, et al. Immunohistochemical detection of cytoplasmic LC3 puncta in human cancer specimens. Autophagy. 2012;8(8):1175–84. doi: 10.4161/auto.20353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American statistical association. 1958;53(282):457–81. [Google Scholar]
- 24.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer chemotherapy reports Part 1. 1966;50(3):163–70. [PubMed] [Google Scholar]
- 25.Cox D. Regression Models and Life-Tables (with discussion) Journal of the Royal Statistical Society, Series B. 1972:34. [Google Scholar]
- 26.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. Journal of Clinical Oncology. 1983;1(11):710–9. doi: 10.1200/JCO.1983.1.11.710. [DOI] [PubMed] [Google Scholar]
- 27.Spearman C. The proof and measurement of association between two things. The American journal of psychology. 1904;15(1):72–101. [PubMed] [Google Scholar]
- 28.Woolson RF, Clarke WR. Statistical methods for the analysis of biomedical data. John Wiley & Sons; 2011. [Google Scholar]
- 29.Ladoire S, Penault-Llorca F, Senovilla L, Dalban C, Enot D, Locher C, et al. Combined evaluation of LC3B puncta and HMGB1 expression predicts residual risk of relapse after adjuvant chemotherapy in breast cancer. Autophagy. 2015;11(10):1878–90. doi: 10.1080/15548627.2015.1082022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang W, Li Q, Song C, Lao L. Knockdown of autophagy-related protein 6, Beclin-1, decreases cell growth, invasion, and metastasis and has a positive effect on chemotherapy-induced cytotoxicity in osteosarcoma cells. Tumor Biology. 2015;36(4):2531–9. doi: 10.1007/s13277-014-2868-y. [DOI] [PubMed] [Google Scholar]
- 31.Xie Z, Xie Y, Xu Y, Zhou H, Xu W, Dong Q. Bafilomycin A1 inhibits autophagy and induces apoptosis in MG63 osteosarcoma cells. Molecular medicine reports. 2014;10(2):1103–7. doi: 10.3892/mmr.2014.2281. [DOI] [PubMed] [Google Scholar]
- 32.Lambert LA, Qiao N, Hunt KK, Lambert DH, Mills GB, Meijer L, et al. Autophagy: a novel mechanism of synergistic cytotoxicity between doxorubicin and roscovitine in a sarcoma model. Cancer research. 2008;68(19):7966–74. doi: 10.1158/0008-5472.CAN-08-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uozaki H, Ishida T, Kakiuchi C, Horiuchi H, Gotoh T, Iijima T, et al. Expression of heat shock proteins in osteosarcoma and its relationship to prognosis. Pathology-Research and Practice. 2000;196(10):665–73. doi: 10.1016/S0344-0338(00)80118-1. [DOI] [PubMed] [Google Scholar]
- 34.Rondeaux P, Galand P, Horman S, Mairesse N. Effects of antisense hsp 27 gene expression in osteosarcoma cells. In Vitro Cellular & Developmental Biology-Animal. 1997;33(9):655–8. doi: 10.1007/s11626-997-0117-z. [DOI] [PubMed] [Google Scholar]
- 35.Morii T, Ohtsuka K, Ohnishi H, Mochizuki K, Satomi K. Inhibition of heat-shock protein 27 expression eliminates drug resistance of osteosarcoma to zoledronic acid. Anticancer research. 2010;30(9):3565–71. [PubMed] [Google Scholar]
- 36.Xie X-b, Yin J-q, Wen L-l, Gao Z-h, Zou C-y, Wang J, et al. Critical role of heat shock protein 27 in bufalin-induced apoptosis in human osteosarcomas: a proteomic-based research. PloS one. 2012;7(10):e47375. doi: 10.1371/journal.pone.0047375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.